Abstract
Background:
Long QT syndrome (LQTS) is caused by the abnormal function of ion channels, which may also affect atrial electrophysiology and be associated with the risk of atrial fibrillation (AF). However, large-scale studies of AF risk among LQTS patients and its relation to LQTS manifestations are lacking. We aimed to assess the risk of AF and its relationship to the LQTS genotype and the long-term prognosis in LQTS patients.
Methods:
Genotype-positive patients with LQTS (784 LQT1, 746 LQT2 and 233 LQT3) were compared with 2,043 genotype-negative family members. Information on the occurrence of AF was based on physician-reported ECG-verified events. Multivariate Cox proportional hazards regression analyses were performed for ages 0 to 60 and after 60 years (reflecting an early and late onset of AF) to assess the risk of incident AF by genotype and the relationship of AF to the risk of cardiac events defined as syncope, documented torsades de pointes, and aborted cardiac arrest or sudden cardiac death.
Results:
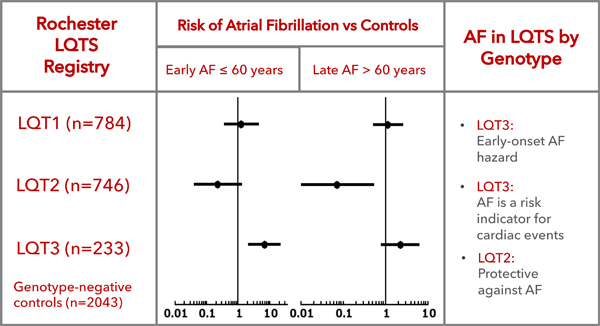
In patients followed from birth to 60 years of age, LQT3 patients had an increased risk of AF compared to genotype-negative family members (HR=6.62, 95%CI 2.04–21.49, p<0.001), while neither LQT1 nor LQT2 demonstrated increased AF risk. After the age of 60 years, LQT2 patients had significantly lower risk of AF compared with genotype-negative controls (HR=0.07, 95%CI 0.01–0.53, p=0.011). AF was a significant predictor of cardiac events in LQT3 patients through the age of 60 (HR=5.38, 95%CI 1.17–24.82, p=0.031).
Conclusions:
Our data demonstrate an increased risk of early age AF in LQT3 patients and also indicate a protective effect of the LQT2 genotype in it’s association with a decreased risk of AF after the age of 60.
Keywords: atrial fibrillation, long QT syndrome, sudden cardiac death, genetics, SCN5A, KCNH2, KCNQ1, Ventricular Fibrillation
Graphical Abstract
Background
Atrial fibrillation (AF) is a common cardiac rhythm disturbance that increases in prevalence from 0.1–0.5% among those <50 years of age to 1–2% in the 50–60 year old age group and 5–7% and higher in patients aged 70 years or older.1–5 In most cases, attributable primarily to the elderly or patients with cardiovascular comorbidities, AF occurs on the basis of well-established risk factors,2 however up to 20% of patients diagnosed with AF are younger than 60 and apart from AF may be ostensibly healthy.
While pathophysiology of AF and its natural course still remain challenging,6 pharmacological interventions aimed at termination of AF episode or prevention of AF recurrence are primarily achieved through interventions aimed at either sodium current, which slows the upstroke of the action potential (such as class IC antiarrhythmic drugs) or potassium current, which leads to prolongation of the repolarization phase of action potential and extension of refractory period of myocardial cells. Since most of the ion channels are represented in both atrial and ventricular myocardium,7, 8 abnormal channel function is likely to affect both ventricular and atrial repolarization processes, which was supported by large cohort studies 9,10 describing the association between the QT interval on surface ECG and the occurrence of AF.
Patients with the inherited Long QT Syndrome, which has an estimated prevalence of 1:2500 in the general population,11 have a prolongation of ventricular repolarization caused by altered expression of function of repolarizing ion channels, mainly “loss-of-function” potassium channels or “gain-of-function” sodium channel mutations, of which SCN5A, KCNQ1 and KCNH2” are the most commonly affected genes, resulting in reduction of net repolarizing currents.12,13–15
Electrophysiological studies performed in patients with LQTS demonstrated prolongation of action potential duration in atrial myocardium and inducible polymorphic atrial tachycardia, phenotypically described as atrial torsades de pointes.16 The suspected causative link between carrying an LQTS mutation and device-detected subclinical AF was further supported by a small-scale case-control study in ICD-treated LQTS patients17 and observations of increased early-onset AF prevalence among genotype-positive LQTS patients in the Mayo Clinic study, which first performed a systematic analysis of the relationships between LQTS and AF.18 Studies reported to date, however, were limited in size, not able to assess genotype-specific aspects of the association between LQTS and AF and did not address late-onset AF. Therefore, our aim was to assess the risk of AF and its relationship to genetic and clinical manifestations of LQTS in a large cohort of patients with the most common variants of congenital LQTS.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study population
Patients in this study were from the Rochester-based LQTS Registry; enrollment into the registry has been previously described.13, 19, 20,21 Patients were selected to the current analysis if they were shown to be carriers of disease causing mutations in KCNQ1 (LQT1, n=784), KCNH2 (LQT2, n=746), or SCN5A (LQT3, n=233).15 Patients were excluded from the study if they had more than one LQTS-associated mutation. A cohort of family members who were genetically tested and found to be negative for LQTS-associated mutations was used as a control group (n=2,043), which was followed up at the discretion of cardiologists who are contributing to the registry. Though systematic screening for AF was not included in the registry protocol, ECG-documented AF was a pre-specified reportable event.
