Abstract
Background
This study assessed whether a quality improvement (QI) process to streamline transfer from a primary stroke center (PSC) to a comprehensive stroke center (CSC) could reduce the delay of reperfusion by mechanical thrombectomy (MT).
Methods
From 2015 to 2017, a QI process was implemented with specific interventions to reduce door-in-to-door-out (DIDO) time in a high volume PSC, and speed up interhospital transfer and inhospital processes at the CSC. Clinical characteristics and time metrics were compared in the QI (2015–2017; n = 157) and pre-QI cohorts (2012–2014; n = 121).
Results
During the QI process, the median symptom onset to reperfusion time was reduced by 50 minutes (367 vs 417 minutes in the pre-QI cohort, p < 0.04), with a substantial 40-minute DIDO reduction (78 vs 118 minutes, p < 0.01), related to the faster administration of IV thrombolysis (median door-to-needle time: 49 vs 82 minutes, p = 0.0001). The door-to-door time was shortened (170 vs 205 minutes, p = 0.002), but not the transfer time (92 vs 87 minutes, p = 0.5). The QI process had no effect on the prehospital phase (77 vs 76 minutes, p = 0.83) and on the time from MRI imaging at the PSC to reperfusion (252 vs 288 minutes, p = 0.12). The rate of modified Rankin Scale score 0–2 at 90 days was comparable in the pre-QI and QI cohorts.
Conclusions
A QI process can reduce the reperfusion therapy delay in a distant CSC; however, we could not demonstrate that it can also improve the outcome of patients who undergo MT.
A meta-analysis of clinical trials confirmed the clinical benefit of mechanical thrombectomy (MT)1 in selected patients with acute ischemic stroke (AIS) and large vessel occlusion (LVO), and indicated that patients' transfer from a primary stroke center (PSC) to a comprehensive stroke center (CSC) for rescue thrombectomy is a valid option. However, the treatment benefit decreases with longer times to reperfusion time.2 Therefore, intrahospital and interhospital workflows need to be improved to facilitate and accelerate MT implementation.3 Here, we assessed whether a 3-year quality improvement (QI) process concerning the intrahospital/interhospital workflow with reduction of the PSC door-in-to-door-out (DIDO) time could reduce the reperfusion delay for patients admitted in a PSC and transferred to a distant CSC (156 km apart; 1.5 hour by ambulance).
Methods
From January 1, 2012 to December 31, 2017, the clinical data of all patients with AIS due to LVO admitted to the PSC were extracted from a prospectively maintained database. Patients admitted to the PSC beyond 4.5 hours after symptom onset were not included in the study analysis because they were judged ineligible for transfer due to the anticipated transport delay that precluded MT implementation within 6 hours from symptom onset. The criteria for patients' transfer to the CSC and procedures are summarized in table 1. The following data were systematically collected: demographic features, pretreatment NIH Stroke Scale (NIHSS) score, Diffusion-Weighted Imaging-Alberta Stroke Program Early CT (DWI-ASPECT) score, and modified Rankin Scale score at 3 months post-MT. The QI process was implemented from January 1, 2015 to December 31, 2017, with specific interventions to reduce DIDO and PSC-to-CSC transfer times, and to streamline the intrahospital workflow at the CSC (for details, see table 1(g) and figure e-1, links.lww.com/CPJ/A105). The following time metrics were compared in the pre-QI cohort (2012–2014) and QI cohort (2015–2017): PSC door to IV thrombolysis (IVT) start (door-to-needle [DTN]), PSC DIDO, PSC-door-out to CSC-door-in (transfer time), PSC door-in to CSC door-in (door-to-door [D2D]), symptom onset to PSC door, symptom onset to IVT start, symptom onset to CSC door, imaging at PSC to reperfusion, MT puncture to reperfusion, and symptom onset to reperfusion. Data were presented using medians, interquartile ranges (IQRs), means, and SDs. Significance threshold was set at 5% and was calculated using the χ2 and Student's t tests, and Kruskal-Wallis analysis of variance.
