Abstract
Epidemiologic studies link increased autism spectrum disorder (ASD) risk to obstetrical conditions associated with inflammation and steroid dysregulation, referred to as prenatal metabolic syndrome (PNMS). This pilot study measured steroid-related biomarkers in early second trimester maternal serum collected during the First and Second Trimester Evaluation of Risk study. ASD case and PNMS exposure status of index offspring were determined through linkage with autism registries and birth certificate records. ASD case (N=53) and control (N=19) groups were enriched for PNMS exposure. Higher estradiol and lower sex hormone binding globulin (SHBG) were significantly associated with increased ASD risk. Study findings provide preliminary evidence to link greater placental estradiol activity with ASD and support future investigations of the prenatal steroid environment in ASD.
Keywords: Autism, biomarkers, prenatal risk factors, metabolic syndrome
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social/communication, restricted areas of interests, repetitive behaviors, and abnormal responses to sensory input (American Psychiatric Association, 2013). ASD affects approximately 2% of the population (Baio et al. 2018), and core ASD symptoms have no proven pharmacologic intervention.
Small studies that compared mean cortisol levels between groups of children with and without ASD suggest the presence of abnormal hypothalamic-pituitary-adrenal (HPA) axis functioning in this population (Corbett et al. 2006; Spratt et al. 2012; Taylor and Corbett, 2014; Tordjman et al. 2014; Zinke et al. 2010, Tomarken et al. 2015). Compared to typically developing children (N=32), children with ASD and intellectual disability (N=55, mean IQ=42) had higher salivary cortisol levels at each time point over a 24-hour period and lower diurnal variation of these cortisol levels (Tordjman et al. 2014). The degree of HPA axis dysregulation as measured by salivary cortisol also coincided with the severity of social and communication impairment (Tordjman et al. 2014). Using a mock MRI scanner paradigm, children with ASD (N=12, mean IQ=77) demonstrated poor stress response habituation and more day-to-day variability of their cortisol circadian rhythm when compared to typically developing children (N=10, mean IQ=114) (Corbett et al. 2006). One study of high functioning children with ASD (N=36, mean IQ=104) found that reduced cortisol variability associated with ASD was primarily attributable to a subgroup (25%) of these children (Tomarken et al. 2015). The nature and prevalence of HPA axis dysfunction among children with ASD remain unclear and continue to be an area of active investigation.
The in utero steroid environment plays a critical role in fetal HPA axis development (Howland et al. 2017; Moisiadis and Matthews, 2014; Montenegro et al. 2019; Reynolds, 2013). Steroid production during pregnancy is coordinated among each component of the maternofetoplacental unit (i.e., mother, placenta, and fetus) (Kallen, 2004; Murphy et al. 2006). Under normal conditions, the fetal adrenal cortex does not acquire the capacity to synthesize cortisol de novo until 23 to 24 weeks gestation. Instead, in early second trimester, the fetal adrenal gland produces dehydroepiandrosterone (DHEA) in response to HPA axis activation (Coulter and Jaffe, 1998; Ishimoto and Jaffe, 2011). Fetal DHEA, along with maternal DHEA, subsequently serves as the substrate for placental estradiol production. In turn, placental estradiol stimulates the fetal adrenal cortex to initiate de novo cortisol synthesis (Albrecht and Pepe, 1999; Coulter and Jaffe, 1998; Watterberg, 2004).
Epidemiologic studies have found associations between obstetrical conditions related to steroid dysregulation and increased ASD risk. These conditions include pre-existing/gestational diabetes, pre-existing/gestational hypertension, and eclampsia/pre-eclampsia collectively referred to as prenatal metabolic syndrome (PNMS) (Hisle-Gorman et al. 2018; Krakowiak et al. 2012; Nahum Sacks et al. 2016; Reynolds, 2013; Wang et al. 2017; Watterberg, 2004). Additionally, pre-pregnancy obesity and weight gain during pregnancy have been used as proxies for aberrant in utero steroid hormone exposure in studies of hormone-sensitive cancers affecting offspring (Petridou et al. 1992, Kinnunen et al. 2004, Lumey, 1998). Pre-pregnancy obesity and increased pregnancy weight gain have also been identified as risk factors for ASD (Bilder et al. 2013, Dodds et al. 2011; Hisle-Gorman et al. 2018; Nahum Sacks et al. 2016; Wang et al. 2017).
Between 1999 and 2002, Utah participated in the NIH-funded, multi-site First and Second Trimester Evaluation of Risk (FASTER) study to compare obstetrical screening approaches for aneuploidy in a large, prospectively-identified cohort (Malone et al. 2005). The Utah FASTER study site collected medical information, maternal serum samples, and ultrasound reports on over 12,000 pregnant women. FASTER’s timing and catchment area overlapped with ASD case ascertainment through Utah’s Registry of Autism and Developmental Disabilities, providing the opportunity to identify maternal serum samples corresponding to offspring with ASD. Linkage with birth certificate data allows for the identification of offspring with PNMS exposure. The aims of this pilot study were to analyze early second trimester maternal serum samples from the FASTER study to (1) characterize maternal serum steroid-related biomarkers associated with ASD during this gestational period and (2) explore how fetal steroidogenic activity may be influenced and reflected in these maternal biomarkers. Case and control samples were enriched for PNMS exposure to examine a potentially additive influence of PNMS on the prenatal steroid environment during pregnancies of offspring with and without ASD.
METHODS
Approval for this study was obtained from the Utah Registry of Autism and Developmental Disabilities (URADD) Oversight Committee, Utah State Office of Education, and Institutional Review Boards of the University of Utah Intermountain Healthcare, Utah Department of Health, and Resource for Genetic and Epidemiologic Research Review Committee, which is an oversight body that regulates Utah Population Database access.
FASTER Parent Study
The FASTER Study was an obstetrical study that ascertained over 12,000 women with singleton pregnancies living along Utah’s Wasatch Front from 1999 to 2002 (Malone et al. 2005). Supplementary consent was obtained from 10,849 Utah FASTER study participants to allow their residual serum samples to be used for additional research studies. The Utah Population Database is a unique, comprehensive medical research resource that accesses many sets of high-quality, population-based, individual-level records (Smith, 2019). Offspring of FASTER study participants who provided this additional consent were linked to participants through birth certificate records resulting in 3,327 male and 3,114 female FASTER offspring identified.
ASD Case Status
In 2016, multiple registries were linked within the Utah Population Database to identify individuals with ASD to investigate ASD birth risk factors. URADD was the primary ASD case source. URADD is administered through the University of Utah Department of Psychiatry with oversight from the Utah Department of Health in cooperation with the Utah State Board of Education. URADD classifies ASD cases using ASD diagnostic billing codes and special education autism exceptionality status (Bakian et al., 2015a; Bakian et al., 2015b). Two FASTER birth years (2000, 2002) overlapped with ASD case finding enhanced through Utah’s participation in the Centers for Disease Control and Prevention’s Autism and Developmental Disabilities Monitoring Network, which uses a record review methodology that has been validated in Utah (Bakian et al. 2015a; CDC, 2007; Yeargin-Allsopp et al. 2003). Additional ASD cases were identified through diagnostic billing codes from the University of Utah and Intermountain Healthcare Enterprise Data Warehouses and statewide hospital discharge summaries housed in the Utah Population Database. Among FASTER offspring who linked to birth certificate records, 168 were identified as ASD cases.
