Abstract
Lipids play a critical role in energy metabolism, and a suite of proteins is required to deliver lipids to tissues. Several of these proteins require an intricate endoplasmic reticulum (ER) quality control (QC) system and unique secondary chaperones for folding. Key examples include apolipoprotein B (apoB), which is the primary scaffold for many lipoproteins, dimeric lipases, which hydrolyze triglycerides from circulating lipoproteins, and the low-density lipoprotein receptor (LDLR), which clears cholesterol-rich lipoproteins from the circulation. ApoB requires specialized proteins for lipidation, dimeric lipases lipoprotein lipase (LPL) and hepatic lipase (HL) require a transmembrane maturation factor for secretion, and the LDLR requires several specialized, domain-specific chaperones. Deleterious mutations in these proteins or their chaperones may result in dyslipidemias, which are detrimental to human health. Here, we review the ER quality control systems that ensure secretion of apoB, LPL, HL, and LDLR with a focus on the specialized chaperones required by each protein.
Ensuring the correct levels of lipids in the circulation is essential for supplying energy to tissues while preventing lipid overload (Ramasamy 2016). In order to reach tissues requiring triglycerides, lipids must overcome the challenge of being insoluble in the blood. Specialized proteins with unique structural characteristics are required to package, transport, and deliver lipids in the harsh extracellular environment.
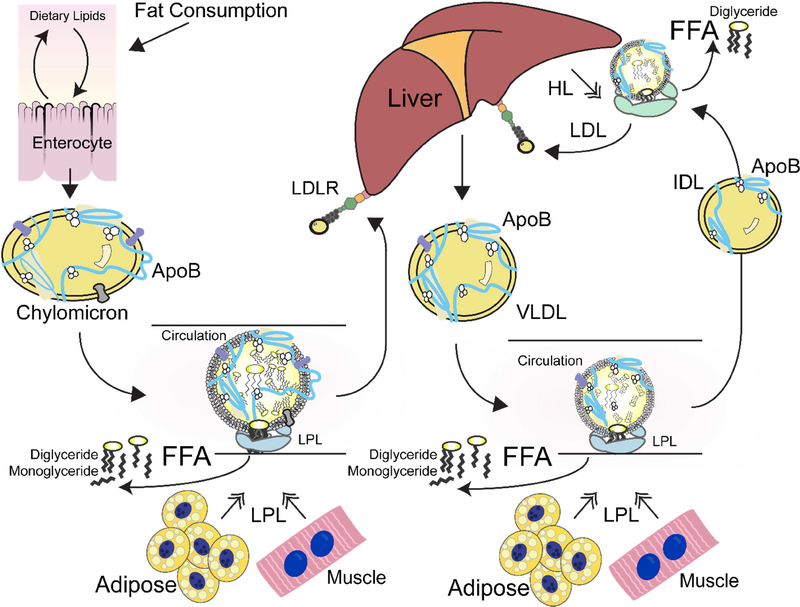
Lipids are packaged and circulated through the blood in the form of lipoproteins. These protein-lipid assemblies have a hydrophobic core and a hydrophilic surface decorated with surface apolipoproteins, loss of which can result in dyslipidemias (Patni et al., 2000). For example, very low-density lipoproteins (VLDL), intermediate density lipoproteins (IDL), low density lipoproteins (LDL), and chylomicrons are defined by the hydrophobic scaffolding protein apolipoprotein B (apoB). VLDL, which are synthesized in the liver and transport endogenous fat, use apoB-100, the full-length version of apoB. However, chylomicrons, which are synthesized in the intestine and transport dietary fat, use apoB-48, the truncated form of apoB (Kane et al. 1980; Glickman et al. 1986).
Once lipoproteins are transported to the circulation, specialized lipases aid in the release of their component lipids. The secreted dimeric lipases lipoprotein lipase (LPL) and hepatic lipase (HL) hydrolyze triglycerides (TGs) from lipoproteins and provide free fatty acids to tissues. LPL is secreted from the parenchyma of adipose and muscle into the capillary lumen and acts on VLDL and chylomicrons (Figure 1). HL is secreted from hepatic cells and acts on remnant lipoproteins and cholesterol-rich high-density lipoproteins (HDL; Musliner et al. 1979; Marques-Vidal et al. 1991). LPL deficiency results in elevated serum triglyceride levels, which is a risk factor for acute pancreatitis (Simha and Garg 2009). Several pathogenic lipase variants have been characterized as causing lipase misfolding or mis-trafficking (Fisher et al. 1997).
Figure 1: Physiological overview of triglyceride metabolism.
The major scaffolding protein apoB is a defining component of lipoprotein particles released from the intestines and liver. Chylomicrons are lipoproteins that are synthesized from the intestine and transport dietary lipids. VLDL are synthesized from the liver and transports endogenous lipids. Both VLDL and chylomicrons contain apoB, and TGs from these lipoproteins are hydrolyzed in the circulation by LPL. HL hydrolyzes TGs from IDL. Remnant lipoproteins are taken up by LDLR.
The low-density lipoprotein receptor (LDLR) is a cell surface receptor that binds to apoB-100 and other apolipoproteins to mediate endocytosis of cholesterol-rich LDL particles and clear them from circulation (Figure 1; Brown and Goldstein 1976). Uptake of LDL by LDLR provides cells with cholesterol to be used, stored, or excreted from the body. Loss of function variants in the LDLR gene cause familial hypercholesterolemia (FH), which is characterized by the inability to clear LDL from the plasma. About half of pathogenic LDLR variants are unable to exit the ER (Hobbs et al. 1992).
Secreting functional apoB, LPL, HL, and LDLR is critical for lipid metabolism and the prevention of dyslipidemias. Proper secretion requires folding and post-translational processing in the ER. The ER QC system acts a proofreader and gatekeeper for proteins exiting the ER. The QC system aids in folding of secreted and membrane proteins and facilitates the degradation of terminally misfolded proteins. The ER contains a unique ion, redox, and chaperone environment that permits several co-translational and post-translational modifications to ensure proper protein folding. These modifications include disulfide bond formation, signal peptide cleavage, N-linked glycosylation, and glycophosphatidylinositol -anchor addition (Ellgaard and Helenius 2003). Terminally misfolded proteins are degraded by proteasomal or autophagic processes. The ER QC system operates at both a general and protein-specific level termed primary and secondary quality control, respectively (Ellgaard et al. 1999).
ApoB, LPL, HL, and LDLR have unique structural characteristics that warrant the assistance of a network of specialized secondary quality control chaperones. Here, we review the network of quality control proteins required for the packaging, transport, and delivery of lipids.
Quality Control of Apolipoprotein B
ApoB is the main scaffolding protein for the lipoproteins VLDL and chylomicrons (Kane et al. 1980). Mutations in apoB or the chaperones required for apoB lipidation can result in failure of apoB-containing lipoproteins to exit the ER (Wetterau et al. 1992; Burnett et al. 2003; Burnett et al. 2007). This causes the dyslipidemias, familial hypobetalipoproteinemia and abetalipoproteinemia (Ramasamy 2016). Familial hypobetalipoproteinemia, which can be caused by mutations in apoB, is inherited in an autosomal codominant pattern and is defined by <5th percentile of plasma LDL cholesterol or total apoB levels (Heiss et al. 1980; Schonfeld 2003). Clinical manifestations include a fatty liver and difficulty absorbing fats and fat-soluble vitamins. Abetalipoproteinemia, which is caused by pathogenic variants in an apoB-specific chaperone, is a rare inherited disorder characterized by the absence of apoB-containing lipoproteins in the plasma (Wetterau et al. 1992; Ramasamy 2016). Symptoms include failure to thrive, deficiencies in fat-soluble vitamins, disturbances in muscle coordination, and retinitis pigmentosa (Ramasamy 2016).
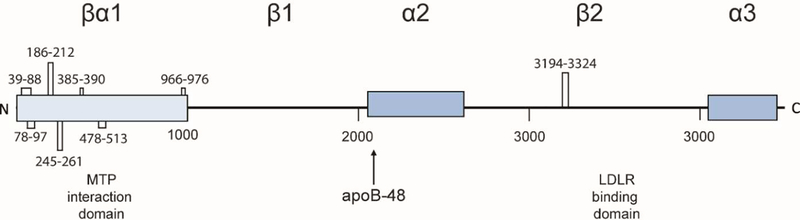
ApoB is a large, monomeric protein composed of five domains with alternating α-helical and β-sheet regions (Segrest et al. 2001). The first 1000 amino acids, referred to as the βα1 domain, consist of β-barrel, α-helical, and C-sheet subdomains and is particularly important for lipid recruitment (Jiang et al. 2006). ApoB is disulfide-rich, and processing of these disulfide bonds is critical for secretion (Shelness and Thornburg 1996; Huang and Shelness 1997; Tran et al. 1998). ApoB-100 contains eight disulfide bonds, seven of which are clustered into the βα1 domain (Figure 2; Yang et al. 1990). ApoB also contains 16 N-linked glycans which are required for secretion (Schumaker et al. 1994). We will describe several ER chaperones involved in lipidation, disulfide bond formation, and glycan-dependent folding of apoB such as microsomal triglyceride transfer protein (MTP), protein disulfide isomerase (PDI), and calnexin (Cnx)/calreticulin (Crt). Several proteins regulate ER degradation in the event of apoB misfolding or failure to undergo lipidation.
Figure 2: ApoB-100 domain map.
ApoB has a pentapartite structure consisting of alternating α-helical and β-strand domains. MTP interactions are localized to the βα1 domain, and LDLR binding sites are localized to the β2 domain. Disulfide linkages with residue numbers are indicated.
