Abstract
Introduction
Electronic cigarettes (e-cigarettes) are a rapidly evolving class of tobacco products intended to deliver nicotine to users. There are many types of e-cigarettes available and the most popular type today in the United States are “pod” based devices that use high nicotine concentration liquids. Understanding the nicotine delivery capabilities of e-cigarettes is imperative for understanding their addictive potential and safety profile, informing regulation, and revealing their potential use as smoking cessation aids.
Areas covered
This review discusses nicotine content of e-cigarettes, effectiveness of nicotine aerosolization by devices, delivery of nicotine to users, and user and device characteristics that impact each of these.
Expert opinion
Modern e-cigarettes have the potential to deliver equal or more nicotine compared to a tobacco cigarette. Future research needs to identify the nicotine delivery profiles likely to benefit public health and the means to regulate them appropriately while also identifying those that are likely to cause harm. Public health benefit accrues if e-cigarettes help smokers quit combustible cigarettes completely. Harm is possible if inadequate nicotine delivery undermines cessation attempts, e-cigarettes facilitate continued combustible cigarette use, long-term e-cigarette use is associated with substantial morbidity/mortality, and/or e-cigarettes increase the initiation of combustible cigarette use among never smokers.
Keywords: e-cigarettes, nicotine, addiction, dependence, tobacco control
1. Introduction
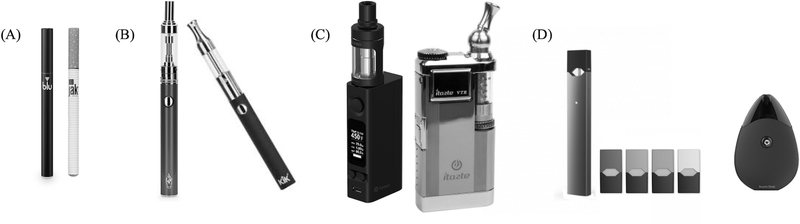
Electronic cigarettes (e-cigarettes) are battery powered devices intended to deliver aerosolized nicotine to their users. E-cigarettes generally consist of a battery, a heating element (often called a “coil” or “atomizer”), and a liquid solution that contains solvents such as propylene glycol (PG) and/or vegetable glycerin (VG), sweeteners and flavorants, and, usually, the pharmacologically active and dependence-producing drug nicotine [1]. E-cigarettes were first produced in China in 2003, and first appeared on the U.S. market in 2006 [2]. Early devices (first generation) are referred to as “cig-a-likes” because they generally resemble a cigarette in size and shape; they are often disposable as the liquid cannot be replenished and battery is not rechargeable (see Figure 1) [3]. Second generation devices resemble pens and sometimes use more powerful and rechargeable batteries with cartridges that can be refilled with liquid (see Figure 1) [3]. Third generation devices (sometimes called “mods”) are larger, allow the user to control many features (e.g., device power, heating temperature) and also are refillable (see Figure 1) [3].The most recent iteration of e-cigarettes, pod-based devices, are smaller than other types and use a disposable or refillable “pod” that contains the heating element and the liquid, which is often more highly concentrated with nicotine than other e-cigarettes. A common feature of these pod devices is the liquid used; it is a lower pH solution and consists of nicotine conjugated with a weak base (referred to as a nicotine salt) (see Figure 1). Pod devices now have the majority of e-cigarette market share in the United States (U.S.) with JUUL alone accounting for 75% of e-cigarette sales in October 2018 (according to manufacturer sales reports) [4–6].
Figure 1.
E-Cigarette Generations. (A) First generation devices (“cig-a-likes”). (B) Second generation devices. (C) Third generation devices (“mods”). (D) Pod-based systems (on the left is the JUUL, and on the right is the Sourin Drop)
In the U.S., e-cigarette use has increased substantially in recent years; between 2014 and 2016, the percentage of adults who had ever used an e-cigarette increased from 12.6% to 15.3% [7]. Among youth, this growth in popularity is especially rapid; between 2011 and 2018, past 30 day use among high school students increased from 11.7% to 20.8% [8]. While the long-term health effects of prolonged e-cigarette use are unknown, these products could be a useful harm reduction tool for cigarette smokers who use e-cigarettes in place of combustible cigarettes, as the full switch from a combustible to e-cigarette decreases smokers’ exposure to multiple known toxicants and carcinogens contained in tobacco smoke [9–11] and can reduce short-term adverse health outcomes in several organ systems [12]. However, if smokers continue to use cigarettes while using e-cigarettes (“dual use”) there is no associated reduction in exposure to toxicants [10]. This pattern of dual use is becoming increasingly common with over half of e-cigarette users also smoking cigarettes in 2015 and 2016 [13]. Additionally, e-cigarette use among previously nicotine-naïve adolescents and young adults may engender nicotine dependence and is associated with the transition to combustible cigarette use [14]. The fact that dependence on tobacco is driven primarily by the pharmacological effects of nicotine is well established [15]. The other potential and unknown public health risk may be an increase in the number of users who initiate e-cigarette use at a young age, would become addicted, and would continue using e-cigarettes for a long period of time, but who may never transition to a combustible cigarette. Therefore, a complete understanding of the capability of e-cigarettes to deliver nicotine is required in order to help establish their potential as a harm reduction tool and as a facilitator of long-term use or transition to combustible cigarette use. This review will cover: (i) nicotine pharmacology; (ii) design components of e-cigarettes; (iii) the nicotine content of e-cigarette liquids; (iv) the nicotine yield in e-cigarette aerosols; and (v) e-cigarette nicotine delivery profile, pharmacokinetics and long-term exposure.
2. Nicotine pharmacology and chemistry
Nicotine (3-(1-methyl-pyrrolidinyl)-pyridine) is a volatile chemical that is the most abundant tobacco alkaloid and the main addictive component of cigarettes and e-cigarettes [16]. After being inhaled, nicotine can be absorbed buccally, in the upper airways, and in the lungs. When absorbed in the lungs, it rapidly enters the pulmonary venous circulation, goes through the heart, enters arterial circulation and moves across the blood-brain barrier. Once in the brain, nicotine binds to nicotinic acetylcholine receptors (nAChRs), which are cholinergic ligand gated ion channels [16]. When nicotine, a chemical with cholinergic agonistic activity, binds to these receptors it opens the channels and allows cations to flow in, activating a signal transduction pathway. In the central nervous system this activation leads to the release of multiple neurotransmitters (dopamine, norepinephrine, serotonin, GABA, glutamate, and endorphins). Dopamine is the predominant neurotransmitter released, and it is associated with pleasure and appetite suppression [16]. The release of these neurotransmitters is critical in the reinforcing and dependence-producing effects of nicotine (for a comprehensive review of nicotine pharmacology: [16]).
The pH of inhaled aerosol plays a large role in nicotine bioavailability [14] and previous research on nicotine chemistry in combustible cigarettes and smokeless tobacco is relevant to e-cigarettes because the pH of e-cigarette liquids can vary, thus potentially influencing users’ nicotine exposure [17–20]. The pH of e-cigarette liquids has been shown to be driven by nicotine concentration [17] (as nicotine concentration increases so does the pH of the liquid), and the pH of an e-cigarette liquid is important in determining the amount of protonated and unprotonated nicotine present. Since nicotine is a weak base (pKa=8.0), at a higher pH, a larger portion of nicotine is unprotonated (“free-base”) and nicotine is more volatile [21]. As a consequence of higher pH, a significant proportion of nicotine is present in a gas phase of inhaled aerosol. This leads to increased absorption in oral cavity and upper respiratory tract but also cause more irritation and sensation of unpleasant taste of nicotine. With lower pH, more nicotine is present in a liquid phase of aerosol leading to limited absorption in upper respiratory tract but also reducing harshness and unpleasant taste. This allows users to inhale deeply without experiencing unpleasant sensations which results in an increased lung deposition of the aerosol and enhanced absorption of nicotine through the lungs. One study found that in a liquid with a pH of 5.16 the proportion of unprotonated/protonated nicotine was 2.5%/97.5%, and in a liquid with a pH of 9.66 the proportion was reversed to 97.7%/2.3% [18].