Data collection
Standard 12-lead resting ECG was acquired at the time of enrolment in the registry. RR and QT intervals were measured on the first recorded ECG and used for calculation of the heart rate corrected QT-interval according to Bazett’s formula (QTc). Clinical data were collected on prospectively designed forms with information on demographic characteristics, personal and family medical history, ECG findings, therapies, including QT-prolonging medications, and events during long-term follow-up, including AF. Information about beta-blocker use was also collected in order to allow for time-dependent assessment of their possible impact on the incidence of cardiac events.
Endpoints
The age at first documentation of AF as reported by a physician contributing to the registry was considered the date of first clinical manifestation of AF for the purpose of this analysis and construction of Kaplan-Meier survival curves. The cardiac event endpoint of the Rochester LQTS registry study was the age at occurrence of a first syncope (defined as transient loss of consciousness that was abrupt in onset and recovery), aborted cardiac arrest requiring defibrillation as a part of resuscitation attempts (ACA) or LQTS-related SCD (abrupt in onset without evident cause, if witnessed or death that was not explained by any other cause if it occurred in a non-witnessed setting including sleep).
The LQTS Registry study was approved by the University of Rochester Research Subject Review Board.
Statistical analysis
Differences in the various characteristics by genotype were evaluated by standard univariate statistical methods. The cumulative probabilities of the first occurrence of AF from birth, for early onset, and after age 60, for late onset, were assessed by the Kaplan-Meier method using the log-rank statistic for significance testing. Multivariate Cox proportional hazards regression analyses were adjusted for gender, QTc, and time-dependent beta-blocker therapy and were used to estimate the association between specific LQTS genotype and risk of AF in the above age ranges as well as the association between incident AF and subsequent cardiac events. Because detection of short-lasting AF episodes may have been affected by the use of implantable cardioverters-defibrillators, the model was also adjusted for time-dependent ICD use. As a secondary analysis, the model was additionally adjusted for co-morbidities associated with AF, such as hypertension, coronary artery disease and diabetes.
For the analysis of cardiac event endpoints the multivariate Cox proportional hazards was again utilized to evaluate the independent association of time-dependent AF with the risk of cardiac events from both birth through age 60 and beyond age 60.
Results
Study population.
The clinical characteristics of the study population are presented in the Table 1 that illustrates comparisons between LQTS carriers by genotype and non-carriers.
Table 1.
Clinical characteristics of studied patients
| Genotype-negative | LQT1 | LQT2 | LQT3 | p-value 1–2-3 | |
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| # of Patients | 2043 | 784 | 746 | 233 | |
| Probands, n(%) | N/A | 136 (17) | 166 (22) | 35 (15) | 0.012 |
