Abstract
T cell immunity undergoes a complex and continuous remodeling with aging. Understanding those dynamics is essential in refining immunosuppression.
Aging is linked to phenotypic and metabolic changes in T-cell immunity, many resulting into impaired function and compromised effectiveness. Those changes may impact clinical immunosuppression with evidences suggesting age-specific efficacies of some (CNI and mTORi) but not necessarily all immunosuppressants.
Metabolic changes of T cells with aging have only recently been appreciated and may provide novel ways of immunosuppression.
Here, we provide an update on changes of T-cell immunity in aging.
Introduction
Aging is linked to systemic changes, compromising physiological reserves and an augmented vulnerability to stressors impacting the immune response to pathogens, cancer, and alloantigen.1,2 Immunosenescence represents a complex and continuous remodeling of specific cellular subpopulations, rather than a uniform corrosion.3 Intriguingly, the impact of aging is more prominent in T-cell immunity while components of innate immunity appear relatively well preserved.4,5 While aging causes alterations in B cells, current reports suggest that these changes are less pronounced.6
T cell immunity plays a critical role in host defenses to pathogens and immune responses against neoplasm.7 During alloimmunity, T cells play a pivotal role for both, in tolerance and rejection.5 In an aging population, dynamics of T-cell immunity have gained clinical relevance.
Effects of aging on T-cell immunity are broad and include T-cell intrinsic and systemic effects with a switch of T cell phenotypes (shifting the ratio of naïve and memory T-cells) and declining thymic outputs.
Here, we review age-specific changes of T cells in detail focusing on the link between T cell senescence and response to immunosuppressants in aging. We also introduce the concept of utilizing age-specific aspects of T cell metabolism to achieve immunosuppression.
Dynamics of T-cell compartments in Aging
Phenotypic changes
With aging, T cell populations shift from dominantly naïve to memory subsets.8 This phenomenon is attributed, at least in part, to an impaired regeneration of naïve T cells as a consequence of thymic involution.
In the thymus, T-cell progenitors develop into mature and functional T cells prior to joining the peripheral T cell pool.9 Clinically, thymic involution begins already by 1 year, with a reduction of naïve T cells by 50% for any 15 year life-time period, leading to a significant reduction in thymic output of naïve T cell in the 60+ populalation.10 Antigen exposure is playing an additional role. In neonates, less than 1% of T cells are antigen experienced; this ratio increases with age and approx. 65% of T cells have encountered antigens when reaching 50–70 years.11,12 Notably, memory T cells are long-lived13 and T cell memory responses show a half-life of 8–15 years.14 Longevity of memory T cell subsets is mainly attributed to self-renewal rather than the life-span of individually T cells.13 In a comparable fashion, naïve T cells may divide and generate daughter T cells with a naïve phenotype. Indeed, increased homeostatic proliferation may compensate, at least in part, for the reduced thymic output in aging.15 Notably, proliferation rates of naïve T cells appear slower than those of memory T cells.16,17
In aging a limited T cell receptor (TCR) repertoire is being observed with T-cell diversity dropping 1000-fold in individuals >70 years.18,19 Moreover, the homeostatic proliferation of naïve T cells may not compensate for the declining diversity of T cell receptors (TCR). Thus, the predominant role of memory T cell subsets, impaired generation of naïve T cells and reduction in T cell diversity shape T cell immunity in aging resulting into an overall compromised response to new antigens.
The repetitive exposure to antigens over a life-time has also been linked to the loss of CD28 expression on T cells. CD28 is a key co-stimulatory surface receptor that plays a critical role in antigen-dependent activation, proliferation and survival of T cells.20 Virtually all human T cells express CD28 at the time of birth. In sharp contrast, by the age of 80, 10–15% of peripheral blood CD4+ T cells and 50–60% of CD8+ T cells lack the expression of CD28.21 Chronic antigen stimulation through complete TCR/CD28 engagement has been shown to trigger the loss of CD28 on T cells, a process that can also be observed in-vitro.22 As an alternative and compensatory pathway of the classical TCR/CD28 activation, senescent T cells increase the de-novo expression of cytotoxic NK cell receptors.23 Intriguingly, increased NK receptor expression has been linked to recurrent antigenic TCR stimulation.24 Thus, T cells appear to switch from specific, adaptive receptor profiles to characteristics that are typically seen in innate immune cells. Indeed, CD28- CD4+ T cells have shown a compromised capacity to proliferate while gaining powerful cytotoxic capacities.25 Similarly, old CD8+ T cells acquire activating killer cell lectin-like (KLR) and killer-cell immunoglobulin-like receptors (KIRs).26
In contrast, the augmented expression of inhibitory receptors including PD-1 and CTLA4 on old T-cells may down-regulate the stimulation of TCRs. Old CD-4 T cells demonstrated an increased expression of inhibitory receptors in old mice (20 mths). Of note, loss of CD28 is accompanied by an increased gene expression of its antagonist, the CTLA-4 receptor26 facilitating a switch towards an overall inhibitory phenotype in old T-cells.
