Abstract
Ordered lipid domains (rafts) are generally considered to be features of eukaryotic cells, but ordered lipid domains formed by cholesterol lipids have been identified in bacteria from the genus Borrelia, and similar cholesterol lipids exist in the bacterium Helicobacter pylori. To determine whether H. pylori lipids could form ordered membrane domains, we investigated domain formation in aqueous dispersions of H. pylori whole lipid extracts, individual H. pylori lipids, or defined mixtures of H. pylori lipids, or other membrane-forming lipids. DPH (1,6-diphenyl-1,3,5-hexatriene) anisotropy measurements were used to assay membrane order and FRET (Förster resonance energy transfer) was used to detect the presence of co-existing ordered and disordered domains. We found that H. pylori membrane lipid extracts spontaneously formed lipid domains. Domain formation was more stable when lipids were extracted from H. pylori cells grown in the presence of cholesterol. Certain isolated H. pylori lipids (by themselves or when mixed with other lipids) also had the ability to form ordered domains. To be specific, H. pylori cholesteryl-6-O-tetradecanoyl-α-D-glucopyranoside (CAG) and cholesterol-6-O-phosphatidyl-α-D-glucopyranoside (CG) had the ability to form and/or stabilize ordered domain formation, while H. pylori phosphatidylethanolamine did not, behaving similarly to unsaturated phosphatidylethanolamines. We conclude that specific H. pylori cholesterol lipids have a marked ability to form ordered lipid domains.
Graphical Abstract

Introduction
Lipid rafts in eukaryotic cells are tightly packed liquid ordered (Lo) state microdomains that are rich in sterol and sphingolipids (1–5). Rafts play important roles in signal transduction, cell polarization, and trafficking (2, 6). They are believed to co-exist with more loosely packed liquid disordered (Ld) domains that are rich in unsaturated lipids (2, 7). The existence and properties of lipid rafts have been well-studied in both lipid vesicles and eukaryotic membranes (8–11). Lipid raft-like domains have also recently been identified in bacteria (12, 13). The existence of lipid domains has been proposed in Escherichia coli (14), Bacillus subtilis (15), Staphylococcus aureus (16) and Borrelia burgdorferi (17) membranes, based on cardiolipin, cholesterol, or cholesterol-like molecules. In the case of Borrelia, which contains membrane cholesterol and cholesterol-derived lipids, ordered state membrane domains are very similar to eukaryotic lipid rafts (17–19). The cholesterol glycolipid acyl cholesterol galactoside (ACGal) is the main ordered domain-forming component, and Borrelia PC can participate in ordered domain formation (20).
An open question is whether lipid rafts exist in other cholesterol-containing bacteria, such as Mycoplasma, Ehrlichia, Anaplasma, Brachyspira, and Helicobacter (21–26). Helicobacter pylori is a gram-negative microaerophilic curved rod colonizing half of the world’s population (27, 28). It is a major etiologic agent in both peptic ulcer disease, and adenocarcinoma of the stomach (29, 30). H. pylori strains produce diverse lipids (21, 22, 31). H. pylori strain NCTC 11638 contains three different cholesterol glycolipids. Their likely structures have been defined (22) (Supplemental Figure 1): cholesterol-α-D-glucopyranoside (CG) has cholesterol attached to glucose through a glycosidic bond, cholesteryl-6-O-tetradecanoyl-α-D-glucopyranoside (CAG) has a 14-carbon saturated acyl chain attached to carbon 6 of glucose on CG, and cholesterol-6-O-phosphatidyl-α-D-glucopyranoside (CPG) is proposed to have a CG moiety attached to a phosphatidic acid via linkage between the phosphate and carbon 6 of glucose (32, 33). Phospholipids, mainly phosphatidylethanolamine (PE), comprise the majority of total H. pylori lipids. (22). Other phospholipids include cardiolipin (CL) and phosphatidylglycerol (PG). There are only tiny amounts of phosphatidylserine and sphingomyelin (22). Saturated acyl chains dominate lipid fatty acyl composition with about half being myristic acid (C14:0) and about 20% a 19-carbon cyclopropane fatty acid (C19:0 cyc). Cyclopropyl groups have properties similar to cis double bonds, and when in phospholipid acyl chains result loosely packed lipid bilayers (34).
H. pylori acquires cholesterol from host cells and in vitro from its culture medium, converting some of it to cholesterol glucosides (35). The enzyme responsible for this, cholesterol-α-glucosyltransferase (CGT), is encoded by hp0421/capJ, and can glucosylate several sterols, but has a marked preference for cholesterol (32). Cholesterol and cholesterol glycolipids are important because they affect H. pylori host interactions (36–38). Deletion of capJ in H. pylori increases internalization by macrophages (38), and H. pylori cells with membranes rich in cholesterol-α-glucoside resist phagocytosis by antigen-presenting cells (35). In vivo, H. pylori cells grown in cholesterol-free medium are unable to colonize the gerbil stomach (37), and an H. pylori ΔcapJ strain exhibited attenuated colonization of the gerbil stomach (36).
The presence of cholesterol glucosides in H. pylori suggests that there might be cholesterol-enriched domains within its membranes. In addition, a homolog of flotillin proteins, which are normally found in the cholesterol-enriched domains of eukaryotic cells, has been found enriched in an H. pylori detergent-resistant membrane (DRM) fraction (39). DRM can arise from ordered domains (40). Since H. pylori cells interact with the lipid rafts of host cell membranes, this interaction might involve interactions between host and bacterial lipid rafts (41–45). However, whether H. pylori or its lipids can form ordered membrane domains has not been directly explored. To analyze the role of H. pylori cholesteryl glucosides and phospholipids in forming lipid rafts, we conducted spectroscopic studies upon various lipid dispersions composed of combinations of H. pylori lipids with other membrane lipids. The abilities of three cholesteryl glucosides and the major phospholipid HPE to support raft-formation were defined. The results indicate that H. pylori lipids can help form membrane domains in vitro.
Materials and Methods
Materials.
