Abstract
The environmental contaminants pentachlorophenol (PCP) and 4, 4′-dichlorodiphenyltrichloroethane (DDT) are detected in some human blood samples at levels as high as 5 µM (PCP) and 260 nM (DDT). Several cancers are associated with exposures to these contaminants. IL-6 is a pro-inflammatory cytokine that when dysregulated stimulates inflammatory diseases and tumor progression. Immune cells exposed to PCP at 0.05–5 µM and DDT at 0.025–2.5 µM showed increased secretion of IL-6 when the cell preparations contained either T lymphocytes or monocytes. Increased IL-6 secretion was due to PCP and DDT induced cellular production of the cytokine and was dependent on MAP kinase signaling pathways (in the case of PCP). Compound-induced increases in IL-6 production were in part due to increases in either the transcription of and/or stability of its mRNA. Thus, both PCP and DDT have the potential to produce chronic inflammation by stimulating production of IL-6 by immune cells.
Keywords: NK cells, Lymphocytes, Monocytes, Pentachlorophenol, Dichlorodiphenyltrichloroethane, Interleukin 6
INTRODUCTION
The pro-inflammatory cytokine interleukin 6 (IL-6) has roles in the immune response, bone remodeling, cellular differentiation, cell proliferation, and hematopoeisis. (Tanaka et al., 2014; Jorcyk et al., 2011; Fernando et al., 2014). It is produced by monocytes, lymphocytes, macrophages, and muscle cells and plays a major role in chronic inflammation and those diseases associated with inflammation including cancer (Coussens & Werb, 2002; Demaria et al., 2010). Abnormal levels of IL-6 are associated with juvenile idiopathic arthritis, systemic lupus erythematosis, Crohn’s disease, bone loss, and cardiovascular disease (Gabay, 2006; Tanake et al., 2014; Choy and Panayi, 2001).
Pentachlorophenol (PCP) and 4, 4′-Dichlorodiphenyltrichloroethane (DDT) are organochlorine compounds that contaminate the environment. Used as a component in insecticides and antifouling paint (Cirelli, 1978), PCP was previously used to treat the wood in log cabins and is currently used to treat wood utility poles (Cline, et.al, 1989). It is present in sediment and mussel tissues from freshwater lakes (Brown et al., 2005). Individuals exposed to PCP have shown increased incidences of blood and kidney cancers (Cooper and Jones, 2008; Demers et al., 2006). Modes of exposure include absorption through skin, inhalation, and consumption of contaminated food or water. Levels of PCP in human serum ranged from 0.26 −5 µM in individuals who lived in PCP-treated log homes (Cline et al., 1989) and 0.15 µM in individuals with no known exposures (Cline et al., 1989; Uhl et al., 1986). DDT is a pesticide used primarily in insect control, mainly mosquitoes. The United States prohibits its use but it continues to be used in a number of developing countries (Rengam 2013; Davis, 2006; WHO 2006; Turusov et al., 2002; Loganathan 2016). DDT is lipophilic and highly stable and thus enters human tissues through its bioaccumulation and biomagnification in the food chain (Koepke et al.,2004; Kodavanti and Loganathan, 2017). Levels of DDT as high as 23, 169 ng/g of lipid (approximately 260 nM) have been found in human blood serum (Koepke et al., 2004; Trejo-Acevedo et al., 2009). Like PCP, exposure to DDT has been associated with a number of cancers (Eskenazi, et al., 2009; Harada et al., 2016).
Increased secretion of pro-inflammatory cytokines, interferon gamma (IFNγ), tumor necrosis factor alpha (TNFα), and interleukin 1beta (IL-1β), from monocytes and peripheral blood lymphocytes can be stimulated by both PCP and DDT exposures (Massawe et al., 2017; Martin & Whalen, 2017). Further studies indicated that these compound-induced increases in IL-1β secretion were due to the ability of both PCP and DDT to stimulate cellular production of IL-1β (not simply increasing release of pre-existing IL-1β) (Martin et al., 2019). Additionally, both PCP and DDT utilized the p38 MAP kinase (MAPK) signaling pathway to increase cellular production of IL-1β (Martin et al., 2019).
The effects of PCP and DDT on the secretion of IL-6 from immune cell have not been studied. In the current study, a range of increasingly complex mixtures of human immune cells (enriched NK cells, a mixture of NK and T lymphocytes, and a mixture of lymphocytes and monocytes) were examined to see if exposure to either PCP or DDT stimulated secretion of IL-6. Further, we examined whether PCP or DDT-induced increases in IL-6 secretion were the result of increased cellular production of IL-6 or simply stimulation of the release of a pre-existing store of the cytokine. Concentrations of PCP and DDT that increased cellular production of IL-6 were further investigated to determine whether this was caused by increases in the mRNA for IL-6. Additionally, the role of MAPK pathways in any PCP-induced or DDT-induced increases in IL-6 production was studied.
MATERIALS AND METHODS
Preparation of peripheral blood mononuclear cells (PBMCs) and Monocyte Depleted (MD)-PBMCs
PBMCs were isolated from leukocyte filters (PALL- RCPL or FLEX) obtained from the Red Cross Blood Bank (Nashville, TN). Leukocytes were obtained from the filters as described in Meyer et al., 2005. PBMCs and MD-PBMCs were then prepared as previously described (Martin et al., 2019). Briefly, the filters were back-flushed with phosphate buffered saline pH 7.4 (PBS) containing containing 5 mM disodium EDTA and 2.5% [w/v] sucrose. The eluent resulting from the back-flush was then layered onto Lymphosep® (1.077g/mL) and centrifuged at 1200g for 30 min. The resulting cells have a yield and viability that is indistinguishable from those prepared from buffy coats.
Preparation of NK cells
NK cells were isolated from buffy coats (source leukocytes from healthy adult donors) purchased from Key Biologics, LLC (Memphis, TN) using the RosetteSep human NK cell enrichment antibody cocktail (Stem Cell Technologies, Vancouver, British Columbia, Canada). This procedure has been described in detail in Brown et al 2018a.
Chemical Preparations, cell treatments, and cell viability
DDT and PCP were purchased from Sigma-Aldrich (St. Louis, MO) and mitogen activated protein kinase (MAPK) inhibitors were purchased from Fischer Scientific (Pittsburgh, PA). DDT and PCP stock solutions were prepared as 100 mM solutions in Dimethyl sulfoxide (DMSO). MAPK inhibitor (PD98059 and SB202190) stock solutions were 50 mM solutions in DMSO. Appropriate dilutions of the stock solutions were prepared as described in Martin &Whalen 2017.
Cells were treated with DDT at concentrations from 0.025–2.5 μM and PCP from 0.05– 5 μM for various length of incubation (dependent on the specific study). For studies examining secretion alone, cells were exposed to either PCP or DDT for 24 h, 48 h, or 6 days. Following the incubation period, cell supernatants were obtained and stored at −70 C. For studies examining cellular production of IL-6, cells were treated with 5–0.05 µM PCP or 2.5–0.025 µM DDT for 10 min, 1 h, 6 h or 24 h. Supernatants were collected to examine secretion of IL-6 from cells and the cell pellets were lysed ( lysis buffer from Active motif, Carlsbad, CA) to examine for intracellular levels of IL-6. The cell lysates and their corresponding supernatants were stored frozen at −70–80 o C up to the point when they were assayed by ELISA (secretion) and Western blot (intracellular). Appropriate controls were included in all studies. The concentrations of PCP and DDT that were examined are in the range of those seen in human blood samples (Cline et al., 1989; Uhl et al., 1986; Koepke et al., 2004; Trejo-Acevedo et al., 2009).