| Male, n (% ) | 938(46) | 330(42) | 329(44) | 102(44) | 0.715 |
| Age at enrolment, years | 23±21 | 26±21* | 25±21* | 26±20 | 0.862 |
| Atrial Fibrillation | |||||
| AF at any time, n (%) | 29(1.4) | 16(2.0) | 3(0.4)* | 17(7.3) | <0.001 |
| AF in probands, n (%) | N/A | 4 (2.9) | 2 (1.2) | 4 (11) | 0.005 |
| Age at1st AFib, yr | 71±11 | 58±17* | 46±16* | 49±21* | 0.393 |
| AF<=60 years, n (%) | 6(0.3) | 6(0.8) | 2(0.3) | 12(5.2)† | <0.001 |
| AF> 60 years, n (%) | 19 (1.1) | 11 (1.4) | 1 (0.1)* | 6 (2.5) | 0.003 |
| ECG, mean±sd | |||||
| RR, sec | 772±218 | 831±216† | 823±245† | 836±245† | 0.702 |
| PR, sec | 147±30 | 152±33† | 148±29 | 157±32† | <0.001 |
| QTc, msec | 422±31 | 480±48† | 480±54† | 478±53† | 0.740 |
| Treatment, n (%) | |||||
| Beta-blockers | 311(15) | 543(69) † | 582(78) † | 142(61) † | <0.001 |
| Class I and III AAD | 20 (1.0) | 13 (1.6) | 7 (0.9) | 38 (16) † | <0.001 |
| LCSD | 0(0) | 8(1) † | 14(2) † | 2(1)* | 0.315 |
| Pacemaker | 25(1) | 33(4) † | 78(10) † | 17(7) † | <0.001 |
| ICD | 12(1) | 105(13) † | 194(26) † | 80(34) † | <0.001 |
| Cardiac Events During Follow Up, n(%) | |||||
| Syncope | 269(13) | 309(39) † | 312(42) † | 64(27) † | <0.001 |
| ACA | 9(0) | 29(4) † | 57(8) † | 18(8) † | 0.002 |
| Sudden Cardiac Death | 0(0) | 13(2) † | 19(3) † | 12(5) † | 0.011 |
| Appropriate Shock | 0(0) | 18(2) † | 37(5) † | 8(3) † | 0.019 |
AAD - antiarrhythmic drugs; ACA – aborted cardiac arrest; AF – atrial fibrillation; ICD – implantable cardioverter-defibrillator; LCSD – left cardiac sympathetic denervation
- p<0.05 in comparison with Genotype-negative group
- p<0.001 in comparison with Genotype-negative group
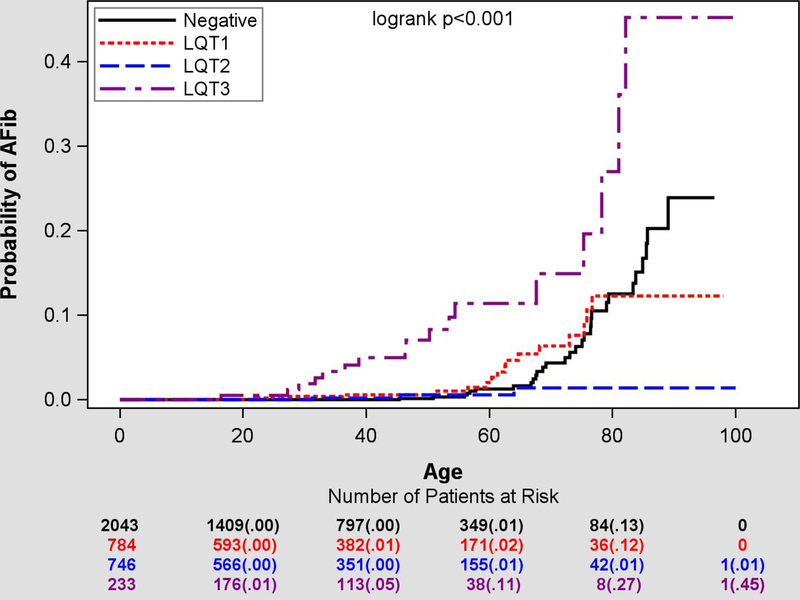
AF at any time was observed in 29 control subjects (1.5%) and in 36 (2.0%) patients with LQTS (Figure 1). However, when early-onset AF ≤60 years was considered, it was observed extremely rarely in the control population (0.3%, n=6) compared to 1.1% (n=20) of LQTS subjects with its cumulative risk of AF by the age of 60 reaching 11% in patients with LQT3 compared to 1–2% prevalence in patients with LQT1 (2%), LQT2 (1%) and genotype-negative controls (1%).
Figure 1.
Cumulative lifetime risk of new-onset early AF by genotype.
LQTS Genotype-Related Risk of AF
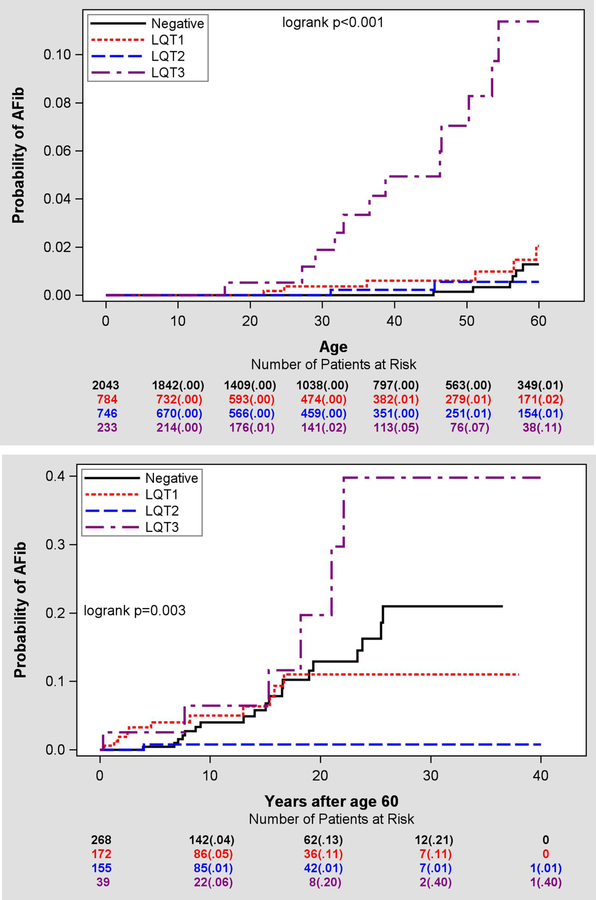
The cumulative probability of AF through the age of 60 by genotype is shown in Figure 2A. The results of the Cox regression analysis of the risk of AF assessed in relation to the genotype-negative family members as a reference group are presented in Table 2. For the early-onset AF by the age ≤60 years, a ten-fold increase risk of AF was observed in patients carrying LQT3 mutations in relation to the control group while patients with other LQTS genotypes did not demonstrate any significant difference from the control group. AF prevalence among men through age 60 was not significantly higher than in women (14% vs 9%, p=0.412) among LQT3 patients.
Figure 2.
A. Cumulative risk of new-onset early AF by genotype. Note significant hazard of early-onset AF associated with LQT3 genotype. B. Cumulative risk of new-onset late AF by genotype. Note negligibly low incidence of AF after 60 years of age in patients with LQT2 genotype.
Table 2.
