Abstract
Congenital abnormalities of the urinary tract are some of the most common human developmental abnormalities. Several genetically engineered mouse models have been developed to mimic these abnormalities and aim to better understand the molecular mechanisms of disease. This atlas has been developed as an aid to pathologists and other biomedical scientists for the identification of abnormalities in the developing murine urinary tract by cataloguing normal structures at each stage of development. Hematoxylin & eosin and immunohistochemical stained sections are provided, with a focus on E10.5–18.5, as well as a brief discussion of postnatal events in urinary tract development. A section on abnormalities in the development of the urinary tract is also provided and molecular mechanisms are presented as supplementary material. Additionally, overviews of the two key processes of kidney development, branching morphogenesis and nephrogenesis, are provided to aid in the understanding of the complex organogenesis of the kidney. One of the key findings of this atlas is the histological identification of the ureteric bud at E10.5, as previous literature has provided conflicting reports on the initial point of budding. Furthermore, attention is paid to points where murine development is significantly distinct from human development, namely in the cessation of nephrogenesis.
Keywords: Atlas, embryo, development, genetically engineered mice, kidney, bladder, urethra
Introduction
In humans, congenital anomalies of the urinary tract occur in approximately 1 in 500 fetal ultrasound examinations and represents 35–45% of all congenital abnormalities. Prevalence data suggests that anomalies specific to the urinary tract occur in roughly 3–6 per 1000 live births1, 2. Although many abnormalities go unnoticed, congenital kidney disease in children is an important cause of illness and death. According to the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Report (2008), major consequences of congenital kidney defects leading to subsequent renal insufficiency include renal aplasia, hypoplasia or dysplasia (15.9%), obstructive uropathy (15.6%), and polycystic kidney disease (2.9%).3 Congenital functional and structural abnormalities of the lower urinary tract, including vesicoureteral reflux, urinary tract obstruction, and abnormalities of the bladder and urethra, account for 20–30% of all prenatally identified anomalies in humans.4
The discovery and understanding of genetic and epigenetic (nongenetic influences on gene expression) causes of congenital urinary tract disorders is becoming increasingly recognized, and in turn the development and use of animal models to recapitulate the diseases have been increasingly utilized. In particular, the development of genetically engineered mouse (GEM) models to target genes of interest has been used to increase knowledge of the molecular mechanisms of these disorders.5 The increasingly widespread use of GEM models to understand normal urinary tract development, as well as the congenital disorders that may occur in humans, requires knowledge of the normal events that occur within the developing urinary system. Therefore, an understanding of the molecular and histological changes in these models is needed. Two tables providing key developmental terms along with definitions, timing and functions are provided for the upper (Table 1) and lower (Table 2) murine urinary tracts. The most common abnormal renal developmental phenotypes found in mice are provided in Table 3, and an online supplemental section on molecular events in early, mid and late gestation is also provided.
Table 1.
Key Definitions/Timing/Functions for Murine Upper Urinary Tract Development
| Term | Definition/Timing/Function |
|---|---|
| Ampulla | Bulbous ending or terminus to the uretic tree in kidney development. Point at which branching morphogenesis occurs.13 |
| Calyx | Single cavity within the kidney in which multiple papillary ducts drain. Several minor calyces can come together to form a major calyx. Formed from rapid ureteric bud division.13 |
| Collecting duct system | Consists of ureter, renal pelvis, calyces, and collecting tubules. Arises from UB branching morphogenesis.13 |
| Collecting tubules | Portion of the collecting duct system into which the nephrons drain. Responsible for water absorption from the urine.12 |
| Comma and S-shaped bodies | Interim structures in the developing nephron. The comma-shaped body elongates, folds and acquires an S shape. The proximal segment of the S-shaped body forms the renal vesicle, the distal segment forms the distal convoluted tubules, and the medial segment forms the proximal convoluted tubules and loop of Henle.13 |
| Common nephric duct | Caudal portion of the Wolffian duct that connects with urogenital sinus. Functions as the initial orifice of ureter connection to the urogenital sinus before repositioning of the ureteral orifice.19 |
| Cortex | Outer zone of the mammalian kidney. Defined as the only renal tissue containing glomeruli.13, 18 |
| Distal tubule | Portion of the nephron between the loop of Henle and the collecting duct. Further responsible for reabsorption of filtrate particularly for regulating potassium, calcium, and magnesium levels as well as water reabsorption.12 |
| Glomerular basement membrane | Specialized basement lamina in the glomeruli that forms between podocytes and endothelial cells.11,12 |
| Glomeruli | Most proximal portion of the nephron. Consists of a capillary tuft surrounded by highly specialized epithelial cells called podocytes. Functions to receive and filter blood.12 |
| Inner medulla | Most interior zone, closest to the papilla, of the mammalian kidney. Includes the papilla.13 |
| Intermediate mesoderm (nephrogenic cord) | Subpopulation of the mesoderm germ layer from which many renal and urinary structures develop.12,13 |
| Intermediate tubule (loop of Henle) | Portion of the nephron between the proximal and distal tubules. Can loop into either the outer or inner medulla. Further responsible for reabsorption of filtrate particularly for regulating potassium, calcium, and magnesium levels as well as water reabsorption.12 |
| Mesonephros | Second of the three mammalian developmental renal structures. Occurs at E9.0, consists of approximately 18 tubules which arise from the nephrogenic cord, of which only the most cranial 4–6 connect to the nephric duct and are functional with nephrons. Degenerates by apoptosis at E14.5. As with the pronephros, the nephric duct remains. In males, some of the cranial tubules remain and give rise to the epididymal ducts of the testis.12,13 |
| Metanephric mesenchyme | Collection of distinct mesenchymal cells that originates from the nephrogenic cord at E10.5. Gives rise to glomeruli, tubular segments of nephron, and interstitium in mature kidney.13 |
| Metanephros | Final of the three developmental renal structures. Ultimately develops into permanent kidneys. Develops from ureteric bud and metanephric mesenchyme at E10.5.12,13 |
| Nephric duct (mesonephric, Wolffian duct) | Arises from the caudal portion of the pronephric duct. Continues caudal growth until connection with the urogenital sinus. Induces tubule formation in the intermediate mesoderm and gives rise to ureteric bud.12,13 |
| Nephron | Functional unit of the kidney. Consisting of glomerulus, proximal tubule, loop of Henle and distal tubule. Connects to the collecting ducts.36 |
| Outer medulla | Second zone in the mammalian kidney. Divided into an inner and outer stripe dependent on the portion of the nephron present. Characterized by a lack of glomeruli.13,18 |
| Papilla | Most interior layer of the kidney into which the collecting duct system drains. Mice have a single, long papilla which can protrude into the ureter.13 |
| Podocytes | Specific epithelial cells located on the glomerular basement membrane within the glomeruli. Extend ‘foot processes’ that come together to form a slit diaphragm for glomerular filtration.11,12 |
| Pronephric duct | Earliest form of an epithelial tube in renal development. Occurs at E8.0 from intermediate mesoderm which undergoes a mesenchymal-to-epithelial transition. The cranial portion begins to regress at the same time as the pronephros, but the caudal portion persists and extends caudally to form the nephric duct.12 |
| Pronephros | Earliest of the three mammalian developmental renal structures. Occurs at E8.0 and develops from the intermediate mesoderm. Consists of non-functional transient tubules which open to the pronephric duct. Regresses at E9.0.12,13 |
| Proximal tubule | Portion of the nephron between glomeruli and loop of Henle. Located in the cortex. Responsible for reabsorbing the majority of filtrate that passes through the glomerular filter.12 |
| Renal capsule | Fibrous capsule that encloses the kidney.13 |
| Renal pelvis | A funnel like structure of urothelium into which the renal papilla drains. Connects to the ureter.86 |
| Renal vesicle | Epithelial nephron precursor structure, formed from metanephric mesenchyme. Elongates to form comma, then S-shaped bodies.13 |
| Ureter | Epithelial tube that connects the kidney to the urinary bladder. Consists of urothelium, lamina propria, smooth muscle, and a layer of adventitia.87 |
| Ureteric bud | Buds from nephric duct at E10.5. Gives rise to collection duct system including ureters, renal pelvis, calyces, and collecting tubules.2,13 |
| Urogenital ridge | Formed from the intermediate mesoderm at E9.5. Bilateral, longitudinal ridges that consist of coelomic surface epithelium and underlying mesenchyme.27 |
Table 2.
Key Definitions/Timing/Functions for Murine Lower Urinary Tract Development
| Term | Definition/Timing/Function |
|---|---|
| Anorectal canal | Arises from the division of the cloaca by the urorectal septum and forms the colon, anus, and rectum.30,31 |
| Bladder neck | The narrow, open end of the bladder between the ureteral orifices and the urethra.30 |
| Bladder trigone | Triangular area at the base of the bladder whose apexes consist of the two ureteral orifices and the urethral orifice. A structurally distinct region of the bladder with endodermal and mesodermal origins.4,6 |
| Cloaca | A caudal expansion that is the original point of termination of the hindgut. Divided by the urorectal septum into the urogenital sinus and the anorectal canal.27 |
| Cloacal membrane | Region of contact of the endodermal cloacal epithelium and the surface ectoderm. Ruptures to form anal opening and the proximal urethral meatus.30 |
| Definitive urethral meatus (male) | Final orifice of the penile urethra. Located at the distal end of the penis. Forms at E17.5.30 |
| Definitive urogenital sinus | Arises from the division of the urogenital sinus at E13.014.0. Definitive urogenital sinus goes on to form the penile urethra in males and the lower portion of the vagina and the vestibule of the vagina in females.4,27,31 |
| Fundus of the bladder | The rounded, blind-end of the bladder.30 |
| Genital tubercle | Early structure of the external genitals. Forms the penis in males and the clitoris in females.88 |
| Hindgut | The caudal portion of the primitive gut. Forms the cloaca and several structures of the lower digestive tract.89 |
| Lamina propria | Thin, membranous layer of the bladder between the urothelium and the smooth muscle layer.60 |
| Membranous urethra (female) | Comprises the entire urethra in females. Derived from the vesicourethral canal.4 |
| Membranous urethra (male) | Region of the urethra between the prostatic urethra and the penile urethra in males. Derived from the vesicourethral canal.4,90 |
| Penile (spongy) urethra (male) | Portion of the urethra within the penis of adult males. Forms from the definitive urogenital sinus.30,31 |
| Prostatic urethra (male) | Region of the urethra proximal to the site of entry of the ejaculatory ducts. Forms from the vesical portion of the urogenital sinus (vesicourethral canal).6 |
| Proximal urethral meatus | Initial orifice of the phallic urethral. Formed from the rupture of the cloacal membrane at E13. Closes in males by E16.5, but remains open in females.30 |
| Sinovaginal bulb (female) | Solid epithelial cord that is derived from the urogenital sinus. Forms the developmental connection between the urethra and the upper vagina. Postnatally, forms a portion of the vulva epithelium.30 |
| Smooth muscle | Muscular outer layer of the bladder that can be contracted voluntarily or involuntarily. Forms from the mesenchyme surrounding the urogenital sinus.28,60 |
| Urachus | The canal that connects the allantois and the urinary bladder in embryos. Site where urine is drained in the embryo prior to development of the urethra.26 |
| Urethra | Epithelial tube connected to the bladder through which urine is excreted. Forms from the endodermal urogenital sinus. In males, consists of prostatic, membranous, and penile regions. In females, consists of only a membranous region.30,31 |
| Urethral plate | The primordia of the external urethra. Develops from an outgrowth of the cloacal epithelium. Forms from the lining of the urethral groove in the genital tubercle of both sexes.6,29,30,60 |
| Urethral sphincter | Part of the urinary continence mechanism. Derived from a condensation of mesenchyme in the urogenital sinus after the division of the cloaca. Consists of smooth muscle lissosphincter and striated muscle rhabdosphincter.4 |
| Urinary bladder | Arises from vesicourethral canal. Functions to store urine from the metanephros before it is release to the urethra. Consists of a urothelial layer, the lamina propria, a layer of smooth muscle, and enclosed by a layer of adventitia.6,7,60 |
| Urogential sinus | Endodermal origins, derived from the hindgut. Further subdivides into the bladder and the definitive urogenital sinus, by E13.0–14.0.27 |
| Urorectal septum | Divides the cloaca into the (ventral) urogenital sinus and the (dorsal) anorectal canal.31 |
| Urothelium | Inner most layer of the bladder. Consists of multilayered epithelium that is impermeable to urine.60 |
| Vesicourethral canal | E13.5 arises from the upper portion of the urogenital sinus, above the original point of contact of the mesonephric ducts. Develops into the bladder and a portion of the urethra.7,27 |
| Vestibule of the vagina (female) | Shallow basin into which the vagina and urethra open. Develops from the definitive urogenital sinus in females.26,31 |
Table 3.