Cell viability was observed at the end of each exposure period. Viability was determined using the trypan blue exclusion method as described previously (Martin et al., 2019). The viability of NK cells, MD-PBMCs, and PBMCs was not affected by any of the concentrations of PCP or DDT at any length of incubation (data not shown). Additionally, treatment with the pathway inhibitors also had no effect on the viability of PBMCs (data not shown).
IL-6 Secretion Assay
Secreted levels of IL-6 were measured using the BD OptEIA™ Human IL-6 enzyme-linked immunosorbent assay (ELISA) kit (BD-Pharmingen, San Diego, CA). The details of the assay are described in Martin et al., 2019 and Brown et al., 2018a.
Western blot
Intracellular levels of IL-6 were determined from cell lysates by Western blot. Details of this procedure have been previously described (Martin et al., 2019).
RNA Isolation and RT-qPCR
RNA was prepared from PBMCs using the RNeasy Mini Kit (Qiagen, Venlo, Netherlands) and RT-qPCR was performed using the QuantiTect SYBR Green RT-PCR kit (Qiagen). The specifics of the RNA isolation and RT-qPCR procedures have been published (Martin et al., 2019).
Statistical Analysis
For statistical analysis, a p value of less than 0.05 indicated a significant difference. ANOVA was used to determine if there were statistically significant differences within a given experimental group. If significance was seen with ANOVA, pair-wise analysis was done using Student’s t test, to compare control to an individual treatment.
RESULTS
Secretion of IL-6 from NK cells exposed to PCP
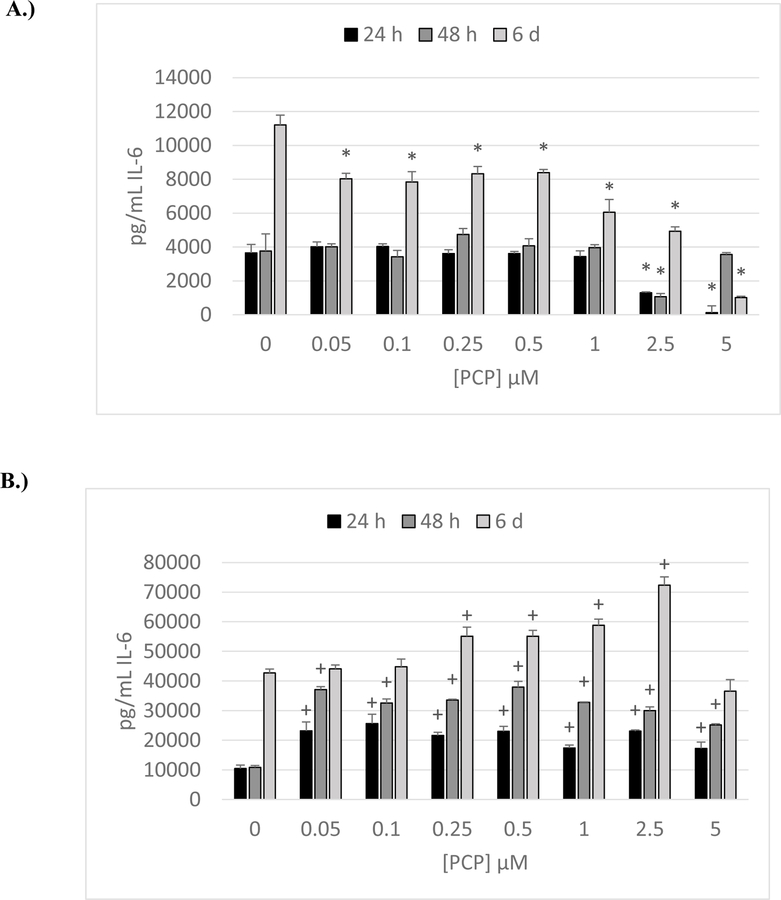
Cells used were obtained from buffy coats (KB= Key Biologic buffy coats). The effects of exposing NK cells to 0 −5 µM PCP for 24 h, 48 h, and 6 days are shown in Figure 1A. Results are from a representative experiment (KB192) and were repeated in cells from 4 donors. There were significant decreases in IL-6 secretion seen at the 5, 2.5, or 1 µM exposures in cells from all donors at each of the lengths of exposure. Cells from all donors showed decreased IL-6 secretion at the 2.5 µM exposure when exposed for 24 h or 48 h. After 6 days of exposure to 2.5 µM PCP there was decreased secretion in cells from 3 of the 4 donors. While cells from the donor in the representative experiment showed decreased IL-6 secretion from NK cells at concentrations of PCP of 0.05, 0.1, 0.25, and 0.5 µM (after 6 days only), concentrations below 1 µM, caused no consistent alterations in secretion of IL-6 (data from all donors) from NK cells.
Figure 1. Effects of 24 h, 48 h and 6-day exposures to PCP on secretion of IL-6 from NK Cells, MD-PBMCs, and PBMCs.
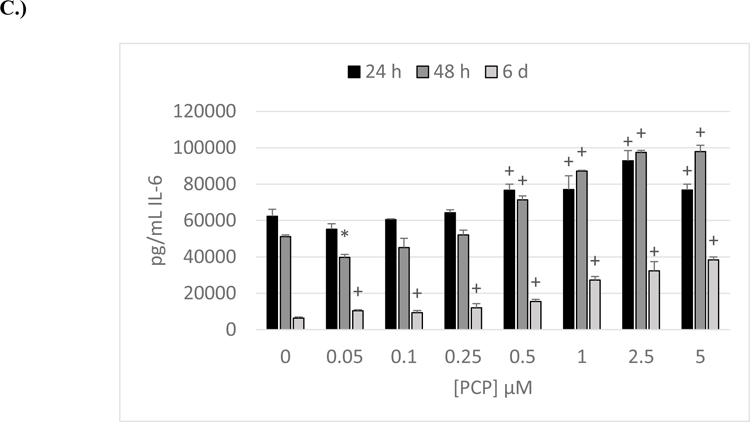
Effects of 24 h, 48 h, and 6 day exposures to 0–5 µM PCP on secretion of IL-6 from NK cells, MD-PBMCs, and PBMCs. A) NK cells (representative experiment of cells from donor KB192. B) MD-PBMCs (representative experiment of cells from donor F243). C) PBMCs (representative experiment of cells from donor F436). * represents significant decreases and + represents significant increases compared to the control (p<0.05).
Effects of PCP Exposure on Secretion of IL-6 by monocyte depleted peripheral blood mononuclear cells (MD-PBMCS)
Cells were obtained from blood filters (F) obtained from the American Red Cross. Figure 1B is a representative experiment (cells from donor F243) of the effects of exposing MD-PBMCs (lymphocyte preparation) to PCP (0–5 µM) on IL-6 secretion. Experiments were repeated in cells from 4 individual donors. In stark contrast to what was seen with NK cells, all four donors showed significant increases at one or more length of incubation at one or more concentration of PCP when compared to the control. PCP concentrations of 0.25–1 µM caused significant increases in IL-6 at one or more length of exposure (24 h, 48 h, and 6 day).
Effects of PCP Exposure on Secretion of IL-6 by PBMCS
The effect of PCP (0–5 µM) exposures on IL-6 secretion from PBMCs (lymphocytes plus monocytes) is shown in Figure 1C. Results were very similar to what was seen with MD-PBMCs, with cells from all donors showing significant increases after either 24 h, 48 h, or 6 days of exposure to at least 1 concentration of PCP.
Effects of PCP Exposures on cellular production (Secretion + Intracellular Levels) of IL-6
10 min and 1 h exposures:
When PBMCs were exposed to 0–5 µM PCP for 10 min or 1 h, there were no consistent increases in production of IL-6 in PBMCs from 4 separate donors (data not shown).
