Abstract
Objective.
To analyze structural, longitudinal MRI findings during the development of accelerated knee osteoarthritis (AKOA) over 4 years.
Materials and Methods.
From the Osteoarthritis Initiative (OAI) knees with no radiographic osteoarthritis (KL 0/1) developing advanced-stage osteoarthritis (KL 3/4; AKOA) within a 4-year (y) timeframe were selected. MRIs were graded using the modified Whole-Organ Magnetic Resonance Imaging Score (WORMS) at the beginning of the 4y timeframe (index visit), at 2y, and 4y follow-up. Morphological and clinical findings associated with KL 3/4 onset within 2y compared to 4y were assessed using generalized estimating equations.
Results.
AKOA was found in 162 knees of 149 subjects (age 63.25±8.3; 103 females; BMI 29.4±3.9). Moderate to severe meniscal lesions WORMS ≥ 3 were present in 25% (41/162) at the index visit, 64% (104/162) at 2y and 93% (151/162) at 4y follow-up. Meniscal extrusion was the most prevalent finding (ranging from 18% at the index visit, 45% at 2y and 94% at 4y follow-up) and root tears were the most common types of tears (9% at the index visit; 22% at 2y and 38% at 4y). Risk factors associated with KL 3/4 onset within 2y included root tears at the index visit (adjusted OR, 2.82; 95% CI: 1.33, 6.00; p=0.007) and incident knee injury (42%, 49/116 vs. 24%, 11/46, p = 0.032).
Conclusion.
Meniscal abnormalities, in particular extrusion and root tears, were the most prevalent morphological features found in subjects with AKOA. These results suggest that meniscal abnormalities have a significant role in accelerated progression of OA.
Keywords: Osteoarthritis, Magnetic Resonance Imaging, Cartilage
Introduction
Knee osteoarthritis (OA) is the most common degenerative disorder of the knee that typically progresses slowly, over the course of many years [1]. However, 3.4% of adults at risk of knee OA have been observed to progress from no knee OA to advanced and end stage knee OA within four years or less [2, 3]. This form of accelerated disease onset with fast structural progression has previously been defined as accelerated knee OA (AKOA) [2, 3]. While several studies have evaluated features associated with structural progression in early structural disease [4-10], the rapid pace of degeneration raises the question as to what distinguishes AKOA from a more standard progression of OA. Moreover, subjects with AKOA were previously found to be more likely to receive a total knee replacement compared to those with no knee OA or compared to those with a more gradual knee OA onset [11]. Clinical risk factors associated with AKOA have been studied previously. Driban et al identified higher age, elevated body mass index (BMI), previous knee injury, and greater coronal tibial slope as risk factors for AKOA [12-14]. Recently, accelerated joint degeneration was also reported as a possible adverse event in patients with painful OA treated with monoclonal antibodies binding and inhibiting nerve growth factor (NGF, tanezumab, fulranumab) [15, 16]. As the accelerated degeneration mainly occurred in subjects using NGF in combination with non-steroidal anti-inflammatory drugs (NSAIDs), it is assumed that the medication induced joint pain reduction may have led to an increase in stress on the already damaged joint [17]. Imaging features frequently observed in knees developing AKOA include meniscal tears such as root and radial tears, meniscal extrusion and subchondral insufficiency fractures [18-20]. Moreover, adults with AKOA were recently characterized by early meniscal damage and subsequently larger bone marrow edema pattern (BMEP) [21]. However, the underlying biological processes causing the rapid degeneration in these subjects remain largely unknown.
The aim of our study was therefore to investigate longitudinal structural MRI findings occurring during AKOA development in knees with no OA (Kellgren-Lawrence (KL) 0/1) developing KL grade 3/4 AKOA in four years or less. Furthermore, we aimed to assess differences between rapid AKOA onset within two years compared to AKOA onset within four years and to assess findings in subjects with bilateral AKOA compared to subjects with unilateral AKOA.
Material and Methods
Study Subjects
We conducted a longitudinal, observational cohort study selecting subjects that met a KL-based AKOA definition, based on knees progressing from no OA (KL 0/1) to advanced-stage OA (KL 3/4) within four years or less [3]. Subjects were participants of the Osteoarthritis Initiative (OAI), a longitudinal, observational cohort study enrolling 4796 subjects with, or at risk of developing knee OA. Informed consent was obtained from all participants; the study was compliant with the Health Insurance Portability and Accountability Act and was approved by the local institutional review boards of all participating centers and in accordance with the 1964 declaration of Helsinki and the later amendments. Participants were recruited from February 2004 until May 2006 and followed over a period of 8 years. Specific datasets used were baseline and annual follow up clinical datasets 0.2.3, 3.2.1, 6.2.2, 8.2.1 and 10.2.2, physical exam and measurements datasets 0.2.2, 3.2.1, 6.2.1, 8.2.1 and 10.2.1, MRI datasets 0.E.2, 3.E.2, 6.E.2, 8.E.2 and 10.E.1, and central radiograph reading datasets kXR_SQ_BU 0.8, 3.7, 6.5, 8.2 and 10.2.
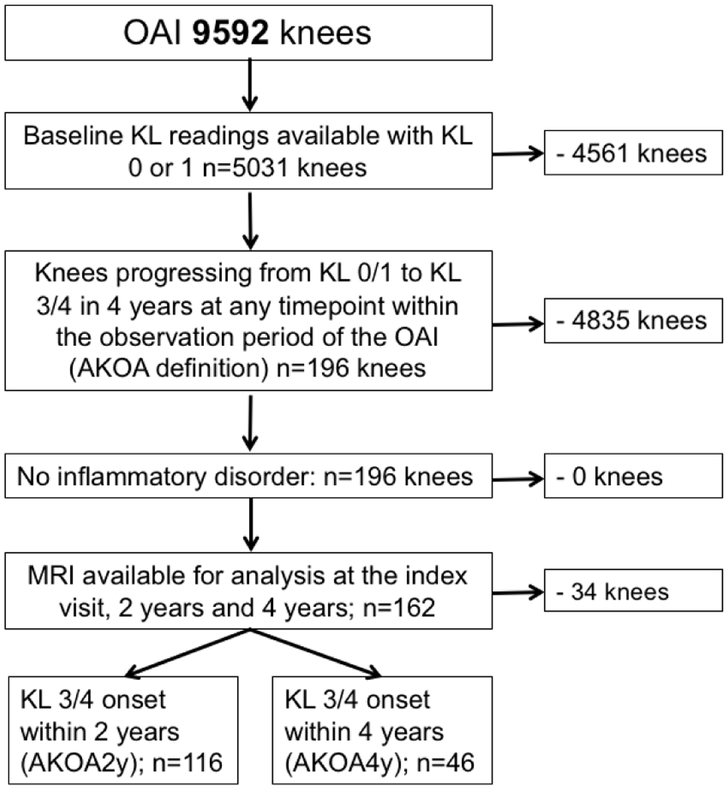
Bilateral knee radiographs were obtained annually from the OAI baseline visit to the 48 month clinic visit, and then at the 72 and 96 month visits [22] and centrally assessed for KL-grades [23]. We used KL grades, when available, from the OAI baseline visit, 24 months, 48 months, 72 months and 96 months to identify knees with accelerated knee OA development (KL 0/1 to KL3/4) within a 4 year period. The starting timepoint of this 4 year timeframe was defined as the index visit, the 4 year timeframe was defined as follow-up period. The knees that met this definition were defined as the index knees. Further selection criteria included MR available for evaluation at the index visit, two and four-years and no underlying inflammatory disorders. The subject selection process is shown in Figure 1.
