Abstract
Several hypothalamic neuronal populations are directly responsive to growth hormone (GH) and central GH action regulates glucose and energy homeostasis. However, the potential role of GH signaling in proopiomelanocortin (POMC) neurons has not been studied yet. Thus, we investigated whether POMC neurons are responsive to GH and if ablation of GH receptor (GHR) or STAT5 in POMC cells leads to metabolic imbalances. Approximately 60% of POMC neurons of the arcuate nucleus exhibited STAT5 phosphorylation after intracerebroventricular GH injection. Ablation of GHR or STAT5 in POMC cells did not affect energy or glucose homeostasis. However, glucoprivic hyperphagia was blunted in male and female GHR knockout mice, and in male POMC-specific STAT5 knockout mice. Additionally, the absence of GHR in POMC neurons decreased glycemia during prolonged food restriction in male mice. Thus, GH action in POMC neurons regulates glucoprivic hyperphagia as well as blood glucose levels during prolonged food restriction.
Keywords: energy metabolism, food restriction, glucose metabolism, hypoglycemia, melanocortin system, POMC
1. Introduction
The arcuate nucleus of the hypothalamus (ARH) contains well-known neuronal populations that regulate energy and glucose homeostasis, including orexigenic neurons that co-express the agouti-related protein (AgRP) and the neuropeptide Y (NPY), as well as anorexigenic neurons that express the proopiomelanocortin (POMC) prohormone (Andermann and Lowell, 2017; Ramos-Lobo and Donato, 2017). POMC is cleaved in bioactive peptides, including α-melanocyte-stimulating hormone (α-MSH), which activates melanocortin receptors 3 and 4 to induce satiety (Coll and Loraine Tung, 2009; Rossi et al., 1998). A key feature of ARH neurons is the capacity to detect different signals that regulate their activity and neuropeptide expression. In this sense, hormones that decrease food intake, such as leptin, insulin or cholecystokinin activate POMC neurons and inhibit AgRP/NPY neurons. On the other hand, humoral factors that induce food intake, i.e. ghrelin, increase the activity of AgRP/NPY neurons (Andermann and Lowell, 2017; Ramos-Lobo and Donato, 2017; Williams et al., 2010). Therefore, POMC and AgRP/NPY ARH neurons regulate food intake and other metabolic aspects through inputs generated by multiple hormonal receptors expressed by these cells.
Growth hormone (GH) is produced by somatotrophs of the pituitary gland. Its classical functions are related to cell proliferation, metabolism, and tissue and somatic growth (Dehkhoda et al., 2018). Interestingly, several pieces of evidence indicate that GH can affect neural circuits that regulate energy metabolism (Kim et al., 2015). For example, GH overexpression in the central nervous system causes hyperphagia and obesity in mice (Bohlooly et al., 2005). Additionally, GH receptor (GHR) deficient mice exhibit blunted feeding response to ghrelin (Egecioglu et al., 2006). Transgenic carp overexpressing GH presents increased food intake associated with higher Agrp hypothalamic expression (Zhong et al., 2013). A recent study also showed that intracerebroventricular (icv) injection of GH increases feeding in mice, inducing upregulation of Agrp and Npy expression in the hypothalamus (Furigo et al., 2019b). Furthermore, GH induces depolarization in a subset of ARH AgRP neurons (Furigo et al., 2019b). Therefore, GH has central orexigenic effects via activation of AgRP/NPY neurons. Accordingly, 95% of ARH AgRP/NPY neurons express the Ghr mRNA (Kamegai et al., 1996) or exhibit phosphorylation of the signal transducer and activator of transcription 5 (pSTAT5) after an acute GH injection (Furigo et al., 2019b).
Despite the evidence that GH acts directly in AgRP/NPY neurons to regulate food intake and energy expenditure, no information exists about the potential role of GH signaling in POMC neurons. Of note, POMC and AgRP/NPY neurons express many hormone receptors concomitantly (Andermann and Lowell, 2017; Ramos-Lobo and Donato, 2017), which makes it plausible to hypothesize that GH may also modulate the metabolism via ARH POMC neurons. Therefore, the objectives of the present study were to determine 1) whether POMC neurons are directly responsive to GH, 2) if ablation of GHR specifically in POMC cells leads to changes in energy and glucose homeostasis, and 3) the consequences of Stat5a/b ablation in POMC neurons since the JAK2/STAT5 pathway is considered the major intracellular signaling pathway induced by the GH/GHR interaction (Dehkhoda et al., 2018; Furigo et al., 2016; Teglund et al., 1998).
2. Materials and methods
2.1. Mice
Genetic deletions of Ghr or Stat5a/b genes in POMC cells were obtained through the breeding of POMC-Cre mouse (Stock No: 005965; The Jackson Laboratory) with animals carrying loxP-flanked Ghr (Furigo et al., 2019b; List et al., 2013) or Stat5a/b alleles (Buonfiglio et al., 2015; Furigo et al., 2018). The Cre expression pattern during development in the POMC-Cre mice has been characterized in previous studies (Padilla et al., 2010; Padilla et al., 2012). All POMC GHR KO and POMC STAT5 KO mice carried the POMC-Cre transgene and were homozygous for the loxP-flanked alleles, whereas control animals were littermates negative for the Cre transgene. To determine whether POMC neurons in the nucleus of the solitary tract (NTS) are responsive to GH, a POMC reporter mouse was produced by breeding the POMC-Cre mouse with the Cre-inducible enhanced GFP-reporter mouse (Stock No: 004178; The Jackson Laboratory). Animals were weaned at 21 days of age and tails were collected for DNA extraction. A conventional PCR was performed on genomic DNA, and PCR products were resolved by agarose gel electrophoresis for identification of mouse genotypes. Mice were maintained in standard conditions of 12h light/dark cycles (lights on at 8:00 am) and controlled temperature (22-24°C), and received potable water and regular chow adlibitum (2.99 kcal/g; 9.4% calories from fat; Quimtia, Brazil), unless described during experiments. The experiments were conducted in male and female mice, according to Ethics Committee on the Use of Animals of the Institute of Biomedical Sciences at the University of São Paulo (protocol #73/2017).
2.2. Evaluation of GH responsive cells in the brain
To determine whether POMC neurons are responsive to GH and to confirm the ablation of GHR/STAT5 in POMC-expressing cells, control (n = 6), POMC GHR KO (n = 3) and POMC STAT5 KO (n = 3) male mice received icv cannulas (Plastic One, USA). After approximately 5 days of recovery, mice received an acute icv injection of porcine GH (6 μg in 2 μl) obtained from Dr. A.F. Parlow (National Hormone and Peptide Program, USA) and after 30 minutes were anesthetized with isoflurane and transcardially perfused with saline followed by 10% buffered formalin solution (~180 mL/mouse). A group of control male mice (n = 4) received an acute icv injection of PBS (2 pl) to determine the basal presence of pSTAT5 immunoreactive cells in the hypothalamus. Collected brains were maintained for 1 hour in formalin for post-fixation, and were transferred to 20% sucrose solution overnight. Brains were cut in a freezing microtome in 30 μm thickness sections and maintained in cryoprotection buffer at −20°C. For double-labeled pSTAT5/α-MSH immunofluorescent reactions, sections were rinsed in 0.02 M potassium PBS, pH 7.4 (KPBS) buffer and incubated for 20 minutes in water solution containing 1% hydrogen peroxide and 1% sodium hydroxide. Sections were rinsed and incubated for 10 min in 0.3% glycine and then 10 min in 0.03% SDS solution. Primary rabbit pSTAT5 antibody (1:1000; Cell Signaling; #9351) and sheep aMSH antibody (1:4000; Millipore, USA #AB5087) were diluted in KPBS buffer containing sodium azide and Triton for 48 hours at 4°C. Subsequently, sections were rinsed in KPBS and incubated in AlexaFluor 488- and 594-conjugated secondary antibodies raised in donkey (1:500, Jackson ImmunoResearch Laboratories) for 90 min at room temperature. Slices were mounted in gelatin-coated slides and covered with Fluoromount G mounting medium (E.M.S.). Photomicrographs were acquired with Zeiss Axiocam HRc camera coupled to a Zeiss Axioimager A1 microscope connected to Axiovision software (Zeiss, Munich, Germany). The number of pSTAT5 or double-labeled pSTAT5/α-MSH cells was counted in two representative rostrocaudal levels of the ARH (Bregma −1.46 and −1.70).
