Abstract
Pachyonychia congenita (PC), a rare genodermatosis, primarily affects ectoderm-derived epithelial appendages and typically includes oral leukokeratosis, nail dystrophy, and very painful palmoplantar keratoderma (PPK). PC dramatically impacts quality of life though it does not affect lifespan. PC can arise from mutations in any of the wound repair-associated keratin genes KRT6A, KRT6B, KRT6C, KRT16, or KRT17. There is no cure for this condition, and current treatment options for PC symptoms are limited and palliative in nature. This review focuses on recent progress made towards understanding the heterogeneity and pathophysiology of PPK lesions, the most prevalent and debilitating of all PC symptoms. In particular, the study of the Krt16 null mouse, which spontaneously develops footpad lesions that mimic several aspects of PC-associated PPK, revealed three main stages of PPK lesion progression. Ahead of lesion onset, keratinocytes in the palmoplantar (footpad) skin of Krt16 null mice exhibit specific defects in differentiation. At the time of PPK onset, there is elevated oxidative stress and hypoactive Keap1-Nrf2 signaling. During active PPK, there is a profound defect in the ability of the epidermis to maintain or return to normal homeostasis. The progress made suggests new avenues to explore for the treatment of PC-based PPK and deepens our understanding of the mechanisms controlling skin tissue homeostasis.
Pachyonychia congenita (PC; OMIM #1672000 and 167210) is a rare genodermatosis with a collection of symptoms primarily affecting ectoderm-derived appendages including oral leukokeratosis, nail dystrophies, sebaceous cysts, natal teeth, and palmoplantar keratoderma (PPK). While PC does not impact lifespan, it dramatically impacts quality of life for affected individuals. For instance, individuals with PC experience severe plantar pain from PPK lesions daily, often making everyday tasks difficult. There is currently no known cure or effective therapeutics for the treatment of PC1.
PC can arise from autosomal dominant mutations in any of five keratin genes including KRT6A, KRT6B, KRT6C, KRT16, or KRT171–5. These keratins are normally expressed in epithelial appendages and are otherwise robustly inducible, e.g., after injury or exposure to environmental stresses, together accounting in part for the clinical presentation of this disorder. Most PC-causing mutations are missense alleles, with occasional mall insertions or deletions in the keratin coding sequence. Until recently, two majors types of PC, type 1 (Jadahsson-Lewandowsky6) and type 2 (Jackson-Lawler7), were recognized based on their prevalent clinical features. Nowadays, five subtypes of PC are recognized based on genetic etiology - for example, PC caused by a KRT6A mutation corresponds to the PC-K6a subtype. Owing in part to the heterogeneity in the clinical presentation of PC (even among patients with very similar alleles), a definitive diagnosis can only be ascertained through sequencing of these keratin genes1,8,9.
The Pachyonychia Congenita Project
The PC Project is a USA public charity that was founded in 2003 and has evolved into a life-changing resource for both individuals with PC and for clinicians and researchers interested in this condition. This organization connects individuals with PC and their families to others with this condition, and to clinicians, translational and basic science researchers. The PC project provides assistance to individuals with PC to attend support meetings and qualify for genetic testing. Further, the PC Project is home to the International PC Research Registry (IPCRR), which gathers extremely valuable data from questionnaires, photos, and notes on genetically-confirmed PC cases. As of January 2019, the IPCRR includes 864 genetically confirmed cases of PC in 49 countries. This PC registry has evolved into a transformative resource for patients, clinicians and researchers working together towards understanding this disorder and developing effective therapeutics. Finally, the PC Project plays a lead role in fostering basic and clinical research on PC. More information about the PC Project and how to get involved can be found on the publicly-available website www.pachyonychia.org.
Asserting a focus on palmoplantar keratoderma
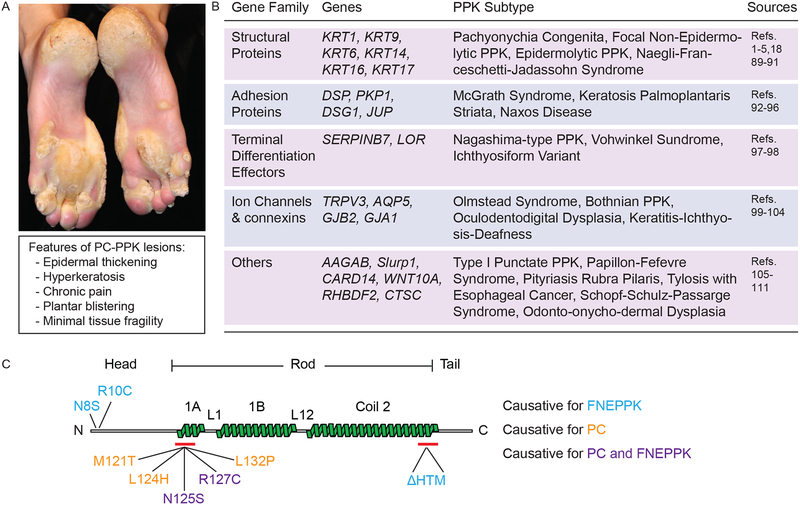
While individuals with PC present with many symptoms of significance, PPK is highly penetrant and reportedly most debilitating1 (Fig. 1A). Virtually all individuals with PC (> 90%1) present with PPK lesions restricted primarily to pressure points in palmar and/or plantar epidermis and consisting of dramatic epidermal thickening and hyperkeratosis3–5,10. PPK lesions are debilitating in part because of the extreme pain associated with them11–13. Interestingly, these lesions do not display signs of keratinocyte fragility and/or lysis. The latter represents a predominant element in epidermolysis bullosa simplex (EBS), a genetically-determined skin blistering condition caused by mutations in either KRT14 or KRT514–17. Keratinocyte fragility also is a dominant pathophysiological feature in epidermolytic PPK, which is often caused by mutations in KRT918, the major differentiation-specific keratin in volar epidermis19,20. The greater complexity of keratin gene expression in volar epidermis likely contributes to maintain keratinocyte structural integrity in spite of mutations in individual genes such as KRT6A-C, KRT16 and KRT17. This said, the pathophysiology of PC-associated PPK is only partially understood at present, reflecting significant limitations related to the low incidence of this orphan disease and the severe pain associated with these lesions21,22. Accordingly, there is no effective treatment for PC-based PPK. The current standard of care for PPK consists of routine removal of calluses followed by treatment with moisturizers23 (see below for details). A deeper understanding of the pathophysiology of PPK might spearhead the development of effective therapeutics for individuals with PC and also inform researchers, clinicians and drug developers on other genetic and clinical subtypes of PPK (Fig. 1B). This text focuses on recent progress made in deciphering the pathophysiology of PC-associated PPK lesions.
