Abstract
Objective
To investigate whether women and men with Parkinson disease (PD) differ in their biochemical and clinical responses to long-term treatment with inosine.
Methods
The Safety of Urate Elevation in Parkinson’s Disease (SURE-PD) trial enrolled 75 people with early PD and baseline serum urate below 6 mg/dL and randomized them to 3 double-blinded treatment arms: oral placebo or inosine titrated to produce mild (6.1–7.0 mg/dL) or moderate (7.1–8.0 mg/dL) serum urate elevation for up to 2 years. Parkinsonism, serum urate, and plasma antioxidant capacity were measured at baseline and repeatedly on treatment; CSF urate was assessed once, at 3 months. Here in secondary analyses results are stratified by sex.
Results
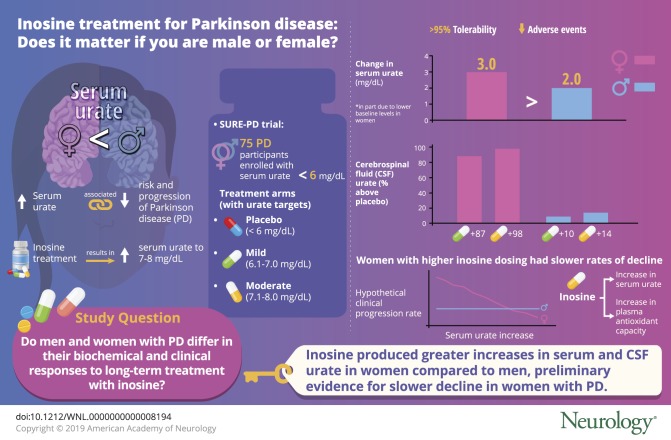
Inosine produced an absolute increase in average serum urate from baseline that was 50% greater in women (3.0 mg/dL) than in men (2.0 mg/dL), consistent with expected lower baseline levels in women. Similarly, only among women was CSF urate significantly greater on mild or moderate inosine (+87% [p < 0.001] and +98% [p < 0.001], respectively) than on placebo (in contrast to men: +10% [p = 0.6] and +14% [p = 0.4], respectively). Women in the higher inosine dosing group showed a 7.0 Unified Parkinson’s Disease Rating Scale (UPDRS) points/year lower rate of decline vs placebo (p = 0.01). In women, slower rates of UPDRS change were associated with greater increases in serum urate (r = −0.52; p = 0.001), and with greater increases in plasma antioxidant capacity (r = −0.44; p = 0.006). No significant associations were observed in men.
Conclusions
Inosine produced greater increases in serum and CSF urate in women compared to men in the SURE-PD trial, consistent with the study's design and with preliminary evidence for slower clinical decline in early PD among women treated with urate-elevating doses of inosine.
Clinicaltrials.gov identifier
Classification of evidence
This study provides Class II evidence that inosine produced greater urate elevation in women than men and may slow PD progression in women.
Serum urate is a reduced risk factor for Parkinson disease (PD)1–5 and a prognostic biomarker of favorable PD progression.6–9 These prospective associations are robust in men, but weaker or absent in women.10–12 One plausible explanation for the difference implicates greater biological effects of urate (or a determinant of urate levels) on PD pathophysiology in men than in women. Sex-specific modulation of disease mechanisms is supported by the well-established lesser risk of PD among women, though clinical progression of PD does not appear to differ consistently by sex.13 Alternatively, assuming similar biological effects of urate in men and women, observational studies may underestimate such effects among women because of their substantially lower urate levels (typically ∼1.0 mg/dL lower than men in both general14 and PD6,7 populations) in combination with a nonlinear relationship between urate concentration and PD outcomes, which is more robust above the population median of serum urate concentration (i.e., >6 mg/dL, at which men outnumber women 6:1 in early PD trials).6,7 Therapy designed to raise serum urate to a fixed target range (e.g., 7–8 mg/dL)15 would produce a greater average increase in serum urate, and, presumably, a greater clinical effect in women than men.
The Safety of Urate Elevation in Parkinson’s Disease (SURE-PD) trial, a randomized, double-blind, placebo-controlled, dose-finding trial of the urate precursor inosine,15 provides an opportunity to explore these complementary hypotheses. Seventy-five participants with early, largely untreated PD and serum urate below 6 mg/dL were randomly assigned to 1 of 3 treatment groups: placebo or inosine dosed to increase serum urate to 6.1–7.0 or 7.1–8.0 mg/dL. Women constituted the majority (55%) of study participants and a similar proportion across the groups (52%, 58%, and 54%, respectively),15 in contrast to the consistent proportion of ∼34% women in de novo PD clinical trials.6,7,16 The higher proportion in SURE-PD was expected due to the exclusion (by design) of consented participants whose screening serum urate was above the population median serum urate value of 6 mg/dL, the vast majority of whom were men (as above).