Common Abnormal Renal Development Phenotypes in Mice
| Renal phenotype | Number of genotypes with a certain phenotype |
|---|---|
| Abnormal Kidney Collecting Duct Morphology | 89 |
| Abnormal Kidney Collecting Duct Epithelium Morphology | 14 |
| Abnormal Kidney Collecting Duct Number | 7 |
| Abnormal Papillary Duct Morphology | 6 |
| Dilated Kidney Collecting Duct | 44 |
| Kidney Collecting Duct Atrophy | 2 |
| Abnormal Kidney Cortex Morphology | 751 |
| Abnormal Kidney Corticomedullary Boundary Morphology | 15 |
| Abnormal Kidney Development | 190 |
| Abnormal Kidney Medulla Development | 6 |
| Abnormal Kidney Mesenchyme Morphology | 48 |
| Abnormal Metanephric Mesenchyme Morphology Absent Metanephric Mesenchyme Increased Metanephric Mesenchyme Apoptosis |
37 5 19 |
| Abnormal Metanephric Ureteric Bud Development | 6 |
| Abnormal Metanephros Morphology | 31 |
| Absent Metanephros | 11 |
| Small Metanephros | 9 |
| Abnormal Nephrogenic Zone Morphology | 22 |
| Absent Nephrogenic Zone | 6 |
| Delayed Kidney Development | 22 |
| Abnormal Kidney Epithelium Morphology | 86 |
| Abnormal Kidney Interstitium Morphology | 100 |
| Abnormal Kidney Medulla Morphology | 102 |
| Abnormal Kidney Inner Medulla Morphology | 9 |
| Abnormal Kidney Medulla Development | 6 |
| Abnormal Kidney Outer Medulla Morphology | 4 |
| Abnormal Kidney Pyramid Morphology (Papilla and Inner Medulla) | 42 |
| Absent Kidney Medulla | 3 |
| Kidney Medulla Atrophy | 13 |
| Kidney Medulla Hypoplasia | 8 |
| Abnormal Kidney Pelvis Morphology | 246 |
| Abnormal Kidney Calyx Morphology | 16 |
| Abnormal Kidney Papilla Morphology | 38 |
| Absent Kidney Papilla | 2 |
| Elongated Kidney Papilla | 1 |
| Kidney Papillary Atrophy | 19 |
| Kidney Papillary Hypoplasia | 7 |
| Short Kidney Papilla | 1 |
| Abnormal Kidney Pelvis Urothelium Morphology | 2 |
| Absent Kidney Pelvis | 2 |
| Double Kidney Pelvis | 2 |
| Hydronephrosis | 207 |
| Abnormal Kidney Size | 716 |
| Abnormal Kidney Weight | 250 |
| Enlarged Kidney | 262 |
| Small Kidney | 462 |
| Abnormal Nephron Morphology | 969 |
| Abnormal Nephron Morphogenesis | 5 |
| Abnormal Nephron Number | 36 |
| Abnormal Renal Corpuscle Morphology | 681 |
| Abnormal Renal Tubule Morphology | 443 |
| Absent Nephron | 3 |
| Dilated Nephron | 2 |
| Absent Kidney | 166 |
| Bifid Kidney | 0 |
| Duplex Kidney | 38 |
| Ectopic Kidney | 15 |
| Fused Kidneys | 6 |
| Single Kidney | 90 |
| Abnormal Ureter Development | 26 |
Main categories of renal phenotypes are indicated in bold, and subcategories are italicized and further indented. The number of genotypes with a certain renal phenotype is indicated. One genotype may have several different renal abnormalities. These data were collected from the Mouse Genome Database, Mouse Genome Informatics web site, The Jackson Laboratory, Bar Harbor, Maine. (http://www.informatics.jax.org/vocab/mp_ontology/MP:0003604), Last accessed May 1, 2019.
A thorough understanding of normal anatomic features and the typical evolution of anatomic structures is needed in order to effectively find and characterize developmental abnormalities, and this atlas aims to help in that endeavor. In order to establish a complete evaluation of the conceptus, it is recommended that sections be made in all three planes: frontal, sagittal, and transverse. Since the urinary tract is a bilaterally symmetrical system, assessment of all structures is generally best done with a frontal section, in which both kidneys, portions of both ureters, and the urinary bladder can be visualized simultaneously. However, since many structures may only be seen on a few sections, it is recommended that serial or step sections be taken through the entire organ in order for a complete and thorough evaluation. Whether one would use serial or step sections, and how many sections one would need to evaluate, would depend on the age and orientation of the embryo and the structure of interest. As with any phenotyping study, it is critical that age- and sex-matched wildtype controls be included for comparison. Additionally, since strain differences may exist, strain-matched controls are advised.
The terms “embryo”, “fetus”, and “conceptus” are sometimes used interchangeably within the literature. For these reasons, definition of these terms is warranted. The term “embryo” is used for the developing human individual from the time of implantation until the time of onset of bone marrow formation in the humerus, which is about the end of the eighth week postconception. After this stage and until birth, the term “fetus” is used. “Conceptus” is a term that can be used for both the embryo and fetus but also includes the embryonic part of the placenta as well as the amnion, chorion, and yolk sac. The “embryo” classification scheme allows for a standardized staging system for human embryos, and distinguishing between these stages may occasionally be of critical importance. Since the mouse has a much shorter gestation period, the designation of “embryo” versus “fetus” is less important, whereas the developmental age post-conception is critically important. For this reason, the term “embryo” is used to define all stages of murine development between fertilization and birth with the stage of development indicated by the gestational age (E0.5 + days postconception).6 Human anatomic terms may be used to discuss certain developmental processes per convention. For anatomic terminologies used for human embryos/fetuses that do not correspond to mouse anatomy, the official international veterinary nomenclature (Nomina Anatomica Veterinaria, sixth edition) may be used and is available online at http://www.wava-amav.org/wava-documents.html (last accessed 5/24/19).
Phenotypic characterization of the mouse embryo is a growing field and requires extensive knowledge of normal developmental stages throughout gestation. Currently, reference material on embryogenesis of the mouse is limited to descriptive anatomic histology atlases7,8 and online references.9,10 This atlas provides a detailed histopathologic assessment of stage-specific events in development throughout gestation in the mouse. Sections include early, mid and late gestational development of the upper and lower urinary tracts, as well as postnatal urinary tract development and an introduction to certain developmental abnormalities. Tables listing the timing of major milestones in upper (Table 4) and lower (Table 5) urinary tract development are provided, and include correlations with figures when appropriate. Detailed, high resolution photomicrographs of normal hematoxylin and eosin (H&E) stained structures of the mouse urinary tract, including the kidney, ureter, urinary bladder, and urethra, are provided throughout this atlas as an aid in the identification of phenotypes relating to abnormal development. A table listing the abbreviations used in the text and figures is also provided (Supplemental Table 1).
Table 4.
General Timeline for Murine Embryonic Upper Urinary Tract Development
| Stage | Events |
|---|---|
| E8.0 | Pronephros forms from intermediate mesoderm.13 |
| E9.0 | Pronephros regresses, mesonephros develops from nephric (Wolffian) duct.13 |
| E10.5 | Ureteric Bud forms. Metanephric mesenchyme forms from intermediate mesoderm or nephrogenic cord. Ureter connects with but does not communicate with the cloaca through the common nephric duct.6,7,12,13,14 |
| E11.0 | Ureteric bud invades metanephric mesenchyme, induces condensation of metanephric mesenchymal cells around duct tip.12 |
| E11.5 | Branching morphogenesis and nephrogenesis begins.12,18 |
| E12.5 | Renal vesicles form from pre-tubular aggregates that undergo mesenchymal to epithelial transition. Common nephric duct shortens and integrates with the urogenital sinus, at the future bladder, to transpose the site of ureteric budding to the urogenital sinus from the Wolffian duct.4,12,19 |
| E13.5 | Increase in the differentiation of the cortical region of the metanephros. A moderate number of renal vesicles and tubules can be seen in the periphery, or cortical region, of the organ. Position of ureteral orifice has repositioned to bladder neck, separate from the common nephric duct, with communication.7,19 |
| E14.5 | Mesonephros begin degeneration, complete apoptosis within 24 hours. Glomeruli can be detected, except in the most peripheral zone. By E14, stromal cells can be seen in the kidney.7,12,18 |
| E15.5 | A morphologically distinct medullary region, devoid of glomeruli, is detectable. The ureteral lumen is open at E15.18,19 |
| E16.5 | Clear demarcation between cortex and medulla. Glomeruli continue to differentiate and localize in the outer cortical region, proximal and distal tubules apparent. Most of medulla is undifferentiated mesenchyme with radially arranged collecting tubules. Differentiation of the ureteral epithelium and mesenchyme occurs. At this point the kidney begins to produce urine.4 |
| E17.5 | Kidneys are in an advanced stage of differentiation and are able to function. Zones of the kidney are clearly demarcated, and mature nephrons are visible. The renal pelvis is now composed of both major and minor calyces, and the ureters are easy to trace from the kidney to the bladder.7,26 |
| E18.5 | More mature glomeruli, large number of proximal and distal tubules in the cortex, large numbers of loops of Henle. |
| Postnatal | Newborn mouse kidney contains ~1600 ureteric tips. Nephrogenesis concludes on postnatal day 4 and the kidney contains approximately 10,000 to 14,000 nephrons.13,18 |
Table 5.
General Timeline for Murine Embryonic Lower Urinary Tract Development
| Stage | Events |
|---|---|
| E9.0 | The expansion of the caudal portion of the hindgut begins as the first distinct event in the development of the cloaca.7 |
| E10.5 | Caudal portion of the hindgut completes dilation to form the cloaca. Common nephric ducts connect but do not communicate with the cloaca. The urorectal septum is visible.7,8,28 |
| E11.5 | Nephric ducts remain blind in their connection with the cloaca. Ventral urogenital sinus portion of the cloaca is identifiable. The genital tubercle is visible adjacent to the cloaca.7,8,29 |
| E12.5 | The cloaca is in the process of being divided by the downward growth of the urorectal septum into the urogenital sinus and the rectum, but septum has not yet reached the cloacal membrane. Common nephric duct shortens and integrates with the urogenital sinus, at the future bladder, to transpose the site of ureteric budding to the urogenital sinus from the Wolffian duct. The cranial apex of the urogenital sinus portion of the cloaca extends toward the umbilicus to form the urachus.4,6,7,8 |
| E13.5 | The cloaca is fully divided by the urorectal septum into the urogenital sinus and the anorectal canal. The cloacal membrane ruptures, forming the anal opening and the proximal urethral meatus. The primitive bladder or vesicourethral canal and the definitive urogenital sinus can also be identified. The urogenital sinus epithelium begins to differentiate to urothelium, while surrounding mesenchyme begins to differentiate into smooth muscle. Ureter now opens to the urogenital sinus separately from Wolffian duct.4,7,27,28,30 |
| E14.5 | Distinct bladder portion of urogenital sinus is identifiable. Male and female primitive urethra are similar in structure.8,32 |
| E15.5 | Bladder becomes fully developed organ with multilayered urothelium. Urethra is identifiable as a distinct structure. In males, the proximal urethral meatus is partially closed and the penile urethra is separated from the ectoderm by an invasion of mesenchyme into the genital tubercle.4,7,26,30 |
| E16.5 | In males, the penile urethra is internalized by the fusion of the urethral folds and the proximal urethral meatus is nearly closed. In females, the proximal urethral meatus remains open at the base of the genital tubercle.30 |
| E17.5 | In males, a mesenchymal growth leads to a bend in the urethra at the glans-body junction, the urethral plate is no longer present, and the definitive urethral meatus has formed at the distal end of the penis. In females, the urethra remains linear, ventral to the clitoris, the urethral plate continues to canalize but remains ventral to the clitoris. The rhabdosphincter can be identified.30 |
| E18.5 | The primitive vagina is fused to the membranous urethra in females though the sinovaginal bulb.30 |
| Postnatal | The urachus regresses after birth and is replaces by the median umbilical ligament. In females, the sinovaginal bulb reaches the base of the clitoris at postnatal day 8, and the vagina separates from the urethra. Remodeling of the bladder occurs resulting in a narrowing of the lamina propria and reorganization of the smooth muscle.7,30,31 |
Materials and Methods
Animals
Embryos from CD-1 IGS mice/Crl:CD1(ICR) timed pregnant dams (Charles River Laboratories, Raleigh, NC) were used for imaging and histopathology. All animal procedures used in this study were approved by the National Institute of Environmental Health Sciences Animal Care and Use Committee.
Staging
The morning the vaginal plug was detected was considered as embryonic day E0.5 in this study. Due to variability of ovulation and conception in the dams, the developmental status of each embryo was validated through external and internal identification of developmental landmarks established in Kaufman (1992). Theiler stage (TS), another morphological criterion used for mouse embryo staging, was also used to evaluate embryonic age.
Collection and Fixation of Embryos
E10.5–E18.5 embryos were collected in the morning. After pregnant dams were euthanized by CO2, the uterus was removed and placed in refrigerated 0.1M phosphate buffered saline (PBS). The embryos were subsequently obtained using a dissection microscope while submerged in PBS, and were then immediately transferred to Bouin’s fixative (Poly Scientific, Bay Shore, NY). Fixation was performed based upon embryonic age; E10.5–11.5 embryos were fixed for two hours, E12.5–16.5 were fixed for four hours, and E17.5–18.5 were fixed for seventy-two hours. A midline incision was made through the thorax and abdomen of E18.5 embryos to ensure adequate perfusion of tissues.
Embedding and Sectioning of Embryos
Following fixation, embryos were rinsed in 70% ethanol saturated with lithium carbonate (Sigma-Aldrich, St. Louis, MO) for three, 30-minute washes and were then processed for paraffin embedding. Embryos younger than E13.5 were embedded in 1% agar before submission to minimize handling during paraffin embedding. For each time point, embryos were embedded on their backs, sides, or heads for sectioning in the respective coronal (frontal), sagittal, or transverse (horizontal) plane. Serial 6-µm sections through the entire embryo were placed on charged slides (A. Daigger & Co., Vernon Hills, IL) and routinely stained with H&E for histopathology review.
Immunohistochemistry
Indirect immunohistochemical staining was performed on formalin-fixed, paraffin-embedded embryos. The tissues were deparaffinized in xylene and rehydrated through a graded series of ethanol. Endogenous peroxidase was blocked by immersing slides in 3% H2O2 for 15 minutes. A summary of the immunohistochemistry reagents is provided in Supplemental Table 2. Detailed protocols are provided on the NIEHS Immunohistochemistry Website (https://www.niehs.nih.gov/research/resources/protocols/protocols-immuno/index.cfm).