6 h exposures:
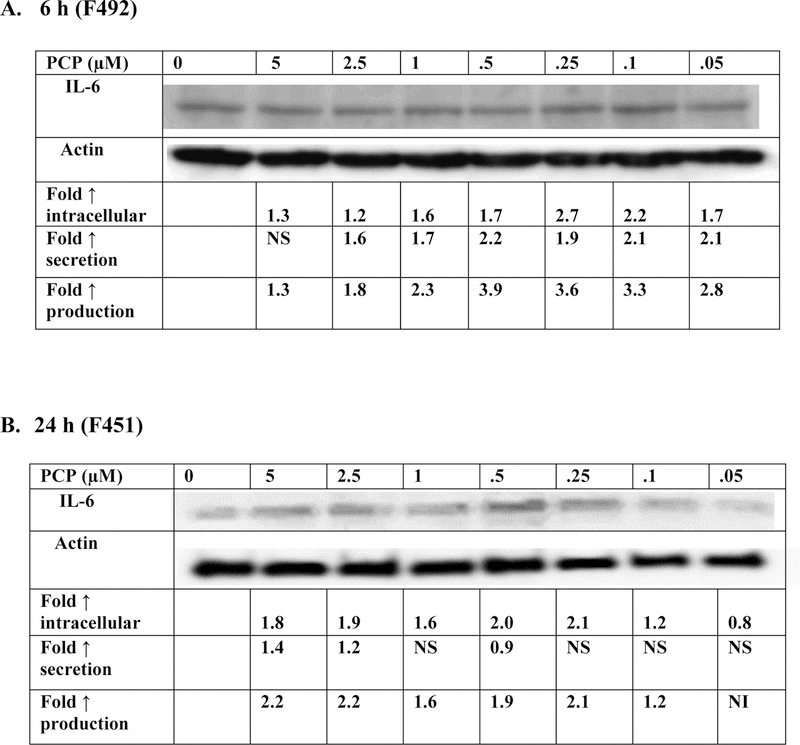
Exposure of PBMCs to PCP of 6 h caused increases in the production of IL-6 (secretion + intracellular levels) in cells from all donors. The magnitude of the increases in IL-6 production by immune cells varied by donor. Additionally, the concentrations at which increased production occurred varied in a donor dependent manner. Data from a representative experiment (cells from donor F492) are shown in Figure 2A. Table 1 gives the results on production from cells prepared from 3 additional donors.
Figure 2. Effect of exposures to PCP on synthesis of IL-6 in PBMCs.
Effects of exposures to 0–5 µM PCP on the production of IL-6 in PBMCs. A) 6 h exposure. The blot is from a representative experiment (F492) with accompanying secretion data. An increase in secretion or in intracellular level is a number greater than 1; a decrease in secretion or intracellular level is a number less than 1. The control is arbitrarily set at 1. A combined fold increase (secretion + intracellular) greater than 1 indicates an increase in production. Changes in fold production for 3 additional experiments are given in Table 1. B) 24 h exposure. The blot is from a representative experiment (F451) with accompanying secretion data. Changes in fold production for 3 additional experiments are given in Table 1.
NS indicates no significant change in secretion (p>0.05)
NI indicates no significant increase in production ( Fold increase ≤1)
Table 1:
Effects of PCP exposures on production of IL-6 in PBMCS after 6 h and 24 h
| Fold ↑ production IL-6 | ||||||||
|---|---|---|---|---|---|---|---|---|
| [PCP] µM | 5 | 2.5 | 1 | 0.5 | 0.25 | 0.1 | 0.05 | |
| Exposure time | donor | |||||||
| 6h | F490 | NI | 2.6 | 2.6 | 2.2 | 2.8 | 3.4 | NI |
| F493 | NI | NI | NI | NI | NI | 1.1 | NI | |
| F494 | 1.3 | 1.2 | 1.5 | 1.5 | 2 | 1.5 | 1.2 | |
| 24 h | F444 | 2.3 | NI | 1.2 | 1.5 | 1.4 | NI | 1.1 |
| F481 | 1.2 | NI | NI | 1.2 | 1.2 | 1.2 | NI | |
| F485 | NI | 1.4 | NI | NI | 1.1 | NI | NI | |
NI indicates no significant increase ( Fold increase ≤1)
24 h exposures:
The effects of 24 h exposures to 0–5 µM PCP on the production of IL-6 are shown in Figure 2B and Table 1. Cells from 4 individual donors were examined. There were increases in production of IL-6 (secretion + intracellular levels) after 24-hours of exposure to PCP in cells from all donors. Cells from donor F451 (Figure 2B) showed increased production when exposed to all by the lowest concentration of (0.05 µM PCP). These increases ranged from 1.2 fold (at 0.1 µM PCP to 2.2 fold (at 5 µM PCP). Cells from each of the donors showed increases in production at 2 or more concentrations of PCP. These results, taken together with those at 10 min, 1h and 6 h, indicate that PCP stimulates cellular production of IL-6 at one or more PCP concentrations within 6 h of exposure.
Effects of Exposures to PCP on IL-6 mRNA Levels in PBMCs
Levels of IL-6 mRNA were measured in cells exposed to PCP for 2 h, 6 h, and 24 h, in order to determine if the increases in IL-6 production seen after 6 h and 24 h were due to increased IL-6 mRNA. Levels of IL-6 mRNA were not consistently increased in cell exposed to PCP for 2 h (Table 2). However, after 6 h of exposure, cells from 3 of the 4 donors tested showed PCP-induced increases in mRNA for IL-6 (Table 2). A similar result was seen after 24 h of exposure to PCP. These results suggest that PCP-induced increases in IL-6 mRNA may account for at least a portion of the increase in IL-6 production seen after 6 h and 24 h exposures.
Table 2:
Effects of 2 h, 6 h, and 24 h exposures to PCP on IL-6 mRNA levels in PBMCs
| 2h | Interleukin 6 mRNA expression (AU) (mean± S.D.) | ||||
|---|---|---|---|---|---|
| [PCP] µM |
F563 | F564 | F565 | F566 | |
| 0 | 46±3 | 64±6 | 30±3 | 18±0.5 | |
| 0.05 | 24±1* | 64±6 | 32±2 | 29±1+ | |
| 0.1 | 30±2* | 73±2 | 35±3 | 28±2+ | |
| 0.25 | 27±0.7* | 65±3 | 30±2 | 23±0.6+ | |
| 0.5 | 32±1* | 72±0.4 | 26±1 | 25±1+ | |
| 1 | 28±6* | 66±1 | 26±1 | 22±1+ | |
| 2.5 | 25±0.2* | 75±16 | 18±2* | 22±1+ | |
| 5 | 17±2* | 41±3* | 14±1* | 17±0.6 | |
| 6h | Interleukin 6 mRNA expression (AU) (mean± S.D.) | ||||
|---|---|---|---|---|---|
|
[PCP]
µM |
F538 | F540 | F541 | F542 | |
| 0 | 23±0.5 | 11±7 | 8±0.6 | 23±1 | |
| 0.05 | 19±0.3* | 14±3 | 45±8+ | 18±0.8* | |
| 0.1 | 25±0.4+ | 18±3 | 27±13 | 15±0.4* | |
| 0.25 | 19±0.3* | 11±0.8 | 28±3+ | 18±0.6* | |
| 0.5 | 24±0.2+ | 17±2 | 28±11 | 16±0.5* | |
| 1 | 15±1* | 16±0.4 | 36±19 | 31±2+ | |
| 2.5 | 12±0.1* | 22±1 | 30±8+ | 43±11 | |
| 5 | 18±0.3* | 12±4 | 21±2+ | 55±18 | |
| 24h | Interleukin 6 mRNA expression (AU) (mean± S.D.) | ||||
|---|---|---|---|---|---|
|
[PCP]
µM |
F525 | F527 | F528 | F530 | F548 |
| 0 | 27±2 | 15±0.1 | 5±1 | 13±1 | 9±2 |
| 0.05 | 21±2* | 14±1 | 19±1+ | 13±1 | 11±1 |
| 0.1 | 31±1+ | 15±2 | 14±3+ | 10±1* | 16±2+ |
| 0.25 | 39±4+ | 16±0.4+ | 11±1+ | 13±1 | 15±1+ |
| 0.5 | 43±3+ | 19±.1+ | 16±3+ | 10±0.4* | 10±1 |
| 1 | 37±1+ | 17±1 | 14±3+ | 7±0.2* | 8±.4 |
| 2.5 | 30±1 | 14±1 | 22±2+ | 9±3 | 9±1 |
| 5 | 25±8 | 11±1* | 19±3+ | 9±0.3 | 5±1* |
Values are mean±S.D. of triplicate determinations.