Figure 1:
Subject selection flowchart.
For a secondary analysis, knees with AKOA were subdivided into KL 3/4 onset within two years (AKOA2y) and KL 3/4 onset within four years (AKOA4y) to assess differences between both AKOA forms. AKOA2y included knees in which either KL 3/4 was already present at two-year follow-up, or knees with KL 0/1 at year two and KL 3/4 at four-year follow-up.
Subject characteristics including age, sex, race, BMI, co-morbid diseases, knee injury and surgery before the index visit or during the follow-up period, family history of knee replacements, Physical Activity Score of the Elderly (PASE) [24] and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score [25] were obtained from clinical and physical exam datasets.
MR Imaging
MRIs were acquired on 3T MRI scanners (Siemens Magnetom Trio; Siemens, Erlangen, Germany) and standard transmit-receive knee coils (USA Instruments, Aurora, Ohio, USA). The acquisition protocol has been published previously [26]. Sagittal intermediate-weighted fat-suppressed turbo spin-echo (TSE) sequences (repetition time/echo time, 3200/30 ms; slice thickness, 3 mm), coronal intermediate-weighted non-fat suppressed TSE sequences (3700/29 ms; slice thickness, 3 mm) and sagittal 3D dual-echo in steady state (DESS) with selective water excitation (WE) (16.3/4.7 ms; slice thickness, 0.7 mm) were the main sequences used for analysis.
Longitudinal structural MRI findings were evaluated in all knees with AKOA at the index visit, at two and at four years. MRIs were graded by one radiologist (S.C.F. 3 years of experience) under supervision of a board certified musculoskeletal radiologist (T.M.L. 24 years of experience), blinded to radiographic OA grade and clinical data, using the modified Whole-Organ Magnetic Resonance Imaging Score (WORMS) [27]. Cartilage lesions (range 0-6) and BMEP (range 0-3) were each graded over 6 subregions (PAT, TRO, MF, LF, MT, LT) and meniscal abnormalities (range 0-4) were graded in 3 medial and 3 lateral subregions: meniscus body, anterior horn and posterior horn. Maximum scores across all subregions were calculated for each WORMS category. In addition, meniscal tears were graded as vertical tears, horizontal tears, flap tears, complex, bucket handle tears, or root tears and presence or absence of meniscal extrusion was documented with a cutoff of 3 mm for the medial and 2 mm for the lateral meniscus [28]. Insufficiency fractures were defined as BMEP with linear signal abnormalities along the subchondral bone as previously demonstrated by Plett et al [29]. Accelerated osteophyte formation (graded according to WORMS) was documented if there were no/minimal osteophytes at the index visit and severe osteophytes at two-four-year follow-up.
Inter-/intrareader reproducibility
Intra-and interreader reproducibility of WORMS grading by our group have been validated in multiple previous studies [30-34]. In these studies, intraclass correlation coefficients were calculated in order to compare WORMS subscores for the meniscus and cartilage. Intraclass correlation coefficients for intrareader reproducibility ranged between 0.80 (0.69-0.95) [32] and 0.96 (0.94-0.97) [31] for the meniscus and between 0.81 (0.68-0.91) [32] and 0.99 (0.98-0.99) [31] for the cartilage. Interreader intraclass correlation coefficients ranged between 0.81 (0.76-0.88) [32] and 0.97 (0.95-0.98) [31] for the meniscus and between 0.79 (0.72-0.868) [33] and 0.97 (0.95-0.98) [31] for the cartilage.
Statistical Analysis
Statistical analysis was performed with Stata v. 14 software (StataCorp, College Station, TX) using a 2-sided 0.05 level of significance. Differences between WOMAC pain scores between the index visit and at four-year follow-up were assessed using paired t-tests. Annual changes in WORMS imaging findings (linear trends) were assessed with generalized estimating equations, predicted values were calculated at each timepoint.
Differences in risk factors (index visit imaging parameters, index visit clinical parameters and knee injury or surgery before the index visit or during follow-up) were assessed between the AKOA groups AKOA2y and AKOA4y using AKOA2y or AKOA4y as the outcome and index visit clinical and imaging parameters as predictors: Pearson’s chi-square tests were used for categorical data, t-tests for numeric variables. Differences in index visit imaging findings were assessed using generalized estimating equations, adjusted for age, sex, race, and BMI. Moreover, differences in index visit imaging parameters and index visit clinical parameters were assessed between knees of subjects developing bilateral AKOA and knees of subjects developing unilateral AKOA using bilateral or unilateral AKOA as the outcome and index visit clinical and imaging parameters as predictors: Pearson’s chi-square tests were used for categorical data, t-tests for numeric variables. Differences in index visit imaging findings were assessed using generalized estimating equations, adjusted for age, sex, race, and BMI.
Results
Subject characteristics
Subject characteristics are demonstrated in Table 1. The majority of knees progressed from KL 0/1 to KL 3/4 within two years (72%, 116/162), compared to four years (28%, 46/162). Within the four-year observation period the WOMAC pain scores of the index knee increased significantly (2.18±2.87 to 3.15±3.48, p=0.003). Before the index visit 22% (36/162) knees reported a knee injury with 11/36 injuries occurring within the 2 years before the index visit. Four percent (6/162) had surgery of the index knee before the index visit, however, none of these occurred within two years before the index visit. During the follow-up period 22% (36/162) of the knees had a reported knee injury and 27% (43/162) underwent surgery. All surgeries performed during the follow-up period were arthroscopic partial meniscectomy surgeries. The two surgeries before the index visit included one arthroscopic partial meniscectomy surgery and one ligament repair surgery.
