Abstract
Aim: To explore the mechanism by which long non-coding RNA (lncRNA) TTN-AS1 regulates osteosarcoma cell apoptosis and drug resistance via the microRNA miR-134-5p/malignant brain tumour domain containing 1 (MBTD1) axis.
Results: The lncRNA TTN-AS1 was highly expressed in osteosarcoma and was associated with poor prognosis. The lncRNA TTN-AS1 promoted cell viability and inhibited apoptosis. MiR-134-5p targeted MBTD1, which was regulated by lncRNA TTN-AS1. MBTD1 was highly expressed in osteosarcoma and was associated with poor prognosis. MBTD1 promoted cell viability and inhibited apoptosis, and knockdown of MBTD1 reversed the cancer-promoting effects of lncRNA TTN-AS1. Downregulation of lncRNA TTN-AS1 reduced drug resistance.
Conclusion: In osteosarcoma, lncRNA TTN-AS1 promoted the expression of MBTD1 by targeting miR-134-5p and regulated cell growth, apoptosis and drug resistance.
Methods: The expression characteristics of genes in osteosarcoma patients were analysed using bioinformatics. Plasmid transfection technology was applied to silence or overexpress lncRNA TTN-AS1, miR-134-5p and MBTD1. Western blotting and quantitative polymerase chain reaction (qPCR) were used to detect protein and RNA, respectively. A cell counting kit 8 (CCK-8) and flow cytometry were used to detect cell viability and apoptosis. The effects of lncRNA TTN-AS1 and MBTD1 on osteosarcoma in vivo were studied by using a tumour burden assay.
Keywords: osteosarcoma, lncRNA TTN-AS1, miR-134-5p, malignant brain tumour domain containing protein 1, resistance, aging and age-related diseases
INTRODUCTION
Osteosarcoma is a musculoskeletal malignancy that originates in the interstitial cell line [1]. The incidence of osteosarcoma is approximately 3 per million, and the incidence of osteosarcoma in men is higher than that in women [2]. The predilection site of osteosarcoma is the medullary ends of the long bones, especially the distal femur and the proximal humerus around the knee joint [3]. The course of osteosarcoma progresses rapidly, and morbidity and mortality are high [4, 5]. Currently, the main treatments for osteosarcoma include surgery, chemotherapy, immunotherapy and gene therapy [6–8]. The strong proliferative capacity and drug resistance of osteosarcoma cells are important factors affecting prognosis [9, 10].
Long non-coding RNA (lncRNA) typically has a length of more than 200 nucleotides [11]. LncRNAs do not have protein translation capabilities, but they play an important role in various processes by directly or indirectly regulating protein expression [12]. LncRNAs can not only directly participate in the regulation of gene expression but can also act as competing endogenous RNAs (ceRNAs) to interact with miRNAs [13, 14]. A microRNA (miRNA) is an evolutionarily conserved single-stranded RNA that typically contains 21–24 nucleotides [15]. MiRNAs play a key role in the post-transcriptional regulation of mRNA by targeting the 3′ untranslated region (UTR) of mRNA, leading to mRNA degradation or translational inhibition [15]. In recent years, many studies have found that lncRNA is involved in the development and progression of osteosarcoma as ceRNA [16–18]. The number of single lncRNAs is large, and the pathways are diverse. Bioinformatics helps to screen for meaningful lncRNAs [19, 20].
In this paper, we found that the lncRNA TTN-AS1 and malignant brain tumour domain containing protein 1 (MBTD1) were overexpressed in osteosarcoma, and they were associated with the poor prognosis of osteosarcoma patients. Based on this, we revealed a novel mechanism by which lncRNA TTN-AS1 regulated proliferation and apoptosis of osteosarcoma cells via miR-134-5p/MBTD1. This may provide new ideas for the discovery of strategies for osteosarcoma treatment.
RESULTS
LncRNA TTN-AS1 is highly expressed in osteosarcoma and is associated with poor prognosis
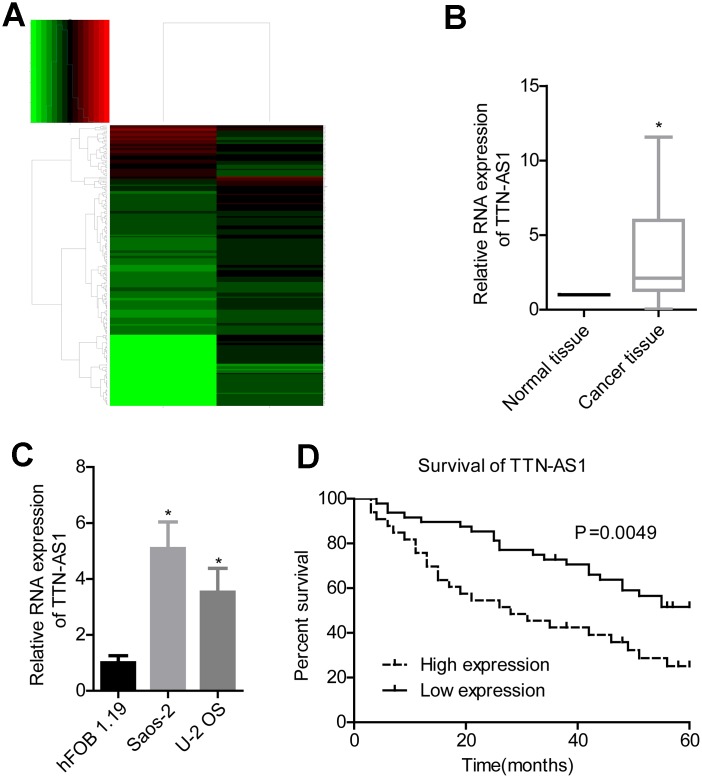
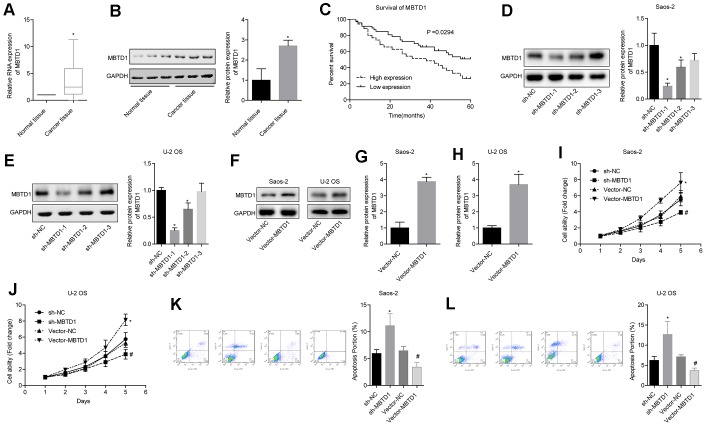
Bioinformatics analysis revealed that lncRNA TTN-AS1 was upregulated in osteosarcoma (Figure 1A). Clinical sample test results also showed that lncRNA TTN-AS1 was highly expressed in tumour tissues (2.08 ± 2.45 vs. 1.00 ± 0.02) (Figure 1B). Overexpression of lncRNA TTN-AS1 was also present in osteosarcoma cell lines (5.09 ± 0.94 and 3.47 ± 1.04 vs. 1.00 ± 0.14) (Figure 1C). Patients with high expression of lncRNA TTN-AS1 had lower survival rates (Figure 1D). Further clinical analysis also showed that a high level of lncRNA TTN-AS1 was associated with higher Enneking stage and remote transfer (Table 1).