Figure 1:
Palmoplantar keratoderma, a genetically heterogeneous disorder. (A) Photograph of PC-based PPK lesions from an individual with a KRT16 L124R mutation. Source: Pachyonychia Congenita Project (www.pachyonychia.org). (B) Table summarizing the diversity of genes which, when mutated, can elicit a PPK clinical presentation. Various clinical subtypes of PPK are accounted for. Additional references for table are 18,89–111. (C) Schematic of select mutations in keratin 16 (K16) protein that are causative for PC, focal non-epidermolytic PPK (FNEPPK), or both PC and FNEPPK. K16 exhibits the tripartite domain structure shared by all IF proteins, with a N-terminal “head” domain, central α-helical “rod” domain, and C-terminal “tail” domain. The central rod domain is comprised of heptad repeat-containing α-helical coils (1A, 1B, Coil 2) separated by non-heptad repeat linkers (L1 and L12). Many attributes of the central rod domain (see red bars) are highly conserved and represent a signature element among IF proteins. Representative mutations that are causative for FNEPPK are in blue text, mutations causative for PC are in gold text, and mutations that are causative for both FNEPPK and PC are in purple text.
A short primer on the nomenclature of PC-associated keratin genes
The original catalog of human keratin proteins devised by Moll et al.24 already recognized the existence of K6 as a type II, and of K16 and K17 as type I, keratins. However, the true diversity of keratin genes and proteins was underestimated until the advent of whole genome sequencing efforts25, which necessitated a revision of the Moll nomenclature26. Per the internationally accepted nomenclature, human genes are designated using capital lettering (e.g., KRT16) and mouse genes are designated using normal size lettering (e.g., Krt16). The multiplicity of K6 sequences was originally uncovered in the human27. Currently, we know of two functional genes in the mouse, Krt6a and Krt6b28, and three functional K6 genes in the human, KRT6A, KRT6B, and KRT6C2,29. By contrast, a single gene codes for each of K1630,31 and K17 proteins32,33 in the human and mouse genomes. The high degree of conservation known to apply to orthologous keratin genes in the mouse and human, in terms of sequence features and regulation, applies to the PC-associated keratin genes29,31,32. This information is relevant to discussing the utilization of transgenic mouse models to study keratin mutation-based human conditions such as PC.
Lessons learned from transgenic mouse models
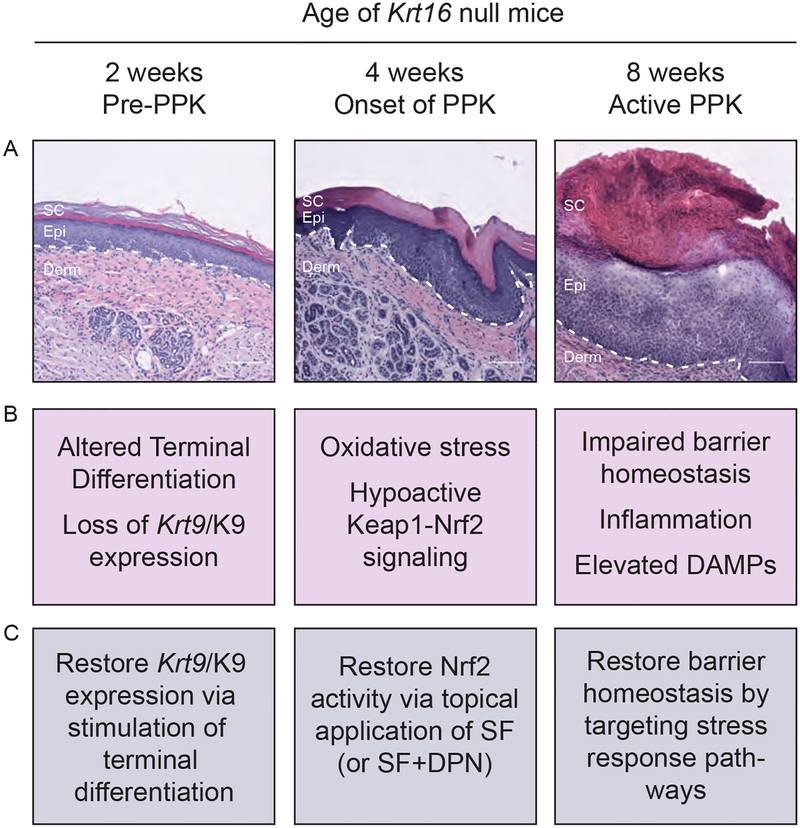
As there are no in vitro human cell culture models that can be used to investigate the cellular and molecular mechanisms underlying PPK pathophysiology or screen potential therapeutics, researchers have relied on the use of transgenic mouse models (summarized in Table 1) to study PC and PPK34. Among the models available, the Krt16 null mouse strain is the only one that spontaneously develops footpad skin lesions mimicking PC-associated PPK lesions. Characterization of Krt16 null mice has revealed three phases in PPK, each with a somewhat unique molecular signature: pre-PPK, PPK onset, and active PPK (Fig. 2).
Table 1:
Mouse models with phenotypes that are potentially relevant to pachyonychia congenita (PC).
| Year | Mouse model | Genetic modification | Main phenotype(s) | References |
|---|---|---|---|---|
| 1996 | Krt6aΔ21P | Deletion of 52 amino acids (residues 125–176) between head and 1A helix domain | Intraepidermal blistering | 82 |
| 1999 | Krt6a transgenic | Truncation deleting the 2B region of the central rod domain | Lethal blister or alopecia | 83 |
| 1999 | Krt6a transgenic | Replacement of E2 by HK1-tag | Hyperkeratosis and late-onset alopecia | 83 |
| 2000 | Krt6a/Krt6b−/− | Deletion of Krt6a and Krt6b locus | Oral lesions | 49 84 |
| 2000 | Krt6a−/− | Deletion of Krt6a locus | Delay of reepithelialization after wounding | 85 |
| 2002 | Krt17−/− | Deletion of Krt17 locus | Age and strain dependent alopecia | 48 |
| 2005 | Krt6a/Krt6b−/−; Krt17−/− | Deletion of Krt6a, Krt6b, and Krt17 locus | Severe cell lysis in nail bed epithelium | 86 |
| 2008 | Krt75 knock-in | Point mutation of codon N158 (corresponding to mutation N171 in PC case) | Defects in hair shaft, nail fragility | 87 |
| 2011 | KRT6A N171K Humanized skin | Bioengineered skin equivalents derived from PC individuals with N171K mutation engrafted onto immunodeficient mice | Acanthosis and epidermal blistering | 88 |
| 2012 | Krt16−/− | Deletion of Krt16 locus | Oral lesions, footpad lesions resembling human PPK | 38,47 |
Figure 2:
Development of PPK-like lesions in Krt16 null footpad skin proceeds in three stages: Pre-PPK (at 2 weeks of age), Onset of PPK (at 4 weeks), and Active-PPK (at 8 weeks). (A) Representative histology of Krt16 null footpad skin at 2-, 4-, and 8-weeks of age. At 2 weeks, the epidermis shows a normal thickness and overall architecture but, upon closer inspection, alterations including the abnormal appearance of the granular layer, crowding of basal keratinocytes, and a decreased nuclear aspect ratio of basal keratinocytes can be seen. At 4 weeks, prior to macroscopic appearance of lesions, mild epidermal thickening is observed. By 8 weeks, there is dramatic thickening of the living epidermis and the stratum corneum, infiltration of immune cells, and limited suprabasal cell lysis. Dotted line is epidermal/dermal junction. Derm = dermis. Epi = epidermis, SC = stratum corneum. Scale bar = 100 μm. Images acquired using a Zeiss microscope with Apotome attachment and processed using Zen 2.3 software. (B) Summary of key molecular changes that occur at 2-, 4-, and 8-weeks of age in Krt16 null footpad skin. (C) Potential therapeutic interventions for each stage of lesion development (see refs. 35,37,39,77). SF = sulforaphane. DPN = Diarylpropionitrile.