2.3. Analysis of neuronal fiber density
Brain sections of 4 months old control, POMC GHR KO and POMC STAT5 KO male mice were obtained as described in section 2.2. Brain sections were subjected to immunofluorescence staining in order to evaluate the integrated optical density (IOD) of POMC or AgRP immunoreactive fibers in brain areas that receive dense innervation from ARH neurons (Bouret et al., 2004; Lima et al., 2016), including the paraventricular nucleus of the hypothalamus (PVH), lateral hypothalamic area (LHA) and dorsomedial nucleus of the hypothalamus (DMH). Brain sections were rinsed in KPBS, followed by incubation in 3% normal donkey serum for 1 hour. Next, sections were incubated overnight in ant-β-endorphin antisera (1:2,000; Phoenix Pharmaceuticals), since β-endorphin is a POMC-derived peptide, or anti-AgRP antisera (1:2,000, Phoenix Pharmaceuticals, Inc.). Subsequently, sections were incubated for 90 min in Alexa Fluor488-conjugated secondary antibody (Jackson ImmunoResearch Laboratories). After rinsing in KPBS, sections were mounted onto gelatin-coated slides and covered using Fluoromount G. The IOD obtained in the PVH, LHA and DMH was analyzed using the ImageJ software (http://rsb.info.nih.gov/ij) and subtracted from the IOD assessed in adjacent areas with low staining (background). One representative rostrocaudal level of LHA (Bregma −1.22) and DMH (Bregma −1.82), and two rostrocaudal levels of PVH were analyzed (Bregma −0.70 and −0.94). The number of POMC neurons in the ARH was also determined (Bregma −1.46 and −1.70).
2.4. Energy metabolism and hormonal analyses
Body weight was recorded weekly for 25-30 weeks. Then, body composition (lean/fat mass) was obtained by time-domain nuclear magnetic resonance using the LF50 body composition mice analyzer (Bruker, Germany). Next, mice were separated in individual cages to determine basal food intake for 2 days. Mice were then placed in the Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, OH, USA) for oxygen consumption (VO2), carbon dioxide production, respiratory exchange rate (RER) and locomotor activity measurements. VO2 was normalized by total body weight. Animals were allowed to adapt to the CLAMS for 2-3 days, followed by 3 days of assessments. Results are expressed as average values of these days. After all in vivo tests, blood was collected in the middle of the light cycle (at 1:00-3:00 pm; lights on at 8 am) to determine serum leptin and corticosterone levels using ELISA assays. Leptin (Quantikine®ELISA #MOB00, R&D, USA) and corticosterone (Arbor Assays, #K014, USA) ELISA kits have a detection limit of 22 pg/mL and 18.6 pg/mL, and an intra- and inter-assay coefficient of variability ≤ 6% and 8%, respectively.
2.5. Glucose metabolism and glucoprivic hyperphagia analyses
The glucose tolerance tests (GTT) and insulin tolerance tests (ITT) were performed in single-housed mice between 1:00-3:00 pm. Food was removed from cage at 10:00 am, and 4h later mice received an intraperitoneal (i.p.) injection of glucose (2 g/kg b.w.) or insulin (1 IU/kg b.w.) and glycemia was evaluated after 20, 40, 60, 90 and 120 minutes. The ITT was performed after 3-4 days of recovery from the GTT. To evaluate the counter-regulatory response to hypoglycemia, mice received an i.p. injection of 2-deoxy-D-glucose (2DG; 0.5 mg/kg b.w.) at 10:00 am and glycemia was measured 0, 20, 40, 60, 90, 120 and 180 minutes afterwards. The 2DG-induced hyperphagia was also evaluated at 10:00 am by recording the cumulative food intake 2, 3 and 4h after PBS or 2DG i.p. injections.
2.6. Energy and glucose metabolism analysis during food restriction
The protocol of food restriction consisted in offering 40% of basal food intake for 5 consecutive days 2 h before lights off. The body weight, body composition and glycemia were determined at baseline and daily during food restriction, at the time food was provided.
2.7. Statistical analysis
Data were expressed as mean ± s.e.m. and GraphPad Prism software was used for analysis. Differences in the number of pSTAT5 positive cells among PBS-injected mice, GH-injected control mice and GH-injected POMC GHR KO mice were determined by one-way ANOVA and Newman-Keuls multiple comparison post-hoc test. Changes in body weight, body composition, blood glucose levels (GTT, ITT, 2DG test and during food restriction), VO2 and RER along time were analyzed by repeated measures two-way ANOVA and the contrasts identified by Bonferroni’s multiple comparisons test. The possible differences in 2DG-induced hyperphagia were determined individually for each time point (2h, 3h and 4h) using repeated measures two-way ANOVA and Bonferroni’s multiple comparisons test since the same animals received PBS and 2DG injections in different days. The remaining comparisons between the control and the conditional KO group were performed using two-tailed Student’s t-test. Differences were considered significant if P ≤ 0.05.
3. Results
3.1. POMC neurons are responsive to GH
To determine whether POMC neurons express functional GHR and therefore can exhibit pSTAT5 after an acute GH injection (Furigo et al., 2017), double-labeled pSTAT5/α-MSH immunofluorescent reactions were performed in mice receiving an icv GH injection. GH injection increased pSTAT5 in both POMC and non-POMC cells in the ARH of control mice (Fig. 1A–C). Of note, 64% of ARH POMC neurons were responsive to GH (Fig. 1A–C). As expected, an acute GH injection also induced pSTAT5 in the ARH of POMC GHR KO mice. However, the number of double-labeled cells was significantly reduced in the conditional knockout mice, compared to GH-injected control animals, whereas the number of non-POMC neurons expressing pSTAT5 was unchanged (Fig. 1A, D–E). PBS-injected mice showed a smaller amount of pSTAT5 positive cells in the ARH, mostly restricted to non-POMC cells (Fig. 1A, F–G). Thus, our genetic ablation was efficient in reducing significantly the number of POMC neurons that are responsive to GH in the ARH. POMC neurons are also found in the NTS (Ellacott et al., 2006). To determine whether NTS POMC neurons are responsive to GH, POMC reporter mice received GH injection. We found that POMC neurons in the NTS are not responsive to GH (Supplementary Fig. 1). Since POMC expression is also found in pituitary corticotropic cells, serum corticosterone levels were determined. Control and POMC GHR KO mice showed similar serum corticosterone levels (Control: 163 ± 15 ng/mL; POMC GHR KO: 183 ± 23 ng/mL; P = 0.4759), indicating that GHR ablation in POMC cells did not affect glucocorticoid secretion.
Fig. 1. POMC neurons in the arcuate nucleus are responsive to GH.
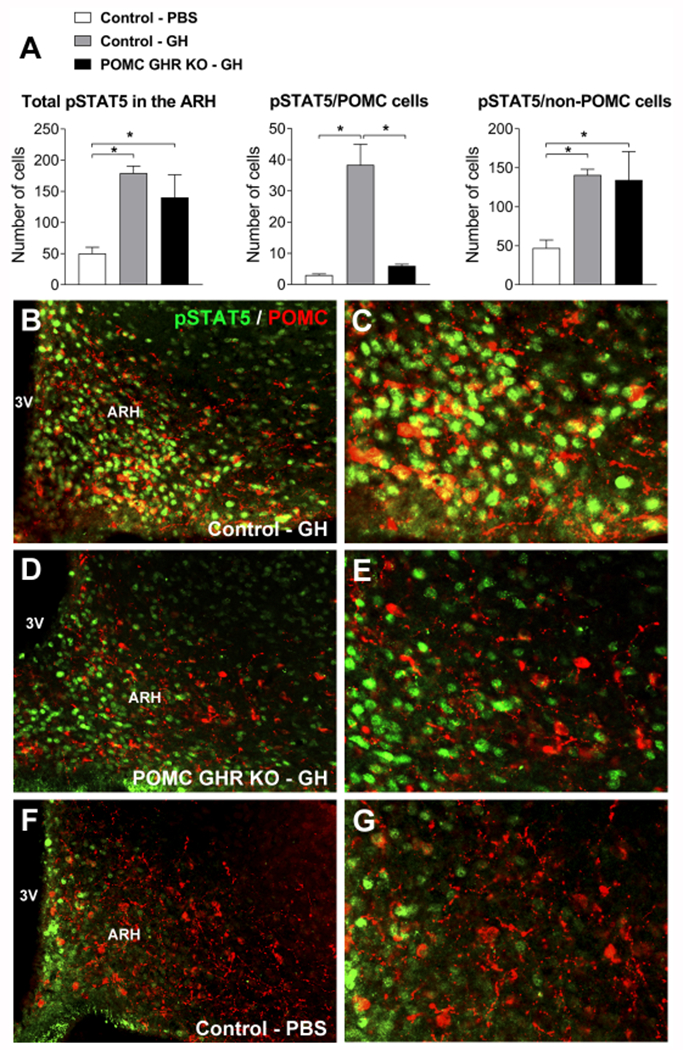
A. Bar graphs showing the number of pSTAT5 cells in the ARH as well as the number of pSTAT5 cells that are POMC and non-POMC in PBS-injected control mice (n = 4), GH-injected control mice (n = 6) and GH-injected POMC GHR KO mice (n = 3). B-G. Epifluorescence photomicrographs showing double-labeling immunofluorescence staining of pSTAT5 (green) and POMC (red) in the arcuate nucleus of the hypothalamus (ARH) after 30 minutes of an icv injection of porcine GH (6 μg in 2 μL) in control mice (B-C), POMC GHR KO mice (D-E) or PBS-injected control mice (F-G). Arrowheads indicate double-labeled neurons. Abbreviation: 3V, third ventricle. Scale bars: B = 100 μm; C = 50 μm. Comparisons between groups were performed using one-way ANOVA and Newman-Keuls multiple comparison post-hoc test. *, P < 0.05.
