Abstract
Background:
The prevalence of subclinical atrial fibrillation (AF) in the elderly general population is unclear. We sought to define the prevalence of subclinical AF in a community-based elderly population, and to characterize subclinical AF and the incremental diagnostic yield of 4 vs 2 weeks of continuous ECG monitoring.
Methods:
We conducted a cross-sectional analysis within the community-based multi-center observational Atherosclerosis Risk in Communities (ARIC) study using visit 6 (2016-2017) data. The 2,616 ARIC study participants who wore a leadless, ambulatory ECG monitor (Zio® XT Patch) for up to 2 weeks were aged 79±5 years, 42% men and 26% black. In a subset, 386 participants without clinically recognized AF wore the monitor twice, each time for up to 2 weeks. We characterized the prevalence of subclinical AF (i.e., AF detected on the Zio® XT Patch without clinically recognized AF) over 2 weeks of monitoring and the diagnostic yield of 4 vs 2 weeks of monitoring.
Results:
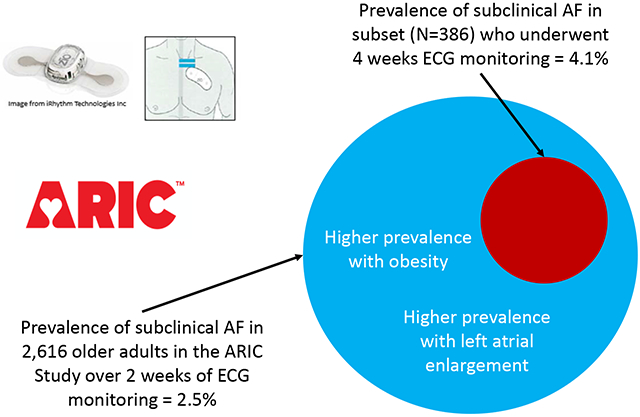
The prevalence of subclinical AF was 2.5%; the prevalence of subclinical AF was 3.3% among white men, 2.5% among white women, 2.1% among black men and 1.6% among black women. Subclinical AF was mostly intermittent (75%). Among those with intermittent subclinical AF, 91% had AF burden ≤10% during the monitoring period. In a subset of 386 participants without clinical AF, 78% more subclinical AF was detected by 4 weeks vs 2 weeks of ECG monitoring.
Conclusions:
In our study, the prevalence of subclinical AF was lower than previously reported and monitoring beyond 2 weeks provided substantial incremental diagnostic yield. Future studies should focus on individuals with higher risk to increase diagnostic yield and consider continuous monitoring duration longer than 2 weeks.
Keywords: atrial fibrillation, elderly, ECG, population studies
Journal Subject Terms: Atrial Fibrillation, Aging, Epidemiology, Electrocardiology (ECG)
Graphical Abstract
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia with an estimated lifetime risk of 1 in 3 among whites1 and 1 in 5 among African Americans.1, 2 AF is associated with an increased risk of stroke, heart failure, mortality and reduced quality of life.3 Importantly, among AF patients, anticoagulant use is associated with a 64% reduction in stroke risk and 20% reduction in mortality.4 AF can be subclinical and is thought to occur in approximately 30% of individuals who experience an ischemic stroke.5 Yet, prevalence estimates of subclinical AF are lacking in an unselected community-based population to inform the role of systematic screening for subclinical AF.6 Current guidelines for AF screening do not recommend ambulatory ECG monitoring but rather an opportunistic screening by pulse palpation or ECG rhythm strip.7 More recently, the U.S. Preventive Services Task Force concluded that the current evidence is insufficient to assess the balance of benefits and harms of screening for AF with ECG.8, 9
To move the field forward, a key piece of information is critically needed: the prevalence of subclinical AF based on contemporary advances in wearable technology in an unselected elderly population. Thus far, 3 European studies have sought to address systematic AF screening—including a stepwise screening program,10 a cluster randomized controlled trial,11 and the STROKESTOP study.12 These screening studies, however, have been limited by using 12-lead ECG or 24-48 hour Holter monitors, which might underestimate the prevalence of subclinical AF.13, 14
Technological innovation in ambulatory ECG monitoring in recent years has permitted a longer monitoring period beyond the traditional 24-48 hours of Holter monitoring and better characterization of AF burden.15-17 The Zio® XT Patch (iRhythm Technologies; San Francisco, CA) is a leadless, ambulatory ECG monitoring device that can record heart rhythm continuously for 2 weeks. Two previous studies have used longer term monitoring but were not conducted in populations designed to estimate the prevalence of subclinical AF in community-dwelling elderly individuals. 18, 19
To overcome the limitations of studies thus far, we estimated the prevalence of subclinical AF among the elderly by implementing the Zio® XT Patch in more than 2,600 participants of the Atherosclerosis Risk in Communities (ARIC) study who self-identified as black or white. As secondary aims, we defined clinical correlates of subclinical AF and characterized the type of AF (intermittent vs. continuous) and AF burden (proportion of monitoring time in AF) among elderly individuals who have subclinical AF. Finally, we estimated the incremental diagnostic yield for subclinical AF from wearing the Zio® XT Patch device for 4 weeks as compared to 2 weeks.
Methods
The data, analytic methods, and relevant study materials can be made available to other researchers where in accordance with ARIC study policies,20 upon request, for the purposes of reproducing results.
Study Population and Design
The ARIC Study21 is a prospective, community-based cohort study, which began in 1987-1989. The 15,792 ARIC study participants at inception were aged 45-64 years and were recruited from 4 U.S. communities (Forsyth County, NC; Jackson, MS; Washington County, MD; suburbs of Minneapolis-St. Paul, MN). Thus far, six study visits have been completed. Continuous follow-up through surveillance of each community’s hospital discharge lists has occurred, as have regular follow-up telephone calls (annual through 2011, thereafter twice yearly). Participants provided written informed consent at each visit, and this study was approved by Institutional Review Boards at all participating institutions. Relevant to this cross-sectional analysis, 4,003 participants attended the visit 6 clinic exam during 2016-2017 when they were aged 75-94 years.
