Abstract
The presence of celiac trunk or hepatic arterial anomaly influences preservation of vascular arterial system and achievement of an R0 resection in the liver and pancreatic resection. The objective of this study is to review the literature, to describe the anomalous arterial variations of the celiac trunk and hepatic artery reiterating the importance of identification of the anomaly.
Keywords: celiac trunk, hepatic arterial anomaly
Introduction
The first description of normal and aberrant celiac trunk anatomy was published in 1756 by Haller. Lipshutz seems to have been the first who suggested a classification of the celiac trunk into four types. Later, Adachi presented a more detailed classification, while the two most commonly used classifications were suggested by Morita and Michels [1], [2], [3], [4], [5]. Knowledge of the variant vascular anatomy of upper gastrointestinal region is valuable to hepato-biliary surgeons to limit operative complications due to unexpected bleeding and to the achievement of R0 resection during liver or biliary tree resection or pancreaticoduodenectomy [6], [7]. It is defined typical ‘normal’, the hepatic artery that arises from celiac trunk and divides into three main branches- the right hepatic, left hepatic and middle hepatic supplying the right, left and the quadrate lobe of the liver respectively. ‘Aberrant’ hepatic is a hepatic arising otherwise than from a typical celiacal hepatic and is of two types accessory and replaced. The term ‘accessory’ hepatics should be used only in those cases where the normal celiacal right or left hepatic is present, and there is an additional artery from other sources. When the normal celiacal right or left hepatic artery is missing the replacing vessel coming from another source supplying the right or left lobe is to be termed as a ‘replaced’ right or left hepatic artery. The coeliac artery, also known as the celiac trunk (CT) or coeliac axis is the first ventral branch of the abdominal aorta. It is a short wide branch about 1.25 cm long, that arises from the aorta at the level of T12–L1 vertebrae immediately below the aortic hiatus of the diaphragm. The normal branching pattern of the CT and the three main branches are left gastric artery (LGA), common hepatic artery (CHA) and splenic artery (SA). The celiac axis and its branches supply the derivatives of foregut, i.e. stomach, spleen, pancreas, liver and part of the duodenum. The most common classical type of branching of CT is known as trifurcation and was first described by Haller in 1756 as tripus Halleri. The incidence of different celiac and hepatic artery anomalies was calculated depending on Uflacker’s and Michel’s classifications. According to the Michels’ studies about the anatomical vascular anomaly, celiaco-mesenteric arterial aberrations is described in 22%-48% of observations [8], [9]. The literature suggests that the normal pattern of hepatic artery anatomy is observed in 55 to 79% of patients [11], [12], [13]. Since Michels published his first report, several studies have reported not only common and rare hepatic artery variants, but also different classifications [14] (Table 1).
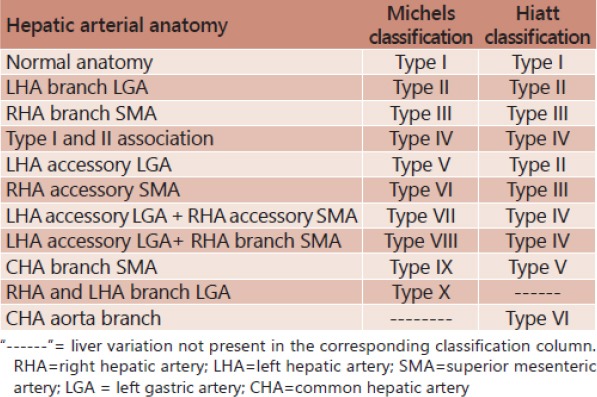
Table 1.
Michels and Hiatt classifications (from CT Angiography for Delineation of Celiac and Superior Mesenteric Artery Variants in Patients, Corinne B. Winston et al.) [28]
Material and Methods
Study selection
A systematic search of the literature from PUBMED databases published between 1766 and 2018 was performed. The following search terms were used: celiac trunk, hepatic artery, anomaly, variants, normal hepatic artery, aberrant, accessory, Michels classification. A manual search was also carried out.
