Abstract
Exosomes are secreted membrane-bound vesicles containing a cargo of curated nucleic acids, proteins, and lipids that can alter gene expression in recipient cells. Toxic agents can alter exosome synthesis and bioactive cargo composition, thus allowing exosomes to serve as biomarkers of exposure and response. While human and animal studies have identified exosome biomarkers of organ toxicity, in vitro models are ideal to examine biological mechanisms of exosome function. Here, we discuss the importance of exosomes in toxicology research and describe applications of in vitro models in advancing our understanding of their role in exposure-associated disease. This discussion of new research frontiers is in commemoration of the invaluable contributions of Dr. Daniel Acosta to the field of in vitro biology and toxicology. Emerging studies have implicated exosomes as mediators of neurodegeneration by shuttling pollutant-induced pathogenic proteins and miRNAs from afflicted neurons to neighboring cells. Exosomes also provide a mechanistic link between inhalation exposures and airway inflammation, remodeling, and systemic effects. Furthermore, exosomes provide the means for toxic agents to initiate oncogenic transformation and create favorable tumor microenvironments. Expansion in this field using in vitro models is essential to unlock the potential applications of exosome biology in toxicology.
Keywords: exosomes, extracellular vesicles, in vitro models, biomarkers, mechanism of action
1. Introduction
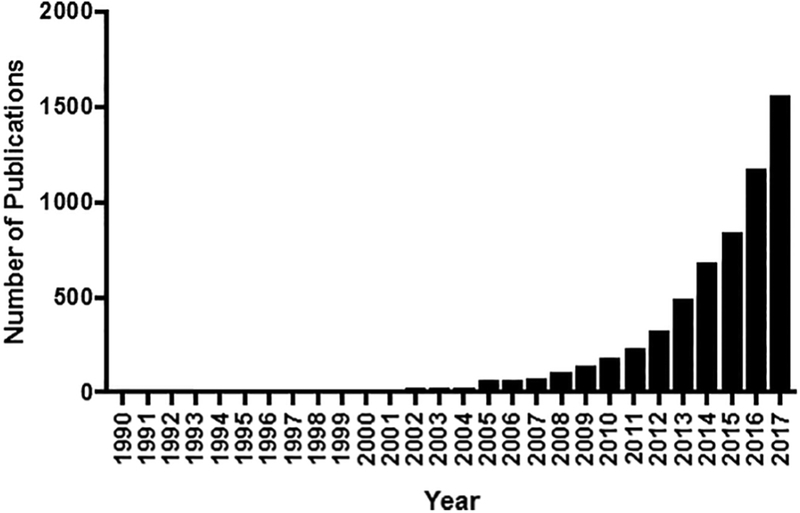
Exosomes have recently transformed from underappreciated extracellular vesicles into central players at the forefront of biological research. First identified in the early 1980’s (Harding & Stahl, 1983; Pan & Johnstone, 1983; Trams et al., 1981), exosomes were initially dismissed as unexciting cellular waste disposal mechanisms. Recent developments have invigorated the field of exosome research, with nearly 2,000 exosome-related papers published in 2017 (Figure 1). Three insights are largely responsible for this renewed interest. First, exosomes contain proteins, lipids, and nucleic acids derived from their parent cell that can act as messengers of the state of the cell of origin. Second, exosomes can migrate from their parent cell into accessible body fluids, such as blood and urine, to serve as proxy for biomonitoring purposes. Lastly, exosomes can deliver their cargo to other cells and alter their physiology, a capability with substantial implications to our understanding of cell-to-cell communication, disease pathology, and development of novel therapeutics.
Figure 1. Number of publications featuring exosomes by year.
Figure generated using PubMed search engine. (https://www.ncbi.nlm.nih.gov/pubmed)
To date, the bulk of exosome research has occurred in the fields of immunology and cancer where central research foci include discovery of novel biomarkers, understanding the role of exosomes in immune modulation, tumor formation, and metastasis, and exosomes as carriers of cancer therapy (Théry et al., 2009). The application of exosome research in toxicology can be equally transformative; though such research is still in its infancy. In this review written to honor the many accomplishments of Dr. Daniel Acosta, Jr., mentor and friend, we summarize critical concepts of exosome biogenesis, composition, and fate, discuss the importance of exosome research in toxicology, and describe the present and future use of in vitro models to advance our understanding of the role of exosomes in disease.