After absorption, nicotine is distributed widely throughout the body, and average steady state distribution of nicotine by volume ranges from 2.2 to 3.3 L/kg [22]. The distribution half-life of nicotine is approximately 8 minutes [22]. Nicotine predominately is metabolized in the liver, and 70% to 80% of nicotine is metabolized to cotinine and trans-3-hydroxycotinine [16]. Nicotine metabolites are excreted in the urine and the following are the major metabolites: cotinine (10–15%), cotinine glucuronide (12–17%), and trans-3’-hydroxycotinine (33–40%). Nicotine metabolites measured in urine account for approximately 90% of the systemic dose of nicotine. The elimination half-life of nicotine is approximately 120 minutes [16].
3. Important design components of E-Cigarettes
E-cigarettes work by the following mechanism: the battery of the device is activated which powers the heating element or coil, the coil is thus heated and then vaporizes some of the liquid adjacent to it. As the user inhales, the vapor is drawn away from the coil and condenses into an aerosol mist. Thus, inhaled e-cigarette emission is an aerosol of liquid particles (liquid phase) suspended in air (gas phase). A wick (within the heating coil) brings fresh liquid to the coil to replace the previously vaporized liquid [23]. The breakdown of e-cigarette liquid chemical components on the heating coil can produce potentially harmful byproducts such as carbonyls [24–26] (which have been correlated with pulmonary disease in smokers [27]).
As noted, the liquid usually consists of some ratio of PG/VG, flavorants, and nicotine (some ratio of protonated/unprotonated). Different e-cigarette batteries range from 3 to 6 V, and some devices (i.e. 3rd generation) allow users to vary the voltage of their device [3]. The resistance of e-cigarette heating coils ranges from <1.0Ω (in so called “sub-ohm devices”) to 6.5Ω [3]. The power of a device is a function of both the voltage of the battery and the resistance of the coils. Generally, first generation devices have a lower voltage battery and/or a higher resistance coil due to size constraints (“cig-a-like” devices are made to be the size of cigarette and are smaller than later generation devices, with the exception of pod mods) [3]. Each of these design characteristics influences e-cigarette nicotine yield in an inhaled aerosol and its dose delivered to the user [3].
4. Nicotine content of E-Cigarette liquids
Although some e-cigarette liquids do not contain nicotine, the majority of users use an e-cigarette with a liquid that does (in 2015, 99.0% of e-cigarette products sold contained nicotine) [28,29]. One systematic review examined the chemicals in e-cigarette cartridges and refill solutions in studies published between 2007 and 2013 [30]. Of the studies examined, six looked at the nicotine content of cartridges (used in first generation devices). These studies found the nicotine content to be between 0 and 21.8 mg/cartridge. Six studies examined the nicotine content in refill solutions and found nicotine content to vary between 0 and 87.2 mg/ml [30].
A variety of other studies have examined the labeled and measured nicotine content of e-cigarette liquids in the US and elsewhere. The results indicate that labeled concentration can range from 0 to 60 mg/ml with most liquids being in the range of 0 to 24 mg/ml [17,31–34], and the measured nicotine content of e-cigarettes can range from “not detected”/ “below level of quantitation” to 52.0 mg/ml [17,31,34,35]. Two studies [33,35] found extremely high measured nicotine concentrations in two samples. One liquid (from a study conducted in South Korea) was found to have 150.3 mg/ml nicotine, and was labeled “pure nicotine” [35]. The other sample was found to have 134.7 mg/ml nicotine and was called “PureNicotineLiquid” by the manufacturer [33]. The authors hypothesized that this product was intended for “do-it-yourself (DIY)” use [33], in which the user dilutes the liquid with solvents like PG and VG and adds flavorants as desired. Results from studies assessing e-cigarette liquid nicotine concentration indicate considerable variability between labeled and measured nicotine content. Measured content often did not match labeled content, with deviations ranging from −41.7% to +52.0% [17,35,36]. In one study, between 10% and 28% of samples had greater than 20% variation between labeled and measured nicotine content [31]. Other studies showed that 10% to 18% of samples had greater than 10% deviation from labeled nicotine content [33,34]. In addition, one study showed that in a sample of liquids labeled 0mg/ml, 91.4% had detectable nicotine ranging from 0 to 23.9 mg/ml [34]. Overall, results indicate considerable variability in liquid nicotine content both within and across different brands of refillable liquids and non-refillable e-cigarettes. Table 1 summarizes the results of these studies.
Table 1.
Labeled and Measured Nicotine Content in E-Cigarettes
| Study | Solution | Labeled Concentration | Measured concentration | % Deviation |
|---|---|---|---|---|
| Lisko et al [18] | Refill solution | 0 to 24 mg/ml | 0 to 20.5 mg/ml | −41.7 to −5.8% |
| Cheng [26] | Cartridge Refill solution |
N/A | 0 to 19.0mg 0 to 87.2mg/ml |
−100 to 100% −100 to 100% |
| Goniewicz et al [27] | Refill solution (US) Refill solution (Poland) |
0 to 36mg/ml 0 to 4mg/ml |
BLQ to 36.6mg/ml ND to 24.7mg/ml |
28.% with ≥20% variation 10% with ≥20% variation |
| Tierney et al [28] | Refill solution | 6 to 24mg/ml | N/A | N/A |
| Davis et al [29] | Refill solution | 0 to 60mg/ml | 0 to 134.7mg/ml | 10% with ≥10% variation |
| Etter and Budgey [30] | Refill solution | 16 to 48mg/ml | 15.5 to 52.0mg/ml | 18% with ≥10% variation |
| Kim et al [31] | Refill solution | N/A | “not detectable” to 17.5mg/ml (“Pure nicotine” 150.3mg/ml) | −32.2 to 3.3% |
| Raymond et al [32] | Refill solution | 0mg/ml 18mg/ml |
0 to 23.9mg/ml 11.6 to 27.4mg/ml |
91.4% with detectable nicotine −35 to 52% |
Clinical studies also have reported the nicotine content of e-cigarette user’s usual brand of liquid. One study of 14 regular e-cigarette users showed an average labeled nicotine content of 7.9 mg/ml and average measured nicotine concentration of 7.4 mg/ml [19]. Another study of 13 regular e-cigarette users showed a labeled e-cigarette liquid concentration between 6 and 24 mg/ml and measured between 5.0 and 15.3 mg/ml [37].
Pod-based devices contain a liquid nicotine solution that, thus far, have higher nicotine concentrations than most other liquids that have been tested. For example, JUUL (see Figure 1) was introduced in 2015 and has since become the most popular e-cigarette available in the U.S. [4,5], though other brands of pod-based devices are available (e.g., Sourin; Bo; Phix). JUUL resembles a computer USB drive and utilizes a “pod” based system in which users are able to buy replaceable pods that contain high concentrations of nicotine in its protonated form (nicotine “salt” liquid) [38]. Nicotine salts are lower in pH and consist of nicotine conjugated with a weak base (e.g., benzoic acid, levulinic acid [39]). The addition of acid in nicotine salts allows manufacturers to greatly increase the concentration of nicotine while apparently avoiding harshness and bitterness [36]. JUUL pods are advertised as having a 5% nicotine solution (by mass) and nicotine concentration has been found to vary between 56.2 and 69 mg/ml [39–41]. The availability of highly-concentrated nicotine-salt solutions seems to be an increasing market trend, as one recent report suggests that an online search showed more than 70 U.S. e-cigarette liquid brands containing more than 5% nicotine [40].