Indicates a significant decrease in secretion and
indicates a significant increase in secretion compared to control cells (cells treated with vehicle alone), p<0.05
Involvement of p44/42 and p38 MAPK pathways in PCP-induced increases in IL-6 production.
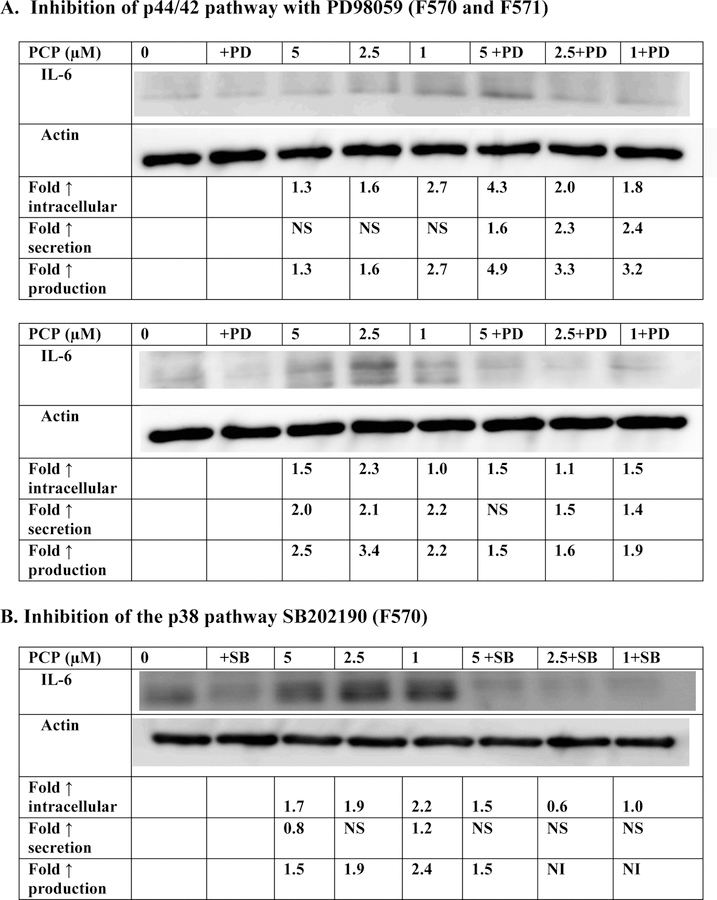
The effects of exposures to 1, 2.5, and 5 μM PCP on production (intracellular + secreted levels) of IL-6 from PBMCs where the p44/42 MAPK pathway is blocked by the MEK inhibitor PD98059 are shown in Figure 3A and Table 3. Cells from three or the four donors showed decreased production of IL-6 when exposed to PCP in the presence of the inhibitor. Overall, the results indicate that the p44/42 MAPK pathway might be utilized by PCP in stimulating IL-6 production in a donor dependent manner. Cells from each of four donors showed lowered PCP-induced IL-6 production when the p38 pathway was inhibited by SB202190 (Figure 3B and Table 3). For example, donor F570 showed 2.4, 1.86, and 1.43 fold increases when PBMCs were exposed to 1, 2.5 and 5 µM PCP in the absence of the p38 inhibitor. When the inhibitor was present, those same PCP exposures caused no increases in production. This indicates that the p38 pathway is being utilized by PCP to stimulate IL-6 production.
Figure 3. Effect of 24 h PCP exposure on synthesis of IL-6 in PBMCs where the p44/42 pathway or p38 pathway are inhibited.
Effects of the inhibition of selected signaling pathways on the production of IL-6 from human PBMCs after 24 h exposures to PCP. A) MEK inhibitor (PD98059). Data is from a 2 experiments (F570 and F571). Data from additional experiments are given in Table 3; B) p38 inhibitor (SB202190). Data is from a representative experiment (F570). Data from additional experiments are given in Table 3. An increase in secretion or in intracellular level is a number greater than 1 a decrease in secretion or intracellular level is a number less than 1. The control is arbitrarily set at 1. Cells treated with inhibitor are compared to control cells treated with inhibitor. A combined fold increase (secretion + intracellular) greater than 1 indicates an increase in production.
NI indicates no significant increase in production (fold increase ≤1); NS indicates no significant change in secretion (p>0.05)
Table 3:
Effects of PCP exposure on the synthesis of IL-6 in PBMCS when the p44/42 or p38 signaling pathways are inhibited.
| Fold ↑ synthesis IL-6 | |||||||
|---|---|---|---|---|---|---|---|
| [PCP] µM | 5 | 2.5 | 1 | 5+I | 2.5+I | 1+I | |
| Inhibitor (I) | donor | ||||||
| PD98059 | F569 | 1.3 | 1.4 | 1.3 | 2.1 | 1.9 | NI |
| F582 | 1.3 | 1.5 | 3.4 | 1.6 | NI | NI | |
| SB202190 | F570 | 1.5 | 1.9 | 2.4 | 1.5 | NI | NI |
| F571 | NI | 1.3 | 2.2 | NI | NI | NI | |
| F584 | 2.7 | 3.3 | 3.3 | 1.3 | 1.4 | 1.4 | |
NI indicates no significant increase (Fold increase ≤1)
Effects of DDT Exposure on Secretion of IL-6 by NK cells
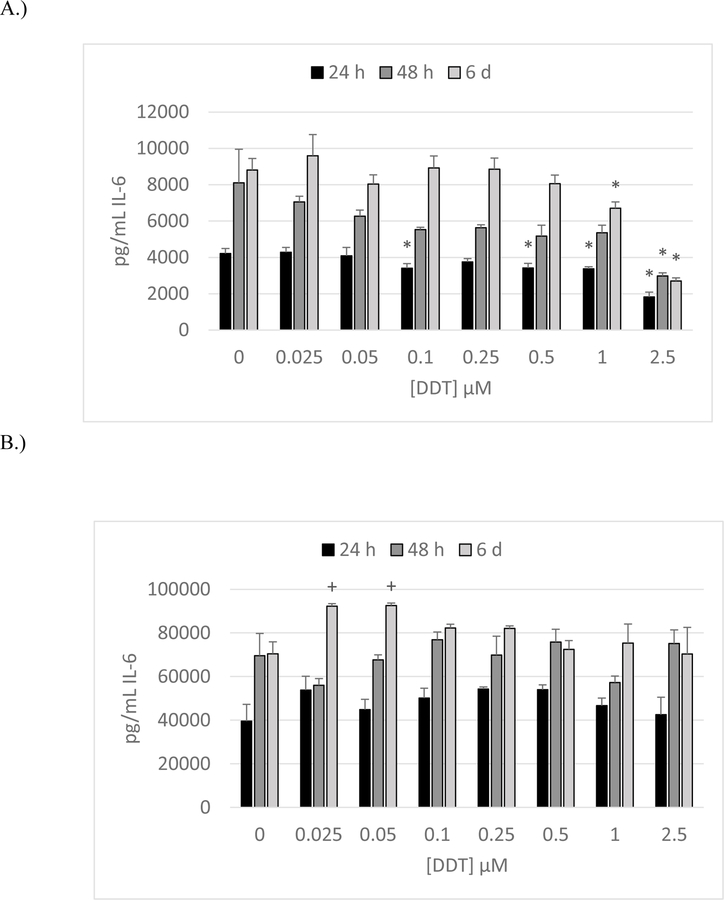
The effects of exposing preparations of enriched NK cells to DDT (0–2.5µM) for 24 h, 48 h, and 6 d are shown in Figure 4A. Cells were prepared from buffy coats obtained from Key Biologics (KB). Data are from a representative experiment using cells from donor KB197. All donors showed significant decreases in IL-6 secretion at 5 µM DDT at one or more length of exposure.