Porcine brain sphingomyelin (bSM); chicken egg sphingomyelin (eSM); 1,2-dimyristoleoyl-sn-glycero-3-phosphocholine (DMoPC); 1,2-dioleoyl--sn-glycero-3-phosphocholine (DOPC); 1,2-dipalmitoyl--sn-glycero-3-phosphocholine (DPPC); 1-palmitoyl-2-oleoyl--sn-glycero-3-phosphocholine-sn-glycero-3-phosphocholine (POPC); cholesterol; 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE); 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE); 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (rhod-DOPE) were purchased from Avanti Polar Lipids (Alabaster AL). 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(1-pyrenesulfonyl) (pyrene-DPPE) was purchased from Molecular Probes, Inc. (Eugene OR). 1,6-diphenyl-1,3,5-hexatriene (DPH) was purchased from Sigma-Aldrich (St. Louis MO). Cholesterol-α-D-glucopyranoside (CG), cholesteryl-6-O-tetradecanoyl-α-D-glucopyranoside (CAG), and cholesterol-6-O-phosphatidyl-α-D-glucopyranoside (CPG) were extracted and isolated from H. pylori strain JP26 as described below. Lipids and probes were dissolved in chloroform and stored at −20°C. The concentrations of commercial unlabeled lipids were quantified by dry weight. Concentration of fluorescent lipids were determined from absorbance in methanol using Ɛpyrene-DPPE=35,000 M−1cm−1 at 350 nm, Ɛrhod-DOPE =95,000 M−1cm−1 at 560 nm, and ƐDPH =84,800 M−1cm−1 at 352 nm, as described (46).
H. pylori culture and lipid extraction, purification, quantitation:
H. pylori strain JP26 was grown at 37 °C in microaerobic conditions (BD GasPak EZ Campy Container System; Beckton Dickinson, Sparks MD) in Brucella broth liquid media or on agar plates (BA, Difco Laboratories, Detroit MI) supplemented with 10% newborn calf serum (NBCS; Serologicals Corporation, Norcross GA) and when desired with 1.5 mM cholesterol/5 mM methyl β cyclodextrin (47). Cell pellets were washed three times with PBS (pH 7.8 ± 0.2) before conducting lipid extraction with CHCl3/methanol using the Bligh and Dyer method (48). The lipid extracts were dried under a nitrogen gas stream and then dissolved in chloroform. Lipids in whole lipid extracts were separated by chromatography on high performance thin layer chromatography (HP-TLC) plates (Millipore). The plates were developed with CHCl3/methanol/water (70/30/5 v/v/v) until solvent migrated to 75% of their height, then dried, and then redeveloped to their full height using hexane/ethyl acetate (60/40 v/v). Multiple lanes with lipid were loaded onto each plate, with about 0.5mg lipid/centimeter lane length. Plates were sprayed with water after chromatography to visualize major lipids. The three major cholesterol glycolipids, CG, CAG, and CPG, and HPE gave well-separated bands, and the silica gel containing them was scraped from the HP-TLC plates. The individual lipids were eluted from the lipid-containing silica gel three times using (at 2x silica gel volume) CHCl3/methanol/water (70/30/5 v/v/v). Supernatants were collected after centrifugation at 5000 rpm for 10 min on a Sorvall RC-5 centrifuge using glass Corex centrifuge tubes. The extracts were passed twice through qualitative filter paper (VWR), and then dried and dissolved in chloroform. Prior studies showed this filtration was sufficient to remove residual silica gel (20). Lipid concentration was measured by dry weight.
Model membrane vesicle preparation:
Bacterial lipids and fluorescent probes were mixed in glass tubes, and then dried under nitrogen for 5–10 min followed by high vacuum for 1 h. PBS heated to 70°C was added to dried lipids that had been preheated to 70°C for 5 min. The samples were then sonicated in a bath sonicator (Laboratory Supplies, Hicksville NY), typically for 5–10 min, but in all cases until dried lipids were no longer stuck to the bottom of the glass tube and samples appeared homogeneously dispersed. Samples were cooled to room temperature before measurements were taken.
Fluorescence measurements:
Fluorescence was measured in a SPEX FluoroLog 3 spectrofluorometer (Jobin-Yvon, Edison NJ), equipped with an automated polarization apparatus, using quartz semi-microcuvettes (excitation path length 10 mm; emission path length 4 mm) as described (49).
Temperature dependence of fluorescence anisotropy—
Samples containing ~50 μM bacterial lipids with 0.1 mol% DPH were prepared as described above. DPH fluorescence anisotropy was used to study membrane physical state (50). Anisotropy was measured from 16°C to 60°C at 4°C intervals. Between intervals, samples were heated and fluorescence anisotropy measured once temperature stabilized as monitored by a probe thermometer (YSI Series 400, probe diameter ~1/16”) placed in a cuvette. Due to the high level of DPH fluorescence, background values were negligible. Mean values and standard deviations calculated from three separate experiments are reported.
Temperature dependence of FRET—
F samples had vesicles composed of a mixture of bacterial lipids and/or other unlabeled lipids, FRET donor, and FRET acceptor. Fo samples were prepared with the same lipids but lacking FRET acceptor. Background samples for F samples lacked donor, while background samples for Fo samples lacked donor and acceptor. The donor/acceptor pair used in the vesicles was 0.1 mol % pyrene-DPPE/5 mol% rhod-DOPE. The pyrene-DPPE fluorescence of each of the F, Fo, and background samples as a function of temperature was measured in a four-sample cuvette holder every 4°C from 16°C to 60°C, with an excitation λ of 350 nm and emission λ of 379 nm (46); F/Fo values were calculated after subtracting backgrounds. Mean values and standard deviations calculated from three separate experiments are reported. Prior studies have shown that the presence of the FRET probes have little effect on domain formation (49, 51).
Laser scanning microscopy:
Giant unilamellar vesicles (GUVs) composed of 1:1 (mol/mol) egg SM/DMoPC were prepared with 30mol% CAG, 1:1 using the electroformation method (52). The fluorescently-labeled lipid rhod-DOPE (0.02 mol%), which preferentially partitions into disordered domains, was included as a marker of domain visualization (53). To prepare GUVs, the desired lipid combinations were dissolved in chloroform at a total lipid concentration of 5 mM, and a small volume (2 μl) was spread on indium tin oxide-coated coverslips. The solvent was then completely evaporated under high vacuum for 1 h and placed in a flow chamber. Then a 2 mm spacer and second coverslip were placed on top of the first coverslip, with contact sites completely sealed by grease. After 200–300 μl distilled water was injected to the chamber, a 1.2 V at 10 Hz was applied for 2 h at 60°C. Samples were observed using the ConfoCor 2 confocal microscope (Carl Zeiss, Germany) immediately after they were cooled to room temperature, and rhod-DOPE fluorescence detected using a 543 nm (red channel) laser.
