Abstract
Background
Females are hypercoagulable and have survival benefit in trauma-induced coagulopathy (TIC). The mechanism for this sex-specific hypercoagulability is unknown. Platelets and platelet function are central in providing hemostatic potential and are the largest contributor to clot strength. Ligands (adenosine diphosphate [ADP] and platelet activating factor [PAF]) bind distinct platelet receptors to potentiate activation and aggregation. We hypothesize that female platelets have a differential response to ADP and PAF, resulting in greater aggregation and activation compared to males, and that estradiol pre-treatment of male or female platelets enhances this activity.
Methods
Platelets were collected from healthy volunteers: pre/post-menopausal females (≤54 years old,>54 years old) and similarly aged males. Platelet aggregometry and flow cytometry (fibrinogen binding capacity) were examined. After treatment with ADP or PAF, platelet aggregation was assessed with Chronolog and activation assessed by CD41 receptor surface expression using flow cytometry. Aggregation and activation were again assessed after platelet pre-treatment with estradiol.
Results
Healthy volunteers included 12 premenopausal and 13 postmenopausal females and 18 similarly aged males. Female platelets (combined pre- and postmenopausal) had increased aggregation with ADP stimulation, as compared to male platelets. Male and female platelets had differential fibrinogen receptor expression, with female platelets (combined pre- and postmenopausal) demonstrating robust activation with ADP versus male platelets with PAF. In the presence of estradiol incubation, male platelets’ activation with PAF approximated that of females (combined pre- and postmenopausal) and activation with PAF was enhanced in both male and female platelets.
Conclusions
Male and female platelets have differential response to stimuli, suggesting sex-dependent signaling and cellular activation. Female platelets have both increased aggregation and activation potential, and estradiol pre-treatment feminizes male platelets to approximate female platelet activation with PAF. These findings offer potential explanation for sex-based differences in hemostatic potential in TIC and question whether donor sex of transfused platelets should be considered in resuscitation. Estradiol may also serve as a novel therapeutic adjunct in TIC.
Level of Evidence:
This is a basic science project and as such, does not require a level of evidence.
Study type:
Original Article.
Keywords: sex dimorphisms, estradiol, trauma-induced coagulopathy, platelets
Background
Sex dimorphisms in coagulation are well-established, with females demonstrating a hypercoagulable profile1,2. On whole blood hemostatic assays, including thrombelastography (TEG), females have shortened phase of enzymatic clotting (reaction time), increased rate of clot propagation (angle), and increased clot strength (maximum amplitude [MA]) compared to males1–3. This hypercoagulability has clinical significance, with severely injured females demonstrating better physiologic response to similar degrees of shock and decreased transfusion requirements compared to their male counterparts in the setting of trauma-induced coagulopathy (TIC)4. We have recently identified that female-specific hypercoagulability is present at baseline, persists following injury, and that when sex is evaluated as an experimental variable in severely injured trauma populations, female sex confers a survival benefit in the setting of depressed MA associated with TIC5.
The mechanism for female-specific hypercoagulability is unknown, but female sex appears to be protective against mortality, even when matched by injury severity, injury mechanism, and shock. It has been hypothesized that sex hormones and platelet biology may be the mechanistic drivers. This is suggested by a higher female MA (an effect predominantly driven by platelets) identified on whole blood clotting assessment (TEG), as compared to males. This is most pronounced in pregnant and peripartum females and those taking hormonal therapy, suggesting a potential sex hormone effect6–10. The presence of sex hormone receptors, including androgens and estrogens, on platelets, as well as estrogen- and androgen-responsive enzymes within platelets, further supports a sex hormone effect on platelets as a basis for female hypercoagulability11–13. The effect of sex hormones on platelet function and their mechanisms of action have not been fully elucidated.
The objective of this study was to compare the platelet function of healthy males and females including platelet aggregation (extent of shape change [ESC]) and activation (fibrinogen receptor binding capacity, i.e. CD41 receptor surface expression) in response to differential stimuli. Adenosine diphosphate (ADP) and platelet-activating factor (PAF) are both important activators of platelets, inducing aggregation and specifically causing robust fibrinogen receptor mobilization via distinct receptors and pathways14–16. We hypothesize that 1) female platelets have increased aggregation and activation with ADP and PAF stimulation compared to males and 2) treatment of platelets with estradiol will enhance male and female platelet aggregation and activation.
Methods
Apheresis platelets from healthy volunteers, specifically from premenopausal and postmenopausal females (menopausal state determined by age cut off of ≤ 54 years, the average age of menopause17) and similarly aged males, were obtained under a Colorado Multiple Institute Review Board approved protocol (COMIRB#00–004). As healthy males or females presented for voluntary donation to a blood donation center, platelets were acquired for each age group: 18–54 years for males and females and > 54 years for males and females. Platelet function was assessed by aggregometry and flow cytometry. For aggregation, 3 ×10^5 platelets/µl were placed in a Chronolog Aggrogometer (Model 490), activated with either 20 µM of ADP or 2 µM of PAF and monitored for extent shape change (ESC) over time. ESC was calculated as a percent (%) of change from baseline (platelet-rich plasma, no stimulant). For flow cytometry, platelets were diluted to a concentration of 60 × 10^3 platelets/µl, activated with 20 µM ADP (Chronolog) for five minutes or 2 µM PAF (Sigma Chemical Co.) for 10 minutes, and fixed with 1% paraformaldehyde. The fixed platelets were then incubated with either a PE-labeled isotype control or PE-labeled CD41 (fibrinogen receptor; BD Bioscience) to assess fibrinogen binding capacity by flow cytometry (BD FACSCanto™ II). Levels of CD41 were measured as mean fluorescent intensity (MFI) with the isotype control subtracted out.