Table 1:
Subject characteristics
| Subject Characteristics | n=162 |
|---|---|
| Age (mean±SD) | 63.25±8.3 |
| Gender | |
| Females [n (%)]* | 103 (63.6) |
| Males [n (%)]* | 59 (36.4) |
| Body mass index (kg/m2) | |
| Index visit (mean±SD) | 29.4±3.9 |
| 4 year follow-up (mean±SD) | 29.9±4.5 |
| Weight change (Index visit – 4 year follow-up) | |
| Weight-loss over 10% [n (%)]* | 10 (6.2) |
| Weight-loss between 5%−10% [n (%)]* | 10 (6.2) |
| Stable weight [n (%)]* | 104 (64.2) |
| Weight-gain between 5%-10% [n (%)]* | 24 (13.8) |
| Weight-gain over 10% [n (%)]* | 14 (8.6) |
| Physical Activity Score for the Elderly (PASE) | |
| Index visit (mean±SD) | 169.5±83.0 |
| 4 year follow-up (mean±SD) | 151.3±82.3 |
| Race | |
| Caucasian [n (%)] * | 138 (85.2) |
| African American [n (%)] * | 23 (14.2) |
| Asian [n (%)]* | 0(0) |
| Other nonwhite [n (%)]* | 1 (0.6) |
| Family history of knee replacement [n (%)]* | 25 (15.4) |
| Diabetes Mellitus at baseline [n (%)]* | 7 (4.3) |
| High blood pressure at baseline [n (%)]* | 28 (17.3) |
| Injury index knee | |
| Before index visit [n (%)]* | 36 (22.2) |
| 2 years before index visit [n (%)]* | 11 (6.8) |
| During follow-up period [n (%)]* | 60 (37.0) |
| Surgery index knee | |
| Before index visit [n (%)]* | 6 (3.7) |
| 2 years before index visit [n (%)]* | 0(0) |
| During follow-up period [n (%)]* | 43 (26.5) |
| WOMAC knee pain index knee | |
| Index visit (mean±SD) | 2.18±2.87 |
| 4 year follow-up (mean±SD) | 3.15±3.48 |
Data are number of knees, with percentage in parentheses
Imaging findings
Longitudinal WORMS imaging findings at the index visit, two years and four years are shown in Table 2. All WORMS imaging categories showed a significant increase between index visit and four years (p<0.001 for all categories). Full-thickness cartilage lesions > 1cm in the tibiofemoral cartilage (WORMS grade ≥5) were not found in any of the knees at the index visit, in 20% (32/162) at two years, and in 61% (99/162) at four years. While 46% (75/162) of the knees had no focal defects and 35% (56/162) had <1 cm partial thickness cartilage defects at the index visit, at four-year follow-up all knees had focal cartilage defects and only 4 knees had <1 cm partial thickness defects. Interestingly, these four cases were all associated with severe meniscal extrusion.
Table 2:
Longitudinal WORMS imaging findings
| Variable | Index visita | 2-yearsa | 4-yearsa | Annual change | p-valueb,c |
|---|---|---|---|---|---|
| Cartilage maximum (max) | 2.90±0.1 | 3.79±0.1 | 4.69±0.1 | 0.45 (0.38-0.51) | <0.001 |
| Score of most severe tibiofemoral cartilage lesion * | |||||
| 0 [n (%)] | 54 (33.3) | 20 (12.3) | 0 (0) | ||
| 1 [n (%)] | 21 (13.0) | 10 (6.6) | 0 (0) | ||
| 2 [n (%)] | 56 (34.6) | 50 (30.9) | 4 (2.5) | ||
| 2.5 [n (%)] | 4 (2.5) | 6 (3.7) | 0 (0) | ||
| 3 [n (%)] | 26 (16.0) | 42 (25.9) | 54 (33.3) | ||
| 4 [n (%)] | 1 (0.6) | 2 (1.2) | 5 (3.1) | ||
| 5 [n (%)] | 0 (0) | 32 (19.8) | 76 (46.9) | ||
| 6 [n (%)] | 0 (0) | 0 (0) | 23 (14.2) | ||
| Meniscus max | 1.62±0.1 | 2.80±0.1 | 3.98±0.1 | 0.59 (0.54-0.64) | <0.001 |
| Score of most severe meniscal lesion * | |||||
| No tear [n (%)] | 28 (17.3) | 8 (4.9) | 0 (0) | ||
| Signal abnormality [n (%)] | 57 (35.2) | 26 (16.0) | 5 (3.1) | ||
| Simple tear [n (%)] | 36 (22.2) | 24 (14.8) | 6 (3.7) | ||
| Complex tear [n (%)] | 30 (18.5) | 55 (34.0) | 32 (19.8) | ||
| Maceration [n (%)] | 11 (6.8) | 49 (30.2) | 119 (73.5) | ||
| Bone marrow edema pattern (BMEP) max | 1.45±0.1 | 1.78±0.1 | 2.10±0.1 | 0.16 (0.12-0.20) | <0.001 |
| Effusion | 0.19±0.1 | 0.51±0.1 | 0.83±0.1 | 0.16 (0.13-0.19) | <0.001 |
Data are number of knees, with percentage in parentheses
Predicted values based on the GEE models
Significant values are in bold (p-value < 0.05)
Linear trend across all timepoints (index visit, 2 and 4 years) calculated using GEE
Moderate to severe meniscal lesions WORMS ≥ grade 3 were present in 25% (41/162) at the index visit, in 64% (104/162) at two-year follow-up and in 93% (151/162) at four-year follow-up. While 53% (85/162) had no tear and 22% (36/162) had a simple tear at the index visit, at four-year follow-up only 3% (5/162) had no tear and only 4% (6/162) had a simple tear. The types of meniscal lesions observed in the medial and lateral meniscus at the index visit, two-years and four-years are summarized in Table 3. Meniscal extrusion was frequently present in the AKOA cohort and found in 19% (30/162) of all AKOA knees at the index visit, 45% (73/162) at two-year follow-up and in 94% (153/162) at four-year follow-up. The most frequent types of observed meniscal tears were meniscal root tears (Fig. 2). These were present in 9% (14/162) at the index visit, 22% (36/162) at two years and in 38% (62/162) at four years. At the four year time point the most common location of root tears was the medial posterior horn in 65% of these cases (40/62), followed by the lateral posterior horn in 19% (12/62), the lateral anterior horn in 15% (9/62), and the medial anterior horn in one knee (2%; 1/62). Twenty-six tears (42%) were graded as full thickness tears and 36 (58%) were graded as partial tears. All meniscal root tears were associated with meniscal extrusion. Interestingly the number of observed root tears increased notably over the observation period, while the number of horizontal tears remained relatively consistent.