Measurement of vesicle size:
Measurement of vesicle size after the fluorescence experiments was carried out at 23°C using a Protein Solutions DynaPro 99 dynamic light-scattering instrument (Wyatt Technology, Santa Barbara CA). Vesicles containing H. pylori lipids were diluted to 5 μM for size measurement. Data were analyzed with Dynamics version 5.25.44 software (Protein Solutions, Inc. Charlottesville VA).
Results
H. pylori lipid separation using high-performance thin-layer chromatography (HP-TLC)
H. pylori membranes contain three kinds of glycolipids, six major phospholipids, and free cholesterol (31), which complicates lipid purification. Total lipid was chromatographed in previous studies (22) using two-dimensional thin-layer TLC plates with CHCl3/methanol/water (65/25/4 v/v/v) and CHCl3/methanol/7N NH4OH (60/35/4.5 v/v/v), or by one dimensional silicic column chromatography using CHCl3/methanol/water (70/30/5 v/v/v). However, these methods do not separate free cholesterol from sterol glycolipids. To circumvent this, we determined that hexane/ethyl acetate (60/40 v/v) could separate free cholesterol from other H. pylori lipids. Figure 1 shows the lipid profile on TLC plates developed in different organic solvents. In agreement with the literature (21, 22, 31), the whole H. pylori lipid extracts contain a significant amount of free cholesterol, three cholesterol glycolipids, and phospholipids (Figure 1A); the major lipid species could be separated after running plates in CHCl3/methanol/water (70/30/5 v/v/v), but this solvent did not separate free cholesterol from CAG. Using a hexane/ethyl acetate (60/40 v/v) solvent, free cholesterol has a higher Rf (about 0.5) than other H. pylori lipids, which (except for very minor bands) barely migrated (Figure 1B). Therefore, we combined these solvent systems (Figure 1C). By comparison to previous work (31) and commercial lipid standards, the major phospholipids and cholesterol glycolipids could be identified. [Note that CAG and ACGal (from Borrelia) have the same mobility on TLC (Figure 1C) due to their very similar chemical structures. Both have cholesterol attached to galactose/glucose through a glycosidic bond, and one acyl chain attached to the carbon 6 of the galactose/glucose (22, 54).
Figure 1. HP-TLC separation of whole lipids from H. pylori strain JP26.
(A) Lipid profile on TLC plates developed in CHCl3/methanol/water (70/30/5 v/v/v). (B) Lipid profile on TLC plates developed in hexane/ethyl acetate (60/40 v/v). (C). Lipid profile on TLC plates first developed up to 3/4 of plate height in CHCl3/methanol/water (70/30/5 v/v/v), then after drying redeveloped to the top of the plate in hexane/ethyl acetate (60/40 v/v). JP26 = lipid extract from H. pylori strain JP26.
Whole H. pylori lipid extracts differ in ability to form ordered state and ordered state domains
We next determined whether H. pylori lipid extracts are able to participate in ordered domain formation in model membrane vesicles by measuring DPH fluorescence anisotropy and FRET in aqueous dispersions of H. pylori whole lipid extracts. First, the temperature dependence of anisotropy was measured for model membranes composed of whole lipid extracts from H. pylori strain JP26 cultured without supplementary cholesterol, or cultured with supplementary cholesterol, or cultured without cholesterol but after 20 mol% cholesterol was added to the lipid extract (Figure 2A). In all three conditions, the H. pylori lipids showed a high level of anisotropy at lower temperatures relative to a standard (DOPC vesicles) for disordered lipid bilayers, indicative of a significant degree of membrane order. (For a fully ordered bilayer DPH anisotropy is in the range 0.30–0.35 (55).) For the lipids from strain JP26 cultured with cholesterol, DPH anisotropy values remained high even when temperature increased, suggesting these vesicles formed the most highly ordered state. This presumably is due to the higher level of both CG and cholesterol in the membranes under these culture conditions (Supplemental Figure 2).
Figure 2. Ability of whole H. pylori lipid extracts to form vesicles with ordered state bilayers or co-existing ordered and disordered domains, as assayed by fluorescence anisotropy and FRET.
(A) DPH fluorescence anisotropy. (B) FRET experiments. Lipid dispersions were composed of: (Squares) H. pylori strain JP26, (Circles) H. pylori strain JP26 cultured with supplemental cholesterol, (Diamonds) H. pylori strain JP26 plus 20 mol% cholesterol, and (Triangles) DOPC. F/Fo is the ratio of fluorescence in vesicles with donor (0.1 mol% pyrene-DPPE) and acceptor (5 mol% rhod-DOPE) to that in vesicles containing only donor, after background values subtracted. In this and the following three figures, lipids were dispersed in PBS at a total concentration of ~50 μM, and mean values from three samples and standard deviations are shown. P-values for difference of mean values at lowest and/or highest temperature calculated using Student’s t-test. ** p < 0.01, *** p < 0.001
Because anisotropy can reveal the overall degree of membrane order but cannot distinguish co-existing ordered and disordered domains, we next used FRET for more direct detection of membrane domains. Pyrene-DPPE was used as a FRET donor that can partition to a significant degree into ordered domains, and rhod-DOPE was used as a FRET acceptor that partitions strongly into disordered domains, as described for Borrelia (20). In this assay, the (partial) segregation of donor and acceptor into separate domains results in weak FRET from donor to acceptor, and thus relatively strong donor fluorescence. This is detected as a higher ratio of fluorescence intensity in the presence of acceptor (F) to fluorescence in the absence of acceptor (Fo) than in vesicles that lack domains and are all in a disordered (Ld) state (49, 56). The fraction of energy transfer equals 1-F/Fo.
It should be noted that the ability to detect ordered domain formation only requires a modest fraction of the FRET donor in the ordered domains, i.e. does not require the FRET donor partition favorably into the ordered domains (51). Prior studies have shown that pyrene-DPPE is able to detect ordered domains about as well as DPH, a probe that partitions about equally between ordered and disordered domains (49).
