Abstract
Integrin α11β1 is a collagen-binding integrin, which is receiving increasing attention in the context of wound healing and fibrosis. Although α11β1 integrin displays similar collagen specificity to α2β1 integrin, both integrins have distinct in vivo functions. In this context, the contribution of α11 subunit cytoplasmic tail interactions to diverse molecular signals and biological functions is largely unknown. In the current study, we have deleted the α11 cytoplasmic tail and studied the effect of this deletion on α11 integrin function. Compared to wild-type cells, C2C12 cells expressing tail-less α11 attached normally to collagen I, but formed fewer focal contacts. α11-tail-less cells furthermore displayed a reduced capacity to invade and reorganize a 3D collagen matrix and to proliferate. Analysis of cell signaling showed that FAK and ERK phosphorylation was reduced in cells expressing tail-less α11. Inhibition of ERK and FAK activation decreased α11-mediated cell proliferation, whereas α11-mediated cell invasion was FAK-dependent and occurred independently of ERK signaling. In summary, our data demonstrate that the integrin α11 cytoplasmic tail plays a central role in α11 integrin-specific functions, including FAK-dependent ERK activation to promote cell proliferation.
Subject terms: Extracellular matrix, Focal adhesion, Integrins, Integrin signalling
Introduction
Integrins are heterodimeric cell surface receptors composed of non-covalently associated α and β subunits, which act as cell surface links to the extracellular matrix (ECM) and to other cells in dynamic cell-cell linkages1. Integrin subunits are composed of different domains with different functions. The extracellular domain of collagen-binding α integrin chains contain an inserted α-I domain, which is responsible for collagen binding without direct involvement of the β subunit. Whereas different integrin β chains display conserved regions including their cytoplasmic tail, the cytoplasmic tails of integrin α chains show little sequence similarity except for the very proximal membrane sequence GFFXR2. It is interesting in this context to note that α11 integrin lacks the conserved GFFXR sequence, but instead the α11-tail contains the GFFRS sequence3. A number of proteins have been found to bind to the conserved GFFXR sequence without displaying specificity for any particular α chain2. The conserved GFFXR site has been demonstrated to bind, for example, SHARPIN4, which keeps integrin in an inactive conformation. Other proteins reported to bind to the conserved membrane proximal region include Rab21, Nischarin and PP2A2. Deletion of GFFXR or mutation of Arginine in the GFFXR sequence render integrins constitutively active, suggested to occur as a result of breakage of a salt linkage between α chains and β chain5. If the deletion occurs beyond the GFFXR sequence, effects vary depending on the nature of the α chain and the cellular background6.
Molecular interactions of the integrin cytoplasmic tails can both regulate inside-out and outside-in signaling as well as strengthening the actin linkages2,7. The NPXY motifs located in the β subunits are important binding sites for talins and kindlins, both taking part in integrin inside-out signaling8,9. These important interactions in turn are regulated through binding of other proteins such as Dok1 and ICAP-1, to the same integrin β chain NPXY motifs. In addition, phosphorylation of the proximal NPXY motif appears to be a molecular switch to regulate tensin binding and localization of α5β1 to fibrillar adhesions10. More recent data have demonstrated that integrin α chains contribute to filamin A-, talin-, and kindlin-binding to the integrin β subunits11–14. Data are thus accumulating with indications that integrin α cytoplasmic tails take an active part in interactions of importance for integrin heterodimer function.
Careful analyses of mice lacking individual collagen-binding integrins show that the collagen-binding integrin receptors are dispensable for normal development, but suggest important roles for these receptors in tissue remodeling events occurring in wound healing, fibrosis and tumor-stroma interactions15. α11β1 integrin is a collagen receptor, which is the latest identified member of the integrin family3,16,17. Although α11 shows an overall sequence homology to other collagen-binding integrin α chains of the β1 subfamily and also displays similar collagen specificity as α2β1 integrin, α11β1 integrin in vivo has functions distinct from the other collagen-binding integrins18–22. This suggests that α11 cytoplasmic tail may regulate α11 functions. The role of cytoplasmic tails of collagen-binding integrins has been studied extensively in the 1990s by the group of Hemler et al. Deletion of the α2 integrin cytoplasmic tail in K562 and RD cells (both requiring integrin activation) demonstrated that the α2 -tail-less integrin showed reduced adhesive activity on collagen I, in a manner suggesting an activation defect23. In chimeric experiments where again the α2 cytoplasmic tail was replaced with the tail of other integrins, it was demonstrated that chimeric α2 integrins with α5-tail (Xα2 Cα5) could mediate collagen gel contraction, whereas chimeric Xα2 Cα4 failed to mediate contraction, but instead promoted cell migration24. Already at this time it was speculated that “α subunit cytoplasmic domains, probably acting in concert with their associated β subunit, also have important but distinct roles and perhaps eventually will be shown to interact with distinct set of intracellular proteins”24. More recent data, using more sensitive assays, analyzing chimeras of all 12 α chains of the β1 integrin subfamily confirm that cytoplasmic tails of integrin α-chains do affect integrin inside-out activation, but that this varies greatly between different integrin α chains25. Deletion experiments are thus not easy to interpret since one also has to consider possible modulatory effects on integrin α/β chain interactions.
In the current study, we have deleted the α11 cytoplasmic tail and studied the effect of this deletion on α11 integrin function. Our data show that the integrin α11 cytoplasmic tail is dispensable for cell attachment but is essential for focal adhesion formation, ERK-dependent cell proliferation, cell migration and reorganization of 3D collagen matrices.
Results
Generation and expression of a human integrin α11-tail-less variant
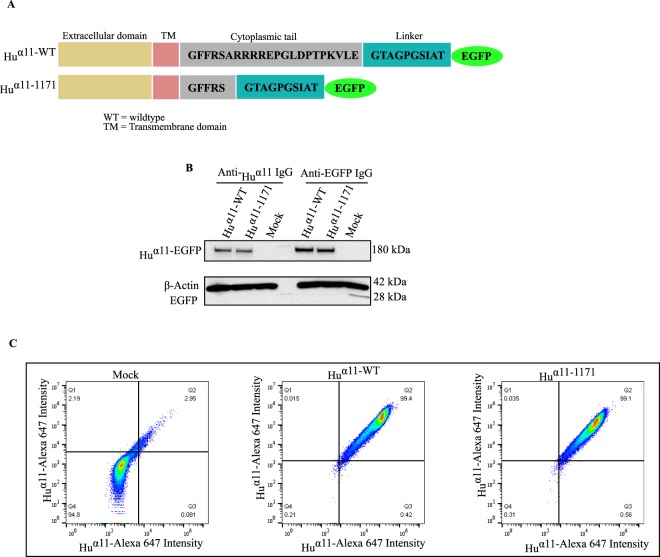
In order to identify the role of the α11 cytoplasmic tail, a mutant variant (Huα11-1171) with a deletion of the terminal 17 amino acids in the cytoplasmic tail of human integrin α11 (Huα11) was generated. Since the antibodies used to detect human α11 react with the cytoplasmic tail26, we have EGFP-tagged the integrin carboxy terminus using a 10 amino acid linker to avoid interference of the EGFP tag. Horwitz et al. pioneered this strategy for integrin α5 and the resultant tagged α5 integrin was characterized in detail without any evidence of artifacts due to the EGFP tag27. Full-length (Huα11-WT) and tail-less (Huα11-1171) Huα11 variants were tagged with enhanced green fluorescence protein (EGFP) and expressed in C2C12 mouse myoblasts, which do not express any collagen-binding integrins28 (Fig. 1A). Based on EGFP intensity, the transfected cells were sorted by flow cytometry with uniform gating to obtain similar expression levels of EGFP. The expression of comparable levels of Huα11-EGFP in the total protein lysates was confirmed by immunoblotting, either with an anti-Huα11 polyclonal antibody or with an anti-EGFP antibody (Fig. 1B). Comparable expression levels of Huα11-EGFP at the cell surface were also confirmed, using mock transfected cells (Mock, empty GFP vector) as a negative control (Fig. 1C).
