Abstract
The inflammasomes are intracellular protein complexes that are assembled in response to a variety of perturbations including infections and injuries. Failure of the inflammasomes to rapidly clear the insults or restore tissue homeostasis can result in chronic inflammation. Recurring inflammation is also provoked by mutations that cause the constitutive assembly of the components of these protein platforms. Evidence suggests that chronic inflammation is a shared mechanism in bone loss associated with aging, dysregulated metabolism, autoinflammatory, and autoimmune diseases. Mechanistically, inflammatory mediators promote bone resorption while suppressing bone formation, an imbalance which over time leads to bone loss and increased fracture risk. Thus, while acute inflammation is important for the maintenance of bone integrity, its chronic state damages this tissue. In this review, we discuss the role of the inflammasomes in inflammation-induced osteolysis.
Keywords: Inflammation, Inflammasomes, Osteoclasts, Bone resorption, NLRP3, Cytokines
Introduction
Nucleotide-binding oligomerization domain-like receptors (NLRs, e.g., NLRP1) or absent in melanoma 2-like receptors (ALRs, e.g., AIM 2) associate with caspase-1 directly or indirectly via apoptosis-associated speck-like protein containing a CARD (ASC) to form intracellular protein complexes called inflammasomes. These macromolecular structures are assembled in response to perturbations caused by microbial products also known as pathogen-associated molecular patterns (PAMPs). For example, anthrax lethal factor, bacterial muramyl dipeptide, and bacterial flagellin induce the nucleation of the NLRP1 inflammasome, NLRP3 inflammasome, and NLRC4 inflammasome, respectively [1, 2]. The inflammasomes are also activated by host endogenous cues from damaged cells or exogenous materials, signals commonly known as danger-associated molecular patterns (DAMPs). In this regard, the NLRP3 inflammasome stands out, owing to its ability to sense a wide range of structurally different molecular entities including crystalline materials, misfolded or aggregated proteins, metabolites, prosthetic implant wear debris, and certain materials found in the environment such as asbestos and silica particles [3–5] (Fig. 1). The NLRC4 inflammasome and AIM2 inflammasome are also activated to some extent by endogenous DAMPs [6–8]. The inflammatory responses induced by PAMPs or DAMPs can be either acute when the perturbation is rapidly resolved, and the homeostasis is restored, or chronic and pathologic when rapid clearance mechanisms fail. Finally, activating mutations in NLRP1, NLRP3, NLRC4, or MEFV cause inflammasome assembly independently of PAMPs or DAMPs [9–15].
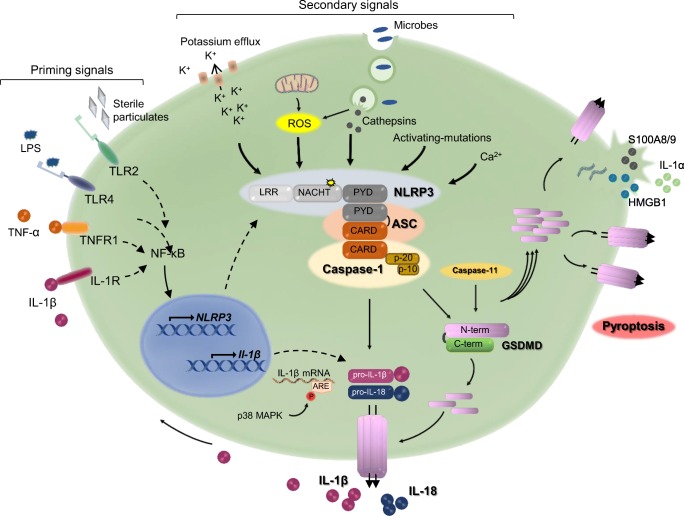
Fig. 1.
Mechanisms of activation of the NLRP3 inflammasome. Activation of the NLRP3 inflammasome involves two steps. Induction of priming signals upon ligation of pathogen recognition receptors (PRRs) such as TLR4 and TLR2, and cytokine receptors including IL-1 receptor (IL-1R; positive feedback) and TNF receptor (TNFR). These signals induce the transcription of NLRP3 and pro-IL-1β through NF-κB; pro-IL-18 is constitutively expressed. Priming of NLRP3 can also be induced by its deubiquitination, independently of de novo protein synthesis (not depicted). pro-IL-1β mRNA are stabilized by p38α MAPK. Increased expression of NLRP3 enables the recruitment of pro-caspase-1 via ASC upon sensing of secondary signals, which are triggered by a wide range of stimuli including K+ efflux, Ca2+ influx, phagocytosis of microorganisms, and particulate materials (causing lysosome destabilization/rupture and release of cathepsins and reactive oxygen species (ROS)), and mitochondrial dysfunction. NLRP3-activating mutations in the NACHT domain cause constitutive activation of this inflammasome. Proximity-induced reaction leads to auto-activation of caspase-1, which then processes pro-IL-1β and pro-IL-18 into IL-1β and IL-18, respectively. Caspase-1 also cleaves GSDMD into GSDMD-N-terminal (N-term) and GSDMD-C-terminal (C-term) fragments. GSDMD-N-term translocates to the plasma membranes where it oligomerizes and forms pores through which IL-1β and IL-18 are secreted. However, excessive pore formation causes pyroptosis, resulting in the release of not only IL-1β and IL-18, but also other mediators such as IL-1α, S100A8/9, and HMGB1. ARE, AU rich elements; ASC, apoptosis-associated speck-like protein containing a CARD; GSDMD, gasdermin D; HMGB1, high-mobility group box 1; IL-1, interleukin-1; LPS, lipopolysaccharide; NF-κB, nuclear factor kappa B; NLRP3, NLR family, pyrin domain containing 3; TLR, toll-like receptor; TNF-α, tumor necrosis factor-α. Stimulation of the noncanonical NLRP3 inflammasome also occurs secondarily to the activation of caspase-11, which also cleaves GSDMD
Caspase-1 processes pro-interleukin-1β (pro-IL-1β) and pro-IL-18 into IL-1β and IL-18, respectively [16]. It also cleaves gasdermin D (GSDMD), generating an N-terminal fragment that translocates from the cytoplasm to the plasma membrane where it forms pores through which IL-1β and IL-18 are secreted [17, 18]. However, excessive pore formation resulting from sustained activation of GSDMD in both infectious and sterile conditions compromises membrane integrity, and ultimately ruptures the cell, releasing pro-inflammatory cytoplasmic contents into the extracellular environment. This form of cell death, termed pyroptosis, is inflammatory and results in the recruitment of immune cells and the perpetuation of inflammation [19, 20]. Caspase-8 and neutrophil elastase can also generate IL-1β and IL-18 whereas caspase-8, caspase-11 (ortholog of human caspase-4 and caspase-5), and neutrophil elastase efficiently process GSDMD [21–28]. Sustained exposure to supra-physiological levels of IL-1β and IL-18, ultimately, inflicts damage to multiple tissues including the skeleton.
Coordinated actions of the osteoclasts and the osteoblasts are essential to maintain bone mass and quality. The osteoclasts remove the old or defective matrix, which is replenished fully by the osteoblasts; this tightly regulated process is known as bone coupling [29]. Several growth factors, including bone morphogenetic proteins (BMPs) and Wnts control the differentiation of the osteoblasts from mesenchymal stem cells whereas the osteoclasts differentiate from myeloid progenitors exposed to signals generated by macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL) [30, 31]. The osteoblast and osteoclast differentiation programs are antagonized and enhanced, respectively, by pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and IL-1β. Excessive bone resorption by the osteoclasts at the expenses of bone formation by the osteoblasts in inflammatory conditions creates an imbalance, which over time leads to bone loss and increased fracture risk [32]. In this article, we review the role of the inflammasomes in bone resorption and highlight the collateral effects of these protein complexes on other skeletal cells.