3.2. Neuronal projections of POMC neurons are not affected by POMC-specific GHR disruption
GH deficient mice or global GHR KO mice exhibit disrupted formation of the projections from POMC and AgRP neurons to key target areas, such as the PVH and DMH (Sadagurski et al., 2015). Therefore, before evaluating possible metabolic changes in POMC GHR KO mice, we determined whether POMC-specific GHR ablation can affect the development of these neuronal projections. Control and POMC GHR KO male mice showed a similar number of POMC cells in the ARH (Fig. 2A and Fig. 3A,E). Additionally, no significant differences between control and POMC GHR KO male mice were observed in the density of POMC innervation in the PVH, LHA and DMH (Fig. 2B–D and Fig. 3B–D, F–H). AgRP innervation was also assessed in these brain areas, but no changes between groups were observed (Fig. 2E–G and Fig. 3I–N). Thus, GH signaling specifically in POMC neurons is not required for the development of the axonal projections of ARH neurons.
Fig. 2. Axonal projections of arcuate nucleus neurons.
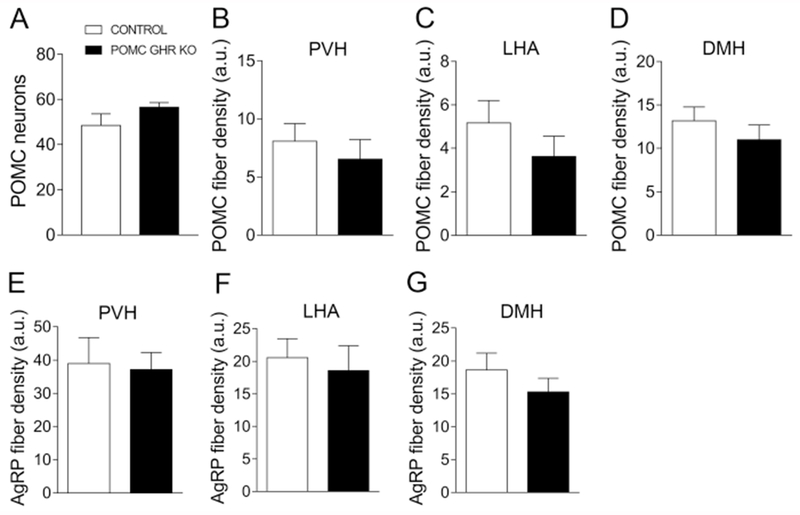
A. Bar graph comparing the number of POMC positive cells in the arcuate nucleus of control (n = 3) and POMC GHR KO male mice (n = 6). B-D. Bar graphs comparing POMC fiber density in the PVH (B), LHA (C) and DMH (D) of control (n = 3) and POMC GHR KO male mice (n = 6). E-G. Bar graphs comparing AgRP fiber density in the PVH (E), LHA (F) and DMH (G) of control (n = 3) and POMC GHR KO male mice (n = 6). Comparisons between groups were performed using two-tailed Student’s t-test.
Fig. 3. Neuronal projections of the arcuate nucleus are not affected by POMC-specific GHR disruption.
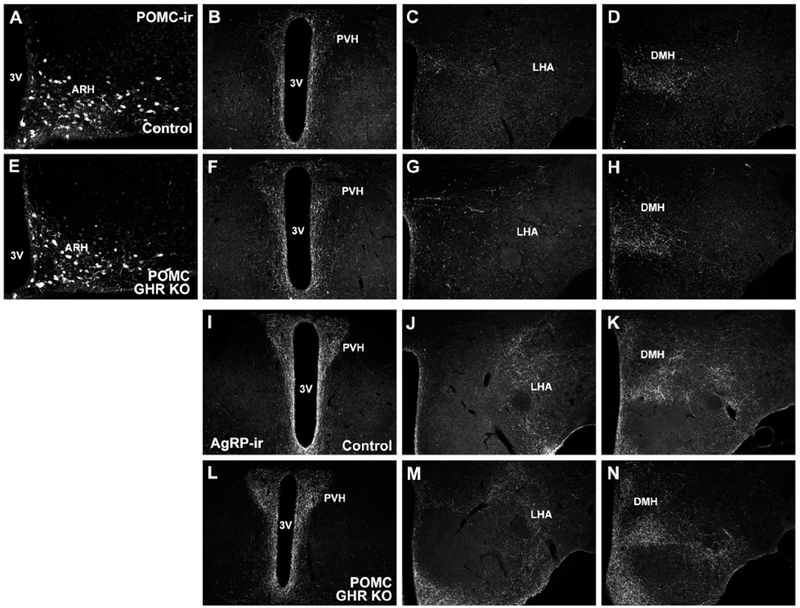
A. Representative photomicrograph showing POMC-immunoreactivity (POMC-ir) in ARH neurons of control mice. B-D. Representative photomicrographs showing POMC innervation in different hypothalamic areas of control mice. E. Representative photomicrograph showing POMC neurons in the ARH of POMC GHR KO mice. F-H. Representative photomicrographs showing POMC innervation in different hypothalamic areas of POMC GHR KO mice. I-N. Representative photomicrographs showing AgRP innervation in different hypothalamic areas of control (I-K) and POMC GHR KO mice (L-N). Abbreviations: 3V, third ventricle; ARH, arcuate nucleus of the hypothalamus; DMH, dorsomedial nucleus of the hypothalamus; LHA, lateral hypothalamic area; PVH, paraventricular nucleus of the hypothalamus. Scale bars: A = 100 μm; B = 200 μm.
3.3. GHR ablation in POMC neurons does not affect energy balance
POMC neurons play an important role in the neuroendocrine control of energy homeostasis (Andermann and Lowell, 2017; Berglund et al., 2012; Coll and Loraine Tung, 2009; Ramos-Lobo and Donato, 2017). Thus, we investigated whether GHR expression in POMC neurons is necessary for the maintenance of energy homeostasis. Ablation of GHR in POMC neurons caused no changes in body weight, lean body mass, body fat mass, food intake, energy expenditure (VO2), RER, ambulatory activity and serum leptin levels in male (Fig. 4A–H) or female mice (Fig. 4I–P).
Fig. 4. GHR ablation in POMC neurons does not affect the energy balance in male and female mice.