Inclusion and exclusion criteria
We retrospectively analysed 100 articles about Hepatic Artery (H.A) and Celiac Trunk Anatomic Variants (CTAV) using Pubmed research. Michels, Uflacker and Hiatt classifications were analyzed (Table 1, 2, 3). The inclusion criteria were: (1) papers are written in English; and (2) manuscript with 100 o most patients. Exclusion criteria: Abstracts, case reports, reviews, low-quality studies and non-comparative studies, and intraoperative data which were unable to be extracted from the published studies were excluded. Here, we describe the most numerous medical records research articles and Michels classification images (Figure 1-9).
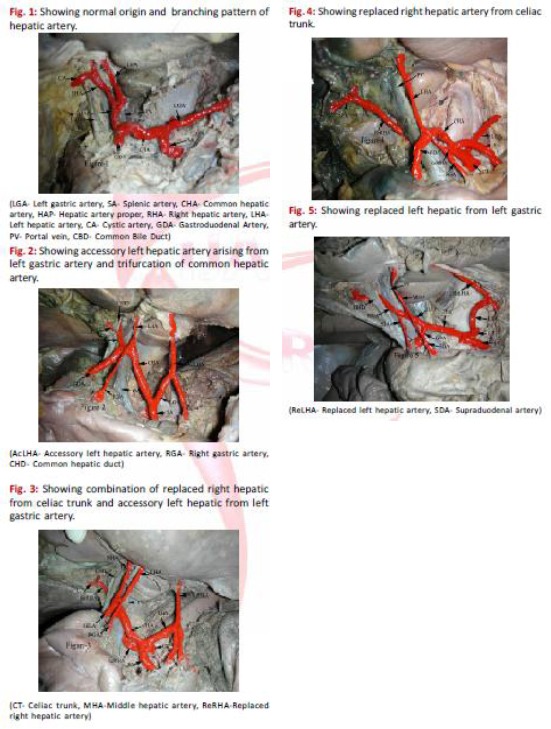
Figure 1.
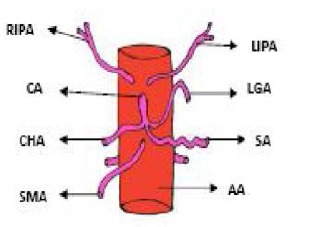
Usual branching pattern of celiac trunk. LGA, left gastric artery; SA, splenic artery; CHA, common hepatic artery; SMA, superior mesenteric artery; RIPA, right inferior phrenic artery; LIPA, left interior phrenic artery; CA, celiac artery; AA, abdominal aorta
Figure 2.
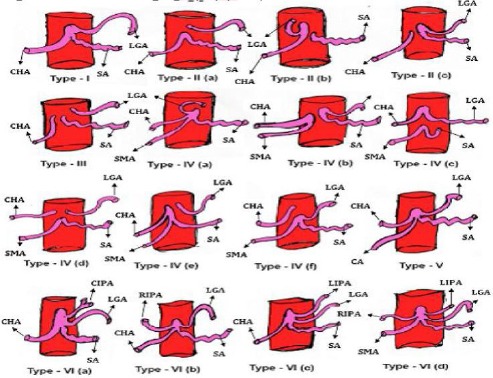
Schematic diagram showing variation in the origin of celiac trunk and its branches. LGA, left gastric artery; SA, splenic artery; CHA, common hepatic artery; SMA, superior mesenteric artery; RIPA, right inferior phrenic artery; LIPA, left interior phrenic artery; CA, celiac artery; AA, abdominal aorta
Figure 3.
