Abstract
Kisspeptin and its receptor, Kiss1r, act in centrally to stimulate reproduction. Recent evidence indicates that kisspeptin is also important for body weight and metabolism, as whole-body Kiss1r KO mice, developed with gene trap technology, display obesity and reduced metabolism. Kiss1r is expressed in brain and multiple peripheral tissues, but it is unknown which is responsible for the metabolic phenotype. Here, we sought to confirm that 1) the metabolic phenotype of the gene trap Kiss1r KOs is due to disruption of kisspeptin signaling and not off-target effects of viral mutagenesis, and 2) the Kiss1r flox line is suitable for creating conditional KOs to study the metabolic phenotype. We used Cre/lox technology (Zp3-Cre/Kiss1r flox) to develop a new global Kiss1r KO (“Kiss1r gKO”) to compare with the original gene trap KO phenotype. We confirmed that deleting exon 2 of Kiss1r from the entire body induces hypogonadism in both sexes. Moreover, global deletion of Kiss1r induced obesity in females, but not males, along with increased adiposity and impaired glucose tolerance, similar to the gene trap Kiss1r KOs. Likewise, Kiss1r gKO females had decreased VO2 and VCO2, likely underlying their obesity. These findings support that our previous results in gene trap Kiss1r KOs are due to disrupted kisspeptin signaling, and further highlight a role for Kiss1r signaling in energy expenditure and metabolism besides controlling reproduction. Moreover, given Kiss1r expression in multiple cell-types, our findings indicate that the Kiss1r flox line is viable for future investigations to isolate specific target cells of kisspeptin’s metabolic effects.
Keywords: kisspeptin, Kiss1, Kiss1r, GPR54, adipose, fat, obesity, metabolism, energy expenditure
Introduction
Kisspeptin (encoded by the gene Kiss1) is a neuropeptide that binds the receptor, Kiss1r (previously termed GPR54). In all mammals, including humans, kisspeptin and its receptor regulate puberty and reproduction by acting in the brain to directly stimulate GnRH secretion, evidenced by findings that humans and rodents with disrupted kisspeptin signaling are infertile, have undeveloped gonads (hypogonadal), and have severely diminished reproductive hormone secretion (reviewed in (1–5)). In addition to being expressed in GnRH neurons in the brain (6–8), Kiss1r is also located in several peripheral tissues, such as adipose tissue, pancreas, adrenal, and gonads (9–14). Likewise, kisspeptin is expressed in several peripheral tissues, including liver, pancreas, gonad, and placenta (12–14). This peripheral expression of both kisspeptin and its receptor suggests that kisspeptin signaling has additional roles besides reproductive regulation. However, until recently, non-reproductive roles of kisspeptin have been largely overlooked.
Our group recently reported that, in addition to stimulating the reproductive axis, kisspeptin signaling also influences body weight (BW), energy balance, and glucose homeostasis. Specifically, compared with WT littermates, global Kiss1r knockout (KO) female mice—lacking kisspeptin signaling in all tissues—displayed significantly greater BWs in adulthood, weighing as much as 30% more than control females (15,16). In addition to becoming obese, Kiss1r KO females also had increased adiposity, higher leptin levels, and substantially impaired glucose tolerance, both on standard chow and high fat diets (15,16). This obesity phenotype was sexually dimorphic, with Kiss1r KO males exhibiting normal BW and glucose regulation out to 6 months of age. The obese Kiss1r KO females did not have increased food intake, but rather they displayed significantly reduced metabolism parameters, including highly reduced metabolic respiratory rates and energy expenditure. These data suggests that their obesity reflects lower daily energy expenditure (15,16) rather than changes in food intake. Importantly, the obesity and metabolic dysfunction in Kiss1r KO females was not just due to their hypogonadal state and absent ovarian estradiol (E2), as long-term ovariectomized Kiss1r KO females still developed obesity, hyperleptinemia, reduced metabolism, and glucose intolerance when compared with long-term ovariectomized WT control females (15,16). In both genotypes, E2 was absent for many month starting from well before puberty, yet the KOs still became heavier and had lower metabolic rates, signifying the phenotype is not just caused by their hypogonadal state.
Because Kiss1r was completely absent from all cells in the body in the global Kiss1r KOs, it remains unknown which specific tissues and cell types, neural or peripheral, are responsible for kisspeptin’s influence on the various metabolic phenotypes. Indeed, peripheral kisspeptin and Kiss1r are present in several metabolic-related peripheral tissues, suggesting a number of potential candidate target sites for kisspeptin’s ability to alter metabolism and energy balance. Moreover, it is possible that different phenotypic impairments (glucose homeostasis impairment, increased adiposity, altered metabolic rate, etc.) observed in global Kiss1r KOs may each reflect absent kisspeptin signaling in different tissues or cell-types, with the net effect of each of these individual tissue-specific changes resulting in the overall obese phenotype. To dissect and isolate the contribution of specific individual cell types/tissues to the various metabolic alterations, future studies might utilize Cre-driven selective knockout of kisspeptin signaling from just one tissue at a time and compare this to the condition in whole-body global KO females. To date, this has not been attempted, and first requires validation that available Kiss1r floxed mouse lines are able to recapitulate the global KO phenotype when used to generate full body KOs.
Multiple strategies have been used to generate mutations in rodent models, including gene targeting by homologous recombination, point mutations or small deletions generated by chemical mutagenesis, and random gene trap mutagenesis applied genome-wide using plasmid, virus, or transposon DNA vectors delivered to ES cells (17–19). Our global Kiss1r KO mouse displaying the obese, metabolic phenotype was created via gene trapping by retroviral mutagenesis. When inserted into an intron of an expressed gene, the gene trap cassette causes transcription to be terminated prematurely, resulting in a fusion transcript encoding a truncated, nonfunctional protein (17,20). Despite other known Kiss1r and Kiss1 KO mouse lines similarly having a hypogonadal, infertile phenotype, confirmation of a metabolic/obese phenotype in these other KO lines has yet to be reported. It is unclear if this is because metabolic parameters have not yet been extensively studied in the other KO lines or if our retroviral gene-trap variant exhibits a metabolic phenotype that is not recapitulated by other genetic models of kisspeptin impairment.