The whole H. pylori lipid extracts showed strong donor fluorescence (high F/Fo) values at low temperature, indicative of weak FRET and co-existing ordered and disordered domains (Figure 2B). F/Fo decreased markedly as temperature increased, indicating at least partial melting of ordered domains, which decreases segregation of FRET donor and acceptor. (It should be noted that changes in F/Fo when temperature was increased to 60°C were found to be reversible when heated samples were cooled to 16°C in representative cases. This confirms that the domains exist at equilibrium.) In contrast, DOPC vesicles showed strong FRET (low F/Fo) that was largely temperature-independent, as expected due to its ability to form only a homogeneous disordered state over the entire experimental range. The temperature-dependence of FRET showed that lipid from strain JP26 cultured without cholesterol supported ordered domain formation at lower temperatures. Similar behavior was observed when we supplemented the lipid extract with 20mol% cholesterol, although there was more ordered domain formation than without supplemental cholesterol at low temperatures. Although an exact “melting temperature” (Tm) is hard to calculate, it is likely that Tm for ordered domain melting in both cases was ≤ 20°C. Lipid extracts from JP26 cultured with supplemental cholesterol also formed ordered domains with a greater thermal stability than those cultured without supplemental cholesterol, as shown by a more elevated F/Fo value that only decreased at temperatures much higher than that for the other H. pylori lipid mixtures (Figure 2B). In fact, the ordered domains also appeared to be more stable than those observed when the lipid extract was simply supplemented with free cholesterol, consistent with the difference seen in the anisotropy results (Figure 2A).
Individual H. pylori lipids differ in ability to form an ordered state and ordered state domains
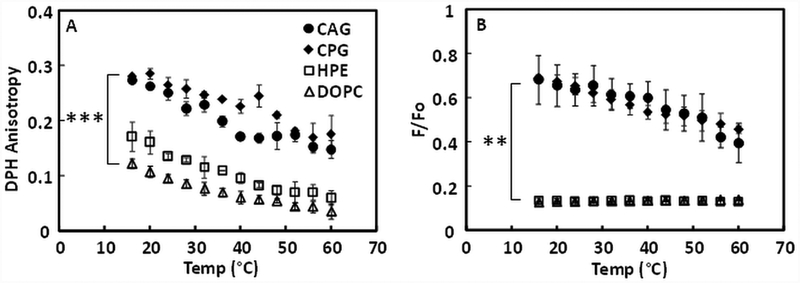
Fluorescence anisotropy and FRET were also used to study the ability of individual H. pylori lipids to form ordered membrane domains. CAG and CPG dispersions take the form of large, presumably unilamellar, lipid vesicles, based on their size (Supplemental Table 1). In vesicles composed of individual H. pylori cholesterol glycolipids, both CAG and CPG formed vesicles with a high degree of order at lower temperature, and DPH anisotropy gradually decreased as temperature increased (Figure 3A). For HPE, the most abundant phospholipid in H. pylori (22), there was only slightly higher anisotropy than DOPC vesicles. Thus, based on anisotropy, CAG and CPG lipids might form ordered domains, but HPE does not.
Figure 3. Ability of individual H. pylori lipids to form ordered state bilayers or co-existing ordered and disordered domains.
(A) DPH fluorescence anisotropy. (B) FRET experiments. P-values for difference of mean values at lowest temperature calculated using Student’s t-test. ** p < 0.01, *** p < 0.001
The ability of individual H. pylori lipids to form ordered domains was examined more directly by FRET (Figure 3B). CAG and CPG vesicles both showed weak FRET (high F/Fo) at low temperature, indicating the presence of co-existing ordered and disordered domains. For both lipids, FRET was temperature-dependent, but remained high at 60°C, indicating that the ordered domains had partly, but not completely, disappeared at high temperature. In contrast, HPE showed a low F/Fo value over the entire temperature range, essentially overlapping with DOPC, consistent with forming only a homogeneous disordered state over the entire temperature range studied. Overall, DPH anisotropy and FRET results are consistent, indicating that CAG and CPG have strong abilities to form ordered state domains, and that HPE cannot form ordered domains by itself.
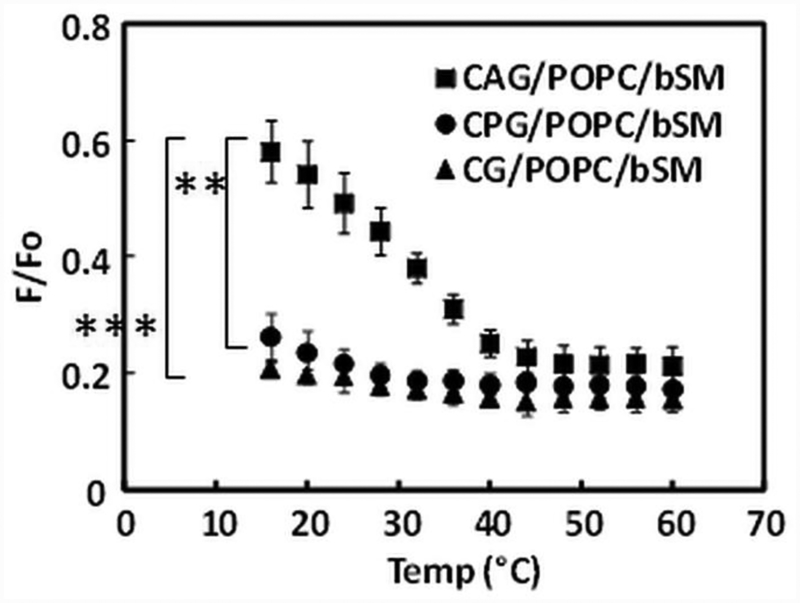
Comparison of CAG, CPG and CG ordered domain-forming ability in lipid mixtures.
The ability of H. pylori cholesterol glycolipids to form ordered domains was then further compared in FRET assays (Figure 4) using lipid mixtures with brain SM and POPC, since SM promotes ordered domain formation, while POPC does not (49). Domain formation is clearly detected in 1:1:1 CAG/bSM/POPC, but is less obvious and less thermally stable with 1:1:1 CPG/bSM/POPC or 1:1:1 CG/bSM/POPC. Thus, of the three cholesterol lipids, CAG appears to have the strongest ability to form ordered domains.
