Abstract
BACKGROUND:
The success of implants is associated first with their osseointegration, and later on with their survival rate. In recent years, many efforts have been exerted to develop implant design, geometry, materials and techniques to enhance the osseointegration process and also to increase the success rate of implant procedures. New techniques, like leukocyte and platelet-rich fibrin (L-PRF) and low-level laser treatment (LLLT), have been developed to enhance the osseointegration around dental implants.
AIM:
This study aims at accelerating bone osseointegration process around dental implant using new techniques to increase the success rate, to allow immediate or early loading of a dental implant, and to make a comparison between the various new techniques in dental implant procedures to figure out which technique will achieve the best results.
METHODS:
The study was conducted on a random sample of 40 male patients. Dental implants were placed in the posterior areas of the lower jaw. Patients were divided randomly into 4 groups; control group, LLLT group, L-PRF group and L-PRF plus LLLT group. They were assessed using cone-beam computed tomography (CBCT).
RESULTS:
The results showed significant differences between all groups over different measured times. All the groups showed improvement in comparison with Normal group, where L-PRF group showed the best result followed by (L-PRF+LLLT) group, while the LLLT group showed the least improvement in comparison with bothL-PRF group and (L-PRF+LLLT) group.
CONCLUSION:
The study demonstrates that L-PRF gives a better performance in the osseointegration around dental implants than LLLT.
Keywords: Dental Implant, Diode Laser, Growth Factors, LLLT, PRF, Cone Beam Computed Tomography
Introduction
The use of dental implants to compensate the loss of teeth has increased through the last 30 years [1], [2]. Dental implants have become a very popular solution due to their high success rate and predictability of the procedure, as well as their relatively few complications [1], [3].
The phenomenon of osseointegration of titanium implants was discovered in 1952 by a Swedish orthopaedic surgeon, P I Brånemark, who defined osseointegration as “a direct structural and functional connection between ordered living bone and the surface of a load-bearing implant” [4]. In recent years, there has been a vast amount of scientific research and development in implant geometry, design, materials and techniques with the objective of further enhancing the success of implant treatment. Most of these developments have focused on how to improve the process of osseointegration through improvements in implant surface and design modifications [5]. New techniques have been developed to enhance the osseointegration around dental implants like L-PRF and LLLT. L-PRF and LLLT have become more and more applicable nowadays in dentistry [5].
Growth factors, which are generally considered a subset of cytokines, refer to the diffusible signaling proteins that stimulate cell growth, differentiation, survival, inflammation, and tissue repair. There are many types of growth factors, one of them is L-PRF which was first described by Choukroun et al., [6] as a new second generation of platelet concentrate. PRF is a simplified processing technique without any complex handling. PRF can be used to promote wound healing, bone regeneration, graft stabilization, wound sealing, and hemostasis. Because the fibrin matrix is better organized, it is able to more efficiently direct stem cell migration and the healing program. Release of growth factors from PRF through in vitro studies and good results from in vivo studies led to optimizing the clinical application of PRF. It was shown that there were better results of PRF over PRP (Platelet-Rich Plasma). Dohan et al., [7] proved a slower release of growth factors from PRF than PRP and observed better healing properties with PRF.
LLLT has been used clinically in the management of several conditions based on its ability to promote stimulatory effects on the biochemical and molecular processes that occur during tissue repair, leading to increased fibroblast and epithelial proliferation, and increased collagen synthesis, which can accelerate the healing process. In addition, LLLT increases the potential for bone repair and remodeling, reduces the inflammation and edema, regulates the immune system, modulates and attenuates the pain, and manages postoperative pain [8], [9], [10], [11], [12], [13], [14]. In dentistry, preclinical findings indicate a positive effect of LLLT on bone repair and osseointegration [15], [16], [17], [18], [19], and this treatment modality has become a well-accepted adjuvant tool to enhance the osseointegration process in cases of rehabilitation involving implant-supported prostheses [16], [17], [18], [19].
Most of the techniques currently available for assessing the osseointegration process, such as histology [20], histomorphometry [21], and X-ray diffraction [22], are invasive and require sample destruction and animal euthanasia. Reproducing them in humans is often difficult [23], [24]. Other technologies for noninvasive assessment of osseointegration include X-ray imaging, cone beam computed tomography (CBCT), multislice computed tomography (CT), micro-computed tomography (μCT) [25], and digital panoramas [26].
Material and Methods
Population and Study Design
The study included 40 randomly selected male patients with missed teeth. Forty dental implants were performed, with one implant per patient. Patients were divided randomly into 4 groups, with 10 patients in each group:
Group A: (Control Group) Implant procedure without addition of growth factors or LLLT.
Group B: Implant procedure with LLLT (Diode laser 808nm).
Group C: Implant procedure with the addition of L-PRF.
Group D: Implant procedure with the combined application of both L-PRF and LLLT.
The study was approved by the NILES’ ethics committee with a registration No. 018006.
