Abstract
BACKGROUND:
Goal-directed fluid therapy (GDFT) improved patient outcomes in various surgical procedures; however, its role during mass brain resection was not well investigated.
AIM:
In this study, we evaluated a simple protocol based on intermittent evaluation of pulse pressure variation for guiding fluid therapy during brain tumour resection.
METHODS:
Sixty-one adult patients scheduled for supratentorial brain mass excision were randomized into either GDFT group (received intraoperative fluids guided by pulse pressure variation) and control group (received standard care). Both groups were compared according to the following: brain relaxation scale (BRS), mean arterial pressure, heart rate, urine output, intraoperative fluid intake, postoperative serum lactate, and length of hospital stay.
RESULTS:
Demographic data, cardiovascular data (mean arterial pressure and heart rate), and BRS were comparable between both groups. GDFT group received more intraoperative fluids {3155 (452) mL vs 2790 (443) mL, P = 0.002}, had higher urine output {2019 (449) mL vs 1410 (382) mL, P < 0.001}, and had lower serum lactate {0.9 (1) mmol versus 2.5 (1.1) mmol, P = 0.03} compared to control group.
CONCLUSION:
In conclusion, PPV-guided fluid therapy during supratentorial mass excision, increased intraoperative fluids, and improved peripheral perfusion without increasing brain swelling.
Keywords: Goal-directed fluid therapy, Neurosurgical operations, Pulse pressure variation, Supratentorial mass excision
Introduction
Neurosurgical operations are characterised by major fluid shift, frequent use of diuretics, and prolonged operative time. The role of fluid therapy in these patients is very critical; hypovolemia might decrease cerebral perfusion; while, fluid over-infusion might swell the brain. Thus, fluid management in these procedures complex and challenging. Evidence on the optimum protocol for intraoperative fluid management in neurosurgical patients is still lacking.
Adequate intracranial volume management is considered a key factor that would overcome the tumour bulk and the surrounding vasogenic oedema facilitating surgical access [1]. Thus, a relaxed brain is one of the targets of intraoperative fluid management during craniotomy [1], [2]. The slack brain would allow proper surgical retraction and consequently, reduces brain retractor ischemia. Brain relaxation scale (BRS) had shown a good correlation with intracranial pressure [3]; thus, an increasing interest was paid to BRS as a simple surrogate for intracranial pressure [1], [2], [4].
Goal-directed hemodynamic therapy (GDT) in the operating room is a term used to describe the use of defined hemodynamic targets to guide intravenous fluid and inotropic therapy [5]. Pulse pressure variation (PPV) is one of the robust dynamic indices of fluid responsiveness which is based on heart-lung interactions [6]. GDT had been frequently investigated in the operating room in high-risk patients especially in major surgery [7], [8], [9]. However, the impact of GDT on patient outcomes, especially BRS, is not well evaluated in brain surgery. In this study, we evaluated PPV-guided fluid management compared to standard fluid management in patients undergoing supratentorial mass excision. We hypothesised that in these procedures, GDT might restrict intraoperative fluid volume, improve brain relaxation, and provide stable patient hemodynamics.
Methods
A randomised, controlled study was conducted in Cairo University hospitals between March 2017 and January 2018, after being approved by the research ethics board (MD-4-2016). Written informed consent was obtained from all subjects who participated in this trial. The study was registered before patient enrolment at clinicaltrials.gov registry system (NCT03033706, principal investigator: Ahmed Hasanin, date of registration: January 27th, 2017).
Sixty-one patients scheduled for supratentorial mass excision were enrolled in the study. Patients less than 18 years old, patients with arrhythmias, pulmonary hypertension, impaired cardiac contractility, impaired liver or kidney function, and patients with body mass index above 40 were excluded. Randomisation was achieved using a computer-generated sequence. Opaque envelopes were used to assign patients in one of the two study groups.