Prenatal Metabolic Syndrome (PNMS) exposure and covariates
Birth risk factors were obtained from Utah birth certificate records. PNMS exposure was defined as the presence of gestational hypertension, gestational diabetes, pre-/eclampsia, pre-existing diabetes and/or pre-existing hypertension. Birth certificates have historically underestimated the rate of pregnancy complications (Dobie et al. 1998). However, the prevalence of PNMS exposure among the FASTER sample approximated that of epidemiologic studies for these conditions (Gillon et al. 2014, Lawrence et al. 2008). Pre-/perinatal characteristics previously associated with ASD and/or PNMS were identified including sex, maternal age, pre-pregnancy maternal body mass index (BMI), pregnancy weight gain, gestational age at birth, and birth weight.
Prenatal maternal serum
Maternal blood samples were collected at 150–186 weeks gestation, between 1999 and 2002. Blood samples were drawn in serum separator tubes, centrifuged within 30 minutes, stored at 4°C, and shipped overnight to a central laboratory where initial FASTER serum studies were performed. Residual serum samples were frozen at −80°C. In 2017, samples were shipped overnight on dry ice, stored at −80°C, thawed on wet ice, and aliquoted into pre-cooled tubes. In total, two thaw/refreeze cycles occurred prior to all of the current study’s serum analyses which were performed in 2018.
Pilot study sample selection
As a pilot study, the sample size was determined based on feasibility and power given funding availability. Because steroid dysregulation occurs more frequently in pregnancies complicated by PNMS, both ASD case and control groups were enriched for PNMS exposure (47% and 42%, respectively). This enrichment provided the opportunity to test a hypothesis that maternal serum steroid biomarker concentrations would follow a dose-response relationship with mean concentrations changing stepwise from the absence of ASD and PNMS to the presence of ASD and PNMS. FASTER pregnancies were divided into the following four groups based on offspring ASD case (ASD+/−) and PNMS exposure (PNMS+/−) status: ASD-/PNMS-, ASD-/PNMS+, ASD+/PNMS- and ASD+/PNMS+. For the ASD- control group, 12 PNMS+ offspring without ASD were randomly selected and matched by sex and birth year to 12 PNMS- offspring without ASD. A larger number of ASD+ case group participants (N=62) were selected relative to the ASD- control groups (N=24) to ensure that all available ASD+/PNMS+ samples were assayed together (N=31). ASD+/PNMS+ participants were matched to ASD+/PNMS- samples (N=31) by sex and birth year. Corresponding early second trimester serum samples (N=77) were located with the following group distributions: 11 ASD-/PNMS-, 8 ASD-/PNMS+, 28 ASD+/PNMS-, and 30 ASD+/PNMS+. Offspring born before 37 weeks gestation (N=5, all ASD+/PNMS+) were removed from analyses to minimize confounding from complications resulting in or arising from preterm birth. Table 1 describes participant characteristics (N=72).
Table 1.
Participant Characteristics
| Characteristics | ASD cases (N=53) | Controls (N=19) | ||
|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | |
| Maternal Age (y) | 28.38 (5.99) | 19–42 | 29.05 (4.98) | 20–38 |
| Gestation Age at sampling (wk)a | 16.7 (1.2) | 14.9–20.6 | 16.4 (0.9) | 15.0–18.1 |
| Gestation Age at birth (wk) | 38.87 (1.29) | 37–42 | 38.79 (1.08) | 37–41 |
| Birth Weight (g) | 3414.91(515.8) | 2296–4590 | 3304.84 (425.65) | 2527–4082 |
| Pre-pregnancy BMI | 26.47 (6.88) | 18.75–55.09 | 25.81 (3.35) | 19.49–31.41 |
| Pregnancy Weight Gain (lbs) | 31.85 (14.4) | (−3)–62 | 27.47 (12.4) | 4–60 |
| N (%) | N (%) | |||
| Birth Year | ||||
| 2000 | 8 (15.1) | - | 2 (10.5) | - |
| 2001 | 16 (30.2) | - | 8 (42.1) | - |
| 2002 | 20 (37.7) | - | 8 (42.1) | - |
| 2003 | 9 (17.0) | - | 1 (5.3) | - |
| PNMS Exposureb | 25 (47.2) | - | 8 (42.11) | - |
| Diabetes | 7 (13.2) | 1 (5.3) | ||
| Hypertension | 18 (34.0) | 7 (36.8) | ||
Gestational age at sample collection was estimated as the difference between the collection date of the sample and date of the last menstrual period (LMP) recorded in birth certificate records. N=63 samples had a collection date available and fell between the LMP and birthdate.
No participant had both diabetes and hypertension exposures.
Serum Analysis
Biomarkers assays were performed for androstenedione, 17-hydroxyprogesterone, DHEA, dehydroepiandrosterone sulfate (DHEAS), free testosterone, total testosterone, sex hormone binding globulin (SHBG), and estradiol in a research laboratory. Serum steroid biomarkers remained intact during extended frozen storage and repeated freeze/thaw cycles (Helzlsouer et al. 1995; Holl et al. 2008). Steroids were measured in 96-well plates; plate loading was conducted by an automated liquid handling system (Gilson Pipetmax). Commercially available kits were used per manufacturer’s instructions, except where noted. Abcam (Boston, MA) ELISA kits measured androstenedione, estradiol, progesterone, total testosterone, and free testosterone. Diametra (Milan, ITA) and RayBiotech (Norcross, GA) ELISA kits quantified DHEA/DHEAS and SHBG, respectively. Estradiol required a 1:2 dilution in assay buffer to ensure samples fell within the dynamic range of the standard curve.
Estimated fetal DHEA (EF-DHEA) calculation
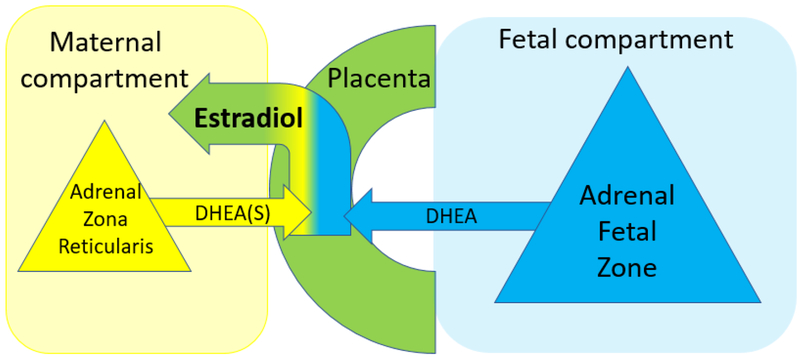
An approximate measure of the fetal adrenal gland’s contribution to placental estradiol production was estimated (referred to as EF-DHEA) using an equation that was developed for this study. This novel calculation (listed below) was based on the following components of established midgestation maternofetoplacental endocrinology: (1) Maternal serum estradiol is produced by the placenta, (2) placental estradiol production volume is determined by the availability of its substrate DHEA, (3) DHEA is provided to the placenta by both the mother and fetus, and (4) maternal DHEA exists primarily in its conjugated form DHEAS (Cunningham, 2010; Mesiano and Jaffe, 1997; Resko et al. 1975; Simpson and Macdonald, 1981; Walsh et al. 1979).
where zEstradiol, zDHEA, and zDHEAS are standardized scores of their respective maternal serum levels. A visual representation of these relationships is depicted in Figure 1.
