Abstract
The freshwater flagellate