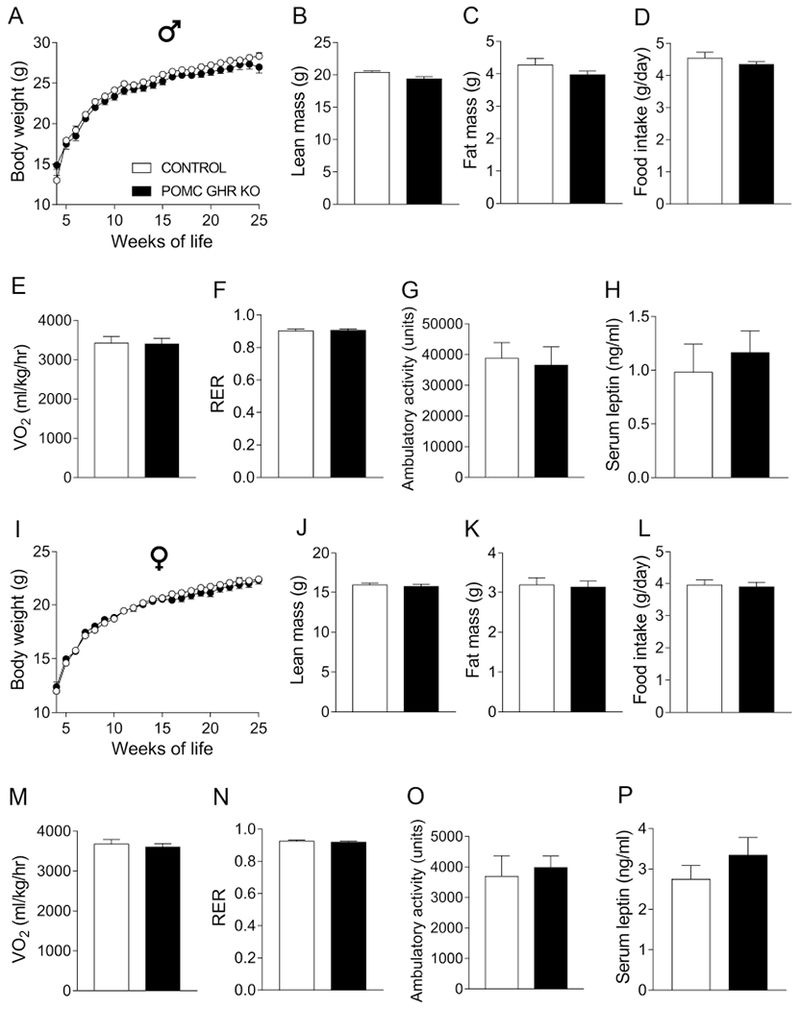
A-H. Body weight (A), lean body mass (B), body fat mass (C), daily food intake (D), oxygen consumption (VO2; E), respiratory exchange ratio (RER; F), ambulatory activity (G) and serum leptin levels (H) of control (n = 12-40) and POMC GHR KO (n = 16-26) male mice. I-P. Body weight (I), lean body mass (J), body fat mass (K), daily food intake (L), VO2 (M), RER (N), ambulatory activity (O) and serum leptin levels (P) of control (n = 16-42) and POMC GHR KO (n = 16-24) female mice. Body weight changes were analyzed by repeated measures two-way ANOVA and the remaining comparisons between groups were performed using two-tailed Student’s t-test.
3.4. Normal glucose homeostasis in POMC GHR KO mice
The melanocortin system, including ARH POMC neurons, is critical for the glucose homeostasis (Berglund et al., 2012; Huo et al., 2009; Rossi et al., 2011). However, GHR ablation in POMC neurons did not affect glucose tolerance or insulin sensitivity in male (Fig. 5A–B) or female mice in comparison to controls (Fig. 5E–F).
Fig. 5. Normal glucose homeostasis, but impaired 2DG-induced hyperphagia in POMC GHR KO mice.
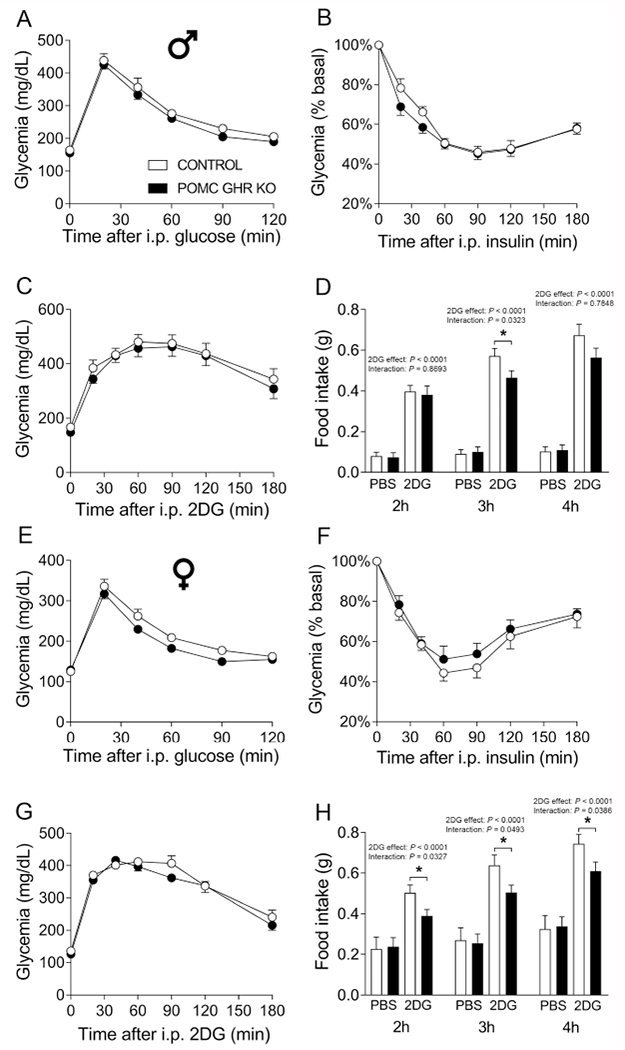
A-C. Blood glucose levels during a glucose tolerance test (GTT, A), insulin tolerance test (ITT, B) and counter-regulatory response induced by 2-deoxy-D-glucose (2DG) infusion (C) in control (n = 16) and POMC GHR KO (n = 16) male mice. D. cumulative food intake 2, 3 and 4 hours after i.p. injection of PBS or 2DG in control (n = 16) and POMC GHR KO (n = 16) male mice. E-G. Blood glucose levels during a GTT (E), ITT (F) and counter-regulatory response induced by 2DG infusion (G) in control (n = 16) and POMC GHR KO (n = 16) female mice. H. cumulative food intake 2, 3 and 4 hours after i.p. injection of PBS or 2DG in control (n = 16) and POMC GHR KO (n = 16) female mice. The data were analyzed by repeated measures two-way ANOVA and the contrasts identified by Bonferroni’s multiple comparisons test. *, P < 0.05 (interaction between 2DG and genotype in each time point).
3.5. Impaired 2DG-induced hyperphagia in POMC GHR KO mice
GH is considered an important counter-regulatory hormone secreted during hypoglycemia (Hussain et al., 2003; Roth et al., 1963; Tennese and Wevrick, 2011; Zhao et al., 2010). Thus, the counter-regulatory response induced by an acute administration of 2DG, a non-metabolized analog of glucose that mimics glucose-deprivation conditions, was evaluated. We found that 2DG infusion produced a similar counter-regulatory response in POMC GHR KO mice, compared to control animals, in both male (Fig. 5C) and female mice (Fig. 5G). The glucoprivic condition produced by 2DG infusion produces an acute hyperphagic behavior (Clegg et al., 2003; Ikeda et al., 1980; Luquet et al., 2007; Ozawa et al., 2011; Thompson et al., 1979). Therefore, the importance of GHR expression in POMC cells for glucoprivic hyperphagia was also evaluated (Fig. 5D and 5H). As expected, male and female control mice showed increased cumulative food intake 2, 3 and 4 hours after 2DG injection, compared to PBS infusion (2DG effect; P < 0.0001 in all time points). Remarkably, while male POMC GHR KO mice showed reduced food intake 3h after 2DG infusion (interaction between 2DG and genotype [F(1,27) = 5.0941, P = 0.0323]), compared to control males (Fig. 5D), POMC GHR KO females presented attenuated 2DG-induced hyperphagia at 2 hours (interaction between 2DG and genotype [F(1,26) = 5.088, P = 0.0327]), 3 hours (interaction between 2DG and genotype [F(1,26) = 4.255, P = 0.0493]) and 4 hours (interaction between 2DG and genotype [F(1,26) = 4.747, P = 0.0386]; Fig. 5H).
3.6. POMC GHR KO male mice exhibit decreased glycemia during prolonged food restriction in males
Circulating GH level increases during food deprivation and plays an important role maintaining glycemia (Zhao et al., 2010) and coordinating adaptive neuroendocrine responses that decrease energy expenditure during this situation (Furigo et al., 2019b). In this sense, we investigated whether GH signaling in POMC neurons is required for some metabolic adaptations that occur during food restriction. Male POMC GHR KO mice showed similar reduction in body weight and lean mass during food restriction, compared to control mice (Fig. 6A–B). However, male POMC GHR KO mice presented decreased glycemia after 5 days of food restriction (interaction between time and genotype [F(5,70) = 3.065, P = 0.0147]; Fig 6C–D). Oxygen consumption and RER were similar between groups during food restriction (Fig. 6E–F). In females, POMC GHR KO mice showed similar body weight, lean and fat mass loss and glycemia during food deprivation (Fig. 6G–J). The 24 h food intake after 5 days of food restriction was analyzed in ad libitum fed male and female mice, and no differences between control and POMC GHR KO mice were observed (Supplementary Fig. 2).
Fig. 6. Decreased glycemia during prolonged food restriction in POMC GHR KO mice.