Figure 4. Comparisons of FRET of raft-forming ability of CAG, CPG and CG in vesicles containing of 1:1:1 mol:mol cholesterol lipids/POPC/brain SM.
P-values for difference of mean values at lowest temperature calculated using Student’s t-test. ** p < 0.01, *** p < 0.001
Role of Helicobacter PE in domain formation
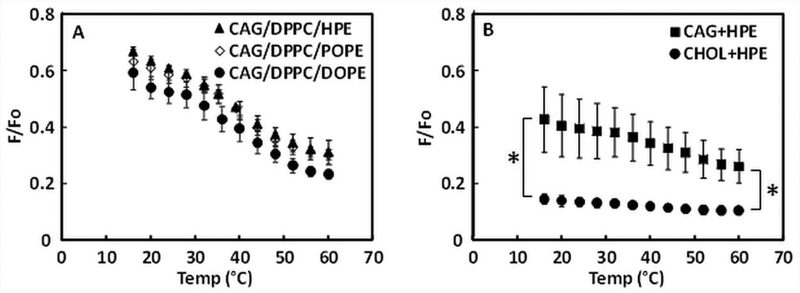
PE is the most abundant H. pylori phospholipid (22), and even though HPE predominantly carries saturated myristic acid (C14:0) and cyclopropane fatty acid (C19:0), the cyclopropane group forms a structure that should interfere with tight membrane bilayer packing. Therefore, HPE might have properties similar to unsaturated PE. To test this, HPE or unsaturated PE were included in the vesicles composed of a mixture of CAG and DPPC (DPPC supports ordered domain formation (57)) to assess ordered domain formation (Figure 5A). Three different types of PE were compared by FRET: HPE, POPE (which has one unsaturated oleoyl acyl chain), and DOPE (which has two oleoyl chains). FRET analysis shows that 1:1:1 CAG/DPPC/HPE, CAG/DPPC/POPE and CAG/DPPC/DOPE have very similar sigmoidal F/Fo vs. temperature curves, indicating that HPE has properties similar to POPE or DOPE molecules.
Figure 5. Ability of PE to form disordered domains in lipid mixtures assayed by FRET.
(A) Comparison of different types of PE in 1:1:1 mixtures. (B) Comparison of 1:1 mixtures. The mean from three samples and standard deviations are shown. P-values for difference of mean values at lowest and highest temperature calculated using Student’s t-test. * p < 0.02
Next, we examined the raft-forming ability of cholesterol and CAG when mixed with HPE in a 1:1 ratio (Figure 5B). CAG/HPE showed weak FRET which increased when temperature increased, indicative of ordered domain formation, while FRET was strong and largely temperature-independent for CHOL/HPE, indicating a lack of ordered domain formation over the temperature range studied. These results indicate that CAG mixed with HPE has a greater ability to form ordered domains than cholesterol mixed with HPE.
CAG can form Lo domains when mixed with other lipids.
When ordered state domains form, they can be either Lo domains which have smooth androunded shapes, or solid-like gel domains which have highly irregular shapes. Although FRET studies do not distinguish between these different types of ordered domains, Lo and gel ordered states can be distinguished by microscopy when domains are sufficiently large to visualize. ACGal from Borrelia forms large Lo domains in GUV containing 1:1 (mol/mol) egg SM/DMoPC (20). Thus, to visualize domain shape, microscopy was performed on GUVs containing H. pylori glycolipids mixed with 1:1 (mol/mol) egg SM/DMoPC (Figure 6). Ordered domains formed with a smooth, rounded boundary in GUV when CAG was present (at 30 mol%). That both CAG and ACGal form Lo domains is not surprising considering their similar chemical structures.
Figure 6. Fluorescence micrographs of GUVs show formation of coexisting Lo and Ld domains in the presence of CAG.
GUVs composed of 1:1 mol:mol eSM/DMoPC with 30 mol% CAG. GUVs contained 0.02 mol% rhod-DOPE as a marker of Ld domains. Left: Cross-section. Middle and Right: 3D reconstructions. The white line is a 1μm scale bar. Note that only Ld domains are stained in each of these vesicles due to the strong partition of the fluorescent probe into the disordered domains.
Domains were not seen in GUVs containing CPG and CG (Supplemental Figure x), presumably because domain size was too small to detect by microscopy.
Discussion
Influence of H. pylori cholesterol glycolipid structure on their ability to participate in ordered domain formation
This study reveals that some membrane lipids of the human gastric bacterium H. pylori have ordered membrane domain/raft-forming properties. Specifically, two major H. pylori cholesterol glycolipids, CAG and CPG, have a strong ability to form membranes in an ordered state, and can form ordered (and co-existing disordered) membrane domains with high thermal stability. CAG, basically a single lipid species, was able to form co-existing ordered and disordered domains over a wide temperature range, consistent with the very gradual melting process shown by DPH anisotropy assay in our results (50). In mixtures with SM and POPC, CAG maintained raft-forming ability, while CPG and CG largely lost this ability. The differences between CAG and CPG are likely to largely reflect differences in their acyl chains (22, 58). As noted above, CAG only contains saturated acyl chains that support ordered domain formation, while CPG has about 50% acyl chains that should not support ordered domain formation (unsaturated acyl chains and acyl chains with cycloproprane rings). Lipid raft levels might vary in different H. pylori strains or under differing growth conditions, because they may differ in relative amounts of CAG and CPG, or in levels of acyl chain unsaturation/branching. That CG has little ability to form lipid rafts presumably reflects steric inhibition of tight packing by the glucose attached to the cholesterol hydroxyl group.
It should be noted that for samples containing H. pylori lipid extracts there was weaker FRET than in DOPC vesicles even at the highest temperature studied, suggesting that ordered domains may have partly persisted in these lipid extracts even at very elevated temperatures. Alternative explanations for weak FRET include a lower fluorescence lifetime for pyrene-DPPE incorporated into the H. pylori lipids, which would make FRET less efficient, an orientation of pyrene-DPPE and rhodamine in H. pylori lipid membranes that is less favorable for efficient FRET than their orientation in DOPC, or that there is partial de-mixing of pyrene-DPPE and rhod-DOPE into different vesicle populations during preparation of the lipid dispersions, so that even when ordered domains fully melt, FRET donor and acceptor remained physically separated, which also would decrease FRET. The latter possibility is unlikely given the observation that DPH anisotropy also indicated that a significant level of high membrane order remains in these lipid mixtures at elevated temperatures.