Figure 1.
The placenta produces estradiol from DHEA of both maternal and fetal origin and shunts over 90% of estradiol into the maternal circulation. The volume of DHEA substrate determines placental estradiol production and subsequently maternal serum estradiol levels. DHEA exists primarily in its conjugated form DHEAS.
Statistical Analysis
The distributions of biomarkers were examined for extreme outliers; one extreme DHEA value was observed and deleted. Distributions subsequently satisfied normality assumptions. Pearson’s correlations were estimated to quantify the association among markers. Complete data were available for all of the covariates except for gestational age at serum collection; missing values (N=9) were imputed using hot-deck imputation (Andridge and Little, 2010).
A series of conditional logistic regression models were fit to measure the association between individual marker concentrations (i.e., androstenedione, 17-hydroxyprogesterone, DHEA, DHEAS, free testosterone, total testosterone, SHBG, and estradiol) and ASD in offspring. As the samples were matched on PNMS status and not on ASD case/control status, the logistic regression models were conditioned on PNMS status. Crude (unadjusted) models were initially formulated, and adjusted models were subsequently fit by incorporating additional covariates including sex, maternal age, pregnancy weight gain, pre-pregnancy BMI, and gestational age at sample collection. Finally, separate crude and multiple conditional logistic regression models were formulated to examine the relationship between EF-DHEA concentrations and ASD risk.
To investigate EF-DHEA and select biomarkers’ predictive value for ASD in offspring, receiver operating characteristic (ROC) curves were constructed. DHEA, estradiol, and SHBG were selected based on their strength of association with ASD from the multiple logistic regression analysis described above. Non-conditional crude and multiple logistic regression models were then formulated for EF-DHEA, DHEA, estradiol, and SHBG and included sex, maternal age, pregnancy weight gain, pre-pregnancy BMI, PNMS status, birth year, and gestational age at sample as covariates. The area under the ROCs from these predictive models were compared using the nonparametric approach of DeLong, Delong and Clarke Pearson (1988).
Post hoc tests were used to investigate differences in biomarker concentrations (i.e., androstenedione, 17-hydroxyprogesterone, DHEA, DHEAS, free testosterone, total testosterone, SHBG, and estradiol) between the ASD and non-ASD samples in the context of PNMS exposure. A Multivariate Analysis of Variance (MANOVA) and a Multivariate Analysis of Covariance (MANCOVA) were formulated to test for differences in all biomarker concentrations in a single model in which the independent variable was a four-level measure reflecting ASD and PNMS exposure status (i.e., ASD-/PNMS-, ASD-/PNMS+, ASD+/PNMS-, and ASD+/PNMS+). The MANCOVA included additional covariates such as sex, maternal age, pregnancy weight gain, pre-pregnancy BMI, and gestational age at sample collection as covariates. After confirming the significance of the overall MANOVA and MANCOVA models based on the F test, separate analysis of variance (ANOVA) and analysis of covariance (ANCOVA) models for each individual steroid-related biomarker were examined. Differences in standardized EF-DHEA concentrations across the four-level ASD/PNMS exposure variable were tested separately using an ANCOVA. The family-wise error rate was controlled for in the significant univariate ANCOVAs using the Scheffé method for post hoc analyses (Falah et al. 2015).
Analyses were conducted in SAS version 9.4 with an alpha of 0.05 selected to assess statistical significance (Resnik et al. 1974).
RESULTS
Steroid-related maternal serum biomarkers and ASD risk
ASD case status was significantly associated with increasing estradiol (AOR=1.21 per 100 pg/ml, 95% CI:1.03–1.42, p=0.02) and decreasing SHBG (AOR=0.34 per 50 nmol/L, 95% CI: 0.17–0.69, p=0.003) (see Table 2) in the adjusted conditional logistic regression analysis.
Table 2.
Association of steroid-related biomarkers with ASD in Utah FASTER participants (N=72)
| Crude models | Adjusted modelsa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Steroid | Mean | Range | SD | OR | 95% CI | P Value | AOR | 95% CI | P Value | |
| Androstenedioneb (ng/ml) | 1.86 | 0.30–5.84 | 1.09 | 0.99 | 0.61–1.59 | 0.96 | 1.01 | 0.59–1.73 | 0.99 | |
| 17-hydroxyprogesteroneb (ng/ml) | 2.37 | 0.81–5.34 | 0.81 | 0.8 | 0.42–1.51 | 0.49 | 0.74 | 0.38–1.47 | 0.39 | |
| DHEAb (pg/ml) | 4.32 | 0.68–20.13 | 2.96 | 0.83 | 0.66–1.04 | 0.11 | 0.76 | 0.57–1.00 | 0.05 | |
| DHEASb (ug/ml) | 1.19 | 0.09–4.37 | 0.82 | 0.67 | 0.36–1.26 | 0.22 | 0.55 | 0.26–1.18 | 0.12 | |
| Free testosteroneb (pg/ml) | 1.23 | 0.06–3.26 | 0.75 | 0.94 | 0.46–1.91 | 0.85 | 0.81 | 0.36–1.82 | 0.61 | |
| Total testosteroneb (ng/ml) | 0.82 | 0.34–1.78 | 0.32 | 2.81 | 0.48–16.46 | 0.25 | 3.15 | 0.37–26.48 | 0.29 | |
| SHBGc (nmol/L) | 117.09 | 57.05–450.50 | 76.87 | 0.43 | 0.26–0.72 | 0.001 | 0.34 | 0.17–0.69 | 0.003 | |
| Estradiold (pg/ml) | 1162.56 | 330.83–2285.98 | 454.05 | 1.2 | 1.04–1.38 | 0.01 | 1.21 | 1.03–1.42 | 0.02 | |
Adjusted for maternal age, newborn’s sex, maternal weight gain during pregnancy, maternal BMI at start of pregnancy, and gestational age at time of serum sample.
OR and AOR are calculated for every 1 unit increase in the steroid
OR and AOR are calculated for every 50 nmol/L increase in SHBG
OR and AOR are calculated for every 100 pg/ml increase in estradiol
EF-DHEA and ASD risk
An 8.0 fold increase in the odds of ASD was associated with every one standard deviation change in EF-DHEA (AOR=8.02, 95% CI: 2.36–27.29, p<0.001) based on the adjusted conditional logistic regression model.