Figure 4. Effects of 24 h, 48 h and 6-day exposures to DDT on secretion of IL-6 from NK Cells, MD-PBMCs, and PBMCs.
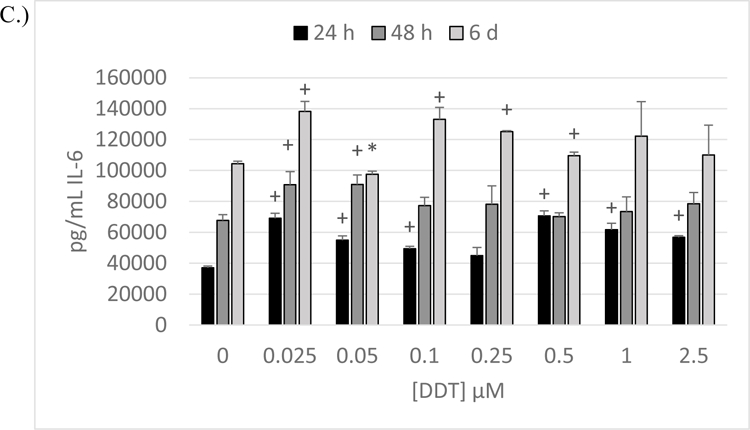
Effects of 24 h, 48 h, and 6 day exposures to 0–2.5 µM DDT on secretion of IL-6 from NK cells, MD-PBMCs, and PBMCs. A) NK cells (representative experiment of cells from donor KB196. B) MD-PBMCs (representative experiment of cells from donor F281). C) PBMCs (representative experiment of cells from donor F197). * represents significant decreases and + represents significant increases compared to the control (p<0.05).
Effects of DDT Exposure on Secretion of IL-6 by monocytes depleted peripheral blood mononuclear cells (MD-PBMCS)
Figure 4B shows the effects of 24 h, 48 h, and 6 d exposures to DDT (0–2.5 µM) on IL-6 secretion from MD-PBMCs. Cells were obtained from blood filters (F= filter obtained from the Red Cross). Cells from donor F281 (representative experiment in Figure 4B) exhibited increased secretion of IL-6 at 0.025 and 0.05 µM DDT after 6 day. Three out of four donors showed significant increases at one or more length of incubation at one or more concentration of DDT when compared to the control. Unlike NK cells, exposure of MD-PBMCs to DDT did not cause any consistent decreases in IL-6 secretion.
Effects of DDT Exposure on Secretion of IL-6 by PBMCS
The effects of exposing PBMCs to DDT for 24 h, 48 h, and 6 d on IL-6 secretion are illustrated in Figure 4C (representative experiment, F197) All donors showed significant increases after 48 h, and 6 days of exposure to at least 1 concentration of DDT. Significant increases occur at all lengths of exposure to one or more concentration of PCP in cells from F197 (Figure 4C).
Effects of DDT Exposures on the Production (Secretion + Intracellular Levels) of IL-6
1 h exposures:
As was seen with PCP, a 1 h exposure of PBMCs to DDT did not cause any consistent increases in the production of IL-6 (data not shown).
6h exposures:
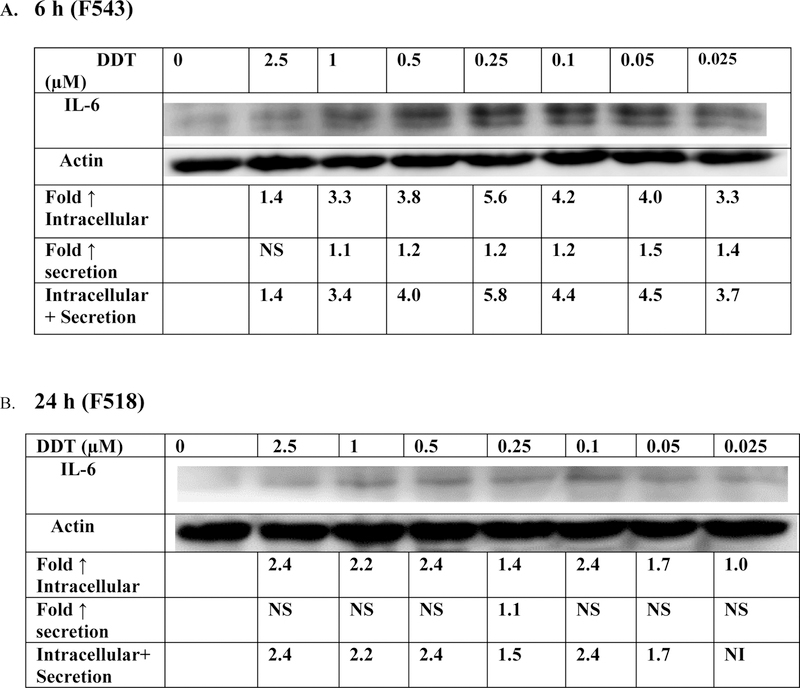
The effects of 6 h exposures to DDT on production (intracellular and secreted levels) of IL-6 in PBMCs were examined in cells from 4 separate donors (Figure 5A and Table 4). Cells from all donors demonstrated increased IL-6 production at one or more concentration of DDT. Cells from donors F536 and F543 shows fold increases ranging from 1.5–3.0 (F536) and 1.4–5.8 (F543).
Figure 5. Effect exposures to DDT on synthesis of IL-6 in PBMCs.
Effects of exposures to 0–2.5 µM DDT on the production of IL-6 in PBMCs. A) 6 h exposure. The blot is from a representative experiment (F543) with accompanying secretion data. An increase in secretion or in intracellular level is a number greater than 1; a decrease in secretion or intracellular level is a number less than 1. The control is arbitrarily set at 1. A combined fold increase (secretion + intracellular) greater than 1 indicates an increase in production. Changes in fold production for 3 additional experiments are given in Table 4. B) 24 h exposure. The blot is from a representative experiment (F518) with accompanying secretion data. Changes in fold production for 3 additional experiments are given in Table 4.