Influence of HPE structure on its ability to participate in ordered domain formation
PE, the most abundant membrane phospholipid in H. pylori, seems unable to participate in ordered domain formation, in contrast to the cholesterol glycolipids or to Borrelia PC. H. pylori HPE species predominantly contain a C14:0 (~50%) and a C19:0 (~30%) cyclopropane acyl chain, two C14:0 acyl chains, or a C14:0 and a C18:1 acyl chain (58). Thus, the fraction of HPE with at least one poorly packing acyl chain, and thus likely to favor formation of a disordered state under our conditions, is likely to be much more than 50% of HPE molecules. Thus, HPE is unlikely to promote ordered domain formation. The similarity in HPE behavior (Figure 5) to that of POPE and DOPE, both of which contain unsaturated acyl chains and should not tend to form ordered domains under our experimental conditions, is consistent with this conclusion.
Implications for other bacteria and potential biomedical applications
Based on these studies it can be concluded that H. pylori lipids have the capacity to form ordered domains/rafts. The raft-forming rules for lipids in H. pylori, and those previously defined in Borrelia (20), agree with those for lipid raft-forming lipids in eukaryotic cells. Because of this, and because rafts form in Borrelia, it is possible that rafts also form in H. pylori. However, demonstrating rafts form in vivo would have to be studied directly in H. pylori, as protein interactions or the distribution of lipids in H. pylori membranes could affect whether rafts actually form.
Another unexplored area is the potential function of rafts in bacteria. In Borrelia, lipid rafts may have a role in membrane integrity and morphology (17), and/or might also help prokaryotes to organize cellular processes (13). Another bacterial raft function might be to promote interaction with host cells (18). Indeed, the two most important host-interaction factors of H. pylori, the cytotoxin-associated gene A product (CagA) and the vacuolation associated gene A (VacA), are translocated from H. pylori cells into host epithelial cell lipid rafts to dysregulate multiple host cell signaling pathways (59). Moreover H. pylori outer membrane proteins (OMPs) including sialic acid-binding adhesin (SabA), blood-group-antigen-binding adhesin (BabA), adherence-associated lipoprotein A and B (AlpA/B), outer inflammatory protein A (OipA), and Helicobacter outer membrane protein Q (HopQ), all play important roles in the bacterial-host interactions (60–62); it is possible that the bacterial lipid rafts regulate these OMP functions. Our studies of H. pylori lipid rafts combined with studies of how lipids and proteins participate in raft formation in H. pylori could aid in studies of their biological functions. H. pylori is characterized by high-level genetic plasticity, rapid genetic adaptation to the individual human host and to gastric environmental constraints (63), and high mutation frequencies leading to antibiotic resistance (64).
Supplementary Material
Highlights.
The ability of H. pylori lipids to form ordered domains (rafts) was measured using FRET.
Mixed H. pylori lipid dispersions form co-existing ordered and disordered domains.
H. pylori cholesterol lipids exhibit a strong tendency to form ordered domains.
H. pylori phosphatidylethanolamine does not exhibit a tendency to form ordered domains.
Acknowledgments
This work was supported by NIH grant GM 122493 (to EL); and DK 090989 (to MJB), and by the C & D and Zlinkoff funds.
Abbreviations:
- SM
sphingomyelin
- bSM
porcine brain sphingomyelin
- eSM
chicken egg sphingomyelin
- CG
cholesterol-α-D-glucopyranoside
- CAG
cholesteryl-6-O-tetradecanoyl-α-D-glucopyranoside
- CG
cholesterol-α-D-glucopyranoside
- CPG
cholesterol-6-O-phosphatidyl-α-D-glucopyranoside
- HPE
H. pylori 1,2-diacyl-sn-glycero-3-phosphoethanolamine
- PE
phosphatidylethanolamine
- CHOL
cholesterol
- DRM
detergent resistant membrane
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- DMoPC
1,2-dimyristoleoyl-sn-glycero-3-phosphocholine
- POPE
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine
- DOPE
1,2-dioleoyl-snglycero-3-phosphoethanolamine
- DPH
1,6-diphenyl-1,3,5-hexatriene
- FRET
Förster resonance energy transfer
- Lo
liquid ordered
- Ld
liquid disordered
- NaDt
sodium dithionite
- pyrene-DPPE
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(1-pyrenesulfonyl)
- rhod-DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl)
- GUVs
giant unilamellar vesicles
- HP-TLC
high performance thin layer chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown DA, and London E (2000) Structure and function of sphingolipid- and cholesterol-rich membrane rafts, The Journal of biological chemistry 275, 17221–17224. [DOI] [PubMed] [Google Scholar]
- 2.Simons K, and Ikonen E (1997) Functional rafts in cell membranes, Nature 387, 569–572. [DOI] [PubMed] [Google Scholar]
- 3.Levental I, and Veatch S (2016) The Continuing Mystery of Lipid Rafts, Journal of molecular biology 428, 4749–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujimoto T, and Parmryd I (2017) Interleaflet Coupling, Pinning, and Leaflet Asymmetry-Major Players in Plasma Membrane Nanodomain Formation, Frontiers in cell and developmental biology 4, 155–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki KGN, Ando H, Komura N, Fujiwara TK, Kiso M, and Kusumi A (2017) Development of new ganglioside probes and unraveling of raft domain structure by single-molecule imaging, Biochimica et biophysica acta. General subjects 1861, 2494–2506. [DOI] [PubMed] [Google Scholar]
- 6.Simons K, and Toomre D (2000) Lipid rafts and signal transduction, Nature reviews. Molecular cell biology 1, 31–39. [DOI] [PubMed] [Google Scholar]
- 7.London E (2002) Insights into lipid raft structure and formation from experiments in model membranes, Current opinion in structural biology 12, 480–486. [DOI] [PubMed] [Google Scholar]
- 8.Brown D (2002) Structure and function of membrane rafts, International journal of medical microbiology : IJMM 291, 433–437. [DOI] [PubMed] [Google Scholar]
- 9.London E (2005) How principles of domain formation in model membranes may explain ambiguities concerning lipid raft formation in cells, Biochim Biophys Acta 1746, 203–220. [DOI] [PubMed] [Google Scholar]