The primary analyses of SURE-PD15 were conducted on the data from all women and men combined because the study was powered to determine the safety, tolerability, and urate-elevating potential of inosine—the primary outcomes—among all participants rather than in any subgroup. Inosine taken orally up to 3.0 g/d (∼1.4 g/d on average) for up to 2 years (18 months on average) was found to be generally safe, well-tolerated, and effective in dose-dependently elevating serum urate by 2.3 and 3.0 mg/dL to achieve the targeted ranges of 6.1–7.0 and 7.1–8.0 mg/dL. Similarly, CSF urate levels measured once (at the 3-month visit) were significantly higher in each inosine treatment arm compared to the placebo arm.
Exploratory secondary analyses were also conducted on long-term clinical outcomes for all SURE-PD participants despite the study's main limitation of inadequate power for detecting modest treatment effects on these measures. Nevertheless, trends toward a slower rate of clinical decline were observed with increasing inosine dosing based on changes in Unified Parkinson’s Disease Rating Scale (UPDRS) scores. These trends were not statistically significant and were not observed for the complementary long-term outcome measure of clinical decline based on time to disability warranting initiation of dopaminergic therapy. Here we explore whether sex differences may have contributed to the effects of inosine on serum and CSF urate levels, and preliminarily on long-term clinical outcomes of the SURE-PD trial.
Methods
The trial design, implementation, and primary results of SURE-PD have been detailed in prior publications15,17 and under its ClinicalTrials.gov registration NCT00833690, and are summarized below. Here the baseline characteristics of study participants and the primary and secondary analyses are stratified by sex.
Participants, sites, and approval
The 75 participants who enrolled at 16 credentialed Parkinson Study Group clinical sites in the United States between 2009 and 2011 met criteria designed to select people with early typical PD not yet requiring addition of symptomatic antiparkinsonian drug therapy, who had serum urate levels below the population median of ∼6 mg/dL. Key eligibility criteria also excluded those at the greatest risk for complications of increased urate levels (e.g., those with a history of gout or uric acid kidney stones) and precluded enrollment of people taking levodopa or other dopamine-replacement medications (except for a stable dose of a monoamine oxidase-B [MAO-B] inhibitor, which was permitted after a protocol amendment in late 2010 to enhance initially slow enrollment). The study protocol was approved by institutional review boards of the administrative and coordination centers and all clinical sites, and executed under a noncommercial investigational new drug (#100896) accepted by the Food and Drug Administration. All participants provided written informed consent.
Intervention, dosing, and follow-up
Eligible participants were randomized 1:1:1 to 3 treatment groups: (1) placebo, (2) inosine titrated to mildly elevate serum urate (to 6.1–7.0 mg/dL), and (3) inosine titrated to moderately elevate serum urate (to 7.1–8.0 mg/dL). Key covariates did not differ appreciably between randomized treatment groups,15 including for men and women separately (data not shown). Treatment was self-administered orally as capsules containing 500 mg of study drug: inosine (active drug) or lactose (placebo) for up to 24 months before a 1-month washout period. Study drug was taken in 1–2 capsule doses from 1–3 times per day based on a titration algorithm targeting the specified elevated urate ranges for the inosine groups, and on an algorithm intended to match the daily capsule intake of active drug for the placebo group. The algorithms entailed dose adjustments in response to levels of serum urate measured at study visits (before which participants took their daily study drug doses), no more than 3 months apart. Participants and site staff were blinded to treatment group assignment and serum urate results.
Outcomes
Prespecified primary outcomes were safety, tolerability, and efficacy for urate elevation. Safety was defined as the absence of serious adverse events (AEs) that warranted terminating an inosine treatment arm or the trial. Tolerability of study drug was defined as the extent to which assigned treatment could continue without prolonged dose reduction due to AEs. Efficacy for urate elevation was considered demonstrated if either CSF urate (measured at the 12-week visit) or serum urate (measured as change from baseline) levels were significantly greater than in the placebo group.
Secondary outcomes
Additional outcomes, which were intended to aid the design of a potential phase 3 clinical efficacy trial, included clinical outcomes based on serial measurements of parkinsonism (UPDRS subscales, determinations of the need for dopaminergic therapy, and nonmotor assessments) and a biomarker assessment of systemic antioxidant capacity.17
Statistical analysis
Serum urate and plasma ferric reducing antioxidant power (FRAP) levels were analyzed in separate shared-baseline, repeated-measures mixed model analyses of variance (ANOVAs) with fixed effects for sex, treatment group, visit (baseline, 2 or 6 months, and end of study), sex × visit, treatment × postbaseline visit, and sex × treatment × postbaseline visit and unstructured within-person covariance among visits. CSF urate levels were log-transformed and analyzed in 2-way ANOVA of sex × treatment. Estimates were back-transformed. Total UPDRS (defined as the sum of parts I–III) scores were analyzed in a shared-baseline, random-slopes mixed model with fixed effects of sex, treatment group, time since baseline, treatment × time, and sex × treatment × time and random participant-specific intercepts and slopes with unstructured covariance. To address whether any observed sex-dependent difference could be explained by other sex-associated baseline characteristics, we also estimated sex-dependent differences in changes in serum urate, plasma FRAP, CSF urate, and UPDRS total score in sensitivity analyses that adjusted for baseline serum urate level, body mass index, use of an MAO-B inhibitor, or use of a thiazide diuretic. Given our limited sample size, we tested each characteristic separately, including in each of the models above terms for the covariate, its 2-way interaction with visit or time, and its 3-way interaction with visit or time and treatment. Missing data, from loss to follow-up, procedural error or omission, or censoring after initiation of dopaminergic therapy for UPDRS scores, were assumed missing at random conditional on the observed data and the modeled within-person covariance structure, yielding unbiased estimates. Pearson correlations were calculated between the empirical Bayes estimate of UPDRS rate of change for each participant from the unadjusted random-slopes model and the increase from the average of all pretreatment serum urate or plasma FRAP levels to the average serum urate from all visits starting after the second inosine titration (6 weeks after baseline) for each participant or to the average plasma FRAP level at the 2 postbaseline visits at which it was measured (6 months and end of study). Times to disability warranting dopaminergic therapy were summarized by Kaplan-Meier product-limit estimates and analyzed by log-rank test, stratifying by treatment group. Analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC) or R (version 3.0.1; R Foundation for Statistical Computing, Vienna, Austria). Inference was based on 2-tailed tests with α = 0.05 without correction for multiple comparisons.