Management of anaesthesia
Patients were pre-medicated with ranitidine (50 mg) and ondansetron (4 mg). Anaesthesia was induced using Propofol 2 mg/kg, atracurium 0.5 mg/Kg, and fentanyl 2 µg/Kg, and maintained using isoflurane (1-1.5%) and atracurium 0.5 mg/Kg/h. After induction of anaesthesia, arterial and right internal jugular central venous catheters were inserted. The endotracheal tube was inserted, and mechanical ventilation was adjusted at a tidal volume of 8 mL/Kg, no PEEP, respiratory rate was adapted to maintain end-tidal CO2 between (30-35 mmHg). Patients’ monitors included: continuous electrocardiograph, continuous invasive arterial pressure monitor, pulse oximetry, end-tidal CO2 monitor, central venous pressure (CVP), and non-invasive blood pressure monitor. Mannitol (0.5 gm/Kg) was administered shortly after induction. Phenytoin (15 mg/Kg) was administered for loading if the patient was not previously loaded. Maintenance bolus of phenytoin (5 mg/Kg) was administered for previously loaded patients. Additional doses of fentanyl (50 µg/dose) were titrated with skin incision, and bur-hole keep means arterial pressure (MAP) and heart rate within 25% of the baseline reading. By the end of the operation; isoflurane was discontinued, and the residual neuromuscular blocking agent was reversed by neostigmine 0.05 mg/Kg and atropine 0.02 mg/Kg. Patients were extubated and admitted to the surgical intensive care unit.
Fluid therapy
All patients received a fluid bolus of 5 ml/kg Tetrastarch (Voluven: Hydroxyethyl starch 130/0.4 in isotonic sodium chloride solution manufactured by Fresenius Kabi Germany) after induction of anaesthesia, then fluid management was as follows:
Control group: in this group, patients received standard fluid management of 4 ml/Kg/hr ringer solution. If MAP decreased by 20% without obvious bleeding, the patient received a rescue fluid bolus of 3 mL/Kg Ringer solution over 5 minutes if CVP was lower than 4 mmHg, and received ephedrine bolus of 9 mg if CVP was higher than 4 mmHg.
GDT group: in this group, patients received a restricted fluid protocol of 1 ml/Kg/hr with concomitant PPV monitoring. The fluid bolus of 3 ml/Kg of ringer solution was administrated whenever PPV was higher than 13%. If the MAP decreased by 20% without obvious bleeding, the patient received ephedrine bolus of 9 mg.
PPV was assessed using invasive blood pressure monitor (GE solar 8000M/I monitor) every 15 minutes. PPV was calculated using the following equation [6]:
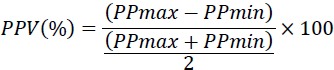
Maximal PP (PPmax) and minimal PP (PPmin) were obtained from the same respiratory cycle. PPV was calculated as the average of measurements obtained over three consecutive respiratory cycles.
In both groups, urine output was calculated and replaced using Ringer’s solution. Blood loss was replaced by tetrastarch solution in the ratio of 1:1. Packed RBCs were transfused if blood haemoglobin was less than 7 g/dL.
Outcomes
Our primary outcome was brain Relaxation scale (BRS). BRS was assessed by the neurosurgeon at 3-time intervals; with dural opening, after two hours, and before dural closure. A 4-point scale [2] was performed as follows: grade 1, perfectly relaxed; grade 2, satisfactorily relaxed; grade 3, firm brain; grade 4, bulging brain. The surgeon was blinded to the study group. The end-tidal CO2 was tightly controlled at 30-35 mmHg during the assessment of BRS.
In addition to demographic data (age, gender, weight, ASA class, incidence of disturbed conscious level “defined as Glasgow Coma Scale below 15” and incidence of preoperative neurological deficit “defined as as upper and/or lower limb weaknesses in contralateral side of the tumor; the presence of dysphasia, visual field defects were considered as neurological deficits as well.”), other outcomes included: Intraoperative fluid requirements, patient position during the operation, arterial-jugular oxygen saturation difference, arterial-Jugular lactate difference, urine output, vital signs (heart rate and MAP) arterial blood gases, intraoperative ephedrine consumption, postoperative electrolytes (Na, K, Mg, and Ca), postoperative serum lactate, postoperative 24-hour urine output, postoperative hemoglobin, and length of hospital stay.
Statistical analysis and sample size calculation
Our primary outcome was brain relaxation scale. A previous study [2] had assumed that a difference in mean BRS of 1 ± 1 is a clinically significant difference. We used a more conservative assumption to detect the same mean difference in BRS with a higher study power (95%). A minimum number of 26 patients per group was calculated Using MedCalc Software version 14.10.2 (MedCalc Sofware bvba, Ostend, Belgium), the minimum calculated number of participants to have a study power of 95% and an alpha error of 0.05 was 27 patients per group.