The present study experimentally tested whether a novel whole-body KO line created with Cre/lox technology could replicate the previous findings in our original global “gene trap” Kiss1r KO line, thereby providing further evidence for a metabolic role of kisspeptin signaling. By crossing our recently-made Kiss1r flox line (21) with zona pellucida 3-Cre (Zp3-Cre) mice, we generated a new total body deletion of Kiss1r. The Zp3-Cre mouse line utilizes Cre expression controlled by regulatory sequences from the mouse Zp3 gene, which is expressed exclusively in the growing oocyte prior to the completion of the first meiotic division (22–25). First, we used this novel Zp3-Cre/Kiss1r flox model of whole body Kiss1r deletion to validate and confirm of our previous report of obesity and metabolic disturbance in global Kiss1r KO mice made using retroviral gene trap technology. Secondly, from a conceptual and technical perspective, we used this novel approach to provide essential proof of feasibility for future Cre/lox studies that aim to selectively knock out Kiss1r in just one specific metabolic cell type or tissue, in order to isolate and study kisspeptin’s site(s) of action underlying the obese phenotype. Herein we report the presence of a full metabolic, obese phenotype, along with severe hypogonadism, in these novel global Kiss1r KO mice generated with Cre/lox technology.
Materials and Methods
Animals
Experiments used a new global (whole-body) Kiss1r KO mouse line generated with Cre/lox technology to ascertain if one could recapitulate the previously-observed reproductive and metabolic phenotypes of our original global Kiss1r KO mice that were created by gene-trap methodology (15,16,26,27). To create the new global KO of Kiss1r, Zp3-Cre female mice (24) were mated with males of a recently created Kiss1r flox line (Kiss1rfl/fl (13,21), allowing recombination of the Kiss1r allele to occur in the oocyte of female offspring. In target-bearing Zp3-Cre mice, Cre-mediated recombination of the target gene occurs in 100% of oocytes, and Cre expression is directed to the time when maternal genes are first transcribed, thereby inactivating target floxed genes in the maturing oocyte. This allowed us to effectively induce germline recombination to delete the target gene, Kiss1r, from the entire embryo (i.e., a global KO). We then selected recombination positive, Cre negative (Zp3-Cre−/−/Kiss1rrec/wt) offspring to further generate global Kiss1r KOs lacking functional Kiss1r in all cells (Zp3-Cre−/−/Kiss1rrec/rec; termed “Kiss1r gKO”) and control littermate mice (Zp3-Cre−/−/Kiss1rrec/wt and Zp3-Cre−/−/Kiss1rwt/wt).
All mice were genotyped and sexed by using PCR of DNA obtained from toe samples at postnatal day 7 (PND7) or tail DNA at weaning (PND 21). Weaned mice were housed 2–3 per cage with mixed genotype in a 12-hour light/12-hour dark cycle, with ad libitum water and standard rodent chow containing 3.5 kcal/g, 45.2% carbohydrates, 11.4% fat, and 17.2% crude protein. All experiments were approved by the Institutional Animal Care and Use Committee from the University of California San Diego.
Reproductive Characteristics
Previously reported global Kiss1r KO mice are well-known to have impaired reproductive status, as evidenced by extremely underdeveloped gonads, reduced anogenital distance (AGD; an indirect measure of testosterone exposure) in males, and correspondingly low circulating estrogen and testosterone levels. For the new global Kiss1r KO mouse line here (Kiss1r gKO), reproductive developmental status was quantified by measuring AGD at week 12 and gonadal weights at sacrifice. For the former, mice were briefly anesthetized with isoflurane and distance measurement was taken from the anus opening to the base of the reproductive organ.
Body Weight Assessment
To assess whether the novel Kiss1r gKOs develop a metabolic phenotype, as occurs in the gene trap global KOs, Kiss1r gKOs of both sexes were analyzed for their BW at multiple ages. Control and experimental littermates were weighed once every two wks, starting at 4 wks of age and ending around 4–5 months old.
Metabolic and Energy Expenditure Analyses
To measure metabolic rates, Kiss1r gKO and control littermates were analyzed at 20–24 weeks of age with indirect calorimetry using equal flow Comprehensive Laboratory Animal Monitoring System (CLAMS) calorimeter system (CLAMS; Columbus Instruments, Columbus, OH). Mice were individually housed 2–3 days prior to the experiment for habituation to being single housed. CLAMS data were collected in clear respiratory chambers (20 × 10 × 12.5 cm) equipped with a sipper tube delivering water and a food tray connected to a balance. The consumption of O2 and production of CO2 was measured by having sample air sequentially passed through O2 and CO2 sensors (Columbus Instruments) for determination of O2 and CO2 content, from which measures of oxygen consumption (VO2) and carbon dioxide production (VCO2) were estimated. Room air was passed through chambers at a flow rate of 0.5 L/min. Exhaust air from each chamber was sampled at 15-min intervals for 1 min. Outdoor air reference values were sampled after every 8 measurements. Gas sensors were calibrated prior to the onset of experiments with primary gas standards containing known concentrations of O2, CO2, and N2 (Airgas Puritan Medical, Ontario, CA). Respiratory exchange ratio (RER) was calculated as the ratio of carbon dioxide production (VCO2) to oxygen consumption (VO2). Energy expenditure (heat formation) was corrected for each mouse’s body mass. Daily food intake was also recorded by the CLAMS system in a subset of mice of each genotype. All data were recorded under ambient room temperature (~24°C) for up to 7 days.