Predictive models of ASD for EF-DHEA and biomarkers
The adjusted multiple logistic regression model to predict ASD outcome measured an area under the ROC of 0.91 (95% CI: 0.82–1.00) for EF-DHEA. Remaining area under the ROCs were 0.87 (95% CI: 0.77–0.98; SHBG), 0.84 (95% CI: 0.72–0.96; estradiol), and 0.83 (95% CI: 0.69–0.98; DHEA) (Figure S1, Table S1). The areas under the ROCs did not differ significantly among the steroid-related biomarkers (p=0.23).
Effects of PNMS exposure on ASD risk associated with biomarkers and EF-DHEA
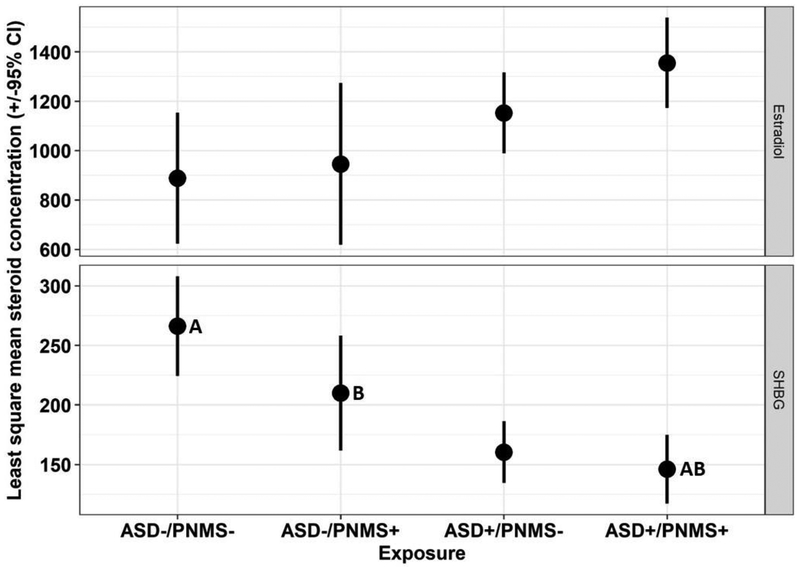
Both the crude MANOVA and MANCOVA yielded a significant overall main effect for ASD/PNMS exposure on serum steroid-related biomarker concentrations (crude Wilks’ Lambda = 0.45, F(24, 171.72) = 2.26, p=0.001; adjusted Wilks’ Lambda=0.44, F(24,157.22)=2.11, p=0.003). Adjusted univariate ANOVA models found significant differences by ASD/PNMS exposure status for SHBG (overall model F(8,63)=3.88 p =0.0009; ASD/PNMS exposure (F(3,63)=8.34, p <0.0001), and estradiol (overall model F(8,62) = 2.27, p = 0.03; ASD/PNMS exposure F(3, 62) = 3.28, p = 0.03) (see Table S2). Following correction for multiple comparisons, statistically significant differences remained in the least square means of SHBG concentrations between ASD+/PNMS+ and ASD-/PNMS- groups (mean difference = −120.60, 95% CI= −195.44 - −44.35, p=0.0004) and the ASD+/PNMS- and ASD-/PNMS- groups (mean difference = −106.08, 95% CI= −176.34 - −35.82, adjusted p=0.0009) (Figure 2).
Figure 2.
Least square mean concentrations (± 95% confidence intervals) of SHBG and estradiol in maternal serum by ASD/PNMS exposure status: ASD-/PNMS-, ASD-/PNMS+, ASD+/PNMS-, ASD+/PNMS+. Least square mean steroid-related biomarker concentrations sharing a letter are significantly different following Scheffé adjustment for multiple comparisons.
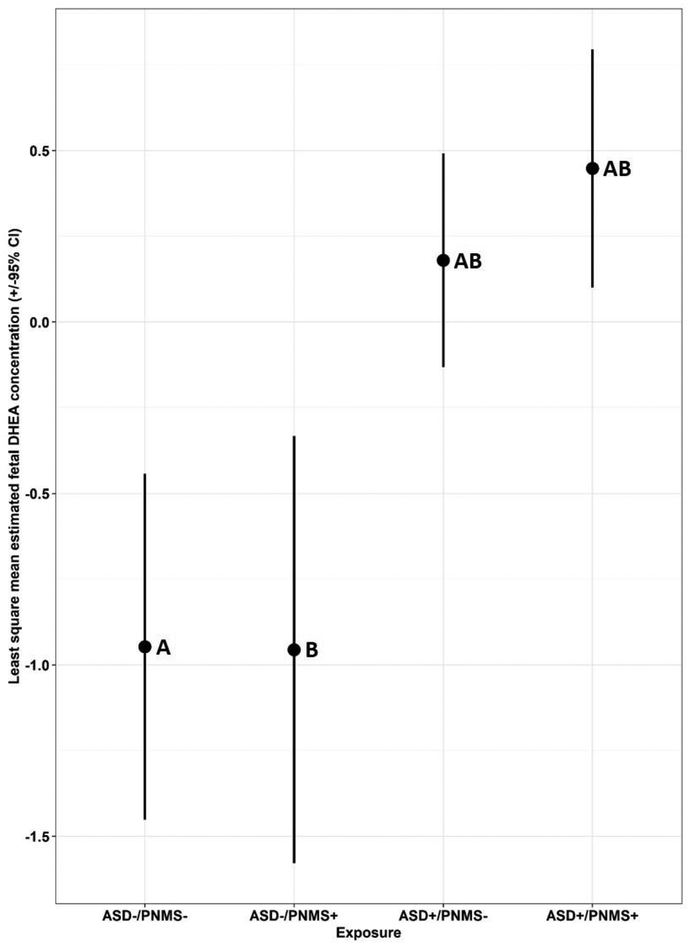
The ANCOVA model identified a significant main effect for ASD/PNMS exposure on EF-DHEA concentrations (F(8,62)=4.71, p=0.0002) and mean differences in EF-DHEA concentrations by ASD/PNMS exposure status (F(3,62)=9.87, p<0.0001). Following corrections for multiple comparisons, statistically significant differences remained in the least square means of EF-DHEA concentrations between ASD+ and ASD- groups regardless of PNMS exposure status (Figure 3).
Figure 3.
Least square mean concentrations (± 95% confidence intervals) of EF-DHEA by ASD/PNMS exposure status: ASD-/PNMS-, ASD-/PNMS+, ASD+/PNMS-, ASD+/PNMS+. Least square mean concentrations sharing a letter are significantly different following Scheffé adjustment for multiple comparisons.
DISCUSSION
ASD among offspring was significantly associated with higher estradiol and lower SHBG levels in early second trimester maternal serum. The collective association of higher estradiol and lower SHBG (which binds biologically active estrogens) in maternal serum of pregnancies with offspring affected by ASD suggests greater estradiol activity during this gestational window (Hammond, 2011). Furthermore, as shown in Figure 2, increasing estradiol levels coincided with decreasing SHBG levels in a dose-response manner according to ASD/PNMS exposure whereby the ASD-/PNMS- group showed the least and the ASD+/PNMS+ group showed the greatest divergence in SHBG versus estradiol least square mean concentrations.