Estradiol (Sigma Aldrich Co.) was dissolved in minimal quantity of 0.9% NaCl. Aggregation and flow cytometry were measured after incubating platelets in 105 pg/ml of estradiol or vehicle control for 15 min at 37oC, a physiologic level in healthy premenopausal females during mid-estrus18.
Statistical analysis was performed in R19. ESC (%) and CD41 (MFI) were compared between sexes with a Mann-Whitney test due to non-normal distribution. ESC and CD41 MFI in the presence of estradiol or normal saline control were compared with the Wilcoxon signed rank test. Significance was determined at p < 0.05. Power analysis was conducted in R to determine a sufficient sample size using an alpha of 0.05, a power of 0.80, a large effect size, and two tails, with an equal allocation of participants into each group. Based on the aforementioned assumptions, the desired sample size was calculated as 20 per group (male or female).
Results
Fifty-three healthy volunteers were included in this study: 12 premenopausal females, 13 postmenopausal females, and 18 similarly aged males (15 young male and 13 older males). The average ages were 30.3 years (range 24–52) in the premenopausal females, 62.2 years (range 58–72) in the postmenopausal females, 38.2 years (range 25–53) in the younger males, and 64.9 years (range 55–71) in the older males.
Platelet aggregation
Platelet aggregation was assessed by extent shape change (ESC) after stimulation with ADP or PAF. Compared to males, female platelets had significantly increased aggregation with ADP stimulation, with a median ESC of 35.5% (32.7–39.2% interquartile range [IQR]) versus 32.4% (27.5–36.6% IQR) in males (p=0.03) (Table 1, Figure 1). There was no difference in platelet aggregation after PAF stimulation between females and males.
Table 1. Aggregation of platelets after ADP or PAF stimulation by sex.
Data presented as median (25–75 interquartile range). P value comparing male and female aggregation using Mann-Whitney* or Kruskal-Wallis† tests as appropriate.
| Stimulant | ESC (%) | |||
|---|---|---|---|---|
| ADP | p value | PAF | p value | |
| By Sex | ||||
| Females (n=23) | 35.5 (31.7−39.2) | 0.03* | 37.0 (31.0−40.0) | 0.49* |
| Males (n=28) | 32.4 (27.5−36.6) | 34.0 (29.9−40.2) | ||
| By Sex and Age | ||||
| Premenopausal females (n=11) | 35.2 (31.7−38.2) | 0.18† | 33.2 (28.2−37.0) | 0.05† |
| Postmenopausal females (n=12) | 36.0 (34.4−39.3) | 39.6 (37.0−41.3) | ||
| Young males (n=15) | 32.8 (28.5−36.7) | 31.2 (30.3−40.2) | ||
| Older males (n=13) | 29.3 (24.7−37.2) | 37.2 (25.1−40.0) | ||
ADP=adenosine diphosphate, PAF=platelet activating factor, ESC=extent shape change
Figure 1. Platelet aggregation, as assessed by extent shape change (ESC), after adenosine diphosphate (ADP) stimulation (a) and platelet activating factor (PAF) simulation (c) and estradiol pre-treatment (b, d).
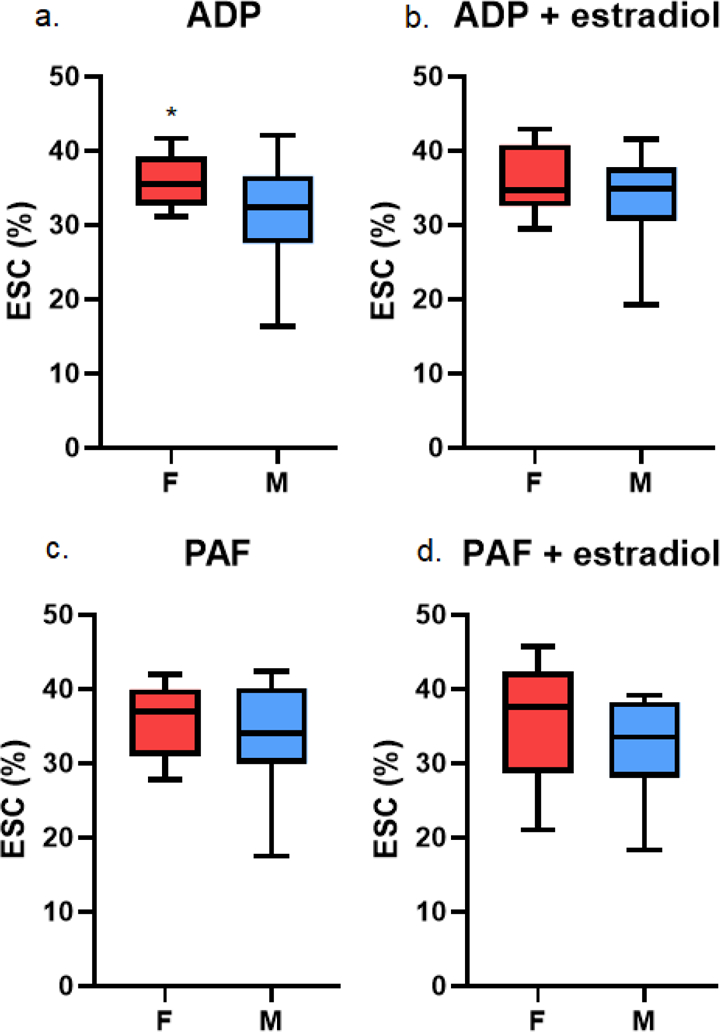
Asterisks indicates p < 0.05.
To look at the effect of age and menopause, we stratified males by age and females by menopausal state. We did not identify an effect on platelet aggregation or activation by age or menopausal state (Table 1).