Table 3:
Meniscal abnormalities
| Index visit | 2-years | 4-years | ||||
|---|---|---|---|---|---|---|
| All* | n=162 | n=162 | n=162 | |||
| Vertical tear | 17 (10.5) | 13 (8.0) | 16 (9.9) | |||
| Horizontal tear | 38 (23.5) | 50 (30.9) | 31 (19.1) | |||
| Flap tear | 3 (1.9) | 11 (6.8) | 14 (8.6) | |||
| Root tear | 14 (8.6) | 36 (22.2) | 62 (38.3) | |||
| Meniscal extrusion | 30 (18.5) | 73 (45.1) | 153 (94.4) | |||
| Medial | Lateral | Medial | Lateral | Medial | Lateral | |
| Anterior horn* | ||||||
| Horizontal tear | 0 (0) | 3 (1.9) | 0 (0) | 4 (2.5) | 0 (0) | 5 (3.1) |
| Root tear | 0 (0) | 3 (1.9) | 1 (0.6) | 4 (2.5) | 1 (0.6) | 9 (5.6) |
| Meniscal body* | ||||||
| Vertical tear | 2 (1.2) | 5 (3.1) | 1 (0.6) | 4 (2.5) | 2 (1.2) | 3 (1.9) |
| Horizontal tear | 5 (3.1) | 4 (2.5) | 7 (4.3) | 5 (3.1) | 0 (0) | 2 (1.2) |
| Flap tear | 1 (0.6) | 2 (1.2) | 7 (4.3) | 2 (1.2) | 5 (3.1) | 4 (2.5) |
| Meniscal extrusion | 14 (8.6) | 16 (9.9) | 42 (25.9) | 31 (19.1) | 111 (68.5) | 42 (25.9) |
| Posterior horn* | ||||||
| Vertical tear | 7 (4.3) | 3 (1.9) | 7 (4.3) | 1 (0.6) | 9 (5.6) | 2 (1.2) |
| Horizontal tear | 16 (9.9) | 10 (6.2) | 24 (14.8) | 10 (6.2) | 12 (7.4) | 12 (7.4) |
| Flap tear | 0 (0) | 0 (0) | 1 (0.6) | 1 (0.6) | 4 (2.5) | 1 (0.6) |
| Root tear | 10 (6.2) | 1 (0.6) | 21 (13.0) | 10 (6.2) | 40 (24.7) | 12 (7.4) |
Types of meniscal abnormalities are listed as number of abnormalities observed, with percentage in parentheses; more than one type of abnormality could occur in one meniscus section of the same knee therefore percentages do not add up to 100%
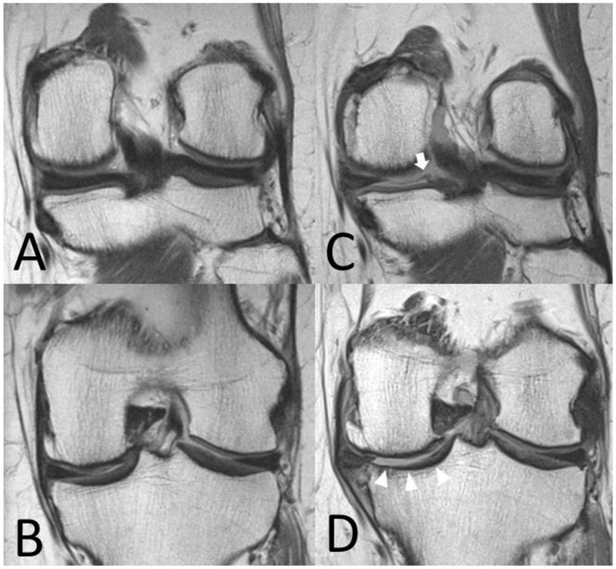
Figure 2:
Coronal intermediate-weighted non-fat suppressed MR images (TR 3700 ms/TE 29 ms) show medial and posterior slices of the left knee of a 63-year old female subject at the index visit (A, B) and 4-year follow-up (C, D). Findings at 4-year follow-up include tearing and elongation of the posterior medial meniscal root (C, white arrow) and meniscal extrusion with partial maceration (D). Note large femoral osteophyte, massive tibial cartilage loss (white arrowheads) and reshaping of the medial tibial plateau.
Osteophyte formation was present in 85% (138/162) of all knees. Of these 54% (88/162) showed rapid osteophyte formation with no or minimal osteophytes progressing to severe osteophytes within four years. In 51% (83/162) the osteophyte had contact with the meniscus, with the appearance of the osteophyte pushing the meniscus further out of the tibiofemoral joint space, as shown in Fig. 3, though sequence and causative relationships remain unknown. In total, 21 knees with insufficiency fractures were identified. These occurred between index visit and year two (7/21) and between year two and year four (14/21).
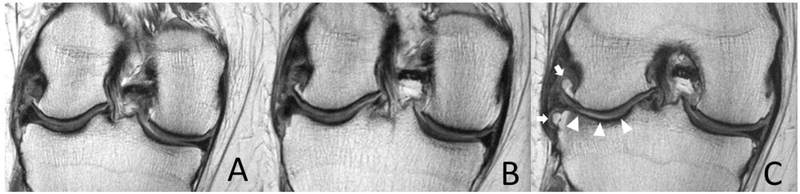
Figure 3:
Coronal intermediate-weighted non-fat suppressed MR images (TR 3700 ms/TE 29 ms) of the corresponding anatomical location (note tibial ACL insertion) show rapid osteophyte development between (A) index visit, (B) 2 years, and (C) 4 years of a 62-year-old male subject with AKOA. The developing osteophytes are seemingly pushing the meniscus further out of the inter-joint space possibly increasing meniscal extrusion (white arrows). Note rapid tibiofemoral cartilage loss (white arrowheads) and meniscus degeneration with increased signal and tearing at 4-year follow-up.
Secondary analysis of knees accelerating in two years
Compared to AKOA4y (KL 3/4 onset within four years), subjects with AKOA2y (KL 3/4 onset within two years) were slightly younger (age: 62.92±8.1 vs. 64.09±8.9; p=0.425) and slightly more active (PASE: 174.8±87.8 vs. 156.17±68.4; p=0.154); however, these findings were not significant. The BMI was similar in both groups (29.3±4.1 vs. 29.7±3.5; p=0.535). Knee injuries during the follow-up period (only including injuries concurrent with the 2y or 4y AKOA timeframe) were significantly more often present in AKOA2y knees (42%, 49/116 vs. 24%, 11/46, p = 0.032). No significant differences were found for sex, race distribution, WOMAC pain score and surgery during the follow-up period.