Lipidation is carried out by MTP
MTP is a chaperone unique to apoB and has been described as the master regulator for apoB lipoprotein secretion (Sirwi and Hussain 2018). MTP was first discovered in liver microsomes and described as a cholesteryl ester and triglyceride transferring protein (Wetterau and Zilversmit 1984). MTP was later characterized as a heterodimer consisting of MTP (M subunit) and PDI (P subunit; Wetterau and Zilversmit 1985). The addition of MTP is sufficient to reconstitute assembly and secretion of apoB containing lipoproteins in non-hepatic and non-intestinal cells (Gordon et al. 1994). Furthermore, an inhibitor of MTP-mediated lipid transport prevented apoB secretion in a concentration dependent manner (Jamil et al. 1996). MTP is thought to co-translationally lipidate apoB, as well as provide triglycerides in the ER lumen for use in lipoprotein assembly (Kulinski et al. 2002). MTP interacts with the N-terminus of apoB. These interactions are reduced as apoB increases in length and becomes more lipidated, suggesting that protein-protein interactions between MTP and apoB may aid in the lipidation of apoB (Hussain et al. 1997).
Loss of function mutations in MTP are a significant source of inherited abetalipoproteinemia. More than 30 MTP variants resulting in abetalipoproteinemia have been characterized (Sani et al. 2011; Barakizou et al. 2016; Ramasamy 2016). Tissue samples from patients with abetalipoproteinemia had decreased triglyceride transfer activity (Wetterau et al. 1992). PDI, the smaller subunit of the MTP complex, is necessary to prevent aggregation and maintain catalytic activity of the lipid transferring M subunit (Wetterau et al. 1991). Several pathogenic variants of the M subunit prevent its interaction with the P subunit, making levels of MTP unstable and resulting in abetalipoproteinemia (Rehberg et al. 1996; Walsh et al. 2016).
In addition to MTP, other lipid transfer proteins may aid in generating apoB-containing lipoproteins. ApoB interacts with cell death-inducing DFF45-like effector B (CIDEB). CIDEB is a membrane protein associating with lipid droplets in the cytoplasm, smooth ER, and Golgi. In the absence of CIDEB, the triglyceride content of apoB-containing lipoproteins decreases. CIDEB is important for both maturation of VLDL and chylomicrons and may interact with both apoB-100 and apoB-48 (Ye et al. 2009; Zhang et al. 2014; Sirwi and Hussain 2018).
Disulfide bond formation is carried out by PDI
ApoB is disulfide-rich, and processing of these disulfide bonds is critical for secretion. ApoB-100 contains eight disulfide bonds, seven of which are clustered into the first 21 percent of the N-terminus (Yang et al. 1990). DTT treatment during the first 20–25 percent of synthesis resulted in ER retention and intracellular turnover of apoB (Shelness and Thornburg 1996). ApoB is sensitive to DTT regardless of lipidation state as shown by studies using both lipid rich and lipid poor forms of apoB (Burch and Herscovitz 2000). This is likely due to the importance of disulfide bond formation for apoB secretion which was demonstrated by studies testing cysteine to serine or alanine mutations (Huang and Shelness 1997; Tran et al. 1998). Disulfide bonds between Cys78 and Cys97 as well as Cys245 and Cys261 appear to be essential in apoB secretion and assembly (Huang and Shelness 1997).
In addition to its chaperone function, the oxidoreductase function of PDI may also be essential for the processing of apoB disulfide bonds. PDI catalyzes the formation of disulfide bonds co-translationally (Carmichael et al. 1977; Bulleid and Freedman 1988). Early studies suggest that the isomerase function of free PDI in the ER was important for apoB secretion (Wang et al. 1997). More recently, knockdown of PDI delayed disulfide bond formation of apoB100. Decreased oxidative folding, which was independent of MTP activity, decreased apoB100 lipidation (Wang et al. 2015). Therefore, formation of disulfide bonds, with the help of PDI, is a requirement for proper apoB lipidation and secretion of apoB-containing lipoproteins.
Glycan processing is essential for secretion
Glycosylation has an essential role in assembly and secretion of apoB, which contains 16 N-linked glycans (Schumaker et al. 1994). Mutagenesis of several of these glycosylated residues impairs secretion and lipoprotein association (Vukmirica et al. 2002). Furthermore, treatment with tunicamycin, which inhibits N-linked glycosylation by preventing peptide oligosaccharide addition, increased degradation of apoB-100. Chaperones that recognize the N-linked glycans of apoB, including Cnx, Crt, and the oxidoreductase ERp57, play an important role in folding and detection of misfolding (Tatu and Helenius 1999; Michalak et al. 2009). When Cnx was inhibited, the amount of apoB undergoing proteasomal degradation increased (Chen et al. 1998).
Export of apoB-containing lipoproteins from the ER
Once nascent lipoproteins are synthesized, they must exit the ER in order to be transported into the circulation. Pre-chylomicrons and pre-VLDL are too bulky to fit into conventional COPII-coated vesicles, which are involved in transporting cargo out of the ER (Ruf and Gould 1998). It was recently discovered that apoB-containing lipoproteins require TANGO1 and TANGO1-like protein (TALI) for ER export. It is hypothesized that TANGO1 and TALI interact with apoB and aid in bringing the bulky lipid cargo to ER exit sites. Binding of TANGO1 and TALI on the cytoplasmic side of Sec23/24, the COPII inner coat, prevents assembly of Sec13/31, the COPII outer coat, to allow continuous fusion of ER-Golgi intermediate compartment membranes and transport of these bulky cargos (Santos et al. 2016). Because TANGO1 is essential for both apoB-containing lipoproteins and collagen, two structurally distinct cargos, it has been proposed that an unidentified protein or chaperone may aid TANGO1 in recognizing different cargos (Saito et al. 2009; Santos et al. 2016). A recent genome-wide association study (GWAS) has implicated TANGO1 in the development of atherosclerosis, underscoring the importance of protein trafficking in these metabolic diseases (Luo et al. 2017)
Degradation by ER-associated degradation or autophagy systems
In the absence of necessary chaperones, apoB can be degraded through a ubiquitin-proteasome pathway. This pathway was first characterized when inhibiting the proteasome increased intracellular levels of ubiquitinated apoB (Yeung et al. 1996). Degradation by ER-associated degradation (ERAD) is primarily due to a lack of apoB lipidation, which is a unique to apoB compared to other ERAD substrates. If the ER lacks sufficient lipids to lipidate apoB or if MTP is not functional, translocation of apoB is arrested and BiP increases its association with apoB in the ER lumen (Rutledge et al. 2009). Ubiquitination is carried out by ubiquitin ligase Gp78 and potentially Hrd1 (Liang et al. 2003). Retrotranslocation is likely carried out by retrotranslocation mediator Derlin-1 with help from Hrd1 and is extracted from the membrane by AAA-ATPase 97 complex (Chen et al. 1998; Ye et al. 2004; Rutledge et al. 2009). An autophagy-based post-ER pathway has also been implicated in apoB degradation (Fisher et al. 2001; Pan et al. 2008).
Quality Control of Lipoprotein Lipase and Hepatic Lipase
The secreted dimeric lipases LPL and HL are essential for the hydrolysis and clearance of TGs in the blood. LPL is responsible for the hydrolysis of TGs in VLDL and chylomicrons, whereas HL hydrolyzes TGs within HDLs (Musliner et al. 1979; Marques-Vidal et al. 1991). Insufficient dimeric lipase levels or activity can result in elevated circulating triglycerides, termed hypertriglyceridemia. Uncontrolled hypertriglyceridemia can contribute to the development of pancreatitis, xanthoma, and ischemic heart disease (Wittrup et al. 1999; Simha and Garg 2009). Specifically, loss of LPL results in elevated VLDL and chylomicron levels, whereas loss of HL leads to elevated levels of circulating HDL (Andersen et al. 2003). Over 100 loss of function mutations have been identified in LPL-deficient patients (Rodrigues et al. 2016). Loss of function mutations can result in misfolding, mis-trafficking, or enzymatic inactivation of LPL or HL.
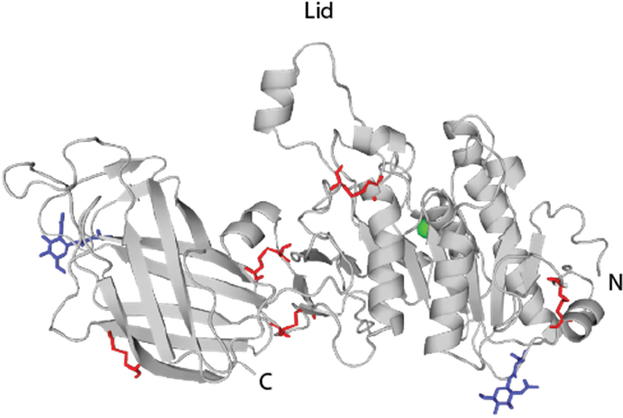
LPL and HL are each composed of a N-terminal domain including an active site, which is covered by a peptide lid, as well as a C-terminal domain that includes a heparin and receptor-binding domain (Wong and Schotz 2002; Griffon et al. 2009). LPL and HL both have five intermolecular disulfides and include two or four N-linked glycans, respectively (Figure 3; Murthy et al. 1996; Miller et al. 2004). In vitro, the LPL C-terminal domain folds quickly, whereas the N-terminal domain folds slowly and tends to form inactive aggregates (Zhang et al. 2005). In vivo, LPL and HL folding is also challenging and both LPL and HL form aggregates in the cell (Ben-Zeev and Doolittle 2004). LPL and HL require lipase maturation factor 1 (LMF1), which prevents aggregated LPL in the ER (Péterfy et al. 2007; Wong and Schotz 2002; Roberts et al. 2018). Here, we will outline both primary and secondary chaperones that glycosylate, fold, and degrade LPL and HL.
Figure 3: Structural features of LPL.
LPL was crystalized in complex with its partner protein GPIHBP1, and a monomer is shown (PDBID: 6OB0; Arora et al. 2019). Ten cysteines that are paired in disulfide bonds are shown in red. Immature N-linked glycans at Asn43 and Asn386 are shown in blue. The Ca2+ ion is shown in green.