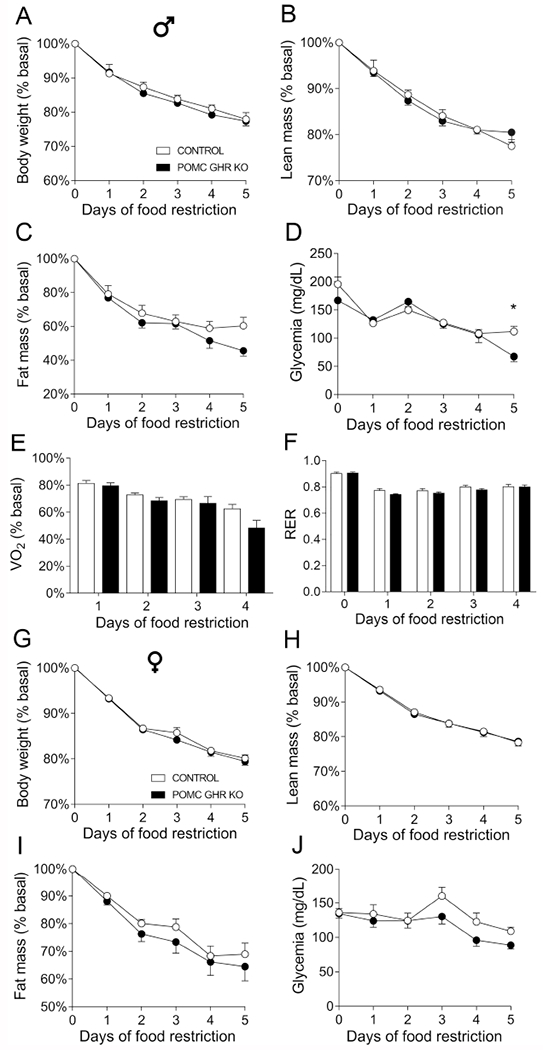
A-F. Changes in body weight (A), lean body mass (B), body fat mass (C), glycemia (D), oxygen consumption (VO2, E) and respiratory exchange ratio (RER, F) in control (n = 8) and POMC GHR KO (n = 8) male mice subjected to 5 days of 60% food restriction. G-J. Changes in body weight (A), lean body mass (B), body fat mass (C) and glycemia (D) in control (n = 7) and POMC GHR KO (n = 8) female mice subjected to 5 days of 60% food restriction. The data were analyzed by repeated measures two-way ANOVA. *, P < 0.05 (Bonferroni’s multiple comparisons test).
3.7. STAT5 ablation in POMC neurons marginally disturbs the energy balance
Inactivation of STAT5 signaling pathway in the central nervous system leads to metabolic imbalances and late-onset obesity (Lee et al., 2008). Additionally, STAT5 is a key downstream intracellular pathway recruited by GHR (Dehkhoda et al., 2018; Furigo et al., 2016; Teglund et al., 1998). Thus, we also investigated whether inactivation of Stat5a/b genes in POMC neurons produces metabolic imbalances. POMC-specific STAT5 knockout (POMC STAT5 KO) mice were produced (Supplementary Fig. 3), as previously described (Buonfiglio et al., 2015; Furigo et al., 2018; Silveira et al., 2017). In this case, while the total number of pSTAT5 cells in the ARH was increased in GH-injected POMC STAT5 KO mice (92 ± 13 cells), compared to PBS-injected mice (50 ± 10 cells; P < 0.05), the number of double-labeled pSTAT5/α-MSH cells remained similar (3.0 ± 0.4 vs. 3.7 ± 0.5; P = 0.32). Serum corticosterone levels were also evaluated and POMC STAT5 KO mice exhibited a tendency towards a higher value (150 ± 14 ng/mL), compared to control mice (96 ± 22 ng/mL; P = 0.0553). However, as seen in POMC GHR KO mice, POMC-specific STAT5 ablation caused no significant changes in the number of POMC neurons in the ARH or in POMC and AgRP innervation of the PVH, LHA and DMH (Supplementary Fig. 4 and Supplementary Fig. 5). POMC STAT5 KO mice also showed similar body weight, lean body mass, body fat mass, food intake and ambulatory activity, compared to control animals, in both males (Fig. 7A–D and 7F) and females (Fig. 7H–K and 7M). However, male POMC STAT5 KO mice exhibited higher serum leptin levels (Fig. 7G), whereas female POMC STAT5 KO mice showed increased energy expenditure, compared to control group (Fig. 7L).
Fig. 7. STAT5 ablation in POMC neurons marginally disturbs the energy balance.
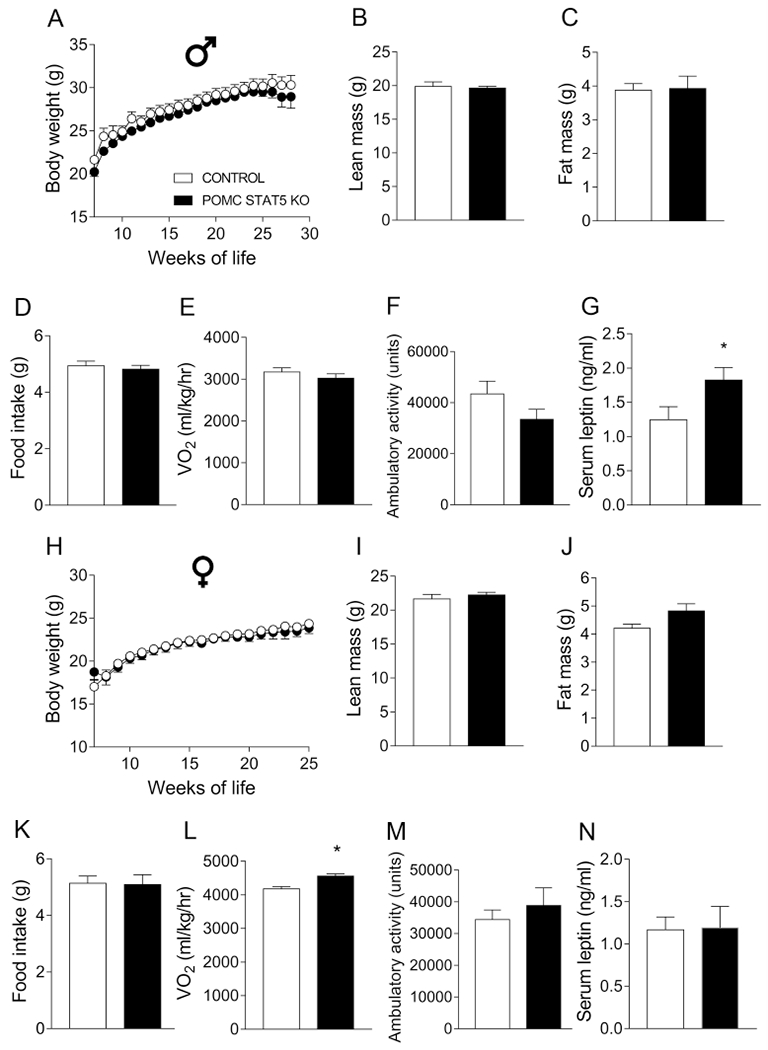
A-G. Body weight (A), lean body mass (B), body fat mass (C), daily food intake (D), oxygen consumption (VO2; E), ambulatory activity (F) and serum leptin levels (G) of control (n = 8-14) and POMC STAT5 KO (n = 9-25) male mice. H-N. Body weight (H), lean body mass (I), body fat mass (J), daily food intake (K), VO2 (L), ambulatory activity (M) and serum leptin levels (N) of control (n = 10-26) and POMC STAT5 KO (n = 10-15) female mice. Body weight changes were analyzed by repeated measures two-way ANOVA and the remaining comparisons between groups were performed using two-tailed Student’s t-test. *, P < 0.05 comparing control and POMC STAT5 KO mice (two-tailed Student’s t-test).
3.8. Ablation of STAT5 in POMC cells does not affect glucose homeostasis, but male POMC STAT5 KO mice present impaired 2DG-induced hyperphagia
STAT5 deletion in POMC neurons did not alter the glycemic response during the GTT and ITT in males (Fig. 8A–B) and females (Fig. 8E–F). Ablation of STAT5 in POMC neurons also produced no changes in the counter-regulatory response to 2DG in both genders (Fig. 8C and 8G). Regarding the feeding response to 2DG, glucoprivic hyperphagia was intact in female POMC STAT5 KO mice (Fig. 8H), whereas male POMC STAT5 KO mice presented impaired 2DG-induced hyperphagia at 2 hours (interaction between 2DG and genotype [F(1,27) = 9.074, P = 0.0056]), 3 hours (interaction between 2DG and genotype [F(1,27) = 5.221, P = 0.0304]) and 4 hours (interaction between 2DG and genotype [F(1,27) = 7.005, P = 0.0134]; Fig. 8D).