Scanning
Digital images were captured from H&E and immunohistochemical stained slides scanned on the Aperio ScanScope AT2 instrument (Leica Biosystems Inc. 1700 Leider Lane, Buffalo Grove, IL 60089) using ImageScope software, version 12.3 (Aperio). Formatting of images for publication was completed using Adobe Photoshop CC version 19.1.0 (Adobe Systems Inc., San Jose, CA).
Upper and Lower Urinary Tract Development during Early, Mid and Late Gestation
Upper Urinary Tract Development During Early Gestation (E8.0–10.5)
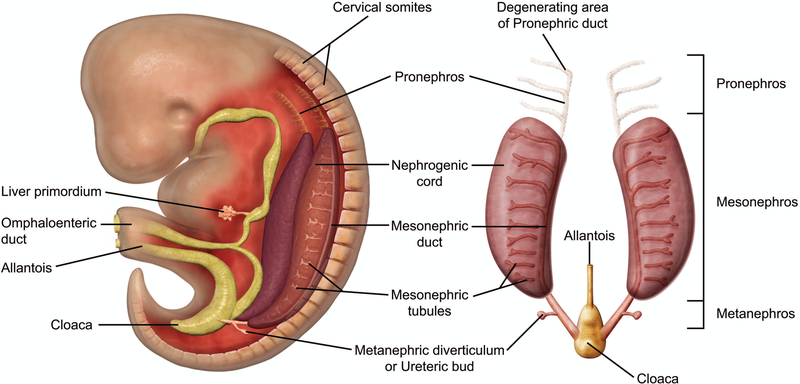
The kidney develops as three portions in a cranio-caudal distribution from the intermediate mesoderm following gastrulation in mammals: the pronephros, mesonephros, and metanephros (Figure 1).11 The Wolffian duct (WD), also known as the nephric or mesonephric duct, grows craniocaudally along the long axis of the embryo, inducing the pronephros and mesonephros formed as anterior segments and the metanephros at the posterior end of the intermediate mesoderm at the level of the mid-hind limb.2 At E8.0, the pronephros is formed from the cranial portion of the intermediate mesoderm, or the nephrogenic cord. This is a nonfunctional, transient organ that degenerates at approximately E9.0; however, the pronephric duct, which forms through a mesenchymal-epithelial transition from the intermediate mesoderm, remains and continues to grow caudally toward the cloaca to form the WD.12,13
Figure 1.
Schematic representation of early kidney development in the mouse embryo. The kidney develops as three portions in a cranio-caudal distribution from the intermediate mesoderm following gastrulation in mammals: the pronephros, mesonephros, and metanephros. The pronephros and mesonephros are transient structures. The pronephros sprouts from the anterior Wolffian duct at around E8.0 and degenerates by E9.0, when the mesonephros begins to develop. The mesonephros consists of the Wolffian duct and a linear array of mesonephric mesenchyme, with only the cranial portion developing into mesonephric tubules. The mesonephros begin to degenerate at around E14.5 and quickly disappears via apoptosis in a caudal to cranial direction. The metanephric mesenchyme arises around E10.5 and the metanephros persists as the definitive adult kidney.
By E9.0, the pronephros has regressed, and the mesonephros has begun to develop within the lateral part of the urogenital ridge.12,13 The mesonephros is composed of approximately 18–26 mesonephric tubules containing renal vesicles. These vesicles arise from the nephrogenic cord, of which only the most cranial 4–6 tubules connect to the WD. Nephrons in the cranial portion of the mesonephros contain rudimentary glomeruli, indicating that the mesonephros has some limited functionality. The mesonephros persists until E14.5, at which time it undergoes apoptosis.12,13
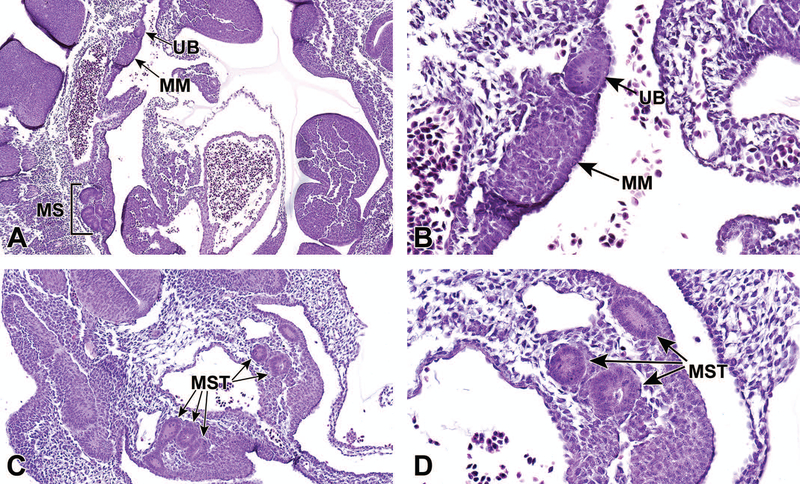
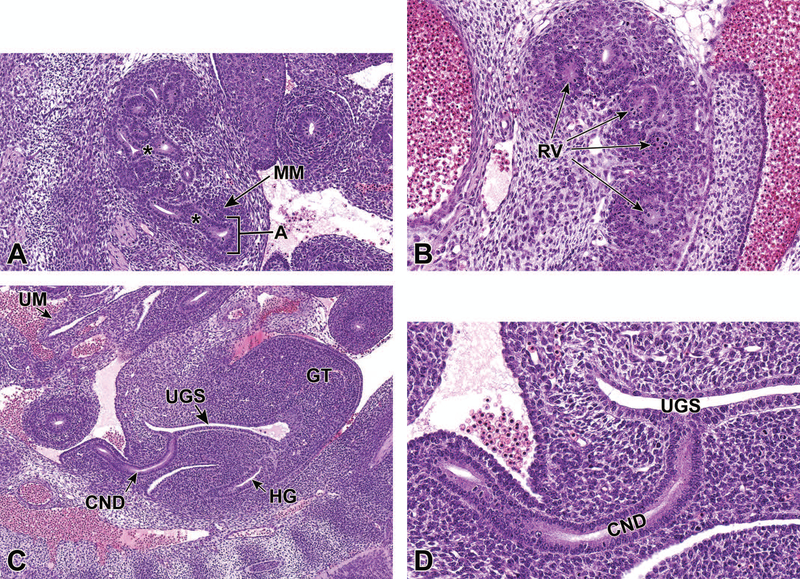
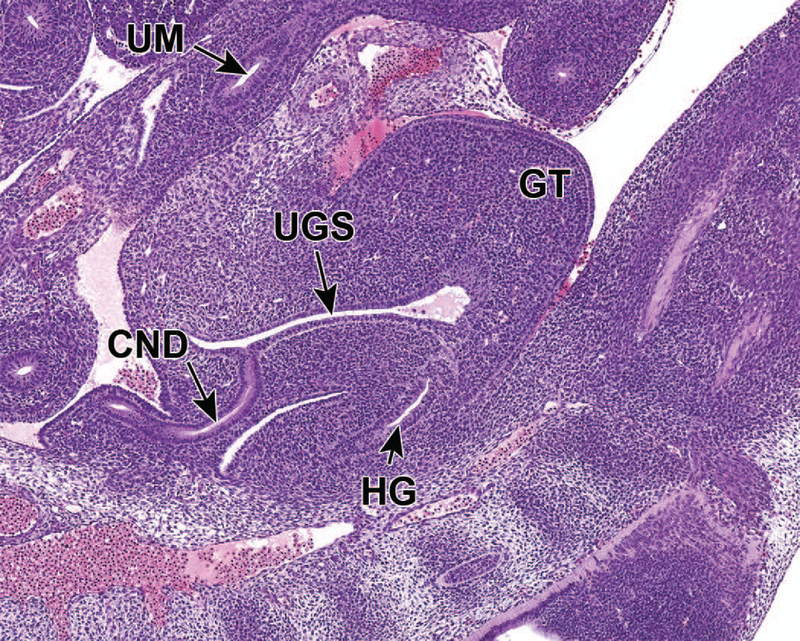
At approximately E10.5, development of the metanephros is initiated by outpouching of the WD just proximal to the contact with the urogenital sinus (UGS). This forms an epithelial thickening that swells and elongates to become the ureteric bud (UB), which grows towards the metanephric mesenchyme (MM) (Figures 2A and 2B).14,15 The MM originates from the intermediate mesoderm at approximately E10.5,12 and contains progenitors of the primitive kidney stroma and nephrons. Reciprocal signals between the UB and the MM are responsible for the differentiation of these two primitive structures into specialized structures. The UB gives rise to the collecting duct system of the mature kidneys, which includes the ureters, renal pelvis, calyces, and collecting tubules. The MM undergoes a mesenchymal-to-epithelial transition to form the glomeruli, tubular segments of the nephron, and interstitium.13 By this time, the ureters have developed from the caudal extension of the UB and make contact, but do not communicate, with the UGS through a common nephric duct (CND) shared with the WD.7,16 As it extends down the trunk, the nephric duct induces the formation of mesonephric tubules in the adjacent nephrogenic cord (Figures 2C and 2D). Only the rostral (cranial) mesonephric tubules become joined to the nephric duct.
Figure 2.
Representative sagittal and transverse sections of an E10.5 embryo during metanephric development. Sagittal sections highlight the ureteric bud (UB) and metanephric mesenchyme (MM) at low (2A) and high (2B) magnifications. Transverse sections depict mesonephric tubules (MST) at low (C) and high (D) magnifications. MS = mesonephros.
Upper Urinary Tract Development During Mid-Gestation (E11.0–15.0) Including Branching Morphogenesis and Nephrogenesis
The primitive functional kidney begins to form through the processes known as branching morphogenesis and nephrogenesis. Branching morphogenesis is characterized by the formation of complex arboriform structures through the reiterative divergence of epithelial tubules during embryogenesis, which in the kidney leads to the formation of the urinary collecting system.17 The invasion of the UB into the MM as a result of various signaling pathways, as well as subsequent branching morphogenesis, are fundamental processes in the establishment of the architecture of the collecting duct system, the number of nephrons comprising the filtration system, and subsequently the functional capacity of the organ.
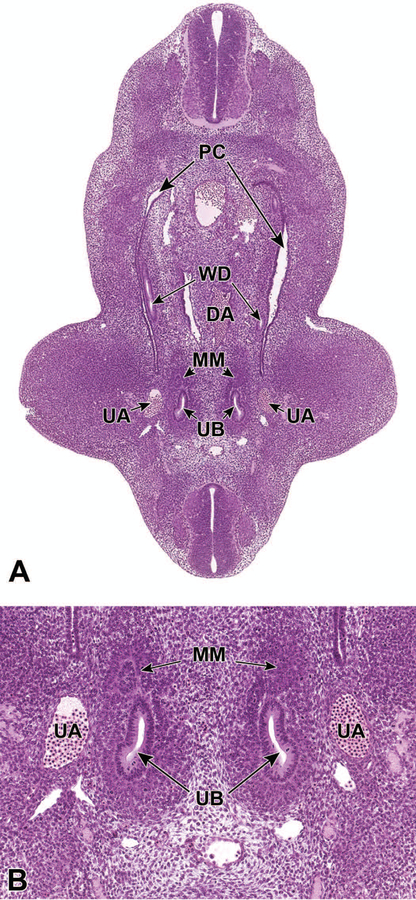
Branching morphogenesis is induced at E11.512,18 as the UB, an outpouching of the posterior WD invades the MM. As the UB elongates and invades the MM, the distal portion of the UB becomes dilated and rounded to form the ampulla, which is surrounded by the MM. Branching of the UB is then induced by signals originating from the MM as the ampulla divides to form new ampullae in a bifurcate, trifurcate, or lateral branching fashion (Figures 3A and 3B).14,15 The invasion of the UB into the MM also leads to activation of the mesenchyme and dedication to the eventual epithelial differentiation, via mesenchymal-to-epithelial transition, and formation of mesonephric tubules that will form the urinary collecting system.1
Figure 3.
Representative transverse sections of an E11.5 embryo. Sections show bifurcation of the ureteric bud (UB) at low (3A) and high (3B) magnifications. DA = dorsal aorta, WD = Wolffian (mesonephric) duct, PC = peritoneal cavity, MM = metanephric mesenchyme, UA = umbilical aorta.