Figure 1.
lncRNA TTN-AS1 is highly expressed in osteosarcoma and is associated with poor prognosis. (A) Bioinformatics analysis of differentially expressed genes in osteosarcoma. (B) QPCR was used to detect the expression level of lncRNA TTN-AS1 in tumour tissues and adjacent tissues. (C) QPCR was applied to detect lncRNA TTN-AS1 levels in osteosarcoma cell lines. (D) 5-year survival analysis was done on osteosarcoma patients with different lncRNA TTN-AS1 levels. *P < 0.05 vs. normal tissue or hFOB 1.19 cell group;
Table 1. Correlation between TTN-AS1 expression and clinicopathological features in OS patients.
| Characteristics | Total number | TTN-AS1 expression | P value | |
| Low (n=29) | High (n=26) | |||
| Age (years) | 0.651 | |||
| <18 | 11 | 4 | 7 | |
| ≥18 | 44 | 25 | 19 | |
| Gender | 0.090 | |||
| Male | 36 | 16 | 20 | |
| Female | 19 | 13 | 6 | |
| Tumor site | 0.385 | |||
| Femur/ Tibia | 18 | 11 | 7 | |
| Other | 37 | 18 | 19 | |
| Enneking stage | 0.041 | |||
| I-II-A | 39 | 24 | 15 | |
| II-B/IVB | 16 | 5 | 11 | |
| Lymph node metastasis | 0.014 | |||
| Absent | 47 | 28 | 19 | |
| Present | 8 | 1 | 7 | |
LncRNA TTN-AS1 promotes cell viability and inhibits apoptosis
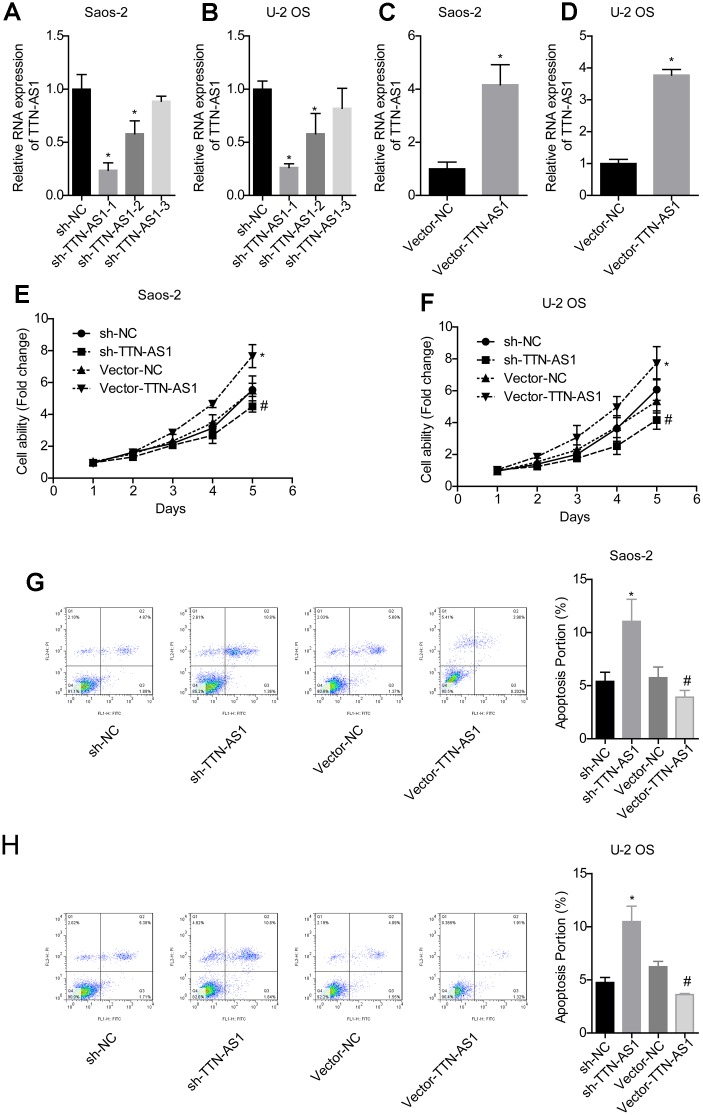
QPCR results showed that the TTN-AS1 level in the sh-TTN-AS1-1 and sh-TTN-AS1-2 groups was lower than that in the sh-NC group (Saos-2: 0.24 ± 0.08 and 0.55 ± 0.17 vs. 1.00 ± 0.19; U-2OS: 0.25 ± 0.04 and 0.54 ± 0.23 vs. 1.00 ± 0.10), while vector-TTN-AS1 significantly upregulated the level of TTN-AS1 (Saos-2: 4.06 ± 0.92 vs. 1.00 ± 0.09; U-2OS: 4.25 ± 0.89 vs. 1.00 ± 0.06), indicating successful transfection (Figure 2A–2D). Sh-TTN-AS1-1 was used for subsequent experiments and referred to as sh-TTN-AS1. The relative cell viability in the vector-TTN-AS1 group (Saos-2: 7.24 ± 0.35; U-2OS: 7.60 ± 0.47) was significantly higher than that of the vector-NC group (Saos-2: 5.28 ± 0.31; U-2OS: 5.88 ± 0.28). In addition, the relative cell viability in the sh-TTN-AS1 group (Saos-2: 4.08 ± 0.11; U-2OS: 4.01 ± 0.15) was lower than that of the sh-NC group (Saos-2: 5.02 ± 0.13; U-2OS: 1.13 ± 0.14) (Figure 2E–2F). The apoptosis rate was increased after transfection with sh-TTN-AS1 (Saos-2: 11.04 ± 2.44%; U-2OS: 10.49 ± 0.20%), and the apoptosis rate was decreased after transfection with vector-TTN-AS1 (Saos-2: 3.92 ± 0.08%; U-2OS: 3.62 ± 0.04%) (Figure 2G–2H). This indicated that lncRNA TTN-AS1 promoted cell viability and inhibited apoptosis.