Body Composition Analyses
Lean and fat mass of a group of Kiss1r gKO females and males was determined using dual energy x-ray absorptiometry (DEXA) at 19–21 weeks old. The mice were fasted for 4–6 hours before measurement. The mice were anesthetized by an ip injection of Fatal-Plus. The DEXA scan was performed with a GE Lunar Pixi Densitometer Machine.
Glucose Tolerance Tests
At 18–20 weeks of age, Kiss1r gKO mice and their control littermates of both sexes underwent glucose tolerance testing (GTT). Mice of both genotypes and sexes were fasted for 6 hours before starting the glucose measurements, with free access to water throughout the experiment. Blood glucose levels were measured using a handheld glucometer before (time 0 min) and after (15, 30, 45, 60, 90, and 120 min) a single ip glucose injection (2g/kg BW dissolved in saline).
Assessment of Kiss1 and Kiss1r gene expression levels in both sexes
qRT-PCR analyses of Kiss1 and Kiss1r genes was compared between normal (i.e., not KOs) adult males and females (8 wks old) for multiple metabolically-relevant tissues, including whole hypothalamus, liver, white and brown adipose (WAT and BAT), and skeletal muscle. Both sexes were GDX 2 wks prior to tissue collection to equalize circulating sex steroids between all the animals. Total RNA was isolated using the phenol/chloroform method and converted to cDNA via reverse transcription (Promega, Sydney, Australia), followed by clean up using the PCR Clean Up Kit (Mo Bio Laboratories, Carlsbad, CA, USA). qRT- PCR was performed in 10-ul reaction volumes and samples were tested in duplicate using a Rotorgene 3000 (Corbett Life Science; Qiagen, Valencia, CA, USA). Reactions used Qiagen SYBR Green PCR Kit (Qiagen, Chadstone Centre, VIC, Australia). Samples were compared with a standard curve (10-fold dilution) and relative gene expression was normalised using a GE Norm algorithm of housekeeping genes peptidylpropyl isomerase A (Ppia), succinate hydrogenase (Sdha) and TATA box binding protein (Tbp). No significant variability was noted in each housekeeping gene.
Statistical Analyses
All data are presented as mean ± SEM. For data at single points (non-repeated measures), single comparisons were made using one- or two-tailed t-tests, as appropriate, and multiple comparisons were performed using one-way ANOVA with Tukey’s post-hoc test. For repeated measures (BWs, GTTs), repeated measures ANOVA was performed, with Bonferroni post-hoc tests directly comparing genotypes at specific points for 3 groups or t-tests for 2 groups. Statistical significance was p<0.05.
Results
Reproductive development is impaired in a new global Kiss1r KO line generated with Cre/lox technology
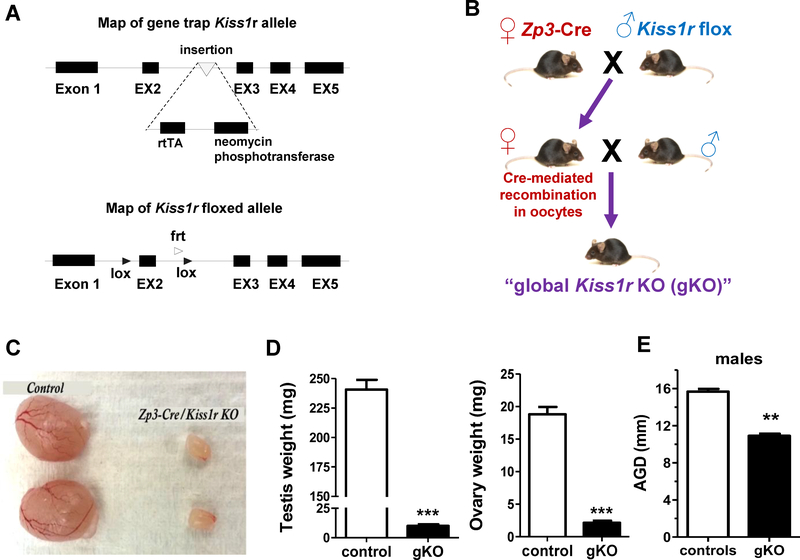
We sought to 1) confirm that disrupting global Kiss1r signaling using Cre/lox technology similarly caused the metabolic phenotypes previously observed in our original gene trap global Kiss1r KOs, and 2) validate that the new Kiss1r flox line is a viable tool for future testing of site-specific kisspeptin metabolic actions. We therefore generated a new global Kiss1r KO line (“Kiss1r gKO”) via germline Cre using Zp3-Cre mice mated to Kiss1r flox mice to delete the second exon of Kiss1r in all cell types throughout the body (Figure 1A and 1B). Given kisspeptin’s established role in promoting reproductive development and fertility, we first assessed gonadal status of these new Kiss1r gKO mice. Adult Kiss1r gKO of both sexes had significantly smaller, undeveloped gonads (testes in males and ovaries in females) compared to WT controls (p<0.001; Figure 1C), indicating that the reproductive axis was impaired, as expected, in our new global Kiss1r KO line. Supporting this, AGD (a testosterone-stimulated somatic measure) in adult Kiss1r gKO males was significantly smaller than in WT male littermates (p<0.01; Figure 1D).
Figure 1:
Development and reproductive characterization of a new global Kiss1r KO mouse made using Cre/lox technology. A) Map of the Kiss1r allele in the original gene-trap Kiss1r KO mouse line and the Kiss1r flox line used in this study. B) Schematic of the simplified strategy for creating the new global Kiss1r KO (“gKO”) by crossing Zp3-Cre and Kiss1r flox strains. C) Image of testes of adult gKO and WT males. D) Ovary and testes weights of adult gKO and control mice (n=6–9/genotype for each sex). E) AGD of adult male gKOs (n=10–15/genotype). **, p<0.01, ***p<0.001.