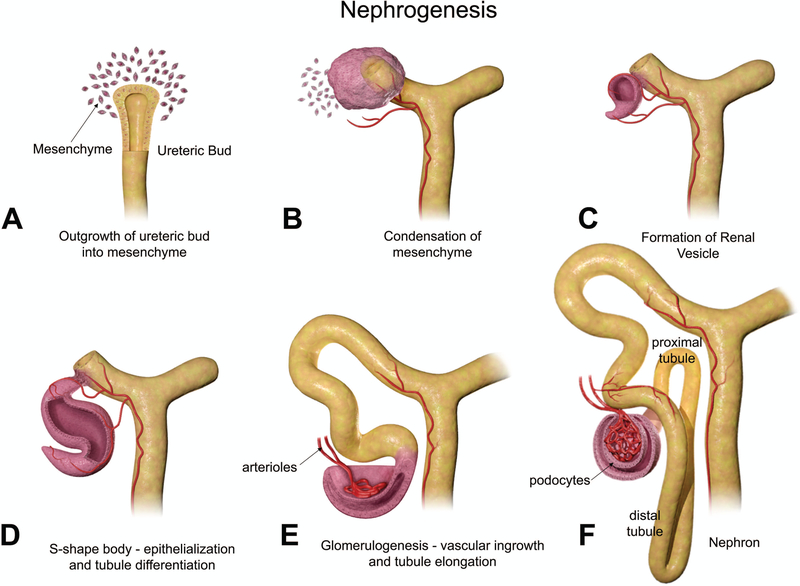
Though mesonephric nephrogenesis occurs by a similar process earlier in embryonic development, metanephric nephrogenesis begins concurrently with branching morphogenesis at E11.5.12,18 Figures 4A–F provides an illustration of the changes in nephron development. As the UB branches, the MM concentrates around the ampulla forming a cap of approximately 4–6 cells, which rapidly proliferates to approximately 30 cells (Figure 4A).12 This cap then undergoes a mesenchymal-to-epithelial transition, resulting in renal vesicles from the MM that express epithelial cell polarity (Figure 4B). The vesicles are surrounded by a basement membrane. The vesicles then elongate and a single cleft is formed to create a comma-shaped body (Figure 4C). During this stage, the distal end of the comma-shaped body fuses with the epithelium of the UB branch, which will form the collecting tubule. Following this, a second cleft forms creating the S-shaped body (Figure 4D), which is patterned along the proximodistal axis. The distal portion, which has fused with the collecting tubule, twists to form the distal convoluted tubule, and the middle portion of the S-shaped body becomes the loop of Henle and the proximal convoluted tubule. Vascular ingrowth and tubule elongation denote the beginning of glomerulogenesis (Figure 4E). The location of the turn in the loop of Henle, either in the cortex or outer medulla or in the inner medulla, determines if nephrons are superficial or juxtamedullary, respectively. The proximal portion of the S-shaped body develops into the renal corpuscle, which contains the glomerulus (Figure 4F).11–13
Figure 4.
Diagrammatic representation of nephrogenesis illustrating the configurational changes as nephrons develop. Reprinted with permission from the Journal of Toxicologic Pathology64.
The development of primitive glomeruli can first be seen in the S-shaped body stage. Endothelial cells infiltrate the proximal portion of the S-shaped body to form a primitive vascular tuft. The endothelial cells are thought to be attracted by the expression of certain proteins from podocyte precursor cells. Upon contact with these endothelial cells, epithelium from the S-shaped body begins to differentiate into podocyte cells, a specific type of epithelium located on the glomerular basement membrane, whose foot processes are important in filtration. It is believed that the development of the mesangial cells that form the structure of the capillary tuft is also influenced by the endothelial cells. The glomerular basement membrane then forms between the differentiating podocytes and endothelial cells as a result of their interactions with one another. The podocytes are located on the adluminal (outside) surface of the glomerular basement membrane. Following this, the podocytes extend their foot processes, forming a slit diaphragm that functions as the pores for glomerular filtration.11,12
By ED 12.0–13.0, the mesonephros has regressed and the WD is left with a few degenerating tubules, the most cranial of which becomes the drainage system of the testis in males. The UB has expanded within the MM to form the primitive renal pelvis, and bifurcation continues to form the intrarenal collecting system (Figure 5A).
Figure 5.
Representative sagittal and transverse sections of an E12.5 embryo. Sections show early branching morphogenesis (5A), early renal vesicles (5B) and the connecting of the common nephric duct to the urogenital sinus (5C and D). Figure 5A sagittal section shows that the ureteric bud has expanded within the metanephric mesenchyme to form the primitive renal pelvis, and bifurcation (asterisks) continues to form the future intrarenal collecting system. A new ampulla (5A) can be seen surrounded by MM. In Figure 5B, renal vesicles (RV), precursors to glomeruli, can be seen in this transverse section. Figure 5C sagittal section depicts the common nephric duct (CND) connecting with the urogenital sinus (UGS). Ureter maturation depends on apoptotic elimination of the CND via apoptosis. Figure 5D, a higher magnification of C, shows that the CND connects with, but does not yet communicate with, the UGS. GT = genital tubercle, HG = hindgut, UM = umbilicus.
Through the process of mesenchymal-to-epithelial transition, MM progenitors differentiate into metanephric tubules and renal vesicles. Metanephric tubules are precursors to renal tubules, while renal vesicles are identified as precursors to glomeruli; both of these structures are precursors to the primitive nephron (Figure 5B).2,17 The ultimate number of nephrons is dictated by the degree of branching morphogenesis during development of the metanephros.2 At this stage, the CND connects with but does not communicate with the UGS (Figures 5C and 5D). The cranial portion of the CND has begun to regress.19 Throughout this period, the metanephros continues to differentiate, enlarge, and ascend from its original location within the pelvic region.
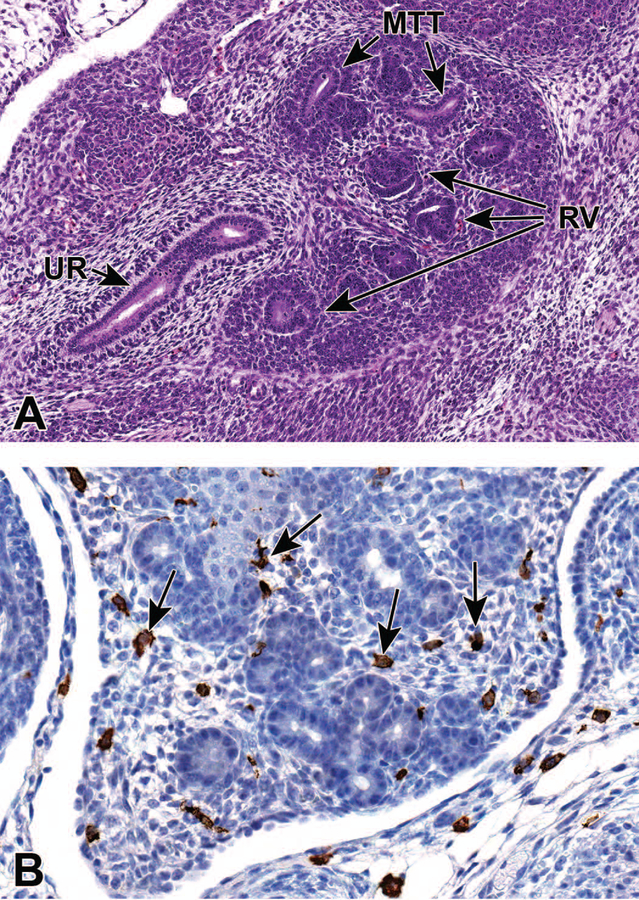
At E13.5 the ureter communicates with the UGS. By E14.0, the CND has completely regressed and the insertion point of the ureter is located in the bladder neck. The CND has now separated from the insertion point of the WD, which remains on the urethra in males.19 Branching morphogenesis and nephrogenesis both continue during this stage. The cortical region becomes more distinct in the periphery of the metanephros, with a moderate number of renal vesicles and metanephric tubules seen histologically, although the distinction can sometimes be challenging with H&E. (Figure 6A).7
Figure 6.
Representative sagittal and frontal sections from an E13.5 embryo. Figure 6A is a sagittal section that shows developing renal vesicles (RV) and metanephric tubules (MTT) within a more distinct cortical region. In Figure 6B, (frontal section) macrophages (arrows), highlighted using an F480 immunohistochemical stain, can be seen interspersed within the interstitial tissue of the metanephros. UR = ureter.
Macrophages in the developing kidney have been reported to facilitate many processes during renal organogenesis, such as branching morphogenesis, angiogenesis and the clearance of cellular debris. They can be seen scattered within the interstitial tissue of the E13.5 metanephros using F480 immunohistochemistry (Figure 6B). In the mouse kidney, the percentage of yolk sac-derived macrophages was found to decrease exponentially from E13.5 to postnatal week 6. Then, between E13.5 and E16.5, the proportion of monocyte-derived macrophages within the kidney progressively increases, and will exceed that of yolk sac-derived macrophages. For a comprehensive overview of the available data regarding the origins, specification, and functions of kidney macrophages in renal development, see the review article by Munro and Hughes.20
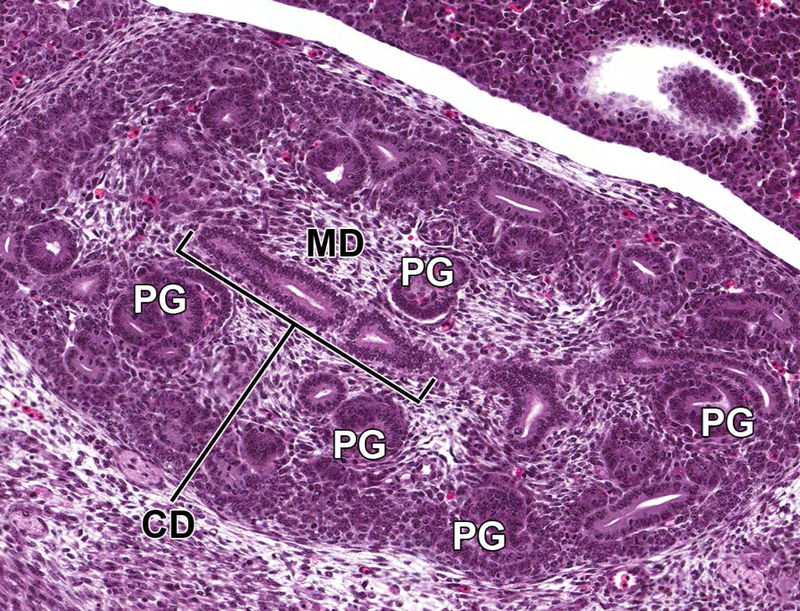
Through E14.0–15.0, the nephrogenic vesicles give rise to primitive glomeruli (Figure 7), which are present within an outer primitive renal cortical region within the metanephros, admixed with nephrogenic aggregates and underlying a peripheral rim of undifferentiated stem cells.6 Deep to this primitive cortex is the inner medullary region that is composed of loose stromal mesenchyme and collecting ducts, as well as a substantial number of primitive glomeruli and collecting tubules. These collecting ducts fuse to form the primitive pelvis, which then drains into the ureter.
Figure 7.
Representative sagittal section of the developing kidney at E14.5. Section shows primitive glomeruli (PG) in the outer cortex, collecting ducts (CD), and early stromal cells in the medulla (MD).
Upper Urinary Tract Development During Late Gestation (E15.5–18.5)
E15.5–16.0 represents an intermediate time frame in development of the distinct zones of the kidney. At E14.5–15.0, the future medullary region contains many glomeruli that, during E15.5–16.0, become concentrated in the periphery of the kidney, the future cortex, and this cortical region will become distinct during E16.5–17.0.7 The interior region that becomes devoid of glomeruli during E16.5–17.0 can now be identified as the distinct medulla (Figure 8A).18 Additionally, the lumens of the ureters are now open, consistent with communication of ureters with the primitive bladder.19
Figure 8.
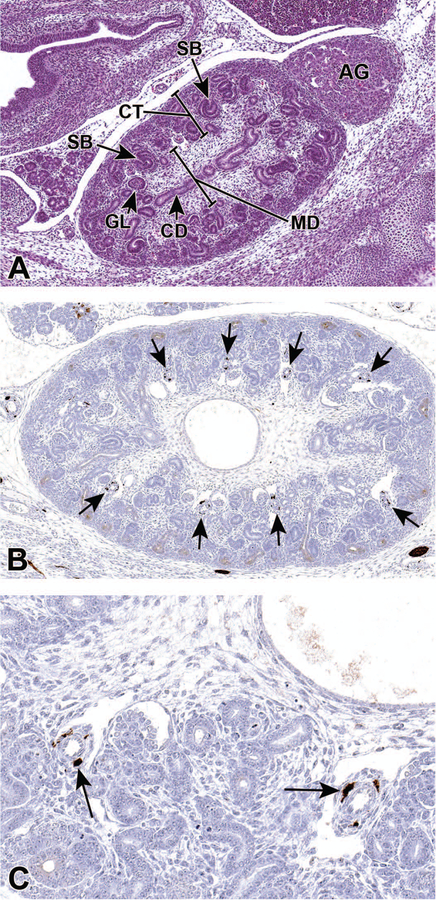
Representative sagittal sections of the developing kidney at E15.5. Figure 8A shows the medulla (MD), which has become more distinct at this stage as it becomes devoid of glomeruli. As the glomeruli continue to develop, a variety of shapes can be appreciated including S shaped bodies (SB; arrows) and more mature glomeruli (GL). In Figure 8B, Class III β-tubulin antibody shows strong immunoreactivity in perivascular cells (arrows) in a kidney from an E15.5 embryo. Figure 8C shows a higher magnification illustrating cytoplasmic localization in specific pericytes (arrows). CD = collecting duct, AG = adrenal gland, CT = cortex.
Class III β-tubulin is used as a marker of specific perivascular cells involved in angiogenesis. 21 Pericytes are functionally associated with regulating blood vessel permeability, vessel diameter, and endothelial cell proliferation.22,23 Figures 8B and 8C illustrate tubulin positive vascular pericytes in an E15.5 developing kidney.
