Abstract
Skin biopsies are commonly used for the assessment of skin pathology in various skin diseases including atopic dermatitis (AD). However, due to the invasive nature of skin biopsies, many patients, particularly children, decline participation. This can lead to potential subject sampling bias as data could be skewed toward more severe, older patients willing to give biopsies. Recently, researchers have began studying the skin with a minimal, non-invasive technique using skin tape strips (STS) to profile the epidermal transcriptome, proteins, and lipids in the skin. However, side by side comparisons of skin biopsy and STS have not been done to assess epidermal penetration. Therefore, 20 STS were collected from the volar surface of forearm from healthy non-atopic subjects and patients with AD, followed by the collection of skin biopsies from adjacent non-taped and taped area of skin. Using H&E staining and immunostaining, we demonstrated that 20 STS reach the upper granular layer of the epidermis. Additionally, we found that the expression of terminal differentiation markers in samples from STS procedure positively correlated with the expression level of these markers in matching skin biopsies. Therefore, STS is a non-invasive and reliable approach to evaluate the expression of skin terminal differentiation markers, which are defective in AD skin.
TO THE EDITOR
Atopic dermatitis (AD) is the most common inflammatory skin disease in the general population. It is characterized by skin barrier defects, and immune defects, (Brunner et al., 2018, Simpson et al., 2018). The pathophysiology of AD skin has been extensively studied using various technologies with skin biopsies (Berdyshev et al., 2018, Gittler et al., 2012, Kim et al., 2016, Lee et al., 2017, Nakatsuji et al., 2016). However, due to the invasive nature of skin biopsies, only a minority of study subjects are willing to undergo this procedure. Additionally, it is difficult to collect skin biopsies from children due to ethical issues and lack of cooperation, despite AD being most prevalent in children. Therefore, many patients, particularly children, decline participation, leading to potential bias as data and our knowledge about AD may be skewed toward more severe patients. Given that the major defect in AD is due to lack of terminal keratinocyte differentiation, we and others have developed a minimal, non-invasive technique using skin tape stripping (STS) to examine skin expression of specific genes, lipids and protein in the stratum corneum (Berdyshev et al., 2018, Broccardo et al., 2009, Di Nardo et al., 2016, Dyjack et al., 2018, Kezic et al., 2011, Li et al., 2017, McAleer et al., 2018). However, there have been no side-by-side studies that compared skin biopsies and STS to determine how deeply the STS procedure can reach into the epidermis. Furthermore, it is not known whether the same information about the stratum corneum (SC) can be derived from the STS as compared to skin biopsies.
Therefore, we performed a STS procedure followed by 2mm skin biopsy of the taped skin versus adjacent non-taped skin. A total of 20 consecutive D-Squame tape discs were applied to the same adjacent site of volar side of the forearm of normal healthy subjects and lesional and nonlesional (5 cm from lesion) skin of AD patients. Twenty consecutive STS did not cause bleeding or scarring. Six normal subjects and six AD patients were enrolled. This study was approved by the Institutional Review Board at National Jewish Health, Denver and written informed consent was obtained from each participant.
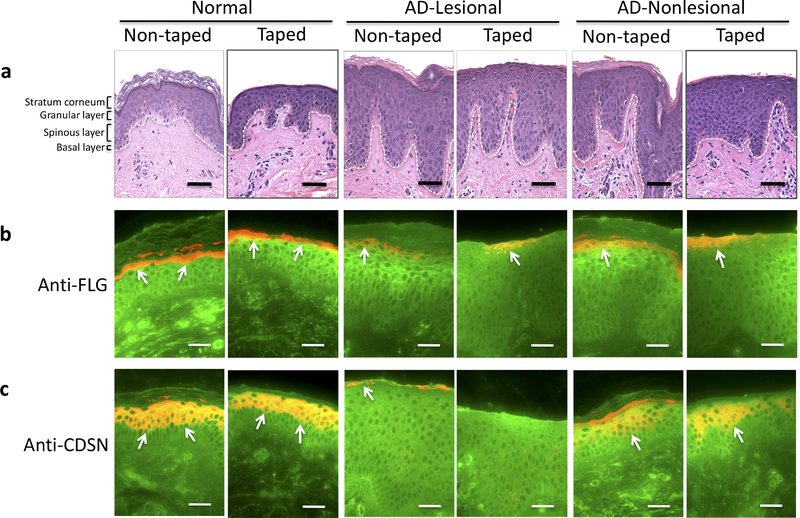
Initially, we performed hematoxylin and eosin (H&E) staining of skin biopsies using a standard method. Representative images (from a total of 6 skin biopsies in each study group) are shown in figure 1, after 20 STS of the skin from a normal subject and from lesional and nonlesional skin of an AD patient. Next, skin biopsies were stained for selected epidermal proteins including filaggrin (FLG) and corneodesmosin (CDSN). FLG and CDSN are terminal differentiation markers that are highly expressed in the upper part of the epidermis (Candi et al., 2005, Lee et al., 2017). Immunostaining demonstrated presence of FLG (Figure 1b) and CDSN (Figure 1c) in the SC and the granular layer of the epidermis. After the STS procedure, FLG (Figure 1b) and CDSN (Figure 1c) from the SC and the upper part of granular layer were removed both in healthy controls and AD subjects. Therefore, data from H&E and immunofluorescence staining suggest that 20 consecutive STS reach the upper part of the granular layer of the epidermis.