Platelet activation
Platelets were assessed for activation by evaluating CD41 receptor surface expression. At baseline (unstimulated control platelets), there was no difference in CD41 receptor surface expression in male versus female platelets. Platelets were stimulated with both ADP and PAF. Female platelets had a robust activation in response to ADP and minimal response to PAF, as evidenced by CD41 receptor surface expression (increase from 2,474 [1,842–3,935 IQR] to 3,236 [2,267–5,050 IQR] after ADP [p=0.02] versus 2,207 [1,508–3,895 IQR] to 2,730 [1,888–4,093 IQR] after PAF [p=0.21]) (Table 2, Figure 2). In contrast, male platelets had minimal activation with ADP stimulation and robust activation with PAF stimulation (CD41 receptor surface expression of 3,123 [1,762–3,643 IQR] to 3,203 [1,712–4,662 IQR] with ADP, p=0.07; 2,957 [1,657–3,708 IQR[ to 3,045 [2,173–5,279 IQR] with PAF, p=0.04) (Table 2, Figure 2).
Table 2. Platelet activation, by CD41 receptor surface expression, of platelets after ADP or PAF stimulation by sex.
Data presented as median (25–75 interquartile range). P value reflective of Wilcoxon signed rank test.
| Stimulant | CD41 Receptor Surface Expression (MFI) | ||||||
|---|---|---|---|---|---|---|---|
| Control | ADP | p value | Control | PAF | p value | ||
| By Sex | |||||||
| Females (n=23) | 2474 (1842−3935) | 3236 (2267−5050) | 0.02 | 2207 (1508−3895) | 2730 (1888−4093) | 0.21 | |
| Males (n=24) | 3123 (1762−3643) | 3203 (1712−4662) | 0.08 | 2957 (1657−3708) | 3045 (2173−5279) | 0.04 | |
| By Sex and Age | |||||||
| Premenopausal females (n=11) | 2363 (1920−3881) | 3236 (2498−3860) | 0.12 | 2411 (1834−3895) | 2776 (1706−4249) | 0.70 | |
| Postmenopausal females (n=12) | 2633 (1483−4378) | 2853 (1830−5065) | 0.13 | 2192 (1464−3884) | 2492 (1981−3956) | 0.11 | |
| Young males (n=12) | 2876 (1402−3256) | 2429 (1658−5021) | 0.15 | 2471 (1129−3441) | 4200 (2614−5968) | 0.002 | |
| Older males (n=12) | 3632 (2053−3944) | 3623 (2074−4571) | 0.30 | 3632 (2053−3944) | 2490 (1391−4921) | 0.73 | |
ADP=adenosine diphosphate, PAF=platelet activating factor
Figure 2. Activation, as assessed by CD41 receptor surface expression (in mean fluorescence intensity [MFI]), in females (red shades) and in males (blue shades) in platelets (a) and estradiol pre-treated platelets (b) after adenosine diphosphate (ADP) stimulation and platelet activating factor (PAF) simulation.
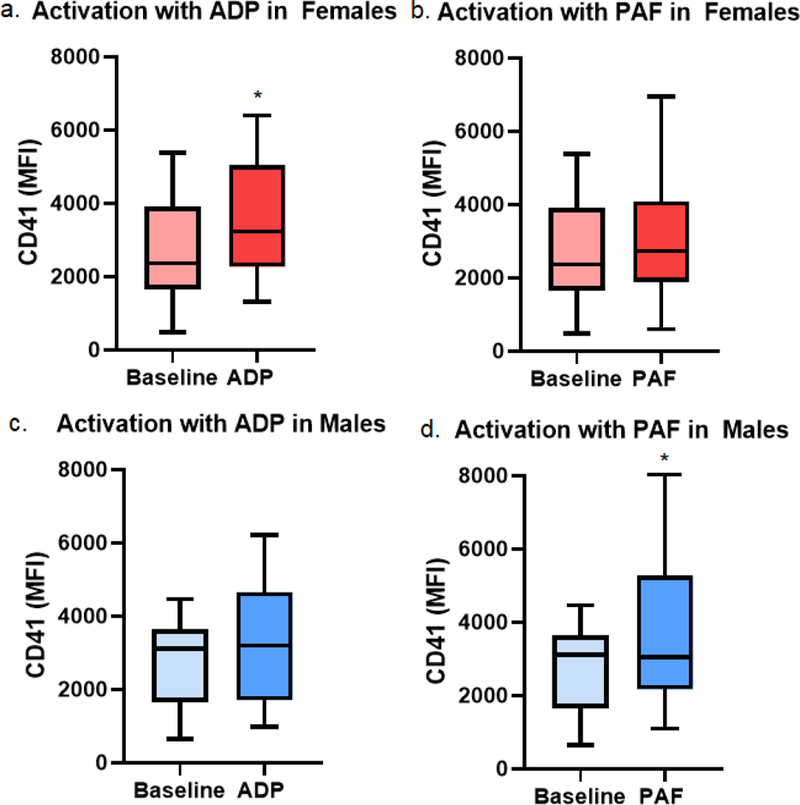
Asterisks indicates p < 0.05 as compared to baseline.
To evaluate the effect of age and menopause, we again stratified males by age and females by menopausal state. No difference was detected in platelet aggregation or activation potential between pre- and postmenopausal females. Upon stratifying males by age, it became evident younger male platelets were the only group to respond with robust platelet activation after PAF stimulation (4,200 [2,614–5,968 IQR] from 2,471 [1,129–3,441 IQR], p=0.002). This activation was not observed in the older males (2,490 [1,391–4,921 IQR] from 3,631 [2,053–3,944 IQR], p=0.73) (Table 2).
Platelet aggregation with estradiol pre-treatment
In a second set of experiments with 21 consecutive donors, platelets were pre-treated with estradiol, and aggregation and activation in response to ADP and PAF were measured, as above. This included four premenopausal females, five postmenopausal females, six younger males, and six older males.