- 10.London E, and Brown DA (2000) Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts), Biochim Biophys Acta 1508, 182–195. [DOI] [PubMed] [Google Scholar]
- 11.Luo C, Wang K, Liu DQ, Li Y, and Zhao QS (2008) The functional roles of lipid rafts in T cell activation, immune diseases and HIV infection and prevention, Cellular & molecular immunology 5, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bramkamp M, and Lopez D (2015) Exploring the existence of lipid rafts in bacteria, Microbiol Mol Biol Rev 79, 81–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez D, and Kolter R (2010) Functional microdomains in bacterial membranes, Genes & development 24, 1893–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mileykovskaya E, and Dowhan W (2000) Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange, Journal of bacteriology 182, 1172–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai F, Shoda M, Harashima R, Sadaie Y, Hara H, and Matsumoto K (2004) Cardiolipin domains in Bacillus subtilis marburg membranes, Journal of bacteriology 186, 1475–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beining PR, Huff E, Prescott B, and Theodore TS (1975) Characterization of the lipids of mesosomal vesicles and plasma membranes from Staphylococcus aureus, Journal of bacteriology 121, 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaRocca TJ, Pathak P, Chiantia S, Toledo A, Silvius JR, Benach JL, and London E (2013) Proving lipid rafts exist: membrane domains in the prokaryote Borrelia burgdorferi have the same properties as eukaryotic lipid rafts, PLoS Pathog 9, e1003353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowley JT, Toledo AM, LaRocca TJ, Coleman JL, London E, and Benach JL (2013) Lipid exchange between Borrelia burgdorferi and host cells, PLoS pathogens 9, e1003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaRocca TJ, Crowley JT, Cusack BJ, Pathak P, Benach J, London E, Garcia-Monco JC, and Benach JL (2010) Cholesterol lipids of Borrelia burgdorferi form lipid rafts and are required for the bactericidal mechanism of a complement-independent antibody, Cell host & microbe 8, 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Z, Toledo AM, Benach JL, and London E (2016) Ordered Membrane Domain-Forming Properties of the Lipids of Borrelia burgdorferi, Biophysical journal 111, 2666–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haque M, Hirai Y, Yokota K, and Oguma K (1995) Steryl glycosides: a characteristic feature of the Helicobacter spp.?, Journal of bacteriology 177, 5334–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirai Y, Haque M, Yoshida T, Yokota K, Yasuda T, and Oguma K (1995) Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis, Journal of bacteriology 177, 5327–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin M, and Rikihisa Y (2003) Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival, Infection and immunity 71, 5324–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith PF (1971) Biosynthesis of cholesteryl glucoside by Mycoplasma gallinarum, Journal of bacteriology 108, 986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trott DJ, Alt DP, Zuerner RL, Wannemuehler MJ, and Stanton TB (2001) The search for Brachyspira outer membrane proteins that interact with the host, Animal health research reviews / Conference of Research Workers in Animal Diseases 2, 19–30. [PubMed] [Google Scholar]
- 26.Livermore BP, Bey RF, and Johnson RC (1978) Lipid metabolism of Borrelia hermsi, Infection and immunity 20, 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moodley Y, Linz B, Bond RP, Nieuwoudt M, Soodyall H, Schlebusch CM, Bernhöft S, Hale J, Suerbaum S, Mugisha L, van der Merwe SW, and Achtman M (2012) Age of the Association between Helicobacter pylori and Man, PLoS pathogens 8, e1002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kusters JG, van Vliet AH, and Kuipers EJ (2006) Pathogenesis of Helicobacter pylori infection, Clinical microbiology reviews 19, 449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cover TL, and Blaser MJ (1992) Helicobacter pylori and gastroduodenal disease, Annual review of medicine 43, 135–145. [DOI] [PubMed] [Google Scholar]
- 30.Peek RM Jr., and Blaser MJ (2002) Helicobacter pylori and gastrointestinal tract adenocarcinomas, Nature reviews. Cancer 2, 28–37. [DOI] [PubMed] [Google Scholar]
- 31.Haque M, Hirai Y, Yokota K, Mori N, Jahan I, Ito H, Hotta H, Yano I, Kanemasa Y, and Oguma K (1996) Lipid profile of Helicobacter spp.: presence of cholesteryl glucoside as a characteristic feature, Journal of bacteriology 178, 2065–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebrun AH, Wunder C, Hildebrand J, Churin Y, Zahringer U, Lindner B, Meyer TF, Heinz E, and Warnecke D (2006) Cloning of a cholesterol-alpha-glucosyltransferase from Helicobacter pylori, The Journal of biological chemistry 281, 27765–27772. [DOI] [PubMed] [Google Scholar]
- 33.Pelz A, Wieland KP, Putzbach K, Hentschel P, Albert K, and Gotz F (2005) Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus, The Journal of biological chemistry 280, 32493–32498. [DOI] [PubMed] [Google Scholar]
- 34.Koynova R, and Caffrey M (1998) Phases and phase transitions of the phosphatidylcholines, Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes 1376, 91–145. [DOI] [PubMed] [Google Scholar]
- 35.Wunder C, Churin Y, Winau F, Warnecke D, Vieth M, Lindner B, Zahringer U, Mollenkopf HJ, Heinz E, and Meyer TF (2006) Cholesterol glucosylation promotes immune evasion by Helicobacter pylori, Nat Med 12, 1030–1038. [DOI] [PubMed] [Google Scholar]
- 36.McGee DJ, George AE, Trainor EA, Horton KE, Hildebrandt E, and Testerman TL (2011) Cholesterol enhances Helicobacter pylori resistance to antibiotics and LL-37, Antimicrobial agents and chemotherapy 55, 2897–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hildebrandt E, and McGee DJ (2009) Helicobacter pylori lipopolysaccharide modification, Lewis antigen expression, and gastric colonization are cholesterol-dependent, BMC microbiology 9, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du SY, Wang HJ, Cheng HH, Chen SD, Wang LH, and Wang WC (2016) Cholesterol glucosylation by Helicobacter pylori delays internalization and arrests phagosome maturation in macrophages, Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi 49, 636–645. [DOI] [PubMed] [Google Scholar]