Figure 2.
lncRNA TTN-AS1 promoted cell viability and inhibited apoptosis. (A–D) QPCR was used to detect the expression level of lncRNA TTN-AS1. (E, F) The cell viability of each group of cells was detected by the CCK-8 method. (G, H) The apoptosis rate of each group of cells was tested using the CCK-8 method. *P < 0.05 vs. sh-NC group; #P < 0.05 vs. vector-NC.
LncRNA TTN-AS1 targets miR-134-5p
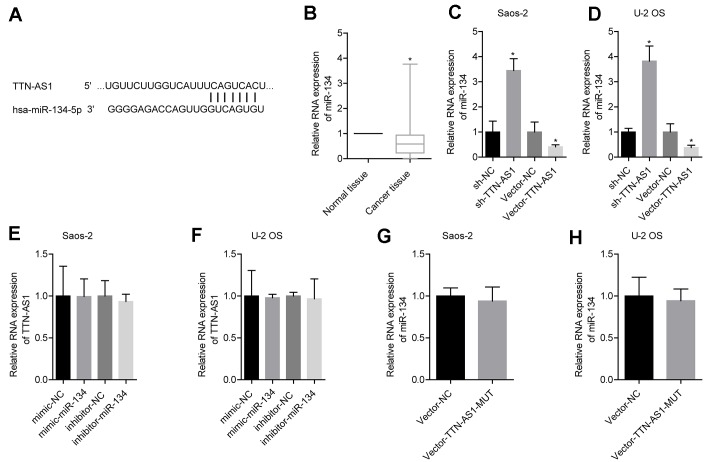
The predicted results from the bioinformatics analysis showed that lncRNA TTN-AS1 targeted miR-134-5p (Figure 3A). Clinical sample test results showed that miR-134-5p was expressed at low levels in osteosarcoma (0.52 ± 0.41) (Figure 3B). Further experimental results showed that upregulation of lncRNA TTN-AS1 inhibited the expression of miR-134-5p, and downregulation of lncRNA TTN-AS1 gave the opposite results (Figure 3C–3D). Promotion or inhibition of miR-134-5p expression had no significant effects on lncRNA TTN-AS1 (Figure 3E–3F). Furthermore, the mutated lncRNA TTN-AS1 did not cause changes in the level of miR-134-5p (Figure 3G–3H). This indicated that lncRNA TTN-AS1 could be a target to regulate the level of miR-134-5p.
Figure 3.
lncRNA TTN-AS1 targeted miR-134-5p. (A) The website predicted that lncRNA TTN-AS1 targeted miR-134-5p. (B) QPCR was used to detect the expression level of miR-134-5p in tumour tissues and adjacent tissues. (C, D) The effects of downregulation or upregulation of lncRNA TTN-AS1 on miR-134-5p are shown. (E, F) The effects of downregulation or upregulation of miR-134-5p on lncRNA TTN-AS1 are shown. (G, H) The effects of transfected mutant lncRNA TTN-AS1 on miR-134-5p are shown. *P < 0.05 vs. normal tissue or vector-NC.
MiR-134-5p targets MBTD1, which is regulated by lncRNA TTN-AS1
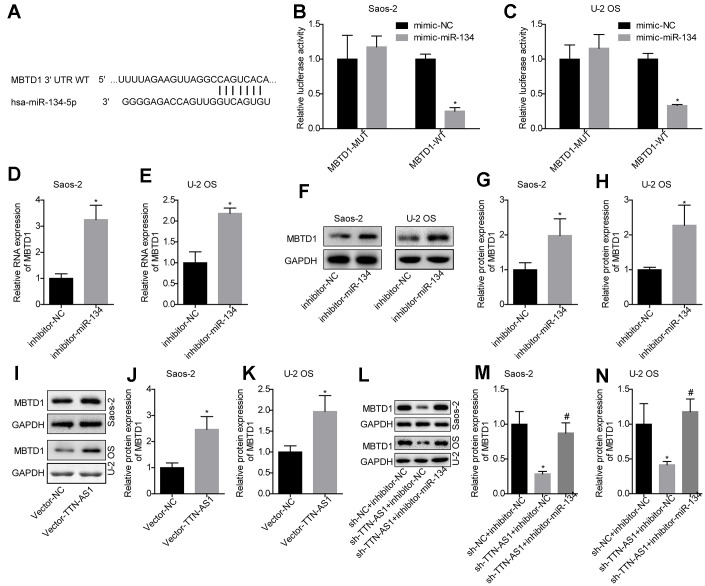
The TargetScan website was used to predict the target gene of miR-134-5p (Figure 4A). The luciferase assay demonstrated that miR-134-5p targeted the 3′-UTR region of MBTD1 (Figure 4B, 4C). Further studies also showed that compared with the inhibitor-NC group, inhibition of miR-134-5p levels could upregulate MBTD1 mRNA and protein levels (Saos-2: 3.14 ± 0.67 and 1.98 ± 0.55; U-2OS: 2.21 ± 0.13 and 2.07 ± 0.57) (Figure 4D–4H). This indicated that miR-134-5p can directly target MBTD1.
Figure 4.