Inclusion Criteria: 1) Patients were males with missed lower posterior teeth; 2) The working areas were edentulous for at least 6 months; 3) The patients’ ages ranges from 30 to 40 years old; and 4) The study was performed on the lower jaw.
Exclusion Criteria: 1) Patients with excessive bone loss; 2) Diseases affecting healing process (e.g. DM, Thyroid disease); 3) Females were excluded to avoid any hormonal changes which may affect the result of the study; and 4) Patients who receive radiotherapy or chemotherapy.
Methods
Group A: (Control Group) Implant procedure without addition of growth factors or LLLT.
Group B: Implant procedure with LLLT (Diode laser 808 nm). LLLT was applied after the implant insertion using diode laser 808 nm. There were six sessions in 2 weeks. The first session started on the same day of the implant insertion. The total energy dose delivered in each session is about 20 J/cm3 in one minute (10 J/cm3 is delivered in 30 seconds buccally and 10 J/cm3 is delivered in 30 seconds lingually) (Figure 1). The power was calculated according to the area to be stimulated, and this depends on the size of the inserted implant. The power was calculated according to this equation: Energy dose (J/cm3) = W.T/cm3.
Figure 1.
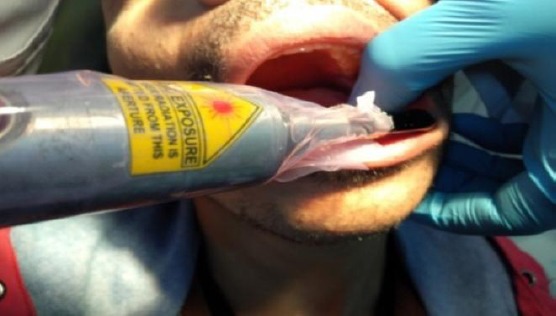
LLLT after implant insertion
Group C: Implant procedure with the addition of L-PRF. The osteotomy site was drilled according to the size of the previously selected implant. Then, whole venous blood (around 5 ml) in each of the two sterile vacutainer tubes (6 ml) was collected without any additives or any anticoagulants. After that the vacutainer tubes were placed in a centrifuge machine.
Adjust the parameters of the centrifuge machine at 3,000 revolutions per minute (rpm) for 10 minutes. After the centrifugation, the following three layers were obtained: Upper part containing straw-colored acellular plasma also called platelet poor plasma (PPP), the bottom of the tube containing red blood cells (RBCs), and the middle part containing the fibrin clot (Figure 2).
Figure 2.
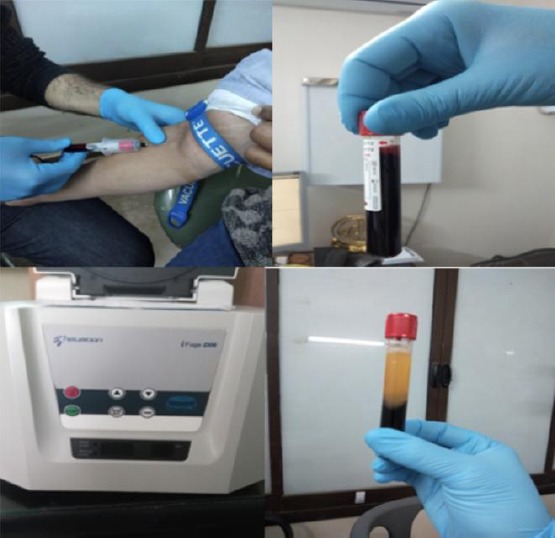
The venous blood was collected and centrifuged
The upper straw-coloured (PPP) layer was then removed and discarded; then the middle fraction was collected, 2 mm below to the lower dividing line, which is the PRF. The formed L-PRF was cut into small pieces using scissors (Figure 3).
Figure 3.
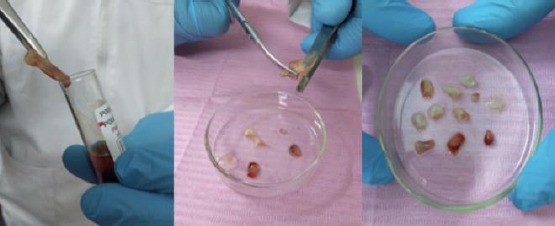
The formed L-PRF was cut into small pieces using scissors
Then, these small pieces of L-PRF were placed inside the osteotomy site before the insertion of the fixture of the prepared implant (Figure 4). Finally, the implant fixture was inserted inside the osteotomy site, which filled with growth factors (Figure 5).
Figure 4.
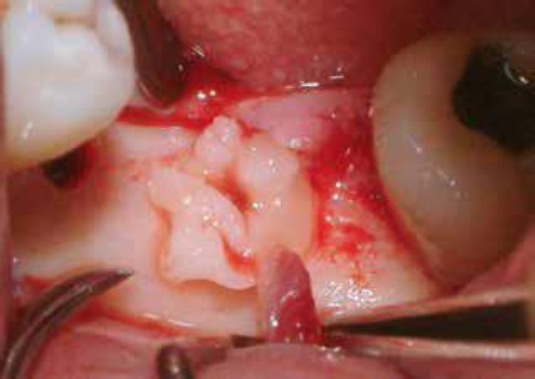
These small pieces of L-PRF were then placed inside the osteotomy site before the insertion of the fixture of the prepared implant
Figure 5.