NI indicates no significant increase in production (fold increase ≤1); NS indicates no significant change in secretion (p>0.05)
Table 4:
Effects of DDT exposures on synthesis of IL-6 in PBMCS after 6 h, and 24 h
| Fold ↑ synthesis IL-6 | ||||||||
|---|---|---|---|---|---|---|---|---|
| [DDT] µM | 2.5 | 1 | 0.5 | 0.25 | 0.1 | 0.05 | 0.025 | |
| Exposure time | donor | |||||||
| 6 h | F535 | NI | NI | 1.4 | 2.3 | 1.5 | 1.3 | NI |
| F536 | 2.3 | 1.6 | 2.2 | 3.0 | 1.6 | 2.5 | 1.5 | |
| F545 | NI | 1.2 | NI | NI | NI | NI | NI | |
| 24 h | F517 | NI | NI | NI | 1.1 | NI | 1.2 | NI |
| F522 | 1.6 | 1.6 | 1.5 | 2.4 | 9.6 | 2.3 | 2.7 | |
| F523 | 1.6 | 1.3 | NI | 1.4 | NI | NI | NI | |
NI indicates no significant increase (Fold increase ≤1)
24h exposures:
The effect of 24-hour exposures to DDT on production of IL-6 are shown in Figure 5B and Table 4. As was seen after a 6 h exposure, cells from all donors exhibited increased IL-6 production after 24 h exposures to DDT. These data indicate that DDT causes significant increases in IL-6 production within 6 h of exposure.
Effects of Exposures to DDT on IL-6 mRNA Levels in PBMCs
Table 5 shows IL-6 mRNA levels in cells exposed to 0–2.5 µM DDT for 2 h and 6 h. Cells from 5 different donors were tested at each time point. There were significant increases in the mRNA for IL-6 after both 2 h and 6 h in each donor at 1 or more concentration of DDT. These data indicate that the increases in IL-6 production that were stimulated by DDT exposures can be attributed to DDT-induced increases in IL-6 mRNA
Table 5:
Effects of 2 h and 6 h exposures to DDT on IL-6 mRNA levels in PBMCs
| 2h | Interleukin 6 mRNA expression (AU) (mean± S.D.) | |||
|---|---|---|---|---|
|
[DDT]
µM |
F577 | F578 | F579 | F581 |
| 0 | 14±7 | 15±0.7 | 25±2 | 11±0.3 |
| 0.025 | 19±2 | 14±2 | 21±2 | 22±1+ |
| 0.05 | 21±2 | 33±3+ | 26±5 | 23±0.8+ |
| 0.1 | 23±1 | 15±1 | 22±3 | 20±0.1+ |
| 0.25 | 23±1 | 42±2+ | 26±2 | 17±1+ |
| 0.5 | 26±1 | 18±1+ | 27±1 | 12±0.2+ |
| 1 | 31±3+ | 20±0.7+ | 30±2+ | 27±3+ |
| 2.5 | 13±9 | 19±0.4+ | 24±1 | 20±0.5+ |
| 6h | Interleukin 6 mRNA expression (AU) (mean± S.D.) | |||
|
[DDT]
µM |
F573 | F574 | F575 | F576 |
| 0 | 17±2 | 6±0.1 | 4±1 | 27±4 |
| 0.025 | 22±3 | 15±0.1+ | 10±2+ | 14±0.2* |
| 0.05 | 25±1+ | 15±0.3+ | 17±2+ | 29±0.5 |
| 0.1 | 23±2+ | 15±1+ | 12±0.6+ | 34±13 |
| 0.25 | 23±2+ | 16±0.6+ | 10±2+ | 40±4+ |
| 0.5 | 22±2+ | 13±0.8+ | 15±1+ | 28±2 |
| 1 | 20±0.4 | 15±0.9+ | 9±2+ | 26±0.8 |
| 2.5 | 22±1+ | 13±0.4 | 8±2 | 33±2 |
Values are mean±S.D. of triplicate determinations.
Indicates a significant decrease in secretion and
indicates a significant increase in secretion compared to control cells (cells treated with vehicle alone), p<0.05
Involvement of p44/42 and p38 MAPK pathways in DDT-induced increases in IL-6 production.
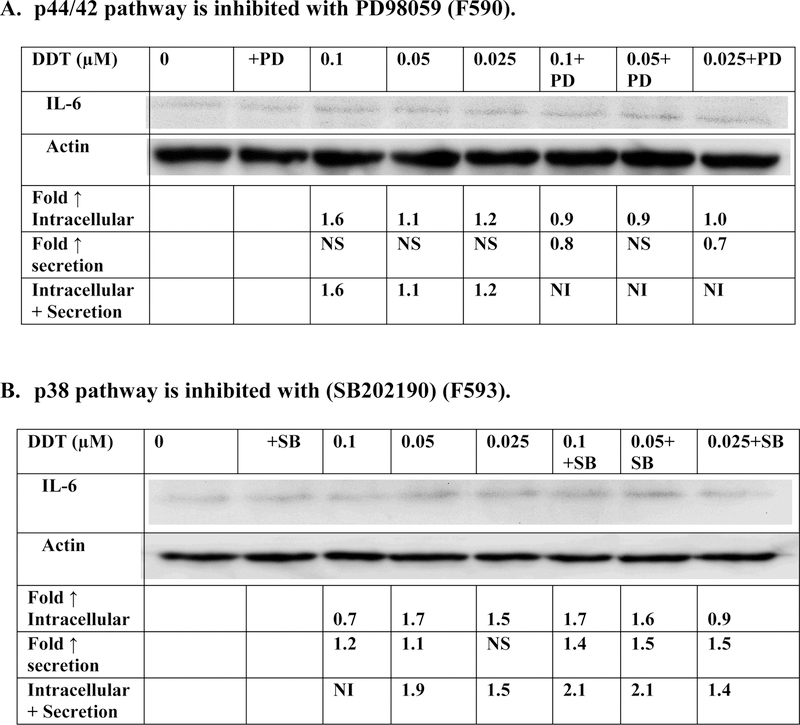
When the p44/42 MAPK pathway was inhibited by PD98059, the DDT-induced increases in IL-6 production were diminished in cells from 3 of the 4 donors (Figure 6A and Table 6). For example cells from donor F590 (Figure 6A) showed increases in IL-6 production of 1.6, 1.1 and 1.2 fold after 6 h exposures to 0.1, 0.05, and 0.025 µM DDT when the p44/42 pathway was not inhibited. However, in the presence of the inhibitor there was no DDT-induced increase in IL-6 production seen at any of the DDT exposures. These results suggest that the p44/42 MAPK pathway may be involved in DDT-induced increases IL-6 production in a donor-dependent manner. The effects of inhibiting the p38 MAPK pathway on DDT-induced IL-6 synthesis are shown in Figure 6B and Table 6. Cells from 2 (F591, F592) of the 4 donors tested showed some decrease in DDT-induced IL-6 production when the p38 pathway was blocked. However, the decrease in DDT-stimulated IL-6 production was less consistent than that seen when the p44/42 pathway was inhibited.
Figure 6. Effect of 6 h DDT exposure on synthesis of IL-6 in PBMCs where the p44/42 pathway or p38 pathway are inhibited.
Effects of the inhibition of selected signaling pathways on the production of IL-6 from human PBMCs after 6 h exposures to DDT. A) MEK inhibitor (PD98059). Data is from a representative experiment (F590). Data from additional experiments are given in Table 6; B) p38 inhibitor (SB202190). Data is from a representative experiment (F593). Data from additional experiments are given in Table 3. An increase in secretion or in intracellular level is a number greater than 1 a decrease in secretion or intracellular level is a number less than 1. The control is arbitrarily set at 1. Cells treated with inhibitor are compared to control cells treated with inhibitor.
