Abstract
Objectives:
Otitis media with effusion (OME) is a common disease of childhood that is largely asymptomatic. However, middle ear fluid can persist for months and negatively impact a child’s quality of life. Many cases of OME remain chronic and require surgical intervention. As biofilms are known to contribute to the persistence of many diseases, this study examined effusions collected from children with chronic OME for presence of essential biofilm structural components, members of the DNABII family of bacterial DNA-binding proteins.
Methods:
Middle ear effusions were recovered from 38 children with chronic OME at the time of tympanostomy tube insertion. A portion of each specimen was submitted for microbiology culture. The remaining material was assessed by immunoblot to quantitate individual DNABII proteins, integration host factor (IHF) and histone-like protein (HU).
Results:
Sixty-five percent of effusions (24/37) were culture-positive for bacterial species or yeast, whereas 35% (13/37) were culture-negative. IHF was detected in 95% (36/38) at concentrations from 2– 481 ng/ μl effusion. HU was detected in 95% (36/38) and quantitated from 13– 5,264 ng/ μl effusion (P≤ 0.05 compared to IHF).
Conclusion:
As DNABII proteins are essential structural components of bacterial biofilms, these data lend further support to our understanding that biofilms are present in the vast majority of chronic middle ear effusions, despite negative culture results. The presence and ubiquity of DNABII proteins in OME specimens indicated that these proteins can serve as an important clinical target for our novel DNABII-directed strategy to treat biofilm diseases such as chronic OME.
Keywords: DNABII proteins, IHF, HU, pediatric otolaryngology, chronic otitis media with effusion
INTRODUCTION
Otitis media with effusion (OME) is a common disease of childhood, and up to 90% of children experience an episode prior to 5 years of age. OME is a leading cause for primary care visits in the United States. Approximately 2.2 million episodes are diagnosed annually, although the true incidence of OME is likely higher, as this disease is largely asymptomatic and many episodes remain undiagnosed.1,2 The annual direct costs of OME to the healthcare system are approximately $4.0 billion annually, and this estimate does not include the sizeable indirect costs associated with lost caregiver productivity.1,3 OME is also the most common cause of hearing loss in children in the developing world, and can affect up to 80% of children at any given point in time.4
OME is characterized by the presence of fluid in the middle ear cavity without signs or symptoms of acute inflammation.1 While the majority of cases recover spontaneously, approximately 25% of episodes persist longer than 3 months and are deemed ‘chronic OME’, and 10% of episodes last longer than 1 year.5,6 Sequelae include significant conductive hearing loss, vestibular problems, and ear discomfort.7 Importantly, OME affects children at a critical age of development and can result in significant speech and language delays, educational deficiencies, poor academic performance, behavioral problems and overall reduced quality of life.7
OME was initially thought to be the outcome of an accumulation of sterile fluid in the middle ear, particularly as the condition does not present with pain, fever, or tympanic membrane inflammation; all signs traditionally associated with acute bacterial infection. However, multiple groups have detected bacterial nucleic acids in culture-negative middle ear effusions via polymerase chain reaction (PCR) assay, thereby demonstrating the highly limited usefulness of traditional culture techniques to confirm the bacterial etiology of OME.8–10 Further, Rayner et al. demonstrated the presence of bacterial mRNA and proteins in culture-negative effusions, which indicated that not only were bacteria present within the middle ear, but they were also metabolically active.11 In an effort to localize the bacteria within the middle ear during disease, Hall-Stoodley et al. examined mucosal biopsies recovered from the middle ears of children with OME and found that bacteria were residing within biofilms adherent to the mucosal surface.12 As further proof, Thornton et al. demonstrated multiple bacterial species, including those commonly associated with OM, in 82% of middle ear biopsies collected from children undergoing ventilation tube insertion for chronic OME or recurrent acute OM.13 Collectively, these and many additional data confirmed that bacteria are indeed present in the middle ears of OME patients and that these bacteria are likely resident within biofilms.