There was no difference in aggregation of pre-treated platelets after ADP or PAF stimulation between females and males (Table 3, Figure 1). Compared to females, males had similar aggregation after ADP stimulation (ESC of 34.9% [30.6–37.8 IQR] versus 34.7% [32.6–40.8 IQR] in females) and PAF stimulation (33.5% [28.0–38.2 IQR] versus 37.6% [28.6–42.4 IQR] in females) (Table 3, Figure 1).
Table 3. Aggregation of native and estradiol pre-treated platelets after ADP or PAF stimulation by sex.
Data presented as median (25–75 interquartile range). P value reflective of Wilcoxon signed rank test.
| Stimulant ± Estradiol | ESC (%) | |||||
|---|---|---|---|---|---|---|
| ADP (Untreated) | ADP (Estradiol) | p value | PAF (Untreated) | PAF (Estradiol) | p value | |
| By Sex | ||||||
| Females (n=9) | 35.6 (34.9−39.6) | 34.7 (32.6−40.8) | 0.30 | 37.0 (30.6−40.3) | 37.6 (28.6−42.4) | 0.82 |
| Males (n=12) | 35.8 (32.7−39.4) | 34.9 (30.6−37.8) | 0.42 | 34.0 (29.9−40.2) | 33.5 (28.0−38.2) | 0.13 |
| By Sex and Age | ||||||
| Premenopausal females (n=4) | 35.6 (31.4−38.8) | 35.0 (30.8−38.6) | 0.12 | 33.2 (28.2−37.0) | 32.8 (22.8−38.5) | 0.89 |
| Postmenopausal females (n=5) | 35.9 (34.9−40.0) | 33.7 (32.6−42.4) | 0.62 | 39.6 (35.2−41.6) | 41.1 (33.4−44.7) | 0.31 |
| Young males (n=6) | 35.0 (31.0−39.4) | 34.0 (29.9−36.2) | 0.44 | 31.2 (30.2−40.8) | 31.7 (29.0−37.8) | 0.16 |
| Older males (n=6) | 37.0 (30.8−40.1) | 37.2 (28.8−39.2) | 0.84 | 37.2 (23.2−40.0) | 36.4 (22.5−38.6) | 0.56 |
ADP=adenosine diphosphate, PAF=platelet activating factor, ESC=extent shape change
Upon stratifying males and females by age and menopausal state respectively, we did not detect an effect by age or menopausal state on platelet aggregation or activation (Table 3).
Platelet activation with estradiol pre-treatment
After estradiol pre-treatment, the robust activation of female platelets with ADP stimulation was diminished (3,533 [2,422–4,682 IQR] from 2,641 [1,682–3,782 IQR], p=0.43) (Table 4, Figure 2). However, estradiol pre-treated female platelet activation significantly increased after PAF stimulation (3,231 [1,832–4,430 IQR] versus 4,581 [3,455–6,447 IQR], p=0.004) (Table 4, Figure 2). Similarly, in male platelets pre-treated with estradiol, activation did not change with ADP stimulation (2,469 [1,828–4,185 IQR] from 3,641 [1,677–4,274 IQR], p=0.99), but significantly increased with PAF stimulation as compared to untreated platelets (2,490 [1,391–4,921 IQR] versus 3,872 [2,195–5,691 IQR], p=0.01) (Table 4, Figure 2).
Table 4. Activation, as assessed by CD41 receptor surface expression, in native and estradiol-incubated platelets after ADP or PAF stimulation by sex.
Data presented as median (25–75 interquartile range). P value reflective of Wilcoxon signed rank test.
| CD41 Receptor Surface Expression (MFI) | ||||||
|---|---|---|---|---|---|---|
| Stimulant ± Estradiol | ADP (Untreated) | ADP (Estradiol) | p value | PAF (Untreated) | PAF (Estradiol) | p value |
| By Sex | ||||||
| Females (n=9) | 3860 (2842−5385) | 3533 (2422−4682) | 0.25 | 3231 (1832−4430) | 4581 (3455−6447) | 0.004 |
| Males (n=12) | 3623 (2074−4571) | 2469 (1828−4185) | 0.52 | 2490 (1391−4921) | 3872 (2195−5691) | 0.009 |
| By Sex and Age | ||||||
| Premenopausal females (n=4) | 4585 (3392−5435) | 3946 (3243−4615) | 0.25 | 2355 (1266−4251) | 4959 (3110−6784) | 0.12 |
| Postmenopausal females (n=5) | 3644 (1866−5267) | 2786 (1712−5163) | 0.81 | 3452 (2472−5531) | 4581 (3111−6080) | 0.06 |
| Young males (n=6) | 3870 (1848−4882) | 2088 (1680−5147) | 0.99 | 2490 (1233−5863) | 4544 (3322−6320) | 0.03 |
| Older males (n=6) | 3203 (1949−4536) | 2977 (1870−3985) | 0.44 | 2434 (1635−4349) | 2443 (2038−5434) | 0.31 |
ADP=adenosine diphosphate, PAF=platelet activating factor
Upon stratifying by age and menopausal state in males and females respectively, there were no differences in activation of female platelets by menopausal state with ADP or PAF stimulation. Younger male platelets treated with estradiol had a robust activation with PAF stimulation compared to platelets without estradiol pre-treatment (3,034 [2,000–4,223 IQR] versus 4,544 [3,322–6,320 IQR], p=0.03), whereas estradiol pre-treatment did not affect platelet activation with PAF in older male platelets (Table 4).