2. Exosome Biogenesis, Composition, and Fate: A Whirlwind Tour
2.1. Exosome Biogenesis
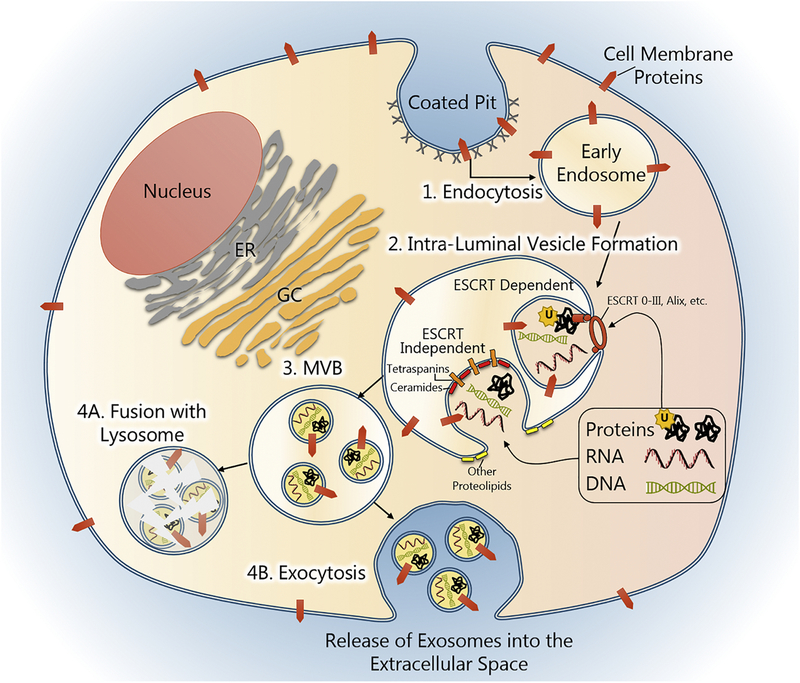
Exosomes are extracellular vesicles of endocytic origin ranging in diameter from 30–150 nM. These features distinguish exosomes from their larger counterparts, microvesicles (100–1,000 nM) and apoptotic bodies (1,000–5,000 nM), which are created from outward budding or blebbing of the cell membrane (György et al., 2011). Exosome formation begins with endocytosis of the plasma membrane and formation of a small membrane-bound vacuole, an early endosome, in the cytoplasm. Components of the cell membrane, including membrane-bound signaling proteins, are captured during this process to become part of the endosomal membrane and ultimately the exosome membrane itself (Théry et al., 2002).
The early endosome matures into a Multi-Vesicular Body (MVB) by inward budding of the endosomal membrane and the formation of small Intra-Luminal Vesicles (ILV), the nascent exosomes. This process is mediated by a subset of proteins called Endosomal Sorting Complexes Required for Transport (ESCRT). In ESCRT-dependent synthesis, ubiquitinated proteins in the cytoplasm are recognized and anchored to the endosomal membrane by ESCRT0, which in turn recruits ESCRT proteins I-III. Additional exosome-associated proteins stabilize this complex, such as ALG-2-interacting protein X (Alix). Together, these proteins form a vesicle-budding machinery that is powered by the ATPase vacuolar protein sorting 4 (Vps4). ILV formation can also occur through ESCRT-independent processes which are facilitated by the coordinate actions of proteolipids and tetraspanins. Membrane constituents such as cholesterol (Strauss et al., 2010; Trajkovic et al., 2008), sphingosine (Yuyama et al., 2012), and ceramides (Bianco et al., 2009; Trajkovic et al., 2008) are thought to encourage membrane curvature and invagination and, in coordination with other proteins such as flotillin, form microdomains rich in tetraspanins, such as CD9, CD63, and CD81. ESCRT-dependent and -independent exosomes can be secreted from the same cell and often contain different types of cargo (Tauro et al., 2013).
2.2. Exosome cargo selection
During MVB formation, exosomes are selectively loaded with proteins and nucleic acids exclusively from the cell membrane, endocytic pathway, and cytosol, but the factors governing cargo selection are not well defined (Théry et al., 2001; Villarroya-Beltri et al., 2014). Protein cargo varies depending on the type of exosome biogenesis. ESCRT-dependent processes select for ubiquitinated cytosolic proteins, but ubiquitination is not required for tetraspanin-mediated ESCRT-independent selection, which targets a wide repertoire of proteins including MHC receptors, metalloproteinases, β-catenin, and viral-associated proteins (Andreu & Yáñez-Mó 2014; Verweij et al., 2011). Various RNA species are found within exosomes, and include approximately 40% miRNAs, 40% piwiRNAs, 3.7% pseudo-genes, 2.4% lncRNAs, 2.1% tRNAs, and 2.1% mRNAs (Y uan et al., 2016). RNAs are thought to be selectively loaded by 30 different types of ribonucleoparticle shuttles based on sequence-specific motifs (Statello et al., 2018); for example, ribonucleoprotein A2B1 (hnRNPA2B1) shuttles miRNAs with the GGAG motif (Villarroya-Beltri et al., 2013). Messenger RNAs are also selectively imported into exosomes, with 3’UTR fragments (Batagov & Kurochkin 2013) and mRNAs bearing particular nucleotide motifs, e.g. CTGCC, being highly represented (Bolukbasi et al., 2012). In addition to RNA, genomic and mitochondrial DNA are also found in exosomes (Guescini et al., 2010; Thakur et al., 2014). While exosome genomic DNA fragments span the entire genome, DNAs that could be harmful to the cell are particularly enriched (Kahlert et al., 2014; Thakur et al., 2014).