It is important to note that there is the potential for nicotine poisoning with dermal, ingestion, and inhalation exposure to e-cigarette nicotine solutions with high nicotine concentrations (such as the “pure nicotine” discussed above). The most common symptoms of nicotine poisoning include nausea and vomiting, however with higher doses tachycardia and seizures can occur. The lethal dose of nicotine has been estimated to be 30 to 60 mg for an adult and 10 mg for a child. [42,43]
5. Nicotine yield in E-Cigarette aerosol
The nicotine yield in e-cigarette aerosols is determined by product characteristics (e.g. power, nicotine concentration, PG/VG ratio) and user behavior (e.g. puff duration, puff velocity). This topic is separate from the amount of nicotine delivered to a user’s blood once it has been aerosolized (discussed in section 6). One method for analyzing the yield from e-cigarettes involves a smoking machine with a predetermined puffing regimen. The nicotine yield in the aerosol generated from these machines can be measured by trapping the aerosol on filter pads or in gas-washing bottles. Trapped nicotine can be extracted from pads with suitable solvents (e.g. ethyl-acetate [44]) and this extract is then analyzed by chromatography methods [44].
An early (2014) review of the literature identified 5 relevant studies and noted varying units of measurement for the characterization of nicotine in e-cigarette aerosols while highlighting the key finding that the amount of nicotine in aerosols varies across products [30]. This review also highlighted the lack of standardization when measuring and reporting nicotine yields in e-cigarette aerosols [30]. Another early study (2013) demonstrated that in a sample of 16 devices, nicotine released in aerosol after 150 puffs (puffing regimen based upon data from e-cigarette users) varied between 0.3 to 8.7 mg and the devices released 31 to 85% of the nicotine in the cartridges. Thus, in this study, there was substantial variability in the consistency and efficacy of nicotine aerosolization [45].
More recently, multiple studies have assessed nicotine emissions from e-cigarettes. Studies have reported nicotine yields from devices in varying ways (per puff, per 15 puffs, per 20 puffs). The literature has shown that the nicotine yield from one puff on a first generation e-cigarette can range from 8 to 33 μg (lower than the 194 to 232 μg/puff of a cigarette) [46]. Two separate studies found diverse performance from e-cigarettes with respect to nicotine yield; they found that the nicotine yield after 15 puffs from different devices ranged from 0.27 to 2.91 mg [26,47]. Also, reports indicate that 2nd and 3rd generation devices can yield 1.01 to 10.61 mg of nicotine in 20 puffs. These studies have shown that yields from e-cigarettes can be comparable to, and can sometimes exceed, the nicotine yield from one tobacco cigarette (1.76 to 2.20 mg per one tobacco cigarette) [26,47,48]. These studies have also reported that, compared to 2nd generation devices, 3rd generation devices produced more aerosol and a more consistent nicotine yield [48]. Additionally, a correlation between nicotine yield and device generation, nicotine concentration, and PG/VG ratio has been reported [26].
The effects of varying puffing topography and device characteristics on nicotine yield have also been investigated. In fact, a mathematical model that can predict nicotine emissions based on the design and operating conditions of an e-cigarette has been reported [23]. This model computes the vaporization rates of individual species present utilizing the unsteady species and energy conservation equations. This model can be used to predict nicotine yield from a given device accounting for variable puffing topography, electrical power, and liquid composition (i.e. nicotine concentration, PG/VG, water content) [23]. In an analysis across 100 different conditions, nicotine yield varied greatly across conditions, ranging from 0.01 to 6.64 mg in 15 puffs, and nicotine yield was correlated positively with device power, puff duration, and nicotine concentration [23,44]. There was no effect of puff velocity [23,44,47] and water content [23] on nicotine yield, and there was a decrease in yield with increasing VG content [23]. The mathematical model was able to predict the individual effects of liquid composition, device power, and puff topography on nicotine yield [23]. Overall, nicotine yield (for a particular concentration) ultimately is determined by how much nicotine-containing liquid is vaporized during a session, thus anything that increases this factor will increase nicotine emissions [24]. Nicotine yield from one device can vary based on many factors (e.g., device power output, liquid nicotine concentration, puff duration/volume), and an important conclusion drawn from these studies is that depending on puff conditions and product features, 15 puffs on an e-cigarette can produce far less or far more nicotine yield than a tobacco cigarette. It is also important to note that the amount of nicotine aerosolized is highly dependent on nicotine concentration. For example, a large amount of aerosol from a liquid with 0.5 mg/ml nicotine will have substantially less nicotine than a small amount of aerosol from a liquid with 60 mg/ml nicotine.
Multiple studies have examined the distribution and size of particles produced by e-cigarettes. Studies have found that there were more nanoparticles present in e-cigarette aerosol compared with cigarette smoke [49], nicotine is evenly distributed in droplets regardless of size [50], and particle concentration after e-cigarette use is 300 to 3000 times higher than in ambient air [51]. Flavor was found to have no effect on particle emission [52]. Another study utilized varying levels of exposure using a smoking machine (low exposure was 7 puffs, and high exposure was 15 puffs) to estimate second hand exposure from e-cigarettes. Among 3 brands of e-cigarettes, the nicotine exposure after use ranged from 0.82 to 6.23 μg/m3. Compared to the emissions from human subjects after 2 cigarettes were smoked in a 30 minute time frame, the emissions after two 5-minute ad lib e-cigarette sessions were far less (31.6 vs. 3.23 μg/m3) [52]. Additionally, levels of exposure to carbonyls have been assessed with studies finding that emissions range from 3.72 to 48.85 μg/15 puffs [26], which is 9 to 807 fold lower than the carbonyl emissions in cigarette smoke [53,54]. The amount of carbonyls released in emissions is positively correlated with device power and PG content [26,54].
Research is starting to emerge regarding the emissions from JUUL and other pod-based devices. Studies of nicotine yield from pod devices differ in their reporting (some report per puff, per 10 puffs, or per 15 puffs), but standardizing across studies indicates that yield for 15 puffs ranges from 1.15 and 2.83 mg of nicotine [39,41,55]. These numbers are greater than yields from previous studies of other e-cigarettes (0.2 to 2.0 mg of nicotine/15 puffs [41,46], and are comparable to a tobacco cigarette which yielded 1.81 mg of nicotine [41]. One study showed that per gram of liquid consumed, 63.72 to 94.21 mg of nicotine are produced. Additionally, 5.25% of nicotine in a JUUL pod is in the free-base form (other e-cigarettes are between 10 and 95% free-base nicotine) [41]. Overall, pod-based devices release more nicotine than other e-cigarettes [39], fewer toxicants than cigarettes (similar levels to other e-cigarettes) [39], and lesser nicotine in the free-base form [41].
