Abstract
CXCR5 mediates homing of both B and follicular helper T (TFH) cells into follicles of secondary lymphoid organs. We found that CXCR5+CD8+ T cells are present in human tonsils and follicular lymphoma, inhibit TFH-mediated B-cell differentiation, and exhibit strong cytotoxic activity. Consistent with these findings, adoptive transfer of CXCR5+CD8+ T cells into an animal model of lymphoma resulted in significantly greater antitumor activity than CXCR5−CD8+ T cells. Furthermore, RNA-Seq-based transcriptional profiling revealed a 77-gene signature unique to CXCR5+CD8+ T cells. The upregulated 33 genes among the 77-gene signature correlated with improved survival in follicular lymphoma patients. We also showed that CXCR5+CD8+ T cells could be induced and expanded ex vivo using IL-23 plus TGF-β, suggesting a possible strategy to generate these cells for clinical application. In summary, our study identified CXCR5+CD8+ T cells as a distinct T-cell subset with ability to suppress TFH-mediated B-cell differentiation, exert strong antitumor activity, and confer favorable prognosis in follicular lymphoma patients.
INTRODUCTION
The differential expression of chemokine receptors on T cells has been associated with their tissue migration and functional polarization.1–5 For example, CXCR5 on follicular helper T cells (TFH) mediates their homing to B-cell follicles, where they provide cognate help to support antigen-driven B cell clonal expansion and somatic hypermutation.6 Follicular B cells in turn promote the maintenance of TFH cells via ICOS-ICOS ligand interaction7, 8, highlighting the importance of the reciprocal crosstalk between T and B cells. CXCL13 has been shown to be the major chemoattractant that directs follicular homing of B cells and TFH cells that express its specific receptor CXCR5.9, 10 Under homeostatic conditions, CXCL13 is preferentially enriched within B-cell follicles of secondary lymphoid organs in mouse and human,11 mainly produced by follicular stromal cells including follicular dendritic cells.12 CXCR5-expressing CD8+ T cells have also been reported to reside in B-cell follicles of human tonsils and likely migrate in response to CXCL13.13
Recently, CXCR5+CD8+ follicular cytotoxic T cells (TFC) have been shown to express high levels of TCF1 and play a major role in controlling chronic LCMV viral infections in animal models.14–17 However, the coexpression of TCF1 and CXCR5 was not evident in tumor-infiltrating T lymphocytes in mouse fibrosarcoma tumor and human melanoma samples,17 suggesting that CXCR5 expression in CD8+ T cells may be differentially regulated in different disease settings. In addition, the frequency of CXCR5+CD8+ T cells in peripheral blood was inversely associated with viral load in HIV patients.16 The immunophenotypic features and transcriptional signatures of the mouse CXCR5+CD8+ T cells were similar to TFH cells, early effector memory precursors, and “exhausted” T cells.14–16 CXCR5+CD8+ T cells have also been shown to be essential for the maintenance of self-tolerance via their regulatory function on TFH activities.18 Whether CXCR5+CD8+ T cells play a role in human cancers is unclear.
In this study, we investigated the role of CXCR5+CD8+ T cells in human follicular lymphoma, the most common indolent non-Hodgkin lymphoma derived from germinal center (GC) B cells. Follicular lymphoma is characterized by aberrant accumulation of malignant GC B cells, mainly caused by overexpression of B cell leukemia/lymphoma 2 (BCL-2) along with other genetic abnormalities.19 The tumor microenvironment of follicular lymphoma consists of a variety of nonmalignant immune cells, including different T cell subsets (TFH, regulatory T cells, CD8+ T cells), macrophages, and follicular dendritic cells, all of which likely impact its pathogenesis and natural history.19–21 Here, we found that CXCR5+CD8+ T cells are more abundant in follicular lymphoma tumors compared with control tonsil samples. CXCR5+CD8+ T cells exhibited high cytotoxic activity, as evidenced by increased expression of IFN-γ, TNF-α, and granzymes, and displayed antitumor efficacy in vitro against human follicular lymphoma cells and in an experimental model of lymphoma. Moreover, they suppressed TFH function. Consistent with this, the gene signature of CXCR5+CD8+ T cells was positively associated with overall survival in follicular lymphoma patients. Together, our results suggest that CXCR5+CD8+ T cells play an important role in the control of human follicular lymphoma.
MATERIALS AND METHODS
Detailed materials and methods included in online supplemental materials.
RESULTS
Frequency and localization of CXCR5+CD8+ T cells
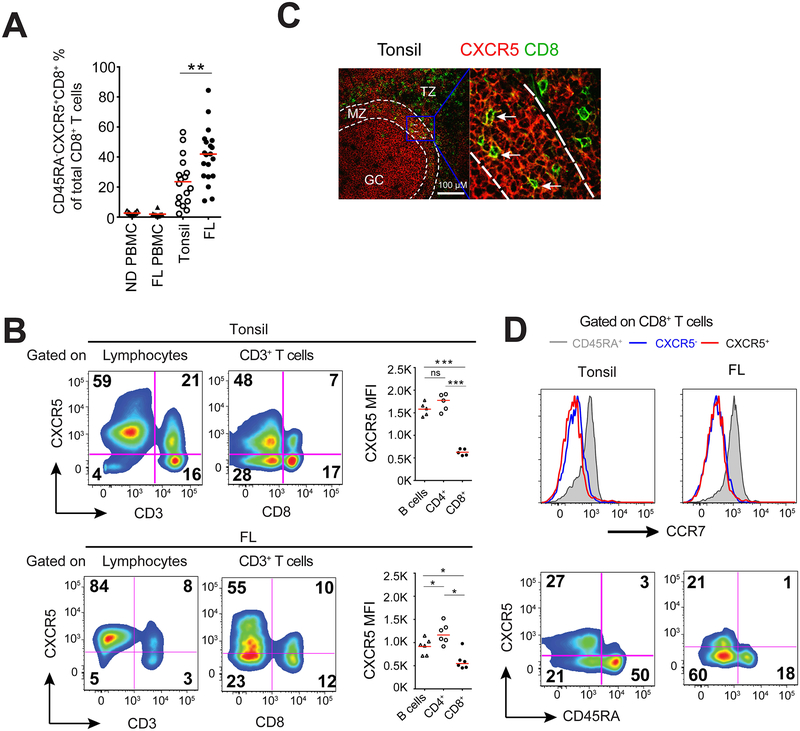
Flow cytometric analysis of single cell suspensions of human follicular lymphoma tumors and tonsils revealed that the proportion of CXCR5+ T cells among CD8+ T cells was approximately 1.5-fold higher in follicular lymphoma specimens compared with those in tonsils (40.96 ± 18.11 % vs. 23.51 ± 16.01, p<0.01) (Figure 1a). Due to the differences in age between tonsil tissues (mostly from children) and follicular lymphoma (median age = 60), we cannot exclude the possibility that these differences are age-dependent. In contrast, CXCR5+CD8+ T cells were barely detectable in peripheral blood mononuclear cells (PBMCs) from both follicular lymphoma patients and healthy donors (Figure 1a), results consistent with previous reports in mice.13–16
Figure 1.