2.3. Exosome Fate
MVBs can: 1) fuse with the plasma membrane and release ILVs (exosomes) into the extracellular space; 2) fuse with a lysosome, destroying their contents; and 3) be retained and become organelles in specialized cell types (e.g. melanosomes in melanocytes). While the factors that dictate MVB fate are unclear, the Rab GTPases are thought to be the principal arbiters. Approximately 63 Rab proteins are differentially distributed throughout cellular compartments and regulate organelle trafficking via cytoskeletal networks and the docking/fusion of membrane-bound entities (Zerial & McBride 2001).
Upon their release, exosomes can be destroyed, excreted, or interact with local and/or distant cells. The factors dictating exosome-cell interactions are being investigated, although adhesion molecules such as integrins and Intercellular Adhesion Molecules (ICAMS), MHC-protein complexes, tetraspanins, and carbohydrate/lectin receptors are thought to be central mediators (Villarroya-Beltri et al., 2014). The delivery of exosome cargo is accomplished by direct exosome fusion with the cell membrane or endocytosis, although in the latter, the incoming exosome is once again housed inside an MVB and subject to other fates, including destruction. Once released inside the cell, exosome cargo can initiate genomic, proteomic and epigenetic changes via translation of mRNAs, gene silencing by miRNAs, and contribution of bioactive lipids (Valadi et al., 2007; Lee et al., 2012 Record et al., 2014).
3. The importance of exosome research in toxicology: Biomarkers
The aforementioned processes governing exosome biogenesis, fate, and export can be altered by cellular stressors (de Jong et al., 2012; Eldh et al., 2010) and chemical exposures (Cordazzo et al., 2014; Harischandra et al., 2018; etc.). Exosome cargo is particularly sensitive to these perturbations, which is a boon to toxicology in several respects. First, exosome cargo profiles can be examined for biomarkers of exposure, exposure-mediated effects, and exposure-associated diseases. To date, in vivo methods such as human clinical studies and animal exposure models have identified novel exosome biomarkers of liver and kidney injury. Current clinical measures of organ injury and disease are frequently based on the levels of specific proteins in the blood (e.g. ALT, AST, creatinine, etc.); however, these clinical readouts often cannot differentiate between the types or extent of acute injury, nor the stage or severity of disease (Johnston 1999; Szabo & Heravi 2017). Recent studies have used exosome miRNA biomarkers from the blood and urine to overcome these limitations. They have found unique exosome profiles associated with alcoholic liver disease, inflammatory liver injury, DILI/APAP injury, and hepatitis-ischemic injury in liver (Bala et al., 2012; Cho et al., 2017) and different types of acute damage in kidney (Zhou et al., 2006; 2008). In some cases, these exosome readouts appear earlier than current clinical chemistries, creating opportunities for early intervention (Beltrami et al., 2012). Other exosome profiling techniques have detected organ-specific damage following radiation exposure (Kulkarni et al., 2016). MicroRNAs, which are highly enriched in exosomes, are also suggested as novel multi-organ drug toxicity indicators, including cardiotoxicity (Nishimura et al., 2015). In summary, exosome biomarkers can differentiate between types of acute damage and exposure-associated diseases. Exosome biomarkers may be detected earlier than traditional read-outs, allowing for early intervention.
4. In vitro exosome studies reveal mechanisms/modes of toxic action in exposure-related diseases
The ability of exposures to alter exosome cargo provides a vehicle for exposed cells to reprogram healthy cells in proximal and distal tissues. Thus, another reason that exosome biology can be valuable to toxicology is that pathogenic exosome release may play a central part in the mechanisms and modes of action connecting toxic agents with adverse exposure outcomes. Identifying these mechanisms using solely in vivo models would prove difficult given the inherent limitations of these systems, including inefficiency, complexity, safety limitations, and difficulty isolating particular tissues. Perhaps the greatest disadvantage is that common animal exposure models (i.e. rodents) allow for procurement of only a limited number of exosomes based on the size of the animal, even in terminal procedures. Alternatively, large numbers of exosomes can be rapidly purified from tissue culture and facilitate more complex mechanistic investigations using specialized in vitro systems, such as primary cultures and organs-on-a-chip. Advances in exosome isolation methods and the availability of commercial isolation kits have increased throughput and eliminated the need for specialized equipment.
The aforementioned advantages have facilitated in vitro studies that have yielded important insights into the roles that exosomes in exposure-related diseases. Research areas where considerable progress has been made are discussed below.
4.1. Exosomes mediate exposure-associated neurodegenerative pathology
In vitro studies have revealed an exosome-based mechanism linking environmental exposure and neurological disease. Misfolded aggregates of proteins such as β-amyloid, tau, and α-synuclein are central drivers of diseases such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis, among others. Exposure to metals, solvents, and pesticides can increase the expression of these proteins in neurons, and their pathogenic forms can be shuttled from affected cells to healthy cells via exosomes (Harischandra et al.; 2017; Emmanouilidou et al., 2010). Pollutants such as manganese (Mn), which increase the abundance of the exosome-generating machinery, such as Rab27a, enhance this pathologic process (Harischandra et al., 2018). Moreover, Mn exposure also increases exosome-mediated export of miRNAs that regulate pathways controlling protein aggregation, autophagy, inflammation, and hypoxia, thus exacerbating disease development (Harischandra et al., 2018). These experiments suggest that an important mechanism of exposure-mediated neurodegeneration is the dissemination of pathogenic proteins and miRNAs from diseased to healthy cells, a process that can be mediated by exosomes. In addition to filling in an important mechanistic gap, these studies may also provide biomarkers to identify individuals with adverse exposure effects.