Glycan processing promotes interactions with Cnx/Crt
Glycosylation is essential for the maturation and secretion of LPL and HL. Glycosylation enables LPL and HL to interact with the lectin chaperones Cnx and Crt (Zhang et al. 2003). During folding in the ER, glucosidases trim glycans on LPL and HL, limiting their interactions with Cnx and Crt (Ben-Zeev et al. 1992; Doolittle et al. 2009) If lectin-dependent folding is incomplete, HL and LPL can be re-glycosylated by the UDP-glucose:glycoprotein glucosyltransferases (UGGTs) I and II (Trombetta et al. 1989; Doolittle et al. 2009; Roberts et al. 2018). Once folded, LPL and HL exit this cycle of glycosylation and folding in the ER and move to the Golgi. In the Golgi, mannosidases trim immature glycans on LPL and HL (Simsolo et al. 1992). Inhibitors of Golgi glycosidases have been used to block LPL secretion, while leaving LPL activity unchanged, indicating that glycan trimming is essential for the secretion of LPL and HL but not their enzymatic activities (Ben-Zeev et al. 1992). Several functional studies have shown the importance of glycosylation. For example, loss of glycosylation at Asn43 abolishes LPL secretion and activity (Buscá et al. 1995) and the addition of a third N-linked glycan increases LPL retention in the ER (Wu et al. 2018)
Disulfide processing requires PDIs and LMF1
LPL and HL each contain five sequential, intramolecular disulfide bonds (Arora et al. 2019). Misfolded LPL tends to form disulfide-linked aggregates in cells (Roberts et al. 2018). PDI family proteins interact with LPL and HL in the ER to promote native disulfide bond formation. HL interacts with the PDI ERp57, which specifically aids in glycoprotein disulfide maturation, as well as the isomerase PDIA1 (Doolittle et al. 2009). LPL maturation is highly dependent on the oxidoreductases ERp44, ERp72, and ERdj5, and knockdown of any of these PDIs greatly reduces LPL secretion (Roberts et al. 2018). However, these chaperones together are not sufficient for dimeric lipase maturation, since lipase maturation factor 1 (LMF1) plays an essential role in lipase maturation.
LMF1 was identified in 1983 when mice with the combined lipase deficiency (cld) mutation displayed reduced levels of circulating LPL and HL and significantly elevated plasma triglycerides (Paterniti et al. 1983). The mutation in cld animals was later mapped to LMF1, an ER resident protein with five transmembrane domains (Péterfy et al. 2007;). LMF1 is essential for the maturation of LPL and HL but not de novo lipase synthesis (Olivecrona et al. 1985). The cld mutation introduces a premature stop in the C-terminal region of LMF1.However, clinical mutations in earlier regions of LMF1 also abolish LPL activity and isolated C-terminal LMF1 is not sufficient for LPL maturation (Babilonia-Rosa and Neher 2014; Serveaux Dancer et al. 2018). A recent investigation has uncovered a role for LMF1 in maintaining ER redox homeostasis. Deletion of LMF1 exacerbates disulfide-dependent LPL aggregation in cells and contributes to a more oxidized ER (Roberts et al. 2018). LMF1 interacts with the LPL partners Cnx and Crt, UGGTs I and II, and several oxidoreductases, as well as components of the ERAD machinery such as Sel1L (Sha et al. 2014; Roberts et al. 2018).
Lysosomal and proteasomal forms of degradation act on dimeric lipases
LPL is degraded by autophagy (Vannier et al. 1989). Misfolded LPL, like the clinical loss of function variant Gly142Glu, is concentrated in the lysosomes to prepare for degradation (Buscá et al. 1996). Unlike LPL, HL is delivered to the proteasome for degradation. HL interacts with components of the ubiquitin-proteasome system including the E3 ubiquitin ligases RNF123 and chaperone BiP. The proteasome inhibitor MG132 reduces HL degradation (Doolittle et al. 2009). It is not yet known what factors contribute to the sorting of LPL and HL for degradation or why their degradation occurs by different pathways.
Quality Control of the Low-Density Lipoprotein Receptor Family
LDLR is the founding member of a family of receptors that bind to and mediate the endocytic uptake of many ligands, including lipoproteins. Other family members include VLDLR, ApoER2, LRP1, LRP1b, LRP4, and Megalin (Dieckmann et al. 2010). The LDLR mediates endocytosis of cholesterol-rich LDL particles in order to clear them from the circulation (Brown and Goldstein 1976). FH is a genetic disorder commonly associated with mutations in LDLR, PSK9, and apoB that can cause early cardiovascular disease due to elevated levels of LDL in the plasma. Over 1,700 variants in the LDLR gene have been identified in patients with a clinical diagnosis of FH, but not all of these variants have been functionally characterized (Chora et al. 2018). Well-characterized LDLR mutations are divided into five classes based on their functional defect (Hobbs et al. 1992). About 50% of the mutants fall into class II, which is defined as LDLR variants that fail to exit the ER (Hobbs et al. 1992). A study of several common class II mutants found that these proteins did not traffic to the Golgi at wild-type levels due to misfolding and degradation by the proteasome (Li et al. 2004).
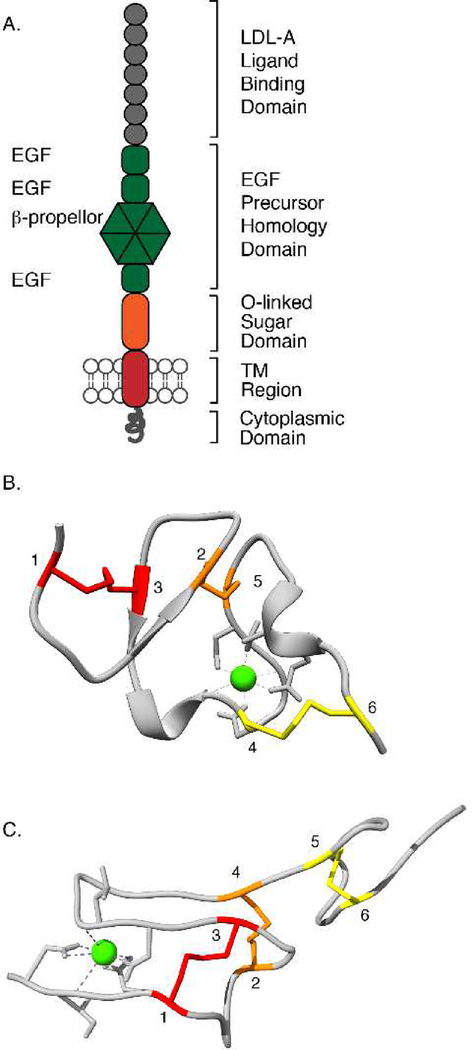
LDLR is a large protein, with 860 residues before signal peptide removal, and is comprised of five structural domains including the LDL receptor ligand-binding type A (LDL-A) modules, epidermal growth factor (EGF) precursor homology region, the O-linked sugar domain, a transmembrane domain and a cytoplasmic region (Figure 4A; Jeon et al. 2001). The LDL-A modules require the assistance of a special chaperone, the LRP-receptor-associated protein (RAP) for ER exit (Herz et al. 1991). The EGF precursor homology region is also the site of frequent class II FH mutations and requires a specialized chaperone, Mesd. The Mesd (mesoderm development) chaperone assists with the folding of a six-bladed β-propeller domain that is contained within the EGF precursor homology region of LDLR family members (Culi et al. 2004). With respect to the O-linked sugar domain, glycosylation of this domain appears to have more of a role in stability once at the cell surface than initial folding (Kozarsky et al. 1988; Iijima et al. 1998). However, the transmembrane region may be critical for LDLR processing in the ER, as some naturally occurring mutations in this domain prevent proper insertion of the LDLR into the membrane (Strom et al. 2015). Finally, the cytoplasmic region of the LDLR plays a role in efficient LDLR exit from the ER, likely by mediating binding to COPII proteins at the ER exit site (Strom et al. 2011). Next, we discuss some of these unique features in detail, focusing on how they render the LDLR particularly dependent on the protein quality control machinery of the ER.
Figure 4: Structural features of the LDLR.
A. Structural motifs of LDLR include the LDL-A ligand binding domain, EGF precursor homology domain, O-linked sugar domain, TM region, and the cytoplasmic domain. B. Structure of human LDL-A module 5 (PDBID: 1AJJ; Fass et al. 1997). shows the three nonsequential disulfide bonds. Cysteines paired in disulfide bonds are colored alike and are numbered sequentially from the N-terminal end of the motif. The Ca2+ ion is shown in green. C. The structure of an LDLR EGF-precursor homology domain is shown (PDBID: 1N7D; Rudenko et al. 2002). Cysteines paired in disulfide bonds are colored alike and numbered sequentially from the N-terminal end of the motif. The Ca2+ ion is shown in green.
Disulfide bond processing is critical for secretion
The 30 mostly nonsequential disulfide bonds in LDLR pose major challenges to its folding. Most disulfide bonds are in the LDL-A modules and the EGF precursor homology region. Disulfide bond processing plays a key role in the proper folding of the seven contiguous LDL-A modules at the N-terminus of LDLR (Esser et al. 1988). Each of these LDL-A modules is about 40 amino acids long and contains six cysteine residues that form three nonsequential disulfide bonds, as well as a Ca2+ ion that is coordinated by acidic residues (Figure 4B; Fass et al. 1997). Disulfide bond formation and Ca2+ coordination are essential for LDL-A folding and are interdependent processes (Atkins et al. 1998; Koduri and Blacklow 2001). Many class II FH mutations are clustered in the LDL-A domains and can be explained by disruption of key disulfide bonds and residues that coordinate a Ca2+ ion in these domains (Hobbs et al. 1992; Fass et al. 1997). The EGF precursor homology domain has two EGF-like repeats followed by a β propeller domain, which is followed by a third EGF-like repeat (Rudenko et al. 2002). Each EGF-like repeat also has three disulfide bonds, two of which are nonsequential (Figure 4C). Two of the EGF-like repeats bind Ca2+, but Ca2+ is not essential for EGF-like repeat folding (Kurniawan et al. 2001).