Visit 6 participants at all 4 sites were invited to wear a Zio® XT Patch and the prescribed wear time was 14 days; exclusion criteria included history of cardiac electronic device implantation or skin allergic reaction to adhesive tape. Age was not an inclusion nor exclusion criterion for the study. At the end of the recording period, participants removed the device and mailed the heart rhythm monitor to iRhythm Technologies Inc., where recorded ECG data were processed using a proprietary algorithm and a report was generated. Of 3,680 visit 6 participants who were eligible to wear the Zio® XT Patch, 2,650 (72.0%) agreed to participate. Of the 2,650 devices, 34 (1.3%) were lost or returned without data resulting in a total of 2,616 Zio® XT Patch devices with analyzable data (Supplemental Figure 1). Further information on other relevant aspects of data collection is provided in the Supplemental Material.
A subset of participants was invited to wear a Zio® XT Patch a second time, also for a prescribed wear time of 2 weeks to assess the incremental diagnostic yield of 4 vs 2 weeks of continuous ECG monitoring. Eligibility criteria for this substudy included no clinical AF (based on ARIC ascertainment through the end of 2017 or self-report of a previous physician diagnosis at visit 6). Our recruitment goal was to consecutively recruit approximately 15% of participants to wear a second patch monitor at the Minneapolis, Washington County, and Jackson field centers. The first device was applied by field center staff during the visit 6 exam. Approximately 4 days after removing the first device, participants were asked to self-apply or come into the study clinic for staff to apply the second device. Three-hundred eighty-six participants without clinical AF wore the device twice, each time for up to 2 weeks.
Atrial Fibrillation
AF diagnosis by the Zio® XT Patch
AF was defined by an irregularly irregular rhythm with absent P-waves lasting ≥30 seconds. AF adjudication by iRhythm was based on a 2-step process. First, the ECG data were interrogated using an FDA-cleared, proprietary algorithm to identify potential AF episodes based on detection of the heart rate, irregularity, and morphology. Next, trained and certified cardiovascular technicians re-examined the detected AF episodes to confirm the diagnoses. A standard report was generated and uploaded to a secure website. The standard report included a diagnosis summary and ECGs, which allowed verification of reported AF. Data on wear time and analyzable time were also obtained from the standard report. In our study, a team of physician ECG readers in EPICARE (Wake Forest University) verified the accuracy of reported AF.
ARIC ascertainment of clinically recognized AF
Clinical AF diagnoses were obtained from 12-lead ECGs at study visits (visits 1-5) and hospital discharge records from 1987 through December 31, 2017.22 All ECG recordings from study visits, automatically coded as AF, were visually rechecked by a cardiologist to confirm the diagnosis.23 Hospitalizations in ARIC are identified by participant or proxy report in follow-up telephone calls and by surveillance of local hospital discharge lists. A trained abstractor obtained and recorded all International Classification of Diseases, Ninth Revision (ICD-9) or Tenth Revision (ICD-10) hospital discharge diagnoses from each hospitalization. AF was defined as the presence of an ICD-9 code of 427.31 or an ICD-10 code of I48.91 in the discharge codes. By physician review of a sample of 125 discharge summaries with ICD codes indicating possible AF, we confirmed the presence of AF in approximately 90%.22
Self-reported clinical AF on ZIO questionnaire
At visit 6, prior to the application of the Zio® XT Patch, participants were asked “Has a doctor said that you have atrial fibrillation?” Participants were also asked to report any symptoms of AF such as palpitations, fluttering sensation in the chest, dyspnea with mild activity, and exercise intolerance. Individuals with symptomatic AF were considered clinical AF.
AF burden
AF burden on the Zio® XT Patch was defined by the percent of analyzable time spent in AF, which we then categorized as no AF (0%), intermittent AF (>0 to <100%) and continuous AF (100%).
Clinical and Subclinical AF
Clinical AF included AF identified by either prior ARIC ascertainment through December 31, 2017 or self-report physician diagnosis of AF at visit 6. Subclinical AF was AF identified on Zio® XT Patch without symptoms of AF and without AF identified by ARIC ascertainment through December 31, 2017 or self-report physician diagnosis of AF at visit 6. Participants wearing the Zio® XT Patch were asked to press a button on the monitor to indicate if they were experiencing symptoms or document in the log the day/time they were experiencing symptoms. Individuals with symptomatic AF were considered clinical AF. Of note, participants with subclinical AF could be in continuous or intermittent AF based on the Zio® XT Patch; importantly, all participants with subclinical AF— whether continuous or intermittent— did not have symptoms of AF or prior diagnosis of AF.
Statistical Analysis
We report the prevalence of AF and its diagnostic status (no AF, subclinical AF, clinical AF) in the entire sample and stratified by race and sex. The prevalence of subclinical AF was estimated among those without clinical AF. We also present demographic and clinical characteristics stratified by AF diagnostic status and used Pearson’s chi-squared tests and Student’s t-tests to compare characteristics across AF diagnostic status. Multinomial logistic regression models were used to provide age, sex and race adjusted odds ratios (95% CI) for each clinical characteristic separately in relation to AF diagnostic status.
For the subset who wore the device twice, we describe characteristics of those who wore the Zio® XT Patch for up to 28 days. Specifically, we report the prevalence of any AF based on ECG monitoring of up to 14 days and AF prevalence based on ECG monitoring of up to 28 days. We estimate the mean duration from device activation before the first episode of intermittent AF detected by the Zio® XT Patch, and graphed the cumulative frequency of AF.
All analyses were conducted using SAS version 9.4 (SAS Institute Inc.; Cary, NC). Figures were generated using Stata version 14.2 (StataCorp LLC; College Station, TX). A two-tailed p-value <0.05 indicated statistical significance.