Mechanisms of bone resorption that are relevant to the inflammasomes
IL-1β plays numerous roles in bone pathophysiology. It stimulates RANKL production by mesenchymal cells (e.g., stromal cells, osteoblasts, and osteocytes), synoviocytes, and T cells directly or indirectly through the regulation of IL-6, IL-17, and TNF-α expression [33–38]. The reciprocal regulation of IL-1β production by these cytokines and the reported RANKL-independent actions of IL-6 and TNF-α in osteoclast differentiation indicate that these responses are complex and multidirectional [39, 40]. Irrespective of the hierarchy of the events, IL-1β and its effectors act in synergy with RANKL to promote osteoclast differentiation and activity while suppressing osteogenesis [32]. IL-1β also stimulates its own synthesis, a positive feedback mechanism that underlies the chronicity of inflammasome actions in bone diseases [33, 41]. On the other hand, while pro-inflammatory actions of IL-18 in skin disorders [42] and infection-associated allergic diseases [43] are well described, the role of this cytokine in bone is ambiguous. Indeed, IL-18 can inhibit or stimulate bone resorption, depending on cell-contexts [44–46].
Secretion of IL-1β through GSDMD-assembled pores by live cells has been reported [18, 26]. This phenomenon referred to as a hyper-reactive state occurs independently of pyroptosis and may be characteristic of diseases of low-grade inflammation as discussed later in this review. GSDMD pores have an inner diameter of 10–20 nm, which is much bigger than the diameter of mature IL-1β and IL-18 (approximately 5 nm) [17, 20, 47–49]. There is thus far no basis for such large pores to facilitate only the secretion of these cytokines, implying that molecules with smaller sizes such as eicosanoids, which are also important regulators of bone resorption may be secreted through GSDMD pores. On the other hand, pyroptosis is presumably prominent in diseases marked by chronic episodes of high-grade inflammation such as inflammasomopathies. In this context, IL-1β and IL-18 are concomitantly secreted alongside alarmins including IL-1α, S100A8/9, HMGB1 [50, 51], and possibly lipid mediators such as eicosanoids [52]; this outcome may underlie the limited efficacy of IL-1 blockers in the treatment of bone diseases. Indeed, not only are these alarmins produced by myeloid cells, synovial cells, and osteoblasts [53, 54], but they are also potent modulators of inflammation and osteoclastogenesis, regulating the expression of RANKL, TNF-α, IL-1β, and IL-6 [33, 55]. Inflammasome signaling also leads to the cleavage of poly(ADP-ribose) polymerase 1 (PARP1) by caspase-7, a response that ultimately promotes the degradation of this negative regulator of osteoclast differentiation and bone resorption [56]. Thus, the inflammasomes are key players in the pathogenesis of inflammatory osteolysis. Understanding the biology of these signaling platforms is essential for the development of effective therapies targeting inflammatory bone loss.
Widespread activities of the inflammasomes in inflammatory osteolysis
Evidence suggests that the inflammasomes are implicated in a wide range of diseases of bone loss driven by sterile and non-sterile inflammation (Table 1).
Table 1.
Inflammasomes and their activators in inflammatory bone diseases
| Activators | Disorders | Description of the activators | Inflammasomes |
|---|---|---|---|
| PAMPs | Periodontitis[[57–59] | Porphyromonas gingivalis | NLRP3, AIM2 |
| Osteomyelitis[[60–62] | Staphylococcus aureus | NLRP3 | |
| Mutations | CAPS[[63–79] | Autosomal dominant | NLRP3, NLRC4, NLRP12 |
| MAS[[11, 12, 80, 81] | Autosomal dominant | NLRC4 | |
| FMF[[82, 83] | Autosomal recessive | Pyrin | |
| DAMPs | Sterile CRMO[[22, 23] | Unknown | NLRP3 |
| Arthritis[[84–88] | Self-DNA, other DAMPs? | NLRP3, AIM2, others? | |
| Metabolic diseases, aging[[5–8, 89–91] | Purine metabolites, fatty acids, other DAMPs? | NLRP3, NLRC4, AIM2, others? | |
| Wear debris osteolysis[[3, 92, 93] | PMMA, CoCrMo, etc. | NLRP3, AIM2 | |
| Crystal-induced arthropathies[[94–97] | MSU crystals (gout), CPPD crystals (pseudogout), BCP crystals | NLRP3 |
Cryopyrin-associated periodic syndromes
Cryopyrin-associated periodic syndromes (CAPS), which include familial cold autoinflammatory syndrome (FCAS), Muckle–Wells syndrome (MWS), and neonatal-onset multisystem inflammatory disease (NOMID) are caused by autosomal dominant mutations in the NACHT domain of NLRP3 [63–65, 98]. Additionally, myeloid-restricted somatic mosaicism and mutations in NLRP12 and NLRC4 may account for the inflammatory responses in CAPS patients negative for mutations in NLRP3 [66]. NLRP3 is believed to switch from a closed and inactive conformation to an open state in response to PAMP- or DAMP-induced cues; NLRP3-activating mutations locked this protein in the active state, leading to the constitutive assembly of the inflammasome [67]. Common features of CAPS include recurring fever episodes, urticaria, conjunctivitis, and joint pain whereas central nervous system complications and arthropathies characterized by bone deformities, bulky epiphyses, leg length discrepancy, and short stature are hallmarks of NOMID, the most severe manifestation of these disorders [66, 68–70]. Consistent with the tumor-like features of bony outgrowths, induced pluripotent stem cells from NOMID patients are more proliferative and exhibit higher differentiation potential than normal cells [71]. Epiphyseal abnormalities undoubtedly predispose NOMID patients to joint instability and subsequent development of osteoarthritis. It is worth noting that skeletal phenotyping is based on radiographic observations and limited histology that reveal heterogeneously calcified bone matrix, and severely disorganized and hypocellular growth plate [70].
Murine models of CAPS in which wild-type Nlrp3 alleles are replaced by murine or human alleles carrying mutations found in patients, reproduce several features of human disorders including early onset of systemic inflammation, skin and joint pathologies, and growth retardation. Cryopyrinopathies are in general more severe in rodents than in humans as mutant mice of all phenotypes (i.e., CAPS, MWS, and NOMID) exhibit short lifespan (3–4 weeks) [72–74]. NOMID mice in which NLRP3 is activated globally exhibit normal patterning of skeletal elements but display hypocellular epiphyses due to massive chondrocyte death, and disorganized growth plate matrix protruding towards the bone marrow cavity [73]. Unexpectedly, conditional activation of NLRP3 in myeloid cells but not in osteochondro-progenitors reproduces the abnormal cartilage features, suggesting that the phenotype is not chondrocyte autonomous [75]. CAPS mice also exhibit severe low bone mass, a phenotype that correlates with a massive expansion of osteoclast precursors, exuberant osteoclastogenesis, and increased osteoclast activity [41, 73, 75–77]. Thus, while the magnitude of bone resorption in CAPS patients is not known, this process is prominent and well characterized in mouse models.
Systemic inflammation and multiple organ pathologies, including bone abnormalities, are entirely prevented in NOMID mice lacking IL-1 receptor [75]. However, persistent residual inflammation is reported in FCAS mice and MWS mice deficient in IL-1 and IL-18 signaling [78, 79]. These findings align with clinical studies, which consistently show that epiphyseal lesions and outgrowths continue to expand for a significant number of NOMID patients on IL-1 biologics despite the resolution of disease-associated inflammatory symptoms [70, 99–101]. Collectively, these observations suggest that IL-1β is not the primary driver of skeletal outcomes in CAPS, and that inflammasome-dependent responses other than IL-1β play a role in these disorders. This view is consistent with findings showing that levels of TNF-α remain elevated in certain CAPS patients on IL-1 blockers, and neutralization of TNF-α activity improves inflammatory endpoints in CAPS mice [78]. This view is further supported by recent evidence indicating that the pathogenesis of NOMID in mice is prevented by (i) genetic ablation of GSDMD and (ii) a novel inhibitor of the interactions of p38α MAPK and MAPK-activated kinase 2, which inhibits not only IL-1β, but also IL-6 and TNF-α [41, 77]. Thus, multiple responses, including pyroptosis, contribute to inflammasomopathies.