CXCR5+CD8+ T cells in human tonsils and follicular lymphoma. (a) Percentages of CD45RA−CXCR5+CD8+ T cells among total CD8+ T cells as determined by flow cytometry in peripheral blood mononuclear cells (PBMC) from normal donors (ND; n=7) and patients with follicular lymphoma (FL; n=10), as well as single cell suspensions of normal human tonsils (n=17) and lymph node biopsies from patients with FL (n=20). Bars represent median. (b) CXCR5 expression on different lymphocyte subsets (CD3− lymphocytes (mostly B cells), CD3+ T cells and CD3+CD8+ and CD3+CD8−(CD4+) T cells) in human tonsil and follicular lymphoma samples. Representative pseudo-color plots and mean fluorescent intensity (MFI) of CXCR5 expression in different lymphocyte subsets are shown. Bars represent median. (c) Confocal microscopic analysis of CD8 (green) and CXCR5 (red) expression in representative tonsil sample. White broken lines indicate the borders between T cell zone (TZ), mantle zone (MZ), and germinal center (GC) in B cell follicle. Arrows indicate CXCR5+CD8+ T cells. Inset (right) shows magnification (60×) of the area outlined in the main image (left). Scale bar, 100 μM. (d) CD45RA (bottom) and CCR7 (top) expression on CD8+ T cell subsets (CD45RA+CXCR5−CD8+, CD45RA−CXCR5−CD8+, CD45RA−CXCR5+CD8+) in tonsil and follicular lymphoma samples. Results are representative of 5 independent samples each.
CXCR5 is required for lymphocytes to enter B-cell follicles, and the density of CXCR5 expression may determine the migration depth and/or residency of the lymphocytes within the follicle. In tonsils and follicular lymphoma samples, the proportion of CXCR5+ cells and CXCR5 fluorescence intensity in CD8+ T cells are much lower than in B cells and CD4+ T cells (Figure 1b). Interestingly, confocal imaging analysis revealed co-localization of CXCR5 and CD8 markers in the mantle zone (MZ), but not in B-cell follicles in tonsil tissues (Figure 1c). Similar findings were reported for mice with chronic viral infections, in which CXCR5+CD8+ T cells were observed in the outer layer of B-cell follicles in lymph nodes and spleens; however, those studies lacked precise subzone analysis.14–16 Of note, most CXCR5+CD8+ T cells are CD45RA− and CCR7− in follicular lymphoma and tonsil samples (Figure 1b and d), indicating an effector/memory phenotype similar to those observed in CXCR5+ TFH22, 23 and previous studies.13 Collectively, these results suggest that CXCR5 expression on a subset of CD8 T cells is associated with their migration ability.
Phenotypic characterization and transcriptional profiling of CXCR5+CD8+ T cells
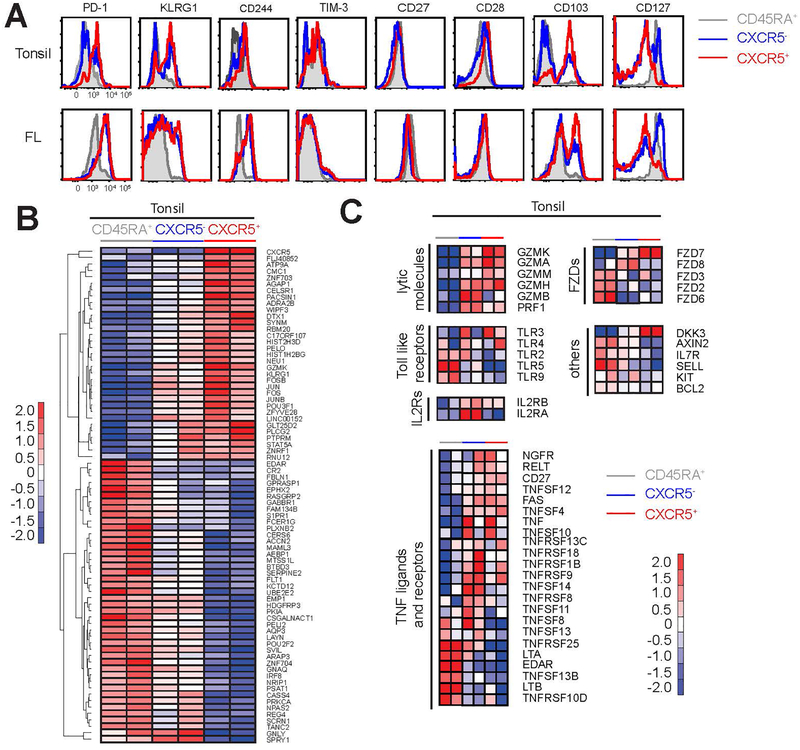
To determine the functional differences among the three subsets of tonsillar CD8+ T cells (CD45RA+CXCR5−, CD45RA−CXCR5− and CD45RA−CXCR5+), we performed phenotypic and transcriptional profiling in both tonsil and follicular lymphoma samples. Immunophenotypic analyses revealed that CXCR5+CD8+ T cells contain a larger proportion of cells that express inhibitory molecules associated with T cell “exhaustion”,24–26 such as PD-1 (PDCD1), killer cell lectin-like receptor G1 (KLRG1), CD244, and Tim-3, than their CXCR5− counterparts (Figure 2a upper panel). These cells also expressed higher levels of markers associated with memory T cell development, such as CD27, CD28, and CD103; but reduced amount of CD127. These data collectively suggested that CXCR5+CD8+ T cells might represent a subset of effector cells that have acquired certain memory T cell characteristics. These results are consistent with transcriptional profiling and phenotypic analysis of murine CXCR5+CD8+ T cells that suggested that these cells are closely related to early effector memory or memory precursor cells.13, 14 This phenotypic profile, however, was not observed in similarly examined follicular lymphoma specimens, presumably because of altered microenvironmental triggers in follicular lymphoma tumors (Figure 2a lower panel).
Figure 2.