Statistical package for social science (SPSS) software, version 15 for Microsoft Windows (SPSS inc., Chicago, iL, USA) was used for data analysis. Continuous data were checked for normality using the Shapiro-Wilk test and was presented as mean (standard deviation) or median (interquartile range) as appropriate. Continuous data were analysed using unpaired t-test or Mann Whitney test as appropriate. Categorical data were presented as frequency (%) and analysed by chi-squared test. Repeated measures were analysed using analysis of variance (ANOVA) for repeated measures with post-hoc pairwise comparisons using the Bonferroni test. A P value less than 0.05 was considered statistically significant.
Results
Seventy-five patients were screened for eligibility. Fourteen patients were excluded (10 patients were excluded from meeting one of the exclusion criteria, and 4 patients declined to participate in the study). Sixty-one patients were available for final analysis (Figure 1).
Figure 1.
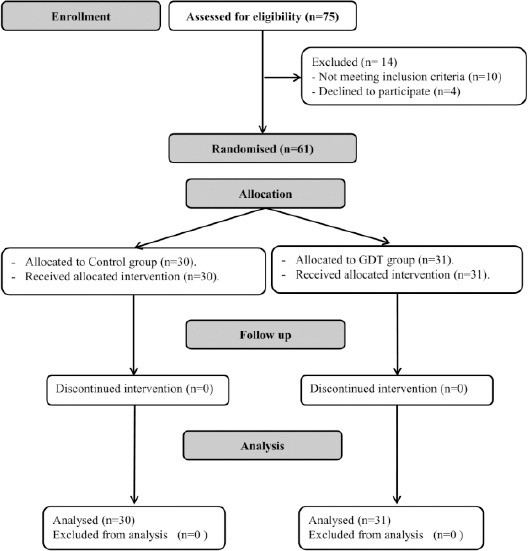
Consort chart showing patient recruitment; GDT: Goal-directed fluid therapy
Both groups were comparable according to demographic data and baseline characteristics (Table 1).
Table 1.
Demographic data and patient characteristics. Data are presented as mean (standard deviation), and frequency (%)
| Control group (n = 30) | GDT group (n = 31) | P value | |
|---|---|---|---|
| Age (years) | 39 (13) | 41 (12) | 0.43 |
| Weight (Kg) | 77 (12) | 76 (10) | 0.85 |
| Male gender | 12 (40%) | 16 (52) | 0.4 |
| ASA class (I/II) | 20/10 | 19/12 | 0.87 |
| DCL | 1 (3%) | 2 (7%) | 1 |
| Increased ICP | 2 (7%) | 4 (13%) | 0.7 |
| Neurological deficit | 3 (10%) | 2 (7%) | 0.7 |
| Convulsions | 3 (10%) | 2 (7%) | 0.6 |
| Tumour type (%) | 0.93 | ||
| Meningioma | 50% | 46% | |
| Glioma | 25% | 18% | |
| Intraventricular tumour | 8% | 14% | |
| Craniopharyngioma | 8% | 5% | |
| Other lesions | 9% | 17% | |
| Largest tumour diameter | 5.9 (3) | 5.4 (1.7) | 0.42 |
| Surgical duration (hours) | 5.3 (1.1) | 5.5 (0.9) | 0.48 |
| Baseline heart rate (bpm) | 82 (7) | 83 (7) | 0.56 |
| Baseline MAP (mmHg) | 88 (9) | 92 (10) | 0.12 |
ASA: American society of anesthesiology classification; DCL: disturbed conscious level; ICP: intra-cranial pressure; MAP: mean arterial pressure.
All patients were operated in a supine, head-up position. The GDT group received a greater number of fluid boluses and had higher total fluid consumption compared to the control group (Table 2).
Table 2.
Fluid management and urine output. Data are presented as mean (standard deviation), and median (quartiles)
| Control group (n = 30) | GDT group (n = 31) | P value | |
|---|---|---|---|
| Infused fluids (mL) | |||
| Total | 3155 (452) * | 0.002 | |
| Crystalloids | 2790 (443) | 2775 (423) * | 0.01 |
| Colloids | 2408 (418) | 380 (51) | 0.85 |
| 383 (61) | |||
| Urine output (mL) | 1410 (382) | 2019 (449) * | < 0.001 |
| Blood loss (mL) | 897 (430) | 887 (377) | 0.93 |
| Blood transfusion (mL) | 885 (363) | 773 (334) | 0.45 |
| Number of fluid boluses | 2 (1.2) | 5 (5.6) * | < 0.001 |
| Number of vasopressor boluses | 0 (0.1) | 0 (0.1) | 0.093 |
denotes statistical significance compared to the control group.