Body weight are elevated in the new global Kiss1r KO line
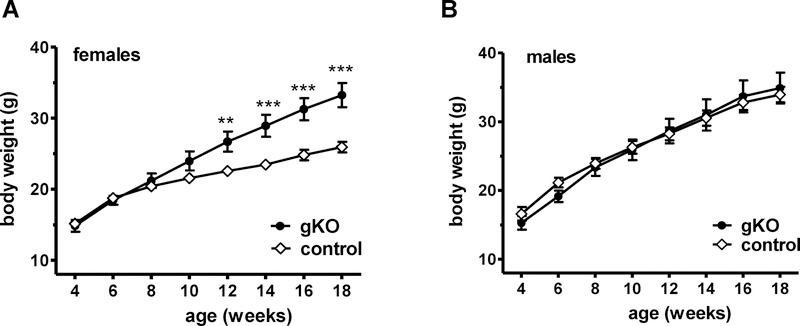
To determine if the new Kiss1r gKOs develop an obesity phenotype like our previous gene trap global Kiss1r KO females, BWs were measured every two weeks starting at week 4 (before puberty). Adult Kiss1r gKO females showed a significantly higher BW compared to WT female littermates (p<0.01; Figure 2A), starting around week 12 and continuing through adulthood. By week 18 of age, Kiss1r gKO females weighed a marked 30% more than WT littermate controls. Conversely, Kiss1r gKO males did not display any BW differences compared to their WT littermates (Figure 2B), similar to the gene trap global KO males.
Figure 2:
Body weights of female and male Kiss1r gKO mice made using Cre/lox technology. A) BWs of gKO and control female mice (n=9–15/genotype). B) BWs of gKO and control male mice (n=7–9/genotype). **, p<0.01, ***p<0.001.
Adiposity is elevated in the new global Kiss1r KO line
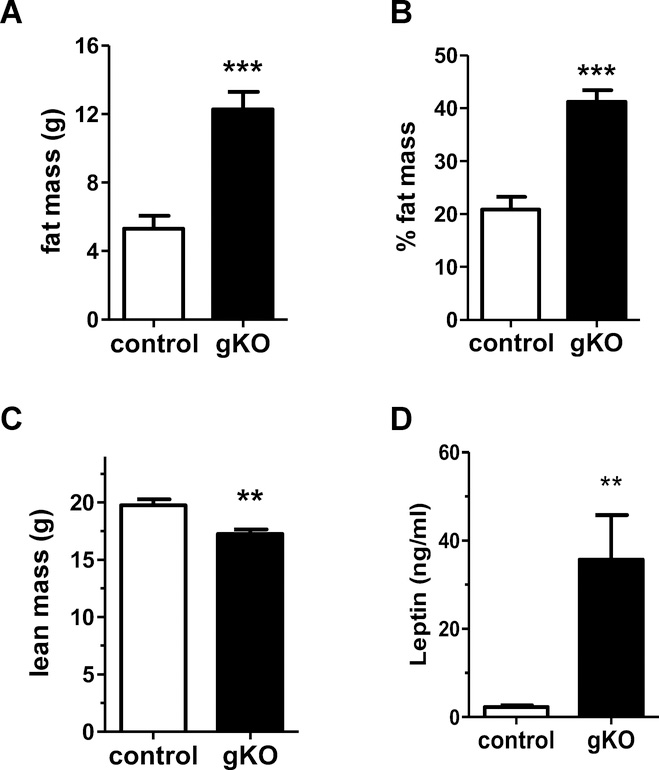
Adult Kiss1r gKO and WT females were analyzed by DEXA to see if their body fat and lean mass composition were altered. gKO females showed a significantly higher fat mass and percent fat mass compared to WT females (p<0.001 for each measure; Figure 3A and 3B), while their lean mass was significantly lower than WT females (p<0.01; Figure 3C). Circulating leptin was also significantly elevated in female Kiss1r gKOs (P<0.01; Figure 3D), matching their elevated fat mass. In line with their normal BWs, Kiss1r gKO males did not show any significant differences in fat mass compared to WT males (data not shown).
Figure 3:
Body composition of adult female gKO and control mice. A) Fat mass, B) percent fat mass, C), lean mass, and D) blood serum leptin levels are all significantly altered in gKO females. n=6–10/genotype. **, p<0.01, ***p<0.001.
Glucose tolerance is impaired in the new global Kiss1r KOs created with Cre/lox technology
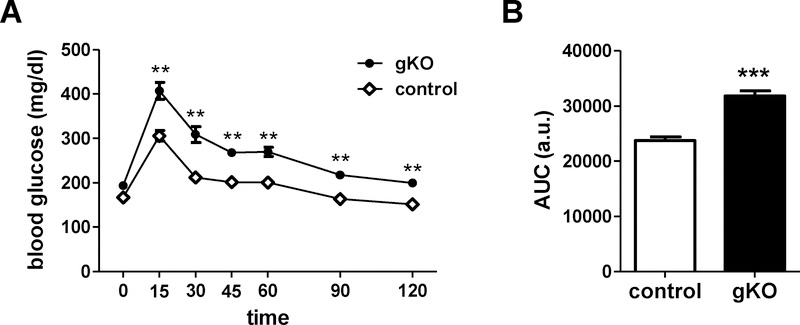
We performed an ip GTT to assess glucose regulation in adult female Kiss1r gKOs. There was a significant genotype difference, with Kiss1r gKOs displaying impaired glucose tolerance (i.e., higher blood glucose levels and slower clearance) relative to WT control females, similar to the gene trap global KOs (p<0.01; Figure 4).