Index visit MR imaging risk factors for KL 3/4 onset within 2 years compared to KL 3/4 onset within 4 years are shown in Table 4. Interestingly, knees with AKOA2y had a significantly higher number of root tears at the index visit (17%; 20/116) compared to knees with AKOA4y (4%; 2/46; p=0.007). All other types of meniscal tears, including meniscus maximum scores were non-significant. Cartilage lesion scores, BMEP and effusion scores were not significantly different in both groups.
Table 4:
Index visit MR imaging findings in AKOA2y and AKOA4y
| Index visit imaging findings | AKOA2y (n=116) |
AKOA4y (n=46; reference) |
Multivariable Regression - adjusted OR |
p-valueb,c |
|---|---|---|---|---|
| Cartilage | ||||
| Cartilage maximum (max; mean±SD) | 3.35±1.5 | 2.78±1.6 | 1.19 (0.97-1.46) | 0.095 |
| Meniscus | ||||
| Meniscus max (mean±SD) | 2.22±1.2 | 1.80±1.4 | 1.21 (0.99-1.49) | 0.061 |
| Horizontal tear [n (%)] | 30 (25.9) | 9 (19.6) | 1.39 (0.81-2.40) | 0.233 |
| Vertical tear [n (%)] | 12 (10.3) | 5 (10.87) | 0.92 (0.47-1.79) | 0.798 |
| Flap tear [n (%)] | 4 (3.4) | 1 (2.2) | 1.28 (0.55-3.02) | 0.568 |
| Root tear [n (%)] | 20 (17.2) | 2 (4.3) | 2.82 (1.33-6.00) | 0.007 |
| Meniscal extrusion [n (%)] | 35 (30.2) | 10 (21.7) | 1.28 (0.69-2.40) | 0.434 |
| Bone marrow edema pattern (BMEP) | ||||
| BMEP max (mean±SD) | 1.63±1.0 | 1.43±0.9 | 1.19 (0.98-1.48) | 0.098 |
| Effusion (mean±SD) | 0.31±0.6 | 0.28±0.6 | 1.10 (0.70-1.72) | 0.677 |
Significant values are in bold (p-value < 0.05)
Adjusted for age, sex, race, BMI
Calculated using GEE
Secondary analysis of knees with bilateral AKOA
Thirteen subjects (26 knees) developed bilateral AKOA. Of these 62% (8/13) subjects developed bilateral AKOA in both knees during the same 4-year timeframe, while 38% (5/13) subjects developed AKOA in both knees but during separate 4-year timeframes. Moreover, 77% (20/26) knees developed AKOA within 2 years while 23% (6/26) knees developed AKOA within 4 years, which is similar to the percentage of knees developing AKOA2y in the entire AKOA cohort (72%, 116/162). Subjects with bilateral AKOA had the same rate of AKOA development (either both knees AKOA2y or both knees AKOA4y) in 69% (9/13). No significant differences were found for age, BMI, sex, race distribution, PASE, injury and surgery during the follow-up period between subjects developing bilateral AKOA and subjects developing unilateral AKOA. Index visit MR imaging findings in knees of subjects developing bilateral AKOA compared to knees of subjects developing unilateral AKOA are shown in Table 5. Knees of subjects developing unilateral AKOA had significantly higher mean cartilage maximum scores at the index visit (3.15±1.5) compared to knees of subjects developing bilateral AKOA (1.89±1.7; p<0.001). Moreover, meniscal extrusion was significantly more often present in knees of subjects developing unilateral AKOA (42/136, 30.9%) compared to knees of subjects developing bilateral AKOA (2/26, 7.7%; p=0.033). Meniscus maximum scores, BMEP and effusion scores were not significantly different in both groups.
Table 5:
Index visit MR imaging findings in knees of subjects developing unilateral and bilateral AKOA
| Index visit imaging findings | Bilateral AKOA (n=26) |
Unilateral AKOA (n=136; reference) |
Multivariable Regression - adjusted OR |
p-valueb,c |
|---|---|---|---|---|
| Cartilage | ||||
| Cartilage maximum (max; mean±SD) | 1.89±1.7 | 3.15±1.5 | 0.55 (0.39-0.77) | <0.001 |
| Meniscus | ||||
| Meniscus max (mean±SD) | 1.58±1.2 | 1.64±1.2 | 1.02 (0.70-1.49) | 0.934 |
| Horizontal tear [n (%)] | 4 (15.4) | 35 (25.7) | 0.53 (0.17-1.67) | 0.274 |
| Vertical tear [n (%)] | 4 (15.4) | 12 (8.8) | 2.37 (0.65-8.65) | 0.600 |
| Flap tear [n (%)] | 2 (7.7) | 3 (2.2) | 5.41 (0.80-5.34) | 0.084 |
| Root tear [n (%)] | 4 (15.4) | 18 (13.2) | 1.36 (0.41-4.57) | 0.614 |
| Meniscal extrusion [n (%)] | 2 (7.7) | 42 (30.9) | 0.20 (0.04-0.88) | 0.033 |
| Bone marrow edema pattern (BMEP) | ||||
| BMEP max (mean±SD) | 1.27±1.2 | 1.49±0.9 | 0.79 (0.50-1.24) | 0.300 |
| Effusion (mean±SD) | 0.15±0.4 | 0.19±0.5 | 2.87 (0.32-2.21) | 0.721 |
Significant values are in bold (p-value < 0.05)
Adjusted for age, sex, race, BMI
Calculated using GEE
Discussion
In this study, we analyzed longitudinal, structural MR imaging findings in knees with AKOA. Meniscal abnormalities and extrusion were the most consistent morphological features present in those with AKOA. Meniscal root tears were the most frequent types of tears in knees with AKOA and rapid osteophyte formation was commonly observed. Moreover, we compared risk factors for rapid KL 3/4 onset within two years (AKOA2y) to KL 3/4 onset within four years (AKOA4y) and found presence of root tears at the index visit, and incident knee injury as significant risk factors for KL 3/4 onset within two years. Interestingly, knees of subjects developing unilateral AKOA had significantly higher index visit cartilage maximum scores and meniscal extrusion was significantly more often present compared to subjects developing bilateral AKOA. These findings indicate that both factors predispose one knee for the development of AKOA but do not predispose subjects for bilateral AKOA development.
Known risk factors for accelerated knee cartilage loss include increased age, elevated BMI, and knee injury [35, 12, 20]. In accordance with these findings the mean BMI was within a high overweight/pre-obese range (29.4 kg/m2). Moreover, incident knee injuries were frequently observed (37% of all knees) and significantly more often present in knees with KL 3/4 onset within two compared four years (42% vs. 24%), underlining the acute damaging potential for meniscus and cartilage tissue. These findings are in accordance to a previous study by Davis et al that found recent knee injuries to predispose knees for the development of AKOA [36]. Previous studies have not associated meniscal resection with AKOA specifically, however, multiple studies have identified it as a risk factor for secondary knee OA [37, 38, 7]. Notably, one third of all AKOA knees had arthroscopic partial meniscal resection during the follow-up period.