The implant was then inserted into the osteotomy site
Group D: Implant procedure with the combined application of both L-PRF and LLLT (Diode laser 808 nm) with the same used protocol applied in the second and third groups.
All patients in the 4 groups were clinically and radiographically assessed using CBCT at baseline one week after the implant insertion (the day of suture removal), followed by another one after 6 weeks from implant insertion, and by another one after 12 weeks from the implant insertion, to evaluate the relative bone density around each implant. Relative bone density (RBD) around the implants was measured using OnDemand software, by inserting a simulated implant at the inserted implant and adjusted to the same dimensions and position, then measured the relative bone density using the verification tool in the software as shown in (Figure 6).
Figure 6.
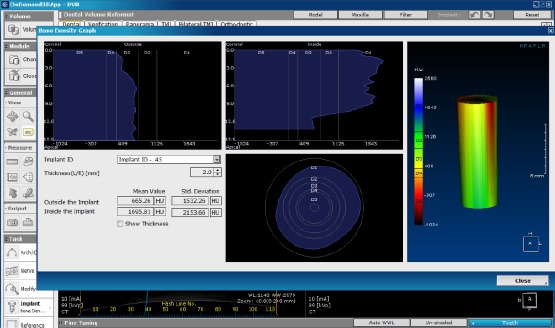
Measuring the relative bone density around the simulated implants using the verification tool in OnDemand software
Diode Laser
Diode laser device class IIIB (Gallium-Aluminum-Arsenide) (808 nm). This device was made in NILES (National Institute of Laser Enhanced Science). The device was calibrated after every 10 cases in NILES.
Growth Factors
Leukocyte and Platelet-Rich Fibrin (L-PRF) products are preparations with leukocytes and with a high-density fibrin network. The preparation is completely natural, which is prepared from the patient’s blood.
Centrifuge
The used centrifuge in this study is NeuationiFuge D06. The maximum speed of this device is 6500 RBM.
Dental Implant
The study used 40 dental implants of Leader system (TiXos dental implant). The dimensions of the used implants varied according to each case.
Cone Beam Machine for Radiographic Evaluation
The patients were examined using the SOREDEX (CRANEX 3Dx). All patients in this study were assessed by the same parameters (10.0 mA) (The time of exposure is 6.1 s) (90 Kv) (Field of view is 6 x 8 cm) (Resolution is high 200 μm).
Results
Descriptive Data
RBD of each implant was measured over one week, 6 weeks and 12 weeks using CBCT. The collected data of the 4 groups were summarised as average and standard deviation values for each group (Figure 7). It is well seen that the 12-week measurements in all groups are the best. The 6-week measurements in for group (A), the measured RBD decreases. On the other hand, the other 3 groups of the different treatments have clear increasing measures; except the laser group with a little increase
Figure 7.
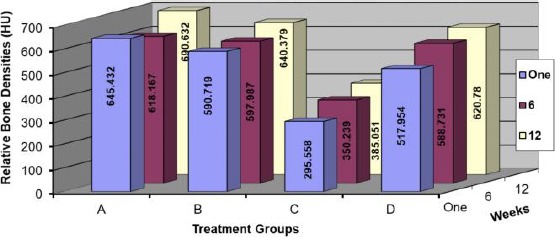
Relative Bone Densities at different groups and times
An illustration of efficiencies overall groups at 6 weeks and also 12 weeks can be seen at figures 8&9; one can see the highest value of the effect of L-PRF and the respective ranking as:
Figure 8.
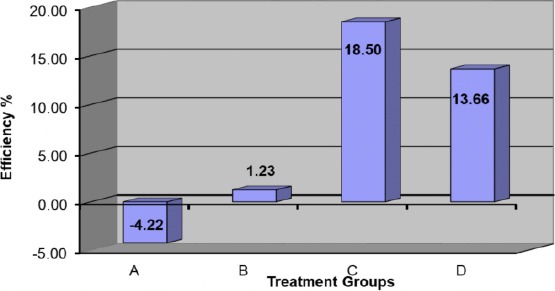
Efficiency of different treatment at 6 weeks
Figure 9.
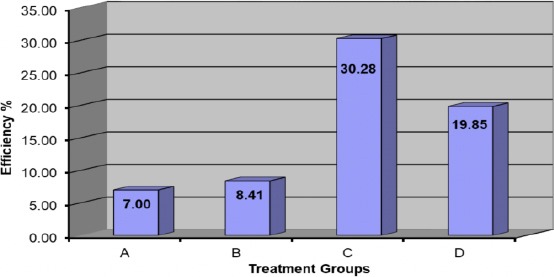
Efficiency of different treatment at 12 weeks
L-PRF group > (L-PRF+LLLT) group > LLLT group > Normal group
To assure the observations, we have to make statistical analysis for these collected data. An ANOVA test, t-test and correlation factor were made over the average values of treatments and the measurement times.