Fig. 8. Normal glucose homeostasis, but impaired 2DG-induced hyperphagia in POMC STAT5 KO male mice.
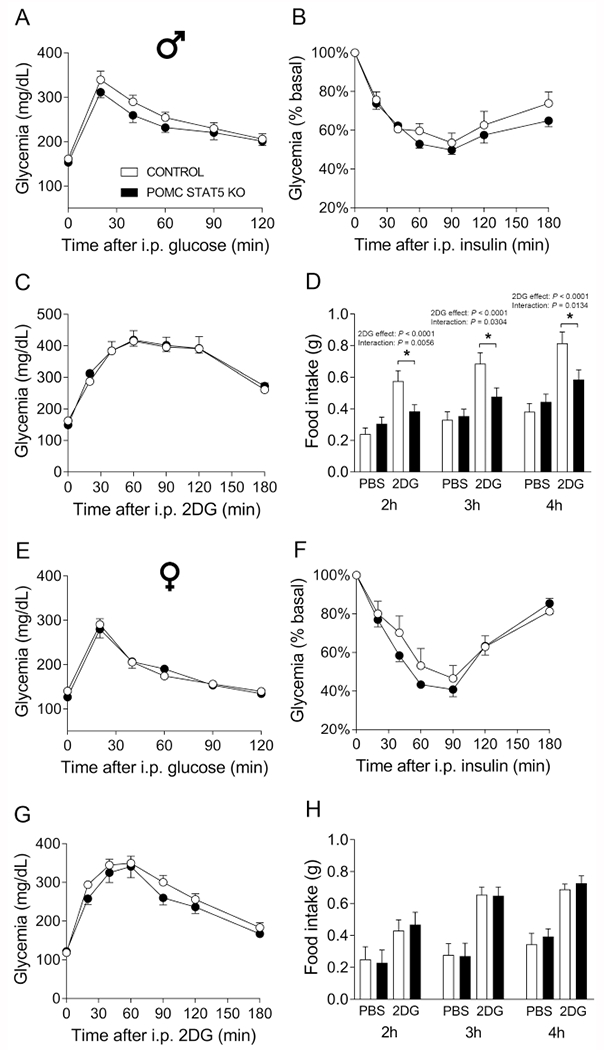
A-C. Blood glucose levels during a glucose tolerance test (GTT, A), insulin tolerance test (ITT, B) and counter-regulatory response induced by 2-deoxy-D-glucose (2DG) infusion (C) in control (n = 14) and POMC STAT5 KO (n = 19) male mice. D. cumulative food intake 2, 3 and 4 hours after i.p. injection of PBS or 2DG in control (n = 14) and POMC STAT5 KO (n = 19) male mice. E-G. Blood glucose levels during a GTT (E), ITT (F) and counter-regulatory response induced by 2DG infusion (G) in control (n = 10) and POMC STAT5 KO (n = 9) female mice. H. cumulative food intake 2, 3 and 4 hours after i.p. injection of PBS or 2DG in control (n = 10) and POMC STAT5 KO (n = 7) female mice. The data were analyzed by repeated measures two-way ANOVA and the contrasts identified by Bonferroni’s multiple comparisons test. *, P < 0.05 (interaction between 2DG and genotype in each time point).
3.9. STAT5 deletion in POMC neurons causes modest effects in the metabolic adaptations during food deprivation
Male and female POMC STAT5 KO mice were also studied during 60% of food restriction. Male POMC STAT5 KO mice presented similar body weight, lean and fat loss during food restriction, compared to control animals (Fig. 9A–C). Additionally, male POMC STAT5 KO mice maintained an equivalent glycemia during food restriction compared to control mice (Fig. 9D). The groups did not present any difference in oxygen consumption during food restriction (Fig. 9E), while a significant interaction between STAT5 deletion and time was observed in the RER during food deprivation ([F(4,39) = 3.355, P = 0.0187]; Fig. 9F). In females, POMC STAT5 KO mice exhibited no changes in body weight, lean and fat mass loss or glycemia, compared to control females (Fig. 9G–J).
Fig. 9. Metabolic changes during food restriction in POMC STAT5 KO male mice.
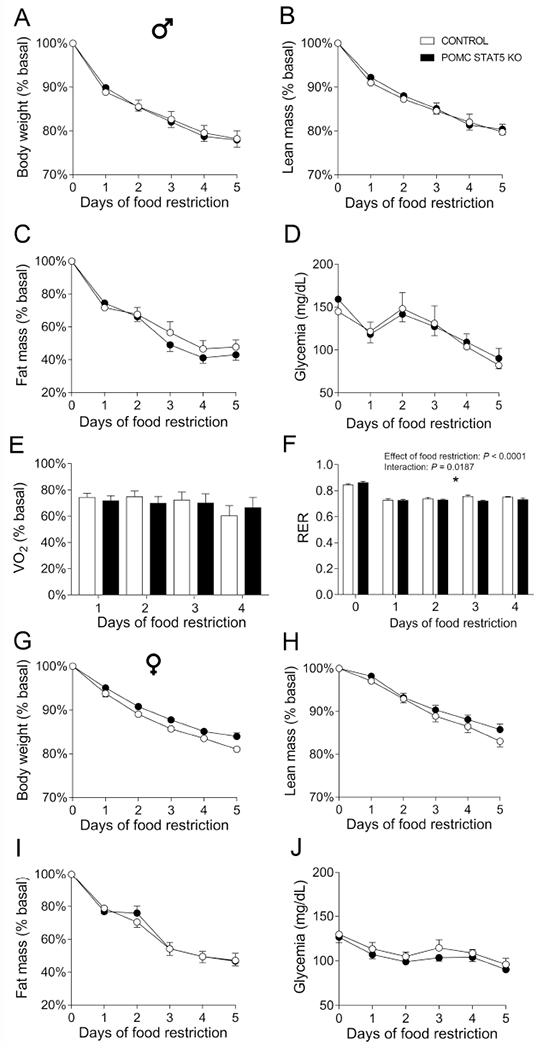
A-F. Changes in body weight (A), lean body mass (B), body fat mass (C), glycemia (D), oxygen consumption (VO2, E) and respiratory exchange ratio (RER, F) in control (n = 7) and POMC STAT5 KO (n = 12) male mice subjected to 5 days of 60% food restriction. G-J. Changes in body weight (A), lean body mass (B), body fat mass (C) and glycemia (D) in control (n = 10) and POMC STAT5 KO (n = 10) female mice subjected to 5 days of 60% food restriction. The data were analyzed by repeated measures two-way ANOVA. *, P < 0.05 (interaction between food restriction and STAT5 ablation).
4. Discussion
POMC and AgRP/NPY neurons are known to regulate the energy balance and glucose homeostasis through the input of various hormones (Andermann and Lowell, 2017; Ramos-Lobo and Donato, 2017). Recently, GH has been shown to be capable of modulating the activity of AgRP/NPY neurons in order to promote neuroendocrine responses to weight loss (Furigo et al., 2019b). However, GH responsive cells in the ARH are not restricted to AgRP/NPY neurons (Furigo et al., 2019b). In the present study, we showed that approximately 60% of ARH POMC neurons are directly responsive to GH since they exhibited pSTAT5 after an acute icv injection of GH. Despite the evidence of functional GHR, the percentage of POMC neurons responsive to GH is lower than that found in AgRP neurons, in which 95% of them express either Ghr mRNA (Kamegai et al., 1996) or GH-induced pSTAT5 (Furigo et al., 2019b). In addition, GHR deletion was efficient in reducing the number of GH-responsive POMC neurons close to levels found in PBS-injected mice.
Until now, POMC-Cre mice have been used in approximately one hundred of published studies (https://www.jax.org/strain/005965). Despite its popularity, there are several confounding factors with this strain that need to be considered. Firstly, POMC is also expressed in the pituitary gland and plays a critical role in the hypothalamic-pituitary-adrenal axis. However, corticotropic cells do not express the GHR in humans and rats (Mertani et al., 1995; Mertani et al., 1994). Additionally, no evidence of important metabolic imbalances or changes in corticosterone levels was found in mice deficient of GHR in POMC cells. On the other hand, POMC STAT5 KO mice showed a non-significant tendency towards higher basal corticosterone levels. Thus, whether STAT5 signaling indeed regulates the function of corticotropic cells still needs to be investigated. POMC neurons are also found in the NTS (Ellacott et al., 2006), but we showed that NTS POMC neurons are not directly responsive to GH, so these cells cannot account for the phenotype observed in POMC GHR KO mice. Another issue regarding POMC-Cre mice is that 25% of AgRP/NPY cells arise from a POMC-expressing lineage (Padilla et al., 2010; Xu et al., 2018). Consequently, a subset of AgRP/NPY neurons is expected to undergo gene deletion, together with POMC neurons. Therefore, part of the phenotype exhibited by POMC GHR KO mice resembles that observed in AgRP-specific GHR KO mice, as previously shown (Furigo et al., 2019b). However, AgRP GHR KO mice exhibit robust metabolic alterations during food restriction (Furigo et al., 2019b), whereas POMC GHR KO mice showed a modest phenotype.