Estradiol in the maternal circulation is synthesized by the placenta; maternal estradiol levels reflect placental estradiol production as most placental estradiol is shunted into the maternal circulation. Placental estradiol plays important regulatory functions within the maternofetoplacental unit, including the facilitation of fetal HPA axis maturation during the second trimester (Pepe and Albrecht, 1995). Because differences in HPA axis functioning have been found in children with ASD, estradiol’s influence on fetal HPA axis maturation may be relevant to ASD.
Fetal HPA axis maturation manifests as the emergence of fetal adrenal de novo cortisol synthesis capacity (Pepe and Albrecht, 1995). Under normal conditions, fetal de novo cortisol synthesis does not begin until 23–24 weeks gestation (Coulter and Jaffe, 1998; Ishimoto and Jaffe, 2011). Small amounts of maternal cortisol in the fetal circulation suppress fetal HPA axis activation through cortisol’s negative feedback on the fetal hypothalamus and pituitary gland (Mesiano and Jaffe, 1997). Cortisol transfer from the mother to the fetus is actively regulated by placental enzymes, which maintain a large gradient between maternal and fetal compartments (Albrecht and Pepe, 1999; Ishimoto and Jaffe, 2011). As term approaches, estradiol strengthens this maternal-fetal cortisol gradient through its actions on these placental enzymes, thereby decreasing the amount of maternal cortisol that transfers to the fetus (Albrecht and Pepe, 1999; Ishimoto and Jaffe, 2011). By reducing maternal cortisol in the fetal circulation, estradiol lessens negative feedback inhibition of the fetal HPA axis, which subsequently stimulates the fetal adrenal gland to initiate de novo cortisol synthesis. De novo cortisol synthesis prepares vital organs for extrauterine life (Albrecht and Pepe, 1999; Ng, 2000; Watterberg, 2004).
Beginning in early second trimester, elevated placental estradiol levels can accelerate de novo cortisol synthesis by increasing the maternal-fetal cortisol gradient prematurely (Albrecht and Pepe, 1999; Coulter and Jaffer, 1998; Pepe et al. 1990; Watterberg, 2004). Therefore, high estradiol activity in early second trimester can facilitate premature maturation of the fetal HPA axis. Because de novo cortisol synthesis stimulates the development of the lungs, skin, and gut, early HPA axis maturation promotes premature newborn survival (Ng, 2000; Watterberg, 2004; Watterberg et al. 1997). Early fetal HPA axis maturation also occurs in the setting of chorioamnionitis, a severe obstetrical inflammatory condition, as demonstrated by increased cortisol secretion and accelerated lung development in exposed preterm newborns (Watterberg et al.1997). Interestingly, elevated proinflammatory biomarkers have been measured in early- and midgestation maternal serum from pregnancies of offspring with ASD (Brown et al. 2014; Goines et al. 2011; Jones et al. 2017, Zerbo et al. 2016). A concurrent investigation of prenatal inflammatory and steroid-related biomarkers is merited to explore whether a pattern of steroid-related biomarkers similar to current study results coincides with pro-inflammatory states during pregnancies associated with ASD.
Higher estradiol activity in pregnancies associated with ASD may indicate a compensatory, rather than pathologic, mechanism because estradiol protects fetal viability during in utero stress (Palmer et al. 1992). Estradiol suppresses inflammation and promotes nutrient and oxygen supply to the fetus by signaling uterine artery dilatation (Magness and Rosenfeld, 1989). The uterine artery experiences its most rapid diameter rise between 10 and 20 weeks gestation (Palmer et al. 1992). This time window overlaps with the period in which the current study’s maternal serum samples were drawn. Low maternal serum estradiol levels have been associated with adverse obstetrical outcomes including the presence and severity of pre-eclampsia, which is a significant cause of placental insufficiency and preterm birth (Jobe et al. 2013; Shin et al. 2018). However, the current study only included term infants, and birthweights between ASD case and control groups were comparable. Repeating this study in offspring born prematurely could elucidate whether higher estradiol continues to be associated with increased ASD risk in the context of greater obstetrical adversity.
To our knowledge, only one prior study has investigated steroid-related ASD biomarkers in midgestation maternal serum. Windham et al. studied a large Californian cohort of pregnancies of offspring with (N=2586) and without (N=600,103) ASD (2016). Windham et al. found a significant, inverse relationship between ASD risk and unconjugated estriol concentrations (Windham et al. 2016). Estriol, the estrogen studied by Windam et al., is used extensively as a component of multiple-marker screening tests for aneuploidy risk stratification. Low estriol, in combination with other biomarker abnormalities, indicates increased aneuploidy risk (Malone et al. 2005; Martin and Cowen, 1990). Historically, obstetrical research and clinical practice have focused on estriol, rather than estradiol, as the estrogen of interest (Martin and Cowen, 1990). As an indicator of fetal steroidogenic activity, estriol’s substrate 16α-Hydroxy-DHEA is almost entirely derived from the fetus while estradiol’s precursor DHEA has both maternal and fetal sources (Cunningham, 2010; Strauss and Barbieri, 2018). Subsequently, maternal serum estriol was proven superior to estradiol in detecting fetal demise as a stand-alone marker (Tulchinsky and Korenman, 1971).
Estradiol was selected as this study’s estrogen of interest because of its advantages over estriol in depicting the in utero environment when measured as one of multiple steroid-related maternal serum biomarkers. Maternal estradiol serum levels exceed those of unconjugated estriol despite higher placental estriol synthesis because estriol is rapidly conjugated and cleared from the maternal circulation (Falah et al. 2015; Goebelsmann and Jaffe, 1971; Guller et al. 1984; Tulchinsky and Hobel, 1973; Tulchinsky and Korenman, 1971). Maternal estradiol levels also capture placental estrogen activity more accurately than estriol because estradiol has significantly higher potency and longer binding duration to estrogen receptors (Andersen et al. 1975; Resnik et al. 1974). Elevated estradiol levels have also demonstrated the capacity to accelerate fetal HPA axis maturation. Considering the strengths and contrasts of these two estrogens, the addition of estriol to the current study’s biomarkers would have provided a valuable context in which to interpret results. Estriol’s exclusion is subsequently a limitation of this study.
Measuring maternal serum steroid biomarkers may feasibly provide the opportunity to explore fetal steroidogenic activity. The placenta produces estradiol from DHEA of both maternal and fetal origin. Based on the established steroid relationships within the maternofetoplacental unit during early 2nd trimester (See Figure 1), the degree to which placental estradiol is produced from fetal DHEA, rather than maternal DHEA, reflects fetal adrenal steroidogenic activity. The inverse relationship between maternal serum DHEA and ASD risk found in the current study suggests that higher maternal serum estradiol levels in pregnancies associated with ASD originate from excess fetal, rather than maternal, DHEA production. Maternal serum estradiol and maternal DHEA substrate availability were used to estimate relative DHEA production by the fetal adrenal gland (i.e., EF-DHEA). Interestingly, EF-DHEA’s predictive value (AUC) for ASD among offspring exceeded, although not statistically significantly, the predictive value of the individual maternal serum steroid-related biomarkers measured in this study. Although EF-DHEA findings are intriguing, this exploration of fetal steroidogenic activity is quite speculative. Scrutiny from fetal endocrinology experts is needed before drawing conclusions from EF-DHEA results.