Figure 4:
A) Glucose regulation of adult gKO female mice is impaired in an ip glucose tolerance test (GTT). B) Area under the curve (AUC) for gKO and control mice during the GTT. n=10–12/genotype. **, p<0.01, ***p<0.001.
Metabolic rates are significantly reduced in the new global Kiss1r KO line
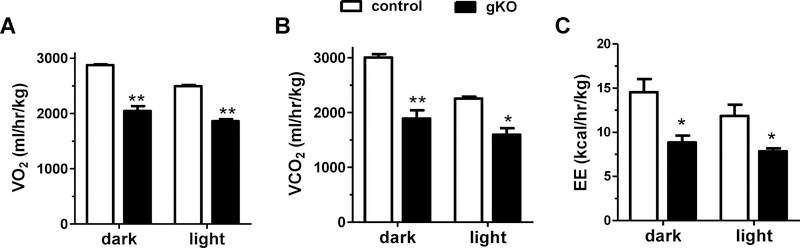
Despite their obesity, mean daily (24 h) food intake was significantly lower in the Kiss1r gKO females than in WT littermate controls (2.41 ± 0.02 vs 3.37 ± 0.08 g/day, p<0.01). To test whether the obesity in the female Kiss1r gKOs may be due instead to diminished metabolism, we used CLAMS metabolic cages to measure metabolic respiratory rates. Adult Kiss1r gKO females had significantly lower levels of both O2 consumption and CO2 production during both the light and dark phases of the light-dark cycle relative to WT female littermates (p<0.01; Figure 5A and B), similar to the gene trap global Kiss1r KO females. Similarly, adult Kiss1r gKO females had significantly lower energy expenditure during both the light and dark phases relative to WT females (p<0.5; Figure 5C).
Figure 5:
Metabolic rates and energy expenditure in controls versus gKO females. A) Oxygen consumption (VO2), B) carbon dioxide production (VCO2), and C) energy expenditure are all significantly decreased in gKOs in both the light and dark periods of the daily light cycle (n=3/genotype). *, p<0.05, **, p<0.01.
Expression patterns of Kiss1 and Kiss1r in metabolic tissues from males and females
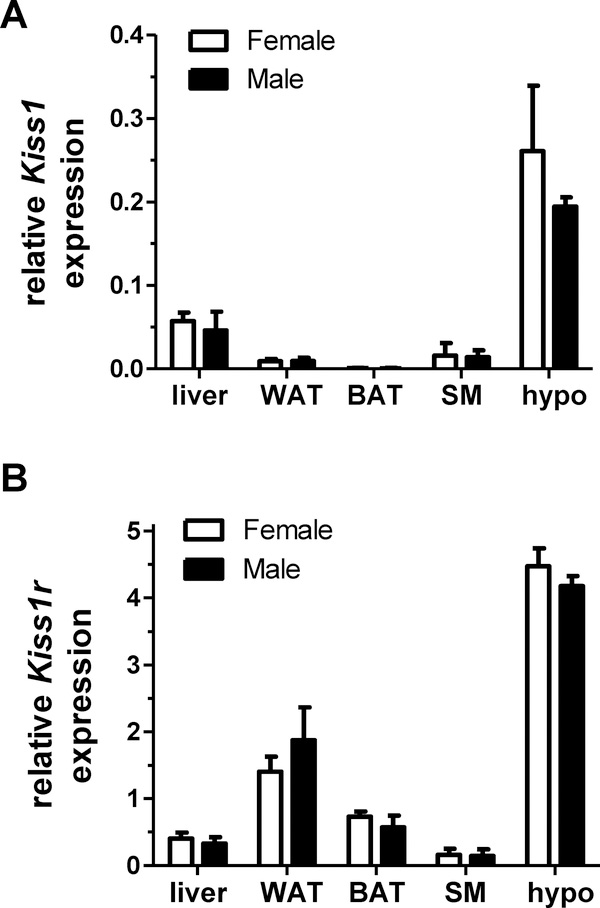
Our results above indicated a major sex difference in the metabolic effects of global Kiss1r deletion: both male and female Kiss1r gKO mice showed hypogonadism, but only female Kiss1r gKOs showed obesity and metabolic impairment. To try and understand the underlying cause of this metabolic sex difference, we compared gene expression levels of Kiss1r, as well as Kiss1 (encoding the ligand, kisspeptin), in several metabolic tissues of male and female mice. In both sexes, both the receptor and ligand mRNAs were detected in the hypothalamus and several peripheral metabolic tissues (Figure 6), as previously reported. In the peripheral tissues, Kiss1 was most highly expressed in liver whereas Kiss1r was expressed more abundantly in WAT and BAT. However, there were no notable sex differences in either Kiss1r or Kiss1 gene expression in any tissue examined (Figure 6), suggesting that the obesity sex difference in gKOs reflects an underlying cause other than differential Kiss1 or Kiss1r expression.
Figure 6:
qPCR gene expression analysis of Kiss1 and Kiss1r levels in several metabolic-relevant tissues, including brain and peripheral tissues, of normal adult GDX male and female mice. A) Kiss1 levels in males and females. B) Kiss1r levels in males and females. There were no sex differences detected for either gene in any tissue examined. WAT, white adipose tissue; BAT, brown adipose tissue; SM, skeletal muscle; hypo, hypothalamus.