Expression of activation markers and transcriptional profile of CXCR5+CD8+ T cells. (a) Expression of activation markers was determined by flow cytometry on CD8+ T cell subsets in tonsil and follicular lymphoma samples. Representative histograms overlays of CD45RA+CXCR5−CD8+ (grey), CD45RA−CXCR5−CD8+ T cells (blue), and CD45RA−CXCR5+CD8+ (red) are shown. (b) CD45RA+CXCR5−CD8+, CD45RA−CXCR5−CD8+, and CD45RA−CXCR5+CD8+ T cell subsets were sorted by flow cytometry from two different tonsil samples and RNA-Seq was performed. Heat map of 77 (33 upregulated and 44 downregulated) differentially expressed genes in CXCR5+ cells versus other two CD8+ T cell subsets is shown. (c) Heat maps showing relative expression of selected genes of interest in the three different CD8+ T cell subsets from tonsil samples.
Identification of a gene signature of CXCR5+CD8+ T cells
To further identify genes that distinguish CXCR5+CD8+ T cells from other CD8+ T cell subsets, we performed RNA-Seq analyses in the three subsets purified from tonsil samples. Relative to CD45RA+CD8+ T cells, CXCR5−CD8+ T cells express 484 upregulated genes and 239 downregulated genes, while CXCR5+CD8+ T cells express 820 upregulated genes and 594 downregulated genes (Supplementary Figure 1a). After exclusion of similarly regulated genes in both subsets, we defined 788 (411 upregulated and 377 downregulated) and 97 (75 upregulated and 22 downregulated) genes that are differentially expressed in CXCR5+CD8+ T cells and their CXCR5− counterparts, respectively (Supplementary Figure 1b–d). When the cutoff value is set as 4-fold change, 77 (33 upregulated and 44 downregulated) genes were selected among the 788 genes as signature genes for CXCR5+CD8+ T cells (Supplementary Figure 1b and Figure 2b). Gene Set Enrichment Analysis (GSEA) showed that this 77-gene signature was associated with enrichment of G-protein coupled receptor protein signaling, TLR signaling, and Wnt/Catenin pathways among others (Supplementary Figure 1e). Of note, CXCR5+CD8+ cells were more similar to TFH cells than CXCR5−CD8+ cells by GSEA (Supplementary Figure 1e). The striking differences between CXCR5− and CXCR5+ subsets are that the latter are more enriched in genes involved in cell mobility and viability, synapse formation, cytoskeleton transport, and cytotoxic capacity than CXCR5− cells (Supplementary Figure 1e). Interestingly, we also observed higher expression levels of Jun, Fos, JunB and FosB in CXCR5+ cells compared with CXCR5− cells (Figure 2b). These transcription factor subunits dimerize to form activating protein 1 (AP-1), which is an upstream transcription factor that regulates differentiation and functions of effector cells including induction of cytokines and cytotoxic molecules.27–29 We also found that the two CD8+ T cells subsets showed differences in the expression of effector molecules such as granzymes and perforin, cytokine receptors, TLRs, and members of the tumor necrosis factor (TNF) superfamily and frizzled (FZD) family (Figure 2c). High levels of AP transcription factor subunits (Jun, Fos, JunB and FosB) and effector proteins also likely imply that CXCR5+CD8+ cells play an active role against infection and tumor.30
CXCR5+CD8+ T cells are transcriptionally distinct
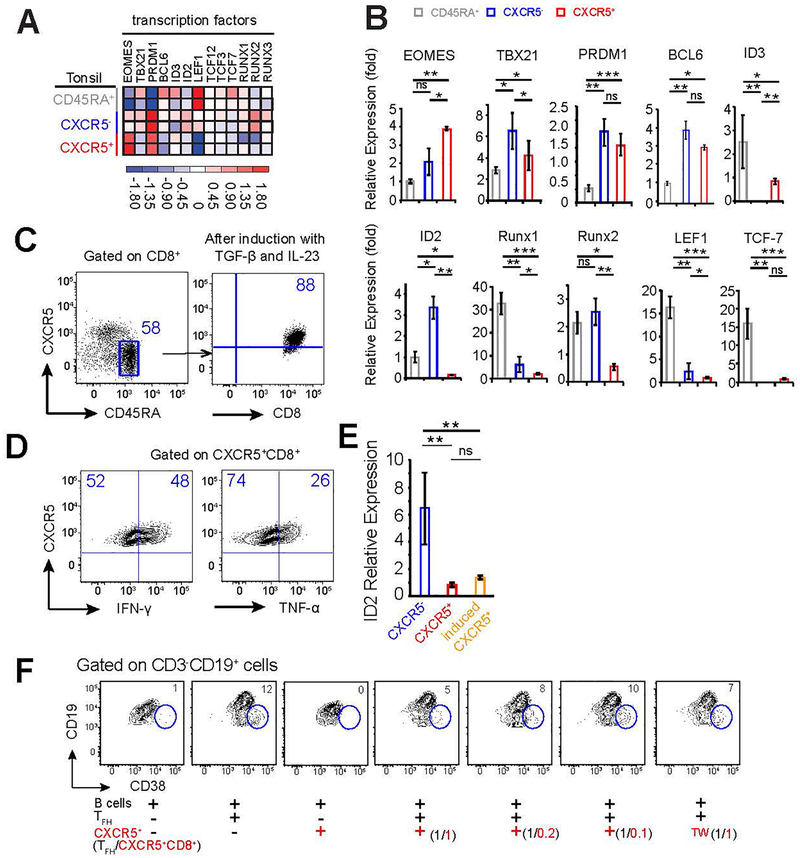
Analysis of the RNA-Seq data for transcription factors associated with development and function of CD8+ T cells showed that CXCR5+CD8+ T cells expressed higher levels of EOMES and ID3, but lower levels of TBX21 (T-bet), BCL6, ID2, LEF1, RUNX1, and RUNX2 compared to CXCR5−CD8+ T cells (Figure 3a). The differential expression of EOMES, TBX21, LEF1, ID2, and RUNX2 was confirmed by quantitative PCR analysis (Figure 3b) and probes used were listed in Table 1. The E-protein and ID protein regulatory axis is important for memory T cell generation and CXCR5 expression in both CD4+ and CD8+ T cells.16, 31 We found that CXCR5+CD8+ and CXCR5−CD8+ T cells express similar levels of ID3, E2A (encoded by Tcf3), and HEB (encoded by Tcf12), but ID2 amounts are significantly lower in the former population (Figure 3a), consistent with findings in murine models.16 Wnt signaling molecules LEF-1 (encoded by Lef1) and TCF-1 (encoded by Tcf7), and RUNX proteins have been known to regulate T-cell fate.32–35 We found that LEF-1 is differentially expressed in the three T-cell subsets, with CD45RA+ being the highest, CXCR5− being intermediate, and CXCR5+ being the lowest (Figure 3a and b). Unlike previously reported results in mouse studies,14, 15 Tcf7 transcripts are similarly expressed at lower level in human CXCR5+ and CXCR5− T cells, in comparison to CD45RA+ population (Figure 3a). RUNX proteins (1–3) are expressed at lower levels in CXCR5+ than those in CXCR5− counterpart (Figure 3a) and it was recently reported that Runx3-mediated Tcf7 repression coordinately enforced acquisition of cytotoxic functions and protected the cytotoxic lineage integrity by preventing TFH-lineage differentiation.36 It is unclear, however, whether the RUNX proteins are involved directly in regulating CXCR5+CD8+ T cell generation.