Higher fetal steroidogenic activity has previously been linked to ASD using early second trimester amniotic fluid samples (Baron-Cohen, et al. 2015). While elevated levels of Δ4 sex steroid pathway components (i.e., testosterone, progesterone, 17a-hydroxyprogesterone, androstenedione) and cortisol were identified, neither estradiol nor estriol were measured. Increased Δ4 sex steroid pathway activity was attributed to fetal production based on sex differences found in amniotic testosterone levels, which do not occur in maternal serum (Baron-Cohen et al. 2015; van de Beek et al. 2004). However, authors did not speculate specifically on the origin of higher amniotic cortisol levels.
This pilot study has several inherent limitations. The generalizability of study results is limited by the small sample size and relative racial and ethnic homogeneity of Utah’s population. Although sufficiently powered to detect differences in steroid concentrations by ASD case status, the confidence intervals for several statistically significant findings were quite broad. The use of immunoassays requires caution regarding the absolute values of steroids measured. However, all samples were measured within a single batch and values were normalized for statistical analysis to account for this potential limitation. As a pilot study, ASD case and control samples were enriched for PNMS exposure to investigate signals of steroid dysregulation in a relatively modest sample size. This analysis included all available ASD+/PNMS+ samples, matched to ASD+/PNMS- samples so that differences between these two ASD+ groups could be examined within one batch. The small ASD- groups (N=11 ASD-/PNMS-; N=8 ASD-/PNMS+) limits this study’s power to consider the influence of PNMS exposure on steroid-related biomarkers in isolation. This study’s PNMS definition reflected the simplistic assumption that ASD risk associated with component conditions is conferred by physiologic elements that are common across these conditions (e.g., inflammation and steroid dysregulation). By collapsing heterogeneous conditions into the dichotomous PNMS exposure category, the differential effect of a single condition or groups of conditions (e.g., hypertension versus diabetes, pre-existing versus gestational onset) could not be determined.
This study’s strengths are derived from Utah’s unique combination of extensive data resources, large obstetrical research cohorts, and strong community support for ASD research. Stakeholders across state, education, and research institutions provided the necessary permissions to identify ASD cases and PNMS exposure among offspring of over 6,000 FASTER study participants. The Utah Population Database provided a trusted site through which linkage could occur between FASTER study participants, birth certificate records, and multiple sources of ASD case ascertainment.
CONCLUSION
Although results require replication in a larger and more diverse population, this study identified prenatal maternal steroid-related biomarkers associated with ASD in a subset of affected offspring enriched for PNMS exposure. The pattern of biomarkers associated with increased ASD risk suggests that higher placental estradiol activity occurred in the early second trimester of these pregnancies. Because elevated estradiol activity during this gestational window may influence fetal HPA axis development, pilot study findings justify future investigations into the in utero steroid environment and fetal HPA axis maturation associated with ASD.
Supplementary Material
Acknowledgments:
We thank the Utah FASTER study participants whose contributions were essential for the success of this study. We appreciate the unique collaboration provided across the University of Utah, Intermountain Healthcare, Utah Registry of Autism and Developmental Disabilities, Utah Department of Health, Utah State Board of Education, and the Pedigree and Population Resource (funded by the Huntsman and Intermountain Healthcare Cancer Foundation).
Funding Sources: This study was supported by University of Utah Neuroscience Initiative. Additional support was received from R01 AG022095 SMITH, KEN R, Early Life Conditions, Survival, and Health: A Pedigree-Based Population Study and R01 MH094400 COON, HILARY, Sequencing Autism Spectrum Disorder Extended Pedigrees.
Footnotes
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the Utah Registry of Autism and Developmental Disabilities Oversight Committee, Utah State Office of Education, and the Institutional Review Boards of the University of Utah, Intermountain Healthcare, Utah Department of Health, and Resource for Genetic and Epidemiologic Research Review Committee, which is an oversight body that regulates Utah Population Database access and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
REFERENCES
- Abbott DH, & Bird IM (2009). Nonhuman primates as models for human adrenal androgen production: function and dysfunction. Reviews in Endocrine & Metabolic Disorders, 10(1), 33–42. 10.1007/s11154-008-9099-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht ED, & Pepe GJ (1999). Central integrative role of oestrogen in modulating the communication between the placenta and fetus that results in primate fecal-placental development. Placenta, 20(2–3), 129–139. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition). American Psychiatric Association; 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Anderson JN, Peck EJ, & Clark JH (1975). Estrogen-induced uterine responses and growth: relationship to receptor estrogen binding by uterine nuclei. Endocrinology, 96(1), 160–167. 10.1210/endo-96-1-160 [DOI] [PubMed] [Google Scholar]
- Andridge RR, & Little RJA (2010). A Review of Hot Deck Imputation for Survey Non-response. International Statistical Review = Revue Internationale de Statistique, 78(1), 40–64. 10.1111/j.1751-5823.2010.00103.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. (2018). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Morbidity and Mortality Weekly Report. Surveillance Summaries (Washington, D.C.: 2002), 67(6), 1–23. 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakian AV, Bilder DA, Carbone PS, Hunt TD, Petersen B, & Rice CE (2015a). Brief report: independent validation of autism spectrum disorder case status in the Utah Autism and Developmental Disabilities Monitoring (ADDM) Network Site. Journal of Autism and Developmental Disorders, 45(3), 873–880. 10.1007/s10803-014-2187-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakian AV, Bilder DA, Coon H, & McMahon WM (2015b). Spatial relative risk patterns of autism spectrum disorders in Utah. Journal of Autism and Developmental Disorders, 45(4), 988–1000. 10.1007/s10803-014-2253-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah MW, Melgaard L, et al. (2015). Elevated fetal steroidogenic activity in autism. Molecular Psychiatry, (20), 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder DA, Bakian AV, Viskochil J, Clark EAS, Botts EL, Smith KR, et al. (2013). Maternal Prenatal Weight Gain and Autism Spectrum Disorders. Pediatrics, 132(5), e1276–e1283. 10.1542/peds.2013-1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Sourander A, Hinkka-Yli-Salomäki S, McKeague IW, Sundvall J, & Surcel H-M (2014). Elevated maternal C-reactive protein and autism in a national birth cohort. Molecular Psychiatry, 19(2), 259–264. 10.1038/mp.2012.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention, Autism and Developmental Disabilities Monitoring Network Surveillance Year 2002 Principal Investigators. (2007). Prevalence of Autism Spectrum Disorders --- Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2002 (MMWR No. 56) (pp. 12–28) [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Abdullah M, Wegelin JA, & Levine S (2006). Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology, 31(1), 59–68. 10.1016/j.psyneuen.2005.05.011 [DOI] [PubMed] [Google Scholar]