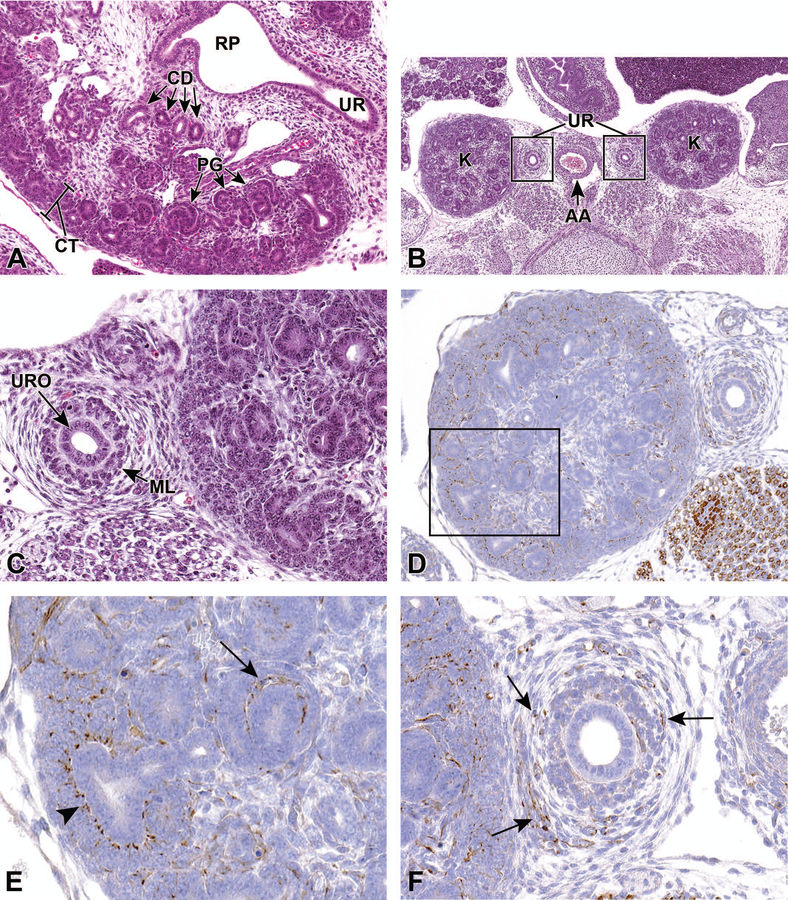
The kidneys become increasingly more rounded by E16.5, with a clear demarcation between the peripheral cortical region and inner medulla by E16.0–17.0. By this stage, 85% of the branching events have already occurred, indicating a slowing in branching morphogenesis.18 Glomeruli continue to differentiate and localize to the outer cortical region of the kidney, and the proximal and distal tubules are apparent within the cortex at this time (Figure 9A). A large portion of the medullary region still consists of undifferentiated mesenchyme with radially-arranged collecting tubules, and undifferentiated metanephric tissue remains subjacent to the renal capsule. At around E16.5, the kidney becomes functional and begins to produce urine.4
Figure 9.
Representative transverse and frontal sections of the developing kidney at E16.5. Primitive glomeruli (PG) in the cortex (CT), collecting ducts (CD) in the medulla, renal pelvis (RP) and the connecting ureter (UR) are shown in a transverse section (9A). A lower magnification frontal section (9B) shows the left and right kidneys (K) and the connecting ureters (UR) on either side of the abdominal aorta (AA). A higher magnification frontal section (9C) shows the multilayered transitional urothelium (URO) within the ureter. The mesenchymal layer (ML) will further differentiate into stromal cells, smooth muscle cells, and adventitial fibroblasts. In frontal sections Figures 9D–F, anti-nestin antibody reveals strong immunoreactivity in the vascular cleft of the developing S-shaped glomeruli, and in the condensing mesenchyme surrounding the ureter at E16.5. Figure 9E is a higher magnification of the boxed region in 9D showing cytoplasmic staining in the vascular cleft of an S-shaped glomerulus (arrow) and in the cortical mesenchyme surrounding ureteric branches (arrowhead). Figure 9F shows cytoplasmic staining in the mesenchymal tissue surrounding a ureter (arrows).
At E16.5, the ureteral epithelial cells differentiate into multilayer urothelium by molecular interactions with ureteral mesenchymal cells (Figures 9B and 9C). Mesenchymal cells will then differentiate into stromal cells, smooth muscle cells, and adventitial fibroblasts. The ureteral smooth muscle is further differentiated into an internal circular and an external longitudinal layer, and the differentiation of the ureteral epithelium and mesenchyme corresponds to the start of urine production in the kidney.4
Nestin, an intermediate filament protein, is widely used as a stem cell marker. Given its interaction with other cytoskeleton proteins, it is suggested to have a role in regulating cellular cytoskeletal structure. Studies have shown that nestin is expressed in progenitors of glomerular endothelial cells and renal progenitors that are derived from metanephric mesenchyme.24 Figures 9D–F show strong anti-nestin immunoreactivity in the vascular cleft of developing glomeruli, cortical mesenchyme surrounding ureteric branches, and in the condensing mesenchyme around the ureter at E16.5. In the adult kidney, expression is only found in differentiated podocytes, suggesting that nestin could be involved in maintaining the structural integrity of podocytes.25
The most defining distinction at E17.5 is the functional ability of the kidney. The kidney now has a significant number of mature nephrons that can function to produce urine, although most of the embryonic excretion continues through the placenta.7,26 The zones of the kidney, including the cortex, inner and outer medulla, and the papilla, are clearly demarcated. Additionally, the renal pelvis, which was previously a quite simple structure, is now composed of major and minor calyces, and the path of the ureters from the kidney to the bladder is easily identified.7
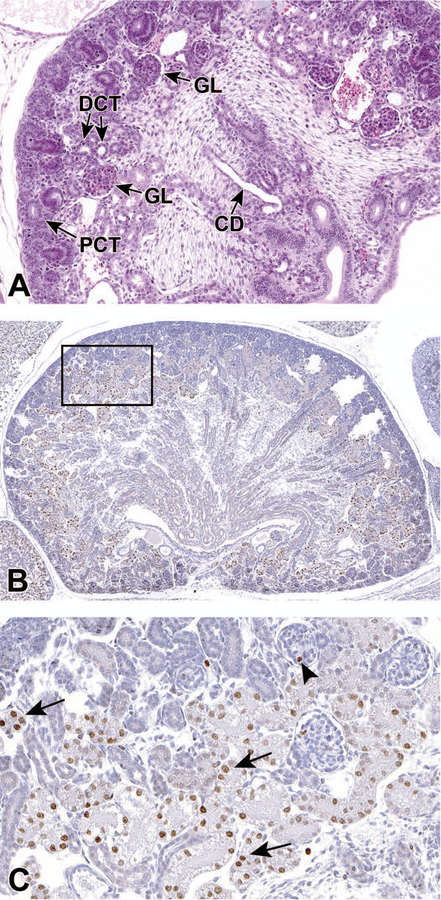
By E18.0, the kidneys are in an advanced stage of differentiation, with increased numbers of more mature glomeruli and larger numbers of proximal and distal convoluted tubules within the renal cortex (Figure 10A). There are also larger numbers of ascending and descending components of the loop of Henle at this point.7
Figure 10.
Representative sagittal and frontal sections of the developing kidney at E18.5. By E18.0, the kidneys are in an advanced stage of differentiation, with increased numbers of more mature glomeruli and larger numbers of proximal and distal convoluted tubules within the renal cortex. Figure 10A is a sagittal section of the developing kidney at E18.5 and shows the collecting duct (CD) and, within the cortex, more mature glomeruli (GL), proximal convoluted tubules (PCT) with brush borders and distal convoluted tubules (DCT). Figure 10B is a frontal section stained with Ki67. Figure10C is a higher magnification of boxed area in 10B and shows a subset of renal tubular cells and occasional glomeruli that are positive for Ki67 at E18.5. Arrows point to positive nuclei in tubular epithelial cells. Arrowhead shows one positive nucleus within a glomerulus.
Ki67 is a nuclear protein that is associated with ribosomal RNA transcription and cellular proliferation. Ki-67 protein is present during all active phases of the cell cycle (G1, S, G2, and mitosis), but is absent in resting (quiescent) cells (G0). Therefore, it is not surprising to find Ki67 positive tubular cells in the developing kidney (Figures 10B and 10C).
Lower Urinary Tract Development During Early Gestation (E8.0–E10.5)
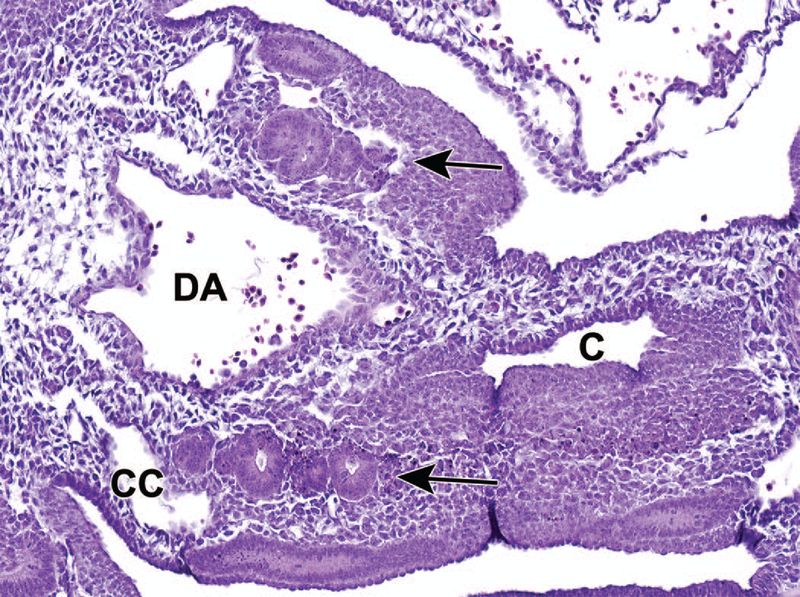
Unlike the upper urinary tract, the lower urinary tract develops from the endoderm germ layer after gastrulation.27 The hindgut differentiates first from the endodermic germ layer at E7.75 and the hindgut diverticulum is fully developed by E8.5. This region is the most caudal portion of the embryonic gut that extends nearly to the caudal extremity of the tail, and develops into several urinary and digestive system structures including the rectum, UGS, and allantois. The most caudal region of the hindgut begins to dilate between E8.5 and E9.0, forming a region that is significantly expanded when compared to the midgut and cranial portions of the hindgut. By E10.5, this expansion can be definitively identified as the cloaca.4,7,26,27 At this time, the urorectal septum (URS) is also visible, but does not yet divide the cloaca into the UGS and the anorectal canal (Figure 11).28
Figure 11.
Representative transverse section of the developing lower urinary tract at E10.5. This section shows the cloaca (C), dorsal aorta (DA), coelomic cavity (CC) and mesonephric tubules (arrows). The most caudal region of the hindgut begins to dilate between E8.5 and E9.0, forming a region that is significantly expanded when compared to the midgut and cranial portions of the hindgut. By E10.5, this expansion can be definitively identified as the cloaca. The urorectal septum has not yet divided the cloaca into the urogenital sinus and anorectal canal.
Lower Urinary Tract Development During Mid Gestation (E11.0–15.0)
Early genitourinary structures continue to take shape during E11.5. The ventral portion of the cloaca, which is destined to become the UGS, becomes more distinct from the anorectal canal by the continued down-growth of the urorectal septum (Figure 12).7,16 Furthermore, the genital tubercle (GT), which is the earliest structure of the future penis and clitoris, is visible as a protrusion adjacent to the cloaca.29
Figure 12.
Representative sagittal section of the developing lower urinary tract at E11.5. The ventral portion of the cloaca, which is destined to become the urogenital sinus, becomes more distinct from the hindgut by the continued down-growth of the urorectal septum and the genital tubercle is visible as a protrusion adjacent to the cloaca. This sagittal section shows the undivided cloaca (C), urorectal septum (URS), hindgut (HG; anorectal canal) and the now-visible genital tubercle (GT).
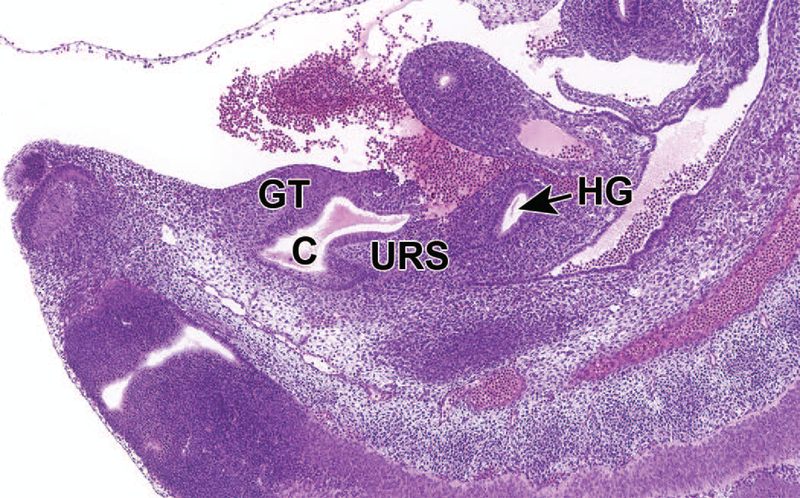
At E12.5, the cloaca is still in the process of being divided by the down-growth of the URS, which has not yet reached the cloacal membrane, the structure that covers the embryonic cloaca during development. Additionally, a portion of the ventral UGS region of the cloaca extends toward the umbilicus to form a tubular structure called the urachus (Figure 13). This structure connects the lumen of the UGS to the allantois.6,26
Figure 13.
Representative sagittal section of the developing lower urinary tract at E12.5. The cloaca is not fully divided until E13.5. A portion of the ventral urogenital sinus (UGS) region of the cloaca extends toward the umbilicus (UM) to form a tubular structure called the urachus (not shown in section). This section shows the transposition of the common nephric duct (CND) to the future bladder wall and the urachus. GT = genital tubercle, HG = hindgut (anorectal canal).