Biofilms contribute to the chronic and recurrent nature of 65–80% of all persistent bacterial infections, including otitis media.14 Children with recurrent acute otitis media are often prescribed multiple rounds of antibiotics, a practice that is one of the major driving forces for the development of bacterial antibiotic resistance worldwide.15 For children with chronic OME, tympanostomy tubes are the mainstay of treatment as they allow for drainage of fluid and facilitate use of topical antibiotic drops during periods of acute infection.7 Biofilm-resident bacteria metabolically quiescent, and are typically 1,000-fold more resistant to antibiotic treatment compared to their planktonic counterparts, which thus explains the ineffectiveness of drugs that target active cellular processes.16 Therefore, development of alternate treatment strategies are required.
Our work has focused on essential structural elements in the biofilm extracellular polymeric substance (EPS), a self-produced matrix which enshrouds and protects the bacteria within these structures.17,18 While the exact composition of the EPS varies among bacterial species, common constituents among biofilms formed by all bacteria tested to date include extracellular DNA (eDNA) and the DNABII family of DNA-binding proteins, integration host factor (IHF) and histone-like protein (HU).17,19,20
Notably, antibodies directed against DNABII proteins: (1) induces collapse of biofilms formed by all 18 species of bacteria tested to date in vitro, including each of the high priority ESKAPE pathogens19,21–24 (2) disrupts biofilms in three unique animal models that include experimental NTHI-induced otitis media19,21,25, Aggregatibacter actinomycetemcomitans-induced periodontitis/peri-implantitis26 and Pseudomonas aeruginosa-induced lung infection25 (3) prevents the development of experimental otitis media in a polymicrobial model of disease25 and (4) prevents the formation of and disrupts pre-formed NTHI biofilms on surgical intranasal resorbable material in vitro27, as well as within both sputum solids collected from patients with cystic fibrosis28 and otorrhea specimens from children with post-tympanostomy tube otorrhea ex vivo.29 Furthermore, there is synergy between anti-DNABII antibody and use of traditional antibiotic treatments; upon collapse of the biofilm structure, the newly released bacteria are 4–8 fold more sensitive to first-line antibiotics for treatment of otitis media as compared to the effects of antibiotics alone on biofilm-resident bacteria.21,30 The aforementioned therapeutic approaches as well as preventative strategies, all of which are directed against the DNABII proteins, form the basis of our ‘DNABII-targeted’ strategy for resolution/ prevention of diseases wherein biofilms contribute to the pathogenesis and chronicity/ recurrence of disease.
Due to the persistent nature of OME and confirmation of biofilms within the middle ears of OME patients, we hypothesized that anti-DNABII-mediated therapy could be used to facilitate disease resolution. Toward achievement of this goal, herein we examined a single middle ear effusion collected from each of 38 children with chronic OME who were undergoing tympanostomy and tube insertion. We analyzed specimens for the presence of DNABII proteins through quantitation of the amount of IHF and HU in each specimen in order to demonstrate the relative abundance of these targets for this therapeutic approach. While previously our lab has utilized immunofluorescence microscopy to demonstrate structural components of bacterial biofilms which consists of a lattice of eDNA and associated DNABII protein localized at the vertices of crossed eDNA strands28,29, this technique is limited by its subjectivity and does not provide data on the relative concentrations of these DNABII proteins. Herein, we demonstrated the presence and relative abundance of DNABII proteins in 95% of clinical chronic OME specimens tested. As such, we are hopeful that this DNABII-directed strategy for disruption of bacterial biofilms will have promise as a highly effective therapeutic against diseases with a biofilm component, including chronic OME.
MATERIALS AND METHODS
Collection and Preparation of Middle Ear Effusions Samples
Institutional Review Board approval was obtained, and 38 pediatric patients with chronic middle ear effusions undergoing tympanostomy tube placement were identified in the pediatric otolaryngology clinic and informed consent obtained. During surgery under anesthesia to place tympanostomy tube(s), a single specimen was collected from each of 38 sequential patients. If the patient had bilateral effusions, only a single specimen was collected unilaterally. Specimens were obtained via a sterile Juhn Tymp-Tap Middle Ear Fluid Aspirator/ Collector (Medtronic Xomed Inc, Jacksonville, FL), labeled according to gender and age of patient, and further characterized according the color of the fluid and consistency of the effusion, i.e. serous, mucoid, purulent, sanguineous. An aliquot of each effusion was submitted for speciation to the Laboratory Services at Nationwide Children’s Hospital. Specimens were designated as negative if they showed no growth or normal cutaneous flora only. Specimens that were culture-negative, but positive for bacteria via Gram stain were designated positive. The effusions were frozen at −80°C for quantitation of DNABII proteins immunoblot analysis. In total 38 specimens were recovered and analyzed herein.