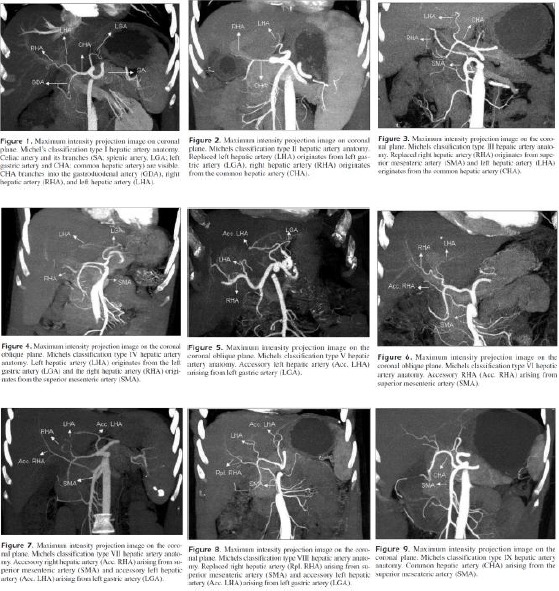
Variations of the celiac trunk and hepatic arteries: a study with 64- detector computed tomographic angiography, H. GÜMÜS et al by European Review for Medical and Pharmacological Sciences (Fig. 1-9)
Figure 4.
Variants in celiac trunk. From Kavitha Kamath. B. Int J Anat Res 201 [8]
Figure 4.
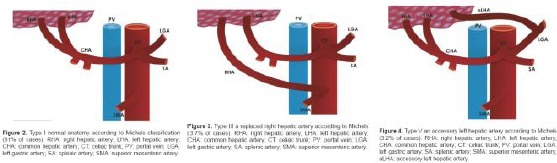
Variants in celiac trunk. From George Noussios J Clin Med Res. 2017;9(4):248-252)
Figure 5.
Variants in celiac trunk. From Soon-Young Song. Radiology: Volume 255: Number 1—April 2010 [27]
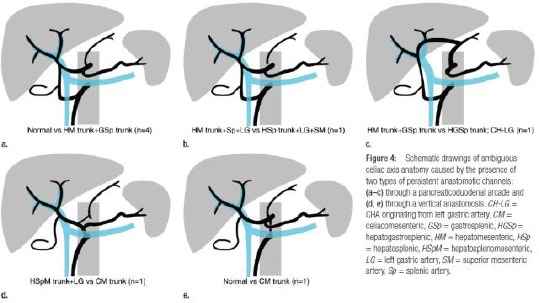
Figure 6.
Schematic drawing illustrates hypothetical anatomic model to summarize possible anatomic courses of celiac axis and CHA variations. Gray indicates normal arterial anatomy. Red indicates possible anatomic course. CA, celiac axis; GD, gastroduodenal artery; ip, intrapacreatic course; LG, left gastric artery; PV, portal vein; SM, superior mesenteric artery; sp, suprapancreatic course; tp, transpancreatic course
Results
Da Fonseca-Neto et al., [15] retrospectively analysed 479 medical records of transplanted adult patients in the 13 years. It was identified as normal hepatic arterial anatomy in 416 donors (86.84%). The other 63 patients (13.15%) showed some variation.
According to the Michels classification, the most frequently observed abnormalities were: right hepatic artery branch of superior mesenteric artery (Type III, n = 27, 5.63%); left hepatic artery branch of the left gastric artery (Type II, n = 13, 2.71%); right hepatic artery arising from the superior mesenteric artery associated with the left hepatic artery arising from the left gastric artery (Type IV, n = 4, 0.83%). Similarly, about Hiatt classification, the most prevalent changes were: right hepatic accessory artery or substitute of the superior mesenteric artery (Type III, n = 28, 6.05%)), followed by liver ancillary left artery or replacement of gastric artery left (Type II, n = 16, 3.34. Fourteen donors (2.92%) showed no anatomical abnormalities defined in classifications, the highest frequency being hepatomesenteric trunk identified in five (01.04%).
H. Gümüs et al., [16] Evaluated 820 patients who underwent angiography of the abdominal aorta. Anatomical findings were grouped according to the Michels classification. Several variations and/or anomalies were noted in 33.2% of the patients (n = 272). The most common abnormality was Michels type III (10.1%), followed by type V (7.3%), type II (4.7%) and others.