Statistical Analysis
The collected data were statistically analysed using ANOVA Test. ANOVA test was made over the average values of treatments and the measurements times, as shown in Table 1.
Table 1.
The resultant data of the ANOVA test overall groups and measurement times
| ANOVA | ||||||
|---|---|---|---|---|---|---|
| Source of Variation | SS | df | MS | F | P-value | F crit |
| Rows | 10551.27 | 2 | 5275.63 | 9.975 | 0.012 | 5.143 |
| Columns | 171042.06 | 3 | 57014.02 | 107.795 | 0.000 | 4.757 |
| Error | 3173.47 | 6 | 528.91 | |||
| Total | 184766.79 | 11 |
The resultant value of P-value is 0.012 (< 0.05) this means that there are significant differences between all groups over different measured times; and to point out the internal variations over the measured times, we can use the (t-test: Paired two samples for means) and the results were as follows: T-test: Paired Two Samples for Means.
Taking into account the previous observation of these tests, they illustrate that there is a significant difference between each time of measurements (one week, 6 weeks and 12 weeks). This proves that both decreasing at 6 weeks (in the normal group) and increasing from one week up to 12 weeks (in all groups) are real, as shown in Table 2.
Table 2.
P-Values of t-test
| Group A | |||
|---|---|---|---|
| 1 Week | 6 Weeks | 12 Weeks | |
| 1 Week | |||
| 6 Weeks | 1.48787E-05 | ||
| 12 Weeks | 1.26072E-08 | 1.90506E-07 | |
| Group B | |||
| 1 Week | 6 Weeks | 12 Weeks | |
| 1 Week | |||
| 6 Weeks | 1.98034E-06 | ||
| 12 Weeks | 8.0504E-09 | 2.75342E-08 | |
| Group C | |||
| 1 Week | 6 Weeks | 12 Weeks | |
| 1 Week | |||
| 6 Weeks | 1.13269E-06 | ||
| 12 Weeks | 1.41573E-06 | 3.56459E-06 | |
| Group D | |||
| 1 Week | 6 Weeks | 12 Weeks | |
| 1 Week | |||
| 6 Weeks | 1.8469E-08 | ||
| 12 Weeks | 2.49878E-10 | 5.706E-07 | |
On the other hand, the difference between the groups over measurement times (6 and 12 weeks) is shown in Table 3 as follows:
Table 3-a.
P-Values of 6 weeks over different Groups
| 6 Weeks | A | B | C | D |
|---|---|---|---|---|
| A | ||||
| B | 0.81217 | |||
| C | 0.00413 | 0.0019 | ||
| D | 0.71282 | 0.9034 | 0.0166 | |
| 12 Weeks | A | B | C | D |
| A | ||||
| B | 0.5688 | |||
| C | 0.0022 | 0.0017 | ||
| D | 0.3938 | 0.8024 | 0.0211 | |
These tests show the following:
- In 6 & 12-week groups, it is well observed that the L-PRF group has a significant difference (< 0.05) with any other groups because of the higher effect of L-PRF.
- The tests between any two groups didn’t show any significant difference except group C due to the positive LLLT effect on osteoclast cells was very clear on RBD during laser bio-stimulation period.
By applying the correlation factor measure, we can note the strong relation between the L-PRF group and (L-PRF+LLLT) group which means that the laser has an inhibitory role on the L-PRF effect (Table 4).
Table 4.
Correlation Factor between the L-PRF group and (L-PRF+LLLT) Group at 6 Weeks and 12 Weeks
| 6 Weeks | 12 Weeks | ||||
|---|---|---|---|---|---|
| C | D | C | D | ||
| C | 1 | C | 1 | ||
| D | -0.736209 | 1 | D | -0.778954 | 1 |
Discussion
Osseointegration is described as an effective interaction between bone tissue and the implant surface [27]. Osseointegration of titanium implants in rats was conducted by Haga et al., This process is characterised by bone resorption in osteoclasts, followed by bone formation by osteoblasts [29]. Few days after the implant placement, osteoclast cells become more prominent in number and inaction than osteoblast cells to start bone resorption. One month after implant placement, the osteoblast cells become more active to start the osseointegration process. From 1.5 to 2.5 months after the placement of implants, the formation of bone tissue by osteoblast cells proceeds in the direction of the damaged bone containing empty osteocytic lacunae, resulting in a reduction in it. The portion of neoformed bone exhibits characteristics of spongy bone. Also, both cell types present reduced volumes, suggesting less cell activity. Three months after implant placement, there was an absence of empty osteocytic lacunae. The area of pre-existent bone has been replaced by neoformed bone containing intact osteocytes. The neoformed bone presents the morphological characteristics of compact bone [28], [30].