Results
The analysis cohort consisted of 2,616 participants (mean ± SD age, 79.2 ± 4.6 years, 42.1% men, and 26.1% black). The characteristics of these 2,616 participants were similar to the entire ARIC visit 6 sample of 4,003 participants (Supplemental Table 1). The median duration of continuous analyzable ECG data was 13.7 days (25th percentile=12.7; 75th percentile=13.9; Range=0.1-14.0), out of a maximum of 14 days. Wear times were excellent: 91.6% wore the device ≥7 days, 86.7% ≥10 days and 81.7% ≥12 days.
Prevalence and Characteristics of Subclinical and Clinical AF
Among the 2,616 monitored by the Zio® XT Patch, AF was detected in 217 (8.3%) participants; 87 (40.0%) participants had intermittent AF while 130 (60.0%) participants had continuous AF. Of the 217 participants with AF detected by the Zio® XT Patch, 57 did not have a previous diagnosis of AF by ARIC ascertainment or self-report at Visit 6; therefore, the overall prevalence of subclinical AF was 2.5% (95% CI: 1.9, 3.2; Table 1; Figure 1). Notably, of the 57 subclinical AF cases, the majority (n=43, 75.4%) had intermittent AF and the minority (n=14, 24.6%) had continuous AF. In addition to the overall prevalence, race- and sex-stratified prevalence estimates are provided: White men had the highest prevalence of subclinical AF (3.3%; 95% CI: 2.0, 4.6), while black women had the lowest prevalence (1.6%; 95% CI: 0.4, 2.8). Supplemental Table 2 shows prevalence estimates of subclinical and clinical AF by categories of CHA2Ds2-VASc score; subclinical AF prevalence was 4.9% (1.4, 8.5) among those with scores ≥6.
Table 1.
Prevalence of Subclinical and Clinical Atrial Fibrillation Among Participants who Wore an ECG Patch Monitor for up to 2 Weeks, Overall and Stratified by Race and Sex: the ARIC Study, 2016-17
| Overall * | White Men | White Women | Black Men | Black Women | |
|---|---|---|---|---|---|
| N | 2616 | 883 | 1044 | 214 | 469 |
| Subclinical AF, N †, | 57 / 2244 ‡ | 24 / 725 | 22 / 891 | 4 / 194 | 7 / 429 |
| Prevalence (95% CI) | 2.5% (1.9, 3.2) | 3.3% (2.0, 4.6) | 2.5% (1.5, 3.5) | 2.1% (0.1, 4.1) | 1.6% (0.4, 2.8) |
| Clinical AF, N | 372 § / 2616 | 158 / 883 | 153 / 891 | 20 / 214 | 40 / 469 |
| Prevalence (95% CI) | 14.2% (12.9, 15.6) | 17.9% (15.4, 20.4) | 14.7% (12.5, 16.8) | 9.4% (5.5, 13.3) | 8.5% (6.0, 11.1) |
| AF detected by Zio® XT | 217 / 2616 | 104 / 883 | 82 / 891 | 11 / 214 | 19 / 469 |
| Patch, N | 8.3% (7.2, 9.4) | 11.8% (9.7, 13.9) | 7.9% (6.2, 9.5) | 5.1% (2.2, 8.1) | 4.1% (2.3, 5.8) |
| Prevalence (95% CI) | |||||
| Intermittent AF, N ∥ | 87 / 2616 | 33 / 883 | 42 / 891 | 3 / 214 | 9 / 469 |
| Prevalence (95% CI) | 3.3% (2.6, 4.0) | 3.7% (2.5, 5.0) | 4.0% (2.8, 5.2) | 1.4% (0.0, 3.0) | 1.9% (0.7, 3.2) |
| Continuous AF, N | 130 / 2616 | 71 / 883 | 40 / 891 | 8 / 214 | 10 / 469 |
| Prevalence (95% CI) | 5.0% (4.1, 5.8) | 8.0% (6.3, 9.8) | 3.8% (2.7, 5.0) | 3.7% (1.2, 6.3) | 2.1% (0.8, 3.4) |
Included in the overall estimates are 6 participants who are neither white nor black.
Subclinical AF: Zio® XT Patch detected AF and no known AF diagnosed based on prior ARIC ascertainment (based on AF hospitalizations and 12-lead ECGs at study visits 1-5) and self-reported no prior AF diagnosis on visit 6 ZIO questionnaire; Clinical AF: AF diagnosed based on prior ARIC ascertainment (based on AF hospitalizations and 12-lead ECGs at study visits 1-5) or self-reported AF diagnosis at visit 6 ZIO questionnaire
The prevalence of subclinical AF was estimated among those without clinical AF, e.g., 57 / (2616-372) = 2.5%.
Of the 372 with clinical AF, 90 (24.2%) were diagnosed by ARIC ascertainment only, 84 (22.6%) by self-report and 198 (53.2%) by both ARIC and self-reported diagnosis.
Intermittent AF: AF burden >0 to <100%; Continuous AF: AF burden = 100%
AF - atrial fibrillation; ECG - electrocardiographic; ARIC - Atherosclerosis Risk in Communities; CI - confidence interval.
Figure 1.
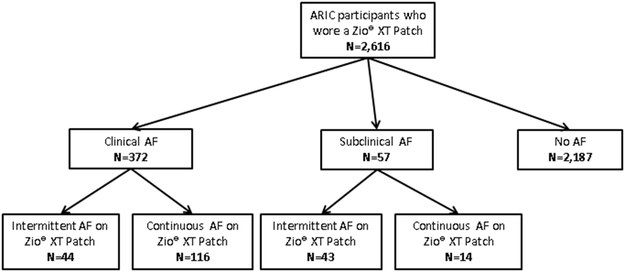
Flow Chart of Atrial Fibrillation Diagnostic Status
*Subclinical AF = Zio® XT Patch detected AF and no known AF diagnosed based on prior ARIC ascertainment and self-reported no prior AF diagnosis on visit 6 ZIO questionnaire; Clinical AF = AF diagnosed based on prior ARIC ascertainment or self-reported AF diagnosis at visit 6 ZIO questionnaire. †Intermittent AF: AF burden >0 to <100%; Continuous AF: AF burden = 100%.
AF - atrial fibrillation; ARIC - Atherosclerosis Risk in Communities.