MiR-134-5p targets MBTD1, which is regulated by lncRNA TTN-AS1. (A) The website predicted that MBTD1 was the target gene of miR-134-5p. (B, C) The luciferase assay confirmed that miR-134-5p targets MBTD1. (D–H) The effects of down-regulating miR-134-5p on MBTD1 mRNA and protein expression levels are shown. (I–K) The effects of upregulation of lncRNA TTN-AS1 on MBTD1 protein expression are shown. (L–N) The effects of knockdown of lncRNA TTN-AS1 and miR-134-5p on MBTD1 protein expression are shown. *P < 0.05 vs. mimic-NC, inhibitor-NC or sh-NC + inhibitor-NC group; #P < 0.05 vs. sh-TTN-AS1 + inhibitor-NC.
The results also showed that upregulation of lncRNA TTN-AS1 promoted MBTD1 protein expression (Saos-2: 2.27 ± 0.44; U-2OS: 1.98 ± 0.48) (Figure 4I–4K). In addition, the results showed that downregulation of lncRNA TTN-AS1 levels inhibited MBTD1 protein expression (Saos-2: 0.26 ± 0.03; U-2OS: 0.38 ± 0.03), while inhibitors partially reversed the inhibitory effects of lncRNA TTN-AS1 on MBTD1 (Saos-2: 0.82 ± 0.41; U-2OS: 1.17 ± 0.42) (Figure 4L–4N). This suggested that lncRNA TTN-AS1 could regulate the expression level of MBTD1 by targeting miR-134-5p.
MBTD1 is highly expressed in osteosarcoma and is associated with poor prognosis, and MBTD1 promotes cell viability and inhibits apoptosis
The level of MBTD1 in cancer tissues was significantly higher than that in normal tissues (Figure 5A, 5B). Osteosarcoma patients with high expression of MBTD1 had worse 5-year survival rates compared to those with low expression of MBTD1 (50.005 vs. 26.43%) (Figure 5C). Western blotting results showed that MBTD1 protein levels in the sh-MBTD1-1 and sh-MBTD1-2 groups were significantly lower than those in the sh-NC group, indicating successful transfection experiments (Figure 5D–5E). sh-MBTD1-1 was used for subsequent experiments and referred to as sh-MBTD1. Cell lines overexpressing MBTD1 were also successfully established, and MBTD1 protein levels were upregulated 3.85 (± 0.38) and 3.67 (± 0.72) times in Saos-2 and U-2OS, respectively (Figure 5G–5H). Further studies showed that overexpression of MBTD1 promoted cell viability and inhibited apoptosis, while downregulation of MBTD1 inhibited cell viability and promoted apoptosis (Figure 5G–5L). This suggested that MBTD1 had a role in promoting tumour growth.
Figure 5.
MBTD1 is highly expressed in osteosarcoma and associated with poor prognosis, and MBTD1 promotes cell viability and inhibits apoptosis. (A, B) The expression level of MBTD1 in osteosarcoma tissues and adjacent tissues is shown. © 5-year survival analysis of osteosarcoma patients with different MBTD1 levels is shown. (D–H) The effects of MBTD1 knockdown or overexpression on MBTD1 protein expression levels are shown. (I–L) The effect of knockdown or overexpression of MBTD1 on cell viability and apoptosis is shown. *P < 0.05 vs. normal tissue or sh-NC group; #P < 0.05 vs. vector-NC.
LncRNA TTN-AS1 inhibited tumour growth via MBTD1.
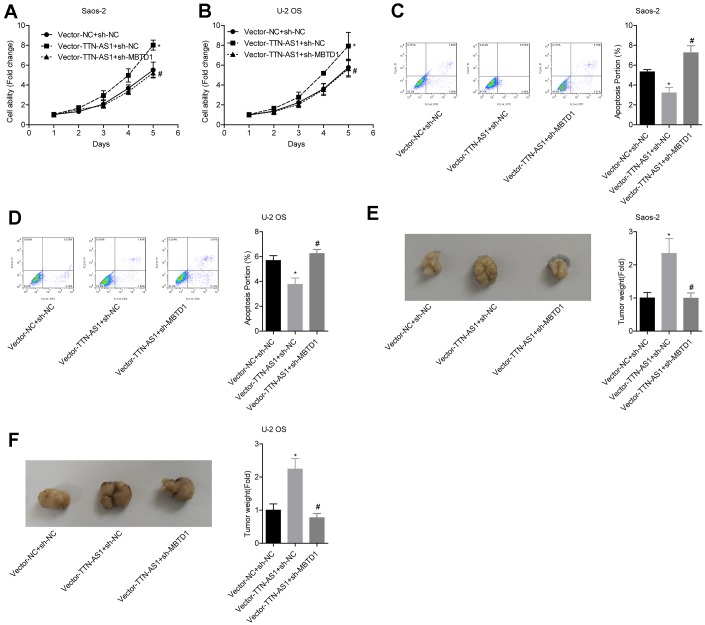
The relative cell viability of the vector-TTN-AS1 + sh-NC group (Saos-2: 7.95 ± 0.27; U-2OS: 7.39 ± 0.66) was significantly higher than that of the vector-NC group; the cell viability of the Vector-TTN-AS1 + sh-MBTD1 group (Saos-2: 4.92 ± 0.18; U-2OS: 4.20 ± 0.31) was lower than that of the Vector-TTN-AS1 + sh-NC group (Figure 6A–6B). The relative apoptosis rate of the Vector-TTN-AS1 + sh-NC group (Saos-2: 3.17 ± 0.62; U-2OS: 3.72 ± 0.48) was significantly lower than that of the vector-NC group; the cell viability of the Vector-TTN-AS1 + sh-MBTD1 group (Saos-2: 7.11 ± 0.64; U-2OS: 6.08 ± 0.40) was higher than that of the Vector-TTN-AS1 + sh-NC group (Figure 6C–6D). These results suggested that knockdown of MBTD1 reversed the cancer-promoting effects of overexpression of lncRNA TTN-AS1. Animal experiments showed that the tumour weight of the vector-TTN-AS1 + sh-NC group (Saos-2: 2.66 ± 0.69; U-2OS: 2.24 ± 0.43) was significantly higher than that of the vector-NC group; the cell viability of the vector-TTN-AS1 + sh-MBTD1 group (Saos-2: 0.99 ± 0.18; U-2OS: 0.76 ± 0.11) was lower than that of the Vector-TTN-AS1 + sh-NC group (Figure 6E–6F).
