Reduced coronary flow reserve (CFR), reflecting coronary microvascular dysfunction, is common in heart failure with preserved ejection fraction (HFpEF)1 and can be caused by a number of factors including extrinsic compression due to myocardial interstitial fibrosis, myocardial hypertrophy with insufficient microvascular supply, diffuse atherosclerosis, coronary microvascular inflammation, and elevated left ventricular (LV) filling pressures. Circulating biomarker correlates of CFR may provide important insights into underlying mechanisms of coronary microvascular dysfunction in HFpEF.
We aimed to uncover a biomarker profile specific to reduced CFR in HFpEF, independent of myocardial hypertrophy, elevated cardiac filling pressures, atrial fibrillation, and epicardial coronary artery disease (CAD). Among 192 with blood samples available of the original 202 patients with a validated diagnosis of HFpEF (LVEF≥40% and without unrevascularized epicardial CAD) in the prospective multinational PROMIS-HFpEF study,1 we measured 265 biomarkers using high-throughput proximity extension assays (Olink Proseek® Multiplex CVD II, III and inflammation 96×96 kits). We excluded 23 biomarkers which had more than 15% of values below the detection limit, leaving us with a total of 242 unique biomarkers for further analyses. CFR was measured with adenosine stress transthoracic Doppler echocardiography and coronary microvascular dysfunction was defined as CFR<2.5. To assess the association between CFR and biomarkers, we used LASSO penalized regression analyses including all biomarkers, age, sex, body mass index, creatinine and study site. Multivariable regression analyses were performed to determine whether associations between biomarkers and CFR (as a continuous variable) were independent of smoking, LV mass index (cardiac hypertrophy), E/e’ (elevated LV filling pressures), and history of atrial fibrillation, revascularized CAD and hypertension. Protein-protein interaction networks were generated using the Genemania plugin in Cytoscape version 3.6.1. Pathway over-representation analyses used data from the gene ontology network with a false discovery rate-controlled p-value of <0.05 and the 242 biomarkers plus additional markers identified by Genemania as background. All analyses were performed using R, version 3.4.0. A 2-sided P-value of <0.05 was considered statistically significant, with correction for multiple testing by the Benjamini-Hochberg method. All study participants gave written informed consent, and the institutional review board at each of the participating sites approved the study. The PROMIS study complies with the Declaration of Helsinki.
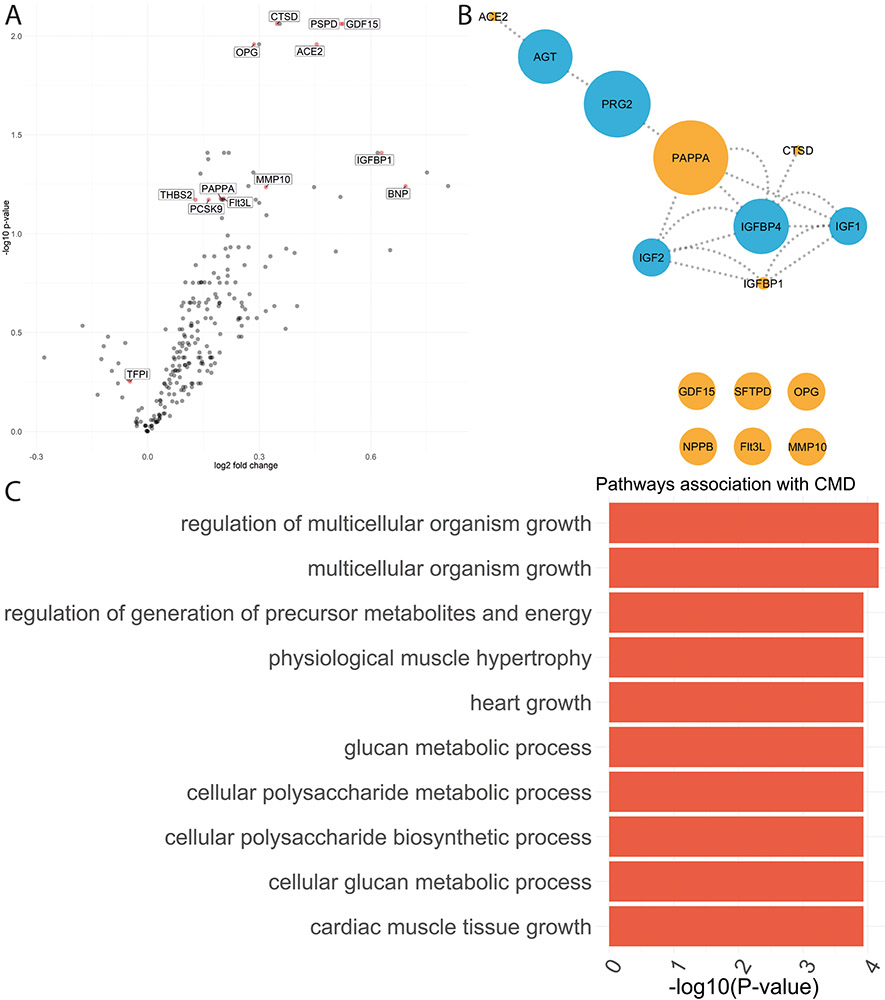
In total, 13 biomarkers were selected for their association with CFR following LASSO regression analysis (penalized beta greater than 0, figure A), of which growth differentiation factor 15 (GDF15), osteoprotegerin (OPG), pulmonary surfactant-associated protein (PSPD), angiotensin converting enzyme 2 (ACE2) and insulin growth factor binding protein 1 (IGFBP1) showed the strongest associations. Two of these, tissue factor pathway inhibitor (TFPI) (P-value =0.18) and PCSK9 (P-value =0.20), lost significance in multivariable regression analyses. Of the 11 remaining biomarkers, 5 (in orange) were hits in our network (Figure B), which was then enriched with additional protein-protein interactions (blue). PAPP-A formed an important hub in the network, suggesting greater biological importance, with similar results for PAPP-A when restricting the analyses to patients with LVEF ≥50%. When translating the network into biological pathways, those relating to cellular metabolism and physiological muscle hypertrophy were over-represented (Figure C). Results were unchanged when excluding biomarkers with either 10% or 20% of measurements below the limit of detection.