Of the 87 participants with intermittent clinical and subclinical AF, the median AF burden (percent monitoring time in AF) was 2.2% (25th percentile=0.6%; 75th percentile=5.5%). The median duration of AF episodes was 2.08 minutes (25th percentile=0.95 minutes, 75th percentile=8.42 minutes). Of note, AF burden and median AF episode duration were inversely associated (Spearman’s r=−0.77). AF burden in this group followed a right-skew distribution whereby 70 (80.5%) participants had an AF burden during the monitoring period ≤10% while 76 (87.4%) had AF burden ≤20% and 84 (96.6%) ≤50% (Supplemental Figure 2). Time to first AF episode during the monitoring period was also right-skewed with a median of 3.5 days (25th percentile=0.8 days; 75th percentile=8.4 days); 39.1%, 69.0%, and 93.1% of participants had the first AF episode detected by Day 2, 7, and 12, respectively (Supplemental Figure 3).
Of the 43 participants with intermittent AF that was subclinical, AF burden also followed a right-skew distribution with 40 (90.7%) having AF burden ≤10% and all having AF burden ≤65% (Supplemental Figure 4). In this group, time to first AF episode during the monitoring period was also right-skewed with a median 4.1 days (25th percentile=1.1; 75th percentile=9.5); 34.9%, 60.5%, and 95.3% of participants had the first AF episode detected by Day 2, 7, and 12, respectively (Figure 2). Of note, among these participants with intermittent subclinical AF, the minimum and maximum ventricular rates (bpm) were mean ± SD 74 ± 20 and 162 ± 36; among those with continuous subclinical AF, the minimum was 40 ± 8 and the maximum was 137 ± 19.
Figure 2.
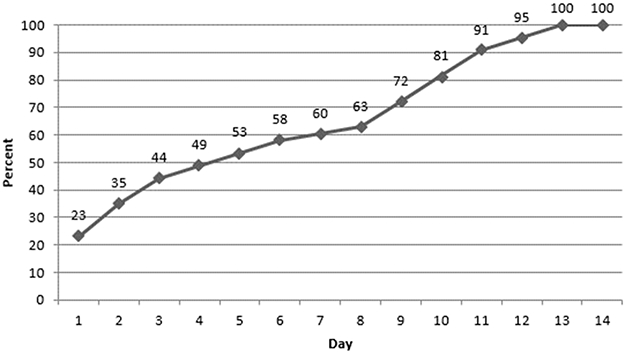
Cumulative Frequency of Intermittent Atrial Fibrillation Detection in Participants with Subclinical Atrial Fibrillation Over 2 Weeks Continuous Ambulatory ECG Monitoring, the ARIC Study, 2016-17, n=43
*Subclinical AF: Zio® XT Patch detected AF and no clinical AF (diagnosed based on prior ARIC ascertainment or self-reported no prior AF diagnosis on visit 6 ZIO questionnaire).
AF - atrial fibrillation; ECG - electrocardiographic; ARIC - Atherosclerosis Risk in Communities.
Clinical Correlates of Subclinical AF
Compared to participants without AF, those with subclinical AF had greater left atrial volume index and CHARGE-AF score (Table 2). The c-statistic of the CHARGE-AF score was 0.677 for clinical AF versus no AF, 0.598 for subclinical AF versus no AF, and 0.592 for clinical AF versus subclinical AF. After adjusting for age, sex and race, those with left atrial volume index ≥34 ml/m2 had higher odds of subclinical AF [OR=2.63 (95% CI: 1.32, 5.23)] than those <34 ml/m2 and those with BMI ≥30 kg/m2 had higher odds of subclinical AF [OR=2.33 (95% CI: 1.09, 4.99)] compared to participants with BMI 18.5-25 kg/m2 (Supplemental Table 3). The prevalence of subclinical AF was 1.5% among those with BMI 18.5-25 kg/m2, 2.2% in 25-30 kg/m2, and 2.7% in >30 kg/m2. Subclinical AF prevalence was 3.8% among those with left atrial volume index ≥34 ml/m2 as compared to 2.0% among those <34 ml/m2.
Table 2.
Clinical Characteristics by Atrial Fibrillation Diagnostic Status (No AF, Subclinical AF, Clinical AF): the ARIC Study, 2016-17
| No AF | Subclinical AF† | Clinical AF | P for subclinical vs no AF‡ |
|
|---|---|---|---|---|
| N | 2187 | 57 | 372 | - |
| Age | 78.9 ± 4.5 | 80.2 ± 4.1 | 81.0 ± 5.2 | 0.03 |
| Male | 894 (40.9) | 28 (49.1) | 179 (48.1) | 0.21 |
| White | 1570 (71.8) | 46 (80.7) | 311 (83.6) | 0.15 |
| Current smoker | 157 (7.3) | 4 (7.0) | 19 (5.2) | 0.94 |
| Current drinker | 1058 (48.9) | 30 (52.6) | 188 (51.0) | 0.58 |
| Physical activity sport index | 2.6 ± 0.8 | 2.5 ± 0.6 | 2.5 ± 0.7 | 0.37 |
| Body mass index, kg/m2 | 28.3 ± 5.3 | 29.4 ± 4.5 | 28.9 ± 5.9 | 0.13 |
| Systolic blood pressure, mmHg | 135.6 ± 18.8 | 132.4 ± 18.5 | 132.5 ± 20.1 | 0.20 |
| Diastolic blood pressure, mmHg | 67.1 ± 10.5 | 67.8 ± 10.0 | 67.3 ± 11.1 | 0.62 |
| Anti-hypertension medication use | 1637 (74.9) | 44 (77.2) | 330 (88.7) | 0.69 |
| Anti-arrhythmia medication use | 5 (0.2) | 0 (0.0) | 41 (11.0) | - |
| Left atrial volume index, mL/m2 § | 24.4 ± 6.7 | 28.1 ± 8.2 | 31.3 ± 13.4 | 0.002 |
| Diabetes | 708 (32.4) | 21 (36.8) | 127 (34.1) | 0.48 |
| Coronary heart disease | 144 (6.6) | 6 (10.5) | 62 (16.7) | 0.24 |
| Heart failure | 132 (6.0) | 3 (5.3) | 80 (21.5) | 0.81 |
| Stroke | 82 (3.8) | 2 (3.5) | 25 (6.7) | 0.92 |
| CHA2DS2-VASc score | 3.8 ± 1.1 | 3.9 ± 1.2 | 4.1 ± 1.3 | 0.50 |
| CHARGE-AF score | 0.15 ± 0.11 | 0.18 ± 0.12 | 0.23 ± 0.16 | 0.04 |
| NT-proBNP, pg/mL§ | 156.6 ± 94.0 | 203.2 ± 222.6 | 405.5 ± 550.3 | 0.14 |
Total n=2616; data are presented as N (%) or mean ± standard deviation
Subclinical AF = Zio® XT Patch detected AF and no known AF diagnosed based on prior ARIC ascertainment and self-reported no prior AF diagnosis on visit 6 ZIO questionnaire; Clinical AF = AF diagnosed based on prior ARIC ascertainment or self-reported AF diagnosis at visit 6 ZIO questionnaire
p-value from Pearson’s chi-squared test for categorical variables or unpaired t-test for continuous variables
Measured at visit 5 (2011-2013)
AF - atrial fibrillation; ARIC - Atherosclerosis Risk in Communities.