Previous studies assessed MRI findings related to AKOA [20, 18, 21]. Roemer et al measured baseline MR features in 20 knees with fast tibiofemoral cartilage loss and 70 knees with slow tibiofemoral cartilage loss over a 30-month period [20]. Meniscal tears and extrusion at baseline were associated with increased risk for slow and fast tibiofemoral cartilage loss, respectively [20]. Driban et al described incident imaging findings in 18 subjects progressing from no radiographic OA (KL 0/1) to severe OA (KL 3/4) within 12 months without injury, or progressing from no radiographic OA before injury to severe OA within 12 months after the injury [18]. Incident imaging findings in this study were medial and lateral meniscal abnormalities, one subchondral fracture and evidence of post-traumatic avascular necrosis [18]. Meniscal abnormalities included horizontal, radial, and complex tears and extrusion [18]. In a subsequent study Driban et al analyzed a larger group of subjects with AKOA (n=125) and compared MR imaging findings to subjects without knee OA and to subjects with a more normal rate of OA development [21]. In this study subjects developing AKOA had >4 times the odds of a destabilizing meniscal pathology two years before KL KL3/4 onset and >7 times the odds of a destabilizing meniscal pathology at the timepoint of KL 3/4 onset, compared to subjects without AKOA [21]. In accordance with our findings, moderate to severe meniscal lesions were identified as one of the key findings associated with AKOA and present in 93% AKOA knees at KL 3/4 onset, demonstrating the important role of the meniscus in preventing cartilage degeneration by providing lubricating properties, shock absorption and significant load distribution [39, 40].
Given that meniscal abnormalities are frequently observed in subjects with and without knee OA [41, 42], we aimed to differentiate types of meniscal tears in detail. Root tears were the most frequent types of tears in knees with AKOA, present in 38% (62/162) at four-year follow-up. Furthermore, root tears were significantly more often present at the index visit in knees with no radiographic OA accelerating within two years compared to knees accelerating within four years (p=0.007). Injuries to the root are assumed to profoundly impact knee health and have previously been associated with increased tibiofemoral cartilage loss [43-45]. As the anterior and posterior meniscal roots substantially contribute to holding the meniscus in its anatomical position [46, 47], partial or complete tears are associated with a loss of meniscal hoop-stress, creating changes in biomechanics shown to be similar to that of a total meniscectomy [48, 43, 45]. The frequency and acute damaging potential of root tears in our AKOA cohort should specifically alert physicians to this type of meniscal damage.
The proportion of incident insufficiency fractures observed in our cohort (13%; 21/162) was smaller compared to the AKOA cohort described by Driban et al (22%; 4/18) [18]. As their study only included knees accelerating to KL 3/4 within 12 months, changes were potentially more acute compared to our study cohort, which included all knees with KL 3/4 onset within a four year timeframe.
A notable number of knees in our cohort with no-minimal osteophytes at the index visit developed severe osteophytes within four years. Periarticular osteophytes are frequently associated with knee OA [49], however, the pace of development observed in our cohort is unusual. Cartilage damage is commonly assumed to initiate osteophyte formation and bone metabolism, detected with 99Tc-DPD-SPECT/CT [50] and NaF PET/CT [51], has been shown to be elevated in tissue subjacent to cartilage lesions. However, in experimental settings osteophyte formation has been observed to begin within three days after induction of the disease process [52], indicating the involvement of additional factors given that no significant cartilage damage would have developed within this limited time period. It has therefore been questioned if osteophytes are a functional adaption to mechanical joint instability or the result of an altered internal joint environment [53-55]. It seems likely that a combination of mechanical and biochemical factors drive the rapid osteophyte formation seen in AKOA. Further research is needed examining alterations in biochemical signaling pathways in AKOA subjects.
We acknowledge that our study has some limitations. No specific information was available concerning the type of mechanical trauma for self-reported knee injury. Furthermore, MR images were acquired at specific time intervals set by the OAI protocol, potentially not capturing important imaging findings occurring directly after knee surgery or injury. As patellofemoral OA was not accounted for in the KL-based AKOA definition, this could have influenced disease progression to some extent. While AKOA was associated with severe meniscal damage in almost all cases, we cannot exclude that other factors such as differences in cartilage composition, OA treatment, other comorbidities or genetic and biochemical factors may have also influenced AKOA development.
In conclusion, meniscal abnormalities were the most consistent morphological feature in knees with AKOA likely have a significant role in accelerated progression of OA. Meniscal resection and knee injuries were also frequently associated with AKOA development. Root tears seem to pose a substantial risk for acute cartilage breakdown, given that they were the most common types of meniscal tears observed in our cohort and significantly associated with accelerated radiographic joint space narrowing within two years compared to four years. Our findings contribute to characterizing the underlying biological processes associated with accelerated cartilage breakdown in AKOA, which is clinically relevant to identify subjects at risk of AKOA and initiate preventative measures.
Acknowledgments:
We would like to thank the participants and staff of the Coordinating Center of the OAI for their invaluable assistance with patient selection, statistical analysis, and technical support. The study was supported by the OAI, a public–private partnership comprising 5 NIH contracts (National Institute of Arthritis and Musculoskeletal and Skin Diseases contracts N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, and N01-AR-2-2262), with research conducted by the OAI Study Investigators. The study was also funded in part by the Intramural Research Program of the National Institute on Aging, NIH. Private funding partners include Merck Research, Novartis Pharmaceuticals, GlaxoSmithKline, and Pfizer; the private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. The analyses in this study were funded through the NIH/NIAMS (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01AR064771 and P50-AR060752).
The analyses in this study were funded through the NIH/NIAMS (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01AR064771 and P50-AR060752). We would like to thank the participants and staff of the Coordinating Center of the OAI for their invaluable assistance with patient selection, statistical analysis, and technical support. The study was supported by the OAI, a public-private partnership comprising 5 NIH contracts (National Institute of Arthritis and Musculoskeletal and Skin Diseases contracts N01-AR-2–2258, N01-AR-2–2259, N01-AR-2–2260, N01-AR-2–2261, and N01-AR-2–2262), with research conducted by the OAI Study Investigators. The study was also funded in part by the Intramural Research Program of the National Institute on Aging, NIH. Private funding partners include Merck Research, Novartis Pharmaceuticals, GlaxoSmithKline, and Pfizer; the private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
Funding information: The analyses in this study were funded through the NIH/NIAMS (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01AR064771 and P50-AR06075)
Footnotes
Disclosures All authors declare they have no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
Sarah C. Foreman, Department of Radiology and Biomedical Imaging, University of California, San Francisco; San Francisco CA, USA.