Figure 1.
H&E and immunofluorescence staining of skin biopsies before and after STS procedure. Skin biopsies were collected from taped and non-taped skin area. Representative images for skin samples from healthy control subjects and AD patients are shown. Skin biopsies from normal healthy subjects, AD-lesional, and AD-nonlesional skin were stained with a standard H&E staining protocol (a). Immunofluorescence staining for FLG (red, b) and CDSN (red, c) are shown. Wheat germ agglutinin-conjugated fluorescein isothiocyanate (green) was used to stain the cytoskeleton. Arrows point to FLG or CDSN staining. All images were collected at original magnification × 400. Bar = 50 μm.
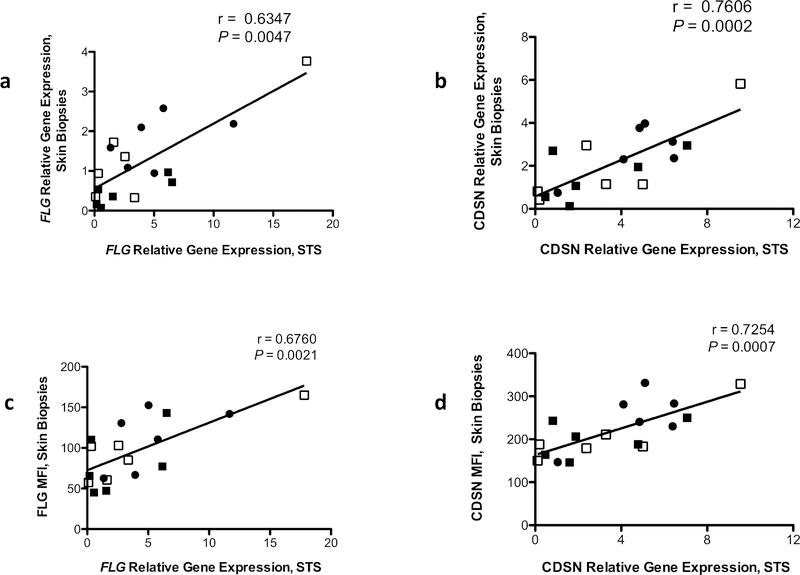
We further examined whether STS procedure is a reliable method to evaluate the expression of epidermal differentiation markers including FLG, CDSN, loricrin (LOR), involucrin (IVL), and keratin (KRT)-1; these targets are mainly expressed in the SC and the upper granular layer of the epidermis and are decreased in AD skin (Broccardo et al., 2009, Candi et al., 2005, Lee et al., 2017). Total RNA was extracted from STS samples and skin biopsies from the matching subjects as described earlier (Dyjack et al., 2018, Nomura et al., 2003). Gene expression of epidermal differentiation markers were evaluated using real time RT-PCR as described (Nomura et al., 2003). Expression of FLG, CDSN, LOR, IVL, and KRT-1 were detected in all RNA samples from both STS and skin biopsies. Importantly, gene expression of FLG (Figure 2a) and CDSN (Figure 2b), LOR (Supplementary Figure 1a), IVL (Supplementary Figure 1b), and KRT-1 (Supplementary Figure 1c) from STS RNA samples and matching skin biopsies showed positive correlations. Additionally, we examined whether the gene expression levels of epidermal differentiation markers from STS correlate with the protein expression levels of these markers in matching skin biopsies. The gene expression levels of FLG and CDSN from STS samples were measured using real time RT-PCR, and the protein levels of FLG and CDSN from skin biopsies were evaluated using immunofluorescence staining. The STS samples gene expression levels of FLG (Figure 2c) and CDSN (Figure 2d) positively correlated with the staining intensities of FLG and CDSN in matching skin biopsies. These findings suggest that STS procedure is a reliable method to evaluate the expression of epidermal differentiation markers.
Figure 2.