6. Nicotine delivery to blood (E-Cigarette pharmacokinetics)
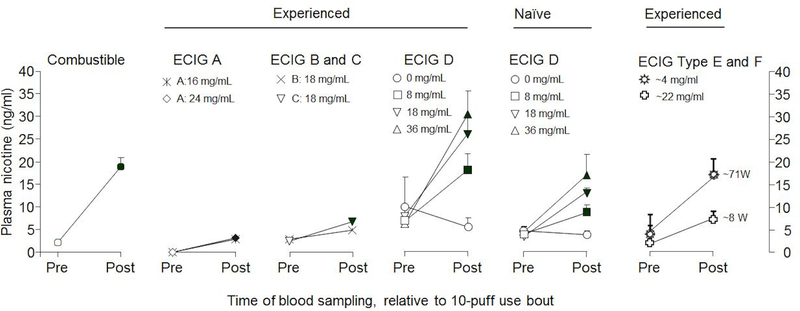
In addition to variability in nicotine content and aerosolization, there is considerable variation across e-cigarettes in their ability to deliver nicotine to users’ blood. As might be expected based on the discussion of nicotine yield above, there are multiple factors that can influence e-cigarette nicotine delivery including device power, liquid nicotine concentration and composition, and user puffing behavior. For reference in the following discussion, the maximum plasma nicotine concentration (Cmax) after smoking a single tobacco cigarette varies between 10 and 30 ng/ml (see Figure 2, left most panel), and the speed of delivery measured by the time of maximum concentration (Tmax) is 5 to 8 minutes from the start of smoking (venous sample) [22,56]. Some e-cigarettes produce a pharmacokinetic curve similar to that for tobacco cigarettes after a regulated puffing session (similar to a bolus dosing of nicotine, with a sharp increase in plasma concentration and a gradual decline after the puffing session is complete) [37]. However, research on nicotine pharmacokinetics during ad lib use of e-cigarettes has shown varying curve shapes, with some users displaying intermittent dosing rather than bolus dosing (i.e. users take a smaller number of puffs more frequently, therefore there is a slower increase in plasma nicotine over the entire course of ad lib use) [57]. This research highlights the variability of use patterns and the resultant varying patterns of nicotine delivery for different users and different devices. Several studies have assessed the influence of device characteristics, including liquid nicotine concentration and power, and users’ puffing behavior (called “puff topography”) and their previous experience with device on nicotine delivery.
Figure 2. Variability in e-cigarette nicotine delivery (adapted from Breland et al. [3]).
Mean (SEM) plasma nicotine concentration from different human laboratory studies and different products with blood sampled before and immediately after a 10-puff product use bout. Combustible cigarette data from [59]. ECIG A is a first generation device [61]. ECIG B is a first generation device, and ECIG C is a third generation device [63]. ECIG D is a second generation device [70,71]. Data for ECIG Type E are from a group of second generation devices (mean power output = ~8 W), and data for ECIG Type F are from a group of third generation devices (mean power output = ~71 W) [35]; note that the higher-powered ECIG Type F delivers more nicotine to the user despite being paired with a lower concentration nicotine liquid (i.e., ~4 mg/ml) [69]. Figure adapted from Breland et al., 2017 [3]. Copyright 2016 New York Academy of Sciences.
6.1. Nicotine delivery from various ENDS devices
Multiple studies that utilized first generation e-cigarettes demonstrated inefficient or negligible nicotine delivery from these devices (see Figure 2). Three studies assessed various brands of first generation e-cigarettes (Ruyan 16 mg cartridge, NPRO 18 mg cartridge, Hydro 16 mg cartridge, and NJOY 26 mg cartridge) [58–60]. These studies utilized a 5 minutes puffing session; one study had participants use the device as they would a tobacco cigarette for 5 minutes [58], and the other two studies had participants take 10 puffs total with a 30 second interval between each puff [59,60]. All of these studies found minimal nicotine delivery from tested devices after 5 minutes of use (no significant increase in plasma nicotine [58] or up to 3.5 ng/ml [60]). A separate study utilizing five different first generation e-cigarettes found that, among e-cigarette users, after 5 minutes of use (10 total puffs, 30 second interval) the nicotine doses delivered by a 16 mg cartridge and a 24 mg cartridge of the same device were similar and significantly less than that from a tobacco cigarette (Figure 2; ECIG A) [61]. However, when a similar study was done among a sample of experienced e-cigarette vapers using their own device (9 to 24 mg/ml nicotine) for 5 minutes (10 total puffs with a 30 second puff interval), plasma nicotine concentration increased significantly from baseline with an average Cmax of 10.3 ng/ml [62]. The results of these studies indicate a significant variability in the nicotine delivery from differing devices, with some not increasing plasma nicotine concentrations significantly [59] and some delivering nicotine at a level approaching that of a tobacco cigarette [62].
One study conducted with experienced users found that after five minutes of controlled use (10 puffs, 30 second interval), the nicotine delivery from a first- and third- generation device (both with 18 mg/ml nicotine) did not differ substantially (see Figure 2; ECIG B and C) [63]. However, two other studies assessing different devices found differences in nicotine delivery based on generation, but they concluded that these devices did not deliver nicotine as effectively as a cigarette [64,65]. The first study was conducted among 11 “dual users” (regularly use both an e-cigarette and a tobacco cigarette) and the following types of devices were tested; six first generation devices ranging from 16 to 48 mg/ml, one second generation device with 20 mg/ml nicotine, and one third generation device with 20 mg/ml nicotine. When comparing devices of similar nicotine strength, advanced generation devices deliver more nicotine than first generation devices. They also found that none of the devices delivered nicotine as effectively as a cigarette (the maximum average Cmax of devices was 13.6 ng/ml with a Tmax of 4 min, while the average Cmax for cigarettes was 17.9 ng/ml with a Tmax of 4 min) [64]. The second study was conducted among 18 inexperienced e-cigarette users trying 6 different devices. The devices included two first generation devices (nicotine concentration 18 and 24 mg/ml, power 4.31W to 5.41W), one second generation device (nicotine 24 mg/ml, power 5.19W), one third generation device (nicotine 24 mg/ml, power 14.1W), one e-Cigar (nicotine 18 mg/ml, power not listed), and one e-Pipe (24 mg/ml, power 14.4W). After a 10 minute regulated session (20 puffs, 30 second intervals), the maximum median Cmax occurred with the third generation device (6.60 ng/ml, higher than the first generation devices), but all devices delivered less nicotine than a tobacco cigarette (median Cmax 18.9 mg/ml) [65].
6.2. Effect of free-base vs. protonated nicotine (salt) formulation on ENDS pharmacokinetics
Very limited research is available assessing the nicotine delivery from pod-based devices that contained nicotine salts. In one study presented at a scientific conference, 13 combustible cigarette smokers achieved significantly lower plasma nicotine concentration after 10 puffs (30 second inter-puff interval) from JUUL (range = 3 to 17 ng/ml) relative to a combustible cigarette (range = 7 to 40 ng/ml) [66]. However, in an unpublished study conducted by the manufacturer of JUUL that involved 24 combustible cigarette smokers who took 10, 3-sec puffs, there was no difference in plasma nicotine concentration for JUUL and a combustible cigarette [67]. Lastly, in a study of 206 young adults who use pod-based devices, median urinary cotinine (the major metabolite of nicotine) was 244.8 ng/ml (higher than reported for adolescent cigarette smokers [68]) [39]. Further research is needed to assess the nicotine delivery from pod-based devices, and from the protonated nicotine liquids that they contain, in order to understand completely their nicotine delivery profile among adults and adolescent users.
6.3. Device power and nicotine delivery
While nicotine concentration, nicotine form (free-base vs. salt) and puff duration are key factors in predicting nicotine delivery, the power output of the device also is important (as with nicotine yield [23,44]). The power of a device (which is a function of battery power and coil resistance) is important in determining the nicotine delivered to a user; this point is highlighted by the differences in nicotine delivery between a low power/ high nicotine device and a high power/ low nicotine device [69]. In a study comparing the nicotine delivery after a 5 minute session (10 total puffs) between second (average nicotine concentration, 22.3 mg/ml; power, 8.6W) and third generation devices (average nicotine concentration, 4.1 mg/ml; power, 71.6W) among experienced users, the more powerful third generation devices delivered more nicotine than second generation devices, even when paired with a liquid that had a lower nicotine concentration (see Figure 2; ECIG E and F). At least in some cases, both generations delivered a similar amount of nicotine compared with a tobacco cigarette [69]. Although generally more nicotine is delivered by higher powered devices, the amount delivered still may not reach the level of a tobacco cigarette [64,65], but some users may be able to compensate for this lower power to increase nicotine intake.