Jan Neumann, Department of Radiology, Technical University of Munich; Munich, Germany.
Gabby B. Joseph, Department of Radiology and Biomedical Imaging, University of California, San Francisco; San Francisco, CA, USA.
Michael C. Nevitt, Department of Epidemiology and Biostatistics, University of California, San Francisco; San Francisco, CA, USA.
Charles E. McCulloch, Department of Epidemiology and Biostatistics, University of California, San Francisco; San Francisco, CA, USA.
Nancy E. Lane, Department of Medicine, University of California, Davis, CA, USA.
Thomas M. Link, Department of Radiology and Biomedical Imaging, University of California, San Francisco; San Francisco, CA, USA.
References
- 1.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driban JB, Eaton CB, Lo GH, Ward RJ, Lu B, McAlindon TE. Association of knee injuries with accelerated knee osteoarthritis progression: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2014;66(11):1673–9. doi: 10.1002/acr.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driban JB, Stout AC, Lo GH, Eaton CB, Price LL, Lu B et al. Best performing definition of accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. Ther Adv Musculoskelet Dis. 2016;8(5):165–71. doi: 10.1177/1759720X16658032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guermazi A, Hayashi D, Roemer FW, Niu J, Quinn EK, Crema MD et al. Brief Report: Partial-and Full-Thickness Focal Cartilage Defects Contribute Equally to Development of New Cartilage Damage in Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis Rheumatol. 2017;69(3):560–4. doi: 10.1002/art.39970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felson DT. Epidemiology of hip and knee osteoarthritis. Epidemiol Rev. 1988;10:1–28. [DOI] [PubMed] [Google Scholar]
- 6.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54(3):795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 7.Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ, Eckstein F, Grago J et al. Partial meniscectomy is associated with increased risk of incident radiographic osteoarthritis and worsening cartilage damage in the following year. Eur Radiol. 2017;27(1):404–13. doi: 10.1007/s00330-016-4361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yau MS, Yerges-Armstrong LM, Liu Y, Lewis CE, Duggan DJ, Renner JB et al. Genome-Wide Association Study of Radiographic Knee Osteoarthritis in North American Caucasians. Arthritis Rheumatol. 2017;69(2):343–51. doi: 10.1002/art.39932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Blizzard L, Jin X, Chen Z, Zhu Z, Han W et al. Quantitative Assessment of Knee Effusion-Synovitis in Older Adults: Association With Knee Structural Abnormalities. Arthritis Rheumatol. 2016;68(4):837–44. doi: 10.1002/art.39526. [DOI] [PubMed] [Google Scholar]
- 10.Kraus VB, Collins JE, Charles HC, Pieper CF, Whitley L, Losina E et al. Predictive Validity of Radiographic Trabecular Bone Texture in Knee Osteoarthritis: The Osteoarthritis Research Society International/Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol. 2018;70(1):80–7. doi: 10.1002/art.40348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis JE, Liu SH, Lapane K, Harkey MS, Price LL, Lu B et al. Adults with incident accelerated knee osteoarthritis are more likely to receive a knee replacement: data from the Osteoarthritis Initiative. Clin Rheumatol. 2018;37(4):1115–8. doi: 10.1007/s10067-018-4025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driban JB, Eaton CB, Lo GH, Price LL, Lu B, Barbe MF et al. Overweight older adults, particularly after an injury, are at high risk for accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. Clin Rheumatol. 2016;35(4):1071–6. doi: 10.1007/s10067-015-3152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Driban JB, Price LL, Eaton CB, Lu B, Lo GH, Lapane KL et al. Individuals with incident accelerated knee osteoarthritis have greater pain than those with common knee osteoarthritis progression: data from the Osteoarthritis Initiative. Clin Rheumatol. 2016;35(6):1565–71. doi: 10.1007/s10067-015-3128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driban JB, Stout AC, Duryea J, Lo GH, Harvey WF, Price LL et al. Coronal tibial slope is associated with accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. BMC Musculoskelet Disord. 2016;17:299. doi: 10.1186/s12891-016-1158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roemer FW, Hayes CW, Miller CG, Hoover K, Guermazi A. Imaging atlas for eligibility and on-study safety of potential knee adverse events in anti-NGF studies (Part 1). Osteoarthritis Cartilage. 2015;23 Suppl 1:S22–42. doi: 10.1016/j.joca.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Hochberg MC. Serious joint-related adverse events in randomized controlled trials of antinerve growth factor monoclonal antibodies. Osteoarthritis Cartilage. 2015;23 Suppl 1:S18–21. doi: 10.1016/j.joca.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg MC, Tive LA, Abramson SB, Vignon E, Verburg KM, West CR et al. When Is Osteonecrosis Not Osteonecrosis?: Adjudication of Reported Serious Adverse Joint Events in the Tanezumab Clinical Development Program. Arthritis Rheumatol. 2016;68(2):382–91. doi: 10.1002/art.39492. [DOI] [PubMed] [Google Scholar]
- 18.Driban JB, Ward RJ, Eaton CB, Lo GH, Price LL, Lu B et al. Meniscal extrusion or subchondral damage characterize incident accelerated osteoarthritis: Data from the Osteoarthritis Initiative. Clin Anat. 2015;28(6):792–9. doi: 10.1002/ca.22590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis JE, Harkey MS, Ward RJ, Mackay JW, Lu B, Price LL et al. Characterizing the distinct structural changes associated with self-reported knee injury among individuals with incident knee osteoarthritis: Data from the osteoarthritis initiative. Clin Anat. 2018;31(3):330–4. doi: 10.1002/ca.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roemer FW, Zhang Y, Niu J, Lynch JA, Crema MD, Marra MD et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252(3):772–80. doi: 10.1148/radiol.2523082197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Driban JB, Davis JE, Lu B, Price LL, Ward RJ, MacKay JW et al. Accelerated Knee Osteoarthritis is Characterized by Destabilizing Meniscal Tears and Pre-Radiographic Structural Disease Burden. Arthritis Rheumatol. 2018. doi: 10.1002/art.40826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal radiology. 2003;32(3):128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 23.Felson DT, Nevitt MC, Yang M, Clancy M, Niu J, Torner JC et al. A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol. 2008;35(10):2047–54. [PMC free article] [PubMed] [Google Scholar]
- 24.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. [DOI] [PubMed] [Google Scholar]