Figure 3.
Expression of transcription factors and induction of CXCR5+CD8+ T cells. (a) Heat map illustrating the relative expression of key transcription factors from RNA-Seq data in CD45RA+CXCR5−CD8+, CD45RA−CXCR5−CD8+, and CD45RA−CXCR5+CD8+ T cell subsets from tonsil samples. (b) mRNA expression of transcription factors determined by quantitative PCR in the three CD8+ T cell subsets. Experiments were repeated at least three times. (c) CD45RA+CXCR5−CD8+ T cells (left plot) were sorted from human tonsils and stimulated with anti-CD3 and anti-CD28 antibodies, and cultured with TGF-β and IL-23 for 2–3 days. Surface expression of CXCR5 was determined by flow cytometry (right plot). (d) CXCR5+CD8+ T cells were induced as described in (c), and stimulated with PMA/Ionomycin for 5 hours with Brefeldin A added for last 3 hours. Production of IFN-γ and TNF-α was determined by intracellular staining and flow cytometry. (e) ID2 expression was determined by qPCR in CD45RA−CXCR5−CD8+ (blue) and CD45RA−CXCR5+CD8+ T cells (red) sorted from human tonsil and CD45RA−CXCR5+CD8+ T cells (orange) induced in vitro as in (c). Data represents 3 independent experiments. (f) Memory B cells sorted from human tonsils were stimulated with SEB and co-cultured in the presence or absence of sorted autologous TFH and/or CXCR5+CD8+ T cells. Differentiation of memory B cells into CD38+CD19int plasmablast cells was determined by flow cytometry after 9 days of co-culture. TW (transwell) indicates cells in the transwell chamber. Ratios of TFH:CXCR5+CD8+ T cells are shown in parentheses. Representative data from one of 6 independent experiments is shown.
Table 1.
Primers used for quantitative PCR.
| Gene | Primer sequence |
|---|---|
| Eomes sense | 5’-CTCTGTGGCTCAAATTCCAC-3’ |
| Eomes anti-sense | 5’-TGGGATTGAGTCCGTTTATG-3’ |
| Tbx21 sense | 5’-GTGACCCAGATGATTGTGCT-3’ |
| Tbx21 anti-sense | 5’-ATATGCGTGTTGGAAGCGT-3’ |
| Prdm1 sense | 5’-TACCTGGTACACACGGGAGA-3’ |
| Prdm1 anti-sense | 5’-GAGATTGCTGGTGCTGCTAA-3’ |
| Bcl6 sense | 5’-ACCCACAGTGACAAACCCTAC-3’ |
| Bcl6 anti-sense | 5’-GGTTTCTCACCGGTATGGAC-3’ |
| Id2 sense | 5’-CAACACGGATATCAGCATCC-3’ |
| Id2 anti-sense | 5’-CACACAGTGCTTTGCTGTCA-3’ |
| Id3 sense | 5’-CGCGTCATCGACTACATTCT-3’ |
| Id3 anti-sense | 5’-GATGACAAGTTCCGGAGTGA-3’ |
| Runx1 sense | 5’-CCGCAGCCATGAAGAACCAG-3’ |
| Runx1 anti-sense | 5’-TCTGCCGATGTCTTCGAGGT-3’ |
| Runx2 sense | 5’-CAGTAGATGGACCTCGGGAA-3’ |
| Runx2 anti-sense | 5’-CCTAAATCACTGAGGCGGTC-3’ |
| Lef1 sense | 5’-ACAGATCACCCCACCTCTTG-3’ |
| Lef1 anti-sense | 5’-TGAGGCTTCACGTGCATTAG-3’ |
| Tcf7 sense | 5’-TCTGCTCATGCATTACCCAC-3’ |
| Tcf7 anti-sense | 5’-AGAGAGAGAGTTGGGGGACA-3’ |
| Gzmh sense | 5’-TCTCAGGCTACCTAGCAGCA-3’ |
| Gzmh anti-sense | 5’-CTTCTGCACTGTCAGCAACA-3’ |
TGF-β induction of CXCR5 expression in CD8+ T cells
Downregulation of ID2 is essential for the generation of murine CXCR5+CD8+ T cells during chronic viral infection.16 ID2 transcription is induced by cytokines IL-2, IL-12 or IL-21 in T cells, and repressed by TGF-β in epithelial cells.31, 37 Furthermore, addition of TGF-β and IL-23 to human naïve CD4+T cells, following priming with anti-CD3/CD28 efficiently promoted the generation of CXCR5+ TFH-like cells ex-vivo.38 Using similar experimental settings, we tested whether TGF-β and IL-23 can facilitate CXCR5+CD8+ T cell differentiation from CD45RA+CXCR5−CD8+ T cells. After three days, the majority (> 80%) of cells in the culture showed elevated CXCR5 expression (Figure 3c). We also observed elevated CXCR5 expression in sorted naïve CD4+ T cell subset (in the same conditions), which is consistent with previous studies (Supplementary Figure 3b).38 The induced CXCR5+CD8+ T cells are functional, as evidenced by their secretion of IFN-γ and TNF-α upon stimulation with PMA/Ionomycin (Figure 3d). Furthermore, we found that ID2 expression was significantly lower in primary and induced CXCR5+CD8+ T cells compared to CXCR5−CD8+ T cells (Figure 3e), indicating downregulation of ID2 may be essential for the upregulation of CXCR5 expression.
Inhibition of Tfh cells by CXCR5+CD8+ T cells
Follicle-resident TFH cells have been suggested to promote immune suppression and tumor growth in human follicular lymphoma via expression of IL-4 and CD40L.39 Due to the anatomic proximity of the two CXCR5+ T cell subsets, we evaluated whether CXCR5+CD8+ T cells affect TFH function. In a co-culture assay with B cells, TFH, and CXCR5+CD8+ T cells, we found that CXCR5+CD8+ T cells inhibited TFH-dependent CD38+CD19int plasmablast cell differentiation in a dose-dependent manner (Figure 3f). Transwell assays showed that CXCR5+CD8+ T cells inhibited TFH function in a cell-cell contact independent manner (Figure 3f). However, it is unknown which soluble factors mediate this function. These results provide novel insights into the cross talk among TFH, CXCR5+CD8+ T cells, and malignant B-cells, and highlight a potential regulatory network in normal lymphoid tissues and in the pathogenesis of follicular lymphoma.