Figure 6.
lncRNA TTN-AS1 inhibits tumour growth via MBTD1. (A–D) The effects of overexpression of lncRNA TTN-AS1 and knockdown of MBTD1 on cell viability and apoptosis are shown. (E, F) The effects of overexpression of lncRNA TTN-AS1 and knockdown of MBTD1 on tumour growth are shown. *P < 0.05 vs. vector-NC + sh-NC group; #P < 0.05 vs. vector-TTN-AS1 + sh-NC.
Downregulation of lncRNA TTN-AS1 reduces drug resistance
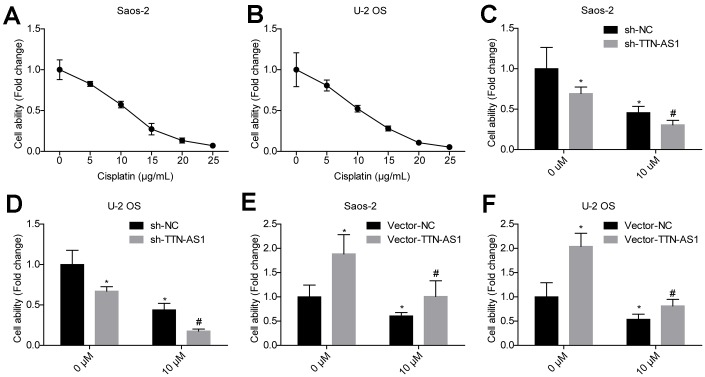
The cell viability of the osteosarcoma cell lines Saos-2 and U2OS treated with different concentrations of cisplatin was examined. The cell viability decreased by approximately 50% when the concentration of cisplatin was 10 μM (Figure 7A–7B). The relative cell viability of the sh-TTN-AS1 group (Saos-2: 0.27 ± 0.02; U-2OS: 0.15 ± 0.01) was significantly lower than that of the sh-NC group (Saos-2: 0.46 ± 0.04; U-2OS: 0.45 ± 0.05) in the presence of 10 μM cisplatin (Figure 7C–7D). The relative cell viability of the vector-TTN-AS1 group (Saos-2: 0.96 ± 0.47; U-2OS: 0.78 ± 0.30) was significantly higher than that of the sh-NC group (Saos-2: 0.75 ± 0.02; U-2OS: 0.51 ± 0.03) in the presence of 10 μM cisplatin (Figure 7E–7F). Upregulation of lncRNA TTN-AS1 partially reverses the inhibitory effect of cisplatin on cells.
Figure 7.
Downregulation of lncRNA TTN-AS1 reduces drug resistance. (A, B) The effects of different concentrations of cisplatin on the viability of osteosarcoma cells are shown. (C, D) Downregulation of lncRNA TTN-AS1 enhanced the inhibitory effects of cisplatin on osteosarcoma cells. (E, F) Upregulation of lncRNA TTN-AS1 attenuated the inhibitory effect of cisplatin on osteosarcoma cells. *P < 0.05 vs. sh-NC and vector-NC group of 0 μM; #P < 0.05 vs. sh-NC and vector-NC group of 10 μM.
DISCUSSION
In this study, we discovered the role of lncRNA TTN-AS1 and MBTD1 in osteosarcoma and analysed the mechanism by which lncRNA TTN-AS1 regulates apoptosis and drug resistance of osteosarcoma cells through the miR-134-5p targeting axis.
The role of lncRNA in osteosarcoma has been confirmed, and both lncRNA and miRNA can be used as markers for the diagnosis and prognosis of osteosarcoma [21–23]. This study found that lncRNA TTN-AS1 was highly expressed in osteosarcoma patients and was associated with poor prognosis. TTN-AS1 is located on chromosome 2: 178,521,183e178,779,963 and was first discovered to be associated with oesophageal squamous cell carcinoma. Patients with high expression of lncRNA TTN-AS1 have a worse prognosis and a higher risk of metastasis [24]. The results of Luo’s study [25] also show that lncRNA TTN-AS1 is overexpressed in lung adenocarcinoma and promotes tumour development by modulating the PTEN/PI3K/AKT pathway. Recent studies show that in cervical cancer, gastric cancer and thyroid cancer, lncRNA TTN-AS1 can act as a ceRNA to promote cancer by targeting miRNA [26–28]. However, the mechanism of action of lncRNA TTN-AS1 in tumours is unclear, and there is no research on lncRNA TTN-AS1 in osteosarcoma. The results of this study showed that lncRNA TTN-AS1 could promote cell viability and inhibit apoptosis, and downregulation of lncRNA TTN-AS1 could reduce drug resistance. This revealed the pro-cancer effects of lncRNA TTN-AS1 on osteosarcoma and the potential for involvement in osteosarcoma drug resistance.
To further explore the mechanism by which lncRNA TTN-AS1 regulated osteosarcoma cell growth and apoptosis, we found through bioinformatics prediction and experimental studies that lncRNA TTN-AS1 targeted miR-134-5p levels as ceRNA and that miR-134-5p inhibited MBTD1 expression by directly binding to the 3′UTR region of MBTD1 mRNA. Studies have found anti-tumour mechanisms of miR-134-5p in non-small cell lung cancer and nasopharyngeal carcinoma [29–31]. MiR-134-5p can also be targeted and regulated by lncRNA [32, 33]. Studies have found that miR-134-5p exerts anti-tumour effects by affecting target gene expression in osteosarcoma [34–36]. This study demonstrated that miR-134-5p was regulated by lncRNA TTN-AS1 to inhibit osteosarcoma growth and apoptosis.
MBTD1 belongs to the polycomb group (PcG) and regulates the transcription process. This study found the role of MBTD1 in tumourigenesis [37]. MBTD1 is associated with low-grade endometrial cancer [38]. However, the role of MBTD1 in osteosarcoma has not been studied. In this study, MBTD1 was highly expressed in osteosarcoma, and high levels of MBTD1 were closely related to malignancy and poor prognosis of osteosarcoma. Cell experiments confirmed that MBTD1 could promote cell viability and inhibit apoptosis. Both the cell experiments and animal experiments confirmed that downregulating the level of MBTD1 reversed the effects of overexpression of lncRNA TTN-AS1 on tumour growth.