In 2-weeks old Krt16 null mice, corresponding to the “pre-PPK stage”, footpad skin keratinocytes exhibit defects in selective aspects of terminal differentiation. At this early time point there are minimal alterations to the skin tissue histology but, already, a dramatic loss of keratin 9 (Krt9/K9) expression has occurred, which then persists throughout lesion progression35. Krt9 occurs exclusively in differentiating keratinocytes of volar skin and represents a predominant marker gene in this setting19,20,36. In contrast to Krt9, several differentiation markers appear to be upregulated in Krt16 null footpad skin, potentially as a compensatory mechanism35. While this partial defect in terminal differentiation is currently unexplained35, it occurs independent and ahead of the oxidative stress phenotype observed at a later stage of progression of PPK-like lesions in this mouse model35,37.
In one-month old Krt16 null mice, corresponding to “onset stage” of PPK-like lesions, footpad skin epidermis displays several features of oxidative stress, including decreased levels of the master cell antioxidant glutathione and decreased expression of glutathione synthesis genes compared to WT controls. Keap1-Nrf2 signaling, a central regulator of the cellular antioxidant response, is markedly attenuated at that time while Nrf2 itself, a transcription factor, is upregulated though ineffective in Krt16 null footpad skin (likely reflecting an attempt to restore redox homeostasis). While difficult to ascertain given restricted access to plantar skin biopsies from PC patients, there is evidence of reduced Nrf2 activity in PC-PPK lesions of individuals with KRT16 mutations37.
In two-months old Krt16 null mice, corresponding to an “active stage” of PPK, there is a profound defect in the ability of footpad skin to maintain or return to normal tissue homeostasis. By this time all Krt16 null mice have spontaneously developed PPK-like lesions on their paws, and while these lesions preferentially arise in areas exposed to the substratum and thus experiencing mechanical stress, they are not associated with keratinocyte fragility38,39. At a molecular level, the Krt16 null footpad lesions exhibit a gross misregulation of many Danger Associated Molecular Patterns (DAMPs) and barrier homeostasis genes, which mimics human PC-based PPK lesions39.
Lessons learned from computational endeavors
Along with targeted molecular analyses, computational analysis of genomic data sets has also provided significant insight into the pathophysiology of PC-based PPK. Systems genetics has been used to explore the role of K16 in regulating the skin’s response to stress. Re-analyzing a powerful systems genetics data set that related the risk of developing skin tumors to the regulation of skin inflammation and barrier function40 revealed a tight link between Krt16, skin barrier genes, and innate immunity effectors including DAMPs39,41. In this data set, moreover, Krt16 expression is significantly correlated with expression of barrier homeostasis and inflammation genes in tail skin, both at baseline and in response to TPA, which acts as a chemical irritant41. The discovery that Krt16 belongs to a network of barrier homeostasis genes pointed to a role for Krt16 in calibrating the skin’s response to barrier-compromising stresses39, which converged nicely with the phenotype of PPK-like lesions exhibited by Krt16 null mice. These efforts lent strong support to the notion that a better understanding of how K16 calibrates the skin’s stress response could be applicable to PC as KRT16 expression is often elevated in PC-based PPK lesions.
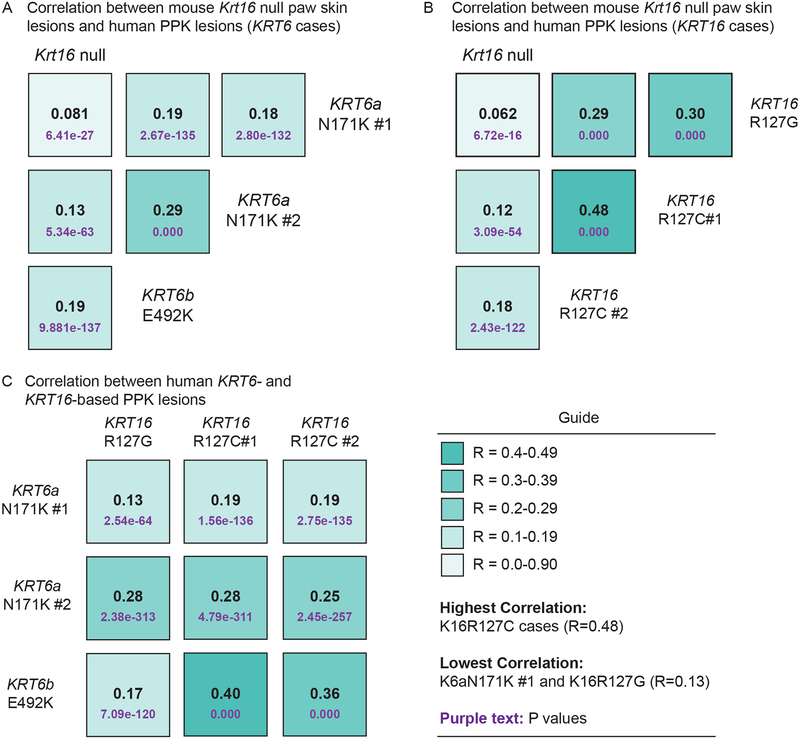
The availability of genome-wide surveys of gene expression from both Krt16 null footpad skin lesions35 and PPK lesions from individuals with PC22 has provided an excellent opportunity to further test the strengths and limitations of the Krt16 null mouse as a valid model for PC-based PPK. Merging the human PPK datasets with the murine Krt16 null footpad lesions dataset, based upon human-mouse orthologous gene pairings, enabled multiple computational analyses35. Pairwise comparisons of global transcriptional changes in Krt16 null footpad lesions and individual PC cases (3 KRT6 cases, 3 KRT16 cases) generated statistically significant positive correlation values in all cases (Fig. 3A–B). Additionally, pairwise comparisons of global transcriptional changes further highlight the high degree of heterogeneity between individual cases involving different keratin mutations, and between cases with the same mutated keratin allele (Fig. 3C). Altogether these comparisons provided a strong case that lesional Krt16 null mouse footpad skin mimics PC-associated PPK lesions at a global gene expression level, reinforcing and extending the notion that the Krt16 null mouse is an appropriate model for the study of pathogenesis of PC-associated PPK lesions.