One key process that occurs during this stage is the remodeling of the connections of the ureters to the cloaca at the point of the future UGS. The ureters, which are derived from the extension of the UBs, initially connect indirectly to the cloaca through the CND which is shared with the WD. At this stage, the CND shortens and dilates to integrate the UGS portion of the cloaca at the site of the future bladder neck. This remodeling transfers the site of ureteric budding from the WD to the epithelium of the cloaca. In males, the WD remains to form the vas deferens, but in females this structure regresses. Through continued apoptosis of the CND (E11–13) and expansion of the future bladder, the ureteral orifices reach their final position on the bladder wall, forming the bladder trigone with the urethral orifice.4,19 This process appears to also be important for opening the ureteral lumen at around E15.19
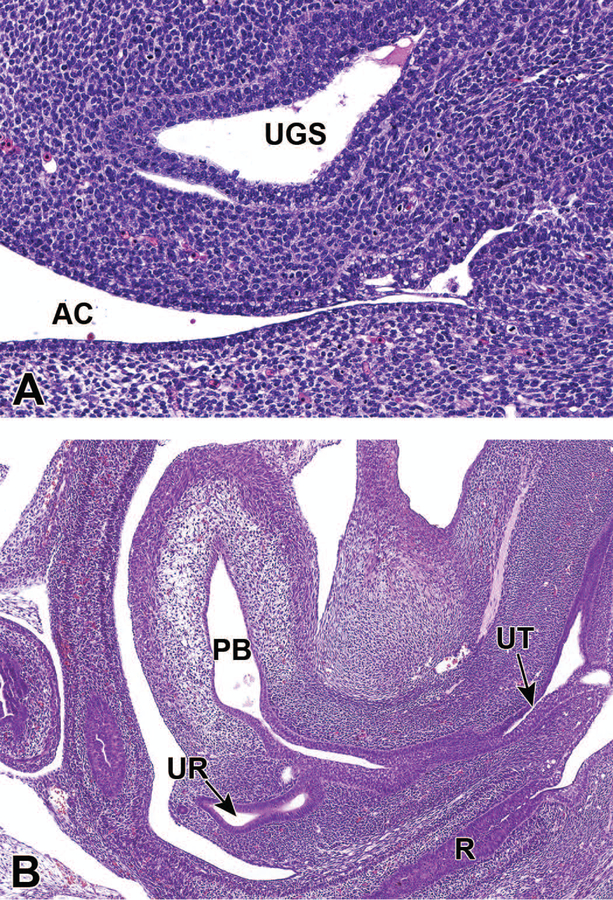
The caudal extension of the URS has finished by E13.5, completing the division of the cloaca into the UGS and the anorectal canal.7 Despite its caudal extension, the URS does not meet the cloacal membrane due to the eventual rupture of the cloacal membrane. This rupture produces the anal opening at the terminus of the anorectal canal, and the proximal urethral meatus at the terminus of the UGS, thus exposing the UGS and dorsal anorectal canal to the exterior.30 At this time, the two distinct regions of the UGS, the upper vesicourethral canal and the lower definitive UGS, are also distinguishable and are defined by their position relative to the point of contact of the nephric ducts to the UGS.7,27 The UGS epithelium also begins to differentiate into urothelium, or the multilayered epithelium distinct to the urinary tract, while the surrounding mesenchyme begins to differentiate into smooth muscle to form the layers of the bladder.4,28 Finally, the ureter now opens completely to the UGS at the site of the future bladder neck, and is now separate from the CND and the WD. Along with the urethral orifice, the ureteral orifices form the bladder trigone on the dorsal bladder neck. The trigone expands in size with the growth of the bladder throughout development.30,31
During E14.5, the vesicourethral canal continues to develop into the primitive bladder and the pelvic portion of the urethra in both males and females (Figure 14). The region of the future bladder is now identifiable as a distinct structure, and the early urethra is similar in structure in both males and females.16,32
Figure 14.
Representative sagittal sections of the developing lower urinary tract at E14.5. Figure 14A shows that the cloacal membrane disintegrated by apoptosis one day earlier at E13.5, completing the division of the cloaca into the urogenital sinus (UGS) and anorectal canal (AC). Figure 14B shows that at E14.5 the vesicourethral canal continues to develop into the primitive bladder (PB) and the pelvic portion of the urethra (UT). The ureter (UR) has also opened completely to the primitive bladder. R = rectum.
Lower Urinary Tract Development During Late Gestation (E15.5–E18.5)
The bladder has reached a mature stage by E15.5, with a multilayered urothelium, smooth muscle, and lamina propria (Figure 15).4 The development of the urethra has become sex-specific at this stage, corresponding to the sex-specific development of the reproductive systems. In males, the proximal urethral meatus is partially closed and the phallic urethra is separated from the ectoderm layer by an invasion of mesenchyme in the genital tubercle; the proximal urethral meatus remains open in females. Furthermore, the anogenital distance, the distance between the urethral meatus and the anus, is longer in males than in females at this stage.30
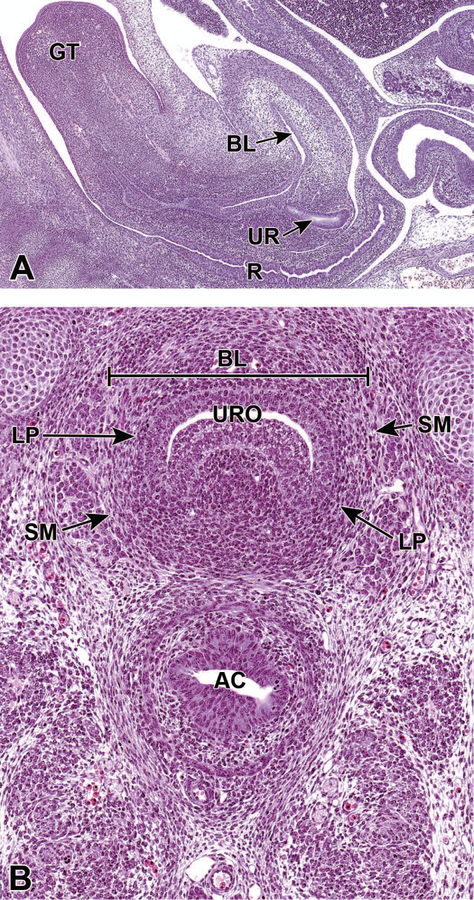
Figure 15.
Representative sagittal and frontal images of the developing lower urinary tract at E15.5. The sagittal section (15A) shows the mature bladder (BL), the ureter (UR) in the region of the neck of the bladder, the rectum (R) and genital tubercle (GT). The frontal section (15B) shows that the urinary bladder has reached a mature stage by E15.5, with a multilayered transitional urothelium (URO), lamina propria (LP), and smooth muscle (SM). AC = anorectal canal.
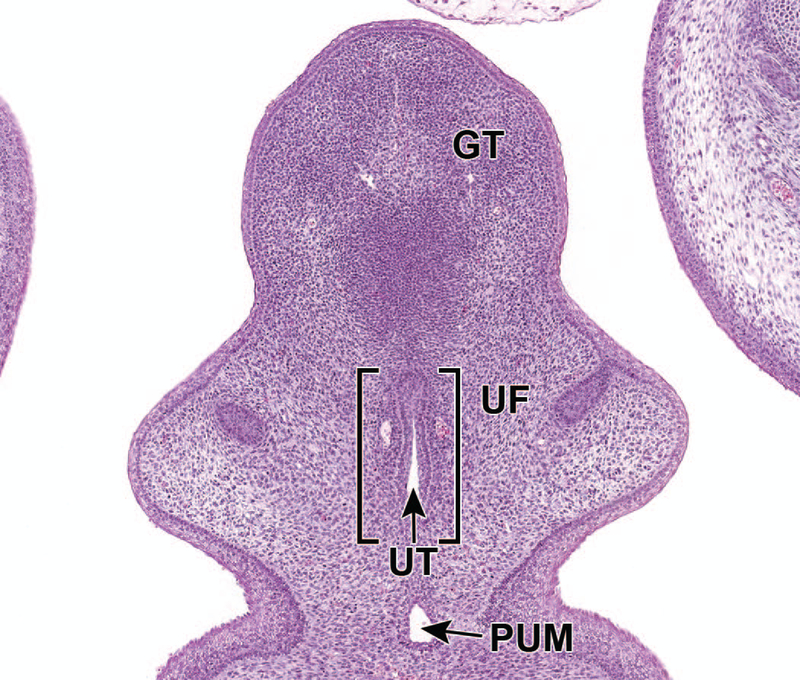
In males, the proximal urethral meatus continues to close during E16.5. Additionally, the urethral folds on either side of the genital tubercle begin to fuse to internalize the penile portion of the urethra (Figure 16). As in the previous stage, the proximal urethral meatus remains open in females.30
Figure 16.
Representative frontal section of the male developing lower urinary tract at E16.5. The proximal urethral meatus (PUM) continues to close during E16.5. Fusion of the urethral folds (UF) in the ventral midline leads to the formation of the urethral groove and subsequently the penile portion of male urethra (UT). GT = genital tubercle.
The distinctions between the male and female urethra continue to become more pronounced at E17.5. A mesenchymal growth leads to a significant bend in the male urethra at the glans-body junctions, while the female urethra remains linear and opens ventrally to the clitoris, which has formed from the genital tubercle. Because the penile urethra has now been completely internalized, the urethral plate is no longer present in males; however, it is still present in females and continues to canalize. At this time, the rhabdosphincter, the portion of the urethral sphincter complex that is composed of striated muscle, can now be seen, although it is unclear when this structure first develops or from where it originates.30
By E18.5, the upper vagina, which is derived from the paramesonephric ducts, has fused to the membranous urethra in females at the sinovaginal bulb (SVB). This connection will persist until after birth.30 Figures 17A and 17B illustrate the male and female urethras, respectively, at this stage of development.
Figure 17.
Representative sagittal sections of the male (17A) and female (17B) developing lower urinary tract at E18.5. Both figures show the urethra (UT) connecting to the bladder (BL). Other structures such as the rectum (R), ejaculatory duct (EJD), penis (P), uterus (UTS), bulbourethral gland (BG) and clitoris (CL) are labeled for orientation.
Postnatal Urinary Tract Development
Mice are born at an immature stage in kidney development. Branching morphogenesis begins to slow at E16.5, and it is suggested that this process terminates between birth and postnatal day (P) 3. This is characterized by the flattening and loss of the ampullae, which serve as the sites for dichotomous branching.33 Nephrogenesis also continues into the postnatal period, which is distinct from humans in which nephrogenesis is completed before birth.13 The number of nephrons at birth has been estimated between 6,000 and 8,000, indicated either by the number of tips and nephrons per tip, or by the number of glomeruli.18,34 However, by the termination of nephrogenesis at approximately P4, the total nephron number is estimated between 10,000 and 14,000.13 Based on analysis of nephron number per bud tip between E14.5 and P4, it was determined that 51% of nephrons form between P0 and P4.34 Postnatal nephrogenesis is also characterized by the induction of multiple nephrons by a single branch tip, as well as an increase in the number of nephrons found on the stalks of the ureteric tree. The cessation of nephrogenesis is understood to correlate with the depletion of the cap mesenchyme, which converts to new nephrons and is completely depleted by P3. This is a result of the conversion of the nephrogenic mesenchyme to nephrons without replenishment of the mesenchyme by progenitor cells. In response to the loss of the cap mesenchyme, Wnt11, which is responsive to GDNF/RET signaling from the mesenchyme, is significantly downregulated in the ureteric branch tips postnatally. However, genes expressed in the branch tips that are responsible for signaling to the mesenchyme, such as Wnt9b, are still strongly expressed. Mesenchyme-to-tip signaling is decreased, but tip-to-mesenchyme signaling is maintained and consistent with the loss of MM by a lack of replenishment. Wnt4, a marker for pretubular aggregates, is present at the UB tips in the early postnatal period; it is undetectable at P5, which is consistent with cessation of nephrogenesis at P4. Taken together, it appears that nephrogenesis ends postnatally in mice as a result of the depletion of the MM. This depletion is due to a burst of nephron formation after birth.33
In the lower urinary tract, the urachus regresses after birth and is replaced by the median umbilical ligament, which connects the apex of the fundus of the bladder to the umbilicus.6,7 This is consistent with the functional ability of the urethra postnatally to excrete urine, although it is distinct from human development in which the urachus regresses before birth.35 In females, the SVB moves caudally postnatally toward the clitoris. The SVB reaches the base of the clitoris at P8, separating the vagina from the urethra; however, the vagina remains closed until puberty, at approximately P28.30 The bladder also demonstrates significant remodeling and growth postnatally. Notably, the thick layer of embryonic lamina propria thins after birth, and the smooth muscle layer reorganizes into two distinct circular and longitudinal layers.31
Abnormal Development of the Upper and Lower Urinary Tracts
Developmental malformations of the kidney and lower urinary tract are uncommon in rodent strains routinely used in non-clinical testing. However, developmental malformations of the human urinary tract are one of the most frequently encountered congenital anomalies comprising around 3–6 per 1000 live births and may be responsible for chronic kidney disease (CKD), end-stage renal kidney disease (ESRD), and represent 20–30% of all prenatally identified birth defects in humans, the most of any organ system.36–38 Furthermore, up to 7% of adults with end-stage renal disease world-wide are attributed to urinary tract anomalies.39 Urinary tract malformations are collectively classified as congenital anomalies of the kidney and urinary tract (CAKUT). The following information on CAKUT is meant to summarize important concepts and not to present a comprehensive or detailed review on the subject.