Macrophage activation syndrome
The NLRC4 inflammasome senses bacterial type III and IV secretion systems and flagellin via NLR family apoptosis inhibitory proteins (NAIPs). As noted above, NLRC4-activating mutations are found in certain FCAS patients. Moreover, recent evidence implicates the NLRC4 inflammasome in the pathogenesis of sterile inflammatory disorders as patients with NLRC4 gain-of-function mutations develop cytopenia, high ferritin levels, hemophagocytosis, and splenomegaly. These symptoms are associated with excessive levels of IL-18 and IL-1β, and recurring fever flares, a phenotype that is reminiscent of macrophage activation syndrome (MAS) [12, 80]. MAS is a frequent complication of systemic juvenile idiopathic arthritis (sJIA), a disease that interferes with healthy skeletal development and bone mass acquisition [102]. Although IL-1β levels are in general lower in MAS compared with CAPS, IL-1 biologics are efficacious in the treatment of sJIA [103, 104]. Consistent with the role of mutated NLRC4 in the development of joint pathologies, transgenic mice expressing constitutively active NLRC4 produce high levels of IL-1β and IL-18, and develop arthritis [81]. The NLRC4 inflammasome is also activated by nucleotide-derived metabolites (e.g., adenine and N-4-acetylcytidine) [6] and fatty acids (e.g., lysophosphatidylcholine and palmitate) [89] and is upregulated by bone-derived DAMPs during osteoclastogenesis [90]. An interplay between the NLRP3 and NLRC4 inflammasomes has been noted in these models of sterile inflammation as well as in response to Salmonella infection [105, 106].
Familial Mediterranean fever
MEFV encodes pyrin, which activates caspase-1 through ASC upon sensing post-translationally modified Rho GTPase [107]. These modifications include phosphorylation, ADP-ribosylation, and glycosylation and occur upon cell exposure to Clostridium toxins and in conditions of mevalonate kinase deficiency or proline-serine-threonine phosphatase-interacting protein 1 (PSTPIP1) gain-of-function [82, 108–110]. Hyper-activation of the pyrin inflammasome by MEFV-activating mutations causes Familial Mediterranean fever (FMF), a disease that is characterized by high levels of IL-1β, IL-6, IL-8, and IL-12, recurring fever episodes, arthritis, and low bone mass [83, 111]. FMF is the most prevalent monogenic autoinflammatory disorder; it affects over 100,000 persons worldwide and causes sporadic and chronic symptoms [98]. FMF mice develop severe systemic inflammation and exhibit massive cartilage and bone erosion [112]. These mice do not display systemic inflammatory symptoms upon deletion of GSDMD, IL-1 receptor, or ASC [112, 113].
Arthritis
Inflammasomes are activated in several autoimmune diseases, including rheumatoid arthritis (RA) and ankylosing spondylitis (AS). Components of the NLRP3 inflammasomes and various cytokines including TNF-α, IL-1β, IL-6, IL-7, and IL-17 are highly expressed in RA [84, 114]. Specific interactions among these cytokines and other inflammatory mediators may drive systemic and focal osteolysis in arthritis [115]. Systemic arthritis and bone loss induced by TNF over-expression are abolished upon ablation of IL-1β signaling despite the presence of synovial inflammation, suggesting that the effects of TNF-α on bone are mediated by IL-1β [116]. These findings positioning IL-1β downstream of TNF-α are in line with the observation that TNF-α-induced RANKL production by murine stromal cells is dependent on IL-1β [85, 117]. As noted above, the upregulation of TNF-α by IL-1β is also well known. Components of the inflammasomes, including NLRP3, ASC, and caspase-1 are upregulated in AS, a disease that is also associated with high levels of IL-1β, TNF-α, IL-6, IL-23, and IL-17 [118]. Bone manifestations in AS include excessive focal bone formation in joints whereas pronounced trabecular bone loss occurs in vertebral bodies [119].
Various inflammasomes are assembled in mouse models of arthritis. For example, NLRP3 and AIM-2 are both upregulated in the synovium of IL-10-deficient mice exposed to antigen-induced arthritis, and osteoclast differentiation from bone marrow cells isolated from these mutant mice is blunted by the inhibitors of NLRP3 and AIM-2 inflammasomes [120]. Moreover, arthritis induced by DNase II deficiency, which is associated with accrual of self-DNA, is attenuated by AIM2 ablation [86, 87]. The complexity of inflammasome functions is underscored by the observations that NLRP3, NLRP1, NLRC4, and caspase-1, but not ASC are dispensable for collagen-induced and antigen-induced arthritis [88, 121]; yet NLRP3 is a key player in joint destruction in a mouse model of A20 deficiency, in which NLRC4 and AIM2 are expendable [122]. Thus, the role of the inflammasomes in experimental arthritis is mouse model-dependent.
Osteomyelitis
Defective innate immune defense mechanisms including the inflammasomes underlie the pathogenesis of periodontitis and osteomyelitis commonly caused by Porphyromonas gingivalis and Staphylococcus aureus, respectively. P. gingivalis–derived PAMPs such as LPS are potent activators of priming signals, which through TLR4 signaling drive the expression of several components of the inflammasomes including IL-1β, NLRP3, AIM2, and caspase-11 [57, 58]. Accordingly, the massive alveolar bone destruction caused by P. gingivalis is attenuated upon loss of NLRP3 [59]. S. aureus bacterial products include peptidoglycans, hemolysins, bacterial lipoproteins, and Panton-Valentine leucocidin stimulate the inflammasomes through TLR2-mediated activation of NF-kB [123]. Moreover, some of these factors promote osteoclastogenesis [124]. The osteoblasts also express the NLRP3 inflammasome [60] though to lower levels compared with myeloid cells [73] and contribute to the pathogenesis of periodontitis and osteomyelitis [61, 125–127].
Autoinflammatory reactions of unknown etiology causes confined chronic non-bacterial osteomyelitis (CNO) or systemic chronic recurrent multifocal osteomyelitis (CRMO). Components of the NLRP3 inflammasome are expressed in osteoclasts in bone specimens from CRMO patients [62]. Mice carrying an inactivating -mutation in the proline-serine-threonine phosphatase-interacting protein 2 gene (Pstpip2) develop a phenotype reminiscent of CRMO, which is associated with over-production of IL-1β, enhanced osteoclastogenesis and bone resorption, responses that depend on IL-1 receptor and IL-1β, but not IL-1α, and are driven by neutrophils [21, 22]. Neutrophils in CRMO mice over-produce IL-1β via redundant actions of caspase-8 and the NLRP3 inflammasome [23].
Metabolic bone diseases
Inflammasomes have been linked to age- and menopause-related osteoporosis [6, 90, 91]. Estrogen profoundly affects the skeleton through various mechanisms including immunomodulation, suppressive effects on the expression of TNF-α and IL-1β, induction of osteoclast apoptosis through ERα, suppression of osteoclast differentiation, inhibition of RANKL production by osteoblasts, T and B cells, and stimulation of osteoprotegerin (OPG) expression [31, 128, 129]. Consistent with increased levels of pro-inflammatory cytokines in conditions of estrogen deficiency, blockade of TNF-α or IL-1β in post-menopausal patients leads to a decrease in the levels of bone resorption markers [130]. Accordingly, inhibition of TNF-α or deletion of NLRP3 protects against ovariectomy-induced bone loss in mice [90, 131].
Aging is associated with low-grade chronic inflammation. This process is referred to as inflammaging and is associated with increased levels of circulating IL-18, IL-1 receptor antagonist, and IL-6 [132]. Inflammasome gene modules including NLRC4 and IL-1β are upregulated in older people compared with younger individuals; persistent expression of these genes correlates with the occurrence of age-related complications, including chronic production of inflammatory cytokines, metabolic dysfunction, and oxidative stress [6]. The NLRP3 inflammasome also modulates age-related inflammation in peripheral tissues and age-related bone loss in mice, though the underlying mechanisms are unknown [91].