T-cell metabolism is impacted by Aging
The metabolism of immune cells is as a critical driver of an effective immune response. Upon classical TCR/CD28 stimulation, metabolic reprogramming towards aerobic glycolysis leads to anabolic growth and the accumulation of T-cell biosynthetic precursors.27 Aerobic glycolysis, although less efficient while providing a faster way for energy generation, enables T cells to respond to high energy demands. In senescent T cells, diminished activities of glycolytic key enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) result in an altered glycolytic flux and impaired breakdown of glucose.28 Inhibitory receptor PD-1 regulates metabolic reprogramming of activated T cells by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation (FAO). In contrast, CTLA4 sustains the metabolic profile of nonactivated cells by inhibiting glycolysis without up-regulation of FAO. Thus, the accumulation of inhibitory receptors with aging may contribute to the impaired glycolysis in old T cells.29 However, underlying molecular mechanism remain unclear.
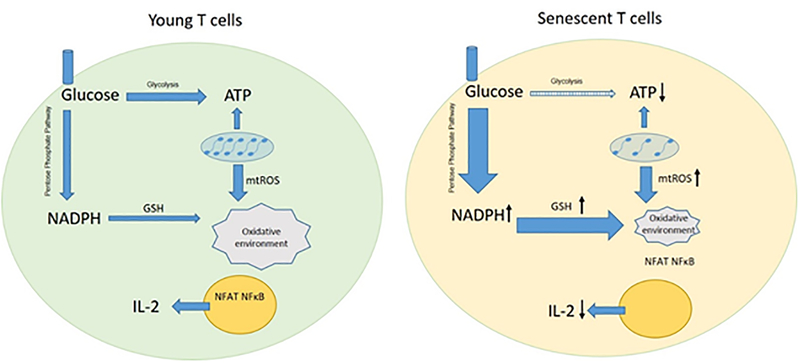
Although T cells utilize aerobic glycolysis to increase the production of ATP upon activation, an increase in mitochondrial respiration represents an additional important component for an effective response.30 With aging, mitochondrial DNA (mtDNA) mutations accumulate and damage mtDNA-encoded proteins, including components of the electron transport chain (ETC), a critical component of mitochondrial respiration.31,32 Moreover, dysfunctional ETC in senescent T cells fail to metabolize ADP into high-energetic ATP. Thus, impaired mitochondrial function and glycolysis lead to a compromised energy production in activated old T cells (Figure 1).33 In support, naïve CD4+ T cells isolated from old mice have shown a compromised capacity to meet metabolic demands when activated, resulting into either reduced lactate production or compromised autophagy.34
Figure 1:
Changes in cellular metabolism of senescent T cells. In activated senescent T cells, ATP production is impaired due to altered glycolytic flux and compromised mitochondrial respiration. Though old T cells have higher mtROS leakage, enhanced pentose phosphate pathway results in a burst of reductive molecule and clearance of mtROS. NFAT and NF-κB fail to translocate into nuclear because of altered cellular redox state. Consequently, the production of IL-2 is impaired.
Alterations in energy generation lead to changes in the cellular redox state linked to the generation of oxidants and reductive molecules. Interestingly, oxidative respiration in mitochondria represents the major source of reactive oxygen species (ROS).35 Moreover, compromised coupling between electron transportation and ATP production gives rise to an augmented mtROS leakage in senescent T cells. The accumulatio of mtROS, however, is met by compensatory mechanisms and cellular H2O2 and superoxide levels appear to increase less in activated old compared to young T cells.36 In support, experimental data have shown that old murine T-lymphocytes are more resistant to oxidative damage.37 TCR-dependent mtROS release is reduced in senescent CD4+ T cells, at least in part linked to an enhanced activity of the anti-oxidative pentose phosphate pathway (PPP)36 and an accumulation of NADPH and precursors of nucleotides. The overwhelming production of NADPH provides senescent T cells with an abundant resource of glutathione, capable to scavenge mtROS. Moreover, inhibition of PPP in senescent T cells reduced intracellular NADPH and GSH concentrations.36 Thus, the relative reduction of mtROS in old T-cells limits the damage of intracellular DNA, proteins and lipids.38,39 Hence, there is a fine balance of uncontrolled oxidative activity linked to various pathological conditions and the potential to compensate mtROS accumulation in old T-cells through protective metabolic pathways40 (Figure 1).
Functional T-cell changes in aging
Memory T cells have less strict requirements for stimulation and can produce a broader set of cytokines.41,42 Besides, CD28- T cells gain an increased expression of lymphocyte function-associated antigen 1(LFA-1) and NKRs further reducing the activation threshold of these cells.43 Yet aging impairs the proliferation and effector function of activated T cells.