In some rodent strains spontaneous congenital renal cysts or hydronephrosis are not uncommon, but most urinary tract anomalies, in general, are infrequently observed in non-clinical rodent studies.40–44 However, urinary tract anomalies in humans represent a much more diverse spectrum of spontaneous malformations which clinically may result in death, CKD, ESRD, hypertension, or remain asymptomatic.45–48
Particularly in humans, malformations of the upper urinary tract represent a large variation in phenotypic anomalies. Major anomalies include renal agenesis, hypoplasia, dysplasia, cystic renal disorders, renal malpositions or ectopia, fusion anomalies, duplex kidneys (double ureters), hydronephrosis, ureter ectopia, renal pelvic-ureter duplications, ureteropelvic junction obstruction, megaureter, and ureteroceles. Major malformations of the lower urinary tract also represent a broad category of phenotypic anomalies including bladder exstrophy, ureterovesical junction incompetence, vesicoureteral reflux, and bladder or urethral anomalies.46,49–52 Because the upper and lower urinary tract are closely linked by developmental origins within the metanephric kidney, lower urinary tract anomalies, especially those leading to urinary tract obstruction (UTO), are frequently observed with kidney anomalies.53 Multiple extrarenal malformations with an abnormal renal component occur with known inherited “syndromic” cases involving other organs and tissues.50,51,54
For the most part, urinary tract anomalies are regarded as a genetically heterogeneous disorder by mutations of single genes, multiple genes, or protein factors involved with early nephrogenic development involving UB branching and differentiation of the MM.38,39,47,55–57 Abnormalities of outgrowth and/or positioning of the UB account for the majority of all developmental defects of the kidney.2 Positioning of the UB is thought to be important in the eventual connection of the ureter to the UGS. High UBs tend to join the UGS in a more posterior position, closer to the urethra, resulting in obstruction of urine flow. UBs that are positioned low on the WD tend to join the UGS in a position that is lateral or anterior to the normal insertion point, leading to reflux of urine.19 Additionally, because the number of nephrons is primarily determined by the number of branches generated during metanephric kidney morphogenesis, defects during this stage may affect nephron development and thus result in a predisposition to CKD.17 However, the developmental pathogenesis of many cases of CAKUT remain unknown.38,48
Investigations using murine models have contributed greatly to the understanding of both mammalian urinary tract development and urinary tract malformations, helping to identify key genes underlying both processes. In general, murine models (transgenic mice) have been used widely to investigate urinary tract anomalies and the number of papers on this topic is extensive. Murine models of urinary tract anomalies often result in similar morphological urinary tract abnormalities as noted in humans or in abnormalities not analogous to humans. For example, one murine model of a mutation of the paired box gene 2 (Pax2), which is a critical gene found in the intermediate mesoderm, may result in kidney and eye malformations comparable to humans with the renal-coloboma syndrome.58 For those interested, information on mouse models can be found in the “GUDMAP: the genitourinary development molecular anatomy project”.59,60 This online resource makes data available from gene disruption studies for investigators who want to further study anatomic and disease development.
Lastly, the developing kidney is sensitive to many nephrotoxic teratogens. Teratogens represent any chemical, drug, toxin, infection, or unfavorable intrauterine environment that results in a developmental malformation.40,61–66 Most teratogenic defects occur during gestational or postnatal periods of age-related susceptibility and are influenced by metabolism, transporter activity, or renal blood flow.40,67
Abnormal Development of the Upper Urinary Tract
Kidney developmental abnormalities may begin as early as the initial stages of nephrogenesis, arise later in fetal development, or even following birth. The two most important phases of nephrogenesis involve the reciprocal inductive events between the UB and the MM, which occurs around E10.5–E11 in the mouse.39 During this period, variations in UB outgrowth, UB branching, or positioning defects account for most of the developmental defects of the kidney and ureter.2,36,68 Therefore, it is extremely important that only one UB arises from the nephric duct and precisely penetrates the MM, otherwise a disruption to the orderly inductive process may ensue, and result in a malformation.37
Major kidney anomalies, including agenesis, hypoplasia, and dysplasia, result from a failure of signaling molecules from the MM or the UB to induce the MM to undergo mesenchymal-to-epithelial transformation. In renal agenesis, defective signaling by the MM results in the lack of UB induction into the MM, resulting in a failure of kidney development, which ultimately leads to the absence of one or both kidneys and corresponding ureters. Obviously, missing both kidneys is incompatible with life, but one kidney can sustain life. In rats, renal agenesis is very rare, as one author (JCS) has observed only two cases of unilateral agenesis in rats from numerous necropsies (unpublished observation).
Hypoplasia, arising from defects of UB branching or poor nutrition during gestation, is characterized by the presence of a structurally smaller, but normal appearing, kidney with fewer nephrons.36 Renal dysplasia is characterized histologically by areas of undifferentiated renal tissue associated with atypical tubules surrounded by a mesenchymal-type stroma. Agenesis, hypoplasia, and dysplasia are uncommon spontaneous findings in rats and mice.69–71
Renal cysts, arising from a variety of causes, are frequently observed in rodents. Numerous renal cystic syndromes occur in humans, which suggests that many different gene mutations may be involved in these developmental disease processes.72 Two familial cystic syndromes of polycystic kidney disease, ARPKD (autosomal recessive) and ADPKD (autosomal dominant), are diseases that lead to renal failure. ADPKD, particularly, is associated with mutations of the PKD1/PKD2 genes coding for polycystin 1.52,72 ARPKD and ADPKD are also considered “ciliopathies” associated with mutations in genes responsible for proper function of the apical cilia lining renal tubule epithelium.50 Both ARPKD and ADPKD have been reported in rats, and are characterized by closely similar morphologies to the disease in humans.73,74 Many mouse models of kidney cystic disease have also been developed and serve as valuable investigative tools into renal cystic disease.72
In rats, hydronephrosis as a genetic malformation appears to be a highly heritable trait.75 Urinary obstruction, as a secondary effect from developmental urinary tract anomalies, often leads to hydronephrosis and significant renal failure in children.76 Importantly, some lesions, such as hydronephrosis, can be transient in both rodents and humans, being present in near term embryos with postnatal resolution.
Renal positioning anomalies and fusion anomalies occur frequently in humans but are rarely reported in rodents. Renal ectopia (kidneys in abnormal locations) such as pelvic kidneys, and fusion anomalies such as horseshoe kidneys or cross-fused ectopia, involve disturbances in the separation of closely developing metanephric blastema during gestation.48,68
Renal tubular dysgenesis (RTD), or impaired proximal tubular development, is a rare anomaly linked to a mutation of genes encoding for the renin-angiotensin system (RAS) which regulates blood pressure and extracellular fluid.77 The morphological hallmark of RTD is the absence or reduction in the number of differentiated proximal tubules, and most patients die during gestation.77 RAS is important in nephrogenesis, and pre- or postnatal exposure to angiotensin converting enzyme or angiotensin receptor inhibitors block RAS expression, resulting in renal developmental anomalies and secondary RTD.61,77
An adverse intrauterine environment has been shown to result in nephron deficits (lower nephron numbers) leading to CKD, hypertension, diabetes, and other renal diseases in later life, which has been referred to as “fetal programming of adult disease”.78,79 A few of the maternal factors known to affect kidney development include undernutrition, vitamin deficiencies, stress, smoking, diabetes, and drug intake.66 Experimental intrauterine growth retardation in rats (uterine horn arterial ligation/low protein diet) or in rabbits (embryos rendered ischemic by vascular ligation) results in morphologic evidence of nephron deficits.80–82 In addition, spontaneously hypertensive rats have reduced numbers of nephrons.83
Abnormal Development of the Lower Urinary Tract
Urinary tract obstruction is common in the human pediatric population, caused by diverse factors targeting different parts of the urinary tract, and has a detrimental effect on the maturing kidney especially tubule function.76,84 Obstruction of urine is particularly serious as it is one of the main causes of ESRD in children.84 Cases of UTO observed in rodents of unknown pathogenesis might have roots in a previously undetected genetic malformation. These cases most likely require careful and detailed study since it has been postulated that urine reflux in mice does not result in major phenotypical consequences and may go unnoticed.49
UB outgrowth and ureter positioning are extremely important in the eventual connection of the ureter to the UGS or future bladder. Therefore, major anomalies associated with UTO may present as ureter ectopia, ureteral duplications, megaureter, ureteroceles (a urine filled ureteric cyst present in the bladder or urethra), ureteropelvic junction obstruction, ureterovesical junction anomalies, and vesicoureteral reflux, all of which can result in hydronephrosis and UTO.51,53,68,76,84
Ureteral ectopia results in insertions of the urethra to the neck of the bladder, to the prostatic urethra, or to the vagina.68 Ureters joining the UGS in a more posterior position closer to the urethra often result in urinary obstruction, while insertions in a more lateral to anterior position lead to vesicoureteral reflux because of vesicoureteral valve malformations and dysfunction.49
Ureteropelvic junction maturation has been recognized as important in the normal function of the pyeloureteral peristaltic machinery for urine transfer.76 Deletions of genes responsible for smooth muscle proliferation and function in the developing urinary tract cause abnormalities of the pelvis and ureter junction resulting in functional obstruction and UTO.85
Conclusions
Studies utilizing transgenic mouse models of human disease often rely on embryogenesis endpoints to study defects in development due to alterations in genes associated with congenital urinary tract defects in humans. These GEM models are powerful tools used to study the effects of alterations of gene targets on phenotypes in a biologic system, and will continue to be utilized by comparative scientists and pathologists. Therefore, a solid knowledge of the events that take place along the developmental path during organogenesis will continue to be a very important skill for comparative and developmental pathologists studying congenital and developmental human disease.
Supplementary Material
Acknowledgements
The authors wish to acknowledge Tina Jones (NIEHS) for slide preparation and sectioning and David Sabio (EPL) for schematic illustrations. We are grateful to Dr. Janice Harvey (NIEHS) for her valuable comments while reviewing the manuscript. We would also like to thank Julie Foley (NIEHS) for her years of dedication and expertise in developing/creating our embryo archive which holds valuable materials used in our embryo atlases. This research was supported [in part] by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Declaration of Conflicting Interests Statement
The author(s) declared no potential, real, or perceived conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Pohl M, Bhatnagar V, Mendoza SA, Nigam SK. Toward an etiological classification of developmental disorders of the kidney and upper urinary tract. Kidney Int 2002;61(1):10–19. [DOI] [PubMed] [Google Scholar]
- 2.Michos O. Kidney development: from ureteric bud formation to branching morphogenesis. Curr Opin Genet Dev 2009;19(5):484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.North American Pediatric Renal Trials and Collaborative Studies. 2008 Annual Report 2008.
- 4.Rasouly HM, Lu W. Lower urinary tract development and disease. Wiley Interdiscip Rev Syst Biol Med 2013;5(3):307–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanna-Cherchi S, Caridi G, Weng PL, et al. Genetic approaches to human renal agenesis/hypoplasia and dysplasia. Pediatr Nephrol 2007;22(10):1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman MH. The anatomical basis of mouse development 1st ed. San Diego, CA: Academic Press; 1999. [Google Scholar]
- 7.Kaufman MH. The atlas of mouse development London: Academic Press; 1992. [Google Scholar]
- 8.Theiler K The house mouse : development and normal stages from fertilization to 4 weeks of age New York: Springer Verlag; 1972. [Google Scholar]
- 9.Petiet AE, Kaufman MH, Goddeeris MM, Brandenburg J, Elmore SA, Johnson GA. High-resolution magnetic resonance histology of the embryonic and neonatal mouse: a 4D atlas and morphologic database. Proc Natl Acad Sci U S A 2008;105(34):12331–12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulik K, Bream PJ, Poe T, Bindra K, Greenwood Genetic Center, UNC Chapel Hill. Normal and Abnormal Mammalian Development https://syllabus.med.unc.edu/courseware/embryo_images/unit-welcome/welcome_htms/akgs.htm. Accessed July, 2017.