Previously, PDGF and PRP were applied around implants to provide bone regeneration and to increase osseointegration [31]. Anil Kumar et al., [32] reported that PRF targeting apoptosis of the osteoclasts, which may have favourable effects on combating bone resorption. Based on these findings, we propose that there is a major role of PRF to limit osteoclastogenesis.
The most challenging issue about LLLT is to define the effective dose. Several studies in the literature promote low-level laser therapy as a useful procedure to improve osseointegration with doses used between 2 and 54 J [33], [34]. Accordingly, we use 20 J/cm3. However, it’s not sensible to decide whether the amount of energy’s being appropriate for biostimulation depending on the dose (Joules) only. Laser spot area should be known to have an opinion about the density of the energy given to the tissue. When the spot area is doubled, the energy density decreases by four times, or if the spot area is halved, the energy density quadruples [33], [35]. So, one should measure the delivered energy regarding the volume of the region and not just the area of the region. In the current study, low-intensity Gallium-Aluminum-Arsenide laser with wavelength 808 nm was used as a regenerative approach to enhance osseointegration and increase the density of bone surrounding the implants. The frequently used lasers in the previous studies range from 670 to 1,064 nm [36]. The energy density delivered to the bone was calculated according to the volume of the implant using the following equation: Energy dose (J/cm3) = W.T/cm3 to ensure the delivery of sufficient energy to the bone surrounding the implant. In this study, laser light was applied from both the buccal and lingual sides. The LLLT group shows improvements in the RBD especially after 6 weeks in comparison with the control group by the previous studies [37], [38], [39].
This group, however, showed lower results of RBD in comparison with L-PRF group. This can be explained by a study by Burcu et al., 2012 [35]. This study used 38 male albino Wistar rats. It aimed at evaluating the effects of 820 nm diode laser on osteoclastic and osteoblastic cell proliferation-activity and RANKL/OPG release during orthodontic tooth movement. The study concluded a very important fact about LLLT, which is Low-level laser therapy is known to be a stimulator of the current biological process in tissue. This fact used to explain why osteoclastic activity varied and increased in laser groups that caused increasing the bone resorption process while osteoblastic activity was similar between groups on the third day of the experiment. At the end of the experiment, because bone formation had already started, the number of osteoblasts was found to have increased in the laser groups.
Moreover, Glinkowski and Pokora indicated that LLLT to bone increased phagocytosis and cytokine (IL-1, TGF-β) synthesis via accelerating macrophage migration [35]. According to Karu et al., the mitochondrial cytochromes absorb the photon energy, and this absorption improves the potential activity of the cells via increasing ATP synthesis [40]. Because osteoclasts are multinuclear cells with mitochondria of high activity [41], they are readily affected by low-level laser radiation. This also explains the higher resorption levels in the irradiated animals. From the previous studies, we can conclude that the decrease in the RBD at 6 weeks in the control group is due to the pronounced activity of the osteoclast cells that are active in the first month after the implant placement. In LLLT group, there was a slight increase in the RBD after 6 weeks because laser biostimulation affects both osteoclast cells positively in the first days after the implant placement and osteoblast cells which become prominent at the end of the first month after the implant placement to compensate the bone loss in the first month and increases the relative bone densities slightly at the end of the 6th week.
Regarding the L-PRF group, the improvement efficiency percentage was the best between the 4 groups due to the great stimulating effect of PRF on healing, stimulation of the osteoblast, and limiting the osteoclastogenesis [32]. In his study, Anil Kumar et al., [32] reported that PRF displayed an inhibitory role in the formation and differentiation of osteoclast cells, and its molecular mechanism of action was related to the apoptosis induction through intrinsic mitochondrial pathway by activating caspase-9, -3 and -7 which are the most prevalent caspases and they are responsible for the majority of apoptotic effects.
Despite the improved RBD in both groups treated with LLLT and L-PRF, when we combine them, the density was improved but to less extent than the group used the L-PRF alone; and this means that the LLLT stimulated the osteoclasts [39], [40] which negatively affected the role of L-PRF slightly. This means that the LLLT has an inhibitory role in the PRF effect.
RBD increases after 12 weeks in the 4 groups because, within the period of 1.5 to 2.5 months after the placement of implants, the activity of the osteoclast cells decreases and the activity of osteoblast cells increases to start the process of bone formation [28], [30].