Classification of evidence
This study investigates whether biochemical and clinical outcomes of the SURE-PD trial may differ by sex, and provides Class II evidence that inosine treatment producing greater increases of serum and CSF urate in women than men with early PD may slow the rate of UPDRS change over 1–2 years to a greater extent among women than men (time × treatment × sex interaction p = 0.007).
Data availability
All individual de-identified participant data from the SURE-PD trial along with its clinical protocol and statistical analysis plan will be shared with qualified researchers upon request to the regulatory sponsor–investigator (Dr. Schwarzschild), the database owner (The Massachusetts General Hospital), or the primary study grantor (The Michael J. Fox Foundation for Parkinson's Research), as feasible. Qualified researchers include those who agree to use the shared study data and materials ethically and exclusively for prespecified biomedical research, the results of which will be made public promptly upon their generation.
Results
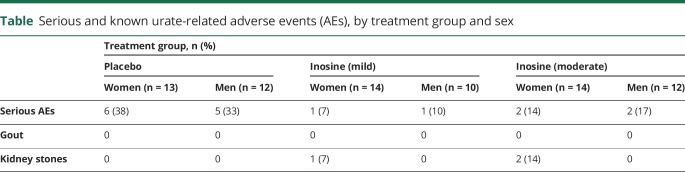
Among the primary outcomes of the SURE-PD trial, study drug tolerability was excellent (>95%) in all 3 groups and its safety in the inosine groups was comparable to if not better than that in the placebo group, as previously reported.15 The distribution of serious AEs (SAEs) between women and men was similar for each treatment group despite the predominance of SAEs in the placebo group (table). However, the risk of kidney stones—the 1 urate-specific AE that likely resulted from inosine treatment—appeared to differ between the sexes, with all 3 occurring in women. Although higher serum urate has been linked to elevated metabolic (glucose and cholesterol) and physiologic (blood pressure) measures, none of these was altered by inosine treatment in SURE-PD overall,15 nor were they modified in men and women separately (data not shown). For example, the change in serum glucose from screening to ∼18 months of treatment with the higher dose of inosine vs placebo was +6.4% in women (p = 0.37) and −6.6% in men (p = 0.33, sex difference p = 0.19).
Table.
Serious and known urate-related adverse events (AEs), by treatment group and sex
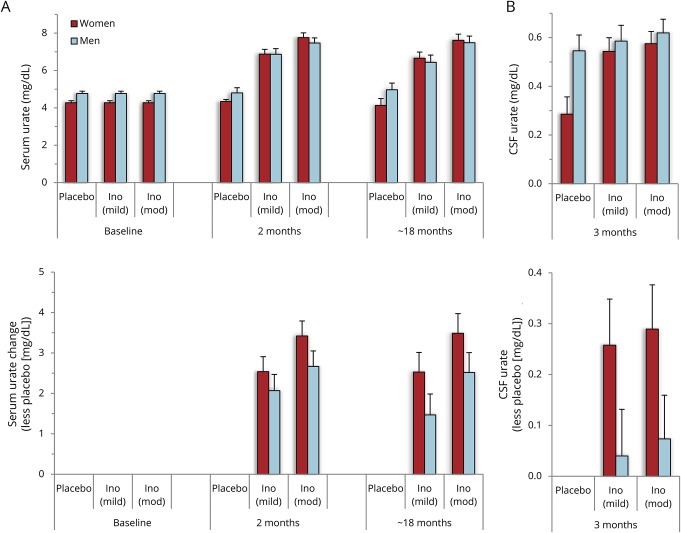
The initial analysis of SURE-PD also demonstrated positive results for the third primary outcome of serum or CSF urate elevation by inosine (see figure 2 in reference 14). However, the increases in serum urate were greater in women than men, and the levels of CSF urate on inosine were greater than on placebo only among women. Although the titration of inosine dosing was equally successful for men and women in achieving the targeted ranges of elevated serum urate (figure 1A), women on average started at a 0.5 mg/dL lower baseline serum urate level (4.3 mg/dL in women and 4.8 mg/dL in men; p < 0.004), contributing to a greater net increase for women than men in either inosine dosing group (1.1 mg/dL [95% confidence interval (CI) −0.36 to 2.5 mg/dL] for mild vs placebo and 1.0 mg/dL [95% CI −0.41 to 2.4 mg/dL] for moderate vs placebo). This represents a ∼70% greater increase in model-estimated serum urate for women compared to men, based on a ∼90% greater increase from baseline to final visit on study drug for women vs men among those assigned to mild inosine dosing vs placebo, and a ∼50% greater increase for women vs men among those assigned to moderate inosine dosing vs placebo. Similarly, the absolute inosine-induced increase in model-estimated serum urate was 50% greater in women than in men: 3.0 vs 2.0 mg/dL increases, respectively, for those in either inosine group compared to the placebo group.