Kuo-Hsien Chiang et al., [17], reviewed 405 patients who underwent upper abdominal arteriography between Aug. 1998 and Sep. 2004. Hepatic artery anatomy was analyzed and classified according to Suzuki’s classification. Single hepatic artery group included 283 of 405 cases (69.9 per cent). Double hepatic artery group included 114 cases (28.1 per cent). Multiple hepatic artery group included 8 cases (2.0 per cent). Seventeen patterns were identified in this study.
MS Ugurel et al., [18] analysed retrospectively 100 patients. There was a coeliac trunk trifurcation in 89% and bifurcation in 8% of the cases. The coeliac trunk was absent in 1%, a hepatosplenomesenteric trunk was seen in 1%, and a splenomesenteric trunk was present in 1%. Hepatic artery variation was present in 48% of patients. Coeliac trunk and/or hepatic arterial variation was present in 23 (39.7%) of the 58 patients with normal renal arteries, and 27 (64.3%) of the 42 patients with accessory renal arteries. There was a statistically significant correlation between renal artery variations and coeliac trunk–hepatic arterial system variations (p50.015). They concluded that MDCT angiography permits a correct and detailed evaluation of hepatic and renal vascular anatomy.
Ahmed M. Osman MD et al., [19] retrospectively analysed 1285 Egyptian patients using triphasic CT or CT aortic angiography. The incidence of different celiac and hepatic artery anomalies was calculated depending on Uflacker’s and Michel’s classifications. They found about 90.5% of the patients showing normal trifurcation pattern of the celiac trunk (Uflacker type I) with the commonest variant was gastro-splenic trunk (Uflacker type V) with 4.3% incidence. The bifurcation pattern was representing 7.7% of cases. Regarding the hepatic artery, 74.3% of the cases showed the normal origin of the hepatic arteries (Michel type I) with the commonest anomaly was Michel type III with 12.5% incidence. Some cases are discovered not previously described by either Uflacker’s or Michel’s classifications. They concluded that good imaging quality of MSCT angiography had proved effectiveness in depicting different celiac trunk and hepatic artery variants.
MK Tharao et al., [20] studied one hundred and two subhepatic regions in adult Kenyans by gross dissection for the pattern of arterial blood supply. Measurements were made for the distance of hepatic bifurcation from the liver. The common hepatic artery (CHA) originated from the celiac trunk in 95.1% and the superior mesenteric artery (SMA) in 4.9% of cases. The mean distance of bifurcation from the liver was 2.6 cm, and the CHA gave rise to 2 hepatic branches in 93.1% of cases. The right hepatic artery (RHA) passed anterior to the CHD in 48%, posterior in 41.2% and 7.8% was related to the common bile duct (CBD). Accessory RHA and left hepatic arteries (LHA) were observed in 10.8% and 9.8% of the cases.
Jonathan R. Hiatt et al., [21], recorded of 1000 patients who underwent liver harvesting for orthotopic transplantation between 1984 and 1993 were reviewed. Arterial patterns in order of frequency included the normal Type 1 anatomy (n = 757), with the common hepatic artery arising from the celiac axis to form the gastroduodenal and proper hepatic arteries and the proper hepatic dividing distally into right and left branches; Type 3 (n = 106), with a replaced or accessory right hepatic artery originating from the superior mesenteric artery; Type 2 (n = 97), with a replaced or accessory left hepatic artery arising from the left gastric artery; Type 4 (n = 23), with both right and left hepatic arteries arising from the superior mesenteric and left gastric arteries, respectively; Type 5 (n = 15), with the entire common hepatic artery arising as a branch of the superior mesenteric; and Type 6 (n = 2), with the common hepatic artery originating directly from the aorta.
A. Ahmed et al., [22], do a retrospective study in which 1000 post-contrast CT scans of the abdomen between July 2011 and June 2012. Out of the 10 Mitchel’s hepatic arterial variations, 9 were seen. Type I was most common, and 678 (67%) patients had it. Type II variation was seen in 178 (17%) patients, type III in 34 (3.4%) type V in 25 (2.5%) patients.