In conclusion, lncRNA TTN-AS1 promoted the expression of MBTD1 by targeting miR-134-5p and regulated cell growth, apoptosis and drug resistance in osteosarcoma. However, further research is needed on the mechanisms by which TTN-AS1 participates in drug resistance and by which MBTD1 promotes the development of osteosarcoma.
MATERIALS AND METHODS
Bioinformatics analysis
The osteosarcoma patient gene data (GSE12865) were downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). A difference analysis was performed on the data. The miRcode website (http://www.mircode.org/) performed a biosignal analysis to predict miRNAs downstream of lncRNA TTN-AS1. The TargetScan website (http://www.targetscan.org/vert_72/) was used to predict downstream target proteins of miR-134-5p.
Osteosarcoma tissues and research
55 osteosarcoma tissues and 9 adjacent tissues were purchased from Alenabio Biotechnology Co., Ltd. (OS208a, Xi’an, China). Quantitative polymerase chain reaction (qPCR) was used to detect lncRNA TTN-AS1, miR-134-5p and MBTD1 mRNA expression levels in cancer tissues and normal tissues. The 5-year survival of patients with high or low expression of lncRNA TTN-AS1 and MBTD1 mRNA was compared. The correlation between lncRNA TTN-AS1 and pathological features of patients with osteosarcoma was analysed. Approval was obtained from the Tongren Hospital Ethics Committee.
Cells culture and transfection
Human osteoblast hFOB 1.19 and osteosarcoma cell lines Saos-2 and U-2OS were purchased from American Type Culture Collection (Manassas, VA, USA) and were grown in RPMI-1640 medium containing 10% foetal bovine serum (FBS, Thermo Fisher, Waltham, USA), 50 U/mL penicillin and 50 μg/mL streptomycin (15070063, Thermo Fisher, Waltham, USA). The plasmid constructs and the corresponding negative control (NC) were built by Shanghai Genepharma Company (China). Plasmid transfection technology was applied to silence or overexpress lncRNA TTN-AS1, miR-134-5p and MBTD1. The cells were transfected with 100 nM RNA oligonucleotides using LipofectamineTM 2000 (Invitrogen, Waltham, USA). Cells were harvested 48 h after transfection for subsequent experiments.
QPCR
Total RNA was acquired using TRIzol (Invitrogen, USA). The cDNA was generated from 1 μg isolated miRNA/mRNA by using an iScriptTMcDNA Synthesis Kit (Bio-Rad, USA). For qPCR processing, the FastStart Universal SYBR Green Master kit (Roche, Switzerland) was used. The reaction system was prepared as follows: 2.5 μL cDNA template, 1 μL forward primer (10 μM), 1 μL reverse primer (10 μM), 10 μL 2 × SYBR Green master mix, 5.5 μL dH2O. QPCR was performed using the following procedure: 2 min at 95°C, 40 cycles of 15 sec at 95°C, 25 sec at 60°C and 60 sec at 72°C. U6 acted as an internal reference for miR-134-5p, and GAPDH served as an internal reference for lncRNA and mRNA. The primers were synthesized by Sangon Biotech (Shanghai, China) and are shown in Table 2.
Table 2. Primers for qPCR.
| Genes | Forward (5′-3′) | Reverse (5′-3′) |
| TTN-AS1 | CCAGACACCTAACCAACTTCC | GTGATCTCATCCCTCTTGCTT |
| miR-134-5p | GAAGCTCATTGGAGACCCTAAC | CAACCTCTAAGTCGTGCTCATAC |
| MBTD1 | CTACAGCCTCCAGCATCACA | CTCATCAGCTGACCCAGACA |
| GAPDH | AGAAGGCTGGGGCTCATTTG | AGGGGCCATCCACAGTCTTC |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
Western blot analysis
The whole-cell protein was extracted by using ice-cold RIPA buffer mixed with protease inhibitors. The protein lysate was separated by SDS-PAGE at 110 V for 100 min and transferred to PVDF membranes at 90 V for 90 min. The PVDF membrane was blocked in 5% non-fat milk for 1 h at room temperature, and antibody (MBTD1, ab170848, Abcam, USA, 71 kD; GAPDH, ab9484, 36 kD) was added at 4°C overnight. The membrane was further probed with the secondary antibody IgG H&L (HRP) (ab6721, Abcam, San Francisco, USA, 1:5000) after washing the membrane with PBST (PBS with 0.2% Tween 20). The protein bands were detected by Pierce ECL Plus western blotting substrate (Thermo Fisher, Waltham, USA) on a ChemiDoc MP instrument (Bio-Rad, California, USA).
Cell counting kit 8 (CCK-8) assay
The cells were seeded in a 96-well culture plate (2 × 104 cells / mL, 100 μL / well), and the blank controls (with medium alone) were set. After hypoxia-reoxygenation, 10 μL of CCK-8 (Beyotime Institute of Biotechnology, Beijing, China) reagent was added to each well, and the culture was continued for 2 h at 37°C. The absorbance values were measured at 450 nm on a microplate reader (Tecan Infinite M200 Micro Plate Reader; LabX, Switzerland), and the corresponding optical density ratio was expressed as cell vitality.
Cell apoptosis
The 2×105 cells were collected in 1.5 mL EP tubes. The supernatant was discarded after centrifugation at 2000 × g at 4°C. Then, 500 μL of binding buffer was added to suspend the cells, and 5 μL of Annexin V-FITC was added and incubated at 4°C for 30 min in the dark. Then, 5 μL of propidium iodide (PI) was added, gently mixed and incubated at room temperature for 5 min. The cell apoptosis rates were detected using an Annexin-V-FITC detection kit (K201-100, BioVision, USA) and flow cytometry (version 10.0, FlowJo, FACS CaliburTM, BD, USA).