Incremental Diagnostic Yield of 4 Weeks vs. 2 Weeks of Continuous Ambulatory Electrographic Monitoring for Subclinical AF
A subset of participants without clinical AF (based on ARIC ascertainment or self-reported physician diagnosis) wore the device a second time (also for up to 2 weeks; resulting in a total of 4 weeks ECG monitoring). The 386 participants were aged 78.5±4.2 years, 45.9% were men and 10.6% were black. Descriptive characteristics of these participants as compared to all visit 6 attendees and those who wore the Zio® XT Patch for only 2 weeks are presented in Supplemental Table 1. Mean recording time was 13.3±1.7 days for the first Zio® XT Patch, 13.1±2.0 for the second patch, and 26.4±2.9 for the combined 4 weeks. Wear times were excellent in this group, with 98.7% wearing the first device ≥7 days, 96.9% for ≥10 days and 94.8% for ≥12 days. Additionally, 97.4% wore the second device ≥7 days, 94.0% for ≥10 days and 91.5% for ≥12 days.
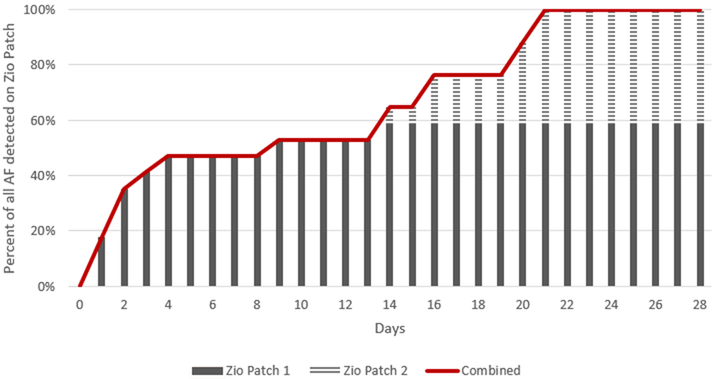
In this subset of 386 participants, 16 had AF detected by the Zio® XT Patch. The prevalence of subclinical AF was 2.3% with 2 weeks of monitoring (95% CI: 0.8, 3.8%; n=9); this increased to 4.1% (2.2, 6.1%; n=16) with 4 weeks of monitoring (Supplemental Table 4). Specifically, 6 participants had AF detected in both the 1st and 2nd Zio® XT Patch, 7 participants did not have AF detected on the 1st Zio® XT Patch but had AF detected on the 2nd Zio® XT Patch, and finally 3 participants did not have AF detected on the 2nd Zio® XT Patch but had AF detected on the 1st Zio® XT Patch. In other words, wearing the Zio® XT Patch for 2 weeks identified 9 cases of AF and increasing monitoring to 4 weeks identified another 7 cases of AF. Thus, 78% (95% CI: 52, 104%) more subclinical AF was detected with 4 weeks versus 2 weeks of monitoring. Across both patch monitoring times, the mean time to first AF episode was 10.3±8.3 days with a median of 11.2 days (25th percentile=2.0, 75th percentile=19.7). Cumulative yield of AF detection over the monitoring time is shown in Figure 3. All 16 participants with subclinical AF identified over the 4 weeks of ECG monitoring were detected by day 21.
Figure 3.
Cumulative Frequency of Subclinical Atrial Fibrillation Detection Over up to 28 Days of Ambulatory ECG Monitoring, ARIC Study, 2016-2017, n=16
*Subclinical AF: Zio® XT Patch detected AF and no clinical (AF diagnosed based on prior ARIC ascertainment or self-reported no prior AF diagnosis on visit 6 ZIO questionnaire).
†Zio 1 (in solid gray) corresponds to subclinical AF detected on the first patch worn for ≤14 days. Zio 2 (in striped gray) corresponds to subclinical AF detected on the second patch worn for ≤14 days. Of the 16 participants with subclinical AF detected up to 28 days monitoring, 9 participants had AF on the first patch (56.3%). All cases were detected by day 21.
AF - atrial fibrillation; ECG - electrocardiographic; ARIC - Atherosclerosis Risk in Communities.
Discussion
Principal findings
Using 2 weeks of continuous ambulatory ECG monitoring in a community-based sample of more than 2,600 elderly black and white individuals aged ≥75 years with a high participation rate, we observed the following key findings. First, the prevalence of subclinical AF in the elderly was 2.5%. The highest prevalence was in white men (3.3%) and the lowest prevalence was in black women (1.6%). Second, clinical characteristics associated with higher prevalence of subclinical AF, compared with no AF, included higher body mass index and left atrial volume index. Third, most subclinical AF cases were intermittent with infrequent episodes. Fourth, 78% more subclinical AF was detected by 4 weeks than by 2 weeks of continuous ECG monitoring. Collectively, our findings have significant implications for the current debate on ECG screening for AF.