4.2. Exosomes link inhalational exposures with pulmonary disease and systemic effects
In vitro studies are revealing that exosomes may mediate adverse processes that facilitate the development of exposure-associated pulmonary diseases such as chronic obstructive pulmonary disease (COPD) and lung fibrosis. Early studies suggest that exposed cells release exosomes that initiate pro-inflammatory and pathogenic gene expression changes in healthy recipient cells. Exosomes from cigarette smoke extract (CSE)-exposed mononuclear cells induce the expression of interleukin 8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1) in normal bronchial epithelial cells (Cordazzo et al., 2014). Moon et al., (2014) also found evidence suggesting that CSE-exposed bronchial epithelial cells release extracellular vesicles that are enriched with full-length CCN family member 1 (CCN1), which promote the subsequent release of vascular endothelial growth factor (VEGF) and IL-8 from naïve cells. Exposure-associated exosome release may also drive airway remodeling and fibrosis: bronchial epithelial cell CSE exposure upregulates the expression and exosome-mediated export of miR-210, which targets autophagy-related 7 (ATG7) and as a result promotes myofibroblast differentiation (Fujita et al., 2015). These exosome-mediated effects provide an important mechanistic link between cigarette smoke exposure and the dysregulation of pulmonary inflammation and airway remodeling that occurs with COPD and lung fibrosis.
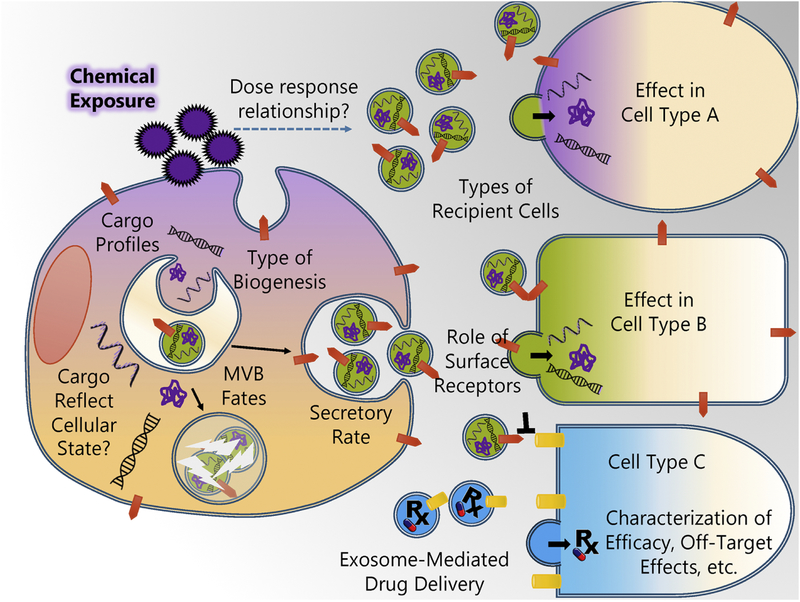
Exosome research may be particularly important in understanding many extra-pulmonary health effects that are associated with inhalational exposures. Exposure-mediated pathogenic exosome release in the setting of inflamed, highly vascularized lung tissue is thought to increase the release and delivery of pro-inflammatory exosomes into the blood stream and cause systemic effects (Wahlund et al., 2017). This hypothesis may help explain the strong correlation between cigarette smoking and extra-pulmonary effects such as rheumatoid arthritis and is supported by recent in vivo findings in which nanoparticles inhaled by mice produced pro-inflammatory exosomes which entered circulation and promoted the maturation of dendritic cells and activation of splenic T cells (Zhu et al., 2012). Future in vitro studies can be used to characterize the interactions and outcomes between exposure-associated pulmonary exosomes and peripheral tissues and organs (Figure 3).
Figure 3. Opportunities for characterizing exposure effects on exosome biology and the pharmacology of exosome-mediated drug delivery using in vitro studies.
In vitro models can be used to investigate how chemical exposures influence various facets of exosome biology, and the cellular pharmacology of exosome-based therapeutics. Cells exposed to chemicals (including pharmacological drugs) can undergo many physiological changes. Exposure-mediated changes in gene expression can modify nucleic acids and proteins available for exosome export. Chemical exposure can also alter various aspects of the endocytic pathway, such as the type of exosome biogenesis (ESCRT dependent versus independent), the selection of cargo, the fate of MVBs, and the rate of exosome secretion. The extent of these alterations may exhibit dose-dependence. Once released, exosomes from exposed cells can interact with a variety of cell and tissue types, but fusion or uptake may be dictated by certain factors (e.g. surface proteins). Exposure-associated exosomes may elicit cell-type specific changes in recipient cells. The biochemical factors that determine the interactions between exposure-associated exosomes and recipient cells may determine the distribution of exposure-mediated effects. These principles also likely apply to exosome-mediated drug delivery. In vitro models can be used to determine how exosome-bound drugs may have different pharmacological profiles from their free-forms.