Figure 3:
Correlation of transcriptional changes between Krt16 null footpad lesions and human PC-based PPK lesions. (A-B) Correlation coefficients (R) calculated from pairwise comparisons of microarray data from Krt16 null footpad lesions35 and human PC-based PPK lesions22 resulting from mutations in KRT6 (A) or KRT16 (B). (C) Correlation coefficients calculated from pairwise comparisons between individual cases of PC-based PPK with either KRT6 or KRT16 mutations. A R value of −1 would convey perfect negative correlation, whereas a R value of 1 conveys perfect correlation between samples. P values for each correlation are denoted in purple text underneath the corresponding R values. Figure adapted from Zieman et al. (2019)35.
A role for keratin imbalances and genetic background in the pathophysiology of PPK
Since the presentation of PC symptoms varies greatly between individuals even with similar or the same mutated keratin allele1,42–44, there likely is a role for genetic background and gene modifiers in the pathophysiology of this condition. Remarkably, similar alleles in KRT16 (N125S and R127C) can elicit a presentation of focal non-epidermolytic PPK vs. full-blown PC44–46 (Fig. 1C), suggesting that the consequences associated with alterations in KRT16 are subject to modifier gene(s) effects. Consistent with this notion, despite the immunological differences between mice and humans, several phenotypic aspects of Krt16 null mice including the PPK-like lesions are modestly impacted by genetic strain background47. Interestingly, select phenotypic traits in Krt17 null mice48 and Krt6a/Krt6b double-null mice49 also exhibit a dependence on genetic background.
In addition to genetic background, imbalances in keratin expression also appear likely to play a significant role in pathophysiology of PC-based PPK. For example, the differentiation-specific keratins KRT2 and KRT9 are both decreased in Krt16 null footpad lesions and human PC-based PPK22,35,50. Of note, mice that are double-null for the differentiation-specific Krt2 and Krt10 develop a keratoderma-like phenotype on footpad skin51 while mice null for Krt9 develop an epidermolytic PPK that closely resembles the corresponding human disorder36. Aside from these (and other) alterations (see35 and22), the expression of KRT6A, KRT6B, KRT16, and/or KRT17 (including the mutated alleles) is dramatically increased in PC-based PPK, as expected given the stress- and wound-sensitive regulation of these genes22. Given the knowledge that K6, K16 and K17 proteins have pleiotropic and context-dependent properties35,37,39,52–56, such alterations in keratin protein levels and balance among them are poised to have a striking impact on the development and evolution of PPK lesions.
Pathophysiological unknowns in PPK and other clinical features of PC
While all clinical manifestations associated with PC are worth a deep investigation, two of them stand out as remaining particularly intriguing at a cellular and molecular level. One is the occurrence of individual or multiple cysts (steatocystoma multiplex; see OMIM entries #184500 and #184510) in PC patients, which are benign fluid filled cysts believed to originate from sebaceous glands and which can occur all over the body and arise preferentially in individuals with mutations in KRT1757–59. These cysts often require surgical drainage or removal as their rupture and/or inflammation pose a risk for infection and can be painful for patients1. Another intriguing manifestation is natal teeth, which refers to presence of teeth in newborns and is also preferentially associated with mutations in KRT171,59. Natal teeth are soft, friable and prone to caries, and are usually lost within the first few months of life1,60–63. Of note, Krt17 is expressed at a very early stage of the development of ectodermal appendages, including the tooth32. Recent studies have shown that genetic variants in the PC-associated keratin genes increase susceptibility to tooth decay64. There is currently no model to study the cystic skin lesions and phenomenon of natal teeth associated with PC.
Limitations of past and current therapeutic strategies for PC
PC-associated PPK has been treated with a combination of keratolytics, pain medication, orthotics, and mechanical removal of calluses23,65. While the keratolytics salicylic acid and urea soften calluses, they cannot control the significant overgrowth associated with most cases of PC-PKK. Pain medication and custom orthotics can partially alleviate discomfort, but do not treat the underlying PPK. Routine mechanical removal of calluses by filing, grinding, or cutting has been the most satisfying treatment for individuals with PC23. Significant efforts are currently under way to develop new and effective therapeutics for the management of these lesions. Two distinct strategies are highlighted here. The first strategy involves the development of short interfering RNAs (siRNAs) that specifically target mutant keratin alleles and reduce their expression. It has shown some promise in a trial of the siRNA TD101, which targets the KRT6A N171K allele, albeit in a single patient21,66,67. In its current form, this approach suffers from the limitation that delivery of such nucleic acid-based therapeutics requires intradermal injections that cause intense pain to the patient. The generation of self-delivery siRNAs for mutant keratin alleles improves the uptake of siRNAs by keratinocytes68 but does not improve penetration through the stratum corneum. Accordingly, a method to deliver siRNA-based therapeutics that involves topical application of therapeutic agents is sorely needed69.
The second strategy to treat PC-based PPK consists of drug-based interventions aimed at reducing mutant keratin gene expression. The mTOR inhibitor rapamycin/sirolimus suppresses K6a expression and, when taken orally, improves PC symptoms70. However, severe side-effects associated with systemic rapamycin treatment prevent it from being a viable long-term treatment for PC. Recently, topical sirolimus treatment of two K6a patients improved PC-PPK without the toxicity of systemic treatment71 but requires additional studies to confirm the safety and efficacy of this treatment. Oral retinoids successfully reduced callus thickness in some PC individuals, but like rapamycin, adverse side effects including increased pain prevent oral retinoids from being a viable long-term treatment for PC72. Statins can also downregulate KRT6A expression73, but so far only oral rosuvastatin has been shown to be effective in a single case of K6a based PC74. Finally, injections of botulinum toxin (Botox) into plantar calluses improved plantar blistering and pain associated with PC-PPK lesions75, but injections are costly and must be performed under anesthesia. While each of these drug-based interventions provide some relief, none of them in present form provide viable long-term treatment strategies for PC-based PPK.