Discussion
Kisspeptin-Kiss1r signaling in the brain is widely acknowledged as a critical activator of the reproductive axis in all mammals, including humans. Kisspeptin’s reproductive effects are achieved through its neural actions, stimulating GnRH neurons, which express Kiss1r. However, recent evidence from our group and others demonstrate that kisspeptin-Kiss1r signaling is also important for non-reproductive processes, including metabolism and energy balance. We recently reported that global Kiss1r KO females, generated via gene trap technology, develop obesity and glucose intolerance in adulthood, due to markedly decreased metabolic rates and energy expenditure that is independent of E2 levels (15,16,26). However, because Kiss1r is expressed in several peripheral tissues, the specific tissue(s) responsible for the observed obese/metabolic phenotype in the global KOs remains undetermined. Here, we demonstrated that the previously-observed obese and metabolic phenotype could be completely reproduced via Cre/lox technology by deleting exon 2 of Kiss1r in the oocyte, thereby producing germline recombination that targets all cells throughout the body (24). Thus, endogenous kisspeptin signaling somewhere, in one or more target cell-types, provides a previously-unrecognized modulation of energy expenditure, body weight, and metabolism.
Our prior studies found that our original gene trap global Kiss1r KO mice are hypogonadal and infertile, similar to other reported mouse knockout models of either Kiss1 or Kiss1r (21,28–31). Our present results, using Cre/lox technology (Zp3-Cre/Kiss1r flox, aka Kiss1r gKO), confirmed that deleting the second exon of Kiss1r from the entire body results in hypogonadal female and male mice. Kiss1r gKO males also had reduced AGD, a testosterone-dependent measure. These findings indicate that Kiss1r signaling in reproductive cells (likely GnRH neurons (21,30)) was disrupted, as expected, validating the Kiss1r flox line. The Cre/lox methodology used here is therefore viable to generate Kiss1r KOs for future reproductive investigations.
Metabolic assessments in the newly generated Kiss1r gKO model also confirmed that lacking Kiss1r from the entire body results in obesity in females, but not males, with higher fat mass and lower lean mass, similar to the original global Kiss1r KO females. Similarly, Kiss1r gKO females also had significantly lower VO2 and VCO2 compared to control females, likely underlying the observed obesity in the Kiss1r gKOs. Daily food intake was not elevated in the Kiss1r gKOs, but rather was decreased relative to controls, as in the gene-trap global KOs. The lower food intake may be a side effect of the elevated leptin in these obese females, as leptin is known to diminish feeding (32–34), but this remains to be tested in future studies. All of these findings confirm that our previous results in the gene trap KO animals are due to disruptions in kisspeptin signaling, and not off target effects of viral mutagenesis. These findings also confirm that Kiss1r signaling is important for not only stimulating the reproductive axis, but also for regulating BW, body composition, and metabolism, at least in females. Importantly, in support of this, a recent clinical study in humans reported metabolic-related effects of kisspeptin (35). In that study, a single infusion of kisspeptin to patients significantly increased insulin secretion and also altered serum metabolites, including reducing small lipid species in the blood (35). These findings in humans are congruent and complementary with our present Kiss1r gKO findings and previous KO reports (15,16), which suggested that functional kisspeptin signaling may normally act to promote insulin action, glucose homeostasis, and prevent elevated adiposity. It will be interesting to see if future clinical studies report alterations in BW after longer-term kisspeptin treatment, and if kisspeptin treatment increases metabolic rates or body temperature in humans.
Given that Kiss1r and kisspeptin are both present in several peripheral metabolic tissues (Figure 6), the exact location(s) of where kisspeptin signaling influences these metabolic and energy balance parameters remains unknown. Of note, recent data indicate that neural energy balance populations, like hypothalamic neuropeptide Y (NPY) and pro-opiomelanocortin (POMC), have unaltered metabolic gene expression in global Kiss1r KO females (26), suggesting that the underlying mechanism giving rise to obesity and reduced metabolic rates in those mice is likely occurring outside the brain in peripheral systems. To test this possibility, future studies can employ Cre/lox technology to conditionally delete Kiss1r specifically from a target cell type or tissue, while maintaining intact Kiss1r elsewhere in the body. The present study confirms that this approach is technically feasible, as the present Kiss1r flox line, when crossed with Zp3-Cre line, was able to be used to fully recapitulate the full metabolic phenotype observed in the global Kiss1r KO mice used in previous studies. In addition to the brain, Kiss1r is expressed in white and brown adipose tissue, liver, gonads, and pancreas, so determining the exact functional target site will require extensive examinations. A previous study found that deletion of Kiss1r from just the pancreas improved glucose metabolism, but that study did not measure body weight, adiposity, or metabolic rates and energy expenditure (13). Thus, it remains unknown if kisspeptin action in the pancreas contributes to BW and metabolic regulation in addition to possible effects on insulin secretion. Regardless, it is important to note that the observed metabolic phenotype of the Kiss1r KOs may not be due to absent kisspeptin signaling in just one tissue. Indeed, actions of kisspeptin in one specific tissue type may contribute to only one or two components of the “overall” metabolic phenotype whereas kisspeptin acting in other specific tissue type may underlie other metabolic aspects. For example, hypothetically, kisspeptin signaling in the pancreas may influence insulin and glucose tolerance, whereas kisspeptin signaling in adipose tissue may influence adiposity or energy expenditure.
Unlike the females, Cre/lox-derived Kiss1r gKO males did not display obesity or alterations in body fat, similar to what was observed in the gene trap global Kiss1r KO males. Despite the absence of a metabolic phenotype, the Kiss1r gKO males did display severe hypogonadism and reduced AGD, signifying that the Cre/lox deletion of Kiss1r signaling was successful. The mechanistic reason for the obesity sex difference similarly observed in both the original global KOs and the new Kiss1r gKOs remains unknown. We tested whether there were sex differences in Kiss1r or Kiss1 expression levels in several tissues but found similar mRNA levels between males and females in all tissues examined. This suggests that the obesity sex difference is not due to sex differences in peripheral kisspeptin production or sex differences in Kiss1r expression in metabolic target tissues, though it remains possible that there are unknown sex differences in these genes in other tissues or cell-types that were not tested in our present study, such as pancreas or adrenals. Investigating this issue will require well-controlled experimental designs given that Kiss1 or Kiss1r expression in the periphery may be influenced by metabolic status, hormones, or other factors (12,13,36–38), as it is in the brain. Alternatively, the obesity sex difference may reflect differences in intra-cellular signaling pathways downstream of Kiss1r in specific metabolic target sites, a possibility for future investigations.