Cox regression analysis of the risk of early (≤60 years) and late (>60years) AF among LQTS mutation carriers by genotype compared to the genotype-negative control subjects (adjusted for gender, QTc, time-dependent beta blocker therapy and time-dependent ICD use)
| Age ≤60 years | Age >60 years | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-value | HR | 95%CI | p-value | |
| LQT1 vs Control | 1,24 | 0.36–4.30 | 0,472 | 1,14 | 0.51–2.55 | 0,748 |
| LQT2 vs Control | 0,22 | 0.04–1.25 | 0.087 | 0,07 | 0.01–0.53 | 0,011 |
| LQT3 vs Control | 6,62 | 2.04–21.49 | <0.001 | 2,23 | 0.79–6.28 | 0,128 |
Assessment of possible association between specific LQT3 mutations and risk of AF yielded inconclusive results. We tested the possibility of AF risk linked to pore mutations in the SCN5A gene and observed significant separation of Kaplan-Meier curves indicating AF risk associated with pore mutations (Supplementary Figure 1A, Supplementary Table 1). Out of 17 LQT3 patients with observed AF, 6 had mutations in the pore region of SCN5A (35%), while 5 of them were located in T370M. However, early AF that was diagnosed before 40 years of age among LQT3 patients was primarily observed in the carriers of non-pore SCN5A mutations. We cannot exclude a possibility that T370M mutation has affinity to AF but there is no literature data supporting it.
LQT1 and LQT2 patients did not show significantly different risks of AF through the age of 60 as compared to control subjects. The relationship between the type of mutation (transmembrane vs non-transmembrane) and AF incidence was tested in LQT1 patients not yielding significant results (Supplementary Figure 1B).
After the age of 60 (Figure 2B), the risk of AF by the age of 80 years was about 13% in control subjects, which was comparable to 11% in LQT1 carriers. In LQT3 carriers, AF occurred in 20% of studied patients by the age of 80 years. In LQT2 carriers there was only 1% risk of AF bye the age of 80 years. Table 2 shows respective hazard ratios after adjustment for gender, QTc, time-dependent beta-blocker treatment and time-dependent ICD placement demonstrating a significantly decreased risk of AF in LQT2 patients relative to control subjects (HR=0.07; p=0.011). With a limited number of 39 LQT3 patients after the age of 60, the hazard ratio for the risk AF in comparison to controls was 2.43 (p=0.089).
Significant differences in regard to late-onset AF risk were observed between LQTS genotypes with both LQT1 (HR 17.62, 95%CI: 2.16–143, p=0.007) and LQT3 (HR 34.48, 95% CI: 4.01–296, p=0.001) showing significantly increased risk of AF after 60 years compared to LQT2 patients. In regard to the early-onset AF, LQT3 patients had significantly elevated risk of AF compared to both LQT1 (HR 7.43, 95%CI: 2.73–20.22, p<0.001) and LQT2 (HR 26.74, 95%CI: 5.92–120.78, p<0.001) mutation carriers.
A sensitivity analyses was done using the Cox regression model additionally adjusted for coronary artery disease, hypertension and diabetes mellitus and resulted in similar hazard ratio estimates demonstrating significant risk associated with LQT3 before 60 years of age (HR 14.26 95%CI 4.06–50.08, p<0.001) and similar protective effect of LQT2 genotype after the age of 60 (HR 0.08, 95%CI 0.01–0.62, p=0.016).
We also evaluated the association between QTc duration and AF in LQT1 and LQT3 carriers and genotype-negative control subjects and found longer QTc being linked to the lower risk of AF in LQT3 patients, but not in LQT1 (Table 3). This analysis could not be conducted in the LQT2 patients since they did not present with AF.
Table 3.
Cox regression analysis of the impact of QTc by 10 ms increase on risk of early (≤60 years) and late (>60years) AF among the carriers of LQT1 and LQT3 mutation carriers and genotype-negative control subjects. (adjusted for gender and time-dependent beta blocker therapy). Interaction p-value LQT1 vs LQT3: p=0.020 for Age≤60 years and p=0.018 for Age>60 years. Interaction between the LQTS genotypes and the control group are not significant in either of the age intervals.
| Age ≤60 years | Age >60 years | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-value | HR | 95%CI | p-value | |
| LQT1 | 1.03 | 0.90–1.17 | 0.674 | 1.01 | 0.90–1.13 | 0.835 |
| LQT3 | 0.82 | 0.72–0.94 | 0.004 | 0.75 | 0.61–0.93 | 0.010 |
| Control | 0.90 | 0.70–1.16 | 0.415 | 0.92 | 0.80–1.06 | 0.231 |
Since beta-blocker therapy, which is commonly administered to patients with LQTS, may affect AF incidence, all Cox regression models were adjusted for time-dependent beta-blocker use. In addition, we have also performed a sensitivity analysis by comparing the probability of AF between the three LQTS genotypes and control group using Kaplan-Meier curve analysis by including only patients who at any time were treated with beta-blockers. Supplementary Figure 2 demonstrate the pattern, which is similar to the one observed for the entire study cohort (Figure 1).
Association between AF and the Risk of Cardiac Events
AF was significantly associated with the risk of cardiac events in the pooled cohort of genotype-positive LQTS patients carrying LQT1, LQT2 or LQT3 mutations before the age of 60 years (HR=9.85, 95%CI 2.37–41.02, p=0.002) and was not observed after the age of 60 (HR=1.03, 95%CI 0.13–7.96, p=0.977). Analysis of the genotype-specific subgroups demonstrated significant association between AF and CE among LQT3 carriers (HR=5.38, 95%CI 1.17–24.82, p=0.031) while the number of events was not sufficient for assessment of risk estimates in the LQT1 and LQT2 subgroups.