Figure 1.
Generation and expression of integrin α11 variants in C2C12 cells. (A) Schematic illustration showing the amino acid sequences in the linker and the cytoplasmic tail of Huα11 variants. (B) Western blot showing total protein expression of Huα11-EGFP in C2C12 cells transfected with Huα11-WT-EGFP and Huα11-1171-EGFP (full size immunoblot is shown in supplementary) (C). FACS analysis of the cell surface expression α11 in Huα11-WT, Huα11-1171 and mock transfected cells (Mock).
Integrin α11 cytoplasmic tail is dispensable for cell adhesion but mediates focal adhesion formation, collagen reorganization, cell migration and cell proliferation
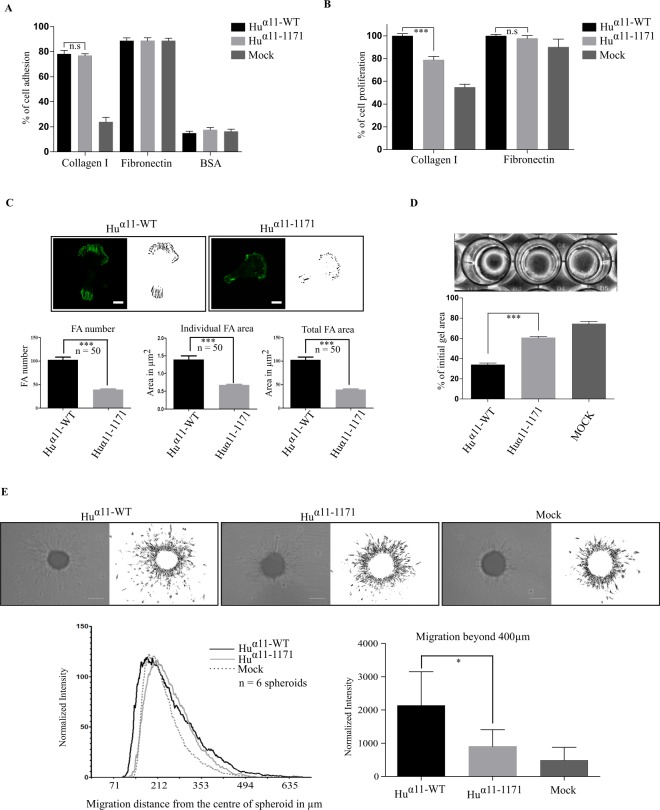
To examine the role of integrin α11 cytoplasmic tail in cell adhesion, Huα11-WT and Huα11-1171 cells were allowed to attach to collagen I or fibronectin using BSA coated wells as negative control. Mock transfected cells did not adhere to collagen, whereas Huα11-WT and Huα11-1171 cells adhered equally well to collagen I and fibronectin. This suggests that the deletion of 17 amino acids in the cytoplasmic tail of Huα11 had no apparent negative influence on integrin activation or cell adhesion to collagen I (Fig. 2A). However, Huα11-1171 cells displayed fewer focal adhesions after 2 hours, with a reduction of 50% in the total area of focal adhesions (Fig. 2C) and 35% reduction in cell spreading (Supplementary Fig. 2). This indicates that cytoplasmic tail of integrin α11 is involved in mediating cell adhesion signaling. To confirm this hypothesis, we assessed the ability of Huα11-1171 cells in mediating: collagen gel reorganization, cell migration and proliferation.
Figure 2.
Integrin α11 cytoplasmic tail is not involved in cell adhesion but mediates focal adhesion formation, collagen reorganization, cell migration and cell proliferation. (A) Role of α11-tail in cell adhesion. Huα11-WT, Huα11-1171 and mock transfected cells (Mock) were allowed to attach on collagen I or fibronectin or BSA in serum-free conditions for 50 mins. Attached cells were fixed, stained with 0.1% crystal violet and absorbance was read at 595 nm. (B) Role of α11-tail in cell proliferation. Huα11-WT, Huα11-1171 and Mock cells were allowed to attach on collagen I or fibronectin in reduced serum condition for 24 hours. Attached cells were fixed, stained with 0.1% crystal violet and absorbance was read at 595 nm. (C) Role of α11 tail in focal adhesion formation. Cells were allowed to attach collagen I for 120 mins. Cells were fixed with 4% PFA and focal adhesions were imaged using TIRF microscopy and quantified. Scale bar: 10 µm. (D) Role of α11-tail in collagen reorganization. Huα11-WT, Huα11-1171 and Mock cells were mixed with collagen I solution and allowed to contract for 16 hours. Gel diameters were measured, and percentage of initial gel area was calculated. (E) Role of α11-tail in spheroid migration. Homospheroids of Huα11-WT, Huα11-1171 and Mock cells were embedded in collagen I gel and spheroid migration was quantified after 24 hours. Radial profile plot depicts the radial cell intensity from the center of the spheroid and the intensity of cells that have migrated beyond 400 µm was calculated. Scale bar: 200 µm. Data shown are pooled from triplicates of at least three independent experiments for cell attachment, cell proliferation and collagen gel contraction. Results were expressed as mean ± standard deviation of at least three replicates from one representative experiment of at least three independent experiments. Statistical significance was assessed by two tailed, unpaired t-tests and P-values are expressed as ***P < 0.001; **P < 0.01 and *P < 0.05.
When cells were allowed to attach on collagen I for 24 hours in low serum conditions, Huα11-1171 cells displayed a significant reduction in cell proliferation compared to Huα11-WT cells, but not on fibronectin, suggesting that the α11 cytoplasmic tail-mediated signaling is involved in the regulation of cell proliferation (Fig. 2B). We further examined the ability of these cells to contract 3D collagen I lattices, a process previously shown to be α2β1- and α11β1- mediated18,28,29. Sixteen hours after the contraction was initiated, the Huα11-1171 cells displayed 50% reduction in collagen contraction compared to Huα11-WT cells (Fig. 2D).
We also investigated the role of the α11 cytoplasmic tail in cell migration using a spheroid migration model in a 3D collagen matrix. The radial cell density profile of the spheroid was analyzed from the center of the spheroid to quantify migrated cells in relation to their distance of migration. Huα11-WT cells migrated out 50% more than Huα11-1171 and mock transfected cells, beyond 400 µm from the center of the spheroid (Fig. 2E). Interestingly, the size of the spheroid core for the Huα11-WT cells was smaller than that observed for spheroids formed from the Huα11-1171 and Mock cells. These results indicate that the α11 cytoplasmic tail is indeed essential to mediate cell proliferation, collagen reorganization and cell migration.