Opportunities for novel therapies
A promising opportunity to complement ongoing efforts to develop effective therapeutics for PC-based PPK would be to target stress response pathways and/or pathways capable of promoting the restoration of normal epidermal differentiation. In Krt16 null mice, topical treatment with the small natural molecule sulforaphane (SF), which activates Nrf2 signaling by modifying Keap176, can prevent PPK-like lesions in male mice37. Addition of the ER-β agonist Diarylpropionitrile (DPN) to the SF treatment regimen is necessary for successful activation of Nrf277 and prevention of PPK-like lesions in female mice. SF is available in pure form or as part of broccoli sprout extract78, can be safely delivered topically, and has shown therapeutic promise in the treatment of EBS arising from mutations in either keratins K5 or K1479,80. The sexual dimorphism in response to SF treatment in mice is a reminder that sex-based differences is an important consideration when developing therapeutics for any disease81. Whether there is a sexual dimorphism in the setting of PC remains an open question.
Another strategy worth considering is to normalize terminal differentiation in volar skin. In male Krt16 null mice treated with SF prior to lesion onset, restoration of Nrf2 activity coincided with induction of Krt9 expression35. Additional efforts should be focused on testing this specific strategy. In the end, the prospect of combining treatment modalities that act to prevent and/or treat active lesions represent an attractive prospect for the treatment of a condition featuring the complexity of PC-associated PPK.
Future directions
Pachyonychia congenita (PC) is a monogenic skin disease with a complex, polygenic presentation. Despite the plethora of challenges that arise in studying this disease, the use of transgenic mouse models and of computational biology has been invaluable and provided novel insight into the pathophysiology of PC-based PPK, one of the most debilitating symptoms for individuals with PC. The study of PPK pathophysiology not only paves the way for researchers to devise therapeutics to treat PC, but also provides an opportunity to better understand the mechanisms that control skin tissue homeostasis.
Bulleted statements:
What’s already known about this topic?
Pachyonychia congenita is a rare genodermatosis caused by mutations in KRT6A, KRT6B, KRT6C, KRT16, KRT17, which are normally expressed in skin appendages and induced following injury.
Individuals with PC present with multiple clinical symptoms that usually include thickened and dystrophic nails, palmoplantar keratoderma (PPK), glandular cysts, and oral leukokeratosis.
The study of PC pathophysiology is made challenging because of its low incidence and high complexity. There is no cure or effective treatment for PC.
What does this study add?
This text reviews recent progress made when studying the pathophysiology of palmoplantar keratoderma (PPK) associated with pachyonychia congenita (PC).
This recent progress points to new possibilities for devising effective therapeutics that may complement current palliative strategies.
Acknowledgements
The authors wish to thank members of the Coulombe laboratory for their support. This work was supported by grant AR044232 from the National Institutes of Health.
Funding Sources:
These studies were supported by grant AR044232 issued to P.A.C. from the National Institute of Arthritis, Musculoskeletal and Skin Disease (NIAMS). A.G.Z. received support from grant T32 CA009110 from the National Cancer Institute.
Footnotes
Conflict of interest disclosures: None declared.
References
- 1.Leachman SA, Kaspar RL, Fleckman P et al. Clinical and pathological features of pachyonychia congenita. The journal of investigative dermatology. Symposium proceedings 2005; 10: 3–17. [DOI] [PubMed] [Google Scholar]
- 2.Wilson NJ, Messenger AG, Leachman SA et al. Keratin K6c mutations cause focal palmoplantar keratoderma. The Journal of investigative dermatology 2010; 130: 425–9. [DOI] [PubMed] [Google Scholar]
- 3.McLean WH, Rugg EL, Lunny DP et al. Keratin 16 and keratin 17 mutations cause pachyonychia congenita. Nature genetics 1995; 9: 273–8. [DOI] [PubMed] [Google Scholar]
- 4.Smith FJ, Jonkman MF, van Goor H et al. A mutation in human keratin K6b produces a phenocopy of the K17 disorder pachyonychia congenita type 2. Human molecular genetics 1998; 7: 1143–8. [DOI] [PubMed] [Google Scholar]
- 5.Bowden PE, Haley JL, Kansky A et al. Mutation of a type II keratin gene (K6a) in pachyonychia congenita. Nature genetics 1995; 10: 363–5. [DOI] [PubMed] [Google Scholar]
- 6.Franklin J Pachyonychia Congenita (Jadassohn and Lewandowski). Proceedings of the Royal Society of Medicine 1939; 32: 263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson AD, Lawler SD. Pachyonychia congenita; a report of six cases in one family, with a note on linkage data. Annals of eugenics 1951; 16: 142–6. [DOI] [PubMed] [Google Scholar]
- 8.Liao H, Sayers JM, Wilson NJ et al. A spectrum of mutations in keratins K6a, K16 and K17 causing pachyonychia congenita. Journal of dermatological science 2007; 48: 199–205. [DOI] [PubMed] [Google Scholar]
- 9.Fu T, Leachman SA, Wilson NJ et al. Genotype-phenotype correlations among pachyonychia congenita patients with K16 mutations. The Journal of investigative dermatology 2011; 131: 1025–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin MT, Levy ML, Bowden PE et al. Identification of sporadic mutations in the helix initiation motif of keratin 6 in two pachyonychia congenita patients: further evidence for a mutational hot spot. Experimental dermatology 1999; 8: 115–9. [DOI] [PubMed] [Google Scholar]
- 11.Krupiczojc MA, O’Toole EA. Plantar pain in pachyonychia congenita. The British journal of dermatology 2018; 179: 11–2. [DOI] [PubMed] [Google Scholar]
- 12.Brill S, Sprecher E, Smith FJD et al. Chronic pain in pachyonychia congenita: evidence for neuropathic origin. The British journal of dermatology 2018; 179: 154–62. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg RL, Coulombe, P.A., Polydefkis, M., Caterina, M.J. Pain mechanisms in heriditary Palmoplantar Keratoderma. The British journal of dermatology 2019; In press. [DOI] [PubMed] [Google Scholar]
- 14.Coulombe PA, Fuchs E. Epidermolysis bullosa simplex. Seminars in dermatology 1993; 12: 173–90. [PubMed] [Google Scholar]
- 15.Coulombe PA, Hutton ME, Letai A et al. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell 1991; 66: 1301–11. [DOI] [PubMed] [Google Scholar]
- 16.Coulombe PA, Hutton ME, Vassar R et al. A function for keratins and a common thread among different types of epidermolysis bullosa simplex diseases. The Journal of cell biology 1991; 115: 1661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coulombe PA, Kerns ML, Fuchs E. Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J Clin Invest 2009; 119: 1784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reis A, Hennies HC, Langbein L et al. Keratin 9 gene mutations in epidermolytic palmoplantar keratoderma (EPPK). Nature genetics 1994; 6: 174–9. [DOI] [PubMed] [Google Scholar]