- Coulter CL, & Jaffe RB (1998). Functional maturation of the primate fetal adrenal in vivo: 3. Specific zonal localization and developmental regulation of CYP21A2 (P450c21) and CYP11B1/CYP11B2 (P450c11/aldosterone synthase) lead to integrated concept of zonal and temporal steroid biosynthesis. Endocrinology, 139(12), 5144–5150. 10.1210/endo.139.12.6333 [DOI] [PubMed] [Google Scholar]
- Cunningham FG (2010). Implantation, embryogenesis, and placental development In Williams obstetrics (23rd ed., pp. 36–77). New York: McGraw-Hill, Medical, c2010. [Google Scholar]
- DeLong ER, DeLong DM, & Clarke-Pearson DL (1988). Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics, 44(3), 837–845. 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- Dobie SA, Baldwin LM, Rosenblatt RA, Fordyce MA, & Andrilla CH (1998). How well do birth certificates describe the pregnancies they report? The Washington State experience with low-risk pregnancies. Matern Child Health J, 2,(3), 145–154. [DOI] [PubMed] [Google Scholar]
- Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S (2011). The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord, 41(7), 891–902. [DOI] [PubMed] [Google Scholar]
- Falah N, Torday J, Quinney SK, & Haas DM (2015). Estriol review: Clinical applications and potential biomedical importance. Clinical Research and Trials, 1(2). 10.15761/CRT.1000109 [DOI] [Google Scholar]
- Gillon TER, Pels A, Dadelszen PV, Macdonell K, & Magee LA (2014). Hypertensive Disorders of Pregnancy: A Systematic Review of International Clinical Practice Guidelines. PLoS ONE, 9(12). doi: 10.1371/journal.pone.0113715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebelsmann U, & Jaffe RB (1971). Oestriol metabolism in pregnant women. Acta Endocrinologica, 66(4), 679–693. [DOI] [PubMed] [Google Scholar]
- Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, et al. (2011). Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Molecular Autism, 2, 13 10.1186/2040-2392-2-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guller S, Bulletti C, Biener A, & Gurpide E (1984). Relative distribution of estrone, estradiol and estriol between fetal and maternal perfusates during perfusions of human term placentas with labelled C19 precursors. Journal of Steroid Biochemistry, 20(4B), 975–979. [DOI] [PubMed] [Google Scholar]
- Hammond GL (2011). Diverse roles for sex hormone-binding globulin in reproduction. Biology of Reproduction, 85(3), 431–441. 10.1095/biolreprod.111.092593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzlsouer KJ, Alberg AJ, Gordon GB, Longcope C, Bush TL, Hoffman SC, & Comstock GW (1995). Serum gonadotropins and steroid hormones and the development of ovarian cancer. JAMA, 274(24), 1926–1930. [PubMed] [Google Scholar]
- Hisle-Gorman E, Susi A, Stokes T, Gorman G, Erdie-Lalena C, Nylund CM (2018). Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatr Res, 84(2), 190–198. doi: 10.1038/pr.2018.23. [DOI] [PubMed] [Google Scholar]
- Holl K, Lundin E, Kaasila M, Grankvist K, Afanasyeva Y, Hallmans G, et al. (2008). Effect of long-term storage on hormone measurements in samples from pregnant women: the experience of the Finnish Maternity Cohort. Acta Oncologica (Stockholm, Sweden), 47(3), 406–412. 10.1080/02841860701592400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland MA, Sandman CA, & Glynn LM (2017) Developmental origins of the human hypothalamic-pituitary-adrenal axis. Expert Rev Endocrinol Metab, 12(50, 321–339. doi: 10.1080/17446651.2017.1356222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H, & Jaffe RB (2011). Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocrine Reviews, 32(3), 317–355. 10.1210/er.2010-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe SO, Tyler CT, & Magness RR (2013). Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension (Dallas, Tex.: 1979), 61(2), 480–487. 10.1161/HYPERTENSIONAHA.111.201624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Croen LA, Yoshida CK, Heuer L, Hansen R, Zerbo O, et al. (2017). Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Molecular Psychiatry, 22(2), 273–279. 10.1038/mp.2016.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen CB (2004). Steroid hormone synthesis in pregnancy. Obstetrics and Gynecology Clinics of North America, 31(4), 795–816, x. 10.1016/j.ogc.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Kinnunen TI, Luoto R, Gissler M, Hemminki E, Hilakivi-Clarke L (2004). Pregnancy weight gain and breast cancer risk. BMC Womens Health, 4(1),7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, & Hertz-Picciotto I (2012). Maternal Metabolic Conditions and Risk for Autism and Other Neurodevelopmental Disorders. Pediatrics, 129(5), e1121–e1128. 10.1542/peds.2011-2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JM, Contreras R, Chen W, & Sacks DA (2008). Trends in the Prevalence of Preexisting Diabetes and Gestational Diabetes Mellitus Among a Racially/Ethnically Diverse Population of Pregnant Women, 1999–2005. Diabetes Care, 31(5):899–904. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- Lumey LH (1998). Prenatal oestrogens and breast cancer. Paediatr Perinat Epidemiol. 12(4), 361–365. [DOI] [PubMed] [Google Scholar]
- Magness RR, & Rosenfeld CR (1989). Local and systemic estradiol-17 beta: effects on uterine and systemic vasodilation. The American Journal of Physiology, 256(4 Pt 1), E536–542. 10.1152/ajpendo.1989.256.4.E536 [DOI] [PubMed] [Google Scholar]
- Malone FD, Canick JA, Ball RH, Nyberg DA, Comstock CH, Bukowski R, … First- and Second-Trimester Evaluation of Risk (FASTER) Research Consortium. (2005). First-trimester or second-trimester screening, or both, for Down’s syndrome. The New England Journal of Medicine, 353(19), 2001–2011. 10.1056/NEJMoa043693 [DOI] [PubMed] [Google Scholar]
- Martin JN, & Cowan BD (1990). Biochemical assessment and prediction of gestational well-being. Obstetrics and Gynecology Clinics of North America, 17(1), 81–93. [PubMed] [Google Scholar]
- Mesiano S, & Jaffe RB (1997). Developmental and functional biology of the primate fetal adrenal cortex. Endocrine Reviews, 18(3), 378–403. 10.1210/edrv.18.3.0304 [DOI] [PubMed] [Google Scholar]
- Moisiadis VG, & Matthews SG (2014). Glucocorticoids and fetal programming part 1: Outcomes. Nat Rev Endocrinol, 10(7), 391–402. doi: 10.1038/nrendo.2014.73. [DOI] [PubMed] [Google Scholar]