Figure 1. Greater effects of inosine on serum and CSF urate in women than men.
(A) Serum urate was measured prior to treatment randomization (baseline), after steady-state dosing of inosine was achieved (2 months visit), and on the last visit on study drug (∼18 months), and values are presented for each of the 3 treatment arms (placebo, and inosine [Ino] dosed to produce a mild or moderate [mod] elevation in serum urate in women and men) (upper panel). Values plotted are point estimates and standard errors from the shared-baseline, repeated-measures, mixed model analysis of variance (ANOVA). (B) CSF urate was measured only once, at the 3-month visit, and is shown for each treatment group, stratified for women and men (upper panel). Values plotted are point estimates and standard errors from the two-way ANOVA.
Consistent with the greater inosine-induced increase in serum urate in women vs men, an inosine-induced increase in urinary urate output in women was approximately double that in men, which may have contributed to the occurrence of kidney stone AEs only in women in this study. Among women on lower and higher dose inosine, daily output of urinary urate at the 12-week visit was 88% and 135% above the output in the placebo group (with mean values in mg/24-hour ± SD of 408 ± 122, 767 ± 324, and 959 ± 492 in placebo, lower inosine, and higher inosine groups, respectively). Among men in the lower and higher dose inosine groups, 24-hour urine urate output at the 12-week visit was 58% and 56% above the output in the placebo group (with values of 615 ± 177, 970 ± 470, and 958 ± 356, respectively). Also in keeping with a greater urate increase in women, they were titrated to a greater number of (500 mg) inosine capsules per day (2.8 ± 1.8 in women vs 2.3 ± 1.4 in men for the mild and moderate inosine dosing arms combined at the end of study; p = 0.30).
In the subset of participants in whom CSF urate was measured (once at the 12-week visit), those in each inosine treatment group had significantly higher levels than those in the placebo group (p < 0.01).15 However, when stratified by sex (figure 1B), CSF urate was significantly greater on mild or moderate inosine (+87% [p < 0.001] and +98% [p < 0.001], respectively) compared to placebo only in women. CSF urate levels among men on mild or moderate inosine were minimally higher (+10% [p = 0.6] and +14% [p = 0.4], respectively), perhaps reflecting high levels among men on placebo (0.55 mg/dL vs 0.29 mg/dL in women) and limited by the small sample size of men with CSF urate values in each group (n = 6–8). Although we previously observed substantially (50%) higher baseline levels of CSF urate in men with PD (0.42 mg/dL, n = 473) than women with PD (0.28 mg/dL, n = 240),7 in SURE-PD the CSF urate levels among men in the placebo group (n = 6) were higher than expected as they were 90% higher than in women in the placebo group (n = 5) (figure 1B) and were also 30% higher in absolute terms compared to previously reported CSF urate levels in men with PD.7
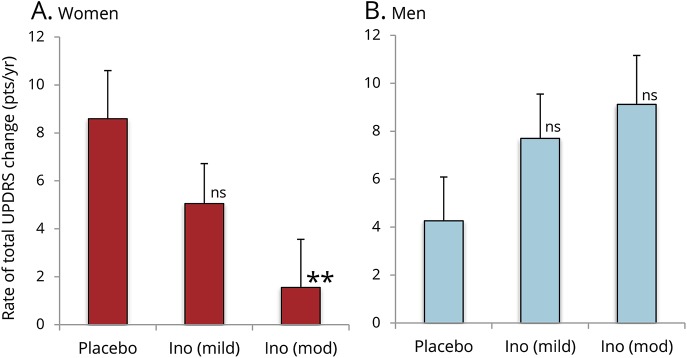
Although the SURE-PD trial was not powered to determine the effects of inosine on clinical progression, an exploratory analysis indicated non-futility of conducting subsequent larger efficacy trials to test the hypothesis that urate-elevating inosine treatment slows clinical decline in PD.15 It also demonstrated a nonsignificant reduction in the rate of clinical deterioration with increasing inosine treatment as assessed by annualized UPDRS score change, though not by the complementary measure of time to disability warranting dopaminergic therapy.15 Stratifying treatment effects on UPDRS score change by sex (figure 2) showed that the trend resulted from a clear inverse association or effect in women but not in men. With randomized assignment to increasing inosine dosing, women showed a dose-dependent decrease in the rate of change in total UPDRS score (parts I–III; p = 0.010), with a 4-fold lower rate of decline in the moderate inosine dosing group compared to the placebo group.