Discussion
This investigation characterizes sex dimorphisms in platelet function, specifically platelet aggregation (as reflected by ESC) and activation (as reflected by fibrinogen receptor surface expression). The results indicate that female and male platelets have sex-specific aggregation and activation potentials. Females had increased platelet aggregation with ADP stimulation as compared to males. Female platelets had robust activation with ADP stimulation, in contrast to male platelets which had increased activation with PAF stimulation, suggesting sex-dependent activation and receptor responses. Estradiol treatment effectively “feminized” the male platelet response to stimulation by PAF, enhancing activation (CD41 receptor surface expression) (schematic for visual representation, Figure 3).
Figure 3. Sex dimorphisms in platelet aggregation and activation potentials and feminization of the male platelet.
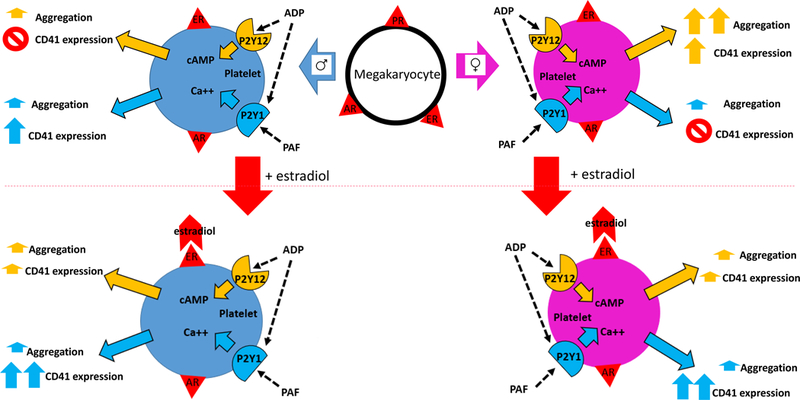
ADP=adenosine diphosphate, PAF=platelet activating factor, ER=estrogen receptor, AR=androgen receptor, PR=progesterone receptor, cAMP=cyclic adenosine monophosphate, Ca++=calcium.
This differential aggregation of platelets may be related to sex hormones, as suggested by both estrogen and testosterone receptors on megakaryocytes and platelets12. The topic of sex dimorphisms in platelet activity has been an area of controversy in the cardiovascular literature, with some studies indicating increased aggregation in females20,21, while others describe the opposite22. Part of this discrepancy may be explained by the stimuli employed, as the results of our investigation indicate that platelets behave differently by sex depending on the stimulating agent. In a study of platelet aggregation over the menstrual cycle in 16 healthy women, there was significant variation in the level of aggregation based on the agonist alone, from ADP to arachidonic acid (AA) to thrombin-receptor activating peptide (TRAP), regardless of the stage of menstrual cycle23. This suggests that circulating sex hormone levels alone do not explain the differential platelet responses, rather type of stimulation, in conjunction with hormones23. In a study of 32 women on estrogen-based hormone replacement therapy, investigators found stimulation of female platelets with ADP, but not thrombin, caused increased intracellular calcium levels24. Our results agree with previous reports of similar platelet aggregation between pre- and postmenopausal females and increased platelet aggregation in females versus males20,21,25. Part of the disagreement in the literature likely lies in the complexity of estradiol’s effects, from genomic to nongenomic, the latter which is of particular interest in anucleate platelets.
In this study, female platelets had increased activation with ADP, whereas male platelets had markedly increased activation with PAF. This differential behavior is likely due to ADP and PAF acting on different receptors and downstream intracellular cascades14. PAF, released from endothelial cells, platelets, and other cellular players, stimulates the P2Y1 receptor, a Gq-coupled receptor, which leads to an increase in intracellular calcium levels through PIP2/IP3 signaling26. The consequent increase in intracellular calcium potentiates shape change and platelet aggregation. In contrast, ADP, released from the dense granules of platelets, stimulates both the P2Y1 receptor and the P2Y12 receptor, the latter of which is a Gi-coupled receptor related to modulation of intracellular cAMP levels27,28. Activation of the P2Y12 receptor results in repressing baseline tonic inhibition, causing a decrease in cAMP levels and a cascade leading to thromboxane A2 production, alpha and dense granule release, expression of P-selection, cross-linking of fibrin, and platelet aggregation. Differential activation of these distinct pathways in male and female platelets may be the reason increased aggregation was observed in the female platelets with ADP stimulation, whereas there was no sex-difference with the less robust platelet stimulation of PAF. While ADP stimulation of P2Y12 is well-established, it is less well-understood about the effects of ADP on other similarly structured class P2Y receptors, including P2YT, which has been shown to be required for full aggregation potentiation and may be involved in the differential effects observed between males and females28. Ultimately, the differential response to ADP and PAF in male and female platelets in this study imply the activation and intracellular signaling of the P2Y1 and P2Y12 are complex and sex-specific. This is also suggested by the literature describing a distinct response to clopidogrel (a P2Y12 receptor inhibitor) by sex, such that females have higher resistance to clopidogrel compared to males29.
Characterization of sex dimorphisms in receptor biology and the roles of sex hormones is essential to understanding the differential performance of severely injured male and female patients in trauma-induced coagulopathy. Recently our group observed that following severe injury, females tolerate depressed clot strength, an effect of platelet and fibrinogen interactions, better than males, conferring a survival benefit for females following trauma5. This differential performance of platelets may be related to distinct responses to ADP and PAF and P2Y receptor signaling. In addition to the platelets themselves, the downstream players from platelet signaling, specifically fibrinogen, may also play a role in the sex-specific performance in TIC and be affected by sex hormones. In a multicenter study of severely injured patients admitted to the intensive care unit (ICU), estradiol was positively correlated with rate of fibrin deposition and cross-linking and overall clot strength in both men and women, effects which may be due to augmentation of P2Y1 and P2Y12 stimulated pathways30. These works underline the critical importance of evaluating sex as a variable in biologic response, clinical research and basic scientific mechanisms. Ultimately, our therapies directed at resuscitation and attenuation of TIC and post-injury inflammation may best be achieved by differential approaches to sex-specific cellular capacities in male and female trauma patients.