Discussion
Main findings
In a large cohort of mutation carriers with the three most common variants of LQTS this study demonstrates significant genotype-specific differences in predisposition to AF dependent on the type of the affected ion channel. Our data confirm earlier suggested AF hazard associated with LQT3 and for the first time demonstrate the protective effect of the LQT2 mutations regarding late-onset AF. While the use of beta-blockers, which may have affected AF incidence, was significantly more common among LQTS patients compared to controls, its use was similar across different LQTS genotypes and could not explain differences in AF predisposition between the groups. Our findings also demonstrate that AF in patients with LQT3 should be considered as a marker of mutation penetrance and an indicator of the increased risk of cardiac events.
Age-related AF prevalence in epidemiological cohorts
The prevalence of AF is known to increase with age and epidemiological studies performed in different cohorts report remarkable similar estimates of AF being below 1% in those younger than 50–60 years and increasing to levels reaching 15% after the age of 80.22,23 There is a general understanding that these estimates are significantly lower than actual AF prevalence due to the paroxysmal nature of the arrhythmia and commonly observed lack of symptoms or unspecific complains that complicate establishing of diagnosis.2 These prevalence estimates are generally drawn from assessments based on symptomatic AF and rely on record linkage with clinical databases containing diagnosis codes for AF.1, 4, 24 Implementation of dedicated AF screening strategies may result in significantly higher prevalence estimates as shown by a Swedish study that utilized thumb-ECG for AF screening.5 In our study, we relied on physician-reported AF, which is similar to the approach applied in the majority of epidemiological studies, on which current AF prevalence estimates are based. In line with previous observations, AF prevalence in the reference population of genotype-negative family members in our study demonstrated age-related increases in AF prevalence being 0.2% at the age of 50 and rising to 1% by 60 years and further up to the level of 5% at 70 and 15% and 80 years. The remarkable agreement between our AF prevalence estimates and the earlier reported epidemiological findings supports the validity of our control group and AF results.
Atrial fibrillation under the age of 60 years in LQTS
AF that occurs at young age in patients without cardiovascular comorbidities or other well-established risk factors is more likely to be related to genetically determined mechanisms than late-onset AF and several single-nucleotide polymorphisms (SNPs) derived from genome-wide association studies (GWAS) have shown their role in predisposition to early onset AF,25 including those affecting the function of potassium channels involved in the repolarization phase of the action potential of cardiac myocytes26–28 and also atrial specific IKur current.29
Our study was focused on assessing the impact of disease-causing mutations in patients with the three most prevalent LQTS genotypes and revealed statistically significant risk associated with carrying LQT3 mutations. The mechanistic rationale for association between mutations in SCN5A and AF may be related to two principal mechanisms. A series of experimental studies on murine models of LQTS demonstrated arrhythmogenic cellular atrial electrophysiology associated with gain-of-function alterations in the INaL current observed as prolongation of action potential duration and increased propensity to early afterdepolarizations (EAD).30, 31 On the other hand, clinical observations presented earlier by our group demonstrated deteriorated atrial conduction in LQT3 patients.32
A number of mutations or rare variants in SCN5A has been reported in patients with either lone AF or AF associated with cardiovascular comorbidities, which co-segregated with familial AF but not necessarily accompanied by QTc prolongation or clinical LQTS.33 In another study on Scandinavian patients with early-onset AF, SCN5A variants previously associated with LQT3 were discovered in patients with early-onset lone AF without QTc prolongation.34 Functional studies performed by the same group demonstrated compromised peak sodium current, increased sustained sodium current, and indicated a possible overlap between the mechanisms underlying LQT3 and lone AF with action potential prolongation as a substrate and EADs as triggers for arrhythmia.
In the context of clinical LQTS, the study performed at the Mayo clinic has provided the first systematic analysis of the possible link between LQTS-causing mutations and early-onset AF.18 Out of 457 LQTS patients who were genotyped at the Mayo clinic, eight had documented AF before age of 50, which was estimated to be 17.5 times higher than the 0.1% population-based prevalence statistic and was slightly more common in LQT1 (5 of 211) than in LQT2 (none of 174) or LQT3 (1 of 59). However, no conclusions could be drawn concerning genotype-specific risk estimates due to the study size limitations, which we were able to overcome. In our study, despite the insignificant differences in the incidence of early onset AF between LQT1/ LQT2 patients and genotype-negative controls, AF manifested at earlier age in LQTS mutation carriers than in controls. Therefore, our study cannot rule out the effect of individual mutations on the risk of early AF, however no systematic relationship could be proven based on our data.
Notably, early onset AF in our genotype-positive LQTS patients was strongly associated with the risk of cardiac events and remained significant in the subgroup of LQT3 patients in whom most of AF cases was reported. These findings suggest that AF in LQT3 mutation carriers should be considered as a manifestation of LQTS and accounted for in risk stratification strategies.
Late-onset AF and the protective effect of LQT2
To the best of our knowledge, no previous study addressed the impact of LQTS mutations on the incidence of late-onset AF and we are the first to report genotype-specific AF risk estimates based on a large-size genotype-positive LQTS cohort. Even in the older age group, there was a trend of an increased incidence of AF in patients with LQT3 (p=0.089), but among patients with LQT1 AF risk did not differ from the one in the reference group and corresponded to the one expected for this age group.