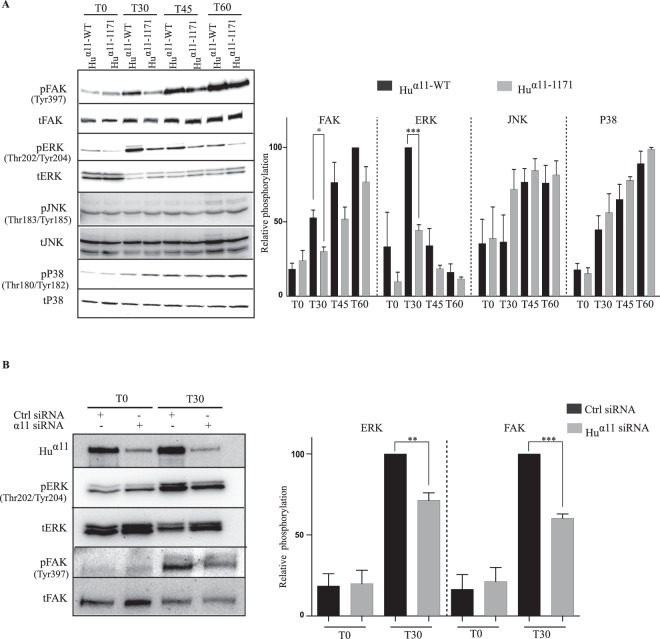
Integrin α11 cytoplasmic tail contributes to FAK and ERK activation
Localization of focal adhesion kinase (FAK) in focal adhesions and its autophosphorylation at Tyrosine residue 397 (FAKY397) is a primary event in integrin signaling leading to cell migration and proliferation30–33. Analysis of FAKY397 activation at different time points revealed that Huα11-1171 cells demonstrated less FAKY397 activation than Huα11-WT cells at 30 and 45 minutes on collagen I (Fig. 3A). Since other collagen-binding integrins have previously been shown to activate MAPK pathways, we investigated activation of ERK, p38 and JNK following attachment of cells to collagen I34–36. Interestingly, Huα11-1171 cells showed a strong reduction in ERK activation compared to Huα11-WT cells on collagen I (Fig. 3A). In contrast, activation of p38 and JNK was not affected by the deletion of the α11 cytoplasmic tail. To confirm the role of integrin α11 in FAK and ERK activation in primary cells, human gingival fibroblasts (hGF) were transfected with control siRNA (Ctrl) or α11 siRNAs (Fig. 3B and supplementary Fig. 3A). The knockdown of α11 did not affect expression of the collagen-binding integrins α1 and α2 chains (Supplementary Fig. 3B,C). Phosphorylated FAKY397 and ERK levels were only reduced in α11 siRNA-treated hGF (Fig. 3B and supplementary Fig. 3A). These results demonstrate that cytoplasmic tail of integrin α11 contributes to FAK and ERK activation.
Figure 3.
Integrin α11 cytoplasmic tail contributes to FAK and ERK activation. (A) Serum-starved Huα11-WT and Huα11-1171 cells were plated on collagen I in serum-free conditions and cells were lysed at different time points (T0, T30, T45 and T60). Total and phosphorylated levels of FAKY397, ERK, p38, JNK were detected by Western blotting and the protein bands were quantified by densitometry analysis (full size immunoblots are shown in supplementary). (B) Human gingival fibroblasts (hGFs) were transfected with control (ctrl) siRNA or α11 siRNA (SMARTpool) and 48 hours post transfection, cells were serum-starved and plated on collagen I in serum-free conditions. After 30 mins, cells were lysed, and the lysates were analyzed by western blotting. Protein bands were quantified by densitometry analysis. Statistical significance was assessed by two tailed, unpaired t-tests and P-values are expressed as ***P < 0.001; **P < 0.01 and *P < 0.05.
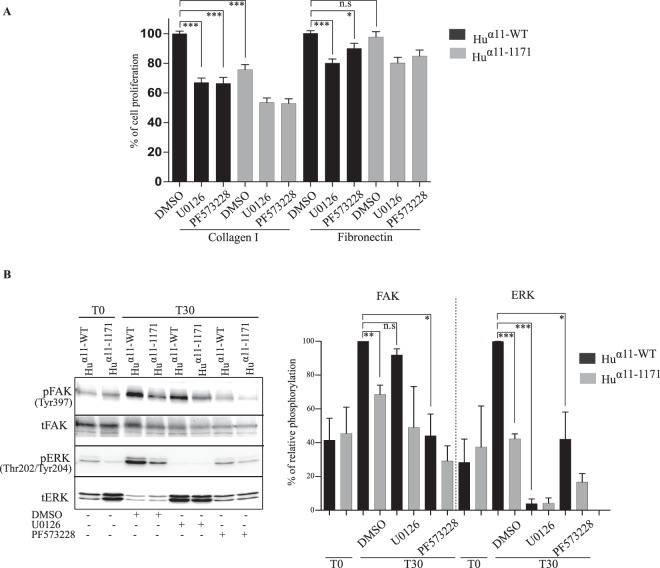
FAKY397 activation is involved in integrin α11 cytoplasmic tail-mediated cell proliferation and migration
We next examined if the reduced cell functions observed with the α11-tail-less cells was due to the defect in FAK and ERK activation. Inhibition of FAKY397 activation by PF573228 inhibited cell proliferation in both Huα11-WT and Huα11-1171 cells, on collagen I. Similarly, inhibition of ERK activation by U0126 also inhibited α11β1-mediated cell proliferation on collagen I, implying that both FAKY397 and ERK activation are required for α11β1-mediated cell proliferation (Fig. 4A). Inhibition of both FAK and ERK activation weakly decreased cell proliferation in Huα11-WT cells on fibronectin, suggesting that cell proliferation mediated by fibronectin-binding integrins is less dependent on these signaling molecules than α11β1-mediated cell proliferation. In order to understand the overlapping function of FAK and ERK in α11β1-mediated cell proliferation, we investigated the relationship between FAK and ERK activation. Inhibition of FAKY397 activation by PF573228 significantly inhibited ERK activation 30 minutes after cells attached to collagen (Fig. 4B). In contrast, inhibition of ERK activation by U0126 did not affect FAKY397 activation, indicating that FAKY397 phosphorylation is required for ERK activation.
Figure 4.
FAKY397 activation is involved in integrin α11 cytoplasmic tail-mediated cell proliferation and migration. (A) Effect of FAK and ERK inhibition in α11-mediated cell proliferation. Huα11-WT and Huα11-1171 cells were allowed to attach on collagen I or fibronectin in presence of either DMSO or U0126 (20 µM) or PF573228 (10 µM) in reduced serum conditions for 24 hours. Attached cells were fixed, stained with 0.1% crystal violet and absorbance was read at 595 nm. Results were expressed as mean ± standard deviation of at least three replicates pooled from three independent experiments. (B) Integrin α11 tail-mediated ERK activation is dependent on FAKY397 activation. Serum starved Huα11-WT and Huα11-1171 cells were treated with DMSO or U0126 or PF573228 and allowed to attach on collagen I for 30 minutes. After 30 minutes, cells were lysed, and the lysates were analyzed for total and phosphorylated levels of FAKY397 and ERK by Western blotting. Protein bands were quantified by densitometry analysis and data shown are pooled from at least three independent experiments (Full size immunoblots are shown in supplementary). Statistical significance was assessed by two tailed, unpaired t-tests and P-values are expressed as ***P < 0.001; **P < 0.01 and *P < 0.05.