NI indicates no significant increase in production (fold increase ≤1); NS indicates no significant change in secretion (p>0.05)
Table 6:
Effects of DDT exposure on the synthesis of IL-6 in PBMCS when the p44/42 or p38 signaling pathways are inhibited.
| Fold ↑ synthesis IL-6 | |||||||
|---|---|---|---|---|---|---|---|
| [DDT] µM | 0.1 | 0.05 | 0.025 | 0.1+I | 0.05+I | 0.025+I | |
| Inhibitor (I) | donor | ||||||
| PD98059 | F591 | 1.6 | 1.9 | 1.2 | NI | NI | NI |
| F592 | 1.6 | 1.7 | 1.9 | 1.5 | 1.1 | NI | |
| F593 | 1.4 | 1.3 | 2.0 | 1.8 | 1.7 | 3.1 | |
| SB202190 | F591 | 2.2 | 2.4 | 2.2 | NI | NI | NI |
| F592 | 1.1 | NI | 1.4 | NI | NI | 1.5 | |
| F595 | 1.5 | 2.0 | 1.8 | 1.8 | 2.4 | 2.4 | |
NI indicates no significant increase ( Fold increase ≤1)
DISCUSSION
PCP and DDT are two organochlorine compounds that very significantly contaminate the environment and make their way into human blood and other tissues (Cirelli, 1978; Cline et al., 1989; Uhl et al., 1986; Brown et al., 2005; Koepke et al., 2004; Trejo-Acevedo et al., 2009). Both have been shown to increase the risk of developing particular malignant tumors (Demers et al., 2006; Cooper & Jones, 2008; Eskenazi, et al., 2009; Harada et al., 2016). These compounds have also been shown to increase the secretion and production of the pro-inflammatory cytokine interleukin 1 beta (IL-1β) from human immune cells (Martin and Whalen, 2017; Martin et al., 2019). Pro-inflammatory cytokines can lead to chronic inflammation which is a significant contributor to a number of diseases including a number of cancers (Coussens & Werb, 2002; Demaria et al., 2010). IL-6 is another cytokine that can contribute to chronic inflammation, and the diseases associated with it, if its levels are not properly regulated (Jorcyk et al., 201; Landskron et al., 2014; Gabay et al.,2006; Nagasaki et al., 2014). Importantly, both PCP and DDT have been shown to increase not just cellular secretion of IL-1β but also its production by the cell (Martin et al., 2019). This effect on cellular production means that exposed cells will have a sustained ability to dispense IL-1β and contribute to an inflammatory state. The current study explores whether PCP and DDT can also stimulate secretion and production of the critical inflammatory cytokine IL-6 from immune cells. The levels of both PCP and DDT that were examined in these studies were near or within the range that is seen in human blood samples (Cline et al., 1989; Uhl et al., 1986; Koepke et al., 2004; Trejo-Acevedo et al., 2009).
Both compounds were capable of increasing IL-6 secretion from cell preparations that contained T-lymphocytes (MD-PBMCs) or T lymphocytes and monocytes (PBMCs). However, the simplest cell preparation of purified NK cells showed no consistent compound-induced increases in IL-6 secretion. These findings are somewhat similar to those seen with another category of environmental contaminants the organotins (TBT and DBT). These compounds were also able to increase the secretion of IL-6 from cell preparations containing T lymphocytes plus monocytes (PBMCs) at certain exposure levels but did not consistently stimulate secretion from purified NK cells or MD-PBMCs (Brown et al., 2018a)
Once we had established that the compounds were able to increase secretion of IL-6 from immune cell preparations that contained T lymphocytes and monocytes, we examined if the increase in secretion was due simply to increased release of pre-existing IL-6 or if the cell was stimulated by the compounds to produce more IL-6. The results indicated that both PCP and DDT were able to stimulate IL-6 production (secreted+intracellular levels) within 6 h of exposure. The compound-stimulated increases maintained out to 24 h. The organotin contaminant, TBT, is also able to stimulate the production of IL-6 (Brown et al. 2018b).
It appears that compound-induced increases in IL-6 mRNA play a role in the increases in IL-6 production seen with exposures to both PCP and DDT. PCP was able to increase the levels of IL-6 mRNA in cells from 3 out of 4 donors after 6 h of exposure and 4 out 5 donors after 24 h. These findings suggest that while increased mRNA for IL-6 occurs in response to PCP exposures it may not completely explain the increases seen in protein production in response to PCP. DDT exposures increased levels of mRNA for IL-6 after both 2 h and 6 h, indicating that the increases in the IL-6 protein induced by DDT may be due to DDT effects on either increased transcription or decreased breakdown of IL-6 mRNA. These results are in contrast to those seen when examining the effects of PCP and DDT on IL-1β mRNA. In those studies, PCP increased the mRNA for IL-1β while DDT did not cause an increase (Martin et al., 2019). As was seen in the current studies with PCP, increases in IL-6 synthesis seen with the organotin contaminant tributylin (TBT) can be only partially explained by increases in mRNA production (Brown et al., 2018b).
IL-6 level are regulated by the p44/42 and p38 mitogen activated protein kinase (MAPK) pathways (Gaestel et al., 2009; Winzen et al.,1999). Thus, we examined if either of these pathways were needed for the increases in IL-6 production that were seen with exposures to either PCP or DDT. PCP appears to require the p38 MAPK pathway to stimulate IL-6 production while DDT appear to need the p44/42 pathway to cause elevations in IL-6 production in cells from the majority of donors. These results differ from what was seen when examining the pathways utilized by PCP and DDT in elevating IL-1β, In those studies both DDT and PCP required the p38 pathway to increase IL-1β production (Martin et al., 2019). However, the organotin compound, TBT, like PCP, appeared to predominantly utilize the p38 pathway to stimulate IL-6 production (Brown et al, 2018b). There are other examples of DDT altering gene expression in both human embryonic kidney epithelial cell line and an endometrial cell line (Frigo et al, 2004).
The role of chronic inflammation in neurodegenerative diseases such as Parkinson’s disease (PD) has been established (Tansey & Goldberg, 2010; Chao et al., 2014). Levels of a number of inflammatory cytokines including IL-6 are elevated in the serum of patients with PD (Chao et al., 2014). Additionally, an association between pesticide exposure and diseases such as PD has been demonstrated (Freire & Koifman, 2012). The results of this study indicate that organochlorine pesticides such as PCP and DDT are able to increase the levels of a critical inflammatory cytokine, IL-6, produced by immune. IL-6 has been shown to associate with neurodegenerative diseases (Chao et al., 2014). Additionally, a previous study showed that both DDT and PCP were also able to elevate the production of the potent pro-inflammatory cytokine IL-1β (Martin et al., 2019). Thus, the capacity of certain pesticides to elevate inflammatory cytokines may be an aspect of the mechanism by which they could elevate risk of neurodegeneration.
To summarize, both PCP and DDT were able to increase the secretion of IL-6 in cell preparations that contained either T lymphocytes (MD-PBMCs) or T lymphocytes and monocytes (PBMCs). More importantly, these compound are able to increase the cellular production of IL-6. PCP-induced increases in IL-6 can be partially explained by PCP’s ability to increase IL-6 mRNA levels. The increases in IL-6 production seen with DDT may be completely due to its ability to increase IL-6 mRNA. The p38 MAPK signaling pathway appears to be most important for PCP-induced increases in IL-6, while neither the p44/42 nor the p38 pathway appeared to be essential to DDT-induced increases in IL-6.