Higher urine output and lower postoperative serum lactate were reported in the GDT group compared to the control group (Table 2, and 4).
Table 3.
Brain relaxation scores. Data are presented as mean (standard deviation), and median (quartiles)
| Control group (n = 30) | GDT group (n = 31) | P value | |
|---|---|---|---|
| BRS-1 | 0.15 | ||
| Mean (SD) | 1.8 (0.7) | 1.54 (0.7) | |
| Median (quartiles) | 2 (1.2) | 1 (1.2) | |
| BRS-2 | 0.66 | ||
| Mean (SD) | 1.9 (0.6) | 1.8 (0.4) | |
| Median (quartiles) | 2 (2.2) | 2 (2,2) | |
| BRS-3 | 0.34 | ||
| Mean (SD) | 1.7 (0.4) | 1.58 (0.5) | |
| Median (quartiles) | 2 (1.2) | 2 (1.2) |
BRS: brain relaxation score, SD: standard deviation.
Table 4.
Postoperative data. Data are presented as mean (standard deviation), and median (quartiles)
| Control group (n = 30) | GDT group (n = 31) | P value | |
|---|---|---|---|
| PH | 7.38 (0.09) | 7.39 (0.06) | 0.56 |
| Lactate (mmol/L) | 2.5 (1.1) | 0.9 (1) * | 0.03 |
| ScVo2 (%) | 75 (6.3) | 74 (5.2) | 0.69 |
| Arterio-jugular Spo2 difference (%) | 23.6 (7.6) | 25.5 (5.1) | 0.26 |
| Arterio-jugular lactate difference (mmol/L) | 0.5 (0.2,0.6) | 0.4 (0.2,0.5) | 0.25 |
| Postoperative urine output (mL) | 1152 (261) | 1277 (269) | 0.07 |
| ICU stay (days) | 2.1 (1.3) | 2.1 (1.2) | 0.93 |
| Hospital stay (days) | 5.7 (1.5) | 5.2 (1.3) | 0.13 |
Scvo2: central venous oxygen saturation; Spo2: arterial oxygen saturation;
denotes statistical significance compared to the control group.
No significant differences were reported between both groups in BRS (Table 3), intraoperative mean arterial pressure (Figure 2), nor heart rate.
Figure 2.
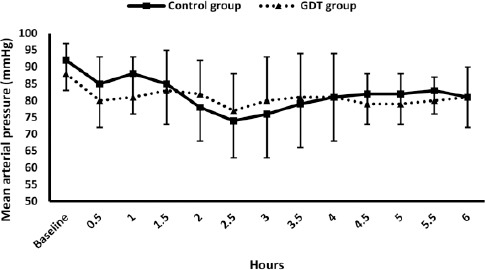
Mean arterial pressure; Markers are means, error bars are standard deviations; * denotes statistical significance compared to the baseline reading within the control group.” Denotes statistical significance compared to the baseline reading within the GDT group; GDT: Goal-directed fluid therapy
Postoperative laboratory investigations (haemoglobin, coagulation profile, and electrolytes), postoperative intensive care unit stay and postoperative hospital stay were comparable between both groups (Table 4).
Discussion
We found that the GDT group received more fluids compared to the control group without impacting BRS. Postoperative serum lactate was lower in the GDT denoting better peripheral organ perfusion compared to the control group. Patients undergoing craniotomy receive brain dehydrating measures; thus, they are vulnerable to intravascular volume depletion. Using PPV as a marker of volume status allowed accurate and early detection of hypovolemia; thus, more fluids were infused in the GDT group compared to the standard therapy group. The relatively better peripheral perfusion in the GDT group is most probably due to adequate and early restoration of intravascular volume using an accurate and reliable hemodynamic target before the occurrence of hypotension. The use of CVP as a measure for fluid responsiveness is declining; however, it is still conventionally used by many physicians [10], [11]. PPV had been reported to be a more accurate index for fluid responsiveness especially in mechanically ventilated patients [6].