Quantitation of DNABII Proteins in Clinical Specimens
To quantitate the amount of each DNABII protein in our panel of 38 chronic OME specimens, an immunoblot assay was performed. First, an equivalent volume of each sample was incubated with an anti-mucolytic agent, 0.1 mg N-acetyl L cysteine (NALC) per ml solution (Sigma-Aldrich, St. Louis, MO) for 30 min in a 37°C water bath to promote both ease of use in this assay system and uniformity of sample constituency. The solution was then centrifuged at 17,000 × g for 10 min at 25°C and supernatants collected for use in the immunoblot assay. Standard curves were generated using known concentrations of native HU and IHF purified from nontypeable Haemophilus influenzae. Purified proteins of known concentration were prepared by serial two-fold dilutions to yield a standard curve that encompassed values from 7.8 ng per slot to 500 ng per slot. Immun-Blot PVDF LF membranes (Bio-Rad, Hercules, CA) were prepared according to manufacturer’s instructions prior to adsorption of purified HU, IHF or a 1:10 dilution of NALC-treated chronic OME samples by vacuum aspiration in a Bio-Dot SF Microfiltration Apparatus (Bio-Rad). Membranes were blocked with 2% normal goat serum (Bethyl Laboratories, Montgomery, TX) plus 2% skim milk (Sigma-Aldrich, St. Louis, MO) in Dulbecco’s phosphate-buffered saline (DPBS) for 1 hr at 25°C. To detect IHF or HU within chronic OME samples, membranes were incubated with polyclonal rabbit anti-IHFNTHI or polyclonal rabbit anti-HUNTHI, respectively, overnight at 4°C. Each polyclonal rabbit serum is specific for its protein target, with minimal cross-reactivity.24 Membranes were washed in DPBS plus 0.05% Tween 20 (DPBS-T) and incubated with 2 μg goat anti-rabbit IgG conjugated to AlexaFluor 594 (Molecular Probes, Eugene, OR) per ml DPBS for 1 hr at 25°C. Membranes were rinsed once with DPBS-T and twice with DPBS prior to air drying, then imaged for 2.3 s with a FluorChem M system (ProteinSimple, San Jose, CA). Mean fluorescent intensity of each band was determined using AlphaView software (Protein Simple) and DNABII proteins within OME samples quantitated by comparison to our standard curves based the relative fluorescence of purified IHF or HU protein bands using GraphPad Prism 6 (GraphPad Software, Inc. La Jolla, CA).
Statistical analysis
Statistical comparison of the amount of protein within the OME specimens was determined by unpaired t-test using GraphPad Prism software. A P-value ≤ 0.05 was considered significant.
RESULTS
Clinical characteristics
In total, 38 specimens were collected from October 2017 through July 2018. Twenty-six of 38 (68%) effusions were collected from male patients, while 12 (32%) were from female patients. Patients ranged from 9 months to 19 years of age. Thirty-three individuals identified as White, while 4 identified as African American and 1 identified as Middle Eastern. Of 38 specimens collected, 21 were characterized as serous with no evidence of mucoid semi-solids, whereas 17 were mucoid.
Microbiology of the middle ear effusions collected
Thirty-eight samples collected were submitted for speciation at Department of Laboratory Medicine Clinical Laboratories at Nationwide Children’s Hospital. Of these, 37 samples yielded culture data (Table 1); no information on culture status or culture data was acquired for one specimen. Thirteen of the 37 specimens (35%) showed either no growth or normal cutaneous flora only, while bacteria or yeast were cultured from the remaining 24 specimens (65%). It was not unexpected that a proportion of specimens were culture-negative, as bacteria resident within a biofilm that is adherent to the mucosal epithelium within the middle ear is not likely to be recovered via aspiration of middle ear fluid that fills the middle ear cavity.11,13,31 Three specimens were polymicrobial.