Figure:
(A) Volcano plot showing biomarkers selected by LASSO penalized regression analyses (red). The Y-axis shows the -Log10 of the false discovery rate corrected p-value for the association of each individual biomarker with coronary microvascular dysfunction (coronary flow reserve <2.5) versus no coronary microvascular dysfunction. The X axis shows the log2 of the fold change of biomarker difference between patients with and without coronary microvascular dysfunction. (B) Results of network analysis for the biomarkers independently associated with coronary microvascular flow reserve (orange) and those predicted in the network (blue). The size of the node reflects the edge betweenness of each biomarker. (C) Bar graph depicting the top 10 overrepresented pathways with the -log10 of the false discovery rate corrected P-value on the X-axis
PAPP-A emerged as a novel key hub in the network associated with reduced CFR in HFpEF, and has been shown to be elevated in patients with unstable atherosclerosis as well as a predictor of cardiovascular events in patients with acute coronary syndrome through extracellular matrix degradation and interaction with IGF-12. Our findings extend the current paradigm of coronary microvascular dysfunction in HFpEF, by suggesting that beyond microvascular inflammation,3 subclinical atherosclerosis may also play an important role. Limitations of this study include possible selection bias with regard to proteins measured, and lack of data on how circulating proteins relate to proteins at the tissue level.
In HFpEF, circulating biomarker profiles suggest that coronary microvascular dysfunction is related to subclinical atherosclerosis (via the PAPP-A pathway) potentially leading to cardiac hypertrophy and metabolic abnormalities.
Acknowledgments
Funding
PROMIS-HFpEF is an AstraZeneca sponsored study. S.J.S. is supported by the U.S. National Institutes of Health grants [R01 HL107577, R01 HL127028, and R01 HL140731] and American Heart Association grants [#16SFRN28780016 and #15CVGPSD27260148]. C.S.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore.
Footnotes
Conflict of Interest Disclosures
S.J.S. has received research grants from Actelion, AstraZeneca, Corvia, and Novartis; and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Cardiora, Eisai, Ironwood, Merck, Novartis, Sanofi, Tenax, and United Therapeutics. C.S.L. has received research support from Boston Scientific, Bayer, Roche Diagnostics, Medtronic, and Vifor Pharma; and has consulted for Astra Zeneca, Bayer, Novartis, Amgen, Merck, Janssen Research & Development LLC, Menarini, Boehringer Ingelheim, Abbott Diagnostics, Corvia, Stealth BioTherapeutics, and Takeda. A.S. received research grants from Academy of Finland and Finnish Foundation for Cardiovascular Research during the conduct of the study; and consulting fees from GE healthcare, Novartis, Abbot, Astra Zeneca. C.H. has received consulting fees from Novartis and MSD. M.L.F., M.A.B., and L.M.G. are all employees of AstraZeneca R&D. All other authors have no disclosures. L.H.L. has received research grants from Novartis, Boehringer-Ingelheim, Vifor Pharma, and AstraZeneca, and consulting fees from Novartis, Merck, Boehringer-Ingelheim, Sanofi, Vifor Pharma, and AstraZeneca.
Data availability: The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
References.
- 1.Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink-Nelson L, Fermer ML, Broberg MA, Gan LM, Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;39:3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayes-Genis A, Conover CA, Overgaard MT, Bailey KR, Christiansen M, Holmes DR, Virmani R, Oxvig C, Schwartz RS. Pregnancy-Associated Plasma Protein A as a Marker of Acute Coronary Syndromes. N Engl J Med. 2001;345:1022–1029. [DOI] [PubMed] [Google Scholar]
- 3.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]