As seen with leptin (Bouret et al., 2004; Ramos-Lobo et al., 2019), GH also appears to be a neurotrophic factor that regulates the development of axonal projections of ARH neurons (Sadagurski et al., 2015). Accordingly, GH deficiency or whole-body GHR deletion leads to reduced POMC and AgRP fiber density in the PVH and DMH (Sadagurski et al., 2015). In the present study, POMC-specific deletion of either GHR or STAT5 caused no changes in POMC and AgRP fiber density in the PVH, LHA and DMH. This divergent finding may be explained by the multiple endocrine, metabolic and growth defects exhibited by GH deficient mice or global GHR KO mice (Duran-Ortiz et al., 2018; Kopchick et al., 2014), which possibly interfered with the formation of these neuronal projections. Thus, additional studies are necessary to investigate the mechanisms behind the neurotrophic effects of GH signaling in the brain.
POMC GHR KO mice showed no alterations in energy balance or glucose homeostasis in basal conditions. Similar results were also observed in AgRP GHR KO mice (Furigo et al., 2019b). Inactivation of GHR in leptin receptor (LepR)-expressing cells caused no effects on energy balance, but led to hepatic insulin resistance (Cady et al., 2017). Since the majority of POMC neurons express the LepR (Lima et al., 2016), the insulin resistance induced by a LepR-specific GHR ablation cannot be explained by AgRP or POMC cells. On the other hand, inactivation of GHR in POMC cells impaired 2DG-induced hyperphagia. Although previous studies have shown that 2DG injection increases the hypothalamic mRNA levels of Npy and Agrp (Fraley et al., 2002; Sergeyev et al., 2000), which could explain the hyperphagia during this situation, other studies provided evidence that AgRP/NPY neurons are not essential for the feeding responses induced by glucoprivation (Luquet et al., 2007). Thus, our findings suggest that GH action in POMC neurons regulates glucoprivic hyperphagia in both male and female mice. Of note, the counter-regulatory response induced by 2DG injection was not affected by GHR ablation in POMC cells, indicating that the known counter-regulatory effect of GH (Furigo et al., 2019a; Hussain et al., 2003; Tennese and Wevrick, 2011) is mediated by other organs or neuronal populations.
As mentioned earlier, food restriction or fasting increases GH levels in humans and mice (Roth et al., 1963; Zhao et al., 2010). The increased GH secretion in those situations requires ghrelin action since ghrelin-deficient mice show attenuated GH secreting during food restriction (Li et al., 2012; Zhao et al., 2010). Defects in GH secretion during food restriction impair the maintenance of glycemia (Li et al., 2012; Zhao et al., 2010). Male POMC GHR KO mice exhibited lower glycemia compared to control mice after 5 days of food restriction, suggesting that GH signaling in POMC cells contributes to maintaining blood glucose levels during prolonged food deprivation.
STAT5 action in the brain regulates food intake, body adiposity, glucose homeostasis and several cognitive and behavioral functions (Buonfiglio et al., 2015; Furigo et al., 2018; Furigo et al., 2016; Lee et al., 2008; Patterson et al., 2012). In order to identify potential intracellular mechanisms recruited by GH to regulate metabolism via POMC neurons, mice carrying a POMC-specific STAT5 ablation was generated. Although only minor metabolic effects were observed in POMC-specific STAT5 KO mice, POMC STAT5 KO mice also exhibited a blunted 2DG-induced hyperphagia, although only in males. Thus, STAT5 is possibly a downstream signaling pathway recruited by GH in POMC cells to induce this effect.
5. Conclusions
Our findings indicate that GH is a hormone that acts on POMC neurons to regulate metabolism, in particular the glucoprivic hyperphagia and blood glucose levels during prolonged food restriction. In addition, STAT5 appears to be a downstream signaling pathway recruited by GH to control 2DG-induced food intake in male mice. Thus, our findings help to explain the mechanisms behind some of the metabolic effects induced by GH. Moreover, we provide additional evidence that the brain should be considered as an important target tissue of GH.
Supplementary Material
Highlights.
Approximately 60% of POMC neurons of the arcuate nucleus are directly responsive to GH
Ablation of GHR or STAT5 in POMC cells does not affect energy and glucose homeostasis
Glucoprivic hyperphagia is attenuated in POMC-specific GHR knockout mice
Absence of STAT5 in POMC cells prevents glucoprivic hyperphagia only in male mice
Acknowledgements
We thank Ana M.P. Campos for the technical assistance. This work was supported by the São Paulo Research Foundation (FAPESP-Brazil) as grants (17/02983-2 to J.D. and 17/21840-8 to R.F.) and scholarships (16/09679-4 to I.F.; 16/20897-3 to F.W.; 17/25281-3 to P.Q. and 17/04006-4 to G.C.), by Pfizer, Inc (2017 Global ASPIRE Young Investigator Research Awards to J.D.) and by NIH (NIA): R01AG059779 to J.J.K. and E.O.L.
Abbreviations:
- 2DG
2-deoxy-D-glucose
- 3V
third ventricle
- AgRP
agouti-related protein
- α-MSH
α-melanocyte-stimulating hormone
- ARH
arcuate nucleus of the hypothalamus
- DMH
dorsomedial nucleus of the hypothalamus
- GH
growth hormone
- GHR
growth hormone receptor
- GTT
glucose tolerance test
- i.p.
intraperitoneal
- icv
intracerebroventricular
- IOD
integrated optical density
- ITT
insulin tolerance test
- KO
knockout
- KPBS
0.02 M potassium PBS
- LepR
leptin receptor
- LHA
lateral hypothalamic area
- NPY
neuropeptide Y
- NTS
nucleus of the solitary tract
- POMC
proopiomelanocortin
- pSTAT5
phosphorylation of the signal transducer and activator of transcription 5
- PVH
paraventricular nucleus of the hypothalamus
- RER
respiratory exchange rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare no conflicts of interest.