In our study, we tried to answer two questions: 1- Would the PPV-guided therapy impact BRS? 2- Would PPV-guided therapy improve the hemodynamic profile and peripheral perfusion? We hypothesised that using GDT might avoid unnecessary excessive fluids; thus, we designed our study to provide a baseline restrictive fluid infusion rate (1 mL/Kg/hour) in the GDFT group with additional fluid boluses according to the PPV values. Surprisingly, the GDT group required more fluids than the control group due to the higher number of fluid boluses.
In our patients, better peripheral perfusion was reported in the GDT group. This was represented by the lower postoperative serum lactate level in addition to higher fluid volume and a smaller number of vasopressor boluses in the GDT group. Serum lactate is an important marker of tissue perfusion [12]. Postoperative serum lactate is a commonly used marker of peripheral perfusion during craniotomy [2], [4], [13], [14]. Elevated serum lactate level is usually associated with poor outcomes [14], especially in patients undergoing craniotomy [16]. Our results are in line with two recent randomised controlled studies. In the first study, Wu et al., [13] had recently compared two-stroke volume variation-based fluid protocols in neurosurgical procedures. Wu et al. had reported that the more liberal fluid protocol (targeting lower stroke volume variation) was associated with lower postoperative serum lactate compared to the restrictive protocol (targeting higher stroke volume variation). In the second study, Sundaram et al. compared PPV-directed therapy to CVP-guided therapy and found that the patients in PPV-guided therapy group received more fluids and had more stable hemodynamic profile compared to the CVP-guided group [17]. Our study showed similar results to the two mentioned studies concerning the higher fluid requirements and the better peripheral perfusion. Furthermore, we had also reported that the higher fluid requirements in the GDT group were not associated with brain swelling.
In a randomised controlled study, Luo et al. had investigated stroke volume variation-guided fluid therapy in brain surgeries [18]. Unlike our study, Luo et al. had given less intraoperative fluids in their study group compared to the control group. Two differences between our study and Luo study might account to the different findings: 1-Their fluid protocol in the control group was not well clarified. 2-They had used higher baseline fluid infusion rate (3 mL/Kg/hour) in their study group compared to our baseline infusion rate (1 mL/Kg/hour).
Two previous studies had evaluated the impact of GDT on BRS; however, both studies aimed to compare colloid and crystalloid solutions in neurosurgical patients [4], [19]; thus, they used GDT in both study groups and did not compare GDT to standard care. Our study is the first to evaluate the impact of GDT, compared to standard care, on BRS in addition to intraoperative fluid requirements and peripheral perfusion.
Usually, the neurosurgical patient is kept as dry as possible. However, to which extent should we restrict fluids? This question has not been adequately answered. The relationship between perioperative fluid administration and patient outcome is U-shaped. Under-transfusion results in cerebral, myocardial, and renal ischemia; while, fluid overload leads to lung congestion, brain oedema, and delayed wound healing [20]. Neurosurgical procedures are characterised by difficult assessment of blood loss under the drapes especially in the presence of irrigating fluids. The frequent use of diuretics makes urine output unreliable index for evaluation of volume status. Thus, it is necessary to use reliable parameters for guiding fluid management. It is highly believed that fluid administration should target clear hemodynamic variables rather than targeting traditional, fixed infusion rate. We chose PPV as a target for fluid management in our patients as it is a simple, accurate dynamic method of fluid responsiveness in mechanically ventilated patients [6]. Based on our findings, we found that using PPV for guiding fluid management in neurosurgical patients is feasible and has promising results. Using PPV would be also beneficial for patients whom anesthesiologists do not prefer central venous line insertion. Our findings might draw attention to the under-determined fluid needs during mass brain resection.
One of the limitations for implementing GDT in the operating room is the need for either complicated algorithms or sophisticated equipment. Introduction of simple protocols for GDT would enable wider application in different settings. Our study has the advantage of the use of a simple GDT protocol based on optimisation of PPV which needs only an arterial line. Most of other GDT protocols are based on either complicated algorithms or sophisticated equipment.
Our study had some limitations: 1-It is a single centre study. 2-All our operations were elective operations. 3-We had a low incidence of disturbed conscious level and increased intracranial pressure; thus, extrapolation of our findings in these groups of patients’ needs more research.
In conclusion, guiding fluid therapy using manual, intermittently calculated PPV during supratentorial mass excision increased intraoperative fluid administration and improved peripheral perfusion without impacting brain relaxation. More research is warranted to reach the optimum strategy and the best cut-off targets.