The ability of the NLRP3 inflammasome to detect a wide variety of endogenous DAMPs is likely an important driver in the development of age- and metabolic-related pathologies. These DAMPs include crystalline cholesterol, extracellular ATP, purine and pyrimidine metabolites, and debris from damaged tissues [6, 133]. For example, metabolites from the purine and pyrimidine pathways stimulate the NLRP3 and NLRC4 inflammasomes in THP-1 cells, activate human platelets and neutrophils in cultures, and promote hypertension and inflammation in mice [6]. The NLRP3 inflammasome may also be involved in hyper-multinucleation of murine osteoclasts caused by purinergic receptor P2X5 signaling [134]. We have shown that bone matrix degradation products regulate the NLRP3 and NLRC4 inflammasomes in cells of the osteoclast lineage [90]. Accordingly, Nlrp3 null mice are protected from bone loss induced by ovariectomy, sustained exposure to parathyroid hormone or RANKL. Treatment of mice with zoledronic acid inhibits inflammasome activation, thus reinforcing the view that endogenous DAMPs are released from the bone matrix during bone resorption, causing autocrine and paracrine effects on osteoclastogenesis [90].
Wear debris-induced osteolysis
Wear particles from articulating prosthetic joint surfaces such as those from cobalt-chromium-molybdenum (CoCrMo) implants induce inflammatory responses that cause aseptic loosening as a result of uncontrolled osteolysis [135]. The osteolytic process is associated with the formation at the implant-bone interface of a cellular membrane enriched in cells of the monocyte-macrophage lineage [136]. Activation of the NF-kB pathway through TLR2 signaling by prosthetic debris promote not only the expression of pro-inflammatory cytokines such as TNF-α, but also priming signals for the NLRP3 and AIM2 inflammasomes. Macrophages can also phagocytose these particles; cellular accumulation of these non-degradable materials enhances the production of reactive oxygen species and the rupture of the phagosomes, which releases cathepsins in the cytoplasm, events that activate the inflammasomes [92]. The size and shape of CoCrMo alloys affect the amplitude of the inflammatory responses [93]. Thus, wear debris can provide both priming and assembly signals that lead to aberrant inflammasome activation. Consistent with the role of the NLRP3 inflammasome in bone damage induced by prosthetic particles, bone resorption induced by polymethylmethacrylate (PMMA) particles is reduced in the absence of caspase-1 [3, 145]. Thus, both the metal and plastic components of the prostheses activate the inflammasomes.
Crystal-induced arthropathies
Endogenous crystalline particles are involved in the pathogenesis of arthropathies. For example, gouty arthritis is caused by precipitation of monosodium urate (MSU) crystals [94], calcium pyrophosphate deposition disease (CPDD) is driven by calcium pyrophosphate dihydrate (CPPD) crystals [94], and degenerative arthropathies such as osteoarthritis and Milwaukee shoulder are the result of abnormal accumulation of basic calcium phosphate (BCP) crystals [137]. Shared mechanisms among these diseases include phagocytosis of crystals by myeloid cells, an event that activates several inflammatory pathways including the inflammasomes, chondrocyte apoptosis, and matrix calcification [138–140]. Bone erosions in gout stems from continuous recruitment of macrophages to tophi [95]; sporadic CPDD is characterized by the presence of CPPD crystals in articular cartilage whereas patients with CPDD familial patterns have low bone mineral density though the extent to which bone resorption is impacted is not known [141]. BCP crystals, particularly hydroxyapatite crystals, promote osteoclast formation in vitro in NLRP3 inflammasome-dependent manner [90, 96, 142]. However, the actions of BCP on NLRP3 inflammasome-mediated skeletal pathology are controversial. Lack of components of the NLRP3 inflammasome prevents the development of neutrophil inflammation in the air-pouch model of synovitis and decreased pathology in the Ank-deficient model of arthritis [97]. By contrast, inflammatory responses induced by the injection of BCP crystals into the knees or intra-peritoneally are independent of the NLRP3 inflammasome [143, 144]. Alternative processing of IL-1β by caspase-8 and pyroptosis may account for these discrepant observations.
Therapeutic perspective
Anti-resorptive drugs such as bisphosphonates and denosumab are efficacious in the prevention of inflammation-associated bone fractures, but they do not impact the course of inflammation (Fig. 2). Thus, inhibition of osteoclast differentiation and/or activity is not sufficient to arrest the damage to the bone surrounding soft tissues such as the synovium in conditions of high-grade inflammation. On the other hand, biologics are successfully used in the clinic to temper down inflammation (Fig. 2). However, biologics have their shortcomings such as high costs, the requirement for parenteral delivery, the development of resistance, and immunosuppression. In addition, the efficacy of these drugs is restrained by redundancy among signaling pathways as they target specific inflammatory instigators. Thus, there is still an unmet medical need for the development of adequate therapeutic strategies; in-depth understanding of the mechanism of action of key inflammatory pathways is required to achieve this goal. Recent breakthroughs revealing that aberrant activities of the inflammasomes cause pyroptosis, a lytic form of cell death that concomitantly unleashes several inflammatory mediators to the extracellular milieu offer novel perspectives for drug discovery. For example, strategies aimed at preventing pyroptosis through selective blockade of individual components of the inflammasomes such as caspase-1, NLRP3, or GSDMD or inhibiting signaling nodes that integrate several inflammatory cues such as p38 MAPK are being fiercely explored.
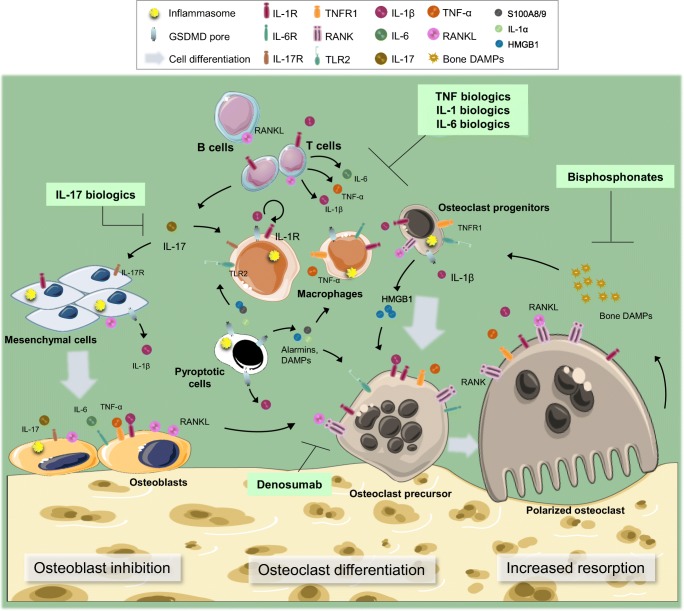
Fig. 2.
Inflammatory osteolysis and therapeutic interventions. Cytokines (e.g., IL-1α, IL-1β, IL-6, IL-17, and TNF-α) stimulate bone resorption directly, acting on the osteoclast lineage or indirectly by inducing RANKL expression by mesenchymal cells, T cells, and B cells. These cytokines also inhibit bone formation. Bone resorption is directly blocked by anti-resorptive drugs such as bisphosphonates and denosumab or indirectly by biologics targeting IL-1α/IL-1β, IL-6, IL-17, or TNF-α. In conditions of low-grade inflammation, bone resorption is amplified by DAMPs that are released from bone matrix
Acknowledgments
We thank Dustin Kress for careful reading of the manuscript. We have tried to cite primary work in most cases, but due to a large amount of research in the area of inflammation, we are sure to have missed some important papers. We apologize in advance to authors who have been omitted. The drawings were modified from https://smart.servier.com
Funding information
This work is supported by NIH/NIAMS AR064755 and AR068972 grants to G.M.