Of note, a recent report (39) from Tena-Sempere’s group analyzed body weight and adiposity phenotype in a different global Kiss1r KO line. They showed lower body weight gain in their KOs of both sexes at early ages (< 6 wks old), which we also previously reported in global Kiss1r KO males (16). Importantly, at older ages, their Kiss1r KO males and females showed normal or increased BW gain, respectively, similar to our prior findings. Moreover, adiposity levels were higher in KOs of both sexes, and on a high fat diet, their female KOs became fatter than WT controls, also matching our prior findings (39). Thus, there was generally good congruence between their findings and both our previous and current report.
In summary, global deletion of Kiss1r from all tissues/cells achieved via Cre/lox technology, results in both impaired reproductive status and diminished energy balance and metabolism in females, the latter of which was correlated with increased adiposity and BW. This confirms our previous findings in gene trap derived global Kiss1r KO mice and further supports a role for Kiss1r signaling in energy expenditure and metabolism in addition to just governing reproduction, findings recently supported by clinical studies in humans. Overall, these findings highlight the complexity and multi-faceted nature of the kisspeptin system throughout the body, and emphasize the need for more tissue- or cell-specific assessments to compare with global whole-body manipulations. Future studies are needed to determine the underlying mechanisms and specific sites of kisspeptin action that lead to obesity and metabolic dysfunction in global KOs, as well as to further define the specific actions of endogenous kisspeptin signaling specifically in target metabolic cell-types.
Highlights.
We used Cre/lox technology to develop a novel global Kiss1r knock out (Kiss1r gKO).
Kiss1r gKO mice of both sexes are severely hypogonadal.
Kiss1r gKO females, but not males, are obese with impaired glucose tolerance.
Kiss1r gKO females show decreased VO2 and VCO2, likely underlying their obesity.
No sex differences were identified in Kiss1 or Kiss1r expression in peripheral tissues.
These data further highlight a key role for Kiss1r signaling in BW and metabolism.
Acknowledgments
Grant Support: This research was supported by NIH grants R01 HD090161, P30 DK063491, U01 HD066432, P50 HD012303, and T32 HD007203.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kauffman AS. Coming of age in the Kisspeptin Era: Sex differences, development, and puberty. Mol Cell Endocrinol 2010; 324:51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oakley AE, Clifton DK, Steiner RA. Kisspeptin Signaling in the Brain. Endocr Rev 2009; 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colledge WH. Transgenic mouse models to study Gpr54/kisspeptin physiology. Peptides 2009; 30:34–41 [DOI] [PubMed] [Google Scholar]
- 4.Herbison AE. The Gonadotropin-Releasing Hormone Pulse Generator. Endocrinology 2018; 159:3723–3736 [DOI] [PubMed] [Google Scholar]
- 5.Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol 2016; 12:452–466 [DOI] [PubMed] [Google Scholar]
- 6.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A 2005; 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbison AE, de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 2010; 151:312–321 [DOI] [PubMed] [Google Scholar]
- 8.Koemeter-Cox AI, Sherwood TW, Green JA, Steiner RA, Berbari NF, Yoder BK, Kauffman AS, Monsma PC, Brown A, Askwith CC, Mykytyn K. Primary cilia enhance kisspeptin receptor signaling on gonadotropin-releasing hormone neurons. Proc Natl Acad Sci U S A 2014; 111:10335–10340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 2001; 276:34631–34636 [DOI] [PubMed] [Google Scholar]
- 10.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001; 411:613–617 [DOI] [PubMed] [Google Scholar]
- 11.Pruszynska-Oszmalek E, Kolodziejski PA, Sassek M, Sliwowska JH. Kisspeptin-10 inhibits proliferation and regulates lipolysis and lipogenesis processes in 3T3-L1 cells and isolated rat adipocytes. Endocrine 2017; 56:54–64 [DOI] [PubMed] [Google Scholar]
- 12.Dudek M, Kolodziejski PA, Pruszynska-Oszmalek E, Sassek M, Ziarniak K, Nowak KW, Sliwowska JH. Effects of high-fat diet-induced obesity and diabetes on Kiss1 and GPR54 expression in the hypothalamic-pituitary-gonadal (HPG) axis and peripheral organs (fat, pancreas and liver) in male rats. Neuropeptides 2016; 56:41–49 [DOI] [PubMed] [Google Scholar]
- 13.Song WJ, Mondal P, Wolfe A, Alonso LC, Stamateris R, Ong BW, Lim OC, Yang KS, Radovick S, Novaira HJ, Farber EA, Farber CR, Turner SD, Hussain MA. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell metabolism 2014; 19:667–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 2001; 276:28969–28975 [DOI] [PubMed] [Google Scholar]
- 15.Tolson KP, Garcia C, Delgado I, Marooki N, Kauffman AS. Metabolism and Energy Expenditure, But Not Feeding or Glucose Tolerance, Are Impaired in Young Kiss1r KO Female Mice. Endocrinology 2016; 157:4192–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toison KP, Garcia C, Yen S, Simonds S, Stefanidis A, Lawrence A, Smith JT, Kauffman AS. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J Clin Invest 2014; 124:3075–3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang W, Hubbard SC, Friedel C, Ruley HE. Enrichment of insertional mutants following retrovirus gene trap selection. Virology 1993; 193:737–747 [DOI] [PubMed] [Google Scholar]