IL-2 is critical for antigen-driven clonal expansion of activated T cells.44 Old CD4+ T cells stimulated with alloantigens have shown an impaired IL-2 production45 while the capacity of CD4+ T cells to produce IL-2 declines with aging.46,47 Of note, compromised CD4+ proliferation can be restored by exogenous IL-2 in an age-independent fashion.48 Similarly, virus-specific old mouse memory CD8+ T cells showed a compromised IL-2 production and limited proliferation. The impaired production of IL-2 in old T cells may also be attributed to an age-specific loss of CD-28 as the costimulatory signal is critical for the activation of T cells and their subsequent production of IL-2.49 Moreover, age-specific changes in T-cell metabolism may also play a role through the inhibition of the nuclear translocation of NFAT and NF-κB, both key transcription factors to produce IL-2.
An imbalance between Th1 and Th2 responses is getting prominent in aging and some reports have linked aging to a declining Th1/Th2 ratio.50–52 Notably, counts for both, Th1 and Th2 cells increase with age, mainly attributed to the memory-dominant phenotype of old T cells. CD28-CD8+ T cells have shown to generate cytotoxic effectors, including granzyme B, perforin (PRF1) and IFN-gamma.53 Nevertheless, there appears to be an age-related defect of CD8+ T cell in response to viral infections or tumor antigens54 with significantly reduced subsets of naïve T-cells. Moreover, a limited TCR diversity contributes largely to age-specific defects of CD8+ T cell response.54 Of note, the generation of CD8+ mediated cell toxicity requires help from CD4+ T cells.55 Thus, the impaired antigen-specific response of CD28-CD8+T cells may be linked, at least in part, to the age-related compromised efficacy of old CD4+ T cells.
Regulatory T cells (Tregs) play a crucial role in the preservation of immune tolerance and prevention of exacerbated immune responses to foreign antigens.56 A lack of Tregs that express CD4, CD25 and FOXP3 have resulted in severe autoimmunity in experimental and clinical models.57 The impact of aging on the number of Tregs remains controversial and both increasing and decreasing numbers of Tregs have been reported.58–62 T-reg functions also change with aging, although not uniformly. CD4+CD25+ Tregs from old mice displayed significantly lower inhibiting capacities on alloantigen-induced delayed-type hypersensitivity responses or on IL-2 and IFNγ production, however prevented the proliferation of effector T cells in an age-independent fashion.60
Responses to Immunosuppressants
Age-related changes in T cell immunity shape a distinct alloimmune response. In general, older organ transplant recipients have less acute rejections. Of relevance, the most frequent causes of death in older transplant recipients are linked to impaired immune responses towards pathogens and tumors making older recipients more susceptible to infections and malignancies.2,3
Calcineurin is a serine-threonine phosphatase that is involved in the transduction of the TCR signal in activated T cells through dephosphorylating NFAT, leading to its translocation from cytoplasm to the nucleus. Cyclosporine A (CsA) and Tacrolimus (TRL) are established CNIs which inhibit calcineurin activity through binding the immunophilins Cycophilin and immunophilin FK506 binding protein 12 (FKBP12), respectively. As a consequence of inhibiting calcineurin, phosphorylated NFAT is not translocated into the nucleus upon TCR stimulation, blocking key genes implicated in T cell activation.63 It’s known that calcineurin activity declines with aging.64 We have observed comparable calcineurin levels in naïve CD4+ T cell from young and old healthy volunteers. However, treatment with TAC resulted into a a dramatically reduced influx of Ca2+ and diminished calcineurin level.65 This age-specific effect on TAC on Ca2+ metabolism and calcineurin pathways leads to a more robust inhibition of IL-2 production with augmented antiproliferative capacities on old CD4+ T cells. Meanwhile, CD8+ T cells depletion did not impact our observed age-specific effects of TAC, indicating the age-specific effects were mainly mediated by CD4+T cells.65 Notably, TAC also exerts age-specific immunosuppression through altered pharmacokinetic and pharmacodynamics. The decline of hepatic cytochrome P450 (CYP450) with aging leads to an augmented first-pass metabolism of TAC.66 A prospective clinical trial in kidney transplant recipients older than 65 years required only half of a body weight–adjusted dosage to achieve therapeutic trough levels.67 Moreover, our experiments in murine transplant model showed a prolonged survival in old recipients even when treated with only half of the TAC dosage applied to younger recipients.65
The mammalian target of rapamycin (mTOR) pathway has been identified as a critical regulator of cellular metabolism. mTOR inhibitors inhibit the activity of mTORC1, blocking the Warburg effect, a critical metabolic step in cancer and immune cells.68 mTOR inhibitors (mTORi) have the capacity to target both, T cells and cancer cells. Thus, anti-tumor capacities of mTOR inhibitors appear of relevance in the design of immunosuppressive regimens in the elderly. A multivariate analysis of 30,000 primary renal transplant recipients revealed that de-novo posttransplant malignancies were significant lower in patients treated with mTORi comparted to CNI-based maintenance immunosuppression.69 Additional prospective trails in nonrenal transplant recipients have been confirmatory.70 However, mTORi have been associated with increased risks of bone marrow suppression and dyslipidemia.71 Old T cells may be more sensitive to changes caused by the inhibition of mTORi. Indeed, animal experiments demonstrated that graft survival following rapamycin treatment was age-dependent and extended in old versus young recipients.72 Intriguingly, this age-specific effect was linked to the significant increase of IFNγ/IL-10 double-positive regulatory type 1 cells.