6.4. Nicotine delivery based on users’ experience
Importantly, user behavior may play a role, as several studies involved participants with little or no e-cigarette experience [e.g. 51,52], while the one that showed more nicotine delivery involved experienced e-cigarette users [62]. Thus, previous experience with tested e-cigarette device among study participants seems to be a critical factor that influences results of pharmacokinetic studies.
In a study of 31 experienced and 31 inexperienced e-cigarette users (Figure 2; ECIG D, taken from preliminary data of the same study with 16 experienced and 16 inexperienced users [70,71]), participants used a 2nd generation e-cigarette with differing liquid nicotine concentration (0, 8, 18, and 36 mg/ml) [72]. In this study, e-cigarette power was held constant while other factors were manipulated, and revealed that, after a 5-minute puffing session (10 puffs, once every 30 seconds), plasma nicotine concentration was related directly to liquid nicotine concentration and user experience. When examining data for the 36 mg/ml liquid, an average plasma nicotine increase of 17.9 ng/ml was observed among the e-cigarette experienced participants, as compared to 6.9 ng/ml for the e-cigarette naïve participants. This difference most likely was due to the fact that e-cigarette experienced participants took longer puffs (mean=5.6 sec) relative to naïve participants (mean=2.9 sec) [72]; puff duration is known to influence e-cigarette nicotine yield [23]. Other studies have produced similar results: when 24 experienced and 23 inexperienced users used a 2nd generation e-cigarette with 18 mg/ml liquid for 5 minutes (10 total puffs), smokers with little e-cigarette experience had lower mean plasma nicotine concentration relative to experienced e-cigarette users, and smokers took shorter puffs than experienced e-cigarette users [73]. The fact that shorter puffs leads to less nicotine delivery may underlie the observation that, with experience, novice e-cigarette users begin to display longer puff durations [74,75].
6.5. E-Cigarette liquid flavor and nicotine delivery
A limited number of studies have been done to assess the different nicotine delivery when flavors are manipulated as the independent variable. One such study examined the differences in nicotine delivery among experienced e-cigarette users using two difference flavored e-liquids [19]. The liquids tested were a tobacco flavor (18 mg/ml) and strawberry flavor (18 mg/ml) and participants took 15 puffs (30 second interval) during the session. Relative to tobacco, strawberry flavored liquid AUC0–180 was significantly greater and Cmax was 22% higher. Although the amount of nicotine inhaled by participants was not statistically different, there was different systemic retention of each flavor. They hypothesized that this difference was due to the lower pH of the strawberry flavor [19]. This limited research suggests differences in nicotine delivery from different flavors, so further research needs to explore the effects of flavor on nicotine delivery due to the popularity of flavors [76], the variety of flavors available to users [77], and the potential for youth initiation due to flavors [78].
6.6. Nicotine delivery from ad lib use of devices
Preliminary work reveals that e-cigarette user ad lib puff topography differs from that seen during regulated puffing (longer puff duration and larger puff volume during ad lib use) [79]. Therefore, in addition to nicotine delivery after regulated puffing sessions, studies have also assessed nicotine delivery during ad lib use of devices. One study demonstrated that during a 90 minute ad lib session, the maximum Cmax among experienced e-cigarette users using their own device (average nicotine concentration 9.4 mg/ml) was 12.8 ng/ml (comparable to a tobacco cigarette), and that the topography of e-cigarette users differed from cigarettes [57]. In a separate study of regular users using their own brand of e-cigarettes (average nicotine concentration 7.9 mg/ml), over a 90-minute ad lib session the average Cmax was 11.5 ng/ml. When these participants were given a device with differing flavors, users changed topography which influenced nicotine exposure [80]. E-cigarette users may compensate for low nicotine liquid concentration by changing puffing topography [70,81–83]. When users puff more intensely on a device to compensate for lower nicotine, there is an increase in carbonyl emissions [84]. Additionally, it has been shown that device power can have an effect on compensatory behavior; one study showed an increase in puff number and duration with lower powered devices, and an increase in nicotine consumption with higher powered devices [85]. These studies demonstrate that experienced users using their own devices as they would in real world environment can deliver nicotine as effectively as a tobacco cigarette, and changes in device and liquid characteristics can change how a device is used [57,80]. Further, due to the differences observed during ad lib and regulated puffing sessions, it is important that subsequent research asses both to gain a better understanding of the nicotine delivery and abuse liability of devices.
7. Conclusion
This review discussed many topics, but a common theme throughout each section was heterogeneity in e-cigarette liquid nicotine content, aerosol nicotine yield and nicotine delivery to users. Some devices contain and/or yield and/or deliver very little nicotine while others have the capability to meet or exceed the nicotine yield/delivery of combustible cigarettes. There are a range of factors that influence e-cigarette nicotine yield and delivery including device characteristics (power), concentration and form of nicotine (free-base vs. protonated) in e-cigarette liquids, other liquid constituents (e.g., PG/VG ratio; flavorants) and user puffing behavior. Generally, relatively low power cig-a-likes that likely deliver little nicotine continue to be marketed and there are third generation devices on the market that may deliver little or a great deal of nicotine depending on the power setting and liquid nicotine concentration chosen by the user, as well as user puffing behavior. Importantly, some users may be unaware of how their choices and puffing behavior influence the delivery of a dependence-producing drug, and that these choices may also influence the delivery of a variety of other non-nicotine toxicants [e.g., carbonyls; 42]. However, as devices continue to evolve and users become more experienced, more effective nicotine delivery is observed in an increasing number of studies. Key areas of future research include: nicotine delivery from pod-based devices and the effect that this may have on youth nicotine dependence, the role and efficacy of e-cigarettes with proven nicotine delivery profiles as cessation aids, and the further development of regulatory targets that would reduce e-cigarette heterogeneity and benefit public health.
8. Expert opinion
E-cigarettes have evolved rapidly since their introduction in the early 2000s. The result of this rapid evolution is the current state in the U.S. and elsewhere, in which a heterogeneous and ever-changing product class is marketed to a large population that likely is under-informed regarding their exposure to nicotine and other toxicants in e-cigarette aerosol. As has been noted, some products, under normal operating conditions, are incapable of delivering nicotine to the user, and, as has been demonstrated in early clinical trials, these products likely are ineffective in maintaining smoking cessation for most smokers [86]. They may also be responsible for the frequent observation that many cigarette smokers use e-cigarettes in addition to, as opposed to instead of, combustible cigarette smoking [13,87,88]. As also noted, other products, also under normal operating conditions, are capable of delivering nicotine at least as effectively as combustible cigarettes. In the hands of current combustible cigarette smokers, and especially in the context of meaningful behavioral support, these products may be useful in smoking cessation [89]. In this study, participants were given a starter pack of e-cigarettes (a second generation e-cigarette with 18 mg/ml liquid) with a recommendation to try other liquids in the strength and flavor of their choice. This study also included behavioral support for a minimum of 4 weeks. The behavioral support aspect of this study is important to note as it has been shown that new e-cigarette users need a period of adjustment to achieve meaningful nicotine delivery [73–75]. The implementation of the advice and behavioral support for smokers using an e-cigarette for cessation is an important area for future research. Support for e-cigarettes as efficacious cessation tools consists of very few randomized controlled clinical trials, and thus more work is needed to establish the extent to which e-cigarettes with a proven nicotine delivery profile can play a role in helping smokers quit.