Figure 2. Rates of long-term change in total Unified Parkinson’s Disease Rating Scale (UPDRS) (parts I–III) scores by treatment group.
(A) Women. (B) Men. Values plotted are from the shared-baseline, random-slopes mixed model (**p = 0.01; ns = nonsignificant compared to placebo). Ino = inosine.
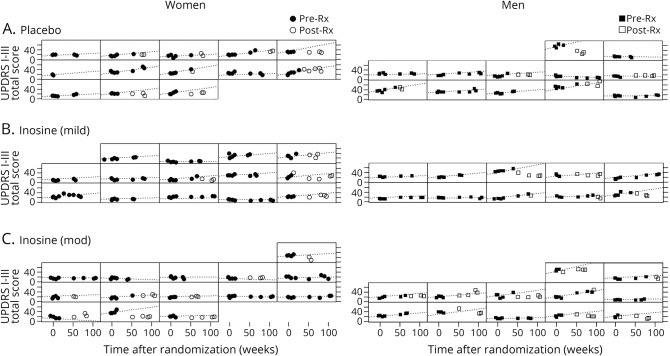
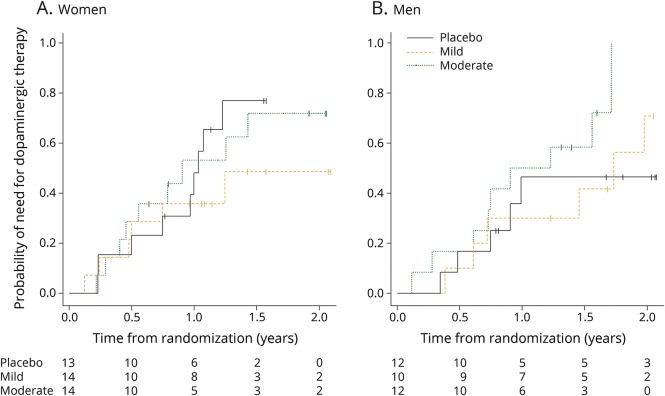
By contrast, there was no decrease in clinical progression with increasing inosine dosing among men. Instead, those in the moderate inosine dosing group showed a 2-fold higher rate compared to placebo (p > 0.05, time × treatment × sex interaction p = 0.007, p = 0.002 to 0.014 in models adjusting for baseline serum urate and other sex-associated baseline characteristics). The difference between sexes in this small sample set can be appreciated by reviewing the raw total UPDRS data graphed over time for each participant among women separately from men (figure 3). Assessment of the complementary measure of time to disability warranting dopaminergic therapy suggested a similar pattern of dissociation by sex, with a weak association of increasing inosine dosing and time to disability in women and a weak inverse association in men (figure 4).
Figure 3. Individual profiles of total Unified Parkinson’s Disease Rating Scale (UPDRS) (parts I–III) scores.
Individual profiles of total UPDRS (parts I–III) scores over months to years, and before (filled symbols) and after (unfilled symbols) need for dopaminergic therapy was determined for each participant randomized to (A) placebo, or to Inosine titrated to a (B) mildly (mild) or (C) moderately (mod) elevated serum urate range, and stratified by sex. Estimated rate (indicated by a dashed line in each profile) of UPDRS total score change prior to initiation of dopaminergic treatment were based on the shared-baseline, random-slopes mixed model. Total UPDRS scores following determination of need for levodopa or other antiparkinsonian medication (indicated by unfilled Post-Rx symbols) often were lower (better) than what was projected (dashed line) rate of change based on total UPDRS scores prior to the determination, consistent with symptomatic benefit in some patients.
Figure 4. Kaplan-Meier plot of time to need for dopaminergic therapy.
(A) Women. (B) Men.
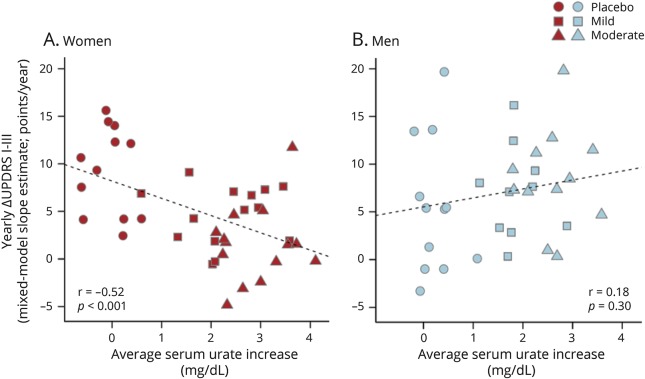
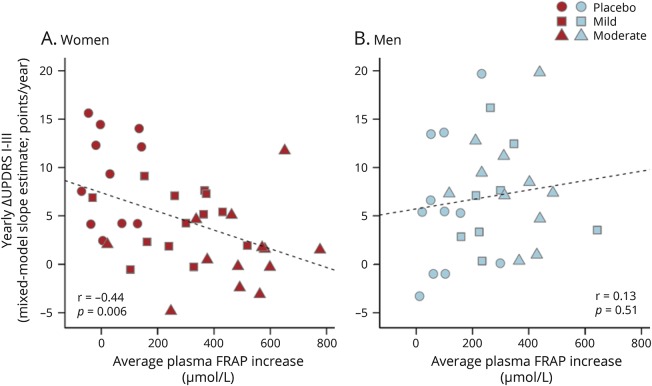
Individual increases in serum urate on study drug also correlated inversely with the rate of clinical deterioration, measured as increase in total UPDRS, in women (r = −0.52; p < 0.001), but not men (r = 0.18; p > 0.05; figure 5). Similarly, individual increases in FRAP, an index of antioxidant capacity in plasma,18 which was also increased by inosine treatment in SURE-PD17 and correlated with serum urate increases in both women (r = 0.87; p < 0.001) and men (r = 0.84; p < 0.001), correlated inversely with the rate of clinical deterioration in women (r = −0.44; p < 0.01), but not men (r = 0.13; p > 0.05; figure 6).