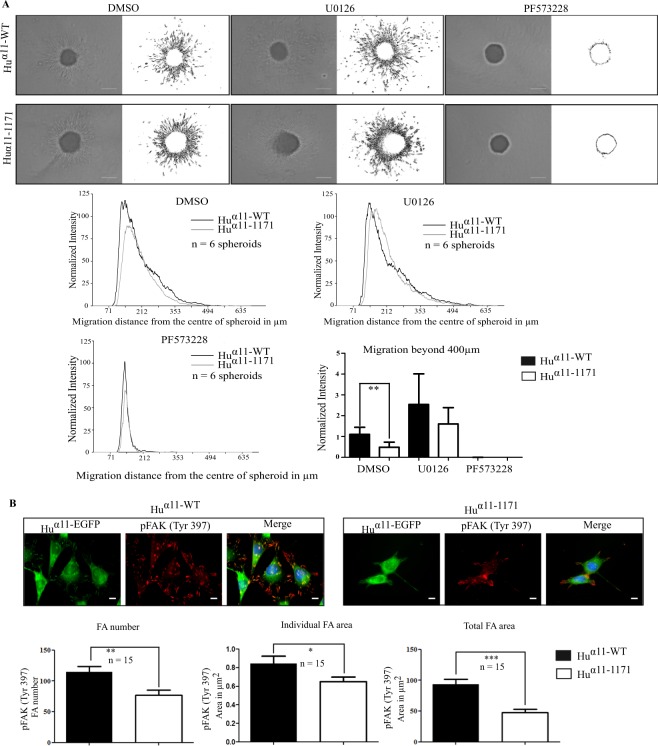
In spheroid migration assays, inhibition of FAKY397 phosphorylation, completely abrogated migration for both Huα11-WT and Huα11-1171 cells (Fig. 5A). Contrariwise, inhibition of ERK in spheroid assay did not inhibit migration beyond 400 µm of both Huα11-WT and Huα11-1171 cells, suggesting that in contrast to cell proliferation, ERK activation is not involved in integrin α11-mediated cell migration in this 3D model. Furthermore, neither inhibition of FAKY397 activation nor ERK activation inhibited serum-stimulated collagen gel contraction (data not shown).
Figure 5.
Integrin α11 cytoplasmic tail is involved in focal adhesion formation and FAKY397 activation. (A) Effect of FAK and ERK inhibition in α11-mediated spheroid migration. Homospheroids of Huα11-WT and Huα11-1171 cells were embedded into collagen I gel and treated with either DMSO or U0126 or PF573228. Spheroid migration was quantified after 24 hours. Radial profile plot depicts the radial cell intensity from the center of the spheroid and the intensity of cells that have migrated beyond 400 µm was calculated. Scale bar: 200 µm. (B) Localization of phospho-FAKY397 in focal adhesions. Huα11-WT and Huα11-1171 cells were allowed to attach on collagen I for 120 minutes. Cells were fixed with 4% PFA and stained for phospho-FAKY397. Focal adhesions positive for phospho-FAKY397 were quantified using ImageJ. Scale bar: 10 µm. Results were expressed as mean ± standard deviation of at least three replicates from one representative experiment of at least three independent experiments. Statistical significance was assessed by two tailed, unpaired t-tests and P-values are expressed as ***P < 0.001; **P < 0.01 and *P < 0.05.
Since FAK is localized in focal adhesions, we investigated the phosphorylation of FAKY397 in focal adhesions. Similar to our previous results with immunoblotting, we observed significantly reduced amounts of phosphorylated FAKY397 positive adhesions for Huα11-1171 cells as compared to Huα11-WT cells (Fig. 5B). These findings suggest that the reduced activation of FAK Y397 in Huα11-1171 cells is due to fewer focal adhesions in these cells per se.
To summarize, our results strongly suggest that the cytoplasmic tail of integrin α11 is essential to stabilize focal adhesions that in turn mediate FAKY397 activation involved in cell proliferation and cell migration.
Discussion
Although integrin cytoplasmic tails are relatively short, they are able to exert control of integrin activity and mediate a number of interactions of importance for integrin function7,8. There are in total more than 20 integrins and various research groups have independently deleted cytoplasmic tails and expressed the mutated variants with different results6,37–39. When integrin β-tails are expressed as chimeric proteins with non-integrin transmembrane- and extracellular parts, the β1 integrin tail sequence can direct chimeric proteins into focal adhesions40. When integrin α-tails are deleted, some α-tail-less heterodimers localize to focal adhesions in a ligand-independent manner41–43 and often become constitutively activated.
In the current study, we have used EGFP-tagged α11 integrins, in a strategy where the tag is separated by a 10 amino acid linker. Like for EGFP tagged α4 and α5 integrins we find no evidence that addition of the tag interferes with integrin function27,44. It could be argued that addition of a tag on tail-less integrin would be more likely to interfere with integrin function than a tag added to full-length integrin. However, independent experiments suggest that the loss of α11 integrin activity seen in the tail-less variant can be duplicated by a specific point mutation in the α11-tail, excluding that non-specific interference of the EGFP-tag (data not shown).
In our present study, we found that deletion of α11 cytoplasmic tail led to reduced focal adhesion formation, reduced cell spreading, reduced collagen gel contraction, reduced cell migration in a 3D context and reduced ERK-dependent cell proliferation, but that the α11-tail was dispensable for cell adhesion under the conditions used. Deletion of α1 cytoplasmic tail in fibroblastic 3T3 cells leaves cell adhesion to collagen IV unaffected but results in ligand-independent focal contact localization45, whereas in endothelial cells α1-tail deletion leads to reduced adhesion to collagen IV36. Deletion of α2 cytoplasmic tail reduces cell adhesion to collagen I in RD rhabdomyosarcoma cells, but this effect can be overcome by ions or ligand concentration, suggesting that a major function of the cytoplasmic α2-tail is to mediate intracellular inside-out activation events, and that α2 deletion effects on cell attachment can be overcome by activating the extracellular domain23. The reduced number of focal contacts seen in α11-tail-less expressing cells probably reflects a contribution of α11-tail to a cytoskeletal linkage, whose nature remains to be established, rather than need for α11-tail in integrin activation. In vivo integrin α2β1 is expressed in platelets and hematopoietic cells46 where integrin activation is essential, whereas α11β1 is mainly expressed on fibroblastic cells26 where β1 integrins are constitutively activated47.
Here we showed that interaction of α11β1 with collagen I mediated ERK signaling. This signaling is thus similar to that observed for α1 (although the preferred ligand for α1β1 is collagen IV48), but is different than for α2β1-mediated signaling, which occurs mainly via p38 in 3D collagen I matrix34. Interestingly, in mouse endothelial cells, limited α2-dependent p38 signaling is observed36. These data suggest for collagen-binding integrins that the presence of cell-dependent factors influence which MAPK signaling pathway will be activated upon collagen ligation. siRNA knockdown of α11 reduced FAK and ERK activation, supporting that α11-mediated ERK signaling is central in fibroblasts, which is the major cell type expressing α11. Previous studies have demonstrated α11-dependent ERK and PI3K phosphorylation in mesenchymal stem cells expressing multiple collagen-binding integrins49. However, in our cell system (C2C12 cells lacking other collagen receptors than the overexpressed α11β1), we failed to detect α11-dependent PI3K activation (data not shown).
Blocking α11-dependent cellular signaling in C2C12 and human gingival fibroblasts cells also blocked ERK-dependent cell proliferation. A majority of the α11-dependent ERK signaling appeared to be dependent on FAK, since FAK inhibition also attenuated the α11-dependent ERK signaling. In the case of α1, FAK independent ERK signaling via Shc has been noted50. Later studies have demonstrated that FAK may enhance and prolong integrin-mediated activation of ERK through p130 (CAS), Crk, and Rap1 in cells expressing B-Raf51. α2-mediated p38 activation has been suggested to depend on specific residues within the α2 integrin subunit cytoplasmic domain52, and independent experiments failed to record FAK activation in 3D collagen gel under conditions of α2-mediated p38 activation34.