Compliance with ethical standards
Conflict of interest
G.M. is a consultant for Aclaris Therapeutics, Inc. Other authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Özören N, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat Immunol. 2006;7(6):576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 2.Guey B, Bodnar M, Manié SN, Tardivel A, Petrilli V. Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function. Proc Natl Acad Sci. 2014;111(48):17254–17259. doi: 10.1073/pnas.1415756111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton L, Paget D, Binder NB, Bohnert K, Nestor BJ, et al. Orthopedic wear debris mediated inflammatory osteolysis is mediated in part by NALP3 inflammasome activation. J Orthop Res. 2013;31(1):73–80. doi: 10.1002/jor.22190. [DOI] [PubMed] [Google Scholar]
- 4.Haneklaus M, O’Neill LAJ. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265(1):53–62. doi: 10.1111/imr.12285. [DOI] [PubMed] [Google Scholar]
- 5.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science (80) 2010;327(5963):296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 6.Furman D, Chang J, Lartigue L, Bolen CR, Haddad F, et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med. 2017;23(2):174–184. doi: 10.1038/nm.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lénárt N, Coutts G, Denes A, Rothwell N, Brough D, et al. AIM2 and NLRC4 inflammasomes contribute with ASC to acute brain injury independently of NLRP3. Proc Natl Acad Sci. 2015;112(13):4050–4055. doi: 10.1073/pnas.1419090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lugrin J, Martinon F. The AIM2 inflammasome: sensor of pathogens and cellular perturbations. Immunol Rev. 2018;281(1):99–114. doi: 10.1111/imr.12618. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman HM, Broderick L. The role of the inflammasome in patients with autoinflammatory diseases. J Allergy Clin Immunol. 2016;138(1):3–14. doi: 10.1016/j.jaci.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Canna SW, Nigrovic PA. Editorial: 21st century storm chasers: defining macrophage activation syndrome. Arthritis Rheum. 2016;68(3):557–560. doi: 10.1002/art.39329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benseler S, DiMattia MA, Gouni S, Biancotto A, O’Shea JJ, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46(10):1140–1146. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romberg N, Al Moussawi K, Nelson-Williams C, Stiegler AL, Loring E, et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet. 2014;46(10):1135–1139. doi: 10.1038/ng.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 14.Jin Y, Birlea SA, Fain PR, Spritz RA. Genetic variations in NALP1 are associated with generalized vitiligo in a Romanian population. J Invest Dermatol. 2007;127(11):2558–2562. doi: 10.1038/sj.jid.5700953. [DOI] [PubMed] [Google Scholar]
- 15.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356(12):1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 16.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Ding J, Wang K, Liu W, She Y, Sun Q, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 18.Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity. 2018;48(1):35–44.e6. doi: 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He WT, Wan H, Hu L, Chen P, Wang X, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25(12):1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35(16):1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassel SL, Janczy JR, Bing X, Wilson SP, Olivier AK, et al. Inflammasome-independent IL-1 mediates autoinflammatory disease in Pstpip2-deficient mice. Proc Natl Acad Sci. 2014;111(3):1072–1077. doi: 10.1073/pnas.1318685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukens JR, Gross JM, Calabrese C, Iwakura Y, Lamkanfi M, et al. Critical role for inflammasome-independent IL-1 production in osteomyelitis. Proc Natl Acad Sci. 2014;111(3):1066–1071. doi: 10.1073/pnas.1318688111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurung P, Burton A, Kanneganti T-D. NLRP3 inflammasome plays a redundant role with caspase 8 to promote IL-1β–mediated osteomyelitis. Proc Natl Acad Sci. 2016;113(16):4452–4457. doi: 10.1073/pnas.1601636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi J, Zhao Y, Wang K, Shi X, Wang Y, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 25.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 26.Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science (80) 2016;352(6290):1232–1236. doi: 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115(46):E10888–E10897. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orning P, Weng D, Starheim K, Ratner D, Best Z, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362(6418):1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sims NA, Gooi JH. Seminars in cell & developmental biology bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19:444–451. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Bellido T, Plotkin LI, Bruzzaniti A. Basic and applied bone biology. Milano: Elsevier; 2014. Bone cells; pp. 27–45. [Google Scholar]
- 31.Novack DV, Mbalaviele G. Osteoclasts-key players in skeletal health and disease. Microbiol Spectr. 2016;4(3):1–19. doi: 10.1128/microbiolspec.MCHD-0011-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mbalaviele G, Novack DV, Schett G, Teitelbaum SL. Inflammatory osteolysis: a conspiracy against bone. J Clin Invest. 2017;127(6):2030–2039. doi: 10.1172/JCI93356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27(1):519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 34.Yun TJ, Chaudhary PM, Shu GL, Frazer JK, Ewings MK, et al. OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J Immunol. 1998;161(11):6113–6121. [PubMed] [Google Scholar]
- 35.Kanematsu M, Sato T, Takai H, Watanabe K, Ikeda K, Yamada Y. Prostaglandin E2 induces expression of receptor activator of nuclear factor-kappa B ligand/osteoprotegrin ligand on pre-B cells: implications for accelerated osteoclastogenesis in estrogen deficiency. J Bone Miner Res. 2000;15(7):1321–1329. doi: 10.1359/jbmr.2000.15.7.1321. [DOI] [PubMed] [Google Scholar]
- 36.Wang R, Zhang L, Zhang X, Moreno J, Luo X, et al. Differential regulation of the expression of CD95 ligand, receptor activator of nuclear factor-kappa B ligand (RANKL), TNF-related apoptosis-inducing ligand (TRAIL), and TNF-alpha during T cell activation. J Immunol. 2001;166(3):1983–1990. doi: 10.4049/jimmunol.166.3.1983. [DOI] [PubMed] [Google Scholar]
- 37.Crotti TN, Smith MD, Weedon H, Ahern MJ, Findlay DM, et al. Receptor activator NF-kappaB ligand (RANKL) expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathy, osteoarthritis, and from normal patients: semiquantitative and quantitative analysis. Ann Rheum Dis. 2002;61(12):1047–1054. doi: 10.1136/ard.61.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinn JM, Horwood NJ, Elliott J, Gillespie MT, Martin TJ. Fibroblastic stromal cells express receptor activator of NF-kappa B ligand and support osteoclast differentiation. J Bone Miner Res. 2000;15(8):1459–1466. doi: 10.1359/jbmr.2000.15.8.1459. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191(2):275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Brien W, Fissel BM, Maeda Y, Yan J, Ge X, et al. RANK-independent osteoclast formation and bone erosion in inflammatory arthritis. Arthritis Rheum. 2016;68(12):2889–2900. doi: 10.1002/art.39837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C, Hockerman S, Jacobsen EJ, Alippe Y, Selness SR, et al. Selective inhibition of the p38α MAPK–MK2 axis inhibits inflammatory cues including inflammasome priming signals. J Exp Med. 2018;215(5):1315–1325. doi: 10.1084/jem.20172063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J, Cho D, Park H. IL-18 and cutaneous inflammatory diseases. Int J Mol Sci. 2015;16(12):29357–29369. doi: 10.3390/ijms161226172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakanishi K. Unique action of interleukin-18 on T cells and other immune cells. Front Immunol. 2018;9:763. doi: 10.3389/fimmu.2018.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Cong X-L, Qin Y-H, He Z-W, He D-Y, Dai S-M. IL-18 upregulates the production of key regulators of osteoclastogenesis from fibroblast-like synoviocytes in rheumatoid arthritis. Inflammation. 2013;36(1):103–109. doi: 10.1007/s10753-012-9524-8. [DOI] [PubMed] [Google Scholar]