- 19.Langbein L, Heid HW, Moll I et al. Molecular characterization of the body site-specific human epidermal cytokeratin 9: cDNA cloning, amino acid sequence, and tissue specificity of gene expression. Differentiation; research in biological diversity 1993; 55: 57–71. [DOI] [PubMed] [Google Scholar]
- 20.Kim D, Hossain MZ, Nieves A et al. To Control Site-Specific Skin Gene Expression, Autocrine Mimics Paracrine Canonical Wnt Signaling and Is Activated Ectopically in Skin Disease. The American journal of pathology 2016; 186: 1140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leachman SA, Hickerson RP, Schwartz ME et al. First-in-human mutation-targeted siRNA phase Ib trial of an inherited skin disorder. Molecular therapy : the journal of the American Society of Gene Therapy 2010; 18: 442–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao YA, Hickerson RP, Seegmiller BL et al. Gene expression profiling in pachyonychia congenita skin. Journal of dermatological science 2015; 77: 156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberg I, Fruchter D, Meilick A et al. Best treatment practices for pachyonychia congenita. Journal of the European Academy of Dermatology and Venereology : JEADV 2014; 28: 279–85. [DOI] [PubMed] [Google Scholar]
- 24.Moll R, Franke WW, Schiller DL et al. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982; 31: 11–24. [DOI] [PubMed] [Google Scholar]
- 25.Hesse M, Magin TM, Weber K. Genes for intermediate filament proteins and the draft sequence of the human genome: novel keratin genes and a surprisingly high number of pseudogenes related to keratin genes 8 and 18. Journal of cell science 2001; 114: 2569–75. [DOI] [PubMed] [Google Scholar]
- 26.Schweizer J, Bowden PE, Coulombe PA et al. New consensus nomenclature for mammalian keratins. The Journal of cell biology 2006; 174: 169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tyner AL, Fuchs E. Evidence for posttranscriptional regulation of the keratins expressed during hyperproliferation and malignant transformation in human epidermis. The Journal of cell biology 1986; 103: 1945–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi K, Yan B, Yamanishi K et al. The two functional keratin 6 genes of mouse are differentially regulated and evolved independently from their human orthologs. Genomics 1998; 53: 170–83. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K, Paladini RD, Coulombe PA. Cloning and characterization of multiple human genes and cDNAs encoding highly related type II keratin 6 isoforms. The Journal of biological chemistry 1995; 270: 18581–92. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg M, RayChaudhury A, Shows TB et al. A group of type I keratin genes on human chromosome 17: characterization and expression. Molecular and cellular biology 1988; 8: 722–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernot KM, Coulombe PA, McGowan KM. Keratin 16 expression defines a subset of epithelial cells during skin morphogenesis and the hair cycle. The Journal of investigative dermatology 2002; 119: 1137–49. [DOI] [PubMed] [Google Scholar]
- 32.McGowan KM, Coulombe PA. Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. The Journal of cell biology 1998; 143: 469–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Troyanovsky SM, Leube RE, Franke WW. Characterization of the human gene encoding cytokeratin 17 and its expression pattern. European journal of cell biology 1992; 59: 127–37. [PubMed] [Google Scholar]
- 34.Chen J, Roop DR. Mouse models in preclinical studies for pachyonychia congenita. The journal of investigative dermatology. Symposium proceedings 2005; 10: 37–46. [DOI] [PubMed] [Google Scholar]
- 35.Zieman A, Poll BG, Ma J et al. Altered keratinocyte differentiation is an early driver of keratin mutation-based palmoplantar keratoderma. Human molecular genetics 2019; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu DJ, Thomson C, Lunny DP et al. Keratin 9 is required for the structural integrity and terminal differentiation of the palmoplantar epidermis. The Journal of investigative dermatology 2014; 134: 754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerns ML, Hakim JM, Lu RG et al. Oxidative stress and dysfunctional NRF2 underlie pachyonychia congenita phenotypes. J Clin Invest 2016; 126: 2356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lessard JC, Coulombe PA. Keratin 16-null mice develop palmoplantar keratoderma, a hallmark feature of pachyonychia congenita and related disorders. The Journal of investigative dermatology 2012; 132: 1384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lessard JC, Pina-Paz S, Rotty JD et al. Keratin 16 regulates innate immunity in response to epidermal barrier breach. Proc Natl Acad Sci U S A 2013; 110: 19537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quigley DA, To MD, Perez-Losada J et al. Genetic architecture of mouse skin inflammation and tumour susceptibility. Nature 2009; 458: 505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quigley DA, Kandyba E, Huang P et al. Gene Expression Architecture of Mouse Dorsal and Tail Skin Reveals Functional Differences in Inflammation and Cancer . Cell reports 2016; 16: 1153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Covello SP, Smith FJ, Sillevis Smitt JH et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. The British journal of dermatology 1998; 139: 475–80. [DOI] [PubMed] [Google Scholar]
- 43.Smith FJ, Corden LD, Rugg EL et al. Missense mutations in keratin 17 cause either pachyonychia congenita type 2 or a phenotype resembling steatocystoma multiplex. The Journal of investigative dermatology 1997; 108: 220–3. [DOI] [PubMed] [Google Scholar]
- 44.Smith FJ, Fisher MP, Healy E et al. Novel keratin 16 mutations and protein expression studies in pachyonychia congenita type 1 and focal palmoplantar keratoderma. Experimental dermatology 2000; 9: 170–7. [DOI] [PubMed] [Google Scholar]
- 45.Shamsher MK, Navsaria HA, Stevens HP et al. Novel mutations in keratin 16 gene underly focal non-epidermolytic palmoplantar keratoderma (NEPPK) in two families. Human molecular genetics 1995; 4: 1875–81. [DOI] [PubMed] [Google Scholar]
- 46.Smith FJ, Liao H, Cassidy AJ et al. The genetic basis of pachyonychia congenita. The journal of investigative dermatology. Symposium proceedings 2005; 10: 21–30. [DOI] [PubMed] [Google Scholar]