In conclusion, applying the findings of the current study, we can conclude that the osseointegration of dental implants can be clearly enhanced using new techniques like LLLT and L-PRF. These new techniques could increase the success rate of dental implants. L-PRF has a more positive effect on the osseointegration of dental implant than LLLT. The combined application of L-PRF and LLLT could enhance the osseointegration of dental implant but to a lower extent than applying L-PRF alone. Moreover, the combined application of L-PRF and LLLT could enhance the osseointegration of dental implant more than applying LLLT alone.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Zohrabian VM, Sonick M, Hwang D, Abrahams JJ. Dental implants. Semin Ultrasound CT MR. 2015;36:415–426. doi: 10.1053/j.sult.2015.09.002. https://doi.org/10.1053/j.sult.2015.09.002 PMid:26589695. [DOI] [PubMed] [Google Scholar]
- 2.Jenny G, Jauernik J, Bierbaum S, Bigler M, Gratz KW, Rucker M, Stadlinger B. A systematic review and meta-analysis on the influence of biological implant surface coatings on peri-implant bone formation. J Biomed Mater Res A. 2016;104:2898–2910. doi: 10.1002/jbm.a.35805. https://doi.org/10.1002/jbm.a.35805 PMid:27301790. [DOI] [PubMed] [Google Scholar]
- 3.Shemtov-Yona K, Rittel D. An overview of the mechanical integrity of dental implants. Biomed Res Int. 2015;2015:547384. doi: 10.1155/2015/547384. https://doi.org/10.1155/2015/547384 PMid:26583117 PMCid:PMC4637045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pal TK. Fundamentals and history of implant dentistry. Journal of the International Clinical Dental Research Organization. 2015;7(3):6. https://doi.org/10.4103/2231-0754.172933. [Google Scholar]
- 5.Gaviria L, Salcido JP, Guda T, Ong JL. Current trends in dental implants. J Korean Assoc Oral MaxillofacSurg. 2014;40(2):50–50. doi: 10.5125/jkaoms.2014.40.2.50. https://doi.org/10.5125/jkaoms.2014.40.2.50 PMid:24868501 PMCid:PMC4028797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choukroun J, Adda F, Schoeffler C, Vervelle A. Uneopportunitéen paro-implantologie:Le PRF. Implantodontie. 2001;42:55–62. [Google Scholar]
- 7.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF):A second-generation platelet concentrate. Part II:Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. https://doi.org/10.1016/j.tripleo.2005.07.009 PMid:16504850. [DOI] [PubMed] [Google Scholar]
- 8.Coelho RC, Zerbinati LP, de Oliveira MG, Weber JB. Systemic effects of LLLT on bone repair around PLLA-PGA screws in the rabbit tibia. Lasers Med Sci. 2014;29:703–708. doi: 10.1007/s10103-013-1384-4. https://doi.org/10.1007/s10103-013-1384-4 PMid:23832178. [DOI] [PubMed] [Google Scholar]
- 9.De Vasconcellos LM, Barbara MA, Deco CP, Junqueira JC, do Prado RF, Anbinder AL, de Vasconcellos LG, Cairo CA, Carvalho YR. Healing of normal and osteopenic bone with titanium implant and low-level laser therapy (GaAlAs):a histomorphometric study in rats. Lasers Med Sci. 2014;29:575–580. doi: 10.1007/s10103-013-1326-1. https://doi.org/10.1007/s10103-013-1326-1 PMid:23624654. [DOI] [PubMed] [Google Scholar]
- 10.Demirkol N, Sari F, Bulbul M, Demirkol M, Simsek I, Usumez A. () Effectiveness of occlusal splints and low-level laser therapy on myofascial pain. Lasers Med Sci. 2015;30:1007–1012. doi: 10.1007/s10103-014-1522-7. https://doi.org/10.1007/s10103-014-1522-7 PMid:24504660. [DOI] [PubMed] [Google Scholar]
- 11.Fronza B, Somacal T, Mayer L, de Moraes JF, de Oliveira MG, Weber JB. () Assessment of the systemic effects of low-level laser therapy (LLLT) on thyroid hormone function in a rabbit model. Int J Oral MaxillofacSurg. 2013;42:26–30. doi: 10.1016/j.ijom.2012.06.017. https://doi.org/10.1016/j.ijom.2012.06.017 PMid:22819694. [DOI] [PubMed] [Google Scholar]
- 12.Gasperini G, Rodrigues de Siqueira IC, Rezende Costa L. Does low-level laser therapy decrease swelling and pain resulting from orthognathic surgery? Int J Oral MaxillofacSurg. 2014;43:868–873. doi: 10.1016/j.ijom.2014.02.015. https://doi.org/10.1016/j.ijom.2014.02.015 PMid:24679851. [DOI] [PubMed] [Google Scholar]
- 13.Park JB, Ahn SJ, Kang YG, Kim EC, Heo JS, Kang KL. Effects of increased low-level diode laser irradiation time on extraction socket healing in rats. Lasers Med Sci. 2015;30:719–726. doi: 10.1007/s10103-013-1402-6. https://doi.org/10.1007/s10103-013-1402-6 PMid:23929563. [DOI] [PubMed] [Google Scholar]