- 45.Martin TJ, Romas E, Gillespie MT. Interleukins in the control of osteoclast differentiation. Crit Rev Eukaryot Gene Expr. 1998;8(2):107–123. doi: 10.1615/critreveukargeneexpr.v8.i2.10. [DOI] [PubMed] [Google Scholar]
- 46.Horwood NJ, Udagawa N, Elliott J, Grail D, Okamura H, et al. Interleukin 18 inhibits osteoclast formation via T cell production of granulocyte macrophage colony-stimulating factor. J Clin Invest. 1998;101(3):595–603. doi: 10.1172/JCI1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci. 2016;113(28):7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finzel BC, Clancy LL, Holland DR, Muchmore SW, Watenpaugh KD, Einspahr HM. Crystal structure of recombinant human interleukin-1β at 2·0 Å resolution. J Mol Biol. 1989;209(4):779–791. doi: 10.1016/0022-2836(89)90606-2. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kevin Tracey DJ, Kanneganti T-D, Walle VM, Vitari AC, Amer AO, et al. Alarmin HMGB1 in endotoxemia inflammasome-dependent release of the. J Immunol. 2019;185:4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang J, Shah R, Robling AG, Templeton E, Yang H, et al. HMGB1 is a bone-active cytokine. J Cell Physiol. 2008;214(3):730–739. doi: 10.1002/jcp.21268. [DOI] [PubMed] [Google Scholar]
- 52.Von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490(7418):107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charoonpatrapong K, Shah R, Robling AG, Alvarez M, Clapp DW, et al. HMGB1 expression and release by bone cells. J Cell Physiol. 2006;207(2):480–490. doi: 10.1002/jcp.20577. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y, Sun W, Gao R, Su Y, Umehara H, et al. The role of high mobility group box chromosomal protein 1 in rheumatoid arthritis. Rheumatology. 2013;52(10):1739–1747. doi: 10.1093/rheumatology/ket134. [DOI] [PubMed] [Google Scholar]
- 55.Bertheloot D, Latz E. HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol. 2017;14(1):43–64. doi: 10.1038/cmi.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang C, Qu C, Alippe Y, Bonar SL, Civitelli R, et al. Poly-ADP-ribosylation-mediated degradation of ARTD1 by the NLRP3 inflammasome is a prerequisite for osteoclast maturation. Cell Death Dis. 2016;7(3):e2153–e2153. doi: 10.1038/cddis.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park E, Na HS, Song YR, Shin SY, Kim YM, Chung J. Activation of NLRP3 and AIM2 inflammasomes by Porphyromonas gingivalis infection. Infect Immun. 2014;82(1):112–123. doi: 10.1128/IAI.00862-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bostanci N, Emingil G, Saygan B, Turkoglu O, Atilla G, et al. Expression and regulation of the NALP3 inflammasome complex in periodontal diseases. Clin Exp Immunol. 2009;157(3):415–422. doi: 10.1111/j.1365-2249.2009.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaguchi Y, Kurita-Ochiai T, Kobayashi R, Suzuki T, Ando T. Regulation of the NLRP3 inflammasome in Porphyromonas gingivalis-accelerated periodontal disease. Inflamm Res. 2017;66(1):59–65. doi: 10.1007/s00011-016-0992-4. [DOI] [PubMed] [Google Scholar]
- 60.McCall SH, Sahraei M, Young AB, Worley CS, Duncan JA, et al. Osteoblasts express NLRP3, a nucleotide-binding domain and leucine-rich repeat region containing receptor implicated in bacterially induced cell death. J Bone Miner Res. 2007;23(1):30–40. doi: 10.1359/JBMR.071002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Josse J, Velard F, Gangloff SC. Staphylococcus aureus vs. osteoblast: relationship and consequences in osteomyelitis. Front Cell Infect Microbiol. 2015;5(November):1–17. doi: 10.3389/fcimb.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scianaro R, Insalaco A, Bracci Laudiero L, De Vito R, Pezzullo M, et al. Deregulation of the IL-1β axis in chronic recurrent multifocal osteomyelitis. Pediatr Rheumatol. 2014;12(1):30. doi: 10.1186/1546-0096-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levy R, Gérard L, Kuemmerle-Deschner J, Lachmann HJ, Koné-Paut I, et al. Phenotypic and genotypic characteristics of cryopyrin-associated periodic syndrome: a series of 136 patients from the Eurofever registry. Ann Rheum Dis. 2014;74(11):2043–2049. doi: 10.1136/annrheumdis-2013-204991. [DOI] [PubMed] [Google Scholar]
- 64.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29(3):301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoffman HM, Gregory SG, Mueller JL, Tresierras M, Broide DH, et al. Fine structure mapping of CIAS1: identification of an ancestral haplotype and a common FCAS mutation, L353P. Hum Genet. 2003;112(2):209–216. doi: 10.1007/s00439-002-0860-x. [DOI] [PubMed] [Google Scholar]
- 66.Cordero MD, Alcocer-Gómez E, Ryffel B. Gain of function mutation and inflammasome driven diseases in human and mouse models. J Autoimmun. 2018;91:13–22. doi: 10.1016/j.jaut.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20(3):319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 68.Goldbach-Mansky R. Current status of understanding the pathogenesis and management of patients with NOMID/CINCA. Curr Rheumatol Rep. 2011;13(2):123–131. doi: 10.1007/s11926-011-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, et al. De novoCIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46(12):3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hill SC, Namde M, Dwyer A, Poznanski A, Canna S, Goldbach-Mansky R. Arthropathy of neonatal onset multisystem inflammatory disease (NOMID/CINCA) Pediatr Radiol. 2007;37(2):145–152. doi: 10.1007/s00247-006-0358-0. [DOI] [PubMed] [Google Scholar]
- 71.Yokoyama K, Ikeya M, Umeda K, Oda H, Nodomi S, et al. Enhanced chondrogenesis of induced pluripotent stem cells from patients with neonatal-onset multisystem inflammatory disease occurs via the caspase 1-independent cAMP/protein kinase A/CREB pathway. Arthritis Rheum. 2015;67(1):302–314. doi: 10.1002/art.38912. [DOI] [PubMed] [Google Scholar]
- 72.Snouwaert JN, Nguyen MT, Repenning PW, Dye R, Livingston EW, et al. An NLRP3 mutation causes arthropathy and osteoporosis in humanized mice. Cell Rep. 2016;17(11):3077–3088. doi: 10.1016/j.celrep.2016.11.052. [DOI] [PubMed] [Google Scholar]
- 73.Bonar SL, Brydges SD, Mueller JL, McGeough MD, Pena C, et al. Constitutively activated NLRP3 inflammasome causes inflammation and abnormal skeletal development in mice. PLoS One. 2012;7(4):4–14. doi: 10.1371/journal.pone.0035979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30(6):860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang C, Xu C-X, Alippe Y, Qu C, Xiao J, et al. Chronic inflammation triggered by the NLRP3 inflammasome in myeloid cells promotes growth plate dysplasia by mesenchymal cells. Sci Rep. 2017;7(1):4880. doi: 10.1038/s41598-017-05033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qu C, Bonar SL, Hickman-Brecks CL, Abu-Amer S, McGeough MD, et al. NLRP3 mediates osteolysis through inflammation-dependent and -independent mechanisms. FASEB J. 2015;29(4):1269–1279. doi: 10.1096/fj.14-264804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao J, Wang C, Yao J-C, Alippe Y, Xu C, et al. Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS Biol. 2018;16(11):e3000047. doi: 10.1371/journal.pbio.3000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGeough MD, Wree A, Inzaugarat ME, Haimovich A, Johnson CD, et al. TNF regulates transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies. J Clin Invest. 2017;127(12):4488–4497. doi: 10.1172/JCI90699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J Clin Invest. 2013;123(11):4695–4705. doi: 10.1172/JCI71543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Canna SW, De Jesus AA, Gouni S, Brooks SR, Marrero B, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46(10):1140–1146. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kitamura A, Sasaki Y, Abe T, Kano H, Yasutomo K. An inherited mutation in NLRC4 causes autoinflammation in human and mice. J Exp Med. 2014;211(12):2385–2396. doi: 10.1084/jem.20141091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park YH, Wood G, Kastner DL, Chae JJ. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol. 2016;17(8):914–921. doi: 10.1038/ni.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ben-Chetrit E, Levy M. Familial Mediterranean fever. Lancet. 1998;351(9103):659–664. doi: 10.1016/S0140-6736(97)09408-7. [DOI] [PubMed] [Google Scholar]