4.3. Exposure-associated exosomes initiate oncogenic programs in healthy cells
Cancer development is associated with exposure to different types of environmental agents, yet the mechanisms and modes of action underlying carcinogenesis are often unknown. A wealth of recent evidence linking exosome and cancer biology is expansive and comprehensively reviewed by Amzi et al. (2013) and Zhang et al. (2015). Key findings have established that exosomes derived from cancer cells induce changes in recipient cells that promote oncogenic transformation, chemotherapeutic resistance, cell survival, and create suitable microenvironments for tumor growth (Amzi et al. 2013; Zhang et al. 2015; Javeed et al., 2017). Given the central role of exosomes in cancer development, it follows that they may also mediate exposure-associated carcinogenesis. Early in vitro studies support this mechanistic link and salient findings are discussed below.
Using in vitro techniques, Munson et al., (2018) discovered that exosomes may be an important mechanistic link between asbestos exposure and mesothelioma. Asbestos exposure alters proteomic profiles in cultured lung epithelial cells and macrophages. When exosomes from these cells are incubated with lung mesothelial cells, they initiate expression changes in numerous genes related to cancer development and epithelial-to-mesenchymal transition. These findings suggest that exosomes are a central mechanism of asbestos-associated mesothelioma and that exosome miRNA cargo may be a biomarker of asbestos-associated disease risk.
Moreover, arsenic-transformed liver epithelial cells secrete exosomes containing miR-155. When these exosomes are incubated with normal liver cells, they cause an inflammatory response mediated by NF-κB (Chen et al., 2017). These in vitro results were corroborated in vivo with the finding that arsenic-exposed individuals also exhibited increased levels of miR-155 in serum exosomes (Chen et al., 2017). Excessive inflammation is thought to create microenvironments conducive to tumor formation (Gonda et al., 2009), thus exosomes may be an important vehicle for arsenic-mediated disease.
Furthermore, toluene, a volatile organic compound, is a major indoor air contaminant that causes a host of adverse responses affecting the eyes, skin, mucous membranes, reproductive system, nervous system, renal system, and respiratory tract (ATSDR, 2015). Studies also suggest that toluene exposure increases cancer risk (Gérin, et al., 1998). The modes of action mediating many of these effects are largely undetermined. Lim et al., (2017) exposed HL-60 human promyelocytic leukemia cells to toluene and analyzed miRNA patterns in cells and exosomes. They found 54 alternatively regulated miRNAs in cells and exosomes that target genes related to cancer development and diseases of the nervous, cardiovascular, and respiratory systems. Exosome-mediated transport and delivery of these miRNAs may offer new insight into how toluene mediates systemic effects and how it may be linked to cancer risk.
5. Exosome-based drug delivery: Implications in pharmacology and toxicology
Research initiatives are underway to harness exosome mediated cell-to-cell communication as a delivery system for therapeutics in varied applications such as vaccination, immunomodulation, and drug delivery (Lener at al., 2015; Fais et al., 2016). The biochemical properties of exosomes or engineered liposome membranes can be used to target delivery to certain cell populations (Ohno et al., 2013; Tian et al., 2014; Tian et al., 2018). In addition to selective delivery, the conveyance of drugs within exosomes or lipophilic particles may in itself produce pharmacological profiles distinct from the free-form of the drug. Exosome-mediated drug delivery enhances the anticancer activity of drugs such as paclitaxel (Kim et al., 2018; Pascucci et al., 2014), acridine orange (Iessi et al., 2017), and doxorubicin (Hadla et al., 2016). The anti-inflammatory activity of curcumin is also found to be enhanced when encapsulated in exosomes (Sun et al., 2016). In addition to altering drug efficacy, exosome-mediated drug delivery may also modify off-target effects. Exosome-associated doxorubicin exhibits reduced cardiotoxicity (Tian et al, 2014; Toffoli et al., 2016) and does not impair liver and kidney function (Tang et al., 2012) compared to free doxorubicin. The implications of these initial studies to toxicology are significant, and call for a reassessment of the pharmacologic profiles of well-characterized drugs in the context of vesicular drug delivery.
It is worth noting that many of the aforementioned findings were identified using in vitro models, thus in vitro toxicology will likely continue to play a large role in deciphering the nuances of exosome-associated pharmacology. In vitro platforms are being used to develop quantitative models describing both the production and pharmacological effects of drug-associated exosomes (Wang et al., 2017). To this end, techniques such as fluorescence lifetime imaging microscopy (FLIM) can be used to assess uptake, intracellular behavior, and the delivery of drug-containing vesicles (Saari et al., 2018). After these initial principles are established, in vivo models can be used to assess drug-associated exosome absorption, distribution, metabolism, and extraction and develop physiologically based pharmacokinetic models.