Prevalence estimates of subclinical AF from studies in selected populations
As summarized in Supplemental Table 5, research using continuous ECG monitoring longer than the traditional 48 hours of Holter monitoring has been conducted in selected population groups, such as patients with a clinical indication (e.g. stroke patients,24, 25 patients with high risk of AF18, 19), or among members of health care insurance databases.18 Notably, a recent pragmatic trial (mHealth Screening to Prevent Strokes; mSToPs) within a large national health plan randomized health insurance members with an increased risk of AF to self-apply the ZioPatch (continuous monitoring for up to 2 weeks), the prevalence of previously undiagnosed AF was nearly 5% based on up to 2 weeks of continuous ECG recording.18 This study, however, was limited by focusing on a health insurance population with a low participation rate (only 0.8% who were eligible for active monitoring agreed to participate) and characteristics of participants compared to non-participants differed, thus the potentially biased prevalence estimates of subclinical AF are likely not generalizable.
Recently, the ASSERT-II trial26 assessed 256 patients aged ≥65 years at neurology or cardiology clinics without a history of AF, but had any of the following: CHA2DS2-VASc score of ≥2, sleep apnea, or BMI >30 kg/m2. Moreover, eligibility also required either left atrial enlargement or increased serum N-terminal pro-B-type natriuretic peptide. Patients were implanted with a subcutaneous monitor and followed for a mean of 16.3±3.8 months. Of 256 patients in the study, subclinical AF was detected in 90 patients (detection rate, 34.4% per year). Although instructive, this study was not based on an unselected community-dwelling elderly population.
Prevalence estimates of subclinical AF from studies conducted in community settings
A recent Scientific Statement from the American Heart Association highlighted crucial knowledge gaps regarding subclinical AF, including the need to estimate the prevalence of subclinical AF and identify its risk factors in community-based populations.27 Studies on ECG screening for AF in community-based populations have been limited by the use of intermittent ECG monitoring as opposed to continuous ECG monitoring. For example, a Swedish study used intermittent ECG recording (20-30 seconds twice daily and in the event of palpitations) over 2 weeks and found the prevalence of subclinical AF among 403 elderly individuals aged 75-76 years without clinical AF to be 7.4%.10 Although participants in this study were community-dwelling, this study was based on symptom-triggered ECG monitoring, as opposed to continuous monitoring. Therefore, the prevalence estimate that was obtained does not reflect the prevalence of subclinical AF in an elderly community-based cohort. In a subsequent investigation in Sweden (STROKESTOP study), based on 2 weeks of intermittent ECG recording, 3% of participants aged 75-76 were found to have previously unknown AF. This study again was limited by the use of intermittent recording and a modest participation rate (54%).12 Additionally, prior studies on ECG screening for AF have been conducted in mostly white populations, which is a significant limitation given known differences in AF risk and prevalence by race. Findings from the MESA Study were recently published, which reported that among the 946 participants who wore a Zio® XT Patch for 12-14 days, 32 cases of AF/flutter were identified during the monitoring period. Notably, participants with known or clinically recognized AF were over-recruited; thus, that study was not designed to define the prevalence of subclinical AF in the community.
Based on our subclinical AF prevalence estimate of 2.5% with 2 weeks of monitoring, the number needed to screen to diagnose 1 patient with subclinical AF is 40 people. If the number needed to treat to prevent 1 stroke is 25 people,28 then the number of elderly Americans needed to screen with up to 2 weeks of ambulatory ECG monitoring to prevent 1 stroke would be 1,000. These estimates will need to be interpreted in the context of other screening efforts; for example, an estimated 235 individuals must be screened by ultrasound to prevent 1 abdominal aortic aneurysm death or 625 individuals must undergo a fecal occult blood test to prevent 1 colorectal cancer death.29 Our prevalence estimate of subclinical AF among elderly people in the community thus provides a key piece of information to shape subsequent conversations on the merits and drawbacks of systematic ambulatory ECG monitoring to screen for subclinical AF in the community. Furthermore, our study also suggests certain subsets of patients with higher prevalence of subclinical AF to target in future randomized controlled trials on screening, e.g., the number needed to screen to prevent 1 stroke among obese individuals and those with left atrial enlargement would be 930 and 660, respectively. It is worth noting that our estimates are based on stroke risk in patients with clinically recognized AF and the assumption that stroke risk is similar across the spectrum of AF burden. Most ARIC participants with subclinical AF have infrequent episodes who may have a lower stroke risk than those with clinical AF or those with frequent AF episodes; as a result, it is possible that the number needed to screen is higher than 1,000.
Characteristics of subclinical AF and diagnostic yield of 4 weeks vs. 2 weeks of ambulatory ECG monitoring
A subset of MESA participants (n=439) wore a Zio® XT Patch twice, each time for up to 2 weeks, and 4% had previously undiagnosed AF/atrial flutter on at least one patch monitor.19 Also similar to our findings in the ARIC study, participants with previously undiagnosed AF had a low AF burden, i.e., proportion of monitoring period spent in AF was ≤20%. Aside from the recent findings from MESA, most studies have compared 14 day wear time (ZioPatch) to 24-48 hours (as would be identified on a Holter monitor) for detection of AF in populations with a clinical indication.30-35 One study reported that the overall diagnostic yield for intermittent AF was greater for 14 days vs. 48 hours with a mean time to first AF episode of ~3 days. This study also reported that the time to first AF episode was inversely related to AF burden, i.e., the lower the AF burden, the longer one would need to monitor to detect AF.35 Several studies have examined the diagnostic yield for AF beyond 2 weeks monitoring; however, this screening generally has been limited to those with prior stroke or transient ischemic attacks: In a meta-analysis of RCTs among those with cryptogenic stroke, AF was identified in 15% of patients with 30 day ECG monitoring but only 4% of patients with 24 hours of monitoring.36
In our study, the vast majority of subclinical AF cases were intermittent (75%). Among those with intermittent subclinical AF, the vast majority had low burden (91% having AF burden ≤10%). Taken together, these findings would suggest that that a single 12-lead ECG or pulse palpation may not be sufficient for screening to detect the vast majority of subclinical AF cases. Furthermore, because most ARIC participants with subclinical AF had low AF burden, longer rather than shorter continuous ECG monitoring would likely have a higher diagnostic yield to detect subclinical AF. This claim was substantiated by findings from our subset of 386 participants who wore the Zio® XT Patch twice for up to 4 weeks total. The prevalence of subclinical AF over a total of 4 weeks of continuous ECG monitoring was higher (4.1% vs 2.3% over 2 weeks) and the additional diagnostic yield for subclinical AF was 78% for 4 weeks vs. 2 weeks. Collectively, these findings raise an important question for the current debate on ECG screening for subclinical AF: the optimal duration for continuous ECG screening. In the subset who wore the Zio® XT Patch twice, all participants with AF identified over 4 weeks monitoring were detected by day 21, consistent with mSTOPs where ~95% of AF cases detected were diagnosed by day 21. However, further research is needed in large community-based studies to definitively answer this question.