Cytotoxic function of CXCR5+CD8+ T cells
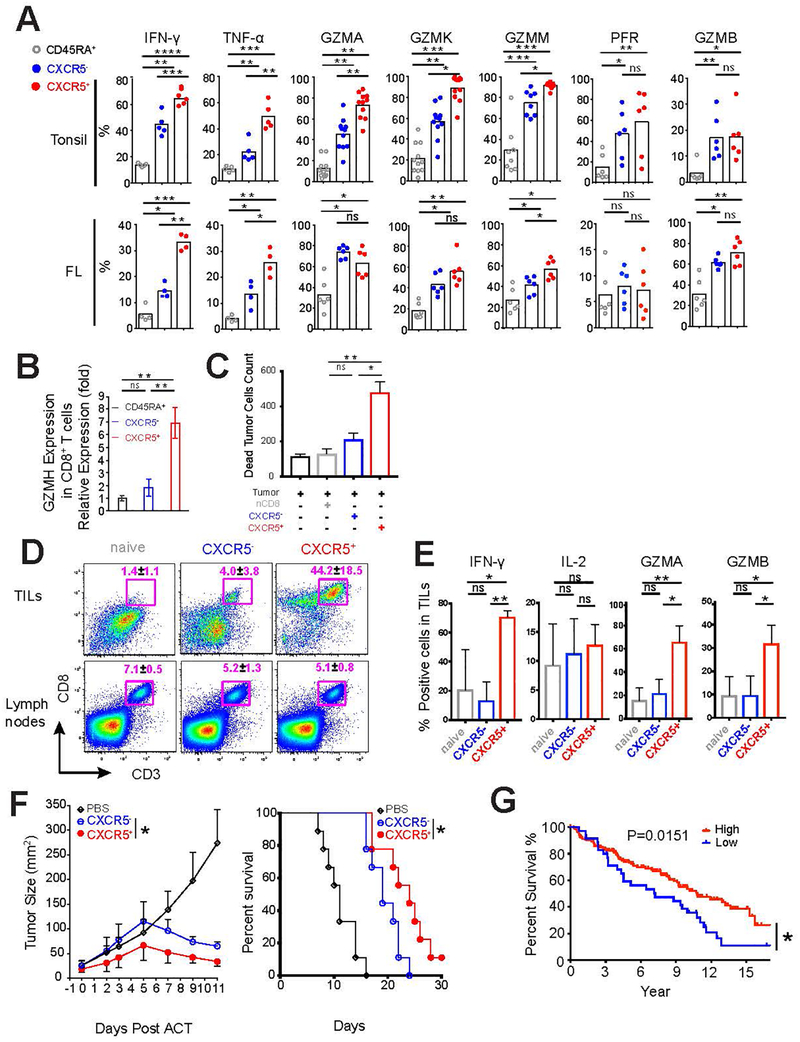
Upon stimulation with PMA and ionomycin, both tonsillar and follicular lymphoma CXCR5+CD8+ T cells produced markedly more effector molecules such as granzymes, perforin, IFN-γ and TNF-α compared with CD45RA+CD8+ and/or CXCR5−CD8+ T cells (Figure 4a and b). In particular, tonsillar CXCR5+CD8+ T cells expressed high levels of granzymes A/K/M/H, but low levels of perforin and granzyme B, compared with CXCR5−CD8+ T cells (Figure 4a and b). The increased abundance of effector proteins in CXCR5+CD8+ T cells correlates well with the elevated expression of EOMES and decreased T-bet (Supplementary Figure 2a–c).40–43 Of note, CXCR5+ compartment in follicular lymphoma showed similar expression of granzymes A/B/K and perforin as CXCR5−CD8+ T cells, which is significantly lower than those in tonsillar CD8+ T cells (Figure 4a and b). These results indicate that unlike tonsillar CXCR5+CD8+ T cells, follicular lymphoma CXCR5+CD8+ T cells may have impaired cytotoxic capability. The functional distinction between marker-defined cell populations isolated from tonsil versus follicular lymphoma has been reported previously, which demonstrated that TFH cells isolated from follicular lymphoma produce distinct effector molecules than normal follicular TFH cells, although, they both support B cell survival and activation.20
Figure 4.
Functional features of CXCR5+CD8+ T cells in human tonsil and follicular lymphoma samples. (a) Intracellular expression of cytokines, granzymes, and perforin were determined in the three CD8+ T cell subsets (CD45RA+CXCR5−CD8+, CD45RA−CXCR5−CD8+, and CD45RA−CXCR5+CD8+) isolated from human tonsils (n=5–11) and follicular lymphoma (n=4–6) after stimulation with PMA/Ionomycin as in figure 3 d. (b) Granzyme H expression in the three CD8+ T cell subsets of human tonsils was analyzed by qPCR. Results are representative of three independent experiments. (c-d) Purified CD45RA+CXCR5−CD8+ T cells from follicular lymphoma samples were activated as described in Figure 3 c and sorted based on CXCR5 expression. Sorted T cell subsets were then co-cultured with autologous tumor cells at the indicated effector : target (E:T) ratios as shown in Figure S3 a. The absolute number (left panel) and percentage (right panel) of CD107a/b+ subset in CXCR5−CD8+ and CXCR5+CD8+ T cells are shown (c). Absolute numbers of dead tumor cells were determined using counting beads, and values are shown for E:T ratio of 10:1 (d). Results are representative of 3 independent experiments (c-d). (e-g) C57BL/6 mice injected with EG7-OVA cells on day 8 were treated with the indicated CD8+ T cell subsets on day 7. The abundance of CD3+CD8+ T cells (e), their expression of IFN- γ, IL-2, granzyme A and B (f) in tumor (TILs) and tumor draining lymph nodes were analyzed. Tumor sizes (left) and survival (right) were monitored (g). Summary data from two independent experiments is shown. (h) Kaplan-Meier curves for overall survival in patients with follicular lymphoma with low (< 25 percentile) or high expression (≥ 25 percentile) of upregulated CXCR5+CD8+ T cell signature genes are shown. P value was calculated using log-rank test.