Ethical approval and consent to participate
Ethical approval from Cairo university hospitals research committee was obtained (MD-4-2016). Written informed consents were obtained from participants before inclusion.
Footnotes
Funding: Cairo University Hospitals, Cairo, Egypt
Competing Interests: The authors have declared that no competing interests exist
Trial registration: clinicaltrials.gov registry system: NCT03033706
Abbreviations: ANOVA: Analysis of variance; BRS: brain relaxation scale; CVP: central venous pressure; MAP: mean arterial pressure; GDT: goal-directed fluid therapy; PPV: pulse pressure variation; SPSS: Statistical package for social science
References
- 1.Quentin C, Charbonneau S, Moumdjian R, et al. A comparison of two doses of mannitol on brain relaxation during supratentorial brain tumor craniotomy:A randomized trial. Anesth Analg. 2013;116(4):862–862. doi: 10.1213/ANE.0b013e318282dc70. https://doi.org/10.1213/ANE.0b013e318282dc70 PMid:23354336. [DOI] [PubMed] [Google Scholar]
- 2.Rozet I, Tontisirin N, Muangman S, et al. Effect of equiosmolar solutions of mannitol versus hypertonic saline on intraoperative brain relaxation and electrolyte balance. Anesthesiology. 2007;107(5):697–697. doi: 10.1097/01.anes.0000286980.92759.94. https://doi.org/10.1097/01.anes.0000286980.92759.94 PMid:18073543. [DOI] [PubMed] [Google Scholar]
- 3.Todd MM, Warner DS, Sokoll MD, et al. A prospective, comparative trial of three anesthetics for elective supratentorial craniotomy. Propofol/fentanyl, isoflurane/nitrous oxide, and fentanyl/nitrous oxide. Anesthesiology. 1993;78(6):1005–1005. doi: 10.1097/00000542-199306000-00002. https://doi.org/10.1097/00000542-199306000-00002 PMid:8512094. [DOI] [PubMed] [Google Scholar]
- 4.Xia J, He Z, Cao X, et al. The brain relaxation and cerebral metabolism in stroke volume variation-directed fluid therapy during supratentorial tumors resection:crystalloid solution versus colloid solution. J Neurosurg Anesthesiol. 2014;26(4):320–320. doi: 10.1097/ANA.0000000000000046. https://doi.org/10.1097/ANA.0000000000000046 PMid:24487733. [DOI] [PubMed] [Google Scholar]
- 5.Bisgaard J, Gilsaa T, Rønholm E, Toft P. Optimising stroke volume and oxygen delivery in abdominal aortic surgery:a randomised controlled trial. Acta Anaesthesiol Scand. 2013;57(2):178–178. doi: 10.1111/j.1399-6576.2012.02756.x. https://doi.org/10.1111/j.1399-6576.2012.02756.x PMid:22897633. [DOI] [PubMed] [Google Scholar]
- 6.Hasanin A. Fluid responsiveness in acute circulatory failure. J intensive care. 2015;3:50. doi: 10.1186/s40560-015-0117-0. https://doi.org/10.1186/s40560-015-0117-0 PMid:26594361 PMCid:PMC4653888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasanin A, Mourad KH, Farouk I, et al. The Impact of Goal-Directed Fluid Therapy in Prolonged Major Abdominal Surgery on Extravascular Lung Water and Oxygenation:A Randomized Controlled Trial. Open Access Maced J Med Sci. 2019;7(8):1276–1276. doi: 10.3889/oamjms.2019.173. https://doi.org/10.3889/oamjms.2019.173 PMid:31110569 PMCid:PMC6514339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benes J, Giglio M, Brienza N, Michard F. The effects of goal-directed fluid therapy based on dynamic parameters on post-surgical outcome:a meta-analysis of randomized controlled trials. Crit Care. 2014;18(5):584. doi: 10.1186/s13054-014-0584-z. https://doi.org/10.1186/s13054-014-0584-z PMid:25348900 PMCid:PMC4234857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearse RM, Harrison DA, MacDonald N, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery:a randomized clinical trial and systematic review. JAMA. 2014;311(21):2181–2181. doi: 10.1001/jama.2014.5305. https://doi.org/10.1001/jama.2014.5305 PMid:24842135. [DOI] [PubMed] [Google Scholar]