6. Conclusions: Reflecting on the past and looking to the future
This review addresses a new frontier in toxicological research. As such, its focus was specifically chosen to honor the achievements and contributions of Dr. Daniel Acosta, Jr. Dr. Acosta was the primary research advisor of Dr. Ramos who then went on to establish a prominent independent career in the field. Dr. Ramos has been training Dr. Bowers and Hassanin, thus extending the long-standing legacy of significant contributions to toxicology made by Dr. Acosta over the course of 40+ years. We honor his achievements and thank him for his kindness and mentoring over many years.
In keeping with Dr. Acosta’s legacy of innovative research in the area of in vitro toxicology, in this review we introduce exosome biology and its role in toxicology. While in vivo studies are essential in understanding factors such as exosome migration, biomarker validity, and drug-associated exosome pharmacokinetics and pharmacodynamics, mechanistic questions and molecular characterization are most certainly best investigated taking advantage of the power of in vitro model systems. Using in vitro models, essential questions can be answered regarding exposure effects on exosome generation, cargo, and MVB fates (Figure 3). Moreover, the effect of drug-associated or exposure-associated exosomes on recipient cells can be assessed using in vitro panels and ‘organ-on-a-chip’ systems. By combining these experimental approaches, the full potential of exosome biology in mediating exposure effects and biomarkers in toxicology can be realized.
Figure 2. Exosome biogenesis.
Exosome biogenesis begins with endocytosis of the cell membrane via receptor-mediated endocytosis, clathrin-coated pits, pinocytosis, etc. This process creates a membrane-bound vacuole called an endosome. The endosomal membrane contains components of the cell membrane, such as receptors and signaling proteins, which were captured during endocytosis. Endosomes form intra-luminal vesicles (ILV), exosomes, when their membranes undergo subsequent invagination and scission. This process is facilitated by endosomal sorting complex required for transport (ESCRT) proteins or ESCRT-independent pathways, which are mediated by tetraspanins and proteolipids. During ILV formation, proteins and nucleic acids are selectively loaded into vesicles. Endosomes containing ILVs, also called multi-vesicular bodies (MVBs), can then merge with a lysosome and have their contents destroyed or be shuttled to the cell membrane and released.
Highlights.
Exosomes can serve as biomarkers of exposure and response and can mediate disease
In vitro models are ideal for investigating exosome-associated mechanisms of action
Exosomes mediate exposure-linked neurodegeneration, pulmonary disease, and cancer
In vitro models are key to unlocking the potential of exosome biology in toxicology
Acknowledgements
This work was funded in part by grants from the University of Arizona Health Sciences, the Southwest Environmental Health Sciences Center (P30 ES006694), and the National Cancer Institute Cancer Center Support Grant (P30 CA023074).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest. In addition, Drs. Bowers and Ramos filed a patent “Exosome-based Liquid Biopsy” pending.
Contributor Information
Emma C. Bowers, Email: ebowers@email.arizona.edu.
Abeer A.I. Hassanin, Email: drabeer2000@gmail.com.
Kenneth S. Ramos, Email: ksramos@email.arizona.edu.
References
- Agency for Toxic Substances and Disease Registry (ATSDR). 2015. Public Health Statement for Toluene Available: https://www.atsdr.cdc.gov/PHS/PHS.asp?id=159&tid=29. Accessed August 2018.