Strengths and Limitations
To our knowledge, this paper provides one of the first community-based estimates on the prevalence of subclinical AF among free-living elderly white and black individuals. Importantly, this estimate is based on continuous ECG monitoring, in contrast to most other studies which involved symptom triggered monitoring. An additional strength is that the ARIC study includes a wealth of detailed characterization and standardized measures for covariates conducted in a research setting as opposed to a clinical setting. We also provide one of the first examinations of the diagnostic yield on subclinical AF from 2 weeks to 4 weeks among elderly individuals without clinical indication for ECG monitoring.
Several limitations should be noted. First, the limited number of intermittent AF cases hindered in-depth subgroup analyses. Second, it is plausible that clinical AF based on ARIC ascertainment may be underestimated as AF managed entirely in the outpatient setting may have been missed by ARIC ascertainment. However, the incidence rates of AF based on ARIC ascertainment are comparable to other population-based studies.22 Third, the ARIC study had up to 5 study ECGs done at ARIC visits 1-5 and results were reported to the participants and their physicians, which could have led to a lower prevalence of subclinical AF by visit 6. However, <10% of AF cases ascertained in ARIC were identified from study ECGs; the vast majority were identified from hospitalization discharge codes.22 Furthermore, even if we excluded AF cases identified by study ECGs from the definition of clinical AF, the prevalence of clinical and subclinical AF remained essentially unchanged at 14.1% and 2.7%, respectively. Fourth, one might also argue that our prevalence estimates may be biased due to conditional participation owing to participants surviving to that time-point, opting to wear an ECG monitor, and agreeing to attend a clinic visit. However, this scenario actually reflects reality; this ARIC sample would closely resemble an elderly population that is amenable to a screening program. Fifth, given the small number of AF events (n=16) in our subsample, we were not able to test whether there were differences in diagnostic yield over 2 versus 4 weeks monitoring based on demographic or clinical characteristics. Finally, this study does not address whether population screening would be cost-effective, when and how often it would have to be done, or whether treating subclinical AF would be cost-beneficial.
Conclusion
This study provides some of the first prevalence estimates of subclinical AF (2.5%) in a community-dwelling elderly population and suggests that extending heart rhythm monitoring beyond 2 weeks will substantially increase the diagnostic yield. The prevalence of subclinical AF in this community-dwelling elderly population was lower than previously reported. These findings can inform future studies testing screening strategies in the community. Future studies that evaluate the role of systematic screening for subclinical AF in the elderly may want to focus on individuals with higher risk (e.g., those who are obese) to increase diagnostic yield and consider potential benefits or drawbacks related to continuous monitoring duration longer than 2 weeks. Future studies should also address other critical knowledge gaps including whether treatment of subclinical AF can prevent sequelae such as ischemic stroke.
Supplementary Material
WHAT IS KNOWN.
The prevalence of subclinical atrial fibrillation (AF) in the elderly have been reported based on selected patient populations or using short-term 24-48 hour Holter monitoring.
However, the prevalence of subclinical AF in the general elderly population based on longer-term continuous heart rhythm monitoring (up to 2 weeks) is unclear.
WHAT THIS STUDY ADDS.
Using a patch monitor that can record heart rhythm continuously for up to 2 weeks, the prevalence of subclinical AF in a community-dwelling elderly population was 2.5%; highest prevalence in white men (3.3%) and lowest prevalence in black women (1.6%).
Higher body mass index and left atrial volume index were associated with higher prevalence of subclinical AF vs. no AF.
Extending the monitoring period to 4 weeks (vs. 2 weeks) increased the detection of subclinical AF by 78%.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding: The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). This work was also supported by grants from the National Heart Lung and Blood Institute [R01HL126637-01A1 (LYC), R01HL141288 (LYC), T32HL007779 (MRR)] and the American Heart Association [16EIA26410001 (AA)]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the American Heart Association.
Non-standard Abbreviations and Acronyms:
- AF
atrial fibrillation
- ARIC
Atherosclerosis Risk in Communities
Footnotes
Disclosures: None.