Another issue discussed above is that, at least in the U.S., there is a robust literature demonstrating that e-cigarette use among previously nicotine-naïve youth is associated with an increased likelihood of initiation of combustible cigarette smoking [14]. There is uncertainty regarding the extent to which the nicotine delivery profile of an e-cigarette is related to this transition, but the recent dramatic surge in e-cigarette use among U.S. adolescents and young adults [8] is a potentially grave threat to public health. In this context, the current popularity of e-cigarette liquids that include protonated nicotine (i.e., nicotine salt liquid) is particularly concerning, especially considering that 34.1% of adolescent e-cigarette users do not know the nicotine concentration of their product [90] and 37% of 15 to 24-year-old JUUL users are unsure if the product contained nicotine [91]. If protonated liquid nicotine eases the inhalation of high nicotine concentration aerosols and thus increases the likelihood of e-cigarette use among previously nicotine-naïve youth, future years are likely to see a dramatic rise in nicotine dependence rates and continued use of vaporized nicotine or, perhaps, a concomitant rise in young adult combustible cigarette use. Moreover, there is a growing body of literature suggesting that e-cigarette use carries health risk: several studies demonstrate that cell function is compromised following exposure to e-cigarette aerosol [92–94] and that animals exposed to e-cigarette aerosols show clear indication of adverse consequences of this exposure [95], including in models related to cardiovascular disease [96,97]. Thus, for people of all ages, e-cigarette use may carry unwanted health risks that will become difficult to avoid in the context of the well-known pattern of compulsive use that is associated with nicotine dependence.
The heterogeneity in e-cigarette nicotine delivery described here is a challenge for policymakers, researchers, and clinicians. E-cigarette regulation, in the U.S. under the authority of the Food and Drug Administration (FDA), has the potential to reduce heterogeneity in the marketplace, but is challenged by the need to balance the public health promise of combustible cigarette cessation with the public health threat of adolescent nicotine dependence, the potential for transition from e-cigarettes to combustible cigarette smoking, and the unknown health risks of long-term e-cigarette use. A potential first step in effective regulation may involve regulating the rate at which nicotine is emitted from an e-cigarette (i.e., nicotine “flux”; [98,99]). Using nicotine flux as a regulatory target, all of the factors that influence it (e.g., device power, liquid nicotine concentration, user behavior) can be accounted for, and conditions that produce a flux that is too low (i.e., ineffective nicotine delivery) or too high (e.g., exceeding the flux of a combustible cigarette) can be made unavailable to consumers. Whether or not nicotine flux is the correct regulatory target, the current state of affairs, in which uninformed consumers use products that may or may not deliver nicotine and a variety of other toxicants, is incompatible with an empirically-based regulatory approach that prioritizes public health.
For researchers, this heterogeneity makes population-level studies of the effects of e-cigarette use – particularly in regard to use as a smoking cessation aid – difficult to interpret. A large-scale survey of cigarette smokers that fails to account for the nicotine delivery profile of individual e-cigarette products might show no effect of e-cigarette use on cessation because most respondents are using ineffective e-cigarettes and do not quit smoking. However, a sub-group of respondents may be using e-cigarettes that deliver nicotine effectively and do quit smoking and these results are undetected. Until there is a reliable and valid method for accounting for e-cigarette heterogeneity in such population-level studies, they may obscure as well as reveal.
For clinicians faced with a cigarette smoker interested in using e-cigarettes to quit smoking, heterogeneity in nicotine delivery means that providing guidance will entail a detailed understanding of the factors that influence it: at a minimum, device power, liquid nicotine concentration, and user behavior. The task is further complicated by the fact that some devices (e.g., those that increase the likelihood of exposure to non-nicotine toxicants) and some liquids (e.g., those that contain or may degrade into non-nicotine toxicants) may increase the health risks of e-cigarette use. In addition, a recent randomized controlled trial [89] highlights the value of behavioral support as an adjunct to e-cigarette use: e-cigarettes that are sold on-line and in convenience stores are unlikely to be accompanied with this valuable and potentially necessary adjunct. Clinicians working with a smoker might consider strongly the value of urging behavioral support as a necessary element of any e-cigarette assisted smoking cessation attempt.
Article highlights.
E-cigarettes are battery powered devices intended to deliver aerosolized nicotine to users.
There is a large amount of variation in the devices and e-cigarette liquids available to users, and the technology continues to evolve rapidly.
There is substantial variation in the nicotine delivery of e-cigarettes based on device, liquid, and user characteristics.
E-cigarettes have the potential to deliver equal or more nicotine compared to a tobacco cigarette.
Nicotine delivery is an important aspect of the potential for e-cigarettes either to benefit or harm public health.
Acknowledgments
Funding
This project was supported in part by NIH National Institute on Drug Abuse (NIDA) grant R01DA037446, NIH National Cancer Institute (NCI) and Food and Drug Administration Center for Tobacco Products (FDA CTP) grant U54CA228110, and NIDA and FDA CTP grant U54DA036105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Declaration of interest
T Eissenberg is a paid consultant in litigation against the tobacco industry and is named on a patent for a device that measures the puffing behavior of electronic cigarette users. M Goniewicz received a research grant from Pfizer and served as a member of the scientific advisory board to Johnson&Johnson, manufacturers of smoking cessation medication. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- [1].Centers for Disease Control and Prevention. Smoking and Tobacco Use; Electronic Cigarettes [Internet]. Cent. Dis. Control Prev. 2019. [cited 2019 May 23]. Available from: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html.
- [2].U.S. Customs and Border Protection. M85579: The tariff classification of a nicotine inhaler and parts from China [Internet]. 2006. [cited 2019 May 23]. Available from: https://rulings.cbp.gov/ruling/M85579.
- [3].Breland A, Soule E, Lopez A, et al. Electronic cigarettes: what are they and what do they do? Ann. N. Y. Acad. Sci. 2017;1394:5–30.** an important review article that highlights e-cigarette epidemiology, device technology, and preclinical and clinical research of health effects.
- [4].Truth Initiative. What is JUUL? [Internet]. Truth Initiat. 2018. [cited 2019 May 23]. Available from: https://truthinitiative.org/news/what-is-juul.
- [5].Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob. Control 2019;28:146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Behind the explosive growth of JUUL [Internet]. Truth Initiat. [cited 2019 Aug 26]. Available from: https://truthinitiative.org/research-resources/emerging-tobacco-products/behind-explosive-growth-juul.
- [7].Bao W, Xu G, Lu J, et al. Changes in Electronic Cigarette Use Among Adults in the United States, 2014–2016. JAMA. 2018;319:2039–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gentzke AS, Creamer M, Cullen KA, et al. Vital Signs: Tobacco Product Use Among Middle and High School Students - United States, 2011–2018. MMWR Morb. Mortal. Wkly. Rep 2019;68:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Goniewicz ML, Gawron M, Smith DM, et al. Exposure to Nicotine and Selected Toxicants in Cigarette Smokers Who Switched to Electronic Cigarettes: A Longitudinal Within-Subjects Observational Study. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2017;19:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Goniewicz ML, Smith DM, Edwards KC, et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw. Open 2018;1:e185937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shahab L, Goniewicz ML, Blount BC, et al. Nicotine, Carcinogen, and Toxin Exposure in Long-Term E-Cigarette and Nicotine Replacement Therapy Users: A Cross-sectional Study. Ann. Intern. Med 2017;166:390–400.**A comprehensive book published by the National Academies of Science, Engineering, and Medicine regarding the public health impact of e-cigarettes.
- [12].National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, et al. Public Health Consequences of E-Cigarettes [Internet]. Eaton DL, Kwan LY, Stratton K, editors. Washington (DC): National Academies Press (US); 2018. [cited 2019 May 23]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK507171/. [PubMed] [Google Scholar]
- [13].Mirbolouk M, Charkhchi P, Kianoush S, et al. Prevalence and Distribution of E-Cigarette Use Among U.S. Adults: Behavioral Risk Factor Surveillance System, 2016. Ann. Intern. Med 2018;169:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Soneji S, Barrington-Trimis JL, Wills TA, et al. Association Between Initial Use of e-Cigarettes and Subsequent Cigarette Smoking Among Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017;171:788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Health C for HP and EO on S and, General USPHSO of the S. The Health Consequences of Smoking: Nicotine Addiction: A Report of the Surgeon General [Internet]. DHHS Publ. No CDC 88–8406. 1988. [cited 2019 May 23]. Available from: https://profiles.nlm.nih.gov/NN/B/B/Z/D/.