In addition to the presence of consecutive disulfide-rich domains, processing of on-pathway but non-native disulfide bonds must occur (Jansens et al. 2002). These transient, non-native disulfides connect distant domains of LDLR in order to compact its structure and potentially prevent the formation of inopportune disulfide bonds with other ER clients. These initial disulfide bonds must be reduced so that final, native disulfides can form. ERdj5 is an ER-resident and PDI family member that is best known for its ability to reduce disulfide bonds in terminally misfolded proteins prior to ER export (Ushioda et al. 2008). ERdj5 processes the non-native disulfide bonds in LDLR such that the correct disulfides can form (Oka et al. 2013). Indeed, depletion of ERdj5 from cells disrupts formation of native disulfide bonds and reduces the secretion of folded LDLR from the ER (Oka et al. 2013). LDLR also requires LMF1, a transmembrane protein that contributes to maintenance of the ER redox environment, for correct folding and secretion to the cell surface (Roberts et al. 2018).
LDLR requires specialized chaperones RAP and Mesd
Whereas most proteins fold with the assistance of general chaperones, most LDLR family receptors also need two private chaperones, RAP and Mesd, for efficient folding, maturation, and transit to the cell surface. RAP is a specialized chaperone that serves to help escort its clients from the ER into the Golgi and to temporary block clients from interacting with their ligands (Herz et al. 1991; Bu et al. 1995). RAP can also promote the folding of LDLR class II mutants that do not efficiently exit the ER (Li et al. 2002). RAP binds to a pair of LDL-A modules in the ER using metal-dependent electrostatic interactions (Fisher et al. 2006). When the RAP-receptor complex reaches the slightly acidic environment of the Golgi, a conformational change occurs. Several solvent-exposed histidine residues become protonated (a “histidine switch”), resulting in release of the LDL-A modules (Lee et al. 2006).
A chaperone named Mesd also plays a role in trafficking of LDLR family members. Mesd is known as Boca in drosophila, where it is important for trafficking of the LDLR family members Arrow and Yolkless (Culi and Mann 2003). Loss of Mesd is early embryonic lethal, making it difficult to study the full impact of its deletion (Wefer et al. 2003). However, in drosophila S2 cells lacking Boca, human LDLR is mis-trafficked (Culi and Mann 2003). Structural studies and deletion analysis show that Mesd specifically promotes folding and trafficking of the β propeller domain found in the EGF precursor homology region (Culi et al. 2004; Koduri and Blacklow 2007).
Misfolded LDLR undergoes proteasomal degradation
Class II mutants of LDLR do not properly fold in the ER and, as a result, do not reach the cell surface. This mutant, misfolded LDLR associates with the ER-resident chaperones Grp78, Grp94, ERp72, and calnexin (Jørgensen et al. 2000; Sørensen et al. 2005). Expression of class II mutants in cultured cells also activates the unfolded protein response (Sørensen et al. 2005). Misfolded LDLR is subsequently degraded via the proteasome. An analysis of several representative class II LDLR mutants showed that these variants were stabilized by proteasomal inhibitors (Li et al. 2004). However, inhibitors of lysosome acidification did not stabilize the mutants (Li et al. 2004).
Conclusion
Distributing lipids throughout the body requires an assortment of secreted macromolecules including lipoproteins, lipases, and receptors. These macromolecules are particularly dependent on both common and specialized ER quality control pathways for maturation in and secretion from their cells of origin (Figure 5). The reasons for their extraordinary need for ER quality control is unique to each component—the difficulty of assembling amphipathic molecules such as lipoproteins, for example, or the challenges of synthesizing receptors in cells that also produce their ligands. Regardless, the end result is that synthesis and trafficking of each component becomes a chain with many links that, if any is broken, can cause dyslipidemia.
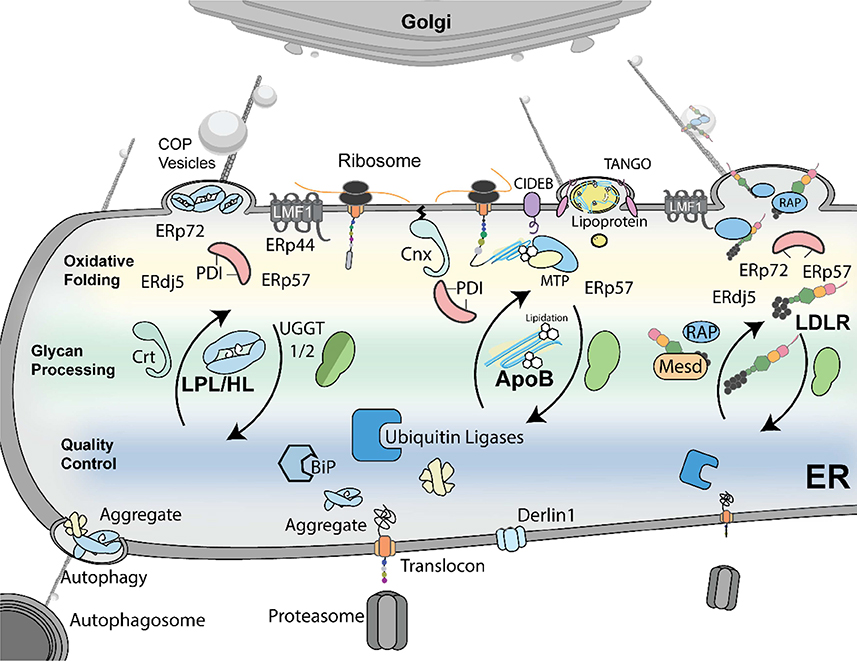
Figure 5: Quality control of LPL/HL, apoB, and LDLR in the ER.
Generalized and highly-specific chaperones are involved in oxidative folding, glycan processing, and quality control in the ER. The PDIs ERdJ5, ERp44, ERp57, ERp72, and PDI are abundant in the ER and facilitate the oxidative folding of LPL, HL, apoB, and LDLR. During oxidative folding, UGGTs glycosylate these folding proteins to promote their interactions with Crt, Cnx, and/or ERp57. In addition to these generalized chaperones, LPL/HL, apoB, and LDLR require unique chaperones to exit the ER. LMF1 is essential for the secretion of mature dimers of LPL and HL. ApoB is co-translationally lipidated and folded by MTP before leaving the ER with the help of TANGO. LDLR interacts with Mesd during folding before being chaperoned from the ER with the help of RAP. In the event of misfolding, Ubiquitin ligases and chaperones like BiP promote aggregate removal and eventual degradation by ERAD or autophagy.
We undoubtedly still have more to learn about the molecular mechanisms and players that ensure quality control of proteins involved in lipoprotein metabolism. For example, how does LMF1 affect redox homeostasis in the ER, and how many clients does it have? Why are LPL and HL so susceptible to misfolding? These and other questions can only be answered through multidisciplinary efforts. GWAS studies coupled with network analysis for dyslipidemic phenotypes can aid in uncovering new causal genes and lead to a better understanding of important proteins involved in these molecular pathways (Leiserson et al. 2013). Subsequent biochemical characterization of pathogenic variants involved in rare dyslipidemias may aid in further understanding mechanisms of ER quality control of proteins important for lipid metabolism (MacArthur et al. 2014).
NMR and crystal structures of LPL, LDLR, RAP, MTP and Mesd, have provided a wealth of information that sheds light on how these proteins function (Fass et al. 1997; Rudenko et al. 2002; Fisher et al. 2006; Köhler et al. 2011; Arora et al. 2019, Biterova et al., 2019). However, there are currently no published, high resolution structures of LMF1, HL, or apoB. Recent advances in cryo-electron microscopy have enabled the elucidation of structures for many previously intractable macromolecules (Lyumkis 2019). This technique may be useful for macromolecules involved in lipoprotein metabolism that have not yet been structurally characterized, due to their size, complexity, or heterogeneous nature. New genetic and structural information will further enhance our understanding of the molecular basis for dyslipidemias. Furthermore, efforts to develop drugs to improve ER proteostasis may provide a therapeutic avenue for rectifying the protein misfolding that causes some cases of dyslipidemias (Gonzalez-Teuber et al., 2019).
Table 1: The Clinical Effects of loss-of-function in Lipoprotein-Binding Proteins Examined in this Review and their Translational Models.
A variety of lipoprotein-associated disorders arise from mutations in lipoprotein binding proteins or their chaperones. Clinical loss-of-function mutations in the genes coding for ApoB, LDLR, LPL, HL, and RAP lead to changes in plasma lipoprotein levels and lipid transport. Similarly, loss-of-function mutations in their specialized chaperones (RAP, LMF1, MTP) lead to similar lipid disorders. Transgenic loss-of-function mouse models have been generated for most of these genes and underscore the importance of these genes in lipid homeostasis.
| Causative Gene | Associated Disorder | Mouse model | Clinical Manifestation | References |
|---|---|---|---|---|
| ApoB | Familial hypobetalipoproteinemia | Embryonic lethality in knockout animals, reduced plasma triglycerides in heterozygous mutants | Reduced plasma total cholesterol, LDL cholesterol, and ApoB levels, hepatic steatosis, fat-soluble vitamin deficiency. | Schonfeld et al. 2003; Chen et al. 2002; Farese et al. 1995 |
| LDLR | Familial hypercholesterolemia | Elevated plasma LDL cholesterol in knockout animals | Elevated plasma LDL cholesterol, increased risk of ischemic heart disease | Hobbs et al. 1992; Usifo et al. 2012; Marks et al. 2003 |
| LPL | Familial LPL deficiency | Severe hypertriglyceridemia, neonatal death | Severe hypertriglyceridemia (chylomicronemia) eruptive xanthomas, increased risk of pancreatitis. | Wittrup et al. 1999; Rodrigues et al. 2016; Weinstock et al. 1995 |
| HL | Hepatic Lipase Deficiency | Mild increase in total cholesterol, increased HDL cholesterol. | Elevated plasma HDL, no change in plasma triglycerides, increased risk of ischemic heart disease. | Homanics et al., 1995. Andersen et al. 2003 |
| RAP | Myopia | Whole-animal knockout causes elevated plasma LDL and TGs | Severe myopia and retinal degeneration. | Aldahmesh et al. 2013; Willnow et al. 1995 |
| LMF1 | Combined Lipase Deficiency | Embryonic lethality in homozygous knockout animals | Reduced post-heparin LPL activity, severe hypertriglyceridemia, increased risk of pancreatitis. | Serveaux Dancer et al. 2018; Peterfy et al. 2007; Ehrhardt et al. 2014 |
| MTP | Abetalipoproteinemia | Whole-animal homozygous knockout is embryonic lethal, heterozygous animals display reduced plasma lipoproteins | Fat malabsorption, hepatomegaly, hypocholesterolemia, low plasma apoB, low plasma triglycerides, fat-soluble vitamin deficiency. | Wetterau et al. 1992; Ramasamy 2016 |
Highlights.