The novel finding is the apparently protective effect of LQT2 mutations in regard to incident AF after the age of 60, who demonstrated only 9% of the risk of AF (i.e. 91% risk reduction) compared to the reference group. Of the 155 LQT2 patients in this age category only one developed AF. Literature on the impact of LQT2 mutations in the context of AF is scarce. Johnson et al. did not observe any AF cases among 174 LQT2 mutations carriers followed up until 50 years of age, which is further supported by our findings.18
To what extent LQT2 mutations affect electrophysiology of atrial myocardium is to a large extent unknown. In a single report by Kirchhof et al., electrophysiology study was performed in ten patients with LQTS, of whom four had LQT2 genotype. Notably, EADs and/or polymorphic atrial tachyarrhythmias were observed in two LQT2 patients who had normal QTc while two carriers of the same mutation (S428L) with QTc of 500 ms and 560 ms did not have either EADs or polymorphic atrial tachyarrhythmias induced during electrophysiological study.
Our finding of a suggested protective effect associated with the LQT2 genotype in regard to the risk of AF is indirectly supported by genotyping studies on patients with lone or early onset AF. KCNH2 variants associated with lone AF have been characterized by slower deactivation and increased repolarizing potassium current with no QTc prolongation observed in affected subects.35 On the other hand, in a study on patients with very early onset of AF performed by Olesen et al. no KCNH2 mutations were identified while the prevalence of mutation in other ion channel related gene was very high.28 Finally, KCNH2 mutations associated with short QT syndrome are well documented, while there is no documentation of association between LQTS-causing mutation in KCNH2 gene and increased risk of AF, in line with findings of the current study.36 It is therefore tempting to speculate that LQT2 mutations associated with loss-of-function effect on the repolarizing IKr channel prolong repolarization of atrial cardiomyocytes and lead to the same effect as class III antiarrhythmic drugs administered for prevention of AF recurrence in patients with paroxysmal AF.
The mechanisms underlying difference in the incidence of AF between patients with LQT1 and LQT2 are not clear at this point. The potassium channels IKs and IKr affected by the mutations associated with the two most common types of LQTS are expressed in both atrial and ventricular myocardium and there is no convincing evidence, which would suggest that selective inhibition of IKs or IKr would result in fundamentally different effect in the atria and ventricles. However, important differences exist between the two channels in regard to their impact on action potential duration (APD), which may explain the differences in regard to the observed association with genotype-dependent AF incidence.
Inhibition of the IKr channel coded by the KCNH2 gene leads to a prolongation of APD and provides the fundamental mechanism of antiarrhythmic action of class III antiarrhythmic drugs. On the other hand, selective inhibition of IKs in humans mimicking loss-of-function mutations associated with LQT1 only minimally affects APD under normal circumstances.37 IKs contribution to the repolarization process becomes apparent during beta-adrenergic stimutation,38 which results in the prolongation of the repolarization phase, associated with increased propensity to EADs thus explaining stress-related triggers in LQT1 patients.32 It is therefore plausible to suggest that loss-of-function KCNQ1 mutations affecting IKs may not result in a consistent APD prolongation in the atrial myocardium as in the case of LQT2, thus not providing a protective mechanism against AF observed among KCNH2 mutation carriers.
Our findings regarding the association between QTc duration and risk of AF indicate that a longer QTc is associated with less AF than with shorter QTc in LQT3 patients whereas such an association was not observed in LQT1 patients and in control subjects. These results imply the existence of non-linear relationship between APD and the risk of AF in SCN5A mutation carriers. We documented in the past that LQT3 patients have prolonged P wave and altered P wave morphology, i.e. well-established markers of increased AF risk, in comparison to healthy controls.32 It is possible that LQT3 patients who have SCN5A mutations also expressed in atria have a protective effect of prolonged atrial APD, which counterbalances the deteriorated atrial conduction that promotes AF in SCN5A mutation carriers. In our study, LQT3 patients with AF had shorter QTc values compared to patients without AF (465 ± 67 vs 479±52 ms, p=0.165), which is in agreement with the study by Olesen et al. who observed early onset AF in SCN5A mutation carriers with unaffected QTc.
Limitations of the study
Our study is based on analysis of the clinical information of patients included in a multi-center registry and therefore has limitations inherent to the registry study design. While relying on the information concerning incident AF provided by participating investigators, no systematic device-based screening for AF was included in the LQTS registry protocol. It is therefore possible that the data we present do not include the incidence of short or mildly symptomatic recurrent AF. However, AF was a prespecified reportable clinical event, which supports the validity of differences observed between the groups. Finally, while observing significant association between AF and cardiac events among patients younger than 60 years, the number of cardiac events was insufficient after the age of 60 in order to appropriately test the relationship between AF and cardiac events.
Conclusion
In a large LQTS cohort, we report a significant association between the LQT3 genotype and the risk of early-onset AF. Additionally, in patients with LQT3, AF is an indicator of an increased risk of cardiac events and should be considered in risk stratification and the choice of primary prevention strategies. On the other hand, patients with the LQT2 genotype have a very low risk of AF during their life time thus suggesting a protective effect of LQT2 mutations against AF.
Supplementary Material
WHAT IS KNOWN
Congenital long QT syndrome is caused by abnormal function of the potassium and sodium ion channels that are expressed both in the ventricular and atrial myocardium
The increased prevalence of atrial fibrillation, particularly at early age, has been reported among carriers of mutations associated with long QT syndrome
WHAT THE STUDY ADDS
LQT3 genotype is significantly associated with early-onset atrial fibrillation
LQT2 genotype is associated with a life-time low risk of atrial fibrillation
In patients with LQT3, atrial fibrillation is an indicator of an increased risk of cardiac events.