Luciferase assay
The sequences containing the wild-type (WT) or mutated (Mut) region MBTD1 were synthesized by Sangon (Shanghai, China) and inserted into a pGL3 vector (Promega Corporation, Madison, USA). For the luciferase reporter assay, miR-134-5p mimics and the respective reporter plasmids were transfected into cells using Lipofectamine 2000 according to the manufacturer’s protocol. After 24 h, the Renilla and Firefly luciferase activities were determined using the Dual-Luciferase Reporter Assay System (Promega Corp.) and a luminometer (Infinite 200 PRO NanoQuant; Tecan Group Ltd., Männedorf, Switzerland).
Tumour burden assay
Specific pathogen-free (SPF) 4-week-old BALB/c nude mice were purchased from the animal centre of Air Force Medical University (Shanghai, China). Saos-2 and U2OS cells were divided into three groups: vector-NC + sh-NC, vector-TTN-AS1 + sh-NC and vector-TTN-AS1 + sh-MBTD1. The mice in each group were injected with 1×106 cells correspondingly. Twenty-eight days after injection, the mice were sacrificed, and tumours were removed to be weighed and photographed. The animal experiment protocol was approved by the Animal Experimentation Ethics Committee of Tongren Hospital.
Statistical analysis
All analyses were conducted using Prism GraphPad 7.0 software. All variables were shown as the mean ± standard deviation (SD). Comparisons among multiple groups were performed using one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Footnotes
CONFLICTS OF INTEREST: The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Kager L, Tamamyan G, Bielack S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol. 2017; 13:357–68. 10.2217/fon-2016-0261 [DOI] [PubMed] [Google Scholar]
- 2.Biazzo A, De Paolis M. Multidisciplinary approach to osteosarcoma. Acta Orthop Belg. 2016; 82:690–98. [PubMed] [Google Scholar]
- 3.Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014; 14:722–35. 10.1038/nrc3838 [DOI] [PubMed] [Google Scholar]
- 4.König M, Osnes TA, Lobmaier I, Bjerkehagen B, Bruland ØS, Sundby Hall K, Meling TR. Multimodal treatment of craniofacial osteosarcoma with high-grade histology. A single-center experience over 35 years. Neurosurg Rev. 2017; 40:449–60. 10.1007/s10143-016-0802-z [DOI] [PubMed] [Google Scholar]
- 5.Meazza C, Scanagatta P. Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther. 2016; 16:543–56. 10.1586/14737140.2016.1168697 [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, He Z, Li Y, Yang Y, Shi J, Liu X, Yuan T, Xia J, Li D, Zhang J, Yang Z. Selection of Surgical Methods in the Treatment of Upper Tibia Osteosarcoma and Prognostic Analysis. Oncol Res Treat. 2017; 40:528–32. 10.1159/000477251 [DOI] [PubMed] [Google Scholar]
- 7.Lee JA, Paik EK, Seo J, Kim DH, Lim JS, Yoo JY, Kim MS. Radiotherapy and gemcitabine-docetaxel chemotherapy in children and adolescents with unresectable recurrent or refractory osteosarcoma. Jpn J Clin Oncol. 2016; 46:138–43. 10.1093/jjco/hyv171 [DOI] [PubMed] [Google Scholar]
- 8.Ferrari S, Serra M. An update on chemotherapy for osteosarcoma. Expert Opin Pharmacother. 2015; 16:2727–36. 10.1517/14656566.2015.1102226 [DOI] [PubMed] [Google Scholar]
- 9.Bhuvaneshwar K, Harris M, Gusev Y, Madhavan S, Iyer R, Vilboux T, Deeken J, Yang E, Shankar S. Genome sequencing analysis of blood cells identifies germline haplotypes strongly associated with drug resistance in osteosarcoma patients. BMC Cancer. 2019; 19:357. 10.1186/s12885-019-5474-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan Y, Xu L, Zhuo N, Ge J, Chen G, Lu XB. The clinical significance of neoadjuvant chemotherapy in improving the drug resistance of osteosarcoma. Minerva Med. 2017; 108:479–81. 10.23736/S0026-4806.17.05122-9 [DOI] [PubMed] [Google Scholar]
- 11.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009; 10:155–59. 10.1038/nrg2521 [DOI] [PubMed] [Google Scholar]
- 12.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009; 136:629–41. 10.1016/j.cell.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 13.de Lara JC, Arzate-Mejía RG, Recillas-Targa F. Enhancer RNAs: Insights Into Their Biological Role. Epigenet Insights. 2019; 12:2516865719846093. 10.1177/2516865719846093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding M, Liu Y, Liao X, Zhan H, Liu Y, Huang W. Enhancer RNAs (eRNAs): New Insights into Gene Transcription and Disease Treatment. J Cancer. 2018; 9:2334–40. 10.7150/jca.25829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaheer U, Faheem M, Qadri I, Begum N, Yassine HM, Al Thani AA, Mathew S. Expression profile of MicroRNA: An Emerging Hallmark of Cancer. Curr Pharm Des. 2019; 25:642–53. 10.2174/1386207322666190325122821 [DOI] [PubMed] [Google Scholar]
- 16.Zhou S, Yu L, Xiong M, Dai G. LncRNA SNHG12 promotes tumorigenesis and metastasis in osteosarcoma by upregulating Notch2 by sponging miR-195-5p. Biochem Biophys Res Commun. 2018; 495:1822–32. 10.1016/j.bbrc.2017.12.047 [DOI] [PubMed] [Google Scholar]
- 17.Zhang E, Li X. LncRNA SOX2-OT regulates proliferation and metastasis of nasopharyngeal carcinoma cells through miR-146b-5p/HNRNPA2B1 pathway. J Cell Biochem. 2019; 120:16575–88. 10.1002/jcb.28917 [DOI] [PubMed] [Google Scholar]