Estradiol incubation feminized male platelet activation with PAF stimulation. These findings suggest estrogen and the estradiol receptor, known to be present and active on the surface of both male and female platelet membranes, have an important role in platelet behavior12. Upon activation, the platelet estradiol receptor ERβ can provoke several intracellular cascades, including involving the PIP2/IP3 signaling pathway, which is the same cascade downstream of P2Y131. Therefore, estradiol may augment the PAF-initiated calcium signaling that ultimately causes increased platelet activation through convergence on and augmentation of the PIP2/IP3 signaling. The reported experiments demonstrate, however, that the signaling pathways are complex (unlikely related to single signaling cascade point) and sex-specific. In this investigation, estradiol pre-treatment of female platelets did not change the activation potential with ADP, perhaps because there is a limitation to the additive effects of the P2Y and ERβ receptors or due to the chronicity of estradiol exposure in females at baseline. Literature has described a lack of responsiveness (and increased bleeding time) to ex vivo estradiol in platelets chronically exposed to the female sex hormones32. In addition, the circulating estradiol in female donors may be relevant. Specifically, there may be a dose response effect with estradiol, such that circulating levels and/or receptor number and type dictate the extent of pro-fibrinogen binding effect of estrogen (whether through changes in levels of fibrinogen receptor, threshold of activation, extent of downstream signaling or augmentation of the fibrinogen receptor effect through amplification of the P2Y receptors). This dose-response effect is suggested in that platelets in premenopausal females are known to have differential fibrinogen receptor activation over the menstrual cycle, glycoprotein IIb-IIIa activation increase during the luteal phase as compared to the follicular phase, the biphasic periodicity of platelet adhesion to type I collagen over the menstrual cycle, and increased fibrinogen contribution to clot strength with increasing doses of in-vitro fertilization sex hormones11,33,34.
This investigation of platelet response to female sex hormones (estradiol) is limited in that we have not similarly evaluated for a sex-specific platelet response to androgens. In a similar investigation focused on the effects of sex hormones, Banerjee et. al. added supraphysiologic levels of testosterone to platelets of healthy male and female donors and found that testosterone increased male ADP-induced platelet aggregation and nitric oxide synthase and thromboxane A2 production35. However, testosterone had no effect on female platelets. Taken together, findings from our current investigation and those from Banerjee et. al. support the concept that sex hormones have differential effects on platelets and suggest that balance of hormonal stimuli should be evaluated.
The feminization of male platelet activation with estradiol highlights a potential role for therapeutic hormone receptor targets and consideration of estradiol therapy in males with TIC and hemorrhagic shock. Administration of estradiol has been linked to abrogation of hemorrhagic shock in female murine models35–38 and in a study of murine hemorrhagic shock in males, estradiol treatment was associated with improved cardiovascular performance and hepatocellular function39. The concept of sex hormones as a therapeutic adjunct in humans has been described in other specialties, including orthotopic liver transplantation in which conjugated estrogen has been shown to reduce transfusion requirements, and in neurotrauma, in which progesterone was attempted as a therapeutic adjunct in traumatic brain injury40,41. The effects of estradiol on coagulation require further evaluation in the in vivo setting to establish efficacy, but the differential platelet receptor performance in males versus females offers novel hypotheses for future mechanistic and clinical investigations. In addition to future resuscitation considerations, these results suggest that current transfusion practices might be enhanced to exploit the differential performance of female platelets. The sex of donor platelets may need to be considered, and preferentially selected for, in blood component resuscitation of TIC after severe injury.
Limitations of our work include a lack of granularity in assessment of oral hormonal therapy and biochemical confirmation of menopausal state. Future investigations could be enhanced by precise documentation of oral contraception or hormone replacement therapy, as well as consideration of biochemical confirmation of estradiol levels. This investigation focused on a singular sex hormone (estradiol) for treatment of platelets. Future investigations to fully characterize sex hormone response of platelets should include expanded sex hormone stimulation with multiple estrogen and androgen players (testosterone, progesterone, estradiol, dihydroepiandrosterone). While our study was adequately powered for a comparison of platelets from males versus females, the smaller sample size to compare the response of platelets from males versus females after estradiol pre-treatment may be underpowered and will be further examined in a larger sample size. Lastly, given our platelets were received from the donor center, we were unable to assess basic demographic factors such as race, which is known to have a relationship with platelet biology. A larger sample size and inclusion of demographic data will be included in future prospective studies.
In sum, male and female platelets have sex-specific aggregation and activation potentials and responses to stimuli, which can be augmented with estrogen pre-treatment and feminize male platelet activation response. These data offer a potential explanation and generate novel hypotheses for sex-based differential performance of platelets in TIC, suggesting that cellular and sex hormone biology impart thrombotic potential and may contribute to patient outcomes following severe injury. Sex dimorphisms in receptor function and platelet behavior offer potential therapeutic targets and ultimately question whether donor sex of transfused platelets should be considered in blood component resuscitation strategies. Future experiments are required to delineate the dose-response of estradiol effects, any potential role of testosterone given the concomitant presence of androgen receptors on platelets, biochemical confirmation of hormonal state in females alongside platelet function testing, and establish normal ranges for ADP- and PAF-induced platelet responsiveness by sex of donor. Estradiol may also serve as a novel therapeutic adjunct in platelet dysfunction and requires additional rigorous in vitro and animal model investigation.
Acknowledgments
Funding
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health (T32 GM008315 and P50 GM049222) and Department of Defense (USAMRAA, W81XWH-12–2-0028), as well as the Foundation for Women and Girls with Bleeding Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other sponsors of the project.
Footnotes
Conflict of Interest
There are no conflicts of interest to report.