- Azmi AS, Bao B, and Sarkar FH 2013. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer and Metastasis Reviews. 32, 623–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu Z, & Yáñez-Mó M 2014. Tetraspanins in extracellular vesicle formation and function. Frontiers in immunology. 5, 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala S, Petrasek J, Mundkur S, et al. 2012. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 56, 1946–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batagov AO, & Kurochkin IV 2013. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3’-untranslated regions. Biology direct. 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrami C, Clayton A, Phillips AO, et al. 2012. Analysis of urinary microRNAs in chronic kidney disease. Biochemical Society Transactions. 40, 875–879; DOI: 10.1042/BST20120090 [DOI] [PubMed] [Google Scholar]
- Bianco F, Perrotta C, Novellino L, et al. 2009. Acid sphingomyelinase activity triggers microparticle release from glial cells. The EMBO journal. 28, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolukbasi MF, Mizrak A, Ozdener GB, et al. 2012. miR-1289 and zipcode-like sequence enrich mRNAs in microvesicles. Mol Ther Nucleic Acids. 1:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Luo F, Liu X, et al. 2017. NF-kB-regulated exosomal miR-155 promotes the inflammation associated with arsenite carcinogenesis. Cancer letters. 388, 21–33. [DOI] [PubMed] [Google Scholar]
- Cho YE, Mezey E, Hardwick JP, et al. 2017. Increased ethanol-inducible cytochrome P450–2E1 and cytochrome P450 isoforms in exosomes of alcohol-exposed rodents and patients with alcoholism through oxidative and endoplasmic reticulum stress. Hepatology communications. 1, 675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordazzo C, Petrini S, Neri T, et al. 2014. Rapid shedding of proinflammatory microparticles by human mononuclear cells exposed to cigarette smoke is dependent on Ca2+ mobilization. Inflamm. Res 63, 539–547. [DOI] [PubMed] [Google Scholar]
- de Jong OG, Verhaar MC, Chen Y, et al. 2012. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. Journal of extracellular vesicles. 1, 18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldh M, Ekström K, Valadi H, et al. 2010. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PloS one. 5, e15353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, et al. 2010. Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. Journal of Neuroscience. 30, 6838–6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais S, O’Driscoll L, Borras FE, Buzas E, Camussi G, et al. 2016. Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS nano. 10, 3886–3899. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Araya J, Ito S, et al. 2015. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J. Extracellular Vesicles 4:28388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérin M, Siemiatycki J, Désy M, et al. 1998. Associations between several sites of cancer and occupational exposure to benzene, toluene, xylene, and styrene: Results of a case-control study in Montreal. American journal of industrial medicine. 34, 144–156. [DOI] [PubMed] [Google Scholar]
- Gonda TA, Tu S, & Wang TC 2009. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell cycle. 8, 2005–2013. [DOI] [PubMed] [Google Scholar]
- Guescini M, Guidolin D, Vallorani L, et al. 2010. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Experimental cell research. 316, 1977–1984. [DOI] [PubMed] [Google Scholar]
- György B, Szabó TG, Pásztói, et al. 2011. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cellular and molecular life sciences. 68, 2667–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadla M, Palazzolo S, Corona G, Caligiuri I, Canzonieri V, et al. 2016. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine.11, 2431–2441. [DOI] [PubMed] [Google Scholar]
- Harding C, & Stahl P 1983. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochemical and biophysical research communications. 113, 650–658. [DOI] [PubMed] [Google Scholar]
- Harischandra DS, Ghaisas S, Rokad D, et al. 2017. Exosomes in toxicology: relevance to chemical exposure and pathogenesis of environmentally linked diseases. Toxicological Sciences. 158, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harischandra DS, Ghaisas S, Rokad D, et al. 2018. Environmental neurotoxicant manganese regulates exosome-mediated extracellular miRNAs in cell culture model of Parkinson’s disease: relevance to α-synuclein misfolding in metal neurotoxicity. Neurotoxicology. 64, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iessi E, Logozzi M, Lugini L, Azzarito T, Federici C, et al. 2017. Acridine Orange/exosomes increase the delivery and the effectiveness of Acridine Orange in human melanoma cells: a new prototype for theranostics of tumors. Journal of Enzyme Inhibition and Medicinal Chemistry. 32, 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javeed N, and Mukhopadhyay D 2017. Exosomes and their role in the micro-/macro-environment: A comprehensive review. Journal of biomedical research. 31, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston DE 1999. Special considerations in interpreting liver function tests. American family physician. 59, 2223–2230. [PubMed] [Google Scholar]
- Kahlert C, Melo SA, Protopopov A, et al. 2014. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. Journal of Biological Chemistry. 289, 3869–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, et al. 2018. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine: Nanotechnology, Biology and Medicine. 14, 195–204. [DOI] [PubMed] [Google Scholar]
- Kulkarni S, Koller A, Mani KM, et al. , E. I 2016. Identifying urinary and serum exosome biomarkers for radiation exposure using a data dependent acquisition and SWATH-MS combined workflow. International Journal of Radiation Oncology* Biology* Physics. 3, 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lener T, Gimona M, Aigner L, Börger V, Buzas E, et al. 2015. Applying extracellular vesicles based therapeutics in clinical trials-an ISEV position paper. Journal of extracellular vesicles. 4, 30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, El Andaloussi S, & Wood MJ 2012. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Human molecular genetics. 21, R125–R134. [DOI] [PubMed] [Google Scholar]
- Lim JH, Song MK, Cho Y, et al. 2017. Comparative analysis of microRNA and mRNA expression profiles in cells and exosomes under toluene exposure. Toxicology in Vitro. 41, 92–101. [DOI] [PubMed] [Google Scholar]