Antitumor function of CXCR5+CD8+ T cells in vivo
Studies in mice have demonstrated that CXCR5+CD8+ T cells constitute a memory reservoir for effector cells in the control of viral replications during chronic viral infection and the mechanisms may involve increased secretion of effector proteins14 and/or limiting the abundance of virally infected TFH and B cells.15 To directly test the tumor-killing ability of CXCR5+CD8+ T cells, we purified follicular lymphoma-infiltrating CD45RA+CXCR5− T cells and expanded them ex-vivo with TGF-β and IL-23 (as described in Figure 3c and Supplementary figure 3a and b). In a co-culture assay with autologous primary follicular lymphoma tumor cells, we observed that CXCR5+CD8+ T cells expressed increased CD107a, a functional marker for CD8 T-cell degranulation,44 and mediated increased tumor killing as compared to CXCR5−CD8+ T cells (Figure 4c, 4d and Supplementary Figure 3a).
To assess CXCR5+CD8+ T cell function in vivo, we employed a syngeneic mouse EG7-OVA T-cell lymphoma model. Briefly, ovalbumin-primed OT1-specific CXCR5+ and CXCR5− CD8+ T cells were sorted by flow cytometry and adoptively transferred into EG7-OVA tumor-bearing TCR−/− mice. We confirmed that immunization with ovalbumin complete Freund’s adjuvant stimulated the generation of CXCR5+ and CXCR5−CD8+ T cells (Supplementary Figure 3c). Seven days following adoptive transfer, we observed dramatically increased accumulation of CXCR5+CD8+ T cells with higher proliferative index at the tumor site compared to naïve or CXCR5−CD8+ T cell subsets (Figure 4e and Supplementary Figure 3d upper panel). Of note, the three T cell subsets showed similar proliferation capability in tumor-draining lymph nodes (Supplementary Figure 3d lower panel). Collectively, our data indicate that highly proliferative CXCR5+CD8+ T cells may have increased ability to migrate into the tumor sites. In agreement with our ex-vivo findings with human T cells (Figure 4c and 4d), adoptively transferred CXCR5+CD8+ T cells expressed higher levels of IFN-γ, and granzymes A and B, compared to naïve or CXCR5− counterparts (Figure 4f). As a consequence, CXCR5+CD8+ T cells showed significantly enhanced antitumor effects than CXCR5−CD8+ T cells, as evidenced by delayed tumor growth and improved survival (Figure 4g). Consistent with previous reports, we found that adoptively transferred CXCR5+ T cells could lose their surface CXCR5 expression following in vivo proliferation, indicating CXCR5 expression is dynamic during T cell activation and differentiation (Figure 4e, 4f and Supplementary Figure 3d).14, 16 Collectively, our data suggest that CXCR5+CD8+ T cells have augmented tumor-control capability, which may result from both efficient migration to tumor site and enhanced cytotoxic function.
CXCR5+CD8+ T cells gene signature associated with favorable clinical outcome
To determine whether CXCR5+CD8+ T cells affect clinical outcome in human cancers, we examined the correlation of the CXCR5+CD8+ T cell gene signature with overall survival using a publicly available gene expression profiling dataset of 187 follicular lymphoma patients that were treated largely with chemotherapy alone.21 Patients were divided into two groups based on the overall expression level of upregulated CXCR5+CD8+ signature genes. We identified 35 cases in the low expression group (<25 percentile) and 152 cases in the high expression group (≥ 25 percentile) (Supplementary Figure 3e). We found that patients in the high expression group had significantly better overall survival compared to those in the low expression group (Figure 4h), suggesting that abundance of CXCR5+CD8+ T cells in follicular lymphoma correlates with favorable clinical outcome. Similar analyses were performed with downregulated CXCR5+CD8+ signature genes, but no correlation with patients’ survival was evident. Prior studies by various groups have shown that gene signatures of immune cell subsets derived from healthy donor blood or tonsillar tissues may be used for analysis of tumor tissues including follicular lymphoma and other malignancies.21, 45–47 Consistent with these reports and our in vitro observations, we found that the gene signature of CXCR5+CD8+ T cells was able to distinguish follicular lymphoma tumor samples into low and high expression groups and had prognostic significance in these patients. Taken together, these in vitro and in vivo results suggest that CXCR5+CD8+ T cells are effective against tumor.
DISCUSSION
In this study, we demonstrated the phenotype, transcription and function of CXCR5+CD8+ T cells in tonsil and follicular lymphoma samples. Relatively little has previously been published about CXCR5+CD8+ T cells in lymphoma, but a previous study found ICOS+CXCR5+CD8+ T cells in only around 10% of lymphoma samples and not in non-neoplastic samples.48 We found that CXCR5+CD8+ T cells showed a phenotype similar to Tfh cells and resided in B cell follicles. With a precursor memory phenotype, CXCR5+CD8+ T cells also exert cytotoxic effects against tumor cells in vitro compared with CXCR5−CD8+ T cells, and suppressed tumor-promoting TFH activities. These characteristics indicated that CXCR5+CD8+ T cells have the ability to migrate into B cell follicles and proliferate and inhibit tumor cells there. Consistent with in vitro data, we found that more CXCR5+CD8+ T cells infiltrated tumors and showed better tumor inhibition than naïve CD8+ T cells and CXCR5−CD8+ T cells in a lymphoma animal model. In contrast to other studies,14–16, 49–51 we did not find the tumor content of CXCR5+CD8+ T cells, enumerated by flow cytometry, to be directly associated with clinical outcome or disease status of follicular lymphoma. However, we did find that higher expression of a gene signature of CXCR5+CD8+ T cells was associated with improved survival.
The strong cytolytic capacity and antitumor effects of CXCR5+CD8+ T cells, compared to CXCR5−CD8+ T cells, are likely due to their higher expression of effector molecules such as IFN-γ, TNF-α, and granzymes, consistent with previous studies,14–16, 50, 52, 53 and their suppression of tumor-promoting TFH activities. However, production of GZMB and perforin by CXCR5+CD8+ T cells did not differ from that of their negative counterpart; the significance of this is uncertain from previous studies.14–16, 49, 50 We found that CXCR5+CD8+ T cells from benign tonsils expressed higher levels of GZMA, K, M and H; therefore, these granzymes might be more likely than GZMB to be involved in the stronger cytotoxicity of CXCR5+CD8+ T cells. However, GZMA and GZMK expression was the same for follicular lymphoma tumor infiltrating CXCR5+ and CXCR5− T cells. More samples are needed to address issues related to the killing capacity of CXCR5+CD8+ T cells, especially since it may be affected by stimulus, tissue origin, disease, and host or patients’ status and species.