The correlation of epidermal differentiation markers expression in samples from STS and those from the matching skin biopsies. Gene and protein expressions of FLG and CDSN were evaluated using real time RT-PCR and immunofluorescence staining, respectively. Correlations of FLG (a) and CDSN (b) between RNA samples from STS procedure and the matching skin biopsies are shown. Significant Pearson correlations between gene levels of FLG (c) and CDSN (d) from STS procedure and staining intensities of FLG and CDSN in the matching subjects are shown. ●= normal healthy skin, ■= AD-lesional skin, □= AD-nonlesional skin
STS procedure has a number of advantages over conventional skin biopsy sampling. 1) STS is non-invasive, the procedure does not cause scarring, is not painful and only minor skin irritation is observed during skin sampling. 2) STS procedure is a reliable method to examine the expression of skin terminal differentiation markers and may be utilized for the examination of defects in terminal differentiation in various skin disorders. 3) STS procedure can be useful for serial skin sampling in clinical trials that examine skin barrier function in response to therapy, as repeated skin biopsies over a short period in the same subject are challenging even in adults. 4) Due to its low cost and non-invasive nature STS allows collection of samples from large patient cohorts and can be useful for disease endotyping, as collected STS can be readily utilized for the lipid, protein and RNA analysis. 5) STS procedure can be useful for skin sampling in various age groups, including infants (Kim et al., 2016, McAleer et al., 2018).
In conclusion, STS maybe be a good standard procedure to evaluate epidermal differentiation markers from the SC and the upper granular layer of the epidermis. Moreover, this method is a safe and reliable strategy to evaluate cellular and molecular characteristics of skin barrier in both children and adults.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by NIH grants AR41256, UL1TR002535 and UL1TR002535. Additionally, the authors wish to acknowledge the Edelstein Family Foundation for their generous support of this work.
ABBREVIATIONS
- AD
Atopic dermatitis
- CDSN
Corneodesmosin
- CT
Cyclic threshold
- DSC
Desmocollin
- FLG
Filaggrin
- H-E
Hematoxylin and eosin
- IVL
Involucrin
- KRT
Keratin
- LOR
Loricrin
- SC
Stratum corneum
- STS
Skin tape stripping
Footnotes
CONFLICT OF INTEREST
We have nothing to declare.
DATA AVAILABILITY
No datasets were generated or analyzed during the current study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Berdyshev E, Goleva E, Bronova I, Dyjack N, Rios C, Jung J, et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight 2018;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broccardo CJ, Mahaffey SB, Strand M, Reisdorph NA, Leung DY. Peeling off the layers: skin taping and a novel proteomics approach to study atopic dermatitis. J Allergy Clin Immunol 2009;124(5):1113–5.e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner PM, Leung DYM, Guttman-Yassky E. Immunologic, microbial, and epithelial interactions in atopic dermatitis. Ann Allergy Asthma Immunol 2018;120(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 2005;6(4):328–40. [DOI] [PubMed] [Google Scholar]
- Di Nardo A, Holmes AD, Muto Y, Huang EY, Preston N, Winkelman WJ, et al. Improved clinical outcome and biomarkers in adults with papulopustular rosacea treated with doxycycline modified-release capsules in a randomized trial. J Am Acad Dermatol 2016;74(6):1086–92. [DOI] [PubMed] [Google Scholar]
- Dyjack N, Goleva E, Rios C, Kim BE, Bin L, Taylor P, et al. Minimally invasive skin tape strip RNA sequencing identifies novel characteristics of the type 2-high atopic dermatitis disease endotype. J Allergy Clin Immunol 2018;141(4):1298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 2012;130(6):1344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kezic S, O’Regan GM, Yau N, Sandilands A, Chen H, Campbell LE, et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy 2011;66(7):934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim BE, Lee J, Han Y, Jun HY, Kim H, et al. Epidermal thymic stromal lymphopoietin predicts the development of atopic dermatitis during infancy. J Allergy Clin Immunol 2016;137(4):1282–5.e4. [DOI] [PubMed] [Google Scholar]
- Lee UH, Kim BE, Kim DJ, Cho YG, Ye YM, Leung DY. Atopic dermatitis is associated with reduced corneodesmosin expression: role of cytokine modulation and effects on viral penetration. Br J Dermatol 2017;176(2):537–40. [DOI] [PubMed] [Google Scholar]
- Li S, Villarreal M, Stewart S, Choi J, Ganguli-Indra G, Babineau DC, et al. Altered composition of epidermal lipids correlates with Staphylococcus aureus colonization status in atopic dermatitis. Br J Dermatol 2017;177(4):e125–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAleer MA, Jakasa I, Hurault G, Sarvari P, McLean WHI, Tanaka RJ, et al. Systemic and stratum corneum biomarkers of severity in infant atopic dermatitis include markers of innate and T helper cell-related immunity and angiogenesis. Br J Dermatol 2019;180(3):586–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Two AM, Chun KA, Narala S, Geha RS, et al. Staphylococcus aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression. J Invest Dermatol 2016;136(11):2192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol 2003;171(6):3262–9. [DOI] [PubMed] [Google Scholar]
- Simpson EL, Villarreal M, Jepson B, Rafaels N, David G, Hanifin J, et al. Patients with Atopic Dermatitis Colonized with Staphylococcus aureus have a Distinct Phenotype and Endotype. J Invest Dermatol 2018;138(10):2224–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.