- 25.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 26.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–41. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rauscher I, Stahl R, Cheng J, Li X, Huber MB, Luke A et al. Meniscal measurements of Tlrho and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology. 2008;249(2):591–600. doi: 10.1148/radiol.2492071870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: is extent associated with severity of degeneration or type of tear? AJR American journal of roentgenology. 2004;183(1):17–23. doi: 10.2214/ajr.183.1.1830017. [DOI] [PubMed] [Google Scholar]
- 29.Plett SK, Hackney LA, Heilmeier U, Nardo L, Yu A, Zhang CA et al. Femoral condyle insufficiency fractures: associated clinical and morphological findings and impact on outcome. Skeletal radiology. 2015;44(12):1785–94. doi: 10.1007/s00256-015-2234-1. [DOI] [PubMed] [Google Scholar]
- 30.Chanchek N, Gersing AS, Schwaiger BJ, Nevitt MC, Neumann J, Joseph GB et al. Association of diabetes mellitus and biochemical knee cartilage composition assessed by T2 relaxation time measurements: Data from the osteoarthritis initiative. J Magn Reson Imaging. 2017. doi: 10.1002/jmri.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann J, Guimaraes JB, Heilmeier U, Joseph GB, Nevitt MC, McCulloch CE et al. Diabetics show accelerated progression of knee cartilage and meniscal lesions: data from the osteoarthritis initiative. Skeletal radiology. 2018. doi: 10.1007/s00256-018-3088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gersing AS, Schwaiger BJ, Heilmeier U, Joseph GB, Facchetti L, Kretzschmar M et al. Evaluation of Chondrocalcinosis and Associated Knee Joint Degeneration Using MR Imaging: Data from the Osteoarthritis Initiative. Eur Radiol. 2017;27(6):2497–506. doi: 10.1007/s00330-016-4608-8. [DOI] [PubMed] [Google Scholar]
- 33.Bucknor MD, Nardo L, Joseph GB, Alizai H, Srikhum W, Nevitt MC et al. Association of cartilage degeneration with four year weight gain−-3T MRI data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2015;23(4):525–31. doi: 10.1016/j.joca.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2012;64(2):248–55. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Driban JB, McAlindon TE, Amin M, Price LL, Eaton CB, Davis JE et al. Risk Factors can Classify Individuals who Develop Accelerated Knee Osteoarthritis: Data from the Osteoarthritis Initiative. J Orthop Res. 2017. doi: 10.1002/jor.23675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis JE, Price LL, Lo GH, Eaton CB, McAlindon TE, Lu B et al. A single recent injury is a potent risk factor for the development of accelerated knee osteoarthritis: data from the osteoarthritis initiative. Rheumatol Int. 2017;37(10):1759–64. doi: 10.1007/s00296-017-3802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sihvonen R, Paavola M, Malmivaara A, Itala A, Joukainen A, Nurmi H et al. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. The New England journal of medicine. 2013;369(26):2515–24. doi: 10.1056/NEJMoa1305189. [DOI] [PubMed] [Google Scholar]
- 38.Zikria B, Hafezi-Nejad N, Roemer FW, Guermazi A, Demehri S. Meniscal Surgery: Risk of Radiographic Joint Space Narrowing Progression and Subsequent Knee Replacement-Data from the Osteoarthritis Initiative. Radiology. 2017;282(3):807–16. doi: 10.1148/radiol.2016160092. [DOI] [PubMed] [Google Scholar]
- 39.Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975(109):184–92. [DOI] [PubMed] [Google Scholar]
- 40.Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411–31. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berthiaume MJ, Raynauld JP, Martel-Pelletier J, Labonte F, Beaudoin G, Bloch DA et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005;64(4):556–63. doi: 10.1136/ard.2004.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale ME, McLaughlin S et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. The Journal of bone and joint surgery American volume. 2003;85-A(1):4–9. [DOI] [PubMed] [Google Scholar]
- 43.Guermazi A, Hayashi D, Jarraya M, Roemer FW, Zhang Y, Niu J et al. Medial posterior meniscal root tears are associated with development or worsening of medial tibiofemoral cartilage damage: the multicenter osteoarthritis study. Radiology. 2013;268(3):814–21. doi: 10.1148/radiol.13122544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carreau JH, Sitton SE, Bollier M. Medial Meniscus Root Tear in the Middle Aged Patient: A Case Based Review. Iowa Orthop J. 2017;37:123–32. [PMC free article] [PubMed] [Google Scholar]
- 45.Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus. Similar to total meniscectomy. The Journal of bone and joint surgery American volume. 2008;90(9):1922–31. doi: 10.2106/JBJS.G.00748. [DOI] [PubMed] [Google Scholar]
- 46.Koenig JH, Ranawat AS, Umans HR, Difelice GS. Meniscal root tears: diagnosis and treatment. Arthroscopy. 2009;25(9):1025–32. doi: 10.1016/j.arthro.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 47.Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124–9. doi: 10.1177/0363546508323254. [DOI] [PubMed] [Google Scholar]
- 48.Crema MD, Roemer FW, Felson DT, Englund M, Wang K, Jarraya M et al. Factors associated with meniscal extrusion in knees with or at risk for osteoarthritis: the Multicenter Osteoarthritis study. Radiology. 2012;264(2):494–503. doi: 10.1148/radiol.12110986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maas O, Joseph GB, Sommer G, Wild D, Kretzschmar M. Association between cartilage degeneration and subchondral bone remodeling in patients with knee osteoarthritis comparing MRI and (99m)Tc-DPD-SPECT/CT. Osteoarthritis Cartilage. 2015;23(10):1713–20. doi: 10.1016/j.joca.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 51.Draper CE, Quon A, Fredericson M, Besier TF, Delp SL, Beaupre GS et al. Comparison of MRI and (1)(8)F-NaF PET/CT in patients with patellofemoral pain. J Magn Reson Imaging. 2012;36(4):928–32. doi: 10.1002/jmri.23682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilbertson EM. Development of periarticular osteophytes in experimentally induced osteoarthritis in the dog. A study using microradiographic, microangiographic, and fluorescent bone-labelling techniques. Ann Rheum Dis. 1975;34(1):12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blom AB, van Lent PL, Holthuysen AE, van der Kraan PM, Roth J, van Rooijen N et al. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage. 2004;12(8):627–35. doi: 10.1016/j.joca.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 54.van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage. 2007;15(3):237–44. doi: 10.1016/j.joca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 55.van Lent PL, Blom AB, van der Kraan P, Holthuysen AE, Vitters E, van Rooijen N et al. Crucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formation. Arthritis Rheum. 2004;50(1):103–11. doi: 10.1002/art.11422. [DOI] [PubMed] [Google Scholar]