Kavitha Kamath. B. [23], studied extrahepatic branching pattern of hepatic arteries in 40 embalmed cadavers. Classical textbook pattern of hepatic arterial anatomy was seen in 30(75%) cases, and ten (25%) cases showed the presence of aberrant hepatic arteries. 12 aberrant hepatic arteries were seen in these ten cases, eight (20%) cases with single aberrant hepatic artery and two (5%) with a combination of aberrant right and left hepatics. Aberrant right hepatic arteries were seen in four (10%) cases, and all of them were replaced right hepatics arising directly from the celiac trunk. Aberrant left hepatic arteries were seen in eight (20%) cases, of which six (15%) were accessory, two (5%) were replaced, and all of them arose from the left gastric artery.
Azhar Perwaiz et al., [24] analysed 200 consecutive pancreaticoduodenectomies finding Fifty-three patients (26.5%) that had arterial anomalies. The complexity of the surgery was determined by the course of these arteries. The mean duration of surgery was 420 ± 32.0 minutes in patients with arterial anomalies versus 370 ± 38.5 minutes in those with normal arterial anatomy (P = 0.005). They concluded that during pancreaticoduodenectomy, arterial anomalies could increase operative complexity but do not usually compromise the safety of the procedure or its oncological outcome.
George Noussios et al., [25], retrieved twenty articles. A total of 19,013 patients were analysed]. 81% of the cases displayed normal anatomy. A replaced right hepatic artery (RHA) arose from the superior mesenteric artery (SMA) in 3.7% of cases, while a replaced left hepatic artery (LHA) stemmed from the left gastric artery in 3% of cases. Both a replaced RHA and a left one were found in 0.8% of cases, while an accessory LHA and an accessory RHA were present in 3.2% and 1.6% of cases, respectively. A common hepatic artery (CHA) originating from the SMA appeared in 1.2% of cases. Finally, 784 cases (4.1%) encountered in were rare unreported anomalies or more commonly unclassified anomalies.
Rafael Lo´pez-Andujar et al., [26], investigated the surgical anatomy of the extrahepatic arterial vascularisation prospectively in 1,081 cadaveric donor livers, transplanted from January 1991 to August 2004. The vascular anatomy of the hepatic grafts was classified according to Michels description. Anatomical variants of the classical pattern were detected in 30% of the livers (n 320). The most common variant was a replaced left artery arising from the left gastric artery (9.7%) followed by a replaced right hepatic artery arising from the superior mesenteric artery (7.8%).
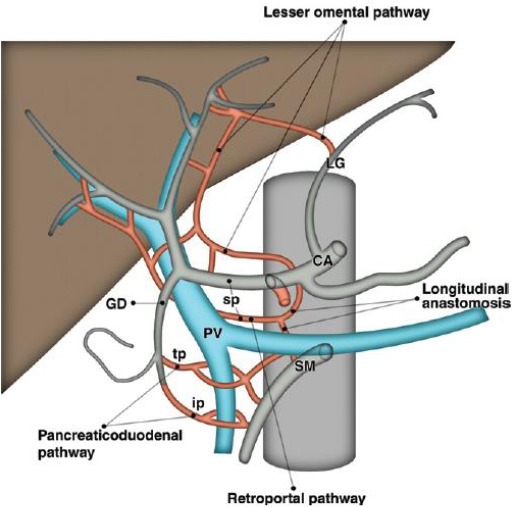
Soon-Young Song et al., [27], retrospectively evaluated 5002 patients who underwent spiral CT and digital subtraction angiography (DSA). Of 15 possible types of CA variation, 13 types were identified. A normal CA was noted in 4457 (89.1%) of the 5002 patients. Twelve types of Celiac Axis (CA) variation were identified in 482 (9.64%) patients. In the remaining 63 (1.26%) patients, the CA anatomy was classified as ambiguous because the Common Hepatic Artery (CHA) was absent owing to separate origins of the hepatic arteries and the gastroduodenal artery (n = 55) or because the origin of the CHA could not be determined owing to persistent anastomotic channels (n = 8). Seven CHAs originating from the normal CA had a retroportal (n = 6) or transpancreatic (n = 1) course. All eight CHAs originating from the left gastric artery passed the fissure of the ligamentum venosum. The 148 CHAs originating from the superior mesenteric artery showed diverse relationships with the pancreas-being supra-, trans-, or intrapancreatic-and the superior mesenteric-portal venous axis-being pre- or retroportal. The 20 CHAs originating from the aorta had a normal suprapancreatic periportal course.