ACKNOWLEDGEMENT
Grants 5T34GM007663 and U54CA163066 from the National Institutes of Health
REFERENCES
- Brown B, Loganathan BG, Owen D 2005. Chlorophenol concentrations in sediment and mussel tissues from selected locations in Kentucky Lake. Chrysalis, Murray State University Undergraduate Research Journal 1: 5–10. [Google Scholar]
- Brown S, Wilburn W, Martin T, & Whalen M 2018a. Butyltin compounds alter secretion of interleukin 6 from human immune cells. Journal of Applied Toxicology 38: 201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Hamza N, Boules M, Wang X, Whalen MM 2018b. Production of interleukin 1 beta and interleukin 6 in human lymphocytes is stimulated by tributyltin. Archives of Toxicology 92:2573–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Wong SC, Tan EK 2014. Evidence of inflammatory system involvement in Parkinson’s disease. BioMed Research International 2014: article ID 308654 9 pages. [DOI] [PMC free article] [PubMed]
- Choy E and Panayi G 2001. Cytokine Pathways and Joint Inflammation in Rheumatoid Arthritis. New England Journal of Medicine 344: 907–916. [DOI] [PubMed] [Google Scholar]
- Cirelli DP 1978. Patterns of pentachlorophenol usage in the United States of America-an overview. In: Rao KR, editor. Pentachlorophenol, chemistry, pharmacology, and environmental toxicology Plenum Press; New York, NY: p. 13–18. [Google Scholar]
- Cline RE, Hill RH, Phillips DL, Needham LL 1989. Pentachlorophenol measurements in body fluids of people in log homes and workplaces. Arch. Environ. Contam. Toxicol 18:475–481. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Jones S 2008. Pentachlorophenol and Cancer Risk: Focusing the Lens on Specific Chlorophenols and Contaminants. Environmental Health Perspectives 116: 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z 2002. Inflammation and cancer. Nature 420: 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MP 2006. With DDT Spraying in Malaysia Can Show the World How to Control Dengue; 21st Century Science and Technology, Summer 2006. pp 53–60.
- Demaria S, Pikarsky E, Karin M, Coussens L, Chen Y, El-Omar E, Trinchieri G, Dubinett S, Mao J, Szabo E, Krieg A, Weiner G, Fox B, Coukos G, Wang E, Abraham R, Carbone M and Lotze M 2010. Cancer and Inflammation: Promise for Biological Therapy. Journal of Immunotherapy 33: 335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers PA, Davies HW, Friesen MC, Hertzman C, Ostry A, Hershler R, et al. 2006. Cancer and occupational exposure to pentachlorophenol and tetrachlorophenol. Cancer Causes Control 17:749–758. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Chevier J, Rosas LG, Anderson HA, Bornman MS, Bouwman H, Chen A, Cohn BA, deJager C, Henshel DS, Leipzig F, Leipzig JS, Lorenz EC, Snedeker SM, Stapleton D 2009. The Pine River Statement: Human Health Consequences of DDT Use. Environ. Health Perspec 117:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando M, Reyes J, Iannuzzi J, Leung G and McKay D 2014. The Pro-Inflammatory Cytokine, Interleukin-6, Enhances the Polarization of Alternatively Activated Macrophages. PLoS ONE 9: p. e94188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C, Koifman S 2012. Pesticide exposure and Parkinson’s disease: Epidemiological evidence of association. Neurotoxicology 33:947–971. [DOI] [PubMed] [Google Scholar]
- Frigo DE, Tang Y, Beckman BS, Scandurro AB, Alam J, Burow ME, McLachlan JA 2004. Mechanism of AP-1-mediated gene expression by select organochlorines through the p38 MAPK pathway. Carcinogenesis 25:249–261. [DOI] [PubMed] [Google Scholar]
- Gabay C 2006. Interleukin-6 and chronic inflammation. Arthritis Research & Therapy, 8(Suppl 2): S3, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaestel M, Kotlyarov A and Kracht M 2009. Targeting innate immunity protein kinase signaling in inflammation. Nature Reviews Drug Discovery 8: 480–499. [DOI] [PubMed] [Google Scholar]
- Harada T, Takeda M, Kojima S, & Tomiyama N 2016. Toxicity and Carcinogenicity of Dichlorodiphenyltrichloroethane (DDT). Toxicological Research 32: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorcyk C, Tawara K and Oxford J 2011. Clinical significance of interleukin (IL)-6 in cancer metastasis to bone: potential of anti-IL-6 therapies. Cancer Management and Research 3:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti PRS, Loganathan BG 2017. Organohalogen Pollutants and Human Health. In: Quah SR and Cockerham WC (eds.) The International Encyclopedia of Public Health, 2nd edition. vol. 5, pp. 359–366. Oxford: Academic Press. [Google Scholar]
- Koepke R, Warner M, Petreas M, Cabria A, Danis R, Hernandez-Avila M, & Eskenazi B 2004. Serum DDT and DDE levels in pregnant women of Chiapas. Mexico. Arch. Environ. Health 59:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. 2014. Chronic Inflammation and Cytokines in the Tumor Microenvironment. Journal of Immunology Research 2014: Article ID 149185, 19 pages, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganathan BG 2016. Persistent organic chemicals in the Pacific Basin countries: An overview. In. Persistent Organic Chemicals in the Environment: Status and trends in the Pacific Basin Countries I. Eds. Loganathan BG, Khim JS, Kodavanti PR, Masunaga S ACS Symposium Series Vol. 1243 American Chemical Society and Oxford University Press; Pp 1–15. [Google Scholar]
- Martin T and Whalen M 2017. Exposures to the environmental toxicants pentachlorophenol and dichlorodiphenyltrichloroethane modify secretion of interleukin 1-beta from human immune cells. Archives of Toxicology 91:1795–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T, Maise J, Gabure S Whalen, M.M. 2019. Exposures to the Environmental Contaminants Pentachlorophenol (PCP) and Dichlorodiphenyltrichloroethane (DDT) Increase Production of the Pro-inflammatory cytokine, Interleukin 1-Βeta (IL-1β), in Human Immune Cells. J. Appl. Toxicol In press [DOI] [PMC free article] [PubMed]
- Massawe R, Drabo L, & Whalen M 2017. Effects of pentachlorophenol and dichlorodiphenyltrichloroethane on secretion of interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα) from human immune cells. Toxicology Mechanisms and Methods 27: 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TP, Zehnter I, Hofmann B, Zaisserer J, Burkhart J, Rapp S, Weinauer F, Schmitz J, Illert WE 2005. Filter Buffy Coats (FBC): A source of peripheral blood leukocytes recovered from leukocyte depletion filters. Journal of Immunological Methods 307: 150–166. [DOI] [PubMed] [Google Scholar]
- Nagasaki T, Hara M, Shiga K, Takeyama H 2014. Relationship between inflammation and cancer progression: review of the recent advances in interleukin-6 signaling and its blockage in cancer therapy. Receptors & Clinical Investigation 1: e202. [Google Scholar]
- Rengam SV 2013. Banned but Still in Use. Letters. PAN Asia and the Pacific February 1, 2013. [Google Scholar]
- Tanaka T and Kishimoto T 2014. The Biology and Medical Implications of Interleukin-6. Cancer Immunology Research 2:288–294. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Goldberg MS 2010. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiology of Disease 37: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo-Acevedo A, Diaz-Barriga F, Carrizales L, Dominguez G, Costilla R, Ize-Lema I, Yarto-Ramirez M, Gavilan-Garcia A, Mejia-Saavedra JJ, & Perez-Maldonado IN 2009. Exposure assessment of persistent organic pollutants and metals in Mexican children. Chemosphere 74:974–980. [DOI] [PubMed] [Google Scholar]
- Turusov V, Rakitsky V, & Tomatis L 2002. Dicholordiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environmental Health Perspectives 110:125–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl S, Schmid P, & Schlatter C 1986. Pharmacokinetics of pentachlorophenol in man. Arch. Toxicol 58:182–186. [DOI] [PubMed] [Google Scholar]
- Winzen R 1999. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. The EMBO Journal 18: 4969–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]