To analyze cell migration in 3D collagen gel we used a spheroid assay. Cell migration53, MMP-induction54 and collagen gel remodeling55 has been shown to depend on ERK signaling in some conditions. In our study, ERK inhibition did not impair cell migration in a collagen matrix. ERK inhibition could attenuate G-protein dependent integrin inhibition as has been reported for α2β1 integrin-dependent cell migration in smooth muscle cells56.
Finally, the collagen gel contraction was not affected by ERK or FAK inhibition suggesting that an alternative signaling pathway is operative in the C2C12 cells overexpressing α11. We have previously demonstrated that TGF-β-dependent contraction of floating collagen lattices by dermal fibroblasts depends on α11- and JNK- signaling19. This signaling pathway might be restricted to dermal fibroblasts or depend on relative levels of crucial components in non-canonical TGF-β signaling pathway being present in the cells. Previous studies have demonstrated that thrombospondin 1 in scleroderma fibroblasts can activate TGF-β to stimulate ERK-dependent collagen contraction57. Since αvβ3 signals via ERK, it is possible that αvβ3 mediates this collagen gel contraction under these conditions58. ERK activation has been shown to stimulate phosphorylation of MLC and in this way contribute to collagen lattice contraction53, but in our experiments pharmacological inhibition of ERK in α11-C2C12 cells failed to inhibit contraction.
In summary, our data suggest that the unique functions of α11 that separates it from other collagen-binding integrins is in part due to its cytoplasmic tail, which is needed for efficient focal contact formation, cell spreading, cell proliferation, cell migration and collagen remodeling.
Materials and Methods
Cell culture
Mouse C2C12 mouse satellite cells were provided by Prof. Anna Starzinski-Powitz (Goethe-Universität, Frankfurt am Main, Germany) and Phoenix 293 cells were provided by Prof. James Lorens, University of Bergen. Primary human gingival fibroblasts (hGF) were isolated from healthy gingival tissues as described earlier59. MRC5 human lung fibroblasts (American Type Culture Collection) were obtained from Robert Lafyatis laboratory (University of Pittsburgh Medical Center, Pittsburgh, PA, USA). Cells were cultured at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM; Gibco®, Invitrogen) with 10% fetal bovine serum (FBS; Gibco®, Invitrogen), 1% penicillin-streptomycin (PEST; Sigma-Aldrich) and 5 µg/ml plasmocin (InvivoGen). Human gingival fibroblasts were grown from biopsies obtained during oral surgery after obtaining informed consent and in accordance with guidelines and regulations at the Department of Prosthetic Dentistry, Karolinska Institute, Stockholm in the 1990s following approval of experimental protocols by local ethics committee at faculty of Odontology, Karolinska institute and were kindly provided by Prof. Kamal Mustafa (University of Bergen)60.
Generation and expression of integrin α11 variants in C2C12 mouse satellite cells
To construct pBABE ITGA11 retroviral expression constructs, pBABE-puro-Itga11 plasmid, pBJ1- Huα11-WT-EGFP and pBJ1- Huα11-1171-EGFP (for detail, see Supplementary information) were used as templates. The ITGA11-EGFP cDNAs from pBJ1-Huα11-WT-EGFP and pBJ1-Huα11-1171-EGFP were excised with XhoI (blunted) and EcoRI and subcloned into pBABE-puro-Itga11 plasmid at BamHI (blunted) and EcoRI sites. The constructs were transfected into Phoenix 293 packaging cell line with X-tremeGENE 9 transfection reagent (Roche Diagnostics GmbH), according to manufacturer’s instructions. The viral supernatant medium was collected after 48 hours post transfection. C2C12 cells, cultured on 6-well plates were infected with viral supernatants containing polybrene at 5 µg/ml by spinfection at 1200 g for 90 minutes. After 36 hours, the culture medium was changed to a selection medium containing 2 µg/ml of puromycin. In addition, the cell populations with similar levels of EGFP intensity were sorted by Fluorescence-activated cell sorting (FACS).
Estimation of cell surface protein expression by FACS
FACS was performed at The Molecular Imaging Centre (MIC), University of Bergen. Cells were detached with Trypsin-EDTA (0.05% Trypsin and 0.02% EDTA; Gibco®, Invitrogen) and neutralized with DMEM containing 10% FBS. The cell suspension was filtered with a 40 µm syringe filter and 2 × 106 cells were used for the analysis. Cells were washed twice with PBS for 5 minutes at 210 × g with phosphate buffered saline (PBS) and the cell pellet was fixed with 2% paraformaldehyde/PBS for 10 minutes. After fixation, cells were washed three times with PBS and blocked with 2% Bovine serum albumin (BSA/PBS) for 30 minutes in room temperature (RT). Cells were incubated with mouse anti- Huα11 IgG (mAb 203E3)61 at a final concentration of 5 µg/ml in 2% BSA/PBS for 60 minutes at 37 °C. After washing three times with PBS, cells were incubated with goat anti-mouse IgG conjugated with Alexa fluor® 647 for 60 minutes in RT. Cells were washed 3X with PBS and analyzed using FACS Accuri for the intensity of Alexa fluor® 647 by using uniform gating for all samples and data was analyzed using FLOWJO computer software for FACS analysis (FLOWJO, LLC).
Cell adhesion assay
Forty-eight-well plates were coated with fibronectin (1 μg/cm2: Sigma-Aldrich) or collagen type I (5 μg/cm2: Bovine PureCol®, Advanced BioMatrix) and incubated for 2 hours at 37 °C. After washing with PBS, the plates were blocked with 2% BSA for 1 hour at 37 °C. Cells were washed three times with DMEM and 1 × 105 cells/well were cultured for 50 minutes at 37 °C. Unattached cells were removed carefully by washing three times with PBS containing Ca2+ and Mg2+. Cells were then fixed with 96% ethanol for 10 minutes at room temperature followed by staining with 0.1% crystal violet for 20 minutes at room temperature. Plates were washed three times with distilled water and the cells were lysed with 1% Triton X-100 for 5 minutes. The lysates were transferred to a 96-well plate and absorbance was read at 595 nm.
Cell proliferation assay
Cells were seeded on 24-well plates coated with fibronectin (1 μg/cm2) or collagen type I (5 μg/cm2) and cultured for 24 hours in DMEM containing 1% FBS. Cells were washed with PBS and fixed with 96% ethanol for 10 minutes at room temperature followed by staining with 0.1% crystal violet for 20 minutes at room temperature. After washing three times with distilled water, the cells were lysed with 1% Triton X-100 for 5 minutes and lysates were transferred to a 96-well plate. The absorbance was read at 595 nm. For inhibition experiments, cells were incubated with U0126 (20 µM; Sigma-Aldrich) or PF573228 (10 µM; Sigma-Aldrich). Data were normalized considering proliferation of wild-type cells as 100% on collagen and fibronectin.
Collagen gel contraction
Collagen gel contraction was performed as described earlier28,59. In brief, 24-well plates were blocked with 2% BSA, overnight at 37 °C and then washed three times with PBS. Collagen solution was prepared by mixing 5 parts of DMEM 2 × (SLM-202-B, Merck Millipore), one part of 0.2 M Hepes at pH.8.0 and 4 parts of Collagen I (3 mg/ml; Bovine PureCol®, Advanced BioMatrix). Collagen solution was then mixed with cells to obtain a final concentration of 1 × 105 cells/ml. To each well, 400 µl of cell-collagen suspension was added and allowed to polymerize for 90 minutes at 37 °C. Polymerized collagen gels were floated with 400 µl of DMEM containing 0.5% FBS. Gel diameters were measured using a ruler and the percentage of the initial gel area was calculated.