- [16].Benowitz NL, Hukkanen J, Jacob P. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol 2009;29–60.*Overview of important chemical and pharmacological aspects of nicotine.
- [17].Lisko JG, Tran H, Stanfill SB, et al. Chemical Composition and Evaluation of Nicotine, Tobacco Alkaloids, pH, and Selected Flavors in E-Cigarette Cartridges and Refill Solutions. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2015;17:1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].El-Hellani A, El-Hage R, Baalbaki R, et al. Free-Base and Protonated Nicotine in Electronic Cigarette Liquids and Aerosols. Chem. Res. Toxicol 2015;28:1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].St Helen G, Dempsey DA, Havel CM, et al. Impact of e-liquid flavors on nicotine intake and pharmacology of e-cigarettes. Drug Alcohol Depend. 2017;178:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stepanov I, Fujioka N. Bringing attention to e-cigarette pH as an important element for research and regulation. Tob. Control 2015;24:413–414. [DOI] [PubMed] [Google Scholar]
- [21].Pankow JF. A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem. Res. Toxicol 2001;14:1465–1481. [DOI] [PubMed] [Google Scholar]
- [22].Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev 2005;57:79–115. [DOI] [PubMed] [Google Scholar]
- [23].Talih S, Balhas Z, Salman R, et al. Transport phenomena governing nicotine emissions from electronic cigarettes: model formulation and experimental investigation. Aerosol Sci. Technol. J. Am. Assoc. Aerosol Res 2017;51:1–11.*This article provides information on the emissions of e-cigarettes based on many different factors. It also derives and tests a mathematical model for predicting nicotine yield in emissions.
- [24].Hutzler C, Paschke M, Kruschinski S, et al. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch. Toxicol 2014;88:1295–1308. [DOI] [PubMed] [Google Scholar]
- [25].Bekki K, Uchiyama S, Ohta K, et al. Carbonyl compounds generated from electronic cigarettes. Int. J. Environ. Res. Public. Health 2014;11:11192–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].El-Hellani A, Salman R, El-Hage R, et al. Nicotine and Carbonyl Emissions From Popular Electronic Cigarette Products: Correlation to Liquid Composition and Design Characteristics. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2018;20:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Talhout R, Schulz T, Florek E, et al. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public. Health 2011;8:613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Etter J-F, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addict. Abingdon Engl 2011;106:2017–2028. [DOI] [PubMed] [Google Scholar]
- [29].Marynak KL, Gammon DG, Rogers T, et al. Sales of Nicotine-Containing Electronic Cigarette Products: United States, 2015. Am. J. Public Health 2017;107:702–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cheng T Chemical evaluation of electronic cigarettes. Tob. Control 2014;23 Suppl 2:ii11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Goniewicz ML, Gupta R, Lee YH, et al. Nicotine levels in electronic cigarette refill solutions: A comparative analysis of products from the U.S., Korea, and Poland. Int. J. Drug Policy 2015;26:583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tierney PA, Karpinski CD, Brown JE, et al. Flavour chemicals in electronic cigarette fluids. Tob. Control 2016;25:e10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Davis B, Dang M, Kim J, et al. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2015;17:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Etter J-F, Bugey A. E-cigarette liquids: Constancy of content across batches and accuracy of labeling. Addict. Behav 2017;73:137–143. [DOI] [PubMed] [Google Scholar]
- [35].Kim S, Goniewicz ML, Yu S, et al. Variations in label information and nicotine levels in electronic cigarette refill liquids in South Korea: regulation challenges. Int. J. Environ. Res. Public. Health 2015;12:4859–4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Raymond BH, Collette-Merrill K, Harrison RG, et al. The Nicotine Content of a Sample of E-cigarette Liquid Manufactured in the United States. J. Addict. Med 2018;12:127–131. [DOI] [PubMed] [Google Scholar]
- [37].St Helen G, Havel C, Dempsey DA, et al. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addict. Abingdon Engl 2016;111:535–544.*Study of multiple e-cigarette types and nicotine delivery.
- [38].Bowen A, XING C. Nicotine salt formulations for aerosol devices and methods thereof [Internet]. 2018. [cited 2019 May 23]. Available from: https://patents.google.com/patent/AU2014262808B2/en?q=nicotine&q=salf&q=formulations&q=aerosol&q=devices&q=methods&q=therof&oq=nicotine+salf+formulations+for+aerosol+devices+and+methods+therof.
- [39].Goniewicz ML, Boykan R, Messina CR, et al. High exposure to nicotine among adolescents who use Juul and other vape pod systems (‘pods’). Tob. Control 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jackler RK, Ramamurthi D Nicotine arms race: JUUL and the high-nicotine product market. Tob. Control 2019; [DOI] [PubMed] [Google Scholar]
- [41].Talih S, Salman R, El-Hage R, et al. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob. Control 2019;*To the best of the authors knowledge, one of the only current publications available that examines JUUL emissions.
- [42].Kim JW, Baum CRM. Liquid Nicotine Toxicity. [Review]. Pediatr. Emerg. Care 2015;31:517–521. [DOI] [PubMed] [Google Scholar]
- [43].Cameron JM, Howell DN, White JR, et al. Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tob. Control 2014;23:77–78. [DOI] [PubMed] [Google Scholar]
- [44].Talih S, Balhas Z, Eissenberg T, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2015;17:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2013;15:158–166. [DOI] [PubMed] [Google Scholar]
- [46].Tayyarah R, Long GA. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul. Toxicol. Pharmacol. RTP 2014;70:704–710. [DOI] [PubMed] [Google Scholar]
- [47].Havel CM, Benowitz NL, Jacob P, et al. An Electronic Cigarette Vaping Machine for the Characterization of Aerosol Delivery and Composition. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2017;19:1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Farsalinos KE, Yannovits N, Sarri T, et al. Protocol proposal for, and evaluation of, consistency in nicotine delivery from the liquid to the aerosol of electronic cigarettes atomizers: regulatory implications. Addict. Abingdon Engl 2016;111:1069–1076. [DOI] [PubMed] [Google Scholar]
- [49].Mikheev VB, Brinkman MC, Granville CA, et al. Real-Time Measurement of Electronic Cigarette Aerosol Size Distribution and Metals Content Analysis. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2016;18:1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Prévôt N, de Oliveira F, Perinel-Ragey S, et al. Nicotine delivery from the refill liquid to the aerosol via high-power e-cigarette device. Sci. Rep 2017;7:2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zervas E, Litsiou E, Konstantopoulos K, et al. Physical characterization of the aerosol of an electronic cigarette: impact of refill liquids. Inhal. Toxicol 2018;1–6. [DOI] [PubMed] [Google Scholar]
- [52].Czogala J, Goniewicz ML, Fidelus B, et al. Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2014;16:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 2014;23:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2014;16:1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Reilly SM, Bitzer ZT, Goel R, et al. Free Radical, Carbonyl, and Nicotine Levels Produced by Juul Electronic Cigarettes. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Patterson F, Benowitz N, Shields P, et al. Individual differences in nicotine intake per cigarette. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol 2003;12:468–471. [PubMed] [Google Scholar]
- [57].St Helen G, Ross KC, Dempsey DA, et al. Nicotine Delivery and Vaping Behavior During ad Libitum E-cigarette Access. Tob. Regul. Sci 2016;2:363–376.*Article discusses the differences in nicotine delivery between regulated and ad lib use, which is an important area of study.