The receptor fusion protein belatacept is composed of the modified Fc domain of the human immunoglobulin IgG1 linked to the extracellular-binding domain cytotoxic T-lymphocyte-associated protein 4(CTLA4).2 Belatacept avidly binds to CD80 on antigen presentation cells, thus blocking CD80 from interacting with CD28 on T cells, preventing a co-stimulatory signal to T cells.73 Clinical trials elucidating age-related mechanism of belatacept are currently lacking. At least in theory, Loss of CD28 and gain of alternative innate receptors may reduce the effect of CTLA4-Ig on old T cells.
With changing pharmacokinetic and -dynamics, antimetabolites and corticosteroids are also expected to have age-specific effects.2
With only limited information from clinical trials in the elderly, recommendations on immunosuppression rely also on the growing knowledge of age-specific alloimmune responses in experimental models.2 Moreover, origin, quality and age of the graft will also impact the selection of immunosuppressants. As an induction treatment, a reduced Thymoglobulin dose or an IL-2 R antibody appears preferable. For maintenance, a reduced Tac dose or a CNI free immunosuppression may be considered. Clinical effects of belatacept in the elderly remain unclear. Base on experimental data, mTORi appear as a promising candidate for CNIs replacement in old recipients.
Changing Patterns of T cell metabolism as a target for effective immunosuppression
T-cell metabolism is an age-specific phenomenon, thus providing a rationale to explore the potential of novel immunosuppressive approaches.
T cell activation leads to rapid cell proliferation and acquisition of effector function. These processes require large amounts of energy and precursors for chromosome duplication, protein biosynthesis and fatty acid synthesis. Metabolic reprogramming is critical for activated T cells in response to the high demand of ATP and substrates during cell division, cytokine synthesis and secretion.74 Upon activation, increased glucose uptake and flux through aerobic glycolysis enables T cells to generate ATP and other essential biomolecules rapidly.74 Meanwhile, augmented metabolism of amino acids, especially glutamine, is also critical to support metabolic reprogramming.75 Glucose is the main fuel source of mitochondria in resting T cells. As glucose is utilized to feed glycolysis upon activation, glutaminolysis is augmented to produce glutamate, serving as an alternative fuel source to maintain the mitochondrial function and metabolism.23 Deprivation of glutamine but not glucose led to an impairment of activation-induced cell growth associated with a reduction of lipid and protein biosynthesiss.76 Moreover, specific T-cell subsets showed distinct metabolic programs. Effector T cells including Th1, Th2, and Th17 have been reported to depend on glucose uptake and glycolysis. By contrast, Tregs and long-lived T memory cells are less dependent upon glycolysis and appear to rely more on lipid oxidation to generate energy.77 These differences indicate metabolic intervention as a promising candidate for selective immunomodulation.78 Indeed, Inhibition of glycolysis promotes the generation of Tregs while reducing the differentiation towards T cell effectors.79 Of additional relevance, a changing pattern of cellular metabolism has been shown in senescent T cells, indicating that targeting of T-cell metabolism may represent an effective approach in age-adapted immunosuppression.
Metabolic interventions on T cells have been shown as an effective therapy. Notably, it’s now clear that the capacity of mTOR inhibitors to impact alloimmune responses is, at least in part, based on effects of metabolic programming.80 Numerous metabolic inhibitors are identified as promising interventions of T cell immunity. 2-DG is a glycolytic inhibitor that targets the rate-limiting enzyme hexokinase(HK) inhibiting the production of glucose-6-phosphate.81 In a murine lupus model, 2DG reduced the production of IFNγ and IL-17 in vitro while preventing disease progress in vivo.82 Acting on the complex I of the mitochondrial respiratory chain, metformin inhibits oxidative respiration that reduces ATP synthesis.83 Subsequent changes in the ATP/ADP ratio activate the AMPK pathway and inhibit mTOR pathways.84 Efforts of using metabolic inhibitors to modify alloimmunity have shown intriguing findings. Combinatorial treatment with metformin and 2DG inhibited TCR/CD28 activation, triggered metabolic reprogramming and prevented IFNγ production. In a fully mismatch skin transplantation model, a single treatment with DON prolonged allograft survival significantly (own unpublished data). In a murine cardiac transplantation model, triple treatment of metformin, 2DG and DON prolonged graft survival significantly with long-term graft acceptance in some animals.85
As old T cells have a compromised mitochondrial glycolysis, inhibitors of glutamine metabolism may be even more effective in old recipients. Indeed, ongoing experiments of our group have shown that a single treatment with DON resulted into long term survival of skin allograft in a strong histoincompatible mouse model.