Lipids are an important macronutrient, and appropriate distribution of lipids to tissues in the body is necessary for health.
Many of the lipoproteins, lipases and cell surface receptors involved in lipid metabolism have an extraordinary dependence on the ER folding and quality control machinery.
Some of the key players in lipoprotein metabolism require specialized chaperones, in addition to general chaperones, to fold and exit the ER.
Inherited mutations in several of these key players affect protein folding, impair normal lipoprotein metabolism, and link protein quality control with dyslipidemia.
Abbreviations used in this article
- apoB
Apolipoprotein B
- ApoER2
Apolipoprotein E receptor 2
- Bip
Binding immunoglobulin protein
- CIDEB
Cell death-inducing DFF45-like effector B
- Cnx
Calnexin
- COP
Coatomer protein
- Crt
Calreticulin
- EGF
Epidermal growth factor
- ER
Endoplasmic reticulum
- ERAD
ER-associated degradation
- ERp44
Endoplasmic reticulum resident protein 44
- ERp57
Endoplasmic reticulum resident protein 57
- ERp72
Endoplasmic reticulum resident protein 72
- FFA
Free fatty acid
- FH
Familial hypercholesterolemia
- GWAS
Genome wide association study
- HL
Hepatic lipase
- IDL
Intermediate-density lipoprotein
- LDLR
Low-density lipoprotein receptor
- LMF1
Lipase maturation factor 1
- LPL
Lipoprotein lipase
- LRP1
LDL Receptor Related Protein 1
- LRP1b
LDL Receptor Related Protein 1b
- LRP4
LDL Receptor Related Protein 4
- Mesd
Mesoderm development protein
- MTP
Microsomal triglyceride transfer protein
- PDI
Protein disulfide isomerase
- QC
Quality control
- RAP
Receptor-associated protein
- TALI
TANGO1-like
- TANGO1
Transport and Golgi organization protein 1
- UGGT
UDP-glucose:glycoprotein glucosyltransferase
- VLDL
Very low-density lipoprotein
- VLDLR
Very low-density lipoprotein receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldahmesh MA, Khan AO, Alkuraya H, Adly N, Anazi S, Al-Saleh AA, ... Alkuraya FS (2013). Mutations in LRPAP1 are associated with severe myopia in Humans. American Journal of Human Genetics, 93(2), 313–320. 10.1016/j.ajhg.2013.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RV, Wittrup HH, Tybjærg-Hansen A, Steffensen R, Schnohr P, Nordestgaard BG. 2003. Hepatic lipase mutations, elevated high-density lipoprotein cholesterol, and increased risk of ischemic heart disease: The Copenhagen City Heart Study. J Am Coll Cardiol. 41:1972–1982. doi: 10.1016/S0735-1097(03)00407-8. [DOI] [PubMed] [Google Scholar]
- Arora R, Nimonkar AV., Baird D, Wang C, Chiu C-H, Horton PA, Hanrahan S, Cubbon R, Weldon S, Tschantz WR, Mueller, Brunner R, Lehr P, Meier P, Ottl J, Voznesensky A, Pandey P, ... Trauger JW. 2019. Structure of lipoprotein lipase in complex with GPIHBP1. Proc Natl Acad Sci. 116:10360–10365. doi: 10.1073/pnas.1820171116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins AR, Brereton IM, Kroon PA, Lee HT, Smith R. 1998. Calcium is essential for the structural integrity of the cysteine-rich, ligand-binding repeat of the low-density lipoprotein receptor. Biochemistry. 37:1662–1670. doi: 10.1021/bi972529n. [DOI] [PubMed] [Google Scholar]
- Babilonia-Rosa MA, Neher SB. 2014. Purification, cellular levels, and functional domains of lipase maturation factor 1. Biochem Biophys Res Commun. 450:423–428. doi: 10.1016/j.bbrc.2014.05.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakizou H, Gannouni S, Messaoui K, Difilippo M, Sassolas A, Bayoudh F. 2016. Abetalipoproteinemia: a novel mutation of microsomal triglyceride transfer protein (MTP) gene in a young Tunisian patient. Egypt J Med Hum Genet. 17:251–254. doi: 10.1016/j.ejmhg.2015.12.003. [DOI] [Google Scholar]
- Ben-Zeev O, Doolittle MH. 2004. Maturation of hepatic lipase: formation of functional enzyme in the endoplasmic reticulum is the rate-limiting step in its secretion. J Biol Chem. 279:6171–6181. doi: 10.1074/jbc.M310051200. [DOI] [PubMed] [Google Scholar]
- Ben-Zeev O, Doolittle MH, Davis RC, Elovson J, Schotz MC. 1992. Maturation of lipoprotein lipase. Expression of full catalytic activity requires glucose trimming but not translocation to the cis-Golgi compartment. J Biol Chem. 267:6219–6227. [PubMed] [Google Scholar]
- Biterova E, Isupov MN, Keegan RM, Lebedev AA, Sohail AA, Liaqat I, Alanen HI, Ruddock LW. 2019. The crystal structure of human microsomal triglyceride transfer protein. Proc Natl Acad Sci U S A. 2019 August 8 pii: 201903029. doi: 10.1073/pnas.1903029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. 1976. Receptor-mediated control of cholesterol metabolism. Science. 191:150–4. [DOI] [PubMed] [Google Scholar]
- Bu G, Geuze HJ, Strous GJ, Schwartz AL. 1995. 39 kDa receptor-associated protein is an ER resident protein and molecular chaperone for LDL receptor-related protein. EMBO J. 14:2269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid NJ, Freedman RB. 1988. Defective co-translational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature. 335:649–651. doi: 10.1038/335649a0. [DOI] [PubMed] [Google Scholar]
- Burch WL, Herscovitz H. 2000. Disulfide bonds are required for folding and secretion of apolipoprotein B regardless of its lipidation state. J Biol Chem. 275:16267–16274. doi: 10.1074/jbc.M000446200. [DOI] [PubMed] [Google Scholar]
- Burnett JR, Shan J, Miskie BA, Whitfield AJ, Yuan J, Tran K, McKnight CJ, Hegele RA, Yao Z. 2003. A novel nontruncating APOB gene mutation, R463W, causes familial hypobetalipoproteinemia. J Biol Chem. 278:13442–52. doi: 10.1074/jbc.M300235200. [DOI] [PubMed] [Google Scholar]
- Burnett JR, Zhong S, Jiang ZG, Hooper AJ, Fisher EA, McLeod RS, Zhao Y, Barrett PHR, Hegele RA, van Bockxmeer FM, Zhang H, Vance DE, McKnight CJ, Yao Z. 2007. Missense mutations in APOB within the betaalpha1 domain of human APOB-100 result in impaired secretion of ApoB and ApoB-containing lipoproteins in familial hypobetalipoproteinemia. J Biol Chem. 282:24270–83. doi: 10.1074/jbc.M702442200. [DOI] [PubMed] [Google Scholar]
- Buscá R, Martínezi M, Vilella E, Pognonec P, Deeb S, Auwerx J, Reina M, Vilaro S. 1996. The mutation gly142 → glu in human lipoprotein lipase produces a missorted protein that is diverted to lysosomes. J Biol Chem. 271:2139–2146. doi: 10.1074/jbc.271.4.2139. [DOI] [PubMed] [Google Scholar]
- Buscá R, Pujana M a, Pognonec P, Auwerx J, Deeb SS, Reina M, Vilaró S. 1995. Absence of N-glycosylation at asparagine 43 in human lipoprotein lipase induces its accumulation in the rough endoplasmic reticulum and alters this cellular compartment. J Lipid Res. 36:939–951. [PubMed] [Google Scholar]
- Carmichael DF, Morin JE, Dixon JE. 1977. Purification and characterization of a thiol:protein disulfide oxidoreductase from bovine liver. J Biol Chem. 252:7163–7. [PubMed] [Google Scholar]
- Chen Y, Le Cahérec F, Chuck SL. 1998. Calnexin and other factors that alter translocation affect the rapid binding of ubiquitin to ApoB in the Sec61 complex. J Biol Chem. 273:11887–11894. doi: 10.1074/jbc.273.19.11887. [DOI] [PubMed] [Google Scholar]
- Chen Z, Fitzgerald RL, Schonfeld G. 2002. Hypobetalipoproteinemic mice with a targeted apolipoprotein (Apo) B-27.6-specifying mutation: in vivo evidence for an important role of amino acids 1254–1744 of ApoB in lipid transport and metabolism of the apoB-containing lipoprotein. J Biol Chem. 277(16):14135–45 [DOI] [PubMed] [Google Scholar]
- Chora JR, Medeiros AM, Alves AC, Bourbon M. 2018. Analysis of publicly available LDLR, APOB, and PCSK9 variants associated with familial hypercholesterolemia: Application of ACMG guidelines and implications for familial hypercholesterolemia diagnosis. Genet Med. 20:591–598. doi: 10.1038/gim.2017.151. [DOI] [PubMed] [Google Scholar]
- Culi J, Mann RS. 2003. Boca, an endoplasmic reticulum protein required for wingless signaling and trafficking of LDL receptor family members in Drosophila. Cell. 112:343–354. doi: 10.1016/S0092-8674(02)01279-5. [DOI] [PubMed] [Google Scholar]