- [58].Bullen C, McRobbie H, Thornley S, et al. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob. Control 2010;19:98–103. [DOI] [PubMed] [Google Scholar]
- [59].Vansickel AR, Cobb CO, Weaver MF, et al. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol 2010;19:1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nides MA, Leischow SJ, Bhatter M, et al. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. Am. J. Health Behav 2014;38:265–274. [DOI] [PubMed] [Google Scholar]
- [61].Yan XS, D’Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regul. Toxicol. Pharmacol. RTP 2015;71:24–34. [DOI] [PubMed] [Google Scholar]
- [62].Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2013;15:267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Farsalinos KE, Spyrou A, Tsimopoulou K, et al. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci. Rep 2014;4:4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hajek P, Przulj D, Phillips A, et al. Nicotine delivery to users from cigarettes and from different types of e-cigarettes. Psychopharmacology (Berl.). 2017;234:773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Voos N, Kaiser L, Mahoney MC, et al. Randomized within-subject trial to evaluate smokers’ initial perceptions, subjective effects and nicotine delivery across six vaporized nicotine products. Addict. Abingdon Engl 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Maloney S, Soule E, Crabtree M, et al. Comparison of Nicotine Delivery and Subjective Effects Profile of JUUL, IQOS, and Combustible Cigarettes. San Francisco, CA; 2019. [Google Scholar]
- [67].Wynne C, Waaka D, Cohen G. Acute Use of Nicotine Salt-based ENDS and Combusted Cigarettes. Baltimore, MD; 2018. [Google Scholar]
- [68].Benowitz NL, Nardone N, Jain S, et al. Comparison of Urine 4-(Methylnitrosamino)-1-(3)Pyridyl-1-Butanol and Cotinine for Assessment of Active and Passive Smoke Exposure in Urban Adolescents. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol 2018;27:254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob. Control 2017;26:e23–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ramôa CP, Hiler MM, Spindle TR, et al. Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: a preliminary report. Tob. Control 2016;25:e6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lopez AA, Hiler MM, Soule EK, et al. Effects of Electronic Cigarette Liquid Nicotine Concentration on Plasma Nicotine and Puff Topography in Tobacco Cigarette Smokers: A Preliminary Report. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2016;18:720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hiler M, Breland A, Spindle T, et al. Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: Influence of liquid nicotine concentration and user experience. Exp. Clin. Psychopharmacol 2017;25:380–392.*A thorough study that examines the nicotine delivery potential of liquids of different nicotine concentration among experienced and inexperienced e-cigarette users.
- [73].Farsalinos KE, Spyrou A, Stefopoulos C, et al. Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naïve users (smokers). Sci. Rep 2015;5:11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lee YH, Gawron M, Goniewicz ML. Changes in puffing behavior among smokers who switched from tobacco to electronic cigarettes. Addict. Behav 2015;48:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hajek P, Goniewicz ML, Phillips A, et al. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2015;17:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Schneller LM, Bansal-Travers M, Goniewicz ML, et al. Use of flavored electronic cigarette refill liquids among adults and youth in the US-Results from Wave 2 of the Population Assessment of Tobacco and Health Study (2014–2015). PloS One. 2018;13:e0202744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zhu S-H, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob. Control 2014;23 Suppl 3:iii3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kong G, Morean ME, Cavallo DA, et al. Reasons for Electronic Cigarette Experimentation and Discontinuation Among Adolescents and Young Adults. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2015;17:847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Spindle TR, Hiler MM, Breland AB, et al. The Influence of a Mouthpiece-Based Topography Measurement Device on Electronic Cigarette User’s Plasma Nicotine Concentration, Heart Rate, and Subjective Effects Under Directed and Ad Libitum Use Conditions. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2017;19:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].St Helen G, Shahid M, Chu S, et al. Impact of e-liquid flavors on e-cigarette vaping behavior. Drug Alcohol Depend. 2018;189:42–48.*To the best of the authors’ knowledge, one of the only available articles that examines nicotine delivery based on e-cigarette liquid flavor.
- [81].Dawkins L, Cox S, Goniewicz M, et al. “Real-world” compensatory behaviour with low nicotine concentration e-liquid: subjective effects and nicotine, acrolein and formaldehyde exposure. Addict. Abingdon Engl 2018;113:1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Dawkins LE, Kimber CF, Doig M, et al. Self-titration by experienced e-cigarette users: blood nicotine delivery and subjective effects. Psychopharmacology (Berl.). 2016;233:2933–2941. [DOI] [PubMed] [Google Scholar]
- [83].Etter J-F. A longitudinal study of cotinine in long-term daily users of e-cigarettes. Drug Alcohol Depend. 2016;160:218–221. [DOI] [PubMed] [Google Scholar]
- [84].Kosmider L, Kimber CF, Kurek J, et al. Compensatory Puffing With Lower Nicotine Concentration E-liquids Increases Carbonyl Exposure in E-cigarette Aerosols. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2018;20:998–1003. [DOI] [PubMed] [Google Scholar]
- [85].Farsalinos K, Poulas K, Voudris V. Changes in Puffing Topography and Nicotine Consumption Depending on the Power Setting of Electronic Cigarettes. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2018;20:993–997. [DOI] [PubMed] [Google Scholar]
- [86].Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet Lond. Engl 2013;382:1629–1637. [DOI] [PubMed] [Google Scholar]
- [87].Schoenborn CA, Gindi RM. Electronic Cigarette Use Among Adults: United States, 2014. NCHS Data Brief 2015;1–8. [PubMed] [Google Scholar]
- [88].QuickStats: Cigarette Smoking Status* Among Current Adult E-cigarette Users,† by Age Group - National Health Interview Survey,§ United States, 2015. MMWR Morb. Mortal. Wkly. Rep 2016;65:1177. [DOI] [PubMed] [Google Scholar]
- [89].Hajek P, Phillips-Waller A, Przulj D, et al. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. N. Engl. J. Med 2019;380:629–637.*New study concluding that e-cigarettes can be effective for smoking cessation versus NRT.
- [90].Morean ME, Kong G, Cavallo DA, et al. Nicotine concentration of e-cigarettes used by adolescents. Drug Alcohol Depend. 2016;167:224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Willett JG, Bennett M, Hair EC, et al. Recognition, use and perceptions of JUUL among youth and young adults. Tob. Control. 2019;28:115–116. [DOI] [PubMed] [Google Scholar]
- [92].Raez-Villanueva S, Ma C, Kleiboer S, et al. The effects of electronic cigarette vapor on placental trophoblast cell function. Reprod. Toxicol. Elmsford N 2018;81:115–121. [DOI] [PubMed] [Google Scholar]
- [93].Ganapathy V, Manyanga J, Brame L, et al. Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PloS One. 2017;12:e0177780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Shaito A, Saliba J, Husari A, et al. Electronic Cigarette Smoke Impairs Normal Mesenchymal Stem Cell Differentiation. Sci. Rep 2017;7:14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Crotty Alexander LE, Drummond CA, Hepokoski M, et al. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 2018;314:R834–R847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Qasim H, Karim ZA, Silva-Espinoza JC, et al. Short-Term E-Cigarette Exposure Increases the Risk of Thrombogenesis and Enhances Platelet Function in Mice. J. Am. Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Olfert IM, DeVallance E, Hoskinson H, et al. Chronic exposure to electronic cigarettes results in impaired cardiovascular function in mice. J. Appl. Physiol. Bethesda Md 1985 2018;124:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Shihadeh A, Eissenberg T. Electronic cigarette effectiveness and abuse liability: predicting and regulating nicotine flux. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2015;17:158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Eissenberg T, Shihadeh A. Nicotine flux: a potentially important tool for regulating electronic cigarettes. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 2015;17:165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]