- 10.Kastrup M, Markewitz A, Spies C, et al. Current practice of hemodynamic monitoring and vasopressor and inotropic therapy in post-operative cardiac surgery patients in Germany:results from a postal survey. Acta Anaesthesiol Scand. 2007;51(3):347–347. doi: 10.1111/j.1399-6576.2006.01190.x. https://doi.org/10.1111/j.1399-6576.2006.01190.x PMid:17096667. [DOI] [PubMed] [Google Scholar]
- 11.Bignami E, Belletti A, Moliterni P, Frati E, Guarnieri M, Tritapepe L. Clinical practice in perioperative monitoring in adult cardiac surgery:is there a standard of care?Results from an national survey. J Clin Monit Comput. 2016;30(3):347–347. doi: 10.1007/s10877-015-9725-4. https://doi.org/10.1007/s10877-015-9725-4 PMid:26089166. [DOI] [PubMed] [Google Scholar]
- 12.Hasanin A, Mukhtar A, Nassar H. Perfusion indices revisited. J Intensive Care. 2017;5(1):24. doi: 10.1186/s40560-017-0220-5. https://doi.org/10.1186/s40560-017-0220-5 PMid:28331621 PMCid:PMC5351209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CY, Lin YS, Tseng HM, et al. Comparison of two stroke volume variation-based goal-directed fluid therapies for supratentorial brain tumour resection:A randomized controlled trial. Br J Anaesth. 2017;119(5):934–934. doi: 10.1093/bja/aex189. https://doi.org/10.1093/bja/aex189 PMid:28981592. [DOI] [PubMed] [Google Scholar]
- 14.Hernández-Palazón J, Fuentes-García D, Doménech-Asensi P, Piqueras-Pérez C, Falcón-Araña L, Burguillos-López S. A comparison of equivolume, equiosmolar solutions of hypertonic saline and mannitol for brain relaxation during elective supratentorial craniotomy. Br J Neurosurg. 2016;30(1):70–70. doi: 10.3109/02688697.2015.1109061. https://doi.org/10.3109/02688697.2015.1109061 PMid:26571037. [DOI] [PubMed] [Google Scholar]
- 15.Fuller BM, Dellinger RP. Lactate as a hemodynamic marker in the critically ill. Curr Opin Crit Care. 2012;18(3):267–267. doi: 10.1097/MCC.0b013e3283532b8a. https://doi.org/10.1097/MCC.0b013e3283532b8a PMid:22517402 PMCid:PMC3608508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brallier JW, Dalal PJ, McCormick PJ, Lin H-M, Deiner SG. Elevated Intraoperative Serum Lactate During Craniotomy Is Associated With New Neurological Deficit and Longer Length of Stay. J Neurosurg Anesthesiol. 2017;29(4):388–388. doi: 10.1097/ANA.0000000000000332. https://doi.org/10.1097/ANA.0000000000000332 PMid:27438799. [DOI] [PubMed] [Google Scholar]
- 17.Sundaram SC, Salins SR, Kumar AN, Korula G. Intra-Operative Fluid Management in Adult Neurosurgical Patients Undergoing Intracranial Tumour Surgery:Randomised Control Trial Comparing Pulse Pressure Variance (PPV) and Central Venous Pressure (CVP) J Clin Diagnostic Res. 2016;10(5):UC01–UC05. doi: 10.7860/JCDR/2016/18377.7850. https://doi.org/10.7860/JCDR/2016/18377.7850 PMid:27437329 PMCid:PMC4948505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo J, Xue J, Liu J, Liu B, Liu L, Chen G. Goal-directed fluid restriction during brain surgery:a prospective randomized controlled trial. Ann Intensive Care. 2017;7(1):16. doi: 10.1186/s13613-017-0239-8. https://doi.org/10.1186/s13613-017-0239-8 PMid:28211020 PMCid:PMC5313491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindroos A-CB, Niiya T, Silvasti-Lundell M, Randell T, Hernesniemi J, Niemi TT. Stroke volume-directed administration of hydroxyethyl starch or Ringer's acetate in sitting position during craniotomy. Acta Anaesthesiol Scand. 2013;57(6):729–729. doi: 10.1111/aas.12105. https://doi.org/10.1111/aas.12105 PMid:23550716. [DOI] [PubMed] [Google Scholar]
- 20.Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002;89(4):622–622. doi: 10.1093/bja/aef220. https://doi.org/10.1093/bja/aef220 PMid:12393365. [DOI] [PubMed] [Google Scholar]