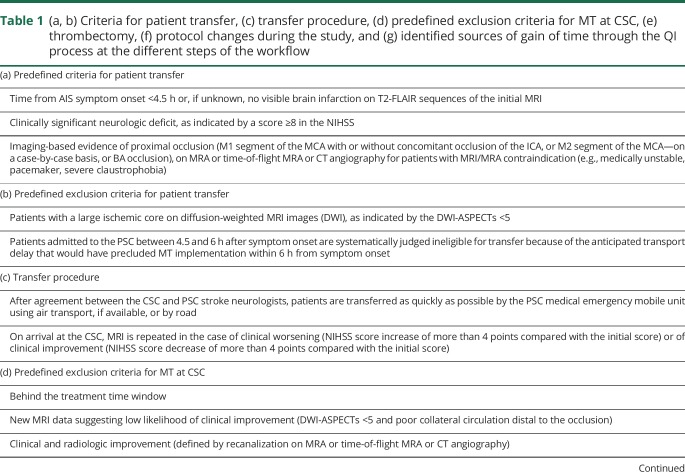
Table 1.
(a, b) Criteria for patient transfer, (c) transfer procedure, (d) predefined exclusion criteria for MT at CSC, (e) thrombectomy, (f) protocol changes during the study, and (g) identified sources of gain of time through the QI process at the different steps of the workflow
Standard protocol approvals, registrations, and patient consents
The QI process was approved by the local ethics committee and was coordinated by the Languedoc-Roussillon Regional Health Agency, France.
Data availability
All data used for the analysis and not published in the article will be shared, in an anonymized manner, with any qualified investigator on simple request.
Results
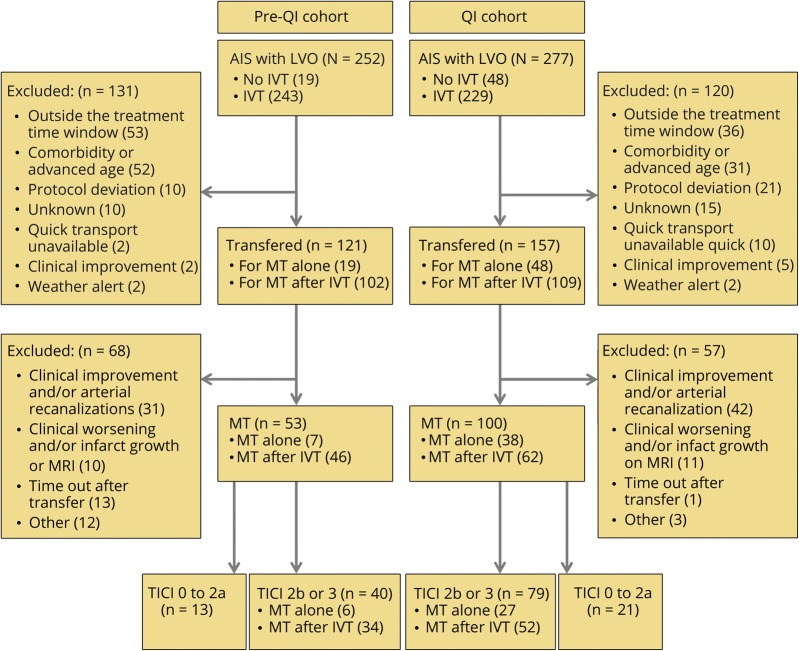
Between January 2012 and December 2017, 529 patients were admitted at the PSC for AIS due to LVO (83 with IVT contraindication and 446 who received IVT), and 278 were transferred to the CSC (transfer rate = 52.6%) for MT alone (n = 67) or “rescue MT after IVT” (n = 211) (figure 1). Their mean age was 73 ± 14 years (range 83), 52.8% were men, and the median baseline NIHSS score was 16 (IQR: 8–21) (table 2).
Figure 1. Study flowchart.
AIS = acute ischemic stroke; IVT = IV thrombolysis; LVO = large vessel occlusion; MT = mechanical thrombectomy; QI = quality improvement; TICI, thrombolysis in cerebral infarction.