Table 1: Identification of microbes within chronic OME samples.
Caption: Tabular presentation of microorganisms identified by traditional culture techniques. Of the 38 specimens sent for speciation, 37 yielded results. Of these 37, 24 (65%) grew bacteria or yeast, while the remaining 13 (35%) showed either no growth or normal cutaneous flora. Three specimens were polymicrobial.
| Organism identified by culture | N |
|---|---|
| Staphylococcus sp. | 11 |
| Corynebacterium-like Gram + bacilli | 3 |
| Haemophilus influenzae | 2 |
| Pseudomonas aeruginosa | 1 |
| Turicella otitidis | 1 |
| Moraxella catarrhalis | 1 |
| Acinetobacter baumaii | 1 |
| Streptococcus sp. | 1 |
| Gram + or Gram – on Gram stain only | 3 |
| Yeast | 3 |
| No growth | 13 |
| No culture data obtained | 1 |
Quantitation of DNABII Proteins in Clinical Specimens by Immunoblot
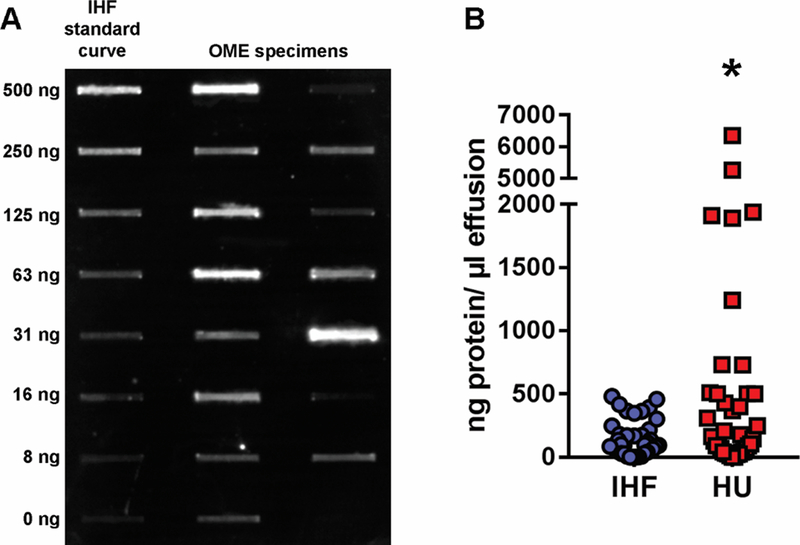
We utilized an objective assay to determine the presence and relative quantity of the DNABII proteins IHF and HU individually, within 38 chronic OME specimens. A representative immunoblot wherein varied amounts of IHF were detected in 16 OME specimens is shown in Fig. 1A. Specific for IHF, 95% (36/38) of specimens were positive for this protein at concentrations from 2 ng/ μl effusion to 481 ng/ μl effusion (Fig. 1B). In regards to HU, 95% (36/38) of specimens were positive and contained 13 ng/ μl effusion to 5,264 ng/ μl effusion. Collectively, among the 38 OME specimens assayed, a significantly greater amount of HU (668 ± 217 ng/ μl effusion) was detected compared to IHF (150 ± 23 ng/ μl effusion), a difference primarily due to 6 of 38 specimens wherein a concentration of greater than 1000 ng HU/ μl effusion was detected.
Figure 1. Quantitation of IHF and HU in effusions recovered from children with chronic OME.