Acknowledgments
Sources of Funding: The study was performed with support from National Institutes of Health grant (No. HL-123483). Dr Platonov was supported by the research grants from The Swedish Heart-Lung foundation (grant #20150574), governmental funding of clinical research from the Swedish Health Care system (ALF) and Fulbright Commission that supported Dr Platonov’s research at the University of Rochester.
Non-standard Abbreviations and Acronyms
- ACA
aborted cardiac arrest
- AF
atrial fibrillation
- APD
action potential duration
- CE
cardiac event
- CI
confidence interval
- EAD
early afterdepolarizations
- ECG
electrocardiogram
- HR
hazard ratio
- ICD
implantable cardioverter-defibrillator
- LQTS
long QT syndrome
- SCD
sudden cardiac death
Footnotes
Disclosures: None
References:
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–378. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 4.Smith JG, Platonov PG, Hedblad B, Engstrom G, Melander O. Atrial fibrillation in the Malmo Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol. 2010;25:95–102. [DOI] [PubMed] [Google Scholar]
- 5.Engdahl J, Andersson L, Mirskaya M, Rosenqvist M. Stepwise screening of atrial fibrillation in a 75-year-old population: implications for stroke prevention. Circulation. 2013;127:930–937. [DOI] [PubMed] [Google Scholar]
- 6.Schotten U, Dobrev D, Platonov PG, Kottkamp H, Hindricks G. Current controversies in determining the main mechanisms of atrial fibrillation. J Intern Med. 2016;279:428–438. [DOI] [PubMed] [Google Scholar]
- 7.Gintant GA. Two components of delayed rectifier current in canine atrium and ventricle. Does IKs play a role in the reverse rate dependence of class III agents? Circ Res. 1996;78:26–37. [DOI] [PubMed] [Google Scholar]
- 8.Lee KS, Lee EW. Ionic mechanism of ibutilide in human atrium: evidence for a drug-induced Na+ current through a nifedipine inhibited inward channel. J Pharmacol Exp Ther. 1998;286:9–22. [PubMed] [Google Scholar]
- 9.Nielsen JB, Graff C, Pietersen A, Lind B, Struijk JJ, Olesen MS, Haunso S, Gerds TA, Svendsen JH, Kober L, et al. J-shaped association between QTc interval duration and the risk of atrial fibrillation: results from the Copenhagen ECG study. J Am Coll Cardiol. 2013;61:2557–2564. [DOI] [PubMed] [Google Scholar]
- 10.Mandyam MC, Soliman EZ, Alonso A, Dewland TA, Heckbert SR, Vittinghoff E, Cummings SR, Ellinor PT, Chaitman BR, Stocke K, et al. The QT interval and risk of incident atrial fibrillation. Heart Rhythm. 2013;10:1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, Gabbarini F, Goulene K, Insolia R, Mannarino S, et al. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz PJ, Crotti L, Insolia R. Long-QT syndrome: from genetics to management. Circ Arrhythm Electrophysiol. 2012;5:868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Robinson JL, Priori SG, Benhorin J, Locati EH, Towbin JA, Keating MT, et al. Influence of the genotype on the clinical course of the long-QT syndrome. International Long-QT Syndrome Registry Research Group. N Engl J Med. 1998;339:960–965. [DOI] [PubMed] [Google Scholar]
- 14.El-Sherif N, Turitto G, Boutjdir M. Congenital Long QT syndrome and torsade de pointes. Ann Noninvasive Electrocardiol. 2017;22:e12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutyifa V, Daimee UA, McNitt S, Polonsky B, Lowenstein C, Cutter K, Lopes C, Zareba W, Moss AJ. Clinical aspects of the three major genetic forms of long QT syndrome (LQT1, LQT2, LQT3). Ann Noninvasive Electrocardiol. 2018;23:e12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirchhof P, Zellerhoff S, Monnig G, Schulze-Bahr E. Pauses after burst pacing provoke afterdepolarizations and torsades de pointes in a patient with long QT syndrome. Heart Rhythm. 2004;1:720–723. [DOI] [PubMed] [Google Scholar]
- 17.Zellerhoff S, Pistulli R, Monnig G, Hinterseer M, Beckmann BM, Kobe J, Steinbeck G, Kaab S, Haverkamp W, Fabritz L, et al. Atrial Arrhythmias in long-QT syndrome under daily life conditions: a nested case control study. J Cardiovasc Electrophysiol. 2009;20:401–407. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JN, Tester DJ, Perry J, Salisbury BA, Reed CR, Ackerman MJ. Prevalence of early-onset atrial fibrillation in congenital long QT syndrome. Heart Rhythm. 2008;5:704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldenberg I, Mathew J, Moss AJ, McNitt S, Peterson DR, Zareba W, Benhorin J, Zhang L, Vincent GM, Andrews ML, et al. Corrected QT variability in serial electrocardiograms in long QT syndrome: the importance of the maximum corrected QT for risk stratification. J Am Coll Cardiol. 2006;48:1047–1052. [DOI] [PubMed] [Google Scholar]