- Culi J, Springer TA, Mann RS. 2004. Boca-dependent maturation of β-propeller/EGF modules in low-density lipoprotein receptor proteins. EMBO J. 23:1372–1380. doi: 10.1038/sj.emboj.7600132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann M, Dietrich MF, Herz J. 2010. Lipoprotein receptors-an evolutionarily ancient multifunctional receptor family. Biol Chem. 391:1341–1363. doi: 10.1515/BC.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle MH, Ben-Zeev O, Bassilian S, Whitelegge JP, Péterfy M, Wong H. 2009. Hepatic lipase maturation: a partial proteome of interacting factors. J Lipid Res. 50:1173–1184. doi: 10.1194/jlr.m800603-jlr200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt N, Bedoya C, & Péterfy M (2014). Embryonic viability, lipase deficiency, hypertriglyceridemia and neonatal lethality in a novel LMF1-deficient mouse model. Nutrition and Metabolism, 11(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A. 2003. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Molinari M, Helenius A. 1999. Setting the standards: quality control in the secretory pathway. Science. 286:1882–1888. [DOI] [PubMed] [Google Scholar]
- Esser V, Limbird LE, Brown MS, Goldstein JL, Russell DW. 1988. Mutational analysis of the ligand binding domain of the low density lipoprotein receptor. J Biol Chem. 263:13282–13290. [PubMed] [Google Scholar]
- Farese RV Jr, Ruland SL, Flynn LM, Stokowski RP, Young SG. 1995. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc Natl Acad Sci U S A. 92(5):1774–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass D, Blacklow S, Kim PS, Berger JM. 1997. Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature. 388:691–693. doi: 10.1038/41798. [DOI] [PubMed] [Google Scholar]
- Fisher C, Beglova N, Blacklow SC. 2006. Structure of an LDLR-RAP Complex reveals a general mode for ligand recognition by lipoprotein receptors. Mol Cell. 22:277–283. doi: 10.1016/j.molcel.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Fisher EA, Pan M, Chen X, Wu X, Wang H, Jamil H, Sparks JD, Williams KJ. 2001. The triple threat to nascent apolipoprotein B. J Biol Chem. 276:27855–27863. doi: 10.1074/jbc.M008885200. [DOI] [PubMed] [Google Scholar]
- Fisher RM, Humphries SE, Talmud PJ. 1997. Common variation in the lipoprotein lipase gene: effects on plasma lipids and risk of atherosclerosis. Atherosclerosis. 135:145–159. doi: 10.1016/S0021-9150(97)00199-8. [DOI] [PubMed] [Google Scholar]
- Glickman RM, Rogers M, Glickman JN. 1986. Apolipoprotein B synthesis by human liver and intestine in vitro. Proc Natl Acad Sci. 83:5296–300. doi: 10.1073/pnas.83.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Teuber V, Albert-Gasco H, Auyeung VC, Papa FR, Mallucci GR, and Hetz C (2019). Small Molecules to Improve ER Proteostasis in Disease. Trends Pharmacol Sci. [DOI] [PubMed] [Google Scholar]
- Gordon DA, Jamil H, Sharp D, Mullaney D, Yao Z, Gregg RE, Wetterau J. 1994. Secretion of apolipoprotein B-containing lipoproteins from HeLa cells is dependent on expression of the microsomal triglyceride transfer protein and is regulated by lipid availability. Proc Natl Acad Sci. 91:7628–32. doi: 10.1073/PNAS.91.16.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffon N, Jin W, Petty TJ, Millar J, Badellino KO, Saven JG, Marchadier DH, Kempner ES, Billheimer J, Glick JM, Rader DJ. 2009. Identification of the active form of endothelial lipase, a homodimer in a head-to-tail conformation. J Biol Chem. 284:23322–23330. doi: 10.1074/jbc.M109.037002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss G, Tamir I, Davis CE, Tyroler HA, Rifkand BM, Schonfeld G, Jacobs D, Frantz ID. 1980. Lipoprotein-cholesterol distributions in selected North American populations: the lipid research clinics program prevalence study. Circulation. 61:302–315. doi: 10.1161/01.CIR.61.2.302. [DOI] [PubMed] [Google Scholar]
- Herz J, Goldstein JL, Strickland DK, Ho YK, Brown MS. 1991. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 266:21232–21238. [PubMed] [Google Scholar]
- Hobbs HH, Brown MS, Goldstein JL. 1992. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1:445–466. doi: 10.1002/humu.1380010602. [DOI] [PubMed] [Google Scholar]
- Homanics GE, de Silva HV, Osada J, Zhang SH, Wong H, Borensztajn J, Maeda N. 1995. Mild dyslipidemia in mice following targeted inactivation of the hepatic lipase gene. J Biol Chem 270:2974–2980 [DOI] [PubMed] [Google Scholar]
- Huang XF, Shelness GS. 1997. Identification of cysteine pairs within the amino-terminal 5% of apolipoprotein B essential for hepatic lipoprotein assembly and secretion. J Biol Chem. 272:31872–6. doi: 10.1074/JBC.272.50.31872. [DOI] [PubMed] [Google Scholar]
- Hussain MM, Bakillah A, Jamil H. 1997. Apolipoprotein B binding to microsomal triglyceride transfer protein decreases with increases in length and lipidation: implications in lipoprotein biosynthesis. Biochem. 36:13060–13067. doi: 10.1021/BI971395A. [DOI] [PubMed] [Google Scholar]
- Iijima H, Miyazawa M, Sakai J, Magoori K, Ito MR, Suzuki H, Nose M, Kawarabayasi Y, Yamamoto TT. 1998. Expression and characterization of a very low density lipoprotein receptor variant lacking the O-linked sugar region generated by alternative splicing. J Biochem. 124:747–755. doi: 10.1093/oxfordjournals.jbchem.a022175. [DOI] [PubMed] [Google Scholar]
- Jamil H, Gordon DA, Eustice DC, Brooks CM, Dickson JK, Chen Y, Ricci B, Chu CH, Harrity TW, Ciosek CP, Biller SA, Gregg RE, Wetterau JR, Gregg RE, Wetterau JR. 1996. An inhibitor of the microsomal triglyceride transfer protein inhibits apoB secretion from HepG2 cells. Proc Natl Acad Sci. 93:11991–5. doi: 10.1073/pnas.93.21.11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansens A, Van Duijn E, Braakman I. 2002. Coordinated nonvectorial folding in a newly synthesized multidomain protein. Science. 298:2401–2403. doi: 10.1126/science.1078376. [DOI] [PubMed] [Google Scholar]
- Jeon H, Meng W, Takagi J, Eck MJ, Springer TA, Blacklow SC. 2001. Implications for familial hypercholesterolemia from the structure of the LDL receptor YWTD-EGF domain pair. Nat Struct Biol. 8:499–504. doi: 10.1038/88556. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Gantz D, Bullitt E, McKnight CJ. 2006. Defining lipid-interacting domains in the N-terminal region of apolipoprotein B. Biochemistry. 45:11799–11808. doi: 10.1021/bi060600w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen MM, Jensen ON, Holst HU, Hansen JJ, Corydon TJ, Bross P, Bolund L, Gregersen N. 2000. Grp78 is involved in retention of mutant low density lipoprotein receptor protein in the endoplasmic reticulum. J Biol Chem. 275:33861–33868. doi: 10.1074/jbc.M004663200. [DOI] [PubMed] [Google Scholar]
- Kane JP, Hardman DA, Paulus HE. 1980. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci. 77:2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koduri V, Blacklow SC. 2001. Folding determinants of LDL receptor type A modules. Biochemistry. 40:12801–12807. doi: 10.1021/bi011344k. [DOI] [PubMed] [Google Scholar]
- Koduri V, Blacklow SC. 2007. Requirement for natively unstructured regions of mesoderm development candidate 2 in promoting low-density lipoprotein receptor-related protein 6 maturation. Biochemistry. 46:6570–6577. doi: 10.1021/bi700049g. [DOI] [PubMed] [Google Scholar]
- Köhler C, Lighthouse JK, Werther T, Andersen OM, Diehl A, Schmieder P, Du J, Holdener BC, Oschkinat H. 2011. The structure of MESD45–184 brings light into the mechanism of LDLR family folding. Structure. 19:337–348. doi: 10.1016/j.str.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky K, Kingsley D, Krieger M. 1988. Use of a mutant cell line to study the kinetics and function of O-linked glycosylation of low density lipoprotein receptors. Proc Natl Acad Sci. 85:4335–4339. doi: 10.1073/pnas.85.12.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulinski A, Rustaeus S, Vance J. 2002. Microsomal triacylglycerol transfer protein is required for lumenal accretion of triacylglycerol not associated with ApoB, as well as for ApoB lipidation. J Biol Chem. 277:31516–31525. doi: 10.1074/jbc.M202015200. [DOI] [PubMed] [Google Scholar]
- Kurniawan ND, Aliabadizadeh K, Brereton IM, Kroon PA, Smith R. 2001. NMR structure and backbone dynamics of a concatemer of epidermal growth factor homology modules of the human low-density lipoprotein receptor. J Mol Biol. 311:341–356. doi: 10.1006/jmbi.2001.4867. [DOI] [PubMed] [Google Scholar]
- Lee D, Walsh JD, Mikhailenko I, Yu P, Migliorini M, Wu Y, Krueger S, Curtis JE, Harris B, Lockett S, Blacklow SC, Strickland DK, Wang YX. 2006. RAP Uses a Histidine Switch to Regulate Its Interaction with LRP in the ER and Golgi. Mol Cell. 22:423–430. doi: 10.1016/j.molcel.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Leiserson MDM, Blokh D, Sharan R, & Raphael BJ (2013). Simultaneous Identification of Multiple Driver Pathways in Cancer. PLoS Computational Biology, 9(5). 10.1371/journal.pcbi.1003054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lu W, Schwartz AL, Bu G. 2002. Receptor-associated protein facilitates proper folding and maturation of the low-density lipoprotein receptor and its class 2 mutants. Biochemistry. 41:4921–4928. doi: 10.1021/bi011894i. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu W, Schwartz AL, Bu G. 2004. Degradation of the LDL receptor class 2 mutants is mediated by a proteasome-dependent pathway. J Lipid Res. 45:1084–1091. doi: 10.1194/jlr.m300482-jlr200. [DOI] [PubMed] [Google Scholar]