- 14.Tang E, Arany P. Photobiomodulation and implants:implications for dentistry. J Periodontal Implant Sci. 2013;43:262–268. doi: 10.5051/jpis.2013.43.6.262. https://doi.org/10.5051/jpis.2013.43.6.262 PMid:24455438 PMCid:PMC3891857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer L, Gomes FV, Carlsson L, Gerhardt-Oliveira M. Histologic and resonance frequency analysis of peri-implant bone healing after low-level laser therapy:an in vivo study. Int J Oral Maxillofac Implants. 2015;30:1028–1035. doi: 10.11607/jomi.3382. https://doi.org/10.11607/jomi.3882 PMid:26394337. [DOI] [PubMed] [Google Scholar]
- 16.Khadra M, Ronold HJ, Lyngstadaas SP, Ellingsen JE, Haanaes HR. Low-level laser therapy stimulates bone-implant interaction:an experimental study in rabbits. Clin Oral Implants Res. 2004;15:325–332. doi: 10.1111/j.1600-0501.2004.00994.x. https://doi.org/10.1111/j.1600-0501.2004.00994.x PMid:15142095. [DOI] [PubMed] [Google Scholar]
- 17.Maluf AP, Maluf RP, BritoCda R, Franca FM, De Brito RB., Jr Mechanical evaluation of the influence of low-level laser therapy in secondary stability of implants in mice shinbones. Lasers Med Sci. 2010;25:693–698. doi: 10.1007/s10103-010-0778-9. https://doi.org/10.1007/s10103-010-0778-9 PMid:20393769. [DOI] [PubMed] [Google Scholar]
- 18.Primo BT, da Silva RC, Grossmann E, Miguens SA, Jr, Hernandez PA, Silva AN., Jr Effect of surface roughness and low-level laser therapy on removal torque of implants placed in rat femurs. J Oral Implantol. 2013;39:533–538. doi: 10.1563/AAID-JOI-D-10-00141. https://doi.org/10.1563/AAID-JOI-D-10-00141 PMid:21534821. [DOI] [PubMed] [Google Scholar]
- 19.Campanha BP, Gallina C, Geremia T, Loro RC, Valiati R, Hubler R, de Oliveira MG. Low-level laser therapy for implants without initial stability. Photomed Laser Surg. 2010;28:365–369. doi: 10.1089/pho.2008.2429. https://doi.org/10.1089/pho.2008.2429 PMid:19860572. [DOI] [PubMed] [Google Scholar]
- 20.Rea M, Lang NP, Ricci S, Mintrone F, Gonzalez Gonzalez G, Botticelli D. () Healing of implants installed in over- or under-prepared sites-an experimental study in dogs. Clin Oral Implants Res. 2015;26:442–446. doi: 10.1111/clr.12390. https://doi.org/10.1111/clr.12390 PMid:24684411. [DOI] [PubMed] [Google Scholar]
- 21.Friedmann A, Friedmann A, Grize L, Obrecht M, Dard M. Convergent methods assessing bone growth in an experimental model at dental implants in the minipig. Ann Anat. 2014;196:100–107. doi: 10.1016/j.aanat.2014.02.001. https://doi.org/10.1016/j.aanat.2014.02.001 PMid:24656913. [DOI] [PubMed] [Google Scholar]
- 22.Lee SW, Hahn BD, Kang TY, Lee MJ, Choi JY, Kim MK, Kim SG. Hydroxyapatite and collagen combination-coated dental implants display better bone formation in the peri-implant area than the same combination plus bone morphogenetic protein-2-coated implants, hydroxyapatite only coated implants, and uncoated implants. J Oral MaxillofacSurg. 2014;72:53–60. doi: 10.1016/j.joms.2013.08.031. https://doi.org/10.1016/j.joms.2013.08.031 PMid:24331565. [DOI] [PubMed] [Google Scholar]
- 23.Ostman PO, Hellman M, Wendelhag I, Sennerby L. Resonance frequency analysis measurements of implants at placement surgery. Int J Prosthodont. 2006;19:77–83. [PubMed] [Google Scholar]
- 24.Pagliani L, Sennerby L, Petersson A, Verrocchi D, Volpe S, Andersson P. The relationship between resonance frequency analysis (RFA) and lateral displacement of dental implants:an in vitro study. J Oral Rehabil. 2013;40:221–227. doi: 10.1111/joor.12024. https://doi.org/10.1111/joor.12024 PMid:23278128. [DOI] [PubMed] [Google Scholar]
- 25.Parsa A, Ibrahim N, Hassan B, van der Stelt P, Wismeijer D. Bone quality evaluation at dental implant site using multislice CT, micro-CT, and cone beam CT. Clin Oral Implants Res. 2015;26:e1–7. doi: 10.1111/clr.12315. https://doi.org/10.1111/clr.12315 PMid:24325572. [DOI] [PubMed] [Google Scholar]
- 26.Barone A, Covani U, Cornelini R, Gherlone E. Radiographic bone density around immediately loaded oral implants:A case series. Clin. Oral Implants Res. 2003;14:610–15. doi: 10.1034/j.1600-0501.2003.00878.x. https://doi.org/10.1034/j.1600-0501.2003.00878.x. [DOI] [PubMed] [Google Scholar]
- 27.Zarb GA, Albrektsson T. Osseointegration - a réquiem for the periodontal ligament? Int J Periodontics Restorative Dent. 1991;11:88–91. [Google Scholar]