Conclusion
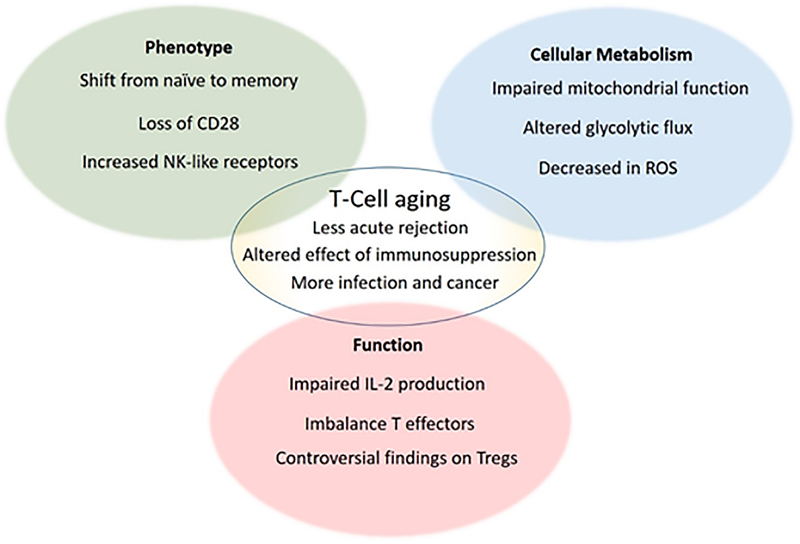
Aging gives rise to a series of modifications in T cells immunity (Figure 2). Overall, T cells compartments undergo a shift to a less efficient response over a life-time.
Figure 2:
T cell aging is characterized by changes in phenotype, alterations in cellular metabolism and impaired function. These alterations contribute at least partially to the clinic phenotype.
In organ transplantation, impaired T cell immunity in aging is linked to less acute rejections and improved graft survival. Old transplant recipients, at the same time, experience more frequent side-effects with higher rates of infections and malignancies. Of note, immunosuppressants, including mTORi and CNIs exert age-specific effects on alloimmune responses.
Alterations of cellular metabolism change with aging and are thus relevant for T-cell immunosenescence. Changes of T-cell metabolisms in aging may represent an interesting and novel immunosuppressive approach in older transplant recipients.
Funding
This work was supported by National Institutes of Health grants R56/R01AG039449. YN was supported by the Chinese Scholarship Council (201606370196) and Central South University. KM and RM were supported by the Osaka Medical Foundation. JI was supported by the Biomedical Education Program and The German Academic Exchange Service.
Abbreviations
- TCR
T cell receptor
- PFKFB3
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
- mtDNA
mitochondrial DNA
- ETS
Electron Transport Chain
- ROS
Reactive Oxygen Species
- PPP
Pentose Phosphate Pathway
- RISP
Rieske Iron Sulfur Protein
- NFAT
Nuclear Factor of Activated T cells
- SOD
Superoxide Dismutase
- NF-κB
Nuclear Factor kappa-light-chain-enhancer of activated B cells
- AMPK
5′ AMP-activated Protein Kinase
- mTORc1
Mammalian Target of Rapamycin Complex 1
- LFA-1
Lymphocyte Function-Associated Antigen 1
- Tregs
Regulatory T cells
- CsA
Cyclosporine A
- TAC
Tacrolimus
- FKBP
FK506 Binding Protein
- PK/PD
Pharmacokinetic and Pharmacodynamic
- CYP450
Hepatic Cytochrome P450
- SRL
Sirolimus
- CTLA-4
Cytotoxic T-lymphocyte-Associated Protein 4
- 2DG
2-Deoxy-D-glucose
- HK
Hexokinase
- DON
6-Diazo-5-oxo-L-norleucine
Footnotes
Disclosure
The authors declare no conflicts of interest.
Reference.