We also demonstrated that CXCR5+CD8+ T cells could be induced in vitro from naïve CD8+ T cells in the presence of TGF-β and IL-23, as previously shown for CD4+ T cells,38 suggesting the possibility that the efficacy of adoptive T cell therapy approaches might be improved by differentiating CD8+ effector T cells into a CXCR5+ phenotype. Furthermore, we and other researchers found that CXCR5+CD8+ T cells characteristically express several genes from the Wnt signaling pathway,14 associated with self-renewal and stem cell like functions. However, we found that TGF-β could not induce CXCR5 expression from mouse naïve CD8+ T cells, similarly found for CD4+ T cells by a previous study.38 From these studies, it also seems that CXCR5 expression, function and development on T cells are more complex in primates than in rodents.
PD-1 is expressed on CXCR5+CD8+ T cells, but the implications of that are uncertain. In mice with LCMV chronic infection, which is used as a model of T-cell exhaustion, two studies reported that CXCR5+CD8+ T cells expressed less PD-1 than did CXCR5−CD8+ T cells;14, 16 in contrast, other studies have found PD-1 expression by both CXCR5− and CXCR5+ CD8+ T cells, with slightly higher expression by the latter, consistent with our findings.15, 49, 50 Jiao found that PD-1/PD-L1 blockade in HIV infection enhanced the function of CXCR5−CD8+ T cells while inhibiting that of CXCR5+CD8+ T cells.50 Their study suggests that our finding of higher PD-1 expression by CXCR5−CD8+ T cells from follicular lymphoma, as compared to that of CXCR5−CD8+ T cells from reactive tonsil samples, may indicate exhaustion.
Rather than being a marker of exhaustion, however, PD-1 expression by CXCR5+CD8+ T cells may be related to their association with follicles. PD-1 expression is characteristic of Tfh cells, which are concentrated toward germinal centers by interaction of their PD-1 with PD-L1 expressed by bystander B cells.54 Therefore, PD-1 and PD-L1 interaction may also enrich CXCR5+CD8+ T cells toward B cell follicles. However, this study found that PD-1 suppressed follicular T cell recruitment through inhibiting PI3K.54 Further study is needed to address PD-1 function on CXCR5+CD8+ T cells, particularly as regards the effect of blocking antibodies, and whether the loss of CCR7 expression and ICOS/ICOSL interaction facilitates movement of CXCR5+CD8+ T cells into follicles or germinal centers and/or their retention there.
We found that CXCR5+CD8+ T cells express a set of unique transcription factors consistent with their function. EOMES, T-bet, Blimp-1 (encoded by PRDM1), and BCL6 are transcriptional factors with reciprocal antagonistic effects (EOMES vs T-bet, Blimp-1 vs BCL6) in the function and lineage differentiation of CD8+ T cells.55–60 The balance among these transcription factors determines T cell fate towards terminal differentiation versus development of memory T cells.41, 42, 55, 57, 58, 61 The EOMEShiT-betdim features of CXCR5+ cells support these cells as an early memory phenotype. By contrast, during chronic infection, EOMEShiCD8+ T cells have been suggested to associate with terminally differentiated progeny with increased cytotoxicity;40 whether a similar phenotype is present during acute infection or tumor settings is unclear.
The plasticity and stability of CXCR5 expression by CD8+ T cells is another feature that needs further study, especially with respect to the potential for adoptive cell therapy. CXCR5 expression is a dynamic process in chronic viral infection.14–16, 49 We found that transferred CXCR5+CD8+ T cells could become CXCR5−CD8+ T cells, consistent with a previous study.14–16, 49 Also, transferred CXCR5+CD8+ T cells proliferated significantly and were enriched within tumors, indicating strong killing ability.
Supplementary Material
ACKNOWLEDGMENT
We thank Dr. Stephen E Ullrich (University of Texas MD Anderson Cancer Center) for technical advice. This work is supported by grants from the Leukemia and Lymphoma Society Quest for Cure Grant (P-QFC-3068–14 to SSN) and NIH National Institute of Allergy and Infectious Diseases (RO1AI109294 to SSW) and Oversea Development Program (SWH2016HWHZ-01 to XL) and generous philanthropic contributions to the University of Texas MD Anderson Moon Shots Program. The South Campus Flow Cytometry and Cell Sorting Core Facility is supported by The University of Texas MD Anderson Cancer Center Support Grant from National Institutes of Health (P30 CA016672) and the Advanced Microscopy Core Facility is supported by National Institutes of Health grant 1S10 RR029552.
Footnotes
DISCLOSURE: All authors declare no conflicts of interest.
Supplementary information is available at Leukemia’s website.
REFERENCES
- 1.Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 2012. May 10; 119(19): 4430–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011. January 28; 34(1): 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 Are Reciprocal and Antagonistic Regulators of T Follicular Helper Cell Differentiation. Science 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 Mediates the Development of T Follicular Helper Cells. Science 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 2009. September 18; 31(3): 457–468. [DOI] [PubMed] [Google Scholar]
- 6.Hardtke S, Ohl L, Forster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood 2005. September 15; 106(6): 1924–1931. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Xu H, Shih C, Wan Z, Ma X, Ma W, et al. T–B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature 2014. October/15/online; 517: 214. [DOI] [PubMed] [Google Scholar]
- 8.Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol 2005. August 15; 175(4): 2340–2348. [DOI] [PubMed] [Google Scholar]
- 9.Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular Helper T Cells. Annu Rev Immunol 2016. May 20; 34: 335–368. [DOI] [PubMed] [Google Scholar]
- 10.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 2006. December; 25(6): 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature 1998. February/19/online; 391: 799. [DOI] [PubMed] [Google Scholar]
- 12.Cyster JG, Ansel KM, Reif K, Ekland EH, Hyman PL, Tang HL, et al. Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev 2000. August; 176: 181–193. [DOI] [PubMed] [Google Scholar]
- 13.Quigley MF, Gonzalez VD, Granath A, Andersson J, Sandberg JK. CXCR5+ CCR7- CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. European journal of immunology 2007. December; 37(12): 3352–3362. [DOI] [PubMed] [Google Scholar]
- 14.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 2016. September 15; 537(7620): 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, et al. CXCR5+ follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol 2016. October 17; 17(10): 1187–1196. [DOI] [PubMed] [Google Scholar]
- 16.He R, Hou S, Liu C, Zhang A, Bai Q, Han M, et al. Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature 2016. September 15; 537(7620): 412–428. [DOI] [PubMed] [Google Scholar]