- 84.Kolly L, Busso N, Palmer G, Talabot-Ayer D, Chobaz V, So A. Expression and function of the NALP3 inflammasome in rheumatoid synovium. Immunology. 2010;129(2):178–185. doi: 10.1111/j.1365-2567.2009.03174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mathews RJ, Robinson JI, Battellino M, Wong C, Taylor JC, et al. Evidence of NLRP3-inflammasome activation in rheumatoid arthritis (RA); genetic variants within the NLRP3-inflammasome complex in relation to susceptibility to RA and response to anti-TNF treatment. Ann Rheum Dis. 2014;73(6):1202–1210. doi: 10.1136/annrheumdis-2013-203276. [DOI] [PubMed] [Google Scholar]
- 86.Baum R, Sharma S, Carpenter S, Li Q-Z, Busto P, et al. Cutting edge: AIM2 and endosomal TLRs differentially regulate arthritis and autoantibody production in DNase II-deficient mice. J Immunol. 2015;194(3):873–877. doi: 10.4049/jimmunol.1402573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jakobs C, Perner S, Hornung V. AIM2 drives joint inflammation in a self-DNA triggered model of chronic polyarthritis. PLoS One. 2015;10(6):e0131702. doi: 10.1371/journal.pone.0131702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ippagunta SK, Brand DD, Luo J, Boyd KL, Calabrese C, et al. Inflammasome-independent role of apoptosis-associated speck-like protein containing a CARD (ASC) in T cell priming is critical for collagen-induced arthritis. J Biol Chem. 2010;285(16):12454–12462. doi: 10.1074/jbc.M109.093252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Freeman L, Guo H, David CN, Brickey WJ, Jha S, Ting JP-Y. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J Exp Med. 2017;214(5):1351–1370. doi: 10.1084/jem.20150237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alippe Y, Wang C, Ricci B, Xiao J, Qu C, et al. Bone matrix components activate the NLRP3 inflammasome and promote osteoclast differentiation. Sci Rep. 2017;7(1):6630. doi: 10.1038/s41598-017-07014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Youm YH, Grant RW, Mccabe LR, Albarado DC, Nguyen KY, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18(4):519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maitra R, Clement CC, Scharf B, Crisi GM, Chitta S, et al. Endosomal damage and TLR2 mediated inflammasome activation by alkane particles in the generation of aseptic osteolysis. Mol Immunol. 2009;47(2–3):175–184. doi: 10.1016/j.molimm.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caicedo MS, Samelko L, McAllister K, Jacobs JJ, Hallab NJ. Increasing both CoCrMo-alloy particle size and surface irregularity induces increased macrophage inflammasome activation in vitro potentially through lysosomal destabilization mechanisms. J Orthop Res. 2013;31(10):1633–1642. doi: 10.1002/jor.22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 95.Schlesinger N, Thiele RG. The pathogenesis of bone erosions in gouty arthritis. Ann Rheum Dis. 2010;69(11):1907–1912. doi: 10.1136/ard.2010.128454. [DOI] [PubMed] [Google Scholar]
- 96.Pazar B, Ea H-K, Narayan S, Kolly L, Bagnoud N, et al. Basic calcium phosphate crystals induce monocyte/macrophage IL-1 secretion through the NLRP3 inflammasome in vitro. J Immunol. 2011;186(4):2495–2502. doi: 10.4049/jimmunol.1001284. [DOI] [PubMed] [Google Scholar]
- 97.Jin C, Frayssinet P, Pelker R, Cwirka D, Hu B, et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc Natl Acad Sci. 2011;108(36):14867–14872. doi: 10.1073/pnas.1111101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Jesus AA, Goldbach-Mansky R. Monogenic autoinflammatory diseases: concept and clinical manifestations. Clin Immunol. 2013;147(3):155–174. doi: 10.1016/j.clim.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neven B, Marvillet I, Terrada C, Ferster A, Boddaert N, et al. Long-term efficacy of the interleukin-1 receptor antagonist anakinra in ten patients with neonatal-onset multisystem inflammatory disease/chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 2010;62(1):258–267. doi: 10.1002/art.25057. [DOI] [PubMed] [Google Scholar]
- 100.Laino ME, Marrocco R, Rigante D, Stabile A, Leone A, et al. Long-term response after 6-year treatment with anakinra and onset of focal bone erosion in neonatal-onset multisystem inflammatory disease (NOMID/CINCA) Rheumatol Int. 2011;31(12):1661–1664. doi: 10.1007/s00296-010-1787-5. [DOI] [PubMed] [Google Scholar]
- 101.Sibley CH, Plass N, Snow J, Wiggs EA, Brewer CC, et al. Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three- and five-year outcomes. Arthritis Rheum. 2012;64(7):2375–2386. doi: 10.1002/art.34409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maruotti N, Corrado A, Cantatore FP. Osteoporosis and rheumatic diseases. Reumatismo. 2014;66(2):125. doi: 10.4081/reumatismo.2014.785. [DOI] [PubMed] [Google Scholar]
- 103.Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial) Ann Rheum Dis. 2011;70(5):747–754. doi: 10.1136/ard.2010.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tarp S, Amarilyo G, Foeldvari I, Christensen R, Woo JMP, et al. Efficacy and safety of biological agents for systemic juvenile idiopathic arthritis: a systematic review and meta-analysis of randomized trials. Rheumatology. 2016;55(4):669–679. doi: 10.1093/rheumatology/kev382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qu Y, Misaghi S, Newton K, Maltzman A, Izrael-Tomasevic A, et al. NLRP3 recruitment by NLRC4 during Salmonella infection. J Exp Med. 2016;213(6):877–885. doi: 10.1084/jem.20132234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Man SM, Hopkins LJ, Nugent E, Cox S, Gluck IM, et al. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc Natl Acad Sci. 2014;111(20):7403–7408. doi: 10.1073/pnas.1402911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu H, Yang J, Gao W, Li L, Li P, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513(7517):237–241. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 108.Haas D, Hoffmann GF. Mevalonate kinase deficiencies: from mevalonic aciduria to hyperimmunoglobulinemia D syndrome. Orphanet J Rare Dis. 2006;1(1):13. doi: 10.1186/1750-1172-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lindor NM, Arsenault TM, Solomon H, Seidman CE, McEvoy MT. A new autosomal dominant disorder of pyogenic sterile arthritis, pyoderma gangrenosum, and acne: PAPA syndrome. Mayo Clin Proc. 1997;72(7):611–615. doi: 10.1016/S0025-6196(11)63565-9. [DOI] [PubMed] [Google Scholar]
- 110.Wise CA, Gillum JD, Seidman CE, Lindor NM, Veile R, et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum Mol Genet. 2002;11(8):961–969. doi: 10.1093/hmg/11.8.961. [DOI] [PubMed] [Google Scholar]
- 111.Ibrahim J-N, Jounblat R, Delwail A, Abou-Ghoch J, Salem N, et al. Ex vivo PBMC cytokine profile in familial Mediterranean fever patients: involvement of IL-1β, IL-1α and Th17-associated cytokines and decrease of Th1 and Th2 cytokines. Cytokine. 2014;69(2):248–254. doi: 10.1016/j.cyto.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 112.Chae JJ, Cho Y-H, Lee G-S, Cheng J, Liu PP, et al. Gain-of-function pyrin mutations induce NLRP3 protein-independent interleukin-1β activation and severe autoinflammation in mice. Immunity. 2011;34(5):755–768. doi: 10.1016/j.immuni.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kanneganti A, Malireddi RKS, Saavedra PHV, Vande Walle L, Van Gorp H, et al. GSDMD is critical for autoinflammatory pathology in a mouse model of familial Mediterranean fever. J Exp Med. 2018;215(6):1519–1529. doi: 10.1084/jem.20172060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Siebert S, Tsoukas A, Robertson J, McInnes I. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacol Rev. 2015;67(2):280–309. doi: 10.1124/pr.114.009639. [DOI] [PubMed] [Google Scholar]