- 47.Zieman A, Coulombe PA. The keratin 16 null phenotype is modestly impacted by genetic strain background in mice. Experimental dermatology 2018; 27: 672–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGowan KM, Tong X, Colucci-Guyon E et al. Keratin 17 null mice exhibit age- and strain-dependent alopecia. Genes & development 2002; 16: 1412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong P, Colucci-Guyon E, Takahashi K et al. Introducing a null mutation in the mouse K6alpha and K6beta genes reveals their essential structural role in the oral mucosa. The Journal of cell biology 2000; 150: 921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rice RH, Durbin-Johnson BP, Salemi M et al. Proteomic profiling of Pachyonychia congenita plantar callus. Journal of proteomics 2017; 165: 132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischer H, Langbein L, Reichelt J et al. Keratins K2 and K10 are essential for the epidermal integrity of plantar skin. Journal of dermatological science 2016; 81: 10–6. [DOI] [PubMed] [Google Scholar]
- 52.Tong X, Coulombe PA. Keratin 17 modulates hair follicle cycling in a TNFalpha-dependent fashion . Genes & development 2006; 20: 1353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Depianto D, Kerns ML, Dlugosz AA et al. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nature genetics 2010; 42: 910–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rotty JD, Coulombe PA. A wound-induced keratin inhibits Src activity during keratinocyte migration and tissue repair. The Journal of cell biology 2012; 197: 381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung BM, Arutyunov A, Ilagan E et al. Regulation of C-X-C chemokine gene expression by keratin 17 and hnRNP K in skin tumor keratinocytes. The Journal of cell biology 2015; 208: 613–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hobbs RP, DePianto DJ, Jacob JT et al. Keratin-dependent regulation of Aire and gene expression in skin tumor keratinocytes. Nature genetics 2015; 47: 933–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McLean WH, Hansen CD, Eliason MJ et al. The phenotypic and molecular genetic features of pachyonychia congenita. The Journal of investigative dermatology 2011; 131: 1015–7. [DOI] [PubMed] [Google Scholar]
- 58.Wilson NJ, Leachman SA, Hansen CD et al. A large mutational study in pachyonychia congenita. The Journal of investigative dermatology 2011; 131: 1018–24. [DOI] [PubMed] [Google Scholar]
- 59.Eliason MJ, Leachman SA, Feng BJ et al. A review of the clinical phenotype of 254 patients with genetically confirmed pachyonychia congenita. Journal of the American Academy of Dermatology 2012; 67: 680–6. [DOI] [PubMed] [Google Scholar]
- 60.Clementi M, Cardin de Stefani E, Dei Rossi C et al. Pachyonychia congenita Jackson-Lawler type: a distinct malformation syndrome. The British journal of dermatology 1986; 114: 367–70. [DOI] [PubMed] [Google Scholar]
- 61.Feinstein A, Friedman J, Schewach-Millet M. Pachyonychia congenita. Journal of the American Academy of Dermatology 1988; 19: 705–11. [DOI] [PubMed] [Google Scholar]
- 62.Munro CS, Carter S, Bryce S et al. A gene for pachyonychia congenita is closely linked to the keratin gene cluster on 17q12-q21. Journal of medical genetics 1994; 31: 675–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terrinoni A, Smith FJ, Didona B et al. Novel and recurrent mutations in the genes encoding keratins K6a, K16 and K17 in 13 cases of pachyonychia congenita. The Journal of investigative dermatology 2001; 117: 1391–6. [DOI] [PubMed] [Google Scholar]
- 64.Duverger O, Carlson JC, Karacz CM et al. Genetic variants in pachyonychia congenita-associated keratins increase susceptibility to tooth decay. PLoS genetics 2018; 14: e1007168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Porter RM, Bravo AA, Smith FJD. Management of Plantar KeratodermasLessons from Pachyonychia Congenita. Journal of the American Podiatric Medical Association 2017; 107: 428–35. [DOI] [PubMed] [Google Scholar]
- 66.Leachman SA, Hickerson RP, Hull PR et al. Therapeutic siRNAs for dominant genetic skin disorders including pachyonychia congenita. Journal of dermatological science 2008; 51: 151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hickerson RP, Smith FJ, Reeves RE et al. Single-nucleotide-specific siRNA targeting in a dominant-negative skin model. The Journal of investigative dermatology 2008; 128: 594–605. [DOI] [PubMed] [Google Scholar]
- 68.Hickerson RP, Flores MA, Leake D et al. Use of self-delivery siRNAs to inhibit gene expression in an organotypic pachyonychia congenita model. The Journal of investigative dermatology 2011; 131: 1037–44. [DOI] [PubMed] [Google Scholar]
- 69.Kaspar RL, Leachman SA, McLean WH et al. Toward a treatment for pachyonychia congenita: report on the 7th Annual International Pachyonychia Congenita Consortium meeting. The Journal of investigative dermatology 2011; 131: 1011–4. [DOI] [PubMed] [Google Scholar]
- 70.Hickerson RP, Leake D, Pho LN et al. Rapamycin selectively inhibits expression of an inducible keratin (K6a) in human keratinocytes and improves symptoms in pachyonychia congenita patients. Journal of dermatological science 2009; 56: 82–8. [DOI] [PubMed] [Google Scholar]
- 71.Teng JMC, Bartholomew FB, Patel V et al. Novel treatment of painful plantar keratoderma in pachyonychia congenita using topical sirolimus. Clinical and experimental dermatology 2018; 43: 968–71. [DOI] [PubMed] [Google Scholar]
- 72.Gruber R, Edlinger M, Kaspar RL et al. An appraisal of oral retinoids in the treatment of pachyonychia congenita. Journal of the American Academy of Dermatology 2012; 66: e193–9. [DOI] [PubMed] [Google Scholar]
- 73.Zhao Y, Gartner U, Smith FJ et al. Statins downregulate K6a promoter activity: a possible therapeutic avenue for pachyonychia congenita. The Journal of investigative dermatology 2011; 131: 1045–52. [DOI] [PubMed] [Google Scholar]
- 74.Abdollahimajd F, Rajabi F, Shahidi-Dadras M et al. Pachyonychia congenita: a case report of a successful treatment with rosuvastatin in a patient with a KRT6A mutation. The British journal of dermatology 2018. [DOI] [PubMed] [Google Scholar]
- 75.Swartling C, Karlqvist M, Hymnelius K et al. Botulinum toxin in the treatment of sweat-worsened foot problems in patients with epidermolysis bullosa simplex and pachyonychia congenita. The British journal of dermatology 2010; 163: 1072–6. [DOI] [PubMed] [Google Scholar]
- 76.Hu C Modification of Keap1 Cysteine Residues by Sulforaphane. 2011; 24: 515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kerns ML, Hakim JMC, Zieman A et al. Sexual Dimorphism in Response to an NRF2 Inducer in a Model for Pachyonychia Congenita. The Journal of investigative dermatology 2018; 138: 1094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Talalay P, Cho CG et al. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A 1992; 89: 2399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kerns ML, DePianto D, Dinkova-Kostova AT et al. Reprogramming of keratin biosynthesis by sulforaphane restores skin integrity in epidermolysis bullosa simplex. Proc Natl Acad Sci U S A 2007; 104: 14460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kerns ML, Guss L, Fahey J et al. Randomized, split-body, single-blinded clinical trial of topical broccoli sprout extract: Assessing the feasibility of its use in keratin-based disorders. Journal of the American Academy of Dermatology 2017; 76: 449–53.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leube RE, Schwarz N. Sex Matters: Interfering with the Oxidative Stress Response in Pachyonychia Congenita. The Journal of investigative dermatology 2018; 138: 1019–22. [DOI] [PubMed] [Google Scholar]