- 11.Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol 2006;22:509–529. [DOI] [PubMed] [Google Scholar]
- 12.Davidson AJ. Mouse kidney development. In: StemBook Cambridge (MA)2008. [Google Scholar]
- 13.Cullen-McEwen L, Sutherland MR, Black MJ. The Human Kidney: Parallels in Structure, Spatial Development, and Timing of Nephrogenesis. In: Little MH, ed. Kidney Development, Disease, Repair and Regeneration San Diego: Academic Press; 2016:27–40. [Google Scholar]
- 14.Bridgewater D, Rosenblum ND. Stimulatory and inhibitory signaling molecules that regulate renal branching morphogenesis. Pediatr Nephrol 2009;24(9):1611–1619. [DOI] [PubMed] [Google Scholar]
- 15.Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation 2006;74(7):402–421. [DOI] [PubMed] [Google Scholar]
- 16.Theiler K The house mouse: atlas of embryonic development New York: Springer-Verlag; 1989. [Google Scholar]
- 17.Shah MM, Sampogna RV, Sakurai H, Bush KT, Nigam SK. Branching morphogenesis and kidney disease. Development 2004;131(7):1449–1462. [DOI] [PubMed] [Google Scholar]
- 18.Cebrian C, Borodo K, Charles N, Herzlinger DA. Morphometric index of the developing murine kidney. Dev Dyn 2004;231(3):601–608. [DOI] [PubMed] [Google Scholar]
- 19.Mendelsohn C. Using mouse models to understand normal and abnormal urogenital tract development. Organogenesis 2009;5(1):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munro DAD, Hughes J. The Origins and Functions of Tissue-Resident Macrophages in Kidney Development. Front Physiol 2017;8:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stapor PC, Murfee WL. Identification of class III beta-tubulin as a marker of angiogenic perivascular cells. Microvasc Res 2012;83(2):257–262. [DOI] [PubMed] [Google Scholar]
- 22.Hellstrom M, Gerhardt H, Kalen M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 2001;153(3):543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997;277(5323):242–245. [DOI] [PubMed] [Google Scholar]
- 24.Wagner N, Wagner KD, Scholz H, Kirschner KM, Schedl A. Intermediate filament protein nestin is expressed in developing kidney and heart and might be regulated by the Wilms’ tumor suppressor Wt1. Am J Physiol Regul Integr Comp Physiol 2006;291(3):R779–787. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Boyle S, Zhao M, et al. Differential expression of the intermediate filament protein nestin during renal development and its localization in adult podocytes. J Am Soc Nephrol 2006;17(5):1283–1291. [DOI] [PubMed] [Google Scholar]
- 26.Rugh R Chronology of Development (8–16 days). In: The mouse : its reproduction and development Oxford [England] ;: Oxford University Press; 1990:149–204. [Google Scholar]
- 27.Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. Mouse urogenital development: a practical approach. Differentiation 2003;71(7):402–413. [DOI] [PubMed] [Google Scholar]
- 28.Islam SS, Mokhtari RB, Kumar S, et al. Spatio-temporal distribution of Smads and role of Smads/TGF-beta/BMP-4 in the regulation of mouse bladder organogenesis. PLoS One 2013;8(4):e61340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haraguchi R, Motoyama J, Sasaki H, et al. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development 2007;134(3):525–533. [DOI] [PubMed] [Google Scholar]
- 30.Georgas KM, Armstrong J, Keast JR, et al. An illustrated anatomical ontology of the developing mouse lower urogenital tract. Development 2015;142(10):1893–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpenter A, Paulus A, Robinson M, et al. 3-Dimensional morphometric analysis of murine bladder development and dysmorphogenesis. Dev Dyn 2012;241(3):522–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baskin LS, Liu W, Bastacky J, Yucel S. Anatomical studies of the mouse genital tubercle. Adv Exp Med Biol 2004;545:103–121. [DOI] [PubMed] [Google Scholar]
- 33.Hartman HA, Lai HL, Patterson LT. Cessation of renal morphogenesis in mice. Dev Biol 2007;310(2):379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Short KM, Combes AN, Lefevre J, et al. Global quantification of tissue dynamics in the developing mouse kidney. Dev Cell 2014;29(2):188–202. [DOI] [PubMed] [Google Scholar]
- 35.Cochard LR, Netter FH. Netter’s atlas of human embryology 1st ed. Teterboro, N.J.: Icon Learning Systems; 2002. [Google Scholar]
- 36.Schedl A. Renal abnormalities and their developmental origin. Nat Rev Genet 2007;8(10):791–802. [DOI] [PubMed] [Google Scholar]
- 37.Little M, Georgas K, Pennisi D, Wilkinson L. Kidney development: two tales of tubulogenesis. Curr Top Dev Biol 2010;90:193–229. [DOI] [PubMed] [Google Scholar]
- 38.Yosypiv IV. Congenital anomalies of the kidney and urinary tract: a genetic disorder? Int J Nephrol 2012;2012:909083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capone VP, Morello W, Taroni F, Montini G. Genetics of Congenital Anomalies of the Kidney and Urinary Tract: The Current State of Play. Int J Mol Sci 2017;18(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frazier KS. Species Differences in Renal Development and Associated Developmental Nephrotoxicity. Birth Defects Res 2017;109(16):1243–1256. [DOI] [PubMed] [Google Scholar]
- 41.Barthold SW, Griffey SM, Percy DH. In: Pathology of laboratory rodents and rabbits 4 ed.: John Wiley & Sons; 2016:102–103, 159. [Google Scholar]
- 42.Seely J, Hard G, Blankenship B. Kidney. In: Suttie AE, Leininger JR, Bradley AE, eds. Boorman’s pathology of the rat : reference and atlas Second ed. London: Academic Press, Elsevier; 2018:125–166. [Google Scholar]
- 43.McInnes EF. Wistar and Sprague–Dawley rats. In: McInnes EF, Mann P, eds. Background Lesions in Laboratory Animals Saint Louis: W.B. Saunders; 2012:28. [Google Scholar]
- 44.Taylor I. Mouse. In: McInnes EF, Mann P, eds. Background Lesions in Laboratory Animals Saint Louis: W.B. Saunders; 2012:57. [Google Scholar]
- 45.Scott JE. Fetal, perinatal, and infant death with congenital renal anomaly. Arch Dis Child 2002;87(2):114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kerecuk L, Schreuder MF, Woolf AS. Renal tract malformations: perspectives for nephrologists. Nat Clin Pract Nephrol 2008;4(6):312–325. [DOI] [PubMed] [Google Scholar]
- 47.Toka HR, Toka O, Hariri A, Nguyen HT. Congenital anomalies of kidney and urinary tract. Semin Nephrol 2010;30(4):374–386. [DOI] [PubMed] [Google Scholar]
- 48.Jain S, Chen F. Developmental pathology of congenital kidney and urinary tract anomalies 2018. [DOI] [PMC free article] [PubMed]
- 49.Uetani N, Bouchard M. Plumbing in the embryo: developmental defects of the urinary tracts. Clin Genet 2009;75(4):307–317. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez MM. Congenital Anomalies of the Kidney and the Urinary Tract (CAKUT). Fetal Pediatr Pathol 2014;33(5–6):293–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colvin R, Chang A. In: Diagnostic Pathology: Kidney Diseases 2 ed. Philadelphia, PA: Elsevier; 2016:828–849. [Google Scholar]
- 52.Woolf AS, Winyard PJ, Hermanns MH, Welham SJ. Maldevelopment of the human kidney and lower urinary tract: an overview. In: The kidney Elsevier; 2003:377–393. [Google Scholar]
- 53.Chen F. Genetic and developmental basis for urinary tract obstruction. Pediatr Nephrol 2009;24(9):1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Risdon R, Woolf A. Development of the Kidney. In: Jennette J, Olson J, Schwartz M, Silva F, eds. Heptinstall’s Pathology of the Kidney 5 ed. Philadelphia: Lippincott-Raven; 1998:1149–1205. [Google Scholar]
- 55.Glassberg KI. Normal and abnormal development of the kidney: a clinician’s interpretation of current knowledge. J Urol 2002;167(6):2339–2350; discussion 2350–2331. [PubMed] [Google Scholar]
- 56.Upadhyay KK, Silverstein DM. Renal development: a complex process dependent on inductive interaction. Curr Pediatr Rev 2014;10(2):107–114. [DOI] [PubMed] [Google Scholar]
- 57.Vivante A, Kohl S, Hwang DY, Dworschak GC, Hildebrandt F. Single-gene causes of congenital anomalies of the kidney and urinary tract (CAKUT) in humans. Pediatr Nephrol 2014;29(4):695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.H Lipschutz J Molecular development of the kidney: A review of the results of gene disruption studies Vol 311998. [DOI] [PubMed] [Google Scholar]
- 59.McMahon AP, Aronow BJ, Davidson DR, et al. GUDMAP: the genitourinary developmental molecular anatomy project. J Am Soc Nephrol 2008;19(4):667–671. [DOI] [PubMed] [Google Scholar]
- 60.Harding SD, Armit C, Armstrong J, et al. The GUDMAP database--an online resource for genitourinary research. Development 2011;138(13):2845–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gomez RA, Sequeira Lopez ML, Fernandez L, Chernavvsky DR, Norwood VF. The maturing kidney: development and susceptibility. Ren Fail 1999;21(3–4):283–291. [DOI] [PubMed] [Google Scholar]
- 62.Solhaug MJ, Bolger PM, Jose PA. The developing kidney and environmental toxins. Pediatrics 2004;113(4 Suppl):1084–1091. [PubMed] [Google Scholar]
- 63.Schreuder MF, Bueters RR, Huigen MC, Russel FG, Masereeuw R, van den Heuvel LP. Effect of drugs on renal development. Clin J Am Soc Nephrol 2011;6(1):212–217. [DOI] [PubMed] [Google Scholar]
- 64.Seely JC. A brief review of kidney development, maturation, developmental abnormalities, and drug toxicity: juvenile animal relevancy. J Toxicol Pathol 2017;30(2):125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cappon G, Hurtt M. Developmental toxicity of the kidney. In: Kapp R, Tyl R, eds. Reproductive Toxicology 3 ed. New York: Informa Healthcare; 2010:193–204. [Google Scholar]
- 66.Moritz KM, Cullen-McEwen LA. Kidney development and fetal programming. In: Early Life Origins of Health and Disease Springer; 2006:130–144. [Google Scholar]
- 67.Gilbert-Barness E. Teratogenic causes of malformations. Ann Clin Lab Sci 2010;40(2):99–114. [PubMed] [Google Scholar]
- 68.Decter RM. Renal duplication and fusion anomalies. Pediatr Clin North Am 1997;44(5):1323–1341. [DOI] [PubMed] [Google Scholar]
- 69.Frazier KS, Seely JC, Hard GC, et al. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol Pathol 2012;40(4 Suppl):14S–86S. [DOI] [PubMed] [Google Scholar]
- 70.Ruehl-Fehlert CI, Deschl U, Kayser M, Hartmann E. Bilateral noncystic renal dysplasia in a Wistar-rat. Exp Toxicol Pathol 2003;54(4):293–299. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki H, Yagi M, Saito K, Suzuki K. Embryonic pathogenesis of hypogonadism and renal hypoplasia in hgn/hgn rats characterized by male sterility, reduced female fertility and progressive renal insufficiency. Congenit Anom (Kyoto) 2007;47(1):34–44. [DOI] [PubMed] [Google Scholar]
- 72.Mulroy S, Boucher C, Winyard PJD, Sandford R. Cystic Renal Diseases. In: Vize PD, Woolf AS, Bard JBL, eds. The Kidney San Diego: Academic Press; 2003:433–450. [Google Scholar]
- 73.Bettini G, Mandrioli L, Morini M. Bile duct dysplasia and congenital hepatic fibrosis associated with polycystic kidney (Caroli syndrome) in a rat. Vet Pathol 2003;40(6):693–694. [DOI] [PubMed] [Google Scholar]
- 74.Kai K, Sato N, Watanabe A, Shiraiwa K, Ogawa S, Kobayashi Y. Polycystic disease of the kidney and liver in Crj: CD (SD) rats. Journal of Toxicologic Pathology 2001;14(1):51–55. [Google Scholar]
- 75.Van Winkle T, Womack J, Barbo W, Davis T. Incidence of hydronephrosis among several production colonies of outbred Sprague-Dawley rats. Laboratory animal science 1988;38(4):402–406. [PubMed] [Google Scholar]
- 76.Chen F. Plumbing the depths of urinary tract obstruction by using murine models. Organogenesis 2009;5(1):297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gubler MC. Renal tubular dysgenesis. Pediatr Nephrol 2014;29(1):51–59. [DOI] [PubMed] [Google Scholar]
- 78.Hoy WE, Hughson MD, Bertram JF, Douglas-Denton R, Amann K. Nephron number, hypertension, renal disease, and renal failure. J Am Soc Nephrol 2005;16(9):2557–2564. [DOI] [PubMed] [Google Scholar]
- 79.Bagby SP. Developmental origins of renal disease: should nephron protection begin at birth? Clin J Am Soc Nephrol 2009;4(1):10–13. [DOI] [PubMed] [Google Scholar]
- 80.Merlet-Benichou C, Gilbert T, Muffat-Joly M, Lelievre-Pegorier M, Leroy B. Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr Nephrol 1994;8(2):175–180. [DOI] [PubMed] [Google Scholar]
- 81.Bassan H, Trejo LL, Kariv N, et al. Experimental intrauterine growth retardation alters renal development. Pediatr Nephrol 2000;15(3–4):192–195. [DOI] [PubMed] [Google Scholar]
- 82.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int 2001;59(1):238–245. [DOI] [PubMed] [Google Scholar]
- 83.Skov K, Nyengaard JR, Korsgaard N, Mulvany MJ. Number and size of renal glomeruli in spontaneously hypertensive rats. J Hypertens 1994;12(12):1373–1376. [PubMed] [Google Scholar]
- 84.Klein J, Gonzalez J, Miravete M, et al. Congenital ureteropelvic junction obstruction: human disease and animal models. Int J Exp Pathol 2011;92(3):168–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang CP, McDill BW, Neilson JR, et al. Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest 2004;113(7):1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krinke G Normative Histology of Organs. In: Hedrich HJ, Bullock GR, eds. The laboratory mouse Amsterdam: Elsevier Academic Press; 2004:133–166. [Google Scholar]
- 87.Frith C, Townsend J, Ayres P. Renal Pelvis, Ureters, Urinary Bladder, Urethra. In: Jones TC, Hard GC, Mohr U, eds. Urinary System 2 ed. Berlin: Springer; 1998:319–322. [Google Scholar]
- 88.Wei W, Howard PS, Macarak EJ. Recombinant insulin-like growth factor-1 activates satellite cells in the mouse urethral rhabdosphincter. BMC Urol 2013;13:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev 2007;87(4):1343–1375. [DOI] [PubMed] [Google Scholar]
- 90.Petiot A, Perriton CL, Dickson C, Cohn MJ. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development 2005;132(10):2441–2450. [DOI] [PubMed] [Google Scholar]
- 91.Davies J. Intracellular and extracellular regulation of ureteric bud morphogenesis. J Anat 2001;198(Pt 3):257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Runck LA, Method A, Bischoff A, et al. Defining the molecular pathologies in cloaca malformation: similarities between mouse and human. Dis Model Mech 2014;7(4):483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ng RC, Matsumaru D, Ho AS, et al. Dysregulation of Wnt inhibitory factor 1 (Wif1) expression resulted in aberrant Wnt-beta-catenin signaling and cell death of the cloaca endoderm, and anorectal malformations. Cell Death Differ 2014;21(6):978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blaschko SD, Cunha GR, Baskin LS. Molecular mechanisms of external genitalia development. Differentiation 2012;84(3):261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.