- 115.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11(3):234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 116.Zwerina J, Redlich K, Polzer K, Joosten L, Kronke G, et al. TNF-induced structural joint damage is mediated by IL-1. Proc Natl Acad Sci. 2007;104(28):11742–11747. doi: 10.1073/pnas.0610812104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wei S, Kitaura H, Zhou P, Patrick Ross F, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115(2):282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim S-K, Cho YJ, Choe J-Y. NLRP3 inflammasomes and NLRP3 inflammasome-derived proinflammatory cytokines in peripheral blood mononuclear cells of patients with ankylosing spondylitis. Clin Chim Acta. 2018;486:269–274. doi: 10.1016/j.cca.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 119.Amarasekara DS, Yu J, Rho J. Bone loss triggered by the cytokine network in inflammatory autoimmune diseases. J Immunol Res. 2015;2015:1–12. doi: 10.1155/2015/832127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Greenhill CJ, Jones GW, Nowell MA, Newton Z, Harvey AK, et al. Interleukin-10 regulates the inflammasome-driven augmentation of inflammatory arthritis and joint destruction. Arthritis Res Ther. 2014;16(4):419. doi: 10.1186/s13075-014-0419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kolly L, Karababa M, Joosten LAB, Narayan S, Salvi R, et al. Inflammatory role of ASC in antigen-induced arthritis is independent of caspase-1, NALP-3, and IPAF. J Immunol. 2009;183(6):4003–4012. doi: 10.4049/jimmunol.0802173. [DOI] [PubMed] [Google Scholar]
- 122.Vande Walle L, Van Opdenbosch N, Jacques P, Fossoul A, Verheugen E, et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature. 2014;512(7512):69–73. doi: 10.1038/nature13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Holzinger D, Gieldon L, Mysore V, Nippe N, Taxman DJ, et al. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J Leukoc Biol. 2012;92(5):1069–1081. doi: 10.1189/jlb.0112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Claro T, Widaa A, McDonnell C, Foster TJ, O’Brien FJ, Kerrigan SW. Staphylococcus aureus protein A binding to osteoblast tumour necrosis factor receptor 1 results in activation of nuclear factor kappa B and release of interleukin-6 in bone infection. Microbiology. 2013;159(Pt_1):147–154. doi: 10.1099/mic.0.063016-0. [DOI] [PubMed] [Google Scholar]
- 125.Kassem A, Henning P, Lundberg P, Souza PPC, Lindholm C, Lerner UH. Porphyromonas gingivalis stimulates bone resorption by enhancing RANKL (receptor activator of NF-κB ligand) through activation of toll-like receptor 2 in osteoblasts. J Biol Chem. 2015;290(33):20147–20158. doi: 10.1074/jbc.M115.655787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Han X, Lin X, Yu X, Lin J, Kawai T, et al. Porphyromonas gingivalis infection-associated periodontal bone resorption is dependent on receptor activator of NF-κB ligand. Infect Immun. 2013;81(5):1502–1509. doi: 10.1128/IAI.00043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Akiyama T, Miyamoto Y, Yoshimura K, Yamada A, Takami M, et al. Porphyromonas gingivalis-derived lysine gingipain enhances osteoclast differentiation induced by tumor necrosis factor-α and interleukin-1β but suppresses that by interleukin-17A: importance of proteolytic degradation of osteoprotegerin by lysine gingipain. J Biol Chem. 2014;289(22):15621–15630. doi: 10.1074/jbc.M113.520510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Faienza MF, Ventura A, Marzano F, Cavallo L. Postmenopausal osteoporosis: the role of immune system cells. Clin Dev Immunol. 2013;2013:1–6. doi: 10.1155/2013/575936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weitzmann MN, Pacifici R. Review series estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116(5):1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Charatcharoenwitthaya N, Khosla S, Atkinson EJ, McCready LK, Riggs BL. Effect of blockade of TNF-α and interleukin-1 action on bone resorption in early postmenopausal women. J Bone Miner Res. 2007;22(5):724–729. doi: 10.1359/jbmr.070207. [DOI] [PubMed] [Google Scholar]
- 131.Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, et al. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A. 2001;98(24):13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 133.Liston A, Masters SL. Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nat Rev Immunol. 2017;17(3):208–214. doi: 10.1038/nri.2016.151. [DOI] [PubMed] [Google Scholar]
- 134.Kim H, Walsh MC, Takegahara N, Middleton SA, Shin HI, et al. The purinergic receptor P2X5 regulates inflammasome activity and hyper-multinucleation of murine osteoclasts. Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cobelli N, Scharf B, Crisi GM, Hardin J, Santambrogio L. Mediators of the inflammatory response to joint replacement devices. Nat Rev Rheumatol. 2011;7(10):600–608. doi: 10.1038/nrrheum.2011.128. [DOI] [PubMed] [Google Scholar]
- 136.Nich C, Takakubo Y, Pajarinen J, Gallo J, Konttinen YT, et al. The role of macrophages in the biological reaction to wear debris from artificial joints. J Long-Term Eff Med Implants. 2016;26(4):303–309. doi: 10.1615/JLongTermEffMedImplants.2017011287. [DOI] [PubMed] [Google Scholar]
- 137.MacMullan P, McMahon G, McCarthy G. Detection of basic calcium phosphate crystals in osteoarthritis. Joint Bone Spine. 2011;78(4):358–363. doi: 10.1016/j.jbspin.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 138.Desai J, Steiger S, Anders H-J. Molecular pathophysiology of gout. Trends Mol Med. 2017;23(8):756–768. doi: 10.1016/j.molmed.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 139.Cheung HS. Calcium crystal effects on the cells of the joint: implications for pathogenesis of disease. Curr Opin Rheumatol. 2000;12(3):223–227. doi: 10.1097/00002281-200005000-00012. [DOI] [PubMed] [Google Scholar]
- 140.So AK, Martinon F. Inflammation in gout: mechanisms and therapeutic targets. Nat Rev Rheumatol. 2017;13(11):639–647. doi: 10.1038/nrrheum.2017.155. [DOI] [PubMed] [Google Scholar]
- 141.Williams CJ, Qazi U, Bernstein M, Charniak A, Gohr C, et al. Mutations in osteoprotegerin account for the CCAL1 locus in calcium pyrophosphate deposition disease. Osteoarthr Cartil. 2018;26(6):797–806. doi: 10.1016/j.joca.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nadra I, Boccaccini AR, Philippidis P, Whelan LC, McCarthy GM, et al. Effect of particle size on hydroxyapatite crystal-induced tumor necrosis factor alpha secretion by macrophages. Atherosclerosis. 2008;196(1):98–105. doi: 10.1016/j.atherosclerosis.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 143.Narayan S, Pazar B, Ea H-K, Kolly L, Bagnoud N, et al. Octacalcium phosphate crystals induce inflammation in vivo through interleukin-1 but independent of the NLRP3 inflammasome in mice. Arthritis Rheum. 2011;63(2):422–433. doi: 10.1002/art.30147. [DOI] [PubMed] [Google Scholar]
- 144.Ea H-K, Chobaz V, Nguyen C, Nasi S, van Lent P, et al. Pathogenic role of basic calcium phosphate crystals in destructive arthropathies. PLoS One. 2013;8(2):e57352. doi: 10.1371/journal.pone.0057352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Clohisy John C., Yamanaka Yasuhiro, Faccio Roberta, Abu-Amer Yousef. Inhibition of IKK activation, through sequestering NEMO, blocks PMMA-induced osteoclastogenesis and calvarial inflammatory osteolysis. Journal of Orthopaedic Research. 2006;24(7):1358–1365. doi: 10.1002/jor.20184. [DOI] [PubMed] [Google Scholar]