- Liang J-S, Kim T, Fang S, Yamaguchi J, Weissman AM, Fisher EA, Ginsberg HN. 2003. Overexpression of the tumor autocrine motility factor receptor Gp78, a ubiquitin protein ligase, results in increased ubiquitinylation and decreased secretion of apolipoprotein B100 in HepG2 cells. J Biol Chem. 278:23984–8. doi: 10.1074/jbc.M302683200. [DOI] [PubMed] [Google Scholar]
- Luo C, Wang F, Ren X, Ke T, Xu C, Tang B, ... Wang QK (2017). Identification of a molecular signaling gene-gene regulatory network between GWAS susceptibility genes ADTRP and MIA3/TANGO1 for coronary artery disease. Biochimica et Biophysica Acta - Molecular Basis of Disease. 10.1016/j.bbadis.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyumkis D (2019). Challenges and opportunities in cryo-EM single-particle analysis. Journal of Biological Chemistry, 294(13), 5181–5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, ... Gunter C (2014). Guidelines for investigating causality of sequence variants in human disease. Nature, 508(7497), 469–476. 10.1038/nature13127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks D, Thorogood M, Neil HAW, & Humphries SE (2003). A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis, 168(1), 1–14. [DOI] [PubMed] [Google Scholar]
- Marques-Vidal P, Azéma C, Collet X, Chap H, Perret BP. 1991. Hepatic lipase-mediated hydrolysis versus liver uptake of HDL phospholipids and triacylglycerols by the perfused rat liver. Biochim Biophys Acta (BBA)/Lipids Lipid Metab. 1082:185–194. doi: 10.1016/0005-2760(91)90193-L. [DOI] [PubMed] [Google Scholar]
- Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. 2009. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 417:651–666. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- Miller GC, Long CJ, Bojilova ED, Marchadier D, Badellino KO, Blanchard N, Fuki IV, Glick JM, Rader DJ. 2004. Role of N-linked glycosylation in the secretion and activity of endothelial lipase. J Lipid Res. 45:2080–2087. doi: 10.1194/jlr.m400162-jlr200. [DOI] [PubMed] [Google Scholar]
- Murthy V, Julien P, Gagné C. 1996. Molecular pathobiology of the human lipoprotein lipase gene. Pharmacol Ther. 70:101–135. doi: 10.1016/0163-7258(96)00005-8. [DOI] [PubMed] [Google Scholar]
- Musliner TA, Herbert PN, Kingston MJ. 1979. Lipoprotein substrates of lipoprotein lipase and hepatic triacylglycerol lipase from human post-heparin plasma. Biochim Biophys Acta-Mol Cell Biol Lipids. 575:277–288. [DOI] [PubMed] [Google Scholar]
- Oka OBV, Pringle MA, Schopp IM, Braakman I, Bulleid NJ. 2013. ERdj5 is the ER reductase that catalyzes the removal of non-native disulfides and correct folding of the LDL receptor. Mol Cell. 50:793–804. doi: 10.1016/j.molcel.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivecrona T, Chernicks SS, Bengtsson-Olivecrona G, Paterniti JR, Brown WV, Scows R. 1985. Combined lipase deficiency ( cld / cld ) in mice. J Biol Chem. 260:2552–2557. [PubMed] [Google Scholar]
- Pan M, Maitin V, Parathath S, Andreo U, Lin SX, Germain C St., Yao Z, Maxfield FR, Williams KJ, Fisher EA. 2008. Presecretory oxidation, aggregation, and autophagic destruction of apoprotein-B: A pathway for late-stage quality control. Proc Natl Acad Sci. 105:5862–5867. doi: 10.1073/pnas.0707460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterniti JR, Brown WV, Ginsberg HN, Artzt K. 1983. Combined lipase deficiency (cld): A lethal mutation on chromosome 17 of the mouse. Science. 221:167–169. doi: 10.1126/science.6857276. [DOI] [PubMed] [Google Scholar]
- Patni N, Ahmad Z, and Wilson DP (2000). Genetics and Dyslipidemia. In Endotext, Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, and Wilson DP, eds. (South Dartmouth (MA)). [Google Scholar]
- Péterfy M, Ben-Zeev O, Mao HZ, Weissglas-Volkov D, Aouizerat BE, Pullinger CR, Frost PH, Kane JP, Malloy MJ, Reue K, Pajukanta P, Doolittle MH. 2007. Mutations in LMF1 cause combined lipase deficiency and severe hypertriglyceridemia. Nat Genet. 39:1483–1487. doi: 10.1038/ng.2007.24. [DOI] [PubMed] [Google Scholar]
- Ramasamy I 2016. Update on the molecular biology of dyslipidemias. Clin Chim Acta. 454:143–185. doi: 10.1016/j.cca.2015.10.033. [DOI] [PubMed] [Google Scholar]
- Rehberg EF, Samson-Bouma ME, Kienzle B, Blinderman L, Jamil H, Wetterau JR, Aggerbeck LP, Gordon DA. 1996. A novel abetalipoproteinemia genotype. Identification of a missense mutation in the 97-kDa subunit of the microsomal triglyceride transfer protein that prevents complex formation with protein disulfide isomerase. J Biol Chem. 271:29945–52. [DOI] [PubMed] [Google Scholar]
- Roberts BS, Babilonia-Rosa MA, Broadwell LJ, Wu MJ, Neher SB. 2018. Lipase maturation factor 1 affects redox homeostasis in the endoplasmic reticulum. EMBO J. 298:2353–2358. doi: 10.15252/embj.201797379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues R, Artieda M, Tejedor D, Martinez A, Konstantinova P, Petry H, Meyer C, Corzo D, Sundgreen C, Klor HU, Gouni-Berthold I, Westphal S, Steinhagen-Thiessen E, Julius U, Winkler K, Stroes E, Vogt A, ... Brunzell JD. 2016. Pathogenic classification of LPL gene variants reported to be associated with LPL deficiency. J Clin Lipidol. 10:394–409. doi: 10.1016/j.jacl.2015.12.015. [DOI] [PubMed] [Google Scholar]
- Rudenko G, Henry L, Henderson K, Ichtchenko K, Brown MS, Goldstein JL, Deisenhofer J. 2002. Structure of the LDL receptor extracellular domain at endosomal pH. Science. doi: 10.1126/science.1078124. [DOI] [PubMed] [Google Scholar]
- Ruf H, Gould BJ. 1998. Size distributions of chylomicrons from human lymph from dynamic light scattering measurements. Eur Biophys J. 28:1–11. doi: 10.1007/s002490050178. [DOI] [PubMed] [Google Scholar]
- Rutledge AC, Qiu W, Zhang R, Kohen-Avramoglu R, Nemat-Gorgani N, Adeli K. 2009. Mechanisms targeting apolipoprotein B100 to proteasomal degradation. Arterioscler Thromb Vasc Biol. 29:579–585. doi: 10.1161/ATVBAHA.108.181859. [DOI] [PubMed] [Google Scholar]
- Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, Polischuk R, Schekman R, Malhotra V. 2009. TANGO1 Facilitates Cargo Loading at Endoplasmic Reticulum Exit Sites. Cell. 136:891–902. doi: 10.1016/j.cell.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Sani MN, Sabbaghian M, Mahjoob F, Cefalù AB, Averna MR, Rezaei N. 2011. Identification of a novel mutation of MTP gene in a patient with abetalipoproteinemia. Ann Hepatol. 10:221–6. [PubMed] [Google Scholar]
- Santos AJM, Nogueira C, Ortega-Bellido M, Malhotra V. 2016. TANGO1 and Mia2/cTAGE5 (TALI) cooperate to export bulky pre-chylomicrons/VLDLs from the endoplasmic reticulum. J Cell Biol. 213:343–54. doi: 10.1083/jcb.201603072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld G 2003. Familial hypobetalipoproteinemia: a review. J Lipid Res. 44:878–83. doi: 10.1194/jlr.R300002-JLR200. [DOI] [PubMed] [Google Scholar]
- Schumaker VN, Phillips ML, Chatterton JE. 1994. Apolipoprotein B and low-density lipoprotein structure: implications for biosynthesis of triglyceride-rich lipoproteins. Adv Protein Chem. 45:205–248. doi: 10.1016/S0065-3233(08)60641-5. [DOI] [PubMed] [Google Scholar]
- Segrest JP, Jones MK, De Loof H, Dashti N. 2001. Structure of apolipoprotein B-100 in low density lipoproteins. J Lipid Res. 42:1346–67. [PubMed] [Google Scholar]
- Serveaux Dancer M, Di Filippo M, Marmontel O, Valéro R, Piombo Rivarola MDC, Peretti N, Caussy C, Krempf M, Vergès B, Mahl M, Marçais C, Moulin P, Charrière S. 2018. New rare genetic variants of LMF1 gene identified in severe hypertriglyceridemia. J Clin Lipidol. 12:1244–1252. doi: 10.1016/j.jacl.2018.06.018. [DOI] [PubMed] [Google Scholar]
- Sha H, Sun S, Francisco AB, Ehrhardt N, Xue Z, Liu L, Lawrence P, Mattijssen F, Guber RD, Panhwar MS, Brenna JT, Shi H, Xue B, Kersten S, Bensadoun A, Péterfy M, Long Q, Qi L. 2014. The ER-associated degradation adaptor protein Sel1 L regulates LPL secretion and lipid metabolism. Cell Metab. 20:458–470. doi: 10.1016/j.cmet.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelness GS, Thornburg JT. 1996. Role of intramolecular disulfide bond formation in the assembly and secretion of apolipoprotein B-100-containing lipoproteins. J Lipid Res. 37:408–19. [PubMed] [Google Scholar]