- 1.Martins PNA, Tullius SG, Markmann JF. Immunosenescence and immune response in organ transplantation. Int Rev Immunol. 2014;33(3):162–173. doi: 10.3109/08830185.2013.829469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krenzien F, ElKahl A, Quante M, et al. A Rationale for Age-Adapted Immunosuppression in Organ Transplantation. Transplantation. 2015;99(11):2258–2268. doi: 10.1097/TP.0000000000000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tu W, Rao S. Mechanisms Underlying T Cell Immunosenescence: Aging and Cytomegalovirus Infection. Front Microbiol. 2016;7:2111. doi: 10.3389/fmicb.2016.02111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cossarizza A, Ortolani C, Monti D, et al. Cytometric analysis of immunosenescence. Cytometry. 1997;27(4):297–313. [DOI] [PubMed] [Google Scholar]
- 5.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033 [DOI] [PubMed] [Google Scholar]
- 6.Pallikkuth S, de Armas L, Rinaldi S, et al. T Follicular Helper Cells and B Cell Dysfunction in Aging and HIV-1 Infection. Front Immunol. 2017;8:1380. doi: 10.3389/fimmu.2017.01380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch S, Larbi A, Dehovanessian E, et al. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinbokel T, Elkhal A, Liu G, et al. G. Immunosenescence and organ transplantation. Transplant Rev (Orlando). 2013;27(3):65–75. doi: 10.1016/j.trre.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer S, Albergante L, Blackburn CC, et al. Thymic involution and rising disease incidence with age. Proc Natl Acad Sci U S A. 2018;115(8):1883–1888. doi: 10.1073/pnas.1714478115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakkis FG, Sayegh MH. Memory T cells: a hurdle to immunologic tolerance. J Am Soc Nephrol. 2003;14(9):2402–2410. [DOI] [PubMed] [Google Scholar]
- 12.Geiger H, Van Zant G. The aging of lympho-hematopoietic stem cells. Nat Immunol. 2002;3(4):329–333. doi: 10.1038/ni0402-329 [DOI] [PubMed] [Google Scholar]
- 13.Macallan DC, Borghans JAM, Asquith B. Human T Cell Memory: A Dynamic View. Vaccines. 2017;5(1):5. doi: 10.3390/vaccines5010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammarlund E, Lewis MW, Hansen SG, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917 [DOI] [PubMed] [Google Scholar]
- 15.Appay V, Sauce D. Naive T cells: the crux of cellular immune aging? Exp Gerontol. 2014;54:90–93. doi: 10.1016/j.exger.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 16.Sachsenberg N, Perelson AS, Yerly S, et al. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J Exp Med. 1998;187(8):1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazenberg MD, Stuart JW, Otto SA, et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART). Blood. 2000;95(1):249–255. [PubMed] [Google Scholar]
- 18.Kohler S, Wagner U, Pierer M, et al. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur J Immunol. 2005;35(6):1987–1994. doi: 10.1002/eji.200526181 [DOI] [PubMed] [Google Scholar]
- 19.Naylor K, Li G, Vallejo AN, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174(11):7446–7452. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Fresnay S, Welty E, et al. Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4-dependent manner. Am J Transplant. 2011;11(8):1599–1609. doi: 10.1111/j.1600-6143.2011.03624.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30(7):306–312. doi: 10.1016/j.it.2009.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205(1):158–169. doi: 10.1111/j.0105-2896.2005.00256.x [DOI] [PubMed] [Google Scholar]
- 23.Seyda M, Elkhal A, Quante M, et al. T Cells Going Innate. Trends Immunol. 2016;37(8):546–556. doi: 10.1016/j.it.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallejo AN, Brandes JC, Weyand CM, et al. Modulation of CD28 expression: distinct regulatory pathways during activation and replicative senescence. J Immunol. 1999;162(11):6572–6579. [PubMed] [Google Scholar]
- 25.Bryl E, Witkowski JM. Decreased proliferative capability of CD4(+) cells of elderly people is associated with faster loss of activation-related antigens and accumulation of regulatory T cells. Exp Gerontol. 2004;39(4):587–595. doi: 10.1016/j.exger.2003.10.029 [DOI] [PubMed] [Google Scholar]
- 26.Chen G, Lustig A, Weng NP. T cell aging: a review of the transcriptional changes determined from genome-wide analysis. Front Immunol. 2013;4:121. doi: 10.3389/fimmu.2013.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212(9):1345–1360. doi: 10.1084/jem.20151159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Fujii H, Mohan SV, et al. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med. 2013;210(10):2119–2134. doi: 10.1084/jem.20130252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patsoukis N, Bardhan K, Chatterjee P, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. doi:ARTN 6692 10.1038/ncomms7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38(2):225–236. doi: 10.1016/j.immuni.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genova ML, Lenaz G. The Interplay Between Respiratory Supercomplexes and ROS in Aging. Antioxid Redox Signal. 2015;23(3):208–238. doi: 10.1089/ars.2014.6214 [DOI] [PubMed] [Google Scholar]