Corinne B. Winston et al., [28], analyzed a CT Angiography (CTA) database from August 2001 to February 2003 that included 394 consecutive CT angiograms in 371 patients, all of whom were under consideration for surgical resection for a suspected pancreatic or hepatobiliary neoplasm One hundred eighty-eight (51%) of the 371 patients had classic arterial anatomy identified at abdominal CT Angiography (CTA). One hundred sixty-two (44%) patients had a single arterial variant identified, and 21 (6%) patients had more than one arterial variant seen. The most common variant identified was a replaced right hepatic artery originating from the superior mesenteric artery (SMA), which was seen in 54 (15%) patients. The second most common variant was a replaced left hepatic artery originating from the left gastric artery, seen in 30 (8%) patients. Variations in the origin of the common hepatic artery were seen in 12 (3%) patients. In six (2%) patients, the common hepatic artery originated from the SMA, and in six (2%) patients, the common hepatic artery originated from the abdominal aorta. A “double hepatic artery,” as described by Fasel et al., and Covey et al., refers to one or both hepatic arteries originating from the celiac axis or the aorta. This was seen in 15 (4%) of the patients in our study population. The right hepatic artery originated from the celiac axis in 13 (4%) patients and from the aorta in one (< 1%) patient. The left hepatic artery originated from the celiac axis in one (< 1%) patient. Accessory hepatic arteries were identified in 16 (4%) patients. We showed some exmples of variants of hepatic artery (From Kavitha Kamath. B. Int J Anat Res 2015) (Figure 1, 2, 3, 4, and 5). We reported the figures reconstruction of celiac axis variants (From Dilli Babu E., Int J Anat Res 2013) (Figure 1, and 2).
Discussion
Modifications of the hepatic branch and the celiac axis, occur in 25% to 75% of cases. According to Michels classification, the most frequent change according to the literature is the type III present in from 6-15.5% of cases. It seems the most important because it has the potential to affect surgical procedures being indispensable its identification. The second most common is type II, reported in literature between 2.5-10%. Type IV is described with an incidence of 1-7.4%. The types VII, VIII, IX and X are rarely described in literature. [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14) This is demonstrated from the figures (from George Noussios J Clin Med Res. 2017; 9(4):248-252) (Figure 2, 3, and 4). We finded also schematic drawings of ambiguous celiac axis anatomy caused by the presence of two types of persistent anastomotic channels (From Soon-Young Song, MD Radiology: Volume 255: Number 1—April 2010) (Figure 4, and 7).
Conclusion
The precise knowledge of the celiac trunk and hepatic artery variants and anomalies is very important pre-surgical, prelaparoscopic or even pre-interventional. Unknowledge these variants produces different technical difficulties, or challenges. It is essential in order to avoid damage, vascular surgical complications. and decrease the morbidity of patients [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28].