- 17.Wu T, Ji Y, Moseman EA, Xu HC, Manglani M, Kirby M, et al. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci Immunol 2016. December 23; 1(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature 2010. September 16; 467(7313): 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kridel R, Sehn LH, Gascoyne RD. Pathogenesis of follicular lymphoma. J Clin Invest 2012. October; 122(10): 3424–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ame-Thomas P, Le Priol J, Yssel H, Caron G, Pangault C, Jean R, et al. Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignant B cells. Leukemia 2012. May; 26(5): 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med 2004. November 18; 351(21): 2159–2169. [DOI] [PubMed] [Google Scholar]
- 22.Forster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein--coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood 1994. August 1; 84(3): 830–840. [PubMed] [Google Scholar]
- 23.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 1996. December 13; 87(6): 1037–1047. [DOI] [PubMed] [Google Scholar]
- 24.Wherry EJ. T cell exhaustion. Nat Immunol 2011. June//print; 12(6): 492–499. [DOI] [PubMed] [Google Scholar]
- 25.Wherry EJ, Ha S-J, Kaech SM, Haining WN, Sarkar S, Kalia V, et al. Molecular Signature of CD8+ T Cell Exhaustion during Chronic Viral Infection. Immunity 2007. October/26/; 27(4): 670–684. [DOI] [PubMed] [Google Scholar]
- 26.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol 2014. February; 35(2): 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez GJ, Pereira RM, Aijo T, Kim EY, Marangoni F, Pipkin ME, et al. The transcription factor NFAT promotes exhaustion of activated CD8(+) T cells. Immunity 2015. February 17; 42(2): 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conze D, Krahl T, Kennedy N, Weiss L, Lumsden J, Hess P, et al. c-Jun NH2-Terminal Kinase (JNK)1 and JNK2 Have Distinct Roles in CD8+ T Cell Activation. The Journal of Experimental Medicine 2002; 195(7): 811–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roychoudhuri R, Clever D, Li P, Wakabayashi Y, Quinn KM, Klebanoff CA, et al. BACH2 regulates CD8(+) T cell differentiation by controlling access of AP-1 factors to enhancers. Nat Immunol 2016. July; 17(7): 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain J, McCaffrey PG, Valge-Archer VE, Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature 1992. April 30; 356(6372): 801–804. [DOI] [PubMed] [Google Scholar]
- 31.Yang CY, Best JA, Knell J, Yang E, Sheridan AD, Jesionek AK, et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat Immunol 2011. December; 12(12): 1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins A, Littman DR, Taniuchi I. RUNX proteins in transcription factor networks that regulate T-cell lineage choice. Nature Reviews Immunology 2009. February/01/online; 9: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med 2011. October; 17(10): 1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gattinoni L, Zhong X-S, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med 2009. July//print; 15(7): 808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. The Journal of Experimental Medicine 2009. January 16, 2009; 206(1): 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shan Q, Zeng Z, Xing S, Li F, Hartwig SM, Gullicksrud JA, et al. The transcription factor Runx3 guards cytotoxic CD8(+) effector T cells against deviation towards follicular helper T cell lineage. Nat Immunol 2017. August; 18(8): 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao Y, Liu X, Zhang W, Deng X, Zhang H, Liu Y, et al. TGF-beta repression of Id2 induces apoptosis in gut epithelial cells. Oncogene 2009. February 26; 28(8): 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitt N, Liu Y, Bentebibel S-E, Munagala I, Bourdery L, Venuprasad K, et al. The cytokine TGF-[beta] co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol 2014. July/27/online; advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rawal S, Chu F, Zhang M, Park HJ, Nattamai D, Kannan S, et al. Cross Talk between Follicular Th Cells and Tumor Cells in Human Follicular Lymphoma Promotes Immune Evasion in the Tumor Microenvironment. The Journal of Immunology 2013; 190(12): 6681–6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 2012. November 30; 338(6111): 1220–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 2003. November 7; 302(5647): 1041–1043. [DOI] [PubMed] [Google Scholar]
- 42.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol 2005. December; 6(12): 1236–1244. [DOI] [PubMed] [Google Scholar]
- 43.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity 2010. January 29; 32(1): 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods 2003. October 1; 281(1–2): 65–78. [DOI] [PubMed] [Google Scholar]
- 45.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 2015. August; 21(8): 938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 2015. May; 12(5): 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000. February 3; 403(6769): 503–511. [DOI] [PubMed] [Google Scholar]
- 48.Le K-S, Amé-Thomas P, Tarte K, Gondois-Rey F, Granjeaud S, Orlanducci F, et al. CXCR5 and ICOS expression identifies a CD8 T-cell subset with TFH features in Hodgkin lymphomas. Blood Advances 2018; 2(15): 1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mylvaganam GH, Rios D, Abdelaal HM, Iyer S, Tharp G, Mavigner M, et al. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proceedings of the National Academy of Sciences 2017; 114(8): 1976–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiao Y-M, Yang H-G, Huang H-H, Tu B, Xing S-J, Mao L, et al. Dichotomous Roles of Programmed Cell Death 1 on HIV-Specific CXCR5+ and CXCR5− CD8+ T Cells during Chronic HIV Infection. Frontiers in Immunology 2017 2017-December-12; 8(1786). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang H, Li L, Han J, Sun Z, Rong Y, Jin Y. CXCR5(+) CD8(+) T Cells Indirectly Offer B Cell Help and Are Inversely Correlated with Viral Load in Chronic Hepatitis B Infection. DNA Cell Biol 2017. April; 36(4): 321–327. [DOI] [PubMed] [Google Scholar]
- 52.Hang J, Linhai L, Jiang H, Zhiwei S, Yihui R, Yun J. CXCR5+ CD8+ T Cells Indirectly Offer B Cell Help and Are Inversely Correlated with Viral Load in Chronic Hepatitis B Infection. DNA and Cell Biology 2017; 36(4): 321–327. [DOI] [PubMed] [Google Scholar]
- 53.Perdomo-Celis F, Taborda NA, Rugeles MT. Follicular CD8(+) T Cells: Origin, Function and Importance during HIV Infection. Front Immunol 2017; 8: 1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi J, Hou S, Fang Q, Liu X, Liu X, Qi H. PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity 2018. August 21; 49(2): 264–274 e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity 2009. August 21; 31(2): 283–295. [DOI] [PubMed] [Google Scholar]
- 56.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 2009. August 21; 31(2): 296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 2012. November//print; 12(11): 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol 2010. February; 11(2): 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin HM, Kapoor V, Guan T, Kaech SM, Welsh RM, Berg LJ. Epigenetic modifications induced by Blimp-1 Regulate CD8(+) T cell memory progression during acute virus infection. Immunity 2013. October 17; 39(4): 661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Welsh RM. Blimp hovers over T cell immunity. Immunity 2009. August 21; 31(2): 178–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams MA, Bevan MJ. Effector and Memory CTL Differentiation. Annual Review of Immunology 2007; 25(1): 171–192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.