Figure 5. Relationships between change in serum urate and estimated rate of clinical decline.
(A) Women. (B) Men. For each participant, the increase in serum urate from baseline to the average of on-treatment values was plotted against the individual's annualized change in total UPDRS score estimated from the random-slopes model shown in figure 2. The placebo group data (circles) appear on the left of each plot given the expected lack of any urate change. Although a clustering of men in the placebo group showed no decline during the trial (filled circles in lower left), contributing to an overall trend toward worsening with increasing urate among men, the trend was not significant. UPDRS = Unified Parkinson’s Disease Rating Scale.
Figure 6. Relationships between change in plasma total antioxidant capacity and rate of clinical decline.
(A) Women. (B) Men. For each participant, the increase in plasma ferric reducing antioxidant power (FRAP) from baseline to the average of on-treatment (6 months and ∼18 months visit) values was plotted against the individual's annualized change in total UPDRS score using the conservative mixed model slope estimate shown in figure 2. The placebo group data (circles) appear on the left of each plot given the lack of FRAP change as expected in this group. Although a clustering of men in the placebo group showed no clinical decline during the trial (filled circles in lower left), contributing to an overall trend toward worsening with increasing FRAP (like urate) among men, the trend was not significant. UPDRS = Unified Parkinson’s Disease Rating Scale.
Discussion
Subanalysis of SURE-PD data stratified by sex demonstrates that inosine produced greater serum and CSF urate elevations in women than in men. The difference in inosine-induced serum urate increase reflects the well-established sex difference in baseline serum urate levels (∼1 mg/dL lower in women,6,7,14 possibly due to estrogen effects on the renal tubule19) in combination with the trial’s design. Although SURE-PD employed the same serum urate eligibility requirements (<6 mg/dL) and target ranges (6.1–7.0 and 7.1–8.0 mg/dL for the 2 inosine treatment groups) irrespective of sex, the expected (and confirmed) lower baseline level in women resulted in their experiencing a greater inosine-induced increase of 1.0 mg/dL to achieve the same targeted range for elevated serum urate. Similarly, CSF urate levels in the inosine groups were significantly greater than on placebo only among women.
If the effects of urate elevation are biologically similar in women and men, then it may be expected that urate-mediated benefits and adverse effects of inosine under the conditions of SURE-PD would be greater in women. Although serious AEs overall were actually less common in the inosine compared to the placebo treatment group in this trial, kidney stones have been the main AE in prior inosine trials (for multiple sclerosis)20,21 and indeed occurred in SURE-PD only in the inosine groups. The episodes of stones in SURE-PD also occurred only in (3) women, despite men typically experiencing kidney stones at twice the incidence as women.22 Thus the occurrence of inosine-associated kidney stones only in women in this study may reflect the greater urate elevations produced by inosine in women or the higher inosine dosages required to achieve those increases, although chance association with women cannot be excluded given the small number of stone AEs.
Similarly, the exploratory analysis of long-term clinical outcomes in SURE-PD when stratified by sex indicated a significant difference by sex with slowing of clinical progression only in women, and an association between slower progression and greater elevation of urate only in women in this study. Mechanistically, the close correlation between greater FRAP as well as serum urate increases and favorable clinical progression in women lends indirect support to the possibility that a disease-modifying benefit of elevating urate is associated with if not mediated by its well-established antioxidant properties.23 Interestingly, in recently reported results of a randomized, placebo-controlled trial of IV urate as a protective treatment of evolving ischemic stroke, stratification of the results by sex in a prespecified secondary analysis of the primary outcome showed evidence that an excellent functional outcome was significantly more likely at 90 days (primary outcome) in women but not in men.24,25 The authors similarly speculated that because women have lower baseline urate levels than do men, “A lower antioxidant capacity in women than in men might have been a factor in the greater benefits seen in women after uric acid replacement …”24
The present data suggest that women would stand to gain at least as much as men from urate-elevating inosine therapy if it were indeed found to protect against long-term clinical decline in PD. The results also suggest an explanation for the seemingly paradoxical epidemiologic data linking higher urate levels to a lower risk1–5,10,11 or slower progression6–9 of PD in men more than women. These association studies demonstrated favorable PD outcomes with increasing serum urate primarily above the population median for serum urate (∼6 mg/dL). Thus women, who represent a relatively small proportion of patients with PD naturally having urate levels above the median, would as a group show a statistically weaker reduction in risk of PD or its progression. Conversely, with their naturally low levels of urate, women may be more likely to experience benefit from targeted urate elevation into a hypothetically “protective” range.