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Haller A. Icones anatomicae in quibus aliquae partes corporis humani delineatae proponuntur et arteriarum potissimum historia continetur. Gottingen. 1756 [Google Scholar]
- 2.Lipshutz B. A Composite Study of the Coeliac Axis Artery. Ann Surg. 1917;65(2):159–159. doi: 10.1097/00000658-191702000-00006. https://doi.org/10.1097/00000658-191702000-00006 PMid:17863663 PMCid:PMC1426316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adachi B. Das Arteriensystem der Japaner. Vol.2. verlag der Kaiserlich- Japanischen Universitat zuKyoto. 1928 [Google Scholar]
- 4.Morita M. Reports and conception of three anomalous cases on the area of the celiac and the superior mesenteric arteries. Igaku Kenkyu (Acta Med) 1935;9:159–172. [Google Scholar]
- 5.Michels NA. Blood supply and anatomy of the upper abdominal organs, with a descriptive atlas. Philadelphia: Lippincott; 1955. pp. 139–143. [Google Scholar]
- 6.Thapa PB, Yonjen TY, Maharjan DK, Shrestha SK. Anatomical Variations of Hepatic Artery in Patients Undergoing Pancreaticoduodenectomy. Nepal Med Coll J. 2016;18(1-2):62–7. [Google Scholar]
- 7.Tharao MK, Saidi H, Kitunguu P, Ogengo JA. Variant anatomy of the hepatic artery in adult Kenyans. Eur J Anat. 2007;11(3):155–161. [Google Scholar]
- 8.Kamath BK. A study of variant hepatic arterial anatomy and its relevance in current surgical practice. Int J Anat Res. 2015;3(01):947–947. https://doi.org/10.16965/ijar.2015.124. [Google Scholar]
- 9.Dilli Babu E, Khrab P. Coeliac trunk variations:review with proposed new classification. Int J Anat Res. 2013;1(3):165–70. [Google Scholar]
- 10.Koops A, Wojciechowski B, Broering DC, Adam G, Krupski- Berdien G. Anatomic variations of the hepatic arteries in 604 selective celiac and superior mesenteric angiographies. Surg Radiol Anat. 2004;26:239–44. doi: 10.1007/s00276-004-0229-z. https://doi.org/10.1007/s00276-004-0229-z PMid:14968265. [DOI] [PubMed] [Google Scholar]
- 11.Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT. Variant hepatic arterial anatomy revisited:digital subtraction angiography performed in 600 patients. Radiology. 2002;224:542–7. doi: 10.1148/radiol.2242011283. https://doi.org/10.1148/radiol.2242011283 PMid:12147854. [DOI] [PubMed] [Google Scholar]
- 12.Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337–47. doi: 10.1016/0002-9610(66)90201-7. https://doi.org/10.1016/0002-9610(66)90201-7. [DOI] [PubMed] [Google Scholar]
- 13.Soin AS, Friend PJ, Rasmussen A, Saxena R, Tokat Y, Alexander GJ, et al. Donor arterial variations in liver transplantation:management and outcome of 527 consecutive grafts. Br J Surg 1996; 83:637–41. doi: 10.1002/bjs.1800830515. https://doi.org/10.1002/bjs.1800830515 PMid:8689206. [DOI] [PubMed] [Google Scholar]
- 14.López-Andújar R, Moya A, Montalvá E, Berenguer M, De Juan M, San Juan F, Pareja E, Vila JJ, Orbis F, Prieto M, Mir J. Lessons learned from anatomic variants of the hepatic artery in 1,081 transplanted livers. Liver transplantation. 2007;13(10):1401–1401. doi: 10.1002/lt.21254. https://doi.org/10.1002/lt.21254 PMid:17902125. [DOI] [PubMed] [Google Scholar]
- 15.Lima HC, Rabelo P, Amorim AG, Lacerda CM. Anatomic Variations of Hepatic Artery:A Study In 479 Liver Transplantations. ABCD. Arquivos Brasileiros de Cirurgia Digestiva (São Paulo) 2017;30(1):35–35. doi: 10.1590/0102-6720201700010010. https://doi.org/10.1590/0102-6720201700010010 PMid:28489166 PMCid:PMC5424684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gümüs H, et al. Variations of the celiac trunk and hepatic arteries:a study with 64-detector computed tomographic angiography European Review for Medical and Pharmacological Sciences. 2013;17:1636–1641. [PubMed] [Google Scholar]