Immunocytofluorescence
Glass bottom dishes (3.5 mm, MatTek) were coated with collagen type I (50 μg/cm2) for 60 minutes at 37 °C. Dishes were washed three times with PBS and 2 × 105 cells were cultured for 2 hours in DMEM with 10% FBS. Cells were then fixed with 4% paraformaldehyde/PBS for 10 minutes at RT and followed by washing three times with PBS for 5 minutes per wash. Focal adhesion images were captured using Nikon Total Internal Reflection Microscope (TIRFM).
For phospho FAKY397 staining experiments, coverslips (12 mm, 1.5 H; Marienfeld) were coated in 24-well plates with collagen type I (50 μg/cm2) for 60 minutes at 37 °C. Cover slips were washed three times and blocked with 2% BSA/PBS for 1 hour at 37 °C. Later, 4 × 104 cells were seeded per well in serum-free conditions. Cells were cultured for 2 hours and fixed with 4% paraformaldehyde/PBS for 10 minutes at RT. Cells were permeabilized in 0.5% Triton X-100 /PBS buffer for 5 minutes and blocked with 5% BSA/PBS containing 0.1% Triton X-100 for 1 hour at RT. Next, cover slips were incubated with polyclonal rabbit anti-phospho FAKY397 IgG (1:400; 44-624, Biosource) in 5% BSA/PBS with 0.1% Triton X-100 for 1 hour at 37 °C. After washing with 0.05% Tween-20/PBS, cover slips were incubated with Alexa fluor® 594 conjugated goat anti-rabbit IgG (1:400, Jackson ImmunoResearch) for 1 hour at RT. Later, coverslips were incubated with DAPI (0.25 μg/ml, Invitrogen) and mounted with ProLong Diamond Antifade mounting medium (Thermo Scientific). Cells were visualized under a Zeiss Axioscope fluorescence microscope and pictures were acquired with a digital AxioCam MRm camera.
Spheroid preparation and migration assay in 3D collagen gel
Homospheroids were made with C2C12 cells using hanging drop method as described earlier62. In short, C2C12 cells were harvested and suspended in culture medium to have a final concentration of 1 × 106 cells/ml. Approximately, 28 drops of cell suspension (25 µl/drop; 2.5 × 104 cells) were made on the lid of a 10 cm Petri dish, containing cell culture medium. The lid was carefully inverted over the Petri dish bottom, without disturbing the drop form. The spheroids were cultured for 3 days under regular cell culture conditions. Collagen I solution was prepared as described in collagen gel contraction assay and 100 µl of collagen I solution was added onto a 96-well plate and incubated for 15 minutes at 37 °C. One spheroid was embedded per well and the spheroid-collagen gel was allowed to polymerize for 90 minutes at 37 °C. After polymerization, 100 µl DMEM was added to each well and cultured for 24 hours. Spheroids were visualized under an inverted light microscope (Leica DMIL) and images were captured. When indicated, DMSO or 20 µM U0126 or 10 µM PF573228 were added to the medium.
SDS-PAGE and western blotting
Cells were seeded on 6-well plates and cultured until confluency. Later, cells were lysed with buffer containing 0.5% Nonidet P-40, 20 mM Tris-HCl pH7.4, 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2 and proteinase inhibitor complete (Roche, Germany). Protein concentration was determined by BCA assay. Protein samples of 20 μg from each clone, mixed with SDS sample buffer (Biorad) containing 2% of 2-β-mercaptoethanol were loaded and separated by 6% SDS-polyacrylamide gel. Then, the purified proteins were transferred to a PVDF membrane using iBlot® system (Invitrogen). Membranes were analyzed with mouse anti-EGFP IgG (1:2000, Clonetech) or polyclonal rabbit anti- Huα11 IgG (PA5-23897, Thermo Fischer Scientific) or mouse anti-β-actin IgG (1:5000, Sigma-Aldrich). Blots were developed using ECL system (Pierce protein research products) and ChemiDoc XRS (Bio-Rad).
For signaling experiments, cells were cultured overnight with reduced serum conditions (1% FBS) and serum starved for 3 hours before the experiments. Trypsinized cells were neutralized with DMEM containing Soyabean trypsin inhibitor (50 µg/ml). Cells were washed three times with DMEM and incubated for 45 minutes on rotator at RT. 106 cells were seeded on to 6-well plates pre-coated with collagen (5 μg/cm2) and blocked with 2% BSA/PBS, as described previously. For T0 samples, cells were lysed directly with 1X SDS sample buffer. For the remaining samples, cells in each were lysed in 1X SDS sample buffer after 30 min (T30), 45 min (T45) and 60 min (T60). Protein lysates were separated by SDS-PAGE and analyzed by western blotting using the following antibodies: Rabbit monoclonal anti-phospho p44/42 MAPK IgG (20G11), rabbit monoclonal anti-p44/42 MAPK IgG (137F5), rabbit anti-FAK IgG (#3285), rabbit monoclonal anti-phospho-SAPK/JNK IgG (81E11), rabbit anti-SAPK/JNK IgG (#9252), mouse monoclonal anti-phospho p38 IgG (28B10), rabbit anti-p38 IgG (#9212) from Cell Signaling Technology and rabbit anti-phospho FAKY397 IgG from Biosource. Relative protein expression was quantified using Image Lab™ Software (Bio-Rad).
Integrin α11 silencing with siRNA
Primary hGFs were harvested and 5 × 105 cells were plated on 10 cm culture dishes 30 min prior to transfection. Cells were transfected with SMARTpool ON-TARGET plus ITGA11 siRNA (L-008000-00-0005, Dharmacon) or Individual ON-TARGET plus ITGA11 (J-008000-10, Dharmacon) or ON-TARGET plus Non-Targeting siRNA (D-001810-02-05, Dharmacon) at a final concentration of 20 nM with HiPerfect transfection reagent. After 48 hours, cells were serum starved for overnight. Cells were prepared as described for signaling experiments. Six-well plates were coated with thin film fibrillar collagen I gel prepared using the collagen I solution described in collagen gel contraction section and allowed to polymerize for 60 min at 37 °C. Cells were harvested and 5 × 105 cells were plated on to each 6-well and lysed as described above for SDS-PAGE and Western blotting analysis. Western blots stained with anti-phospho p44/42 MAPK IgG were reprobed with a custom-made mouse monoclonal anti- Huα11 IgG, mAb 210F4 (Supplementary Fig. 1) to confirm the silencing of ITGA11. In addition, membranes were analyzed with rabbit monoclonal anti-human α2 (EPR 5788, Abcam), mouse monoclonal anti-human α1 antibody (MAB 5676, R&D Systems), mouse GAPDH antibody 6C5 (sc-32233, Santa cruz biotechnology) and mouse anti-β-actin IgG (AC-74, Sigma-Aldrich) to confirm the unchanged levels of integrin α1 or α2 protein. MRC5 protein lysates were used as positive controls.
Image analysis
Focal adhesions were quantified as described previously63. Briefly, raw images were subjected to background correction with a rolling ball radius of 50 using ImageJ. Image contrast was enhanced using ImageJ plugin CLAHE and threshold adjusted. The number of focal adhesions, area of individual focal adhesion and total area of focal adhesions were quantified for a single cell. Cell spreading was quantified by measuring the cell surface area, which was in turn calculated by drawing the cell boundary using ImageJ. Spheroid images were also subjected to background correction and contrast enhancement using CLAHE plugin in ImageJ. Threshold adjusted images were used to quantify the radial cell density profile from the center of the spheroid, using the Radial Profile plugin from ImageJ. The cell densities of migrated cells at different distant points were used to quantify the distance of migration from the center of the spheroid as described earlier64.