The sex-stratified results of SURE-PD have practical implications for future clinical trials investigating urate elevation in neurology. Although the identification of urate as an inverse PD risk factor primarily in men raised the possibility of conducting initial inosine trials exclusively in men with PD, the current data argue strongly for the inclusion of women as well as men in full efficacy trials of urate-elevating strategies for PD and other disorders of neuronal injury or degeneration. One might even argue based on the present data with long-term benefit apparent only in women to focus exclusively on women in subsequent phase 3 trials for PD, stroke, and other neurologic diseases. However, the greatest limitation of the current subanalysis of SURE-PD clinical progression data is that it is substantially underpowered for drawing conclusions about disease-modifying efficacy of inosine for all participants (with only ∼25 participants per treatment group), let alone for women or men separately. Accordingly, the lack of evidence for attenuated progression in the subset of men does not constitute evidence against such an effect in men, and it does not warrant their exclusion from subsequent clinical testing, especially in light of the robust epidemiologic links in men.
Other limitations of our study include its uncertain relevance to those with higher serum urate because of the eligibility criterion restricting enrollment to those with a serum urate level of ≥6 mg/dL (which excluded 43% of consented participants prior to randomization). However, this exclusion was based on safety concerns (over urate elevation in participants with higher urate levels at baseline) rather than any evidence that the role of urate in PD pathophysiology diminishes at higher concentrations. Another study limitation is the lack of a direct measure of oxidative damage as a biomarker of the potential to benefit from inosine treatment given a putative antioxidant mechanism of action. Nevertheless, documentation of elevated urate and antioxidant capacity (FRAP) represents at least indirect evidence of target engagement by inosine. Moreover, the study's restriction to a PD subpopulation with lower serum urate provides an early example of a precision medicine strategy26—based on targeting only patients with PD with diminished serum urate and therefore likely with diminished peripheral antioxidant capacity and possibly greater oxidative damage, who thus may benefit more from bolstering antioxidant capacity.
The demonstration of significantly greater absolute increases in urate in women than in men, in part due to the design of SURE-PD, encourages consideration of sex-specific serum urate target ranges (i.e., a higher one for men) in planning a phase 3 trial of inosine for PD. However, the dearth of available data on clinical progression in PD with serum urate levels >8 mg/dL in combination with the markedly increased risk (to ≥1% annual incidence) of gout in people who chronically exceed this level27 suggest that a conservative target for sustained elevation of serum urate at this point remains no more than 8.0 mg/dL for both men and women. Overall, the present post hoc analysis of sex differences in SURE-PD outcomes strengthens the evidence that the biological relationship between urate and PD is as important in women as in men, and may be most prudently incorporated into future efficacy trials by ensuring the prespecified inclusion of secondary analysis by sex.
Acknowledgment
The authors thank the Parkinson Study Group SURE-PD Investigators and contributors. Their complete listings can be found in reference 14. The authors thank Roseanna Battista for her assistance in organizing the manuscript submission and the study participants and their families for their contributions.
Glossary
- AE
adverse event
- ANOVA
analysis of variance
- CI
confidence interval
- FRAP
ferric reducing antioxidant power
- MAO-B
monoamine oxidase-B
- PD
Parkinson disease
- SAE
serious adverse event
- SURE-PD
Safety of Urate Elevation in Parkinson’s Disease
- UPDRS
Unified Parkinson’s Disease Rating Scale
Appendix. Coinvestigators

Footnotes
Editorial, page 611
Class of Evidence: NPub.org/coe
Author contributions
Michael A. Schwarzschild: drafting/revising the manuscript, data acquisition, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, study supervision, obtaining funding. Eric A. Macklin: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, statistical analysis. Rachit Bakshi: data acquisition, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Shamik Bhattacharyya: drafting/revising the manuscript, data acquisition, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Robert Logan: data acquisition, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Alberto J. Espay: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data. Albert Hung: drafting/revising the manuscript, accepts responsibility for conduct of research and approval, acquisition of data. Grace Bwala: data acquisition, accepts responsibility for conduct of research and final approval, acquisition of data. Christopher G. Goetz: drafting/revising the manuscript, accepts responsibility for conduct of research and final approval, acquisition of data, study supervision. David S. Russell: drafting/revising the manuscript, data acquisition, accepts responsibility for conduct of research and final approval. John Goudreau: drafting/revising the manuscript, data acquisition, accepts responsibility for conduct of research and final approval, acquisition of data. Sotirios Andreas Parashos: drafting/revising the manuscript, data acquisition, accepts responsibility for conduct of research and final approval. Marie-Helene Saint-Hilaire: drafting/revising the manuscript, data acquisition, accepts responsibility for conduct of research and final approval, acquisition of data. Alice Rudolph: drafting/revising the manuscript, accepts responsibility for conduct of research and final approval, study supervision. Joshua Hare: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, obtaining funding. Gary Curhan: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Alberto Ascherio: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval.