- 39.Hutton ML, D’Costa K, Rossiter AE, Wang L, Turner L, Steer DL, Masters SL, Croker BA, Kaparakis-Liaskos M, and Ferrero RL (2017) A Helicobacter pylori Homolog of Eukaryotic Flotillin Is Involved in Cholesterol Accumulation, Epithelial Cell Responses and Host Colonization, Front Cell Infect Microbiol 7, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroeder R, London E, and Brown D (1994) Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior, Proc Natl Acad Sci U S A 91, 12130–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang HJ, Cheng WC, Cheng HH, Lai CH, and Wang WC (2012) Helicobacter pylori cholesteryl glucosides interfere with host membrane phase and affect type IV secretion system function during infection in AGS cells, Molecular microbiology 83, 67–84. [DOI] [PubMed] [Google Scholar]
- 42.Schraw W, Li Y, McClain MS, van der Goot FG, and Cover TL (2002) Association of Helicobacter pylori vacuolating toxin (VacA) with lipid rafts, The Journal of biological chemistry 277, 34642–34650. [DOI] [PubMed] [Google Scholar]
- 43.Raghunathan K, Foegeding NJ, Campbell AM, Cover TL, Ohi MD, and Kenworthy AK (2018) Determinants of raft partitioning of the Helicobacter pylori pore-forming toxin VacA, Infection and immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuo C-H, and Wang W-C (2003) Binding and internalization of Helicobacter pylori VacA via cellular lipid rafts in epithelial cells, Biochem Biophys Res Commun 303, 640–644. [DOI] [PubMed] [Google Scholar]
- 45.Fahimi F, Tohidkia MR, Fouladi M, Aghabeygi R, Samadi N, and Omidi Y (2017) Pleiotropic cytotoxicity of VacA toxin in host cells and its impact on immunotherapy, Bioimpacts 7, 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiederschain GY (2011) The Molecular Probes handbook. A guide to fluorescent probes and labeling technologies, Biochemistry (Moscow) 76, 1276–1276. [Google Scholar]
- 47.Zhang XS, Tegtmeyer N, Traube L, Jindal S, Perez-Perez G, Sticht H, Backert S, and Blaser MJ (2015) A specific A/T polymorphism in Western tyrosine phosphorylation B-motifs regulates Helicobacter pylori CagA epithelial cell interactions, PLoS pathogens 11, e1004621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bligh EG, and Dyer WJ (1959) A rapid method of total lipid extraction and purification, Canadian journal of biochemistry and physiology 37, 911–917. [DOI] [PubMed] [Google Scholar]
- 49.Pathak P, and London E (2011) Measurement of lipid nanodomain (raft) formation and size in sphingomyelin/POPC/cholesterol vesicles shows TX-100 and transmembrane helices increase domain size by coalescing preexisting nanodomains but do not induce domain formation, Biophysical journal 101, 2417–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lentz BR, Barenholz Y, and Thompson TE (1976) Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 2. Two-component phosphatidylcholine liposomes, Biochemistry 15, 4529–4537. [DOI] [PubMed] [Google Scholar]
- 51.Pathak P, and London E (2015) The Effect of Membrane Lipid Composition on the Formation of Lipid Ultrananodomains, Biophysical journal 109, 1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin Q, and London E (2013) Altering hydrophobic sequence lengths shows that hydrophobic mismatch controls affinity for ordered lipid domains (rafts) in the multitransmembrane strand protein perfringolysin O, The Journal of biological chemistry 288, 1340–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiantia S, Schwille P, Klymchenko AS, and London E (2011) Asymmetric GUVs prepared by MbetaCD-mediated lipid exchange: an FCS study, Biophysical journal 100, L1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schroder NW, Schombel U, Heine H, Gobel UB, Zahringer U, and Schumann RR (2003) Acylated cholesteryl galactoside as a novel immunogenic motif in Borrelia burgdorferi sensu stricto, The Journal of biological chemistry 278, 33645–33653. [DOI] [PubMed] [Google Scholar]
- 55.Cheng H-T, Megha, and London E (2009) Preparation and properties of asymmetric vesicles that mimic cell membranes: effect upon lipid raft formation and transmembrane helix orientation, The Journal of biological chemistry 284, 6079–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sachl R, Johansson LB, and Hof M (2012) Forster resonance energy transfer (FRET) between heterogeneously distributed probes: application to lipid nanodomains and pores, International journal of molecular sciences 13, 16141–16156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suga K, and Umakoshi H (2013) Detection of Nanosized Ordered Domains in DOPC/DPPC and DOPC/Ch Binary Lipid Mixture Systems of Large Unilamellar Vesicles Using a TEMPO Quenching Method, Langmuir 29, 4830–4838. [DOI] [PubMed] [Google Scholar]
- 58.Shimomura H, Hosoda K, Hayashi S, Yokota K, and Hirai Y (2012) Phosphatidylethanolamine of Helicobacter pylori Functions as a Steroid-Binding Lipid in the Assimilation of Free Cholesterol and 3-Hydroxl Steroids into the Bacterial Cell Membrane, Journal of bacteriology 194, 2658–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bridge DR, and Merrell DS (2013) Polymorphism in the Helicobacter pylori CagA and VacA toxins and disease, Gut Microbes 4, 101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oleastro M, and Ménard A (2013) The Role of Helicobacter pylori Outer Membrane Proteins in Adherence and Pathogenesis, Biology 2, 1110–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuo Y, Kido Y, and Yamaoka Y (2017) Helicobacter pylori Outer Membrane Protein-Related Pathogenesis, Toxins 9, E101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kao CY, Sheu BS, and Wu JJ (2016) Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis, Biomedical journal 39, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Atherton JC, and Blaser MJ (2009) Coadaptation of Helicobacter pylori and humans: ancient history, modern implications, The Journal of clinical investigation 119, 2475–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Björkholm B, Sjölund M, Falk PG, Berg OG, Engstrand L, and Andersson DI (2001) Mutation frequency and biological cost of antibiotic resistance in Helicobacter pylori, Proceedings of the National Academy of Sciences 98, 14607–14612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.