- Moon HG, Kim SH, Gao J, et al. 2014. CCN1 secretion and cleavage regulate the lung epithelial cell functions after cigarette smoke. American Journal of Physiology-Lung Cellular and Molecular Physiology. 307, L326–L337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P, Lam YW, Dragon J, et al. 2018. Exosomes from asbestos-exposed cells modulate gene expression in mesothelial cells. The FASEB Journal, fj-201701291RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Kondo C, Morikawa Y, et al. 2015. Plasma miR-208 as a useful biomarker for drug-induced cardiotoxicity in rats. J Appl Toxicol. 35,173–80 [DOI] [PubMed] [Google Scholar]
- Ohno SI, Takanashi M, Sudo K, Ueda S, Ishikawa A, et al. 2013. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Molecular Therapy. 21, 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BT, & Johnstone RM 1983. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 33, 967–978. [DOI] [PubMed] [Google Scholar]
- Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, et al. 2014. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. Journal of Controlled Release. 192, 262–70. [DOI] [PubMed] [Google Scholar]
- Pavkovic M, Riefke B, Ellinger-Ziegelbauer H. 2014. Urinary microRNA profiling for identification of biomarkers after cisplatin-induced kidney injury. Toxicology. 324, 147–57 [DOI] [PubMed] [Google Scholar]
- Record M, Carayon K, Poirot M, et al. 2014. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 1841, 108–120. [DOI] [PubMed] [Google Scholar]
- Saari H, Lisitsyna E, Rautaniemi K, Rojalin T, Niemi L, et al. 2018. FLIM reveals alternative EV-mediated cellular up-take pathways of paclitaxel. Journal of Controlled Release. 284, 133–143. [DOI] [PubMed] [Google Scholar]
- Statello L, Maugeri M, Garre E et al. 2018. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PloS one. 13, e0195969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss K, Goebel C, Runz H, et al. 2010. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick typeC disease. J Biol Chem. 285, 26279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, et al. 2010. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Molecular Therapy. 18, 1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, & Momen-Heravi F 2017. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nature Reviews Gastroenterology & Hepatology. 14, 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K, Zhang Y, Zhang H, Xu P, Liu J, et al. 2012. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nature communications. 3, 1282. [DOI] [PubMed] [Google Scholar]
- Tauro BJ, Greening DW, Mathias RA, et al. 2013. Two distinct populations of exosomes are released from LIM1863 colon carcinomacell-derived organoids. Mol Cell Proteomics. 12, 587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur BK, Zhang H, Becker A, et al. 2014. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell research. 24, 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Boussac M, Véron P, et al. 2001. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. The Journal of Immunology. 166, 7309–7318 [DOI] [PubMed] [Google Scholar]
- Théry C, Zitvogel L, Amigorena S 2002. Exosomes: composition, biogenesis and function. Nature Reviews Immunology. 2, 569. [DOI] [PubMed] [Google Scholar]
- Théry C, Ostrowski M, & Segura E 2009. Membrane vesicles as conveyors of immune responses. Nature reviews immunology. 9, 581. [DOI] [PubMed] [Google Scholar]
- Tian T, Zhang HX, He CP, Fan S, Zhu YL, et al. 2018. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 150, 137–149. [DOI] [PubMed] [Google Scholar]
- Tian Y, Li S, Song J, Ji T, Zhu M, et al. 2014. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 35, 2383–2390. [DOI] [PubMed] [Google Scholar]
- Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endo-somes. 2008. Science. 319, 1244–7. [DOI] [PubMed] [Google Scholar]
- Toffoli G, Hadla M, Corona G, Caligiuri I, Palazzolo S, et al. 2015. Exosomal doxorubicin reduces the cardiac toxicity of doxorubicin. Nanomedicine. 10, 2963–2971. [DOI] [PubMed] [Google Scholar]
- Trams EG, Lauter CJ, Salem N Jr, et al. 1981. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 645:63–70 [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, et al. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 9, 654. [DOI] [PubMed] [Google Scholar]
- Verweij FJ, van Eijndhoven MA, Hopmans ES, et al. 2011. LMP1 association with CD63 in endosomes andsecretion via exosomes limits constitutive NF-kappaB activation. EMBO. J2011 30, 2115–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C, Baixauli F, Gutiérrez-Vázquez C, et al. 2014. Sorting it out: regulation of exosome loading. Seminars in cancer biology. 28, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, et al. 2013. Sumoylated hnRNPA2B1 controls thesorting of miRNAs into exosomes through binding to specific motifs. NatCommun;4:2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlund CJ, Eklund A, Grunewald J, et al. 2017. Pulmonary extracellular vesicles as mediators of local and systemic inflammation. Frontiers in cell and developmental biology. 5, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yeung BZ, Cui M, Peer CJ, Lu Z, et al. 2017. Exosome is a mechanism of intercellular drug transfer: Application of quantitative pharmacology. Journal of Controlled Release. 268, 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T, Huang X, Woodcock M, et al. 2016. Plasma extracellular RNA profiles in healthy and cancer patients. Scientific reports. 6, 19413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama K, Sun H, Mitsutake S, Igarashi Y. 2012. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J Biol Chem. 287, 10977–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, & McBride H 2001. Rab proteins as membrane organizers. Nature reviews Molecular cell biology. 2, 107. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yuan X, Shi H, Wu L, Qian H, and Xu W 2015. Exosomes in cancer: small particle, big player. Journal of hematology & oncology. 8, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Cheruvanky A, Hu X, et al. 2008. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney international. 74, 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Pisitkun T, Aponte A, et al. 2006. Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney international. 70, 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Li Y, Shi J, et al. 2012. Exosomes as extrapulmonary signaling conveyors for nanoparticle-induced systemic immune activation. Small. 8, 404–412. [DOI] [PubMed] [Google Scholar]