Table 2.
Demographic and clinical characteristics
Comparison of the demographic and clinical characteristics did not identify any difference between the QI and the pre-QI cohorts, except for the mean age (71 years in the QI cohort and 67 years in the pre-QI cohort, p = 0.0038) (table 1). During the QI process, the median PSC workflow was shortened by 40 minutes compared with the pre-QI period (median DIDO: 78 for the QI vs 118 minutes for the pre-QI cohort, p < 0.01), because of the faster IVT administration (median DTN time: 49 vs 82 minutes, p = 0.0001) (table 2). The median D2D time improved (170 vs 205 minutes, p = 0.002), although transfer time did not change (92 vs 87 minutes, p = 0.5). Finally, the symptom onset to reperfusion time was reduced by 50 minutes in the QI cohort (median 367 vs 417 minutes, p < 0.04). The QI process did not modify the prehospital time (77 vs 76 minutes, p = 0.83), the transfer time (92 vs 87 minutes, p = 0.5), and the imaging to reperfusion time (252 vs 288 minutes, p = 0.12) (table 3 and figure 2). Good functional outcome (rate of mRs score = 0–2) at 90 days was not different between cohorts (table 2 and figure e-2, links.lww.com/CPJ/A105).
Table 3.
Main median times
Figure 2. Comparison of the main relevant times (in minutes, reported as medians) before and during the QI process.
CSC = comprehensive stroke center; D2D = door-to-door (PSC-door-in-CSC-door-in); DIDO, door-in to door-out; DTN = door-to-needle; IVT = IV thrombolysis; PSC = primary stroke center; QI = quality improvement.
Discussion
In patients with AIS and LVO, any delay in arterial reperfusion worsens the clinical outcomes.2 The risk of delayed reperfusion is higher for patients who arrive at PSCs without an on-site interventional neuroradiology service,4–7 with negative effects on their prognosis.8 It has been shown that DIDO times are usually too long and must be shortened to improve MT access.3 During our QI process, we reduced DIDO time by 40 minutes, and the D2D time by 35 minutes. DIDO and D2D times seemed to be influenced mainly by changes in the DTN time (figure 2). Conversely, our QI process did not manage to shorten several unavoidable, time-consuming tasks (imaging data transfer to CSC, CSC referral, transfer organization, and transfer time) after imaging and thrombolysis. On the other hand, intrahospital procedures were implemented to reduce the CSC workflow, with a gain of 9 and 17 minutes (not significant) before and after arterial puncture, respectively, contributing to the overall 50-minute gain.
The clinical effect of this faster workflow is not documented in our series; however, we observed a trend toward a better functional outcome. A larger sample is needed to confirm this observation. Although the median symptom onset to recanalization time was substantially reduced (from 417 minutes in the pre-QI cohort to 367 minutes in QI cohort), MT was still performed in a median time window of low therapeutic efficiency (i.e., >6 hours after symptom onset).4 Except for the DIDO time, the QI process did not shorten the subsequent transfer delays, which represent more than 60% of the symptom onset to reperfusion time in our configuration. Because of geographical and organizational considerations, alternative options to further reduce the symptom onset to reperfusion time in our situation are limited. Direct access to the CSC, without stopping first at the PSC, would result in an almost 2-hour loss for patients undergoing IVT, and this option is not suitable for our mountainous area at 156 km from the CSC. Indeed, a statistical model showed that when the transfer time between PSC and CSC is longer than 45 minutes, the “drip and ship” option provides the most favorable outcome scenario.9 Moreover, it would be difficult to further reduce the DIDO time when using multimodal MRI screening, although, to our knowledge, our 78-minute DIDO time is the fastest ever reported time with this screening modality. A 75-minute DIDO time (top 15th centile) was proposed as key performance index for PSC workflow with CT screening.3 Moving from MRI to CT screening could result in a larger DIDO reduction. However, the potential gain of 20 minutes should be balanced with the loss of the comprehensive information provided by multimodal MRI that can be used for clinical decision-making and prognostic purposes.10 Ultimately, conversion of distant high-volume PSCs with sufficient clinical expertise into CSCs that can perform MT could be a rational and effective approach to provide MT within acceptable time frames for large catchment areas that are far away from existing CSCs.