- 20.Migdalovich D, Moss AJ, Lopes CM, Costa J, Ouellet G, Barsheshet A, McNitt S, Polonsky S, Robinson JL, et al. Mutation and gender-specific risk in type 2 long QT syndrome: implications for risk stratification for life-threatening cardiac events in patients with long QT syndrome. Heart Rhythm. 2011;8:1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zareba W, Moss AJ, Locati EH, Lehmann MH, Peterson DR, Hall WJ, Schwartz PJ, Vincent GM, Priori SG, Benhorin J, et al. Modulating effects of age and gender on the clinical course of long QT syndrome by genotype. J Am Coll Cardiol. 2003;42:103–109. [DOI] [PubMed] [Google Scholar]
- 22.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 23.Jogu HR, O’Neal WT, Broughton ST, Shah AJ, Zhang ZM, Soliman EZ. Frontal QRS-T Angle and the Risk of Atrial Fibrillation in the Elderly. Ann Noninvasive Electrocardiol. 2017;22:e12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busch MC, Gross S, Alte D, Kors JA, Volzke H, Ittermann T, Werner A, Kruger A, Busch R, Dorr M, et al. Impact of atrial fibrillation detected by extended monitoring-A population-based cohort study. Ann Noninvasive Electrocardiol. 2017;22:e12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olesen MS, Holst AG, Jabbari J, Nielsen JB, Christophersen IE, Sajadieh A, Haunso S, Svendsen JH. Genetic loci on chromosomes 4q25, 7p31, and 12p12 are associated with onset of lone atrial fibrillation before the age of 40 years. Can J Cardiol. 2012;28:191–195. [DOI] [PubMed] [Google Scholar]
- 26.Jabbari J, Olesen MS, Holst AG, Nielsen JB, Haunso S, Svendsen JH. Common polymorphisms in KCNJ5 [corrected] are associated with early-onset lone atrial fibrillation in Caucasians. Cardiology. 2011;118:116–120. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen JB, Bentzen BH, Olesen MS, David JP, Olesen SP, Haunso S, Svendsen JH, Schmitt N. Gain-of-function mutations in potassium channel subunit KCNE2 associated with early-onset lone atrial fibrillation. Biomark Med. 2014;8:557–570. [DOI] [PubMed] [Google Scholar]
- 28.Olesen MS, Andreasen L, Jabbari J, Refsgaard L, Haunso S, Olesen SP, Nielsen JB, Schmitt N, Svendsen JH. Very early-onset lone atrial fibrillation patients have a high prevalence of rare variants in genes previously associated with atrial fibrillation. Heart Rhythm. 2014;11:246–251. [DOI] [PubMed] [Google Scholar]
- 29.Christophersen IE, Olesen MS, Liang B, Andersen MN, Larsen AP, Nielsen JB, Haunso S, Olesen SP, Tveit A, Svendsen JH, et al. Genetic variation in KCNA5: impact on the atrial-specific potassium current IKur in patients with lone atrial fibrillation. Eur Heart J. 2013;34:1517–1525. [DOI] [PubMed] [Google Scholar]
- 30.Lemoine MD, Duverger JE, Naud P, Chartier D, Qi XY, Comtois P, Fabritz L, Kirchhof P, Nattel S. Arrhythmogenic left atrial cellular electrophysiology in a murine genetic long QT syndrome model. Cardiovasc Res. 2011;92:67–74. [DOI] [PubMed] [Google Scholar]
- 31.Dautova Y, Zhang Y, Sabir I, Grace AA, Huang CL. Atrial arrhythmogenesis in wild-type and Scn5a+/delta murine hearts modelling LQT3 syndrome. Pflugers Arch. 2009;458:443–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zareba W, Sattari MN, Rosero S, Couderc JP, Moss AJ. Altered atrial, atrioventricular, and ventricular conduction in patients with the long QT syndrome caused by the DeltaKPQ SCN5A sodium channel gene mutation. Am J Cardiol. 2001;88:1311–1314. [DOI] [PubMed] [Google Scholar]
- 33.Darbar D, Kannankeril PJ, Donahue BS, Kucera G, Stubblefield T, Haines JL, George AL Jr., Roden DM. Cardiac sodium channel (SCN5A) variants associated with atrial fibrillation. Circulation. 2008;117:1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olesen MS, Yuan L, Liang B, Holst AG, Nielsen N, Nielsen JB, Hedley PL, Christiansen M, Olesen SP, Haunso S, et al. High prevalence of long QT syndrome-associated SCN5A variants in patients with early-onset lone atrial fibrillation. Circ Cardiovasc Genet. 2012;5:450–459. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi K, Konno T, Tada H, Tani S, Liu L, Fujino N, Nohara A, Hodatsu A, Tsuda T, Tanaka Y, et al. Functional Characterization of Rare Variants Implicated in Susceptibility to Lone Atrial Fibrillation. Circ Arrhythm Electrophysiol. 2015;8:1095–1104. [DOI] [PubMed] [Google Scholar]
- 36.Hong K, Bjerregaard P, Gussak I, Brugada R. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J Cardiovasc Electrophysiol. 2005;16:394–396. [DOI] [PubMed] [Google Scholar]
- 37.Jost N, Virag L, Comtois P, Ordog B, Szuts V, Seprenyi G, Bitay M, Kohajda Z, Koncz I, Nagy N, et al. Ionic mechanisms limiting cardiac repolarization reserve in humans compared to dogs. J Physiol. 2013;591:4189–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jost N, Virag L, Bitay M, Takacs J, Lengyel C, Biliczki P, Nagy Z, Bogats G, Lathrop DA, Papp JG, et al. Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation. 2005;112:1392–1399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.