- 17.Kuo-Hsien C, et al. Angiographic Evaluation of Hepatic Artery Variations in 405 cases. Chin J Radiol. 2005;30:75–81. [Google Scholar]
- 18.Ugurel MS, Battal B, Bozlar U, Nural MS, Tasar M, Ors F, Saglam M, Karademir I. Anatomical variations of hepatic arterial system, coeliac trunk and renal arteries:an analysis with multidetector CT angiography. The British journal of radiology. 2010;83(992):661–661. doi: 10.1259/bjr/21236482. https://doi.org/10.1259/bjr/21236482 PMid:20551256 PMCid:PMC3473504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osman AM, Abdrabou A. Celiac trunk and hepatic artery variants:A retrospective preliminary MSCT report among Egyptian patients. The Egyptian Journal of Radiology and Nuclear Medicine. 2016;47(4):1451–1451. https://doi.org/10.1016/j.ejrnm.2016.09.011. [Google Scholar]
- 20.Tharao MK, Saidi H, Kitunguu P, Julius OA. Variant anatomy of the hepatic artery in adult Kenyans. European Journal of Anatomy. 2019;11(3):155–61. [Google Scholar]
- 21.Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Annals of surgery. 1994;220(1):50–50. doi: 10.1097/00000658-199407000-00008. https://doi.org/10.1097/00000658-199407000-00008 PMid:8024358 PMCid:PMC1234286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed A. Hepatic Arterial Variations And Its Implications On Hepatobiliary Surgery Awais Ahmed, Rashed Nazir, Rayyan Pervez, Umar Amin, Sadia Babar, Atif Rana. Department of Radiology, Shifa International hospital, Islamabad, Pakistan. European Congress of Radiology. 2013 [Google Scholar]
- 23.Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT. Variant hepatic arterial anatomy revisited:digital subtraction angiography performed in 600 patients. Radiology. 2002;224(2):542–542. doi: 10.1148/radiol.2242011283. https://doi.org/10.1148/radiol.2242011283 PMid:12147854. [DOI] [PubMed] [Google Scholar]
- 24.Perwaiz A, Singh A, Singh T, Chaudhary A. Incidence and Management of Arterial Anomalies in Patients Undergoing Pancreaticoduodenectomy. JOP. J Pancreas. 2010;11(1):25–30. [PubMed] [Google Scholar]
- 25.Noussios G, Dimitriou I, Chatzis I, Katsourakis A. The main anatomic variations of the hepatic artery and their importance in surgical practice:review of the literature. J Clin Med Res. 2017;9(4):248–248. doi: 10.14740/jocmr2902w. https://doi.org/10.14740/jocmr2902w PMid:28270883 PMCid:PMC5330766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López-Andújar R, Moya A, Montalvá E, Berenguer M, De Juan M, San Juan F, Pareja E, Vila JJ, Orbis F, Prieto M, Mir J. Lessons learned from anatomic variants of the hepatic artery in 1,081 transplanted livers. Liver transplantation. 2007;13(10):1401–1401. doi: 10.1002/lt.21254. https://doi.org/10.1002/lt.21254 PMid:17902125. [DOI] [PubMed] [Google Scholar]
- 27.Song SY, Chung JW, Yin YH, Jae HJ, Kim HC, Jeon UB, Cho BH, So YH, Park JH. Celiac axis and common hepatic artery variations in 5002 patients:systematic analysis with spiral CT and DSA. Radiology. 2010;255(1):278–278. doi: 10.1148/radiol.09090389. https://doi.org/10.1148/radiol.09090389 PMid:20308464. [DOI] [PubMed] [Google Scholar]
- 28.Winston CB, Lee NA, Jarnagin WR, Teitcher J, DeMatteo RP, Fong Y, Blumgart LH. CT angiography for delineation of celiac and superior mesenteric artery variants in patients undergoing hepatobiliary and pancreatic surgery. American Journal of Roentgenology. 2007;189(1):W13–9. doi: 10.2214/AJR.04.1374. https://doi.org/10.2214/AJR.04.1374 PMid:17579128. [DOI] [PubMed] [Google Scholar]