- Montenegro YHA, Nascimento DQ, Assis TO, & Santos-Lopes SSD (2019). The epigenetics of the hypothalamic-pituitary-adrenal axis in fetal development. Ann Hum Genet, 83(4), 195–213. doi: 10.1111/ahg.12306 [DOI] [PubMed] [Google Scholar]
- Murphy VE, Smith R, Giles WB, & Clifton VL (2006). Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocrine Reviews, 27(2), 141–169. 10.1210/er.2005-0011 [DOI] [PubMed] [Google Scholar]
- Nahum Sacks K, Friger M, Shoham-Vardi I, Abokaf H, Spiegel E, Sergienko R, Landau D, Sheiner E (2016). Prenatal exposure to gestational diabetes mellitus as an independent risk factor for long-term neuropsychiatric morbidity of the offspring. Am J Obstet Gynecol, 215(3), 380.e1–7. doi: 10.1016/j.ajog.2016.03.030. [DOI] [PubMed] [Google Scholar]
- Ng PC (2000). The fetal and neonatal hypothalamic–pituitary–adrenal axis. Archives of Disease in Childhood - Fetal and Neonatal Edition, 82(3), F250–F254. 10.1136/fn.82.3.F250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, & Moore LG (1992). Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstetrics and Gynecology, 80(6), 1000–1006. [PubMed] [Google Scholar]
- Pepe G, & Albrecht E (1995). Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocrine Reviews, 16(5), 608–645. [DOI] [PubMed] [Google Scholar]
- Pepe GJ, Waddell BJ, & Albrecht ED (1990). Activation of the baboon fetal hypothalamic-pituitary-adrenocortical axis at midgestation by estrogen-induced changes in placental corticosteroid metabolism. Endocrinology, 127(6), 3117–3123. 10.1210/endo-127-6-3117 [DOI] [PubMed] [Google Scholar]
- Petridou E, Katsouyanni K, Hsieh CC, Antsaklis A, Trichopoulos D (1992). Diet, pregnancy estrogens and their possible relevance to cancer risk in the offspring. Oncology, 49(2), 127–132. [DOI] [PubMed] [Google Scholar]
- Resko JA, Pleom JG, & Stadelman HL (1975). Estrogens in fetal and maternal plasma of the rhesus monkey. Endocrinology, 97(2), 425–430. 10.1210/endo-97-2-425 [DOI] [PubMed] [Google Scholar]
- Resnik R, Killam AP, Battaglia FC, Makowski EL, & Meschia G (1974). The Stimulation of Uterine Blood Flow by Various Estrogens. Endocrinology, 94(4), 1192–1196. 10.1210/endo-94-4-1192 [DOI] [PubMed] [Google Scholar]
- Reynolds RM (2013). Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis−-2012 Curt Richter Award Winner. Psychoneuroendocrinology, 38(1), 1–11. 10.1016/j.psyneuen.2012.08.012 [DOI] [PubMed] [Google Scholar]
- Shin YY, Jeong JS, Park M-N, Lee J-E, An S-M, Cho W-S, et al. (2018). Regulation of steroid hormones in the placenta and serum of women with preeclampsia. Molecular Medicine Reports, 17(2), 2681–2688. 10.3892/mmr.2017.8165 [DOI] [PubMed] [Google Scholar]
- Simpson ER, & MacDonald PC (1981). Endocrine physiology of the placenta. Annual Review of Physiology, 43, 163–188. 10.1146/annurev.ph.43.030181.001115 [DOI] [PubMed] [Google Scholar]
- Smith KR (n.d.). Pedigree and Population Resource: Utah Population Database. Retrieved from https://healthcare.utah.edu/huntsmancancerinstitute/research/updb/
- Spratt EG, Nicholas JS, Brady KT, Carpenter LA, Hatcher CR, Meekins KA, et al. (2012). Enhanced cortisol response to stress in children in autism. Journal of Autism and Developmental Disorders, 42(1), 75–81. 10.1007/s10803-011-1214-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J, Barbieri R, & Gargiulo A (2018). Yen & Jaffe’s Reproductive Endocrinology (8th ed.). Philadelphia, PA: Elsevier; Retrieved from https://www.elsevier.com/books/yen-and-jaffes-reproductive-endocrinology/9780323479127 [Google Scholar]
- Taylor JL, & Corbett BA (2014). A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology, 49, 207–228. 10.1016/j.psyneuen.2014.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarken AJ, Han GT, Corbett BA (2015) Temporal patterns, heterogeneity, and stability of diurnal cortisol rhythms in children with autism spectrum disorder. Psychoneuroendocrinology, 62, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, Kermarrec S, Bonnot O, Geoffray M-M, Brailly-Tabard S, et al. (2014). Altered circadian patterns of salivary cortisol in low-functioning children and adolescents with autism. Psychoneuroendocrinology, 50, 227–245. 10.1016/j.psyneuen.2014.08.010 [DOI] [PubMed] [Google Scholar]
- Tulchinsky D, & Hobel CJ (1973). Plasma human chorionic gonadotropin, estrone, estradiol, estriol, progesterone, and 17α-hydroxyprogesterone in human pregnancy. American Journal of Obstetrics & Gynecology, 117(7), 884–893. [DOI] [PubMed] [Google Scholar]
- Tulchinsky D, & Korenman SG (1971). The plasma estradiol as an index of fetoplacental function. Journal of Clinical Investigation, 50(7), 1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Beek C, Thijssen JHH, Cohen-Kettenis PT, van Goozen SHM, & Buitelaar JK (2004). Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: what is the best source of information to investigate the effects of fetal hormonal exposure? Hormones and Behavior, 46(5), 663–669. 10.1016/j.yhbeh.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Walsh SW, Wolf RC, & Robinson JA (1979). Estrogens in the Uteroovarian, Uterine, and Peripheral Plasma in Pregnant Rhesus Monkeys’. Biology of Reproduction, 20, 606–610. [DOI] [PubMed] [Google Scholar]
- Wang C, Geng H, Liu W, Zhang G (2017). Prenatal, perinatal, and postnatal factors associated with autism: A meta-analysis. Medicine (Baltimore), 96(18), e6696. doi: 10.1097/MD.0000000000006696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterberg KL, Scott SM, & Naeye RL (1997). Chorioamnionitis, cortisol, and acute lung disease in very low birth weight infants. Pediatrics, 99(2), E6. [DOI] [PubMed] [Google Scholar]
- Watterberg Kristi L. (2004). Adrenocortical function and dysfunction in the fetus and neonate. Seminars in Neonatology: SN, 9(1), 13–21. 10.1016/j.siny.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Windham GC, Lyall K, Anderson M, & Kharrazi M (2016). Autism Spectrum Disorder Risk in Relation to Maternal Mid-Pregnancy Serum Hormone and Protein Markers from Prenatal Screening in California. Journal of Autism and Developmental Disorders, 46(2), 478–488. 10.1007/s10803-015-2587-2 [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, & Murphy C (2003). Prevalence of autism in a US metropolitan area. JAMA, 289(1), 49–55. [DOI] [PubMed] [Google Scholar]
- Yu CKH, Papageorghiou AT, Bindra R, Spencer K, & Nicolaides KH (2004). Second-trimester sex hormone-binding globulin and subsequent development of pre-eclampsia. The Journal of Maternal-Fetal & Neonatal Medicine: The Official Journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians, 16(3), 158–162. 10.1080/14767050400013297 [DOI] [PubMed] [Google Scholar]
- Zerbo O, Traglia M, Yoshida C, Heuer LS, Ashwood P, Delorenze GN, et al. (2016). Maternal mid-pregnancy C-reactive protein and risk of autism spectrum disorders: the early markers for autism study. Translational Psychiatry, 6(4), e783 10.1038/tp.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinke K, Fries E, Kliegel M, Kirschbaum C, & Dettenborn L (2010). Children with high-functioning autism show a normal cortisol awakening response (CAR). Psychoneuroendocrinology, 35(10), 1578–1582. 10.1016/j.psyneuen.2010.03.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.