- 18.Friedel RH, Plump A, Lu X, Spilker K, Jolicoeur C, Wong K, Venkatesh TR, Yaron A, Hynes M, Chen B, Okada A, McConnell SK, Rayburn H, Tessier-Lavigne M. Gene targeting using a promoterless gene trap vector (“targeted trapping”) is an efficient method to mutate a large fraction of genes. Proc Natl Acad Sci U S A 2005; 102:13188–13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedel RH, Soriano P. Gene trap mutagenesis in the mouse. Methods in enzymology 2010; 477:243–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Melchner H, Reddy S, Ruley HE. Isolation of cellular promoters by using a retrovirus promoter trap. Proc Natl Acad Sci U S A 1990; 87:3733–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novaira HJ, Sonko ML, Hoffman G, Koo Y, Ko C, Wolfe A, Radovick S. Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol Endocrinol 2014; 28:225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epifano O, Liang LF, Familari M, Moos MC Jr., Dean J Coordinate expression of the three zona pellucida genes during mouse oogenesis. Development 1995; 121:1947–1956 [DOI] [PubMed] [Google Scholar]
- 23.Hinsch KD, Hinsch E. The zona pellucida ‘receptors’ ZP1, ZP2 and ZP3. Andrologia 1999; 31:320–322 [PubMed] [Google Scholar]
- 24.Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Current biology : CB 1997; 7:148–151 [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Valdivia R, Jeong J, Mukherjee A, Soyal SM, Li J, Ying Y, Demayo FJ, Lydon JP. A mouse model to dissect progesterone signaling in the female reproductive tract and mammary gland. Genesis 2010; 48:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Bond JP, Tolson KP, Nasamran C, Kauffman AS, Smith JT. Unaltered Hypothalamic Metabolic Gene Expression in Kiss 1r Knockout Mice Despite Obesity and Reduced Energy Expenditure. J Neuroendocrinol 2016; 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dror T, Franks J, Kauffman AS. Analysis of Multiple Positive Feedback Paradigms Demonstrates a Complete Absence of LH Surges and GnRH Activation in Mice Lacking Kisspeptin Signaling. Biol Reprod 2013; 88:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 2007; 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 29.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr., Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr., Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med 2003; 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 30.Kirilov M, Clarkson J, Liu X, Roa J, Campos P, Porteous R, Schutz G, Herbison AE. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nature communications 2013; 4:2492. [DOI] [PubMed] [Google Scholar]
- 31.Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci 2007; 27:8826–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rentsch J, Levens N, Chiesi M. Recombinant ob-gene product reduces food intake in fasted mice. Biochemical and biophysical research communications 1995; 214:131–136 [DOI] [PubMed] [Google Scholar]
- 33.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995; 269:540–543 [DOI] [PubMed] [Google Scholar]
- 34.Weigle DS, Bukowski TR, Foster DC, Holderman S, Kramer JM, Lasser G, Lofton-Day CE, Prunkard DE, Raymond C, Kuijper JL. Recombinant ob protein reduces feeding and body weight in the ob/ob mouse. J Clin Invest 1995; 96:2065–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izzi-Engbeaya C, Comninos AN, Clarke SA, Jomard A, Yang L, Jones S, Abbara A, Narayanaswamy S, Eng PC, Papadopoulou D, Prague JK, Bech P, Godsland IF, Bassett P, Sands C, Camuzeaux S, Gomez-Romero M, Pearce JTM, Lewis MR, Holmes E, Nicholson JK, Tan T, Ratnasabapathy R, Hu M, Carrat G, Piemonti L, Bugliani M, Marchetti P, Johnson PR, Hughes SJ, James Shapiro AM, Rutter GA, Dhillo WS. The effects of kisspeptin on beta-cell function, serum metabolites and appetite in humans. Diabetes, obesity & metabolism 2018; 20:2800–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pita J, Barrios V, Gavela-Perez T, Martos-Moreno GA, Munoz-Calvo MT, Pozo J, Rovira A, Argente J, Soriano-Guillen L. Circulating kisspeptin levels exhibit sexual dimorphism in adults, are increased in obese prepubertal girls and do not suffer modifications in girls with idiopathic central precocious puberty. Peptides 2011; 32:1781–1786 [DOI] [PubMed] [Google Scholar]
- 37.Pita J, Rado-Peralta S, Gavela-Perez T, Aragon I, Barrios V, Rovira A, Argente J, Soriano-Guillen L. Plasma kisspeptin levels are elevated in cord blood and present sexual dimorphism in the adult population: relation with leptin, gonadotropins and anthropometrical data. Peptides 2011; 32:983–988 [DOI] [PubMed] [Google Scholar]
- 38.Chakravarthi VP, Khristi V, Ghosh S, Yerrathota S, Dai E, Roby KF, Wolfe MW, Rumi MAK. ESR2 Is Essential for Gonadotropin-Induced Kiss1 Expression in Granulosa Cells. Endocrinology 2018; 159:3860–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velasco I, Leon S, Barroso A, Ruiz-Pino F, Heras V, Torres E, Leon M, Ruohonen ST, Garcia-Galiano D, Romero-Ruiz A, Sanchez-Garrido MA, Olhsson C, Castellano JM, Roa J, Poutanen M, Pinilla L, Vazquez MJ, Tena-Sempere M. Gonadal Hormone-Dependent vs. -Independent Effects of Kisspeptin Signaling in the Control of Body Weight and Metabolic Homeostasis. Metabolism: clinical and experimental 2019; [DOI] [PubMed] [Google Scholar]