- 28.Haga M, Fujii N, Nozawa-Inoue K, Nomura S, Oda K, Uoshima K, et al. Detailed process of bone remodeling after achieve-ment of osseointegration in a rat implantation model. Anat Rec. 2009;292:38–47. doi: 10.1002/ar.20748. https://doi.org/10.1002/ar.20748 PMid:18727113. [DOI] [PubMed] [Google Scholar]
- 29.Raisz LG, Rodan GA. Embryology and cellular biology of bone. Metabolic bone disease and clinically related disorders. Academic Press. 1998:1–22. https://doi.org/10.1016/B978-012068700-8/50002-5. [Google Scholar]
- 30.Fujii N, Kusakari H, Maeda T. A histological study on tissue responses to titanium implantation in rat maxilla:the process of epithelial regeneration and bone reaction. J Periodontol. 1998;69:485–95. doi: 10.1902/jop.1998.69.4.485. https://doi.org/10.1902/jop.1998.69.4.485 PMid:9609380. [DOI] [PubMed] [Google Scholar]
- 31.Anitua E, Orive G, Aguirre JJ, Ardanza B, Andia I. 5-year clinical experience with BTI dental implants:Risk factors for implant failure. J ClinPeriodontol. 2008;35:724–732. doi: 10.1111/j.1600-051X.2008.01248.x. https://doi.org/10.1111/j.1600-051X.2008.01248.x PMid:18616758. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, Mahendra J, Samuel S, Govindraj J, Loganathan T, Vashum Y, Mahendra L, Krishnamoorthy T. Platelet-rich fibrin/biphasic calcium phosphate impairs osteoclasts differentiation and promotes apoptosis by the intrinsic mitochondrial pathway in chronic periodontitis. J Periodontol. 2018;0:1–11. doi: 10.1002/JPER.17-0306. https://doi.org/10.1002/JPER.17-0306 PMid:29958327. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki K, Shimizu NV. Effects of low-energy irradiation on bone remodelling during experimental tooth movement in rats. Lasers Surg Med. 2000;26:282–291. doi: 10.1002/(sici)1096-9101(2000)26:3<282::aid-lsm6>3.0.co;2-x. https://doi.org/10.1002/(SICI)1096-9101(2000)26:3<282::AID-LSM6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 34.Youssef M, Ashkar S, Hamade E, Gutknecht N, Lampert F, Mir M. The effect of low-level laser therapy during orthodontic movement:a preliminary study. Lasers Med Sci. 2008;23(1):27–27. doi: 10.1007/s10103-007-0449-7. https://doi.org/10.1007/s10103-007-0449-7 PMid:17361391. [DOI] [PubMed] [Google Scholar]
- 35.BurcuAyseAltan Oral, Sokucu Mahmud M. Ozkut, SevincInan. Metrical and histological investigation of the effects of low-level laser therapy on orthodontic tooth movement. Lasers Med Sci. 2012;27:131–140. doi: 10.1007/s10103-010-0853-2. https://doi.org/10.1007/s10103-010-0853-2 PMid:21038101. [DOI] [PubMed] [Google Scholar]
- 36.Diniz JS. Effect of low-power gallium-aluminum arsenium laser therapy (830 nm) in combination with bisphosph bisphosphonate treatment on osteopenic bone structure:an experimental animal study. Lasers Med Sci. 2009;24(3):347–347. doi: 10.1007/s10103-008-0568-9. https://doi.org/10.1007/s10103-008-0568-9 PMid:18648870. [DOI] [PubMed] [Google Scholar]
- 37.Lopes CB, Pinheiro AL, Sathaiah S, Duarte J, Cristinamartins M. Infrared laser light reduces loading time of dental implants:a Raman spectroscopic study. Photomed Laser Surg. 2005;23:27–31. doi: 10.1089/pho.2005.23.27. https://doi.org/10.1089/pho.2005.23.27 PMid:15782028. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu N, Mayahara K, Kiyosaki T, Yamaguchi A, Ozawa Y, Abiko Y. Low-intensity laser irradiation stimulates bone nodule formation via insulin-like growth factor-I expression in rat calvarial cells. Lasers Surg Med. 2007;39(6):551–551. doi: 10.1002/lsm.20521. https://doi.org/10.1002/lsm.20521 PMid:17659585. [DOI] [PubMed] [Google Scholar]
- 39.Mikhail FF, El-Din M, Ibrahim T, Zekry K, Nemat A, Nasry S. Effect of Laser Therapy on the Osseointegration of Immediately Loaded Dental Implants in Patients under Vitamin C, Omega-3 and Calcium Therapy. Open Access Maced J Med Sci. 2018;6(8):1468–1468. doi: 10.3889/oamjms.2018.291. https://doi.org/10.3889/oamjms.2018.291 PMid:30159079 PMCid:PMC6108810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J PhotochemPhotobiol. 1999;49:1–17. doi: 10.1016/S1011-1344(98)00219-X. https://doi.org/10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 41.Boyle WJ, et al. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. https://doi.org/10.1038/nature01658 PMid:12748652. [DOI] [PubMed] [Google Scholar]