- Simha V, Garg A. 2009. Inherited lipodystrophies and hypertriglyceridemia. Curr Opin Lipidol. 20:300–308. doi: 10.1097/MOL.0b013e32832d4a33. [DOI] [PubMed] [Google Scholar]
- Simsolo RB, Ong JM, Kern PA. 1992. Characterization of lipoprotein lipase activity, secretion, and degradation at different sites of post-translational processing in primary cultures of rat adipocytes. J Lipid Res. 33:1777–84. [PubMed] [Google Scholar]
- Sirwi A, Hussain MM. 2018. Lipid transfer proteins in the assembly of apoB-containing lipoproteins. J Lipid Res. 59:1094–1102. doi: 10.1194/jlr.R083451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen S, Bakken KS, Kulseth MA, Ranheim T, Leren TP. 2005. Retention of mutant low density lipoprotein receptor in endoplasmic reticulum (ER) leads to ER stress. J Biol Chem. 281:468–476. doi: 10.1074/jbc.m507071200. [DOI] [PubMed] [Google Scholar]
- Strom TB, Laerdahl J, Leren TP. 2015. Mutation p.L799R in the LDLR, which affects the transmembrane domain of the LDLR, prevents membrane insertion and causes secretion of the mutant LDLR. Hum Mol Genet. 24:5836–5844. doi: 10.1093/hmg/ddv304 [DOI] [PubMed] [Google Scholar]
- Strom TB, Tveten K, Holla OL, Cameron J, Berge KE, Leren TP. 2011. Characterization of residues in the cytoplasmic domain of the LDL receptor required for exit from the endoplasmic reticulum. Biochem Biophys Res Commun. 415:642–645. doi: 10.1016/j.bbrc.2011.10.127. [DOI] [PubMed] [Google Scholar]
- Tatu U, Helenius A. 1999. Interaction of newly synthesized apolipoprotein B with calnexin and calreticulin requires glucose trimming in the endoplasmic reticulum. Biosci Rep. 19:189–96. [DOI] [PubMed] [Google Scholar]
- Tran K, Borén J, Macri J, Wang Y, McLeod R, Avramoglu RK, Adeli K, Yao Z. 1998. Functional analysis of disulfide linkages clustered within the amino terminus of human apolipoprotein B. J Biol Chem. 273:7244–7251. doi: 10.1074/JBC.273.13.7244. [DOI] [PubMed] [Google Scholar]
- Trombetta SE, Bosch M, Parodi AJ. 1989. Glucosylation of glycoproteins by mammalian, plant, fungal, and trypanosomatid protozoa microsomal membranes. Biochemistry. 28:8108–8116. doi: 10.1021/bi00446a022. [DOI] [PubMed] [Google Scholar]
- Ushioda R, Hoseki J, Araki K, Jansen G, Thomas DY, Nagata K. 2008. ERdj5 is required as a disulfide reductase for degradation of misfolded proteins in the ER. Science. 321:569–572. doi: 10.1126/science.1159293. [DOI] [PubMed] [Google Scholar]
- Usifo E, Leigh SE, Whittall RA, Lench N, Taylor A, Yeats C, Orengo CA, Martin AC, Celli J, Humphries SE. 2012. Low-density lipoprotein receptor gene familial hypercholesterolemia variant database: update and pathological assessment. Ann Hum Genet. 387–401. doi: 10.1111/j.1469-1809.2012.00724.x. [DOI] [PubMed] [Google Scholar]
- Vannier C, Sylviane D, Pradines-Figuères A, Ailhaud G. 1989. Biosynthesis of lipoprotein lipase in cultured mouse adipocytes. J Biol Chem. 264:13206–13216. [PubMed] [Google Scholar]
- Vukmirica J, Nishimaki-Mogami T, Tran K, Shan J, McLeod RS, Yuan J, Yao Z. 2002. The N-linked oligosaccharides at the amino terminus of human apoB are important for the assembly and secretion of VLDL. J Lipid Res. 43:1496–1507. doi: 10.1194/JLR.M200077-JLR200. [DOI] [PubMed] [Google Scholar]
- Walsh MT, Di Leo E, Okur I, Tarugi P, Hussain MM. 2016. Structure-function analyses of microsomal triglyceride transfer protein missense mutations in abetalipoproteinemia and hypobetalipoproteinemia subjects. Biochim Biophys Acta - Mol Cell Biol Lipids. 1861:1623–1633. doi: 10.1016/j.bbalip.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Wang L, Fast DG, Attie AD. 1997. The enzymatic and non-enzymatic roles of protein-disulfide isomerase in apolipoprotein B secretion. J Biol Chem. 272:27644–51. doi: 10.1074/JBC.272.44.27644. [DOI] [PubMed] [Google Scholar]
- Wang S, Park S, Kodali VK, Han J, Yip T, Chen Z, Davidson NO, Kaufman RJ. 2015. Identification of protein disulfide isomerase 1 as a key isomerase for disulfide bond formation in apolipoprotein B100. Mol Biol Cell. 26:594–604. doi: 10.1091/mbc.E14-08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefer S, Brown K, DeRossi C, Wines ME, Rosenquist T, Holdener BC. 2003. Mesd Encodes an LRP5/6 Chaperone Essential for Specification of Mouse Embryonic Polarity. Cell. 112:355–367. doi: 10.1016/S0092-8674(03)00045-X. [DOI] [PubMed] [Google Scholar]
- Weinstock PH, Bisgaier CL, Aalto-Setala K, Radner H, Ramakrishnan R, Levak-Frank S, Essenburg AD, Zechner R, Breslow JL. 1995. Severe hypertriglyceridemia, reduced high density lipoprotein, and neonatal death in lipoprotein lipase knockout mice. Mild hypertriglyceridemia with impaired very low density lipoprotein clearance in heterozygotes. J Clin Invest. (6):2555–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, Gay G, Rader DJ, Gregg RE. 1992. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 258:999–1001. doi: 10.1126/science.1439810. [DOI] [PubMed] [Google Scholar]
- Wetterau JR, Combs KA, Spinner SN, McLean LR, Aggerbeck LP. 1991. Protein disulfide isomerase appears necessary to maintain the catalytically active structure of the microsomal triglyceride transfer protein. Biochemistry. 30:9728–9735. doi: 10.1021/bi00104a023. [DOI] [PubMed] [Google Scholar]
- Wetterau JR, Zilversmit DB. 1984. A triglyceride and cholesteryl ester transfer protein associated with liver microsomes. J Biol Chem. 259:10863–6. [PubMed] [Google Scholar]
- Wetterau JR, Zilversmit DB. 1985. Purification and characterization of microsomal triglyceride and cholesteryl ester transfer protein from bovine liver microsomes. Chem Phys Lipids. 38:205–22. [DOI] [PubMed] [Google Scholar]
- Willnow TE, Armstrong SA, Hammer RE, & Herz J (1995). Functional expression of low density lipoprotein receptor-related protein is controlled by receptor-associated protein in vivo. Proceedings of the National Academy of Sciences of the United States of America, 92(10), 4537–4541. 10.1073/pnas.92.10.4537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittrup HH, Tybjærg-Hansen A, Nordestgaard BG. 1999. Lipoprotein lipase mutations, plasma lipids and lipoproteins, and risk of ischemic heart disease: A meta-analysis. Circulation. 99:2901–2907. doi: 10.1161/01.CIR.99.22.2901. [DOI] [PubMed] [Google Scholar]
- Wong H, Schotz MC. 2002. The lipase gene family. J Lipid Res. 43:993–999. doi: 10.1194/jlr.r200007-jlr200. [DOI] [PubMed] [Google Scholar]
- Wu MJ, Wolska A, Roberts BS, Pearson EM, Gutgsell AR, Remaley AT, & Neher SB (2018). Co-expression of Novel Furin-Resistant LPL Variants with LMF1 Enhances LPL Secretion and Activity. Journal of Lipid Research, 1(Dc), jlr.D086793. 10.1194/jlr.D086793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Kim TW, Weng SA, Lee BR, Yang ML, Gotto AM. 1990. Isolation and characterization of sulfhydryl and disulfide peptides of human apolipoprotein B-100. Proc Natl Acad Sci. 87:5523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Li JZ, Liu Y, Li X, Yang T, Ma X, Li Q, Yao Z, Li P. 2009. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by Interacting with Apolipoprotein B. Cell Metab. 9:177–190. doi: 10.1016/j.cmet.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA. 2004. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature. 429:841–847. doi: 10.1038/nature02656. [DOI] [PubMed] [Google Scholar]
- Yeung SJ, Chen SH, Chan L. 1996. Ubiquitin-proteasome pathway mediates intracellular degradation of apolipoprotein B. Biochemistry. 35. doi: 10.1021/BI9618777. [DOI] [PubMed] [Google Scholar]
- Zhang L-J, Wang C, Yuan Y, Wang H, Wu J, Liu F, Li L, Gao X, Zhao Y-L, Hu P-Z, Li P, Ye J. 2014. Cideb facilitates the lipidation of chylomicrons in the small intestine. J Lipid Res. 55:1279–1287. doi: 10.1194/jlr.M046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lookene A, Wu G, Olivecrona G. 2005. Calcium triggers folding of lipoprotein lipase into active dimers. J Biol Chem. 280:42580–42591. doi: 10.1074/jbc.M507252200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wu G, Tate CG, Lookene A, Olivecrona G. 2003. Calreticulin promotes folding/dimerization of human lipoprotein lipase expressed in insect cells. J Biol Chem. 278:29344–29351. doi: 10.1074/jbc.M300455200. [DOI] [PubMed] [Google Scholar]