- 18.Liu CW, Liu D, Peng D. Long non-coding RNA ZFAS1 regulates NOB1 expression through interacting with miR-646 and promotes tumorigenesis in osteosarcoma. Eur Rev Med Pharmacol Sci. 2019; 23:3206–16. 10.26355/eurrev_201904_17679 [DOI] [PubMed] [Google Scholar]
- 19.Yousef M, Showe L, Showe M. A study of microRNAs in silico and in vivo: bioinformatics approaches to microRNA discovery and target identification. FEBS J. 2009; 276:2150–56. 10.1111/j.1742-4658.2009.06933.x [DOI] [PubMed] [Google Scholar]
- 20.Cui J, Li D, Zhang W, Shen L, Xu X. Bioinformatics analyses combined microarray identify the deregulated microRNAs in oral cancer. Oncol Lett. 2014; 8:218–22. 10.3892/ol.2014.2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Meng W, Cui H. LncRNA CBR3-AS1 predicts unfavorable prognosis and promotes tumorigenesis in osteosarcoma. Biomed Pharmacother. 2018; 102:169–74. 10.1016/j.biopha.2018.02.081 [DOI] [PubMed] [Google Scholar]
- 22.Cong C, Wang W, Tian J, Gao T, Zheng W, Zhou C. Identification of serum miR-124 as a biomarker for diagnosis and prognosis in osteosarcoma. Cancer Biomark. 2018; 21:449–54. 10.3233/CBM-170672 [DOI] [PubMed] [Google Scholar]
- 23.Pang PC, Shi XY, Huang WL, Sun K. miR-497 as a potential serum biomarker for the diagnosis and prognosis of osteosarcoma. Eur Rev Med Pharmacol Sci. 2016; 20:3765–69. [PubMed] [Google Scholar]
- 24.Lin C, Zhang S, Wang Y, Wang Y, Nice E, Guo C, Zhang E, Yu L, Li M, Liu C, Hu L, Hao J, Qi W, Xu H. Functional Role of a Novel Long Noncoding RNA TTN-AS1 in Esophageal Squamous Cell Carcinoma Progression and Metastasis. Clin Cancer Res. 2018; 24:486–98. 10.1158/1078-0432.CCR-17-1851 [DOI] [PubMed] [Google Scholar]
- 25.Luo J, Liu Z. Long non-coding RNA TTN-AS1 promotes the progression of lung adenocarcinoma by regulating PTEN/PI3K/AKT signaling pathway. Biochem Biophys Res Commun. 2019; 514:140–47. 10.1016/j.bbrc.2019.04.050 [DOI] [PubMed] [Google Scholar]
- 26.Dong MM, Peng SJ, Yuan YN, Luo HP. LncRNA TTN-AS1 contributes to gastric cancer progression by acting as a competing endogenous RNA of miR-376b-3p. Neoplasma. 2019; 66:564–75. 10.4149/neo_2018_180927N721 [DOI] [PubMed] [Google Scholar]
- 27.Cui Z, Luo Z, Lin Z, Shi L, Hong Y, Yan C. Long non-coding RNA TTN-AS1 facilitates tumorigenesis of papillary thyroid cancer through modulating the miR-153-3p/ZNRF2 axis. J Gene Med. 2019; 21:e3083. 10.1002/jgm.3083 [DOI] [PubMed] [Google Scholar]
- 28.Chen P, Wang R, Yue Q, Hao M. Long non-coding RNA TTN-AS1 promotes cell growth and metastasis in cervical cancer via miR-573/E2F3. Biochem Biophys Res Commun. 2018; 503:2956–62. 10.1016/j.bbrc.2018.08.077 [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, Wu Y, Xiao J, Zou J. Long Non-Coding RNA Prostate Cancer-Associated Transcript 7 (PCAT7) Induces Poor Prognosis and Promotes Tumorigenesis by Inhibiting mir-134-5p in Non-Small-Cell Lung (NSCLC). Med Sci Monit. 2017; 23:6089–98. 10.12659/MSM.907904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Tao Z, Qu J, Zhou X, Zhang C. Long non-coding RNA PCAT7 regulates ELF2 signaling through inhibition of miR-134-5p in nasopharyngeal carcinoma. Biochem Biophys Res Commun. 2017; 491:374–81. 10.1016/j.bbrc.2017.07.093 [DOI] [PubMed] [Google Scholar]
- 31.Qin Q, Wei F, Zhang J, Li B. miR-134 suppresses the migration and invasion of non-small cell lung cancer by targeting ITGB1. Oncol Rep. 2017; 37:823–30. 10.3892/or.2017.5350 [DOI] [PubMed] [Google Scholar]
- 32.Jiang F, Qi W, Wang Y, Wang W, Fan L. lncRNA PEG10 promotes cell survival, invasion and migration by sponging miR-134 in human bladder cancer. Biomed Pharmacother. 2019; 114:108814. 10.1016/j.biopha.2019.108814 [DOI] [PubMed] [Google Scholar]
- 33.Shi C, Wang M. LINC01118 Modulates Paclitaxel Resistance of Epithelial Ovarian Cancer by Regulating miR-134/ABCC1. Med Sci Monit. 2018; 24:8831–39. 10.12659/MSM.910932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen CL, Zhang L, Jiao YR, Zhou Y, Ge QF, Li PC, Sun XJ, Lv Z. miR-134 inhibits osteosarcoma cell invasion and metastasis through targeting MMP1 and MMP3 in vitro and in vivo. FEBS Lett. 2019; 593:1089–101. 10.1002/1873-3468.13387 [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Wang Z, Li J, Sang X. Diallyl disulfide suppresses FOXM1-mediated proliferation and invasion in osteosarcoma by upregulating miR-134. J Cell Biochem. 2018. [Epub ahead of print] 10.1002/jcb.28003 [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Lv Z, Xu J, Chen C, Ge Q, Li P, Wei D, Wu Z, Sun X. MicroRNA-134 inhibits osteosarcoma angiogenesis and proliferation by targeting the VEGFA/VEGFR1 pathway. FEBS J. 2018; 285:1359–71. 10.1111/febs.14416 [DOI] [PubMed] [Google Scholar]
- 37.Mills AA. Throwing the cancer switch: reciprocal roles of polycomb and trithorax proteins. Nat Rev Cancer. 2010; 10:669–82. 10.1038/nrc2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dewaele B, Przybyl J, Quattrone A, Finalet Ferreiro J, Vanspauwen V, Geerdens E, Gianfelici V, Kalender Z, Wozniak A, Moerman P, Sciot R, Croce S, Amant F, et al. Identification of a novel, recurrent MBTD1-CXorf67 fusion in low-grade endometrial stromal sarcoma. Int J Cancer. 2014; 134:1112–22. 10.1002/ijc.28440 [DOI] [PubMed] [Google Scholar]