- 82.Takahashi K, Coulombe PA. A transgenic mouse model with an inducible skin blistering disease phenotype. Proc Natl Acad Sci U S A 1996; 93: 14776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wojcik SM, Imakado S, Seki T et al. Expression of MK6a dominant-negative and C-terminal mutant transgenes in mice has distinct phenotypic consequences in the epidermis and hair follicle. Differentiation; research in biological diversity 1999; 65: 97–112. [DOI] [PubMed] [Google Scholar]
- 84.Wojcik SM, Longley MA, Roop DR. Discovery of a novel murine keratin 6 (K6) isoform explains the absence of hair and nail defects in mice deficient for K6a and K6b. The Journal of cell biology 2001; 154: 619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wojcik SM, Bundman DS, Roop DR. Delayed wound healing in keratin 6a knockout mice. Molecular and cellular biology 2000; 20: 5248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong P, Domergue R, Coulombe PA. Overcoming functional redundancy to elicit pachyonychia congenita-like nail lesions in transgenic mice. Molecular and cellular biology 2005; 25: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen J, Jaeger K, Den Z et al. Mice expressing a mutant Krt75 (K6hf) allele develop hair and nail defects resembling pachyonychia congenita. The Journal of investigative dermatology 2008; 128: 270–9. [DOI] [PubMed] [Google Scholar]
- 88.Garcia M, Larcher F, Hickerson RP et al. Development of skin-humanized mouse models of pachyonychia congenita. The Journal of investigative dermatology 2011; 131: 1053–60. [DOI] [PubMed] [Google Scholar]
- 89.Kimonis V, DiGiovanna JJ, Yang JM et al. A mutation in the V1 end domain of keratin 1 in non-epidermolytic palmar-plantar keratoderma. The Journal of investigative dermatology 1994; 103: 764–9. [DOI] [PubMed] [Google Scholar]
- 90.Arin MJ, Longley MA, Kuster W et al. An asparagine to threonine substitution in the 1A domain of keratin 1: a novel mutation that causes epidermolytic hyperkeratosis. Experimental dermatology 1999; 8: 124–7. [DOI] [PubMed] [Google Scholar]
- 91.Lugassy J, Itin P, Ishida-Yamamoto A et al. Naegeli-Franceschetti-Jadassohn syndrome and dermatopathia pigmentosa reticularis: two allelic ectodermal dysplasias caused by dominant mutations in KRT14. American journal of human genetics 2006; 79: 724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Armstrong DK, McKenna KE, Purkis PE et al. Haploinsufficiency of desmoplakin causes a striate subtype of palmoplantar keratoderma. Human molecular genetics 1999; 8: 143–8. [DOI] [PubMed] [Google Scholar]
- 93.Rickman L, Simrak D, Stevens HP et al. N-terminal deletion in a desmosomal cadherin causes the autosomal dominant skin disease striate palmoplantar keratoderma. Human molecular genetics 1999; 8: 971–6. [DOI] [PubMed] [Google Scholar]
- 94.McGrath JA, McMillan JR, Shemanko CS et al. Mutations in the plakophilin 1 gene result in ectodermal dysplasia/skin fragility syndrome. Nature genetics 1997; 17: 240–4. [DOI] [PubMed] [Google Scholar]
- 95.Whittock NV, Smith FJ, Wan H et al. Frameshift mutation in the V2 domain of human keratin 1 results in striate palmoplantar keratoderma. The Journal of investigative dermatology 2002; 118: 838–44. [DOI] [PubMed] [Google Scholar]
- 96.McKoy G, Protonotarios N, Crosby A et al. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet (London, England) 2000; 355: 2119–24. [DOI] [PubMed] [Google Scholar]
- 97.Kubo A, Shiohama A, Sasaki T et al. Mutations in SERPINB7, encoding a member of the serine protease inhibitor superfamily, cause Nagashima-type palmoplantar keratosis. American journal of human genetics 2013; 93: 945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maestrini E, Monaco AP, McGrath JA et al. A molecular defect in loricrin, the major component of the cornified cell envelope, underlies Vohwinkel’s syndrome . Nature genetics 1996; 13: 70–7. [DOI] [PubMed] [Google Scholar]
- 99.Lin Z, Chen Q, Lee M et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. American journal of human genetics 2012; 90: 558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lind L, Lundstrom A, Hofer PA et al. The gene for diffuse palmoplantar keratoderma of the type found in northern Sweden is localized to chromosome 12q11-q13. Human molecular genetics 1994; 3: 1789–93. [DOI] [PubMed] [Google Scholar]
- 101.Blaydon DC, Lind LK, Plagnol V et al. Mutations in AQP5, encoding a water-channel protein, cause autosomal-dominant diffuse nonepidermolytic palmoplantar keratoderma. American journal of human genetics 2013; 93: 330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Richard G, White TW, Smith LE et al. Functional defects of Cx26 resulting from a heterozygous missense mutation in a family with dominant deaf-mutism and palmoplantar keratoderma. Human genetics 1998; 103: 393–9. [DOI] [PubMed] [Google Scholar]
- 103.van Steensel MA, Spruijt L, van der Burgt I et al. A 2-bp deletion in the GJA1 gene is associated with oculo-dento-digital dysplasia with palmoplantar keratoderma. American journal of medical genetics. Part A 2005; 132a: 171–4. [DOI] [PubMed] [Google Scholar]
- 104.Gong XQ, Shao Q, Lounsbury CS et al. Functional characterization of a GJA1 frameshift mutation causing oculodentodigital dysplasia and palmoplantar keratoderma. The Journal of biological chemistry 2006; 281: 31801–11. [DOI] [PubMed] [Google Scholar]
- 105.Giehl KA, Eckstein GN, Pasternack SM et al. Nonsense mutations in AAGAB cause punctate palmoplantar keratoderma type Buschke-Fischer-Brauer. American journal of human genetics 2012; 91: 754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fischer J, Bouadjar B, Heilig R et al. Mutations in the gene encoding SLURP-1 in Mal de Meleda. Human molecular genetics 2001; 10: 875–80. [DOI] [PubMed] [Google Scholar]
- 107.Vanderhooft SL, Francis JS, Holbrook KA et al. Familial pityriasis rubra pilaris. Archives of dermatology 1995; 131: 448–53. [PubMed] [Google Scholar]
- 108.Nagy N, Wedgeworth E, Hamada T et al. Schopf-Schulz-Passarge syndrome resulting from a homozygous nonsense mutation in WNT10A. Journal of dermatological science 2010; 58: 220–2. [DOI] [PubMed] [Google Scholar]
- 109.Adaimy L, Chouery E, Megarbane H et al. Mutation in WNT10A is associated with an autosomal recessive ectodermal dysplasia: the odonto-onycho-dermal dysplasia. American journal of human genetics 2007; 81: 821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blaydon DC, Etheridge SL, Risk JM et al. RHBDF2 mutations are associated with tylosis, a familial esophageal cancer syndrome. American journal of human genetics 2012; 90: 340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Toomes C, James J, Wood AJ et al. Loss-of-function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosis. Nature genetics 1999; 23: 421–4. [DOI] [PubMed] [Google Scholar]