Our study has some limitations. First, data came from a single center with a small number of patients and some features, such as age, MT time window and occlusion location, changed during the study. Second, confounding factors, such as age, could have influenced the outcome. Moreover, some other baseline characteristics, such as etiology and patient's history, could not be compared between cohorts because they were rarely available in the pre-QI cohort. Third, our shorter times could also be explained by improved efficiency because the teams progressively gained experience in patient management. Nevertheless, our study demonstrates that a QI process to streamline the intrahospital/interhospital workflow can accelerate the implementation of distant reperfusion therapy, with a 50-minute reduction of the median symptom onset to reperfusion time.
TAKE-HOME POINTS
→ A QI process to streamline the intrahospital/interhospital workflow can accelerate the implementation of distant reperfusion therapy, with a 50-minute reduction of the median symptom onset to reperfusion time.
→ A substantial 40-minute reduction of DIDO is attainable, mainly through DTN time reduction; conversely, the QI process had little effect on the other time metrics after imaging and thrombolysis at the PSC.
→ A QI process on its own is not sufficient for early reperfusion at distant or very distant PSC.
Appendix. Authors

Footnotes
Editorial 368
Study funding
No targeted funding reported.
Disclosure
The authors report no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Goyal M, Menon BK, Dippel DW, et al. . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, van der Lugt A, et al. . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316:1279–1288. [DOI] [PubMed] [Google Scholar]
- 3.Ng FC, Low E, Andrew E, et al. . Deconstruction of interhospital transfer workflow in large vessel occlusion: real-world data in the thrombectomy era. Stroke 2017;48:1976–1979. [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Jadhav AP, Bonafe A, et al. . Analysis of workflow and time to treatment and the effects on outcome in endovascular treatment of acute ischemic stroke: results from the SWIFT PRIME randomized controlled trial. Radiology 2016;279:888–897. [DOI] [PubMed] [Google Scholar]
- 5.Venema E, Boodt N, Berkhemer OA, et al. . Workflow and factors associated with delay in the delivery of intra-arterial treatment for acute ischemic stroke in the MR CLEAN trial. J Neurointerv Surg 2018;10:424–428. [DOI] [PubMed] [Google Scholar]
- 6.Sablot D, Gaillard N, Smadja P, Bonnec JM, Bonafe A. Thrombectomy accessibility after transfer from a primary stroke center: analysis of a three-year prospective registry. Int J Stroke 2017;12:519–523. [DOI] [PubMed] [Google Scholar]
- 7.Menon BK, Sajobi TT, Zhang Y, et al. . Analysis of workflow and time to treatment on thrombectomy outcome in the endovascular treatment for small core and proximal occlusion ischemic stroke (ESCAPE) randomized, controlled trial. Circulation 2016;133:2279–2286. [DOI] [PubMed] [Google Scholar]
- 8.Froehler MT, Saver JL, Zaidat OO, et al. . Interhospital transfer before thrombectomy is associated with delayed treatment and worse outcome in the STRATIS registry (systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke). Circulation 2017;136:2311–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holodinsky JK, Williamson TS, Kamal N, Mayank D, Hill M, Goyal M. Drip and ship versus direct to comprehensive stroke center: conditional probability modeling. Stroke 2017;48:233–238. [DOI] [PubMed] [Google Scholar]
- 10.Sablot D, Ion I, Khlifa K, et al. . Target door-to-needle time for tissue plasminogen activator treatment with magnetic resonance imaging screening can be reduced to 45 min. Cerebrovasc Dis 2018;45:245–251. [DOI] [PubMed] [Google Scholar]
- 11.Hacke W, Kaste M, Fieschi C, et al. . Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke. Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–1251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used for the analysis and not published in the article will be shared, in an anonymized manner, with any qualified investigator on simple request.