Caption: Panel A, representative immunoblot to demonstrate varied amount of IHF detected in a panel of 16 OME specimens. Panel B, quantitation of IHF (blue circles) and HU (red circles). Among the 38 OME specimens collected, 95% were positive for both IHF and HU proteins by quantitative immunoblot analysis, and overall, a significantly greater amount of HU was detected, compared to IHF (P≤ 0.05). These data indicated that the immunoblot was highly sensitive in ability to both reveal the presence of DNABII proteins within the chronic OME samples, as well as quantitate them. *, P≤ 0.05
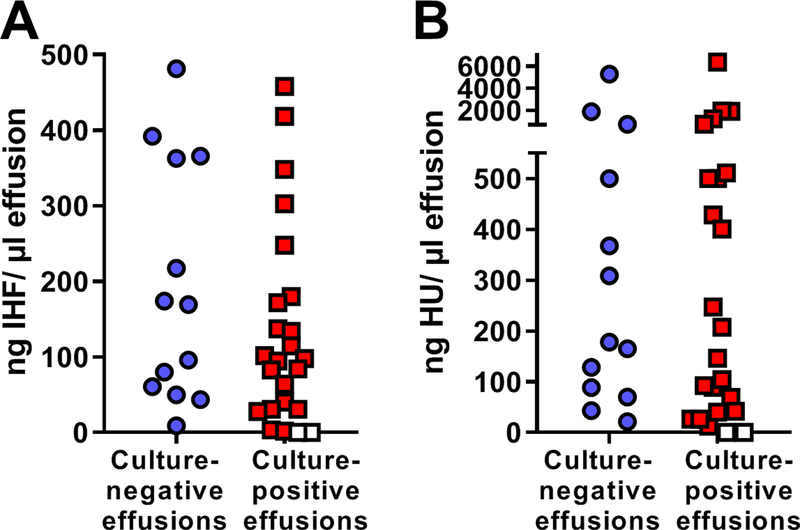
Furthermore, of the 13 specimens that were culture-negative, all 13 showed presence of IHF (Fig. 2A) and HU (Fig. 2B) via quantitative immunoblot assay and this range of concentrations (9 to 481 ng IHF/ μl effusion and 22 to 5264 ng HU/ μl effusion) was not statistically different from that obtained for culture-positive specimens (2 to 458 ng IHF/ μl effusion and 12 to 6352 ng HU/ μl effusion). In summary, DNABII proteins, components that are critical to the structural integrity of bacterial biofilms, were present in 95% of all effusions tested, even when these specimens had been deemed culture-negative.
Figure 2. Lack of correlation between culture status of each chronic OME specimen and quantity of IHF (Panel A) or HU (Panel B) as determined by immunoblot assay.
Caption: Of 13 samples that yielded negative culture results, all 13 were positive for the DNABII proteins IHF and HU by quantitative immunoblot assay, as depicted by the blue circles. Red squares indicate the concentration of IHF or HU detected by quantitative immunoblot in the 24 specimens that yielded positive culture results. White squares depict specimens wherein the quantitative immunoblot assay yielded zero IHF or HU, but positive culture results for bacteria or yeast were obtained. No significant difference in quantity of either IHF or HU was calculated between specimens that were culture-positive versus those that were culture-negative. In sum, DNABII proteins were shown to be present by objective immunoblot assay even in the 13 specimens that had been deemed culture-negative.
DISCUSSION
The pathogenesis of chronic OME is incompletely understood. Chronic OME often follows an episode of acute or recurrent otitis media and is characterized by the persistence of middle ear fluid without signs or symptoms of acute inflammation for greater than 3 months.1 OME is a multifactorial condition that results from the interplay of chronic inflammation from bacterial or viral infections, deficiencies in innate immunity, genetics and mucosal hyperplasia.2 However, more recent work has demonstrated that the chronicity and recurrence of OME is also due to the presence of viable bacteria within a biofilm as shown in animal models and in clinical specimens. Hall-Stoodley et al. demonstrated biofilms in biopsy specimens from the middle ears of children undergoing tympanostomy tube placement for chronic OME and/or recurrent otitis media, but not in those of control children undergoing cochlear implantation.12 Later, Van Hoecke et al. detected the presence of Haemophilus influenzae-specific biofilm structures in culture-positive but not culture-negative middle ear effusions using fluorescence in situ hybridization.32 Ours is the first investigation to identify and quantitate two essential biofilm structural components, IHF and HU, within effusions recovered from children with chronic OME.