Statistical analysis
Statistical significance was assessed by using two tailed, unpaired t-tests as indicated in the figure legends and P < 0.05 considered statistically significant. Statistical analysis and all graphs were done using GraphPad Prism 5 software (GraphPad Inc, USA). Data normalization was done based on maximum value in each experiment and data from three independent experiments was pooled together, and average ± standard deviation was calculated for each cell type and condition. For cell adhesion assay, data normalization was done based on the average absorbance values of the fibronectin-coated wells in each experiment.
Supplementary information
Acknowledgements
The authors wish to thank Mona Grønning for excellent technical assistance and Brith Bergum at the Flow Cytometry Core Facility, University of Bergen, for helping with flow cytometry and cell sorting. The authors also wish to thank Prof. Kamal Mustafa for providing access to the TIRF microscope at the Tissue Engineering Group, Department of Clinical Dentistry, University of Bergen. P.E. was financed by a PhD scholarship from The Faculty of Medicine and Dentistry, University of Bergen. This project was supported by the Western Norway Regional Health Authority (ID 911899), Centre of Cancer Biomarkers (Centre of Excellence funded by Research council of Norway, Project No 223250), Norwegian-Polish EEA grant (ID 202952) and The Norwegian Centre for International Cooperation in Education (SIU) (PNA-2014/10057).
Author contributions
P.E. took part in experiment planning, performed majority of experiments, analyzed all the experiments, wrote the manuscript; J.A. contributed to cell proliferation and siRNA knockdown experiments; N.L. contributed to spheroid and siRNA knockdown experiments; C.Z. participated in experiment design and data interpretation; D.G. supervised, funded, designed the project, interpreted the data and wrote the manuscript.
Data availability
No datasets were generated or analyzed during the current study.
Competing interests
Donald Gullberg is a named inventor on a patent filed by the University of Bergen for α11 monoclonal antibody mAb 210 F4 (PCT/ EP2019/051716).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-51689-6.
References
- 1.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morse EM, Brahme NN, Calderwood DA. Integrin cytoplasmic tail interactions. Biochemistry. 2014;53:810–820. doi: 10.1021/bi401596q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velling T, Kusche-Gullberg M, Sejersen T, Gullberg D. cDNA cloning and chromosomal localization of human alpha11 integrin. A collagen-binding, I domain-containing, beta1-associated integrin alpha-chain present in muscle tissues. J. Biol. Chem. 1999;274:25735–25742. doi: 10.1074/jbc.274.36.25735. [DOI] [PubMed] [Google Scholar]
- 4.Rantala JK, et al. SHARPIN is an endogenous inhibitor of beta1-integrin activation. Nat Cell Biol. 2011;13:1315–1324. doi: 10.1016/0026-0495(88)90105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Toole TE, et al. Modulation of the affinity of integrin alpha IIb beta 3 (GPIIb-IIIa) by the cytoplasmic domain of alpha IIb. Science. 1991;254:845–847. doi: 10.1126/science.1948065. [DOI] [PubMed] [Google Scholar]
- 6.O’Toole TE, et al. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legate KR, Fassler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122:187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- 8.Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 9.Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013;14:503–517. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pankov R, et al. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, et al. Structural mechanism of integrin inactivation by filamin. Nat Struct Mol Biol. 2015;22:383–389. doi: 10.1038/nsmb.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Wang Z, Thinn AM, Ma YQ, Zhu J. The dual structural roles of the membrane distal region of the alpha-integrin cytoplasmic tail during integrin inside-out activation. J Cell Sci. 2015;128:1718–1731. doi: 10.1242/jcs.160663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Franceschi N, Ivaska J. Integrin bondage: filamin takes control. Nat Struct Mol Biol. 2015;22:355–357. doi: 10.1038/nsmb.3024. [DOI] [PubMed] [Google Scholar]
- 14.Li H, et al. Structural basis of kindlin-mediated integrin recognition and activation. Proc Natl Acad Sci USA. 2017;114:9349–9354. doi: 10.1073/pnas.1703064114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeltz C, Gullberg D. The integrin-collagen connection - a glue for tissue repair? J. Cell Sci. 2016;129:653–664. doi: 10.1242/jcs.180992. [DOI] [PubMed] [Google Scholar]
- 16.Zeltz C, Lu N, Gullberg D. Integrin alpha11beta1: a major collagen receptor on fibroblastic cells. Adv Exp Med Biol. 2014;819:73–83. doi: 10.1007/978-94-017-9153-3_5. [DOI] [PubMed] [Google Scholar]
- 17.Gullberg D, Velling T, Sjoberg G, Sejersen T. Up-regulation of a novel integrin alpha-chain (alpha mt) on human fetal myotubes. Dev Dyn. 1995;204:57–65. doi: 10.1002/aja.1002040108. [DOI] [PubMed] [Google Scholar]
- 18.Popova SN, et al. Alpha11 beta1 integrin-dependent regulation of periodontal ligament function in the erupting mouse incisor. Mol Cell Biol. 2007;27:4306–4316. doi: 10.1128/MCB.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz JN, et al. Reduced granulation tissue and wound strength in the absence of alpha11beta1 integrin. J Invest Dermatol. 2015;135:1435–1444. doi: 10.1038/jid.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navab R, et al. Integrin alpha11beta1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non-small cell lung cancer. Oncogene. 2016;35:1899–1908. doi: 10.1038/onc.2015.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulz JN, et al. New developments on skin fibrosis - Essential signals emanating from the extracellular matrix for the control of myofibroblasts. Matrix Biol. 2018;68–69:522–532. doi: 10.1016/j.matbio.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Romaine, A. et al. Overexpression of integrin alpha11 induces cardiac fibrosis in mice. Acta Physiol (Oxf)222, 10.1111/apha.12932 (2018). [DOI] [PubMed]
- 23.Kawaguchi S, Hemler ME. Role of the alpha subunit cytoplasmic domain in regulation of adhesive activity mediated by the integrin VLA-2. J Biol Chem. 1993;268:16279–16285. [PubMed] [Google Scholar]
- 24.Chan BM, et al. Distinct cellular functions mediated by different VLA integrin alpha subunit cytoplasmic domains. Cell. 1992;68:1051–1060. doi: 10.1016/0092-8674(92)90077-p. [DOI] [PubMed] [Google Scholar]
- 25.Thinn AMM, Wang Z, Zhu J. The membrane-distal regions of integrin alpha cytoplasmic domains contribute differently to integrin inside-out activation. Sci Rep. 2018;8:5067. doi: 10.1038/s41598-018-23444-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popova SN, et al. The mesenchymal alpha11beta1 integrin attenuates PDGF-BB-stimulated chemotaxis of embryonic fibroblasts on collagens. Dev Biol. 2004;270:427–442. doi: 10.1016/j.ydbio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153:1427–1440. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiger CF, Fougerousse F, Grundstrom G, Velling T, Gullberg D. alpha11beta1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Dev Biol. 2001;237:116–129. doi: 10.1006/dbio.2001.0363. [DOI] [PubMed] [Google Scholar]
- 29.Klein CE, et al. Integrin alpha 2 beta 1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol. 1991;115:1427–1436. doi: 10.1083/jcb.115.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- 31.Parsons JT, et al. Focal adhesion kinase: structure and signalling. J Cell Sci Suppl. 1994;18:109–113. doi: 10.1242/jcs.1994.Supplement_18.16. [DOI] [PubMed] [Google Scholar]