- 32.Ogasawara Y, Nakayama K, Tarnowka M, et al. Mitochondrial DNA spectra of single human CD34+ cells, T cells, B cells, and granulocytes. Blood. 2005;106(9):3271–3284. doi: 10.1182/blood-2005-01-0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weyand CM, Yang Z, Goronzy JJ. T-cell aging in rheumatoid arthritis. Curr Opin Rheumatol. 2014;26(1):93–100. doi: 10.1097/BOR.0000000000000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattoo H, Faulkner M, Kandpal U, et al. Naive CD4 T cells from aged mice show enhanced death upon primary activation. Int Immunol. 2009;21(11):1277–1289. doi: 10.1093/intimm/dxp094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 36.Yang Z, Shen Y, Oishi H, et al. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci Transl Med. 2016;8(331):331ra338. doi: 10.1126/scitranslmed.aad7151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohmiller JJ, Roellich KM, Toledano A, et al. Aged murine T-lymphocytes are more resistant to oxidative damage due to the predominance of the cells possessing the memory phenotype. J Gerontol A Biol Sci Med Sci. 1996;51(2):B132–140. [DOI] [PubMed] [Google Scholar]
- 38.Goronzy JJ, Li G, Yu M, et al. Signaling pathways in aged T cells - a reflection of T cell differentiation, cell senescence and host environment. Semin Immunol. 2012;24(5):365–372. doi: 10.1016/j.smim.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finkel T Signal transduction by reactive oxygen species. J Cell Biol. 2011;194(1):7–15. doi: 10.1083/jcb.201102095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Meo S, Reed TT, Venditti P, et al. Harmful and Beneficial Role of ROS 2017. Oxid Med Cell Longev. 2018;2018:5943635. doi: 10.1155/2018/5943635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang H, Patel DD, Manton KG. The immune system in aging: roles of cytokines, T cells and NK cells. Front Biosci. 2005;10:192–215. [DOI] [PubMed] [Google Scholar]
- 42.Zanni F, Vescovini R, Biasini C, et al. Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: a contribution to understand the relationship between inflammation and immunosenescence. Exp Gerontol. 2003;38(9):981–987. [DOI] [PubMed] [Google Scholar]
- 43.Yung R, Powers D, Johnson K, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97(12):2866–2871. doi: 10.1172/JCI118743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachmann MF, Oxenius A. Interleukin 2: from immunostimulation to immunoregulation and back again. EMBO Reports. 2007;8(12):1142–1148. doi: 10.1038/sj.embor.7401099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen H, Tesar BM, Du W, et al. Aging impairs recipient T cell intrinsic and extrinsic factors in response to transplantation. PLoS One. 2009;4(1):e4097. doi: 10.1371/journal.pone.0004097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elrefaei M, Blank KJ, Murasko DM. Decreased IL-2, IFN-gamma, and IL-10 production by aged mice during the acute phase of E55+ retrovirus infection. Virology. 2002;299(1):8–19. [DOI] [PubMed] [Google Scholar]
- 47.Gong Z, Liu T, Wan Y, et al. Decreased c-rel activation contributes to aberrant interleukin-2 expression in CD4+T cells of aged rats. Mol Immunol. 2014;61:1–6. doi: 10.1016/j.molimm.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 48.Haynes L, Linton PJ, Eaton SM, et al. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190(7):1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seyda M, Quante M, Uehara H, et al. Immunosenescence in renal transplantation: a changing balance of innate and adaptive immunity. Curr Opin Organ Transplant. 2015;20(4):417–423. doi: 10.1097/MOT.0000000000000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shearer GM. Th1/Th2 changes in aging. Mech Ageing Dev. 1997;94(1–3):1–5. [DOI] [PubMed] [Google Scholar]
- 51.Uciechowski P, Kahmann L, Plümäkers B, et al. TH1 and TH2 cell polarization increases with aging and is modulated by zinc supplementation. Exp Gerontol. 2008;43(5):493–498. doi: 10.1016/j.exger.2007.11.006 [DOI] [PubMed] [Google Scholar]
- 52.Li SP, Miller RA. Age-associated decline in IL-4 production by murine T lymphocytes in extended culture. Cell Immunol. 1993;151(1):187–195. doi: 10.1006/cimm.1993.1230 [DOI] [PubMed] [Google Scholar]
- 53.Tarazona R, DelaRosa O, Alonso C, et al. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000;121(1–3):77–88. [DOI] [PubMed] [Google Scholar]
- 54.Effros RB, Cai Z, Linton PJ. CD8 T cells and aging. Crit Rev Immunol. 2003;23(1–2):45–64. [DOI] [PubMed] [Google Scholar]
- 55.Bennett SRM, Carbone FR, Karamalis F, et al. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dias S, D’Amico A, Cretney E, et al. Effector Regulatory T Cell Differentiation and Immune Homeostasis Depend on the Transcription Factor Myb. Immunity. 2017;46(1):78–91. doi: 10.1016/j.immuni.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 57.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10(12):849–859. doi: 10.1038/nri2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qian T, Ricordi C, Inverardi L, et al. Intrathymic tolerance and age. Transplant Proc. 1995;27(6):3391. [PubMed] [Google Scholar]