Previous work has already shown that biofilms formed by nontypeable Haemophilus influenzae contain eDNA organized into a mesh-like lattice with bacterial DNABII DNA-binding proteins located at the vertices of crossed strands of eDNA.33 Furthermore, DNABII proteins are a critical component for structural stability of the biofilm. Incubation of biofilms with antibodies against DNABII proteins results in their catastrophic collapse in vitro and their rapid clearance in experimental models of otitis media19,21,25, which indicates that these proteins can also serve as important therapeutic targets.
While the precise roles of each DNABII protein is not known, HU and IHF are differentially expressed with intracellular HU steady state levels being greatest during late exponential growth and IHF at its peak during early stationary phase. Hence the distinction in extracellular steady levels may be linked to the predominant growth phase of the extant biofilm. Notably, the 13 culture-negative specimens that were analyzed by immunoblot assay all contained the DNABII proteins IHF and HU. Therefore, culture status alone, which is already known to not be an accurate assessment of whether bacteria are the causative agents of chronic OME, is similarly not an accurate indicator of whether DNABII proteins may be present within the middle ear of these patients.
Whereas immunofluorescent microscopy has previously been used to demonstrate the presence of an eDNA lattice with associated DNABII proteins within otorrhea specimens29, the immunoblot technique employed in this study was able to not only reveal the presence of DNABII proteins within the chronic OME samples, but also to quantitate them. As the DNABII proteins serve as critical structural elements within bacterial biofilms, collectively, our data lend further support to the hypothesis that bacterial biofilms are present in the majority of chronic middle ear effusions, despite negative culture results.
Currently, the mainstay of treatment for OME that persists longer than three months is insertion of tympanostomy tubes.1 Tympanostomy tubes allow for drainage of middle ear fluid, reduce symptoms of OME (including associated conductive hearing loss), and permit use of topical antibiotic drops during periods of acute infection, obviating the need for systemic antibiotic therapy. However, placement of tympanostomy tubes and tailored topical antibiotic treatment are often insufficient to treat chronic OME. In as many as 50% of patients with chronic OME, disease recurs after extrusion of ventilation tubes and repeat surgery may be required.34 An additional complicating factor is the risk of post-tympanostomy tube otorrhea, the incidence of which is up to 74% within 12 months. Post-tympanostomy tube otorrhea can also be very difficult to treat, and failure of topical antibiotic therapy may lead to the additional use of oral antibiotic therapy and possibly tympanostomy tube removal.35 In an earlier report, we showed that eDNA and DNABII proteins are present in post-tympanostomy tube otorrhea.29
Our work herein demonstrated that prior to the placement of tympanostomy tubes and potential development of post tympanostomy tube otorrhea, biofilms containing DNABII proteins are present chronic middle ear effusions. As many of our previous works indicate, antibodies directed against the DNABII proteins disrupt biofilms that contribute significantly to persistent middle ear disease. Moreover, those bacteria that are newly released from the biofilm via exposure to anti-DNABII serum are highly susceptible to antibiotics. As such, antibodies that target DNABII DNA-binding proteins have potential to serve as a novel therapy for children with chronic OME, particularly given that many of these children will have tympanostomy tubes in place that could serve as a conduit for delivery of these antibodies. Moreover, given that children with tympanostomy tubes frequently experience post-tympanostomy tube otorrhea, we reason that if administered prophylactically at the time of tube placement, a DNABII-targeted therapy could perhaps work in a preventative capacity and when administered during episodes of active otorrhea, in a therapeutic capacity. If proven successful, since OME is one of the leading causes of primary care visits and indications for surgery in the pediatric population, as well as the leading causes for hearing loss worldwide, this novel therapeutic approach has potential to relieve an enormous disease burden and/or obviate the need for multiple surgeries.