- 32.Schaller MD, et al. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/MCB.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horton ER, et al. Modulation of FAK and Src adhesion signaling occurs independently of adhesion complex composition. J Cell Biol. 2016;212:349–364. doi: 10.1083/jcb.201508080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivaska J, et al. Integrin alpha2beta1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the alpha2 cytoplasmic tail. J Cell Biol. 1999;147:401–416. doi: 10.1083/jcb.147.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klekotka PA, Santoro SA, Zutter M. M. alpha 2 integrin subunit cytoplasmic domain-dependent cellular migration requires p38 MAPK. J Biol Chem. 2001;276:9503–9511. doi: 10.1074/jbc.M006286200. [DOI] [PubMed] [Google Scholar]
- 36.Abair TD, et al. Functional analysis of the cytoplasmic domain of the integrin {alpha}1 subunit in endothelial cells. Blood. 2008;112:3242–3254. doi: 10.1182/blood-2007-12-126433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan BM, et al. Distinct cellular functions mediated by different VLA integrin a subunit cytoplasmic domains. Cell. 1992;68:1051–1060. doi: 10.1016/0092-8674(92)90077-P. [DOI] [PubMed] [Google Scholar]
- 38.Kassner PD, Kawaguchi S, Hemler ME. Minimum alpha chain cytoplasmic tail sequence needed to support integrin-mediated adhesion. J Biol Chem. 1994;269:19859–19867. [PubMed] [Google Scholar]
- 39.Li X, et al. Requirements for the cytoplasmic domain of the aPS1, aPS2 and bPS integrin subunits during Drosophila development. Development. 1998;125:701–711. doi: 10.1242/dev.125.4.701. [DOI] [PubMed] [Google Scholar]
- 40.LaFlamme SE, Akiyama SK, Yamada KM. Regulation of fibronectin receptor distribution. J Cell Biol. 1992;117:437–447. doi: 10.1083/jcb.117.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briesewitz R, Epstein MR, Marcantonio EE. Expression of native and truncated forms of the human integrin a1 subunit. J. Biol. Chem. 1993;268:2989–2996. [PubMed] [Google Scholar]
- 42.Ylanne J, et al. Distinct functions of integrin alpha and beta subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J Cell Biol. 1993;122:223–233. doi: 10.1083/jcb.122.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawaguchi S, Bergelson JM, Finberg RW, Hemler ME. Integrin alpha 2 cytoplasmic domain deletion effects: loss of adhesive activity parallels ligand-independent recruitment into focal adhesions. Mol Biol Cell. 1994;5:977–988. doi: 10.1091/mbc.5.9.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinco KA, He W, Yang J. T. alpha4beta1 integrin regulates lamellipodia protrusion via a focal complex/focal adhesion-independent mechanism. Mol Biol Cell. 2002;13:3203–3217. doi: 10.1091/mbc.02-05-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Briesewitz R, Kern A, Marcantonio EE. Ligand-dependent and -independent integrin focal contact localization: the role of the alpha chain cytoplasmic domain. Mol Biol Cell. 1993;4:593–604. doi: 10.1091/mbc.4.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zutter MM, Santoro SA. Widespread histologic distribution of the alpha 2 beta 1 integrin cell-surface collagen receptor. Am J Pathol. 1990;137:113–120. [PMC free article] [PubMed] [Google Scholar]
- 47.Bharadwaj M, et al. alphaV-class integrins exert dual roles on alpha5beta1 integrins to strengthen adhesion to fibronectin. Nat Commun. 2017;8:14348. doi: 10.1038/ncomms14348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pozzi A, Wary KK, Giancotti FG, Gardner HA. Integrin alpha1beta1 mediates a unique collagen-dependent proliferation pathway in vivo. J Cell Biol. 1998;142:587–594. doi: 10.1083/jcb.142.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Popov C, et al. Integrins alpha2beta1 and alpha11beta1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death Dis. 2011;2:e186. doi: 10.1038/cddis.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/S0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- 51.Barberis L, et al. Distinct roles of the adaptor protein Shc and focal adhesion kinase in integrin signaling to ERK. J. Biol. Chem. 2000;275:36532–36540. doi: 10.1074/jbc.M002487200. [DOI] [PubMed] [Google Scholar]
- 52.Xu J, Zutter MM, Santoro SA, Clark RA. A three-dimensional collagen lattice activates NF-kappaB in human fibroblasts: role in integrin alpha2 gene expression and tissue remodeling. J Cell Biol. 1998;140:709–719. doi: 10.1083/jcb.140.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klemke RL, et al. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ronziere MC, et al. Integrin alpha1beta1 mediates collagen induction of MMP-13 expression in MC615 chondrocytes. Biochim Biophys Acta. 2005;1746:55–64. doi: 10.1016/j.bbamcr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Kagami S, et al. PDGF-BB enhances alpha1beta1 integrin-mediated activation of the ERK/AP-1 pathway involved in collagen matrix remodeling by rat mesangial cells. J Cell Physiol. 2004;198:470–478. doi: 10.1002/jcp.10433. [DOI] [PubMed] [Google Scholar]
- 56.Wang XQ, Lindberg FP, Frazier WA. Integrin-associated protein stimulates alpha2beta1-dependent chemotaxis via Gi-mediated inhibition of adenylate cyclase and extracellular-regulated kinases. J Cell Biol. 1999;147:389–400. doi: 10.1083/jcb.147.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y, et al. Thrombospondin 1 is a key mediator of transforming growth factor beta-mediated cell contractility in systemic sclerosis via a mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)-dependent mechanism. Fibrogenesis Tissue Repair. 2011;4:9. doi: 10.1186/1755-1536-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weyts FA, Li YS, van Leeuwen J, Weinans H, Chien S. ERK activation and alpha v beta 3 integrin signaling through Shc recruitment in response to mechanical stimulation in human osteoblasts. J Cell Biochem. 2002;87:85–92. doi: 10.1002/jcb.10278. [DOI] [PubMed] [Google Scholar]
- 59.Barczyk MM, et al. A role for alpha11beta1 integrin in the human periodontal ligament. J Dent Res. 2009;88:621–626. doi: 10.1177/0022034509339291. [DOI] [PubMed] [Google Scholar]
- 60.Mustafa K, Silva Lopez B, Hultenby K, Wennerberg A, Arvidson K. Attachment and proliferation of human oral fibroblasts to titanium surfaces blasted with TiO2 particles. A scanning electron microscopic and histomorphometric analysis. Clin Oral Implants Res. 1998;9:195–207. doi: 10.1034/j.1600-0501.1998.090307.x. [DOI] [PubMed] [Google Scholar]
- 61.Zeltz, C. et al. alpha11beta1 Integrin is Induced in a Subset of Cancer-Associated Fibroblasts in Desmoplastic Tumor Stroma and Mediates In Vitro Cell Migration. Cancers (Basel)11, 10.3390/cancers11060765 (2019). [DOI] [PMC free article] [PubMed]
- 62.Lu N, Karlsen TV, Reed RK, Kusche-Gullberg M, Gullberg D. Fibroblast alpha11beta1 integrin regulates tensional homeostasis in fibroblast/A549 carcinoma heterospheroids. PLoS One. 2014;9:e103173. doi: 10.1371/journal.pone.0103173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horzum U, Ozdil B, Pesen-Okvur D. Step-by-step quantitative analysis of focal adhesions. MethodsX. 2014;1:56–59. doi: 10.1016/j.mex.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blacher S, et al. Cell invasion in the spheroid sprouting assay: a spatial organisation analysis adaptable to cell behaviour. PLoS One. 2014;9:e97019. doi: 10.1371/journal.pone.0097019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.