Study funding
The study is not industry-sponsored. This study is funded by The Michael J. Fox Foundation for Parkinson's Research (MJFF2768) with additional support from the NIH 5U01NS090259, a Jane & Alan Batkin Fellowship, and for biostatistical services from the Harvard NeuroDiscovery Center.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM. Observations on serum uric acid levels and the risk of idiopathic Parkinson's disease. Am J Epidemiol 1996;144:480–484. [DOI] [PubMed] [Google Scholar]
- 2.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol 2005;58:797–800. [DOI] [PubMed] [Google Scholar]
- 3.Weisskopf MG, O'Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson's disease. Am J Epidemiol 2007;166:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen C, Guo Y, Luo W, Lin C, Ding M. Serum urate and the risk of Parkinson's disease: results from a meta-analysis. Can J Neurol Sci 2013;40:73–79. [DOI] [PubMed] [Google Scholar]
- 5.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol 2016;15:1257–1272. [DOI] [PubMed] [Google Scholar]
- 6.Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol 2008;65:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ascherio A, LeWitt PA, Xu K, et al. ; Parkinson Study Group DATATOP Investigators. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol 2009;66:1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moccia M, Picillo M, Erro R, et al. Presence and progression of non-motor symptoms in relation to uric acid in de novo Parkinson's disease. Eur J Neurol 2015;22:93–98. [DOI] [PubMed] [Google Scholar]
- 9.Pellecchia MT, Savastano R, Moccia M, et al. Lower serum uric acid is associated with mild cognitive impairment in early Parkinson's disease: a 4-year follow-up study. J Neural Transm 2016;123:1399–1402. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Mosley TH, Alonso A, Huang X. Plasma urate and Parkinson's disease in the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 2009;169:1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao X, O'Reilly ÉJ, Schwarzschild MA, Ascherio A. Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology 2016;86:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortese M, Riise T, Engeland A, Ascherio A, Bjørnevik K. Urate and the risk of Parkinson's disease in men and women. Parkinsonism Relat Disord 2018;52:76–82. [DOI] [PubMed] [Google Scholar]
- 13.Dahodwala N, Pei Q, Schmidt P. Sex differences in the clinical progression of Parkinson's disease. J Obstet Gyneol Neonatal Nurs 2016;45:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odden MC, Amadu AR, Smit E, Lo L, Peralta CA. Uric acid levels, kidney function, and cardiovascular mortality in US adults: National Health and Nutrition Examination Survey (NHANES) 1988-1994 and 1999-2002. Am J Kidney Dis 2014;64:550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkinson Study Group SURE-PD Investigators, Schwarzschild MA, Ascherio A, et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol 2014;71:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkinson Study Group QE3 Investigators, Beal MF, Oakes D, et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol 2014;71:543–552. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharyya S, Bakshi R, Logan R, Ascherio A, Macklin EA, Schwarzschild MA. Oral inosine persistently elevates plasma antioxidant capacity in Parkinson's disease. Mov Disord 2016;31:417–421. [DOI] [PubMed] [Google Scholar]
- 18.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996;239:70–76. [DOI] [PubMed] [Google Scholar]
- 19.Nicholls A, Snaith ML, Scott JT. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br Med J 1973;1:449–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz CE, Spitsin S, Zimmerman V, et al. The treatment of multiple sclerosis with inosine. J Altern Complement Med 2009;15:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonsette RE, Sindic C, D'hooghe MB, et al. ; ASIIMS study group. Boosting endogenous neuroprotection in multiple sclerosis: the Association of Inosine and Interferon-beta in relapsing-remitting Multiple Sclerosis (ASIIMS) trial. Mult Scler 2010;16:455–462. [DOI] [PubMed] [Google Scholar]
- 22.Pearle MS, Calhoun EA, Curhan GC. Urologic Diseases of America Project: urolithiasis. J Urol 2005;173:848–857. [DOI] [PubMed] [Google Scholar]
- 23.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 1981;78:6858–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chamorro Á, Amaro S, Castellanos M, et al. ; URICO-ICTUS Investigators. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol 2014;13:453–460. [DOI] [PubMed] [Google Scholar]
- 25.Llull L, Laredo C, Renú A, et al. Uric acid therapy improves clinical outcome in women with acute ischemic stroke. Stroke 2015;46:2162–2167. [DOI] [PubMed] [Google Scholar]
- 26.Espay AJ, Schwarzschild MA, Tanner CM, et al. Biomarker-driven phenotyping in Parkinson's disease: a translational missing link in disease-modifying clinical trials. Mov Disord 2017;32:319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi HK, Mount DB, Reginato AM, American College of Physicians. American physiological society. Pathogenesis of gout. Ann Intern Med 2005;143:499–516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All individual de-identified participant data from the SURE-PD trial along with its clinical protocol and statistical analysis plan will be shared with qualified researchers upon request to the regulatory sponsor–investigator (Dr. Schwarzschild), the database owner (The Massachusetts General Hospital), or the primary study grantor (The Michael J. Fox Foundation for Parkinson's Research), as feasible. Qualified researchers include those who agree to use the shared study data and materials ethically and exclusively for prespecified biomedical research, the results of which will be made public promptly upon their generation.