- 59.Nobori S, Shimizu A, Okumi M, et al. Thymic rejuvenation and the induction of tolerance by adult thymic grafts. Proc Natl Acad Sci U S A. 2006;103(50):19081–19086. doi: 10.1073/pnas.0605159103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao L, Liguang S, Wang H, et al. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol. 2007;81(6):1386–1394. doi: 10.1189/jlb.0506364 [DOI] [PubMed] [Google Scholar]
- 61.Sharma S, Dominguez AL, Lustgarten J High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177(12):8348–8355 [DOI] [PubMed] [Google Scholar]
- 62.Trzonkowski P, Szmit E, Myśliwska J, et al. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of CTL and NK cells in humans-impact of immunosenescence. Clin Immunol. 2006;119(3):307–316. doi: 10.1016/j.clim.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 63.Cello JP. Acquired immunodeficiency syndrome cholangiopathy: spectrum of disease. Am J Med. 1989;86(5):539–546. [DOI] [PubMed] [Google Scholar]
- 64.Pahlavani MA, Vargas DM. Age-related decline in activation of calcium/calmodulin-dependent phosphatase calcineurin and kinase CaMK-IV in rat T cells. Mech Ageing Dev. 1999;112(1):59–74. [DOI] [PubMed] [Google Scholar]
- 65.Krenzien F, Quante M, Heinbokel T, et al. Age-Dependent Metabolic and Immunosuppressive Effects of Tacrolimus. Am J Transplant. 2017;17(5):1242–1254. doi: 10.1111/ajt.14087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warrington JS, Greenblatt DJ, von Moltke LL. Age-related differences in CYP3A expression and activity in the rat liver, intestine, and kidney. J Pharmacol Exp Ther. 2004;309(2):720–729. doi: 10.1124/jpet.103.061077 [DOI] [PubMed] [Google Scholar]
- 67.Jacobson PA, Schladt D, Oetting WS, et al. Lower calcineurin inhibitor doses in older compared to younger kidney transplant recipients yield similar troughs. Am J Transplant. 2012;12(12):3326–3336. doi: 10.1111/j.1600-6143.2012.04232.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel CH, Powell JD. Targeting T cell metabolism to regulate T cell activation, differentiation and function in disease. Curr Opin Immunol. 2017;46:82–88. doi: 10.1016/j.coi.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kauffman HM, Cherikh WS, Cheng Y, et al. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80(7):883–889. [DOI] [PubMed] [Google Scholar]
- 70.Fischer L, Klempnauer J, Beckemaum S, et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation--PROTECT. Am J Transplant. 2012;12(7):1855–1865. doi: 10.1111/j.1600-6143.2012.04049.x [DOI] [PubMed] [Google Scholar]
- 71.Vincenti F, Schena FP, Paraskevas S, et al. A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant. 2008;8(2):307–316. doi: 10.1111/j.1600-6143.2007.02057.x [DOI] [PubMed] [Google Scholar]
- 72.Quante M, Heinokel T, Edtinger K, et al. Rapamycin Prolongs Graft Survival and Induces CD4+IFN-gamma+IL-10+ Regulatory Type 1 Cells in Old Recipient Mice. Transplantation. 2018;102(1):59–69. doi: 10.1097/TP.0000000000001902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beyersdorf N, Kerkau T, Hunig T CD28 co-stimulation in T-cell homeostasis: a recent perspective. Immunotargets Ther. 2015;4:111–122. doi: 10.2147/ITT.S61647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Le Bourgeois T, Strauss L, Aksoylar HI et al. Targeting T Cell Metabolism for Improvement of Cancer Immunotherapy. Front Oncol. 2018;8:237. doi: 10.3389/fonc.2018.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang R, Dillon CP, Shi LZ, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35(6):871–882. doi: 10.1016/j.immuni.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huynh A, DuPage M, Priyadharshini B, et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol. 2015;16(2):188–196, doi: 10.1038/ni.3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wahl DR, Byersdorfer CA, Ferrara JL, et al. Distinct metabolic programs in activated T cells: opportunities for selective immunomodulation. Immunol Rev. 2012;249(1):104–115. doi: 10.1111/j.1600-065X.2012.01148.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi LZ, Wang R, Huang G, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208(7):1367–1376. doi: 10.1084/jem.20110278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19(3):373–379. doi: 10.1016/j.cmet.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao Q, Chu Z, Zhu L., et al. 2-Deoxy-d-Glucose Treatment Decreases Anti-inflammatory M2 Macrophage Polarization in Mice with Tumor and Allergic Airway Inflammation. Front Immunol. 2017;8:637. doi: 10.3389/fimmu.2017.00637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yin Y, Choi SC, Xu Z, et al. Glucose Oxidation Is Critical for CD4+ T Cell Activation in a Mouse Model of Systemic Lupus Erythematosus. J Immunol. 2016;196(1):80–90. doi: 10.4049/jimmunol.1501537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Biondani G, Peyron JF. Metformin, an Anti-diabetic Drug to Target Leukemia. Front Endocrinol (Lausanne). 2018;9:446. doi: 10.3389/fendo.2018.00446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andrzejewski S, Gravel SP, Pollak M, et al. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:12. doi: 10.1186/2049-3002-2-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee CF, Lo YC, Cheng CH, et al. Preventing Allograft Rejection by Targeting Immune Metabolism. Cell Rep. 2015;13(4):760–770. doi: 10.1016/j.celrep.2015.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]