References:
- 1.Mou L, Norby FL, Chen LY, O’Neal WT, Lewis TT, Loehr LR, Soliman EZ, Alonso A. Lifetime Risk of Atrial Fibrillation by Race and Socioeconomic Status: ARIC Study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol. 2018;11:e006350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weng LC, Preis SR, Hulme OL, Larson MG, Choi SH, Wang B, Trinquart L, McManus DD, Staerk L, Lin H, et al. Genetic Predisposition, Clinical Risk Factor Burden, and Lifetime Risk of Atrial Fibrillation. Circulation. 2018;137:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, McManus DD, Dupuis J, Ellinor PT, Benjamin EJ. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011;124:1982–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67. [DOI] [PubMed] [Google Scholar]
- 5.Friberg L, Rosenqvist M, Lindgren A, Terent A, Norrving B, Asplund K. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke. 2014;45:2599–605. [DOI] [PubMed] [Google Scholar]
- 6.Moran PS, Flattery MJ, Teljeur C, Ryan M, Smith SM. Effectiveness of systematic screening for the detection of atrial fibrillation. Cochrane Database Sys Reviews. 2013:CD009586. doi: 10.1002/14651858.CD009586.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50:e1–e88. [DOI] [PubMed] [Google Scholar]
- 8.Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr., Kemper AR, Kubik M, et al. Screening for Cardiovascular Disease Risk With Electrocardiography: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:2308–2314. [DOI] [PubMed] [Google Scholar]
- 9.Jonas DE, Kahwati LC, Yun JY, Middleton J, Coker-Schwimmer M, Asher GN. Screening for atrial fibrillation with electrocardiography: Evidence report and systematic review for the us preventive services task force. JAMA. 2018;320:485–498. [DOI] [PubMed] [Google Scholar]
- 10.Engdahl J, Andersson L, Mirskaya M, Rosenqvist M. Stepwise Screening of Atrial Fibrillation in a 75-Year-Old Population Implications for Stroke Prevention. Circulation. 2013;127:930–937. PMID:23343564. [DOI] [PubMed] [Google Scholar]
- 11.Fitzmaurice DA, Hobbs FDR, Jowett S, Mant J, Murray ET, Holder R, Raftery JP, Bryan S, Davies M, Lip GYH, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. Brit Med J. 2007;335:383–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass Screening for Untreated Atrial Fibrillation: The STROKESTOP Study. Circulation. 2015;131:2176–84. [DOI] [PubMed] [Google Scholar]
- 13.Ball J, Carrington MJ, McMurray JJ, Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. 2013;167:1807–24. [DOI] [PubMed] [Google Scholar]
- 14.Mittal S, Movsowitz C, Steinberg JS. Ambulatory external electrocardiographic monitoring: focus on atrial fibrillation. J Am Coll Cardiol. 2011;58:1741–9. [DOI] [PubMed] [Google Scholar]
- 15.Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis. 2013;56:224–9. [DOI] [PubMed] [Google Scholar]
- 16.Fung E, Jarvelin MR, Doshi RN, Shinbane JS, Carlson SK, Grazette LP, Chang PM, Sangha RS, Huikuri HV, Peters NS. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol. 2015;6:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittal S, Movsowitz C, Steinberg JS. Ambulatory External Electrocardiographic Monitoring: Focus on Atrial Fibrillation. J Am Coll Cardiol. 2011;58:1741–1749. [DOI] [PubMed] [Google Scholar]
- 18.Steinhubl SR, Waalen J, Edwards AM, Ariniello LM, Mehta RR, Ebner GS, Carter C, Baca-Motes K, Felicione E, Sarich T, Topol EJ. Effect of a home-based wearable continuous ecg monitoring patch on detection of undiagnosed atrial fibrillation: The mstops randomized clinical trial. JAMA. 2018;320:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heckbert SR, Austin TR, Jensen PN, Floyd JS, Psaty BM, Soliman EZ, Kronmal RA. Yield and consistency of arrhythmia detection with patch electrocardiographic monitoring: The Multi-Ethnic Study of Atherosclerosis. J Electrocardiol. 2018;51:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ARIC Internal Data Distribution Agreement. 2017. Available at: https://www2.cscc.unc.edu/aric/distribution-agreements. Accessed August 15, 2019.
- 21.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 22.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC Jr. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, Vaid H, O’Donnell M, Laupacis A, Côté R, et al. Atrial Fibrillation in Patients with Cryptogenic Stroke. N Engl J Med. 2014;370:2467–2477. [DOI] [PubMed] [Google Scholar]
- 25.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, et al. Cryptogenic stroke and underlying atrial fibrillation. New Engl J Med. 2014;370:2478–86. [DOI] [PubMed] [Google Scholar]
- 26.Healey JS, Alings M, Ha AC, Leong-Sit P, Birnie DH, de Graaf JJ, Freericks M, Verma A, Wang J, Leong D, et al. Subclinical Atrial Fibrillation in Older Patients. Circulation. 2017;136:1276–1283. [DOI] [PubMed] [Google Scholar]
- 27.Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, Noseworthy PA, Perez MV, Turakhia MP. Atrial Fibrillation Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e623–e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguilar MI, Hart R. Oral anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Sys Reviews. 2005:Cd001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saquib N, Saquib J, Ioannidis JP. Does screening for disease save lives in asymptomatic adults? Systematic review of meta-analyses and randomized trials. Int J Epidemiol. 2015;44:264–77. [DOI] [PubMed] [Google Scholar]
- 30.Solomon MD, Yang J, Sung SH, Livingston ML, Sarlas G, Lenane JC, Go AS. Incidence and timing of potentially high-risk arrhythmias detected through long term continuous ambulatory electrocardiographic monitoring. BMC Cardiovasc Disord. 2016;16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med. 2014;15:194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turakhia MP, Ullal AJ, Hoang DD, Than CT, Miller JD, Friday KJ, Perez MV, Freeman JV, Wang PJ, Heidenreich PA. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol. 2015;38:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg MA, Samuel M, Thosani A, Zimetbaum PJ. Use of a noninvasive continuous monitoring device in the management of atrial fibrillation: a pilot study. Pacing Clin Electrophysiol. 2013;36:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett PM, Komatireddy R, Haaser S, Topol S, Sheard J, Encinas J, Fought AJ, Topol EJ. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127:95 e11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turakhia MP, Hoang DD, Zimetbaum P, Miller JD, Froelicher VF, Kumar UN, Xu X, Yang F, Heidenreich PA. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am J Cardiol. 2013;112:520–4. [DOI] [PubMed] [Google Scholar]
- 36.Dussault C, Toeg H, Nathan M, Wang ZJ, Roux JF, Secemsky E. Electrocardiographic monitoring for detecting atrial fibrillation after ischemic stroke or transient ischemic attack: systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2015;8:263–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.