This work was presented at the 37th Annual Meeting of the Western Trauma Association, March 3–8, 2019 in Snowmass, Colorado.
References
- 1.Scarpelini S, Rhind SG, Nascimento B, Tien H, Shek PN, Pheng HT, Huang H, Pinto R, Speers V, Reis M, et al. Normal range values for thromboelastography in healthy adult volunteers. Brazilian Journal of Medical and Biological Research 2009;42(12):1210–1217. [DOI] [PubMed] [Google Scholar]
- 2.Gorton HJ, Warren ER, Simpson NA, Lyons GR, Columb MO. Thromboelastography identifies sex-related differences in coagulation. Anesthesia and Analgesia 2000;91(5):1279–1281. [DOI] [PubMed] [Google Scholar]
- 3.Roeloffzen WW, Kluin-Nelemans HC, Mulder AB, Veeger NJ, Bosman L, de Wolf JT. In normal controls, both age and gender affect coagulability as measured by thrombelastography. Anesthesia and Analgesia 2010;110(4):987–994. [DOI] [PubMed] [Google Scholar]
- 4.Deitch EA, Livingston DH, Lavery RF, Monaghan SF, Bongu A, Machiedo GW. Hormonally active women tolerate shock-trauma better than do men: a prospective study of over 4000 trauma patients. Annals of Surgery 2007;246(3):447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman JR, Moore EE, Samuels JM, Cohen MJ, Sauaia A, Sumislawski JJ, Ghasabyan A, Chandler J, Banerjee A, Silliman CC, et al. Trauma resuscitation considerations: sex matters. Journal of the American College of Surgeons 2018; In Press. [DOI] [PMC free article] [PubMed]
- 6.Misz M, Beck P, Flora-Nagy M. Effect of hormonal contraceptives on the coagulation system with special reference to its inhibitors. Orvosi Hetilap 1985;126(43):2635–2640. [PubMed] [Google Scholar]
- 7.von Kaulla E, Droegemueller W, Aoki N, von Kaulla KN. Effect of estrogens on postpartum hypercoagulability and antithrombin 3 activity. American Journal of Obstetrics and Gynecology 1972;113(7):920–926. [DOI] [PubMed] [Google Scholar]
- 8.von Kaulla E, Droegemueller W, von Kaulla KN. Conjugated estrogens and hypercoagulability. American Journal of Obstetrics and Gynecology 1975;122(6):688–692. [DOI] [PubMed] [Google Scholar]
- 9.Zahn CM, Gonzalez DI Jr., Suto C, Kennedy S, Hines JF. Low-dose oral contraceptive effects on thromboelastogram criteria and relationship to hypercoagulability. American Journal of Obstetrics and Gynecology 2003;189(1):43–47. [DOI] [PubMed] [Google Scholar]
- 10.Bremme KA. Haemostatic changes in pregnancy. Best Practice & Research Clinical Haematology 2003;16(2):153–168. [DOI] [PubMed] [Google Scholar]
- 11.Faraday N, Goldschmidt-Clermont PJ, Bray PF. Gender differences in platelet GPIIb-IIIa activation. Thrombosis and Haemostasis 1997;77(4):748–754. [PubMed] [Google Scholar]
- 12.Khetawat G, Faraday N, Nealen ML, Vijayan KV, Bolton E, Noga SJ, Bray PF. Human megakaryocytes and platelets contain the estrogen receptor beta and androgen receptor (AR): testosterone regulates AR expression. Blood 2000;95(7):2289–2296. [PubMed] [Google Scholar]
- 13.Chen LY, Mehta JL. Further evidence of the presence of constitutive and inducible nitric oxide synthase isoforms in human platelets. Journal of Cardiovascular Pharmacology 1996;27(1):154–158. [DOI] [PubMed] [Google Scholar]
- 14.Gremmel T, Yanachkov IB, Yanachkova MI, Wright GE, Wider J, Undyala VV, Michelson AD, Frelinger AL, Przyklenk K. Synergistic Inhibition of Both P2Y1 and P2Y12 Adenosine Diphosphate Receptors As Novel Approach to Rapidly Attenuate Platelet-Mediated Thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology 2016;36(3):501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keating FK, Schneider DJ. The influence of platelet activating factor on the effects of platelet agonists and antiplatelet agents in vitro. Journal of Thrombosis and Thrombolysis 2009;28(1):38–45. [DOI] [PubMed] [Google Scholar]
- 16.Storey RF, Newby LJ, Heptinstall S. Effects of P2Y(1) and P2Y(12) receptor antagonists on platelet aggregation induced by different agonists in human whole blood. Platelets 2001;12(7):443–447. [DOI] [PubMed] [Google Scholar]
- 17.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas 2008;61(1–2):4–16. [DOI] [PubMed] [Google Scholar]
- 18.Elmlinger MW, Kuhnel W, Ranke MB. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clinical Chemistry and Laboratory Medicine 2002;40(11):1151–1160. [DOI] [PubMed] [Google Scholar]
- 19.dplyr: Hadley Wickham, Romain François, Lionel Henry and Kirill Müller (2018). dplyr: A Grammar of Data Manipulation. R package version 0.7.7 https://CRAN.R-project.org/package=dplyr.
- 20.Boudoulas KD, Montague CR, Goldschmidt-Clermont PJ, Cooke GE. Estradiol increases platelet aggregation in Pl(A1/A1) individuals. American Heart Journal 2006;152(1):136–139. [DOI] [PubMed] [Google Scholar]
- 21.Miller CH, Rice AS, Garrett K, Stein SF. Gender, race and diet affect platelet function tests in normal subjects, contributing to a high rate of abnormal results. British Journal of Haematology 2014;165(6):842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu GJ, Lee JJ, Chou DS, Jayakumar T, Hsiao G, Chen WF, Sheu JR. Inhibitory signaling of 17beta-estradiol in platelet activation: the pivotal role of cyclic AMP-mediated nitric oxide synthase activation. European Journal of Pharmacology 2010;649(1–3):140–149. [DOI] [PubMed] [Google Scholar]
- 23.Melamed N, Yogev Y, Bouganim T, Altman E, Calatzis A, Glezerman M. The effect of menstrual cycle on platelet aggregation in reproductive-age women. Platelets 2010;21(5):343–347. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Martinez MC, Labios M, Hermenegildo C, Tarin JJ, O’Connor E, Cano A. The effect of hormone replacement therapy on Ca2+ mobilization and P-selectin (CD62P) expression in platelets examined under flow cytometry. Blood Coagulation & Fibrinolysis 2004;15(1):1–8. [DOI] [PubMed] [Google Scholar]
- 25.Singla A, Bliden KP, Jeong YH, Abadilla K, Antonino MJ, Muse WC, Mathew DP, Bailon O, Tantry US, Gurbel PA. Platelet reactivity and thrombogenicity in postmenopausal women. Menopause 2013;20(1):57–63. [DOI] [PubMed] [Google Scholar]
- 26.Jones S, Evans RJ, Mahaut-Smith MP. Ca2+ influx through P2X1 receptors amplifies P2Y1 receptor-evoked Ca2+ signaling and ADP-evoked platelet aggregation. Molecular Pharmacology 2014;86(3):243–251. [DOI] [PubMed] [Google Scholar]
- 27.Cattaneo M P2Y12 receptors: structure and function. Journal of Thrombosis and Haemostasis 2015;13 Suppl 1:S10–16. [DOI] [PubMed] [Google Scholar]
- 28.Eckly A, Gendrault JL, Hechler B, Cazenave JP, Gachet C. Differential involvement of the P2Y1 and P2YT receptors in the morphological changes of platelet aggregation. Thrombosis and Haemostasis 2001;85(4):694–701. [PubMed] [Google Scholar]
- 29.Hobson AR, Qureshi Z, Banks P, Curzen N. Gender and responses to aspirin and clopidogrel: insights using short thrombelastography. Cardiovascular Therapeutics 2009;27(4):246–252. [DOI] [PubMed] [Google Scholar]
- 30.Gee AC, Sawai RS, Differding J, Muller P, Underwood S, Schreiber MA. The influence of sex hormones on coagulation and inflammation in the trauma patient. Shock 2008;29(3):334–341. [DOI] [PubMed] [Google Scholar]
- 31.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, et al. Estrogen receptors: how do they signal and what are their targets. Physiological Reviews 2007;87(3):905–931. [DOI] [PubMed] [Google Scholar]
- 32.Valera MC, Gratacap MP, Gourdy P, Lenfant F, Cabou C, Toutain CE, Marcellin M, Saint Laurent N, Sie P, Sixou M, et al. Chronic estradiol treatment reduces platelet responses and protects mice from thromboembolism through the hematopoietic estrogen receptor alpha. Blood 2012;120(8):1703–1712. [DOI] [PubMed] [Google Scholar]
- 33.Tarantino MD, Kunicki TJ, Nugent DJ. The estrogen receptor is present in human megakaryocytes. Annals of the New York Academy of Sciences 1994;714:293–296. [DOI] [PubMed] [Google Scholar]
- 34.Harnett MJ, Bhavani-Shankar K, Datta S, Tsen LC. In vitro fertilization-induced alterations in coagulation and fibrinolysis as measured by thromboelastography. Anesthesia and Analgesia 2002;95(4):1063–1066. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee D, Mazumder S, Bhattacharya S, Sinha AK. The sex specific effects of extraneous testosterone on ADP induced platelet aggregation in platelet-rich plasma from male and female subjects. International Journal of Laboratory Hematology 2014;36(5):e74–77. [DOI] [PubMed] [Google Scholar]
- 36.Deitch EA, Ananthakrishnan P, Cohen DB, Xu DZ, Feketeova E, Hauser CJ. Neutrophil activation is modulated by sex hormones after trauma-hemorrhagic shock and burn injuries. American Journal of Physiology Heart and Circulatory Physiology 2006;291(3):H1456–1465. [DOI] [PubMed] [Google Scholar]
- 37.Machiedo GW, Zaets S, Berezina T, Xu DZ, Spolarics Z, Deitch EA. Red blood cell damage after trauma-hemorrhage is modulated by gender. The Journal of Trauma 2004;56(4):837–844. [DOI] [PubMed] [Google Scholar]
- 38.Doucet D, Badami C, Palange D, Bonitz RP, Lu Q, Xu DZ, Kannan KN, Colorado I, Feinman R, Deitch EA. Estrogen receptor hormone agonists limit trauma hemorrhage shock-induced gut and lung injury in rats. PLOS ONE 2010;5(2):e9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizushima Y, Wang P, Jarrar D, Cioffi WG, Bland KI, Chaudry IH. Estradiol administration after trauma-hemorrhage improves cardiovascular and hepatocellular functions in male animals. Annals of Surgery 2000;232(5):673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skolnick BE, Maas AI, Narayan RK, van der Hoop RG, MacAllister T, Ward JD, Nelson NR, Stocchetti N. A clinical trial of progesterone for severe traumatic brain injury. The New England Journal of Medicine 2014;371(26):2467–2476. [DOI] [PubMed] [Google Scholar]
- 41.Frenette L, Cox J, McArdle P, Eckhoff D, Bynon S. Conjugated estrogen reduces transfusion and coagulation factor requirements in orthotopic liver transplantation. Anesthesia and Analgesia 1998;86(6):1183–1186. [DOI] [PubMed] [Google Scholar]