CONCLUSION
Chronic OME is a common disorder in childhood with large socioeconomic impacts and negative developmental implications for young children. Herein, we showed that 95% of chronic middle ear effusion specimens examined via quantitation contained essential biofilm components, members of the DNABII-family of DNA-binding proteins. Moreover, the aforementioned proteins were detected in serous and mucoid effusions through immunoblot techniques, even when the specimens were culture-negative. The presence and ubiquity of these DNABII DNA-binding proteins in this panel of clinical samples indicated that these bacterial proteins provide an important additional clinical target for our novel DNABII-directed strategy to treat biofilm diseases such as chronic otitis media with effusion. Such therapies could have wide-reaching potential to serve in both therapeutic and preventative capacities for the vast population of children worldwide who are affected by OME.36
Acknowledgements:
We are grateful to the patients and their families for their agreement to participate in this study, without whom we could not have conducted this research. We also thank our colleagues at the Research Institute at Nationwide Children’s Hospital and Department of Otolaryngology at Nationwide Children’s Hospital for their insight and technical expertise. This work was funded by NIH/ NIDCD R01 DC11818 to L.O.B. and departmental funds from C.A.E.
Footnotes
Conflict of Interest Statement: None.
REFERENCES
- 1.Rosenfeld RM, Shin JJ, Schwartz SR et al. Clinical Practice Guideline: Otitis Media with Effusion (Update). Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2016; 154:S1–S41. [DOI] [PubMed] [Google Scholar]
- 2.Kubba H, Pearson JP, Birchall JP. The aetiology of otitis media with effusion: a review. Clinical otolaryngology and allied sciences 2000; 25:181–194. [DOI] [PubMed] [Google Scholar]
- 3.Shekelle P, Takata G, Chan LS et al. Diagnosis, natural history, and late effects of otitis media with effusion. Evidence Report/Technology Assessment (Summary) 2002:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qureishi A, Lee Y, Belfield K, Birchall JP, Daniel M. Update on otitis media - prevention and treatment. Infection and drug resistance 2014; 7:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson IG, Dunleavey J, Bain J, Robinson D. The natural history of otitis media with effusion--a three-year study of the incidence and prevalence of abnormal tympanograms in four South West Hampshire infant and first schools. The Journal of laryngology and otology 1994; 108:930–934. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld RM, Kay D. Natural history of untreated otitis media. The Laryngoscope 2003; 113:1645–1657. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld RM, Schwartz SR, Pynnonen MA et al. Clinical practice guideline: Tympanostomy tubes in children. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2013; 149:S1–35. [DOI] [PubMed] [Google Scholar]
- 8.Post J, P Ra, A Jj, et al. Molecular analysis of bacterial pathogens in otitis media with effusion. Jama 1995; 273:1598–1604. [PubMed] [Google Scholar]
- 9.Gok U, Bulut Y, Keles E, Yalcin S, Doymaz MZ. Bacteriological and PCR analysis of clinical material aspirated from otitis media with effusions. International journal of pediatric otorhinolaryngology 2001; 60:49–54. [DOI] [PubMed] [Google Scholar]
- 10.Matar GM, Sidani N, Fayad M, Hadi U. Two-step PCR-based assay for identification of bacterial etiology of otitis media with effusion in infected Lebanese children. Journal of clinical microbiology 1998; 36:1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rayner MG, Zhang Y, Gorry MC, Chen Y, Post JC, Ehrlich GD. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. Jama 1998; 279:296–299. [DOI] [PubMed] [Google Scholar]
- 12.Hall-Stoodley L, Hu FZ, Gieseke A et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. Jama 2006; 296:202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornton RB, Rigby PJ, Wiertsema SP et al. Multi-species bacterial biofilm and intracellular infection in otitis media. BMC pediatrics 2011; 11:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Department of H, Human S. No Title, 2002.