References
- Andermann ML, Lowell BB, 2017. Toward a Wiring Diagram Understanding of Appetite Control. Neuron 95, 757–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED, Vianna CR, Donato J Jr., Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, Coppari R, Elmquist JK, 2012. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest 122, 1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlooly YM, Olsson B, Bruder CE, Linden D, Sjogren K, Bjursell M, Egecioglu E, Svensson L, Brodin P, Waterton JC, Isaksson OG, Sundler F, Ahren B, Ohlsson C, Oscarsson J, Tornell J, 2005. Growth hormone overexpression in the central nervous system results in hyperphagia-induced obesity associated with insulin resistance and dyslipidemia. Diabetes 54, 51–62. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB, 2004. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci 24, 2797–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonfiglio DC, Ramos-Lobo AM, Silveira MA, Furigo IC, Hennighausen L, Frazao R, Donato J Jr., 2015. Neuronal STAT5 signaling is required for maintaining lactation but not for postpartum maternal behaviors in mice. Horm Behav 71, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady G, Landeryou T, Garratt M, Kopchick JJ, Qi N, Garcia-Galiano D, Elias CF, Myers MG Jr., Miller RA, Sandoval DA, Sadagurski M, 2017. Hypothalamic growth hormone receptor (GHR) controls hepatic glucose production in nutrient-sensing leptin receptor (LepRb) expressing neurons. Mol Metab 6, 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Edwards GL, Martin RJ, 2003. Central insulin potentiates eating elicited by 2-deoxy-D-glucose. Physiol Behav 78, 331–336. [DOI] [PubMed] [Google Scholar]
- Coll AP, Loraine Tung YC, 2009. Pro-opiomelanocortin (POMC)-derived peptides and the regulation of energy homeostasis. Mol Cell Endocrinol 300, 147–151. [DOI] [PubMed] [Google Scholar]
- Dehkhoda F, Lee CMM, Medina J, Brooks AJ, 2018. The Growth Hormone Receptor: Mechanism of Receptor Activation, Cell Signaling, and Physiological Aspects. Front Endocrinol (Lausanne) 9, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Ortiz S, Noboa V, Kopchick JJ, 2018. Disruption of the GH receptor gene in adult mice and in insulin sensitive tissues. Growth Horm IGF Res 38, 3–7. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Bjursell M, Ljungberg A, Dickson SL, Kopchick JJ, Bergstrom G, Svensson L, Oscarsson J, Tornell J, Bohlooly YM, 2006. Growth hormone receptor deficiency results in blunted ghrelin feeding response, obesity, and hypolipidemia in mice. Am J Physiol Endocrinol Metab 290, E317–325. [DOI] [PubMed] [Google Scholar]
- Ellacott KL, Halatchev IG, Cone RD, 2006. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology 147, 3190–3195. [DOI] [PubMed] [Google Scholar]
- Fraley GS, Dinh TT, Ritter S, 2002. Immunotoxic catecholamine lesions attenuate 2DG-induced increase of AGRP mRNA. Peptides 23, 1093–1099. [DOI] [PubMed] [Google Scholar]
- Furigo IC, de Souza GO, Teixeira PDS, Guadagnini D, Frazao R, List EO, Kopchick JJ, Prada PO, Donato J Jr., 2019a. Growth hormone enhances the recovery of hypoglycemia via ventromedial hypothalamic neurons. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, fj201901315R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furigo IC, Melo HM, Lyra ESNM, Ramos-Lobo AM, Teixeira PDS, Buonfiglio DC, Wasinski F, Lima ER, Higuti E, Peroni CN, Bartolini P, Soares CRJ, Metzger M, de Felice FG, Donato J Jr., 2018. Brain STAT5 signaling modulates learning and memory formation. Brain Struct Funct 223, 2229–2241. [DOI] [PubMed] [Google Scholar]
- Furigo IC, Metzger M, Teixeira PD, Soares CR, Donato J Jr., 2017. Distribution of growth hormone-responsive cells in the mouse brain. Brain Struct Funct 222, 341–363. [DOI] [PubMed] [Google Scholar]
- Furigo IC, Ramos-Lobo AM, Frazao R, Donato J Jr., 2016. Brain STAT5 signaling and behavioral control. Mol Cell Endocrinol 438, 70–76. [DOI] [PubMed] [Google Scholar]
- Furigo IC, Teixeira PDS, de Souza GO, Couto GCL, Romero GG, Perello M, Frazao R, Elias LL, Metzger M, List EO, Kopchick JJ, Donato J Jr., 2019b. Growth hormone regulates neuroendocrine responses to weight loss via AgRP neurons. Nat Commun 10, 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjorbaek C, 2009. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab 9, 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain K, Hindmarsh P, Aynsley-Green A, 2003. Spontaneous hypoglycemia in childhood is accompanied by paradoxically low serum growth hormone and appropriate cortisol counterregulatory hormonal responses. J Clin Endocrinol Metab 88, 3715–3723. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Nishikawa K, Matsuo T, 1980. Feeding responses of Zucker fatty rat to 2-deoxy-D-glucose, norepinephrine, and insulin. Am J Physiol 239, E379–384. [DOI] [PubMed] [Google Scholar]
- Kamegai J, Minami S, Sugihara H, Hasegawa O, Higuchi H, Wakabayashi I, 1996. Growth hormone receptor gene is expressed in neuropeptide Y neurons in hypothalamic arcuate nucleus of rats. Endocrinology 137, 2109–2112. [DOI] [PubMed] [Google Scholar]
- Kim JH, Leggatt RA, Chan M, Volkoff H, Devlin RH, 2015. Effects of chronic growth hormone overexpression on appetite-regulating brain gene expression in coho salmon. Mol Cell Endocrinol 413, 178–188. [DOI] [PubMed] [Google Scholar]
- Kopchick JJ, List EO, Kelder B, Gosney ES, Berryman DE, 2014. Evaluation of growth hormone (GH) action in mice: discovery of GH receptor antagonists and clinical indications. Mol Cell Endocrinol 386, 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Muenzberg H, Gavrilova O, Reed JA, Berryman D, Villanueva EC, Louis GW, Leinninger GM, Bertuzzi S, Seeley RJ, Robinson GW, Myers MG, Hennighausen L, 2008. Loss of cytokine-STAT5 signaling in the CNS and pituitary gland alters energy balance and leads to obesity. PLoS One 3, e1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RL, Sherbet DP, Elsbernd BL, Goldstein JL, Brown MS, Zhao TJ, 2012. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J Biol Chem 287, 17942–17950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima LB, Metzger M, Furigo IC, Donato J Jr., 2016. Leptin receptor-positive and leptin receptor-negative proopiomelanocortin neurons innervate an identical set of brain structures. Brain Res 1646, 366–376. [DOI] [PubMed] [Google Scholar]
- List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H, Vesel C, Junnila RK, Frank SJ, Masternak MM, Bartke A, Kopchick JJ, 2013. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol 27, 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Phillips CT, Palmiter RD, 2007. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides 28, 214–225. [DOI] [PubMed] [Google Scholar]
- Mertani HC, Pechoux C, Garcia-Caballero T, Waters MJ, Morel G, 1995. Cellular localization of the growth hormone receptor/binding protein in the human anterior pituitary gland. J Clin Endocrinol Metab 80, 3361–3367. [DOI] [PubMed] [Google Scholar]
- Mertani HC, Waters MJ, Jambou R, Gossard F, Morel G, 1994. Growth hormone receptor binding protein in rat anterior pituitary. Neuroendocrinology 59, 483–494. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Arima H, Watanabe M, Shimizu H, Ito Y, Banno R, Sugimura Y, Ozaki N, Nagasaki H, Oiso Y, 2011. Repeated glucoprivation delayed hyperphagic responses while activating neuropeptide Y neurons in rats. Peptides 32, 763–769. [DOI] [PubMed] [Google Scholar]
- Padilla SL, Carmody JS, Zeltser LM, 2010. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med 16, 403–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla SL, Reef D, Zeltser LM, 2012. Defining POMC Neurons Using Transgenic Reagents: Impact of Transient Pomc Expression in Diverse Immature Neuronal Populations. Endocrinology 153, 1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CM, Villanueva EC, Greenwald-Yarnell M, Rajala M, Gonzalez IE, Saini N, Jones J, Myers MG Jr., 2012. Leptin action via LepR-b Tyr1077 contributes to the control of energy balance and female reproduction. Mol Metab 1, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Lobo AM, Donato J Jr., 2017. The role of leptin in health and disease. Temperature 4, 258–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Lobo AM, Teixeira PD, Furigo IC, Melo HM, de Lyra E Silva NM, De Felice FG, Donato J Jr., 2019. Long-term consequences of the absence of leptin signaling in early life. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK, 2011. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 13, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR, 1998. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology 139, 4428–4431. [DOI] [PubMed] [Google Scholar]
- Roth J, Glick SM, Yalow RS, Bersonsa, 1963. Hypoglycemia: a potent stimulus to secretion of growth hormone. Science 140, 987–988. [DOI] [PubMed] [Google Scholar]
- Sadagurski M, Landeryou T, Cady G, Kopchick JJ, List EO, Berryman DE, Bartke A, Miller RA, 2015. Growth hormone modulates hypothalamic inflammation in long-lived pituitary dwarf mice. Aging cell 14, 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyev V, Broberger C, Gorbatyuk O, Hokfelt T, 2000. Effect of 2-mercaptoacetate and 2-deoxy-D-glucose administration on the expression of NPY, AGRP, POMC, MCH and hypocretin/orexin in the rat hypothalamus. Neuroreport 11, 117–121. [DOI] [PubMed] [Google Scholar]
- Silveira MA, Furigo IC, Zampieri TT, Bohlen TM, de Paula DG, Franci CR, Donato J Jr., Frazao R, 2017. STAT5 signaling in kisspeptin cells regulates the timing of puberty. Mol Cell Endocrinol 448, 55–65. [DOI] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang DM, Brown M, Bodner S, Grosveld G, Ihle JN, 1998. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93, 841–850. [DOI] [PubMed] [Google Scholar]
- Tennese AA, Wevrick R, 2011. Impaired hypothalamic regulation of endocrine function and delayed counterregulatory response to hypoglycemia in Magel2-null mice. Endocrinology 152, 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CI, Zagon IS, McLaughlin PJ, 1979. Hypophagia follows the initial hyperphagia produced by 2-deoxy-D-glucose in rats. Physiol Behav 23, 187–190. [DOI] [PubMed] [Google Scholar]
- Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK, 2010. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci 30, 2472–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bartolome CL, Low CS, Yi X, Chien CH, Wang P, Kong D, 2018. Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature 556, 505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS, 2010. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci U S A 107, 7467–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Song Y, Wang Y, Zhang T, Duan M, Li Y, Liao L, Zhu Z, Hu W, 2013. Increased food intake in growth hormone-transgenic common carp (Cyprinus carpio L.) may be mediated by upregulating Agouti-related protein (AgRP). Gen Comp Endocrinol 192, 81–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