- 15.Tristram S, Jacobs MR, Appelbaum PC. Antimicrobial resistance in Haemophilus influenzae. Clinical microbiology reviews 2007; 20:368–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slinger R, Chan F, Ferris W et al. Multiple combination antibiotic susceptibility testing of nontypeable Haemophilus influenzae biofilms. Diagnostic microbiology and infectious disease 2006; 56:247–253. [DOI] [PubMed] [Google Scholar]
- 17.Gunn JS, Bakaletz LO, Wozniak DJ. What is on the Outside Matters: The Role of the Extracellular Polymeric Substance of Gram-negative Biofilms in Evading Host Immunity and as a Target for Therapeutic Intervention. J Biol Chem 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flemming H-C, Wingender J. The biofilm matrix. Nature Reviews Microbiology 2010; 8:623–623. [DOI] [PubMed] [Google Scholar]
- 19.Goodman SD, Obergfell KP, Jurcisek JA et al. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal immunology 2011; 4:625–637. [DOI] [PubMed] [Google Scholar]
- 20.Swinger KK, Rice PA. IHF and HU: flexible architects of bent DNA. Current opinion in structural biology 2004; 14:28–35. [DOI] [PubMed] [Google Scholar]
- 21.Brockson ME, Novotny LA, Mokrzan EM et al. Evaluation of the kinetics and mechanism of action of anti-integration host factor-mediated disruption of bacterial biofilms. Molecular microbiology 2014; 93:1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devaraj A, Justice SS, Bakaletz LO, Goodman SD. DNABII proteins play a central role in UPEC biofilm structure. Molecular microbiology 2015; 96:1119–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devaraj A, Buzzo J, Rocco CJ, Bakaletz LO, Goodman SD. The DNABII family of proteins is comprised of the only nucleoid associated proteins required for nontypeable Haemophilus influenzae biofilm structure. MicrobiologyOpen 2018; 7:e00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novotny LA, Amer AO, Brockson ME, Goodman SD, Bakaletz LO. Structural stability of Burkholderia cenocepacia biofilms is reliant on eDNA structure and presence of a bacterial nucleic acid binding protein. PloS one 2013; 8:e67629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novotny LA, Jurcisek JA, Goodman SD, Bakaletz LO. Monoclonal antibodies against DNA-binding tips of DNABII proteins disrupt biofilms in vitro and induce bacterial clearance in vivo. EBioMedicine 2016; 10:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freire MO, Devaraj A, Young A et al. A Bacterial Biofilm Induced Oral Osteolytic Infection Can be Successfully Treated by Immuno-Targeting an Extracellular Nucleoid Associated Protein. Mol Oral Microbiol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandstetter KA, Jurcisek JA, Goodman SD, Bakaletz LO, Das S. Antibodies directed against integration host factor mediate biofilm clearance from Nasopore. The Laryngoscope 2013; 123:2626–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gustave JE, Jurcisek JA, McCoy KS, Goodman SD, Bakaletz LO. Targeting bacterial integration host factor to disrupt biofilms associated with cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society 2013; 12:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Idicula WK, Jurcisek JA, Cass ND et al. Identification of biofilms in post-tympanostomy tube otorrhea. The Laryngoscope 2016; 126:1946–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chambers JR, Cherny KE, Sauer K. Susceptibility of Pseudomonas aeruginosa Dispersed Cells to Antimicrobial Agents Is Dependent on the Dispersion Cue and Class of the Antimicrobial Agent Used. Antimicrobial agents and chemotherapy 2017; 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Post JC, Stoodley P, Hall-Stoodley L, Ehrlich GD. The role of biofilms in otolaryngologic infections. Curr Opin Otolaryngol Head Neck Surg 2004; 12:185–190. [DOI] [PubMed] [Google Scholar]
- 32.Van Hoecke H, De Paepe A-S, Lambert E et al. Haemophilus influenzae biofilm formation in chronic otitis media with effusion. European Archives of Oto-Rhino-Laryngology 2016; 273:3553–3560. [DOI] [PubMed] [Google Scholar]
- 33.Bakaletz LO. Bacterial biofilms in otitis media: evidence and relevance. The Pediatric infectious disease journal 2007; 26:S17–19. [DOI] [PubMed] [Google Scholar]
- 34.Mandel EM, Rockette HE, Bluestone CD, Paradise JL, Nozza RJ. Myringotomy with and without tympanostomy tubes for chronic otitis media with effusion. Arch Otolaryngol Head Neck Surg 1989; 115:1217–1224. [DOI] [PubMed] [Google Scholar]
- 35.Boston M, McCook J, Burke B, Derkay C. Incidence of and risk factors for additional tympanostomy tube insertion in children. Archives of Otolaryngology–Head & Neck Surgery 20 [DOI] [PubMed] [Google Scholar]
- 36.Organization GWH. Global costs of unaddressed hearing loss and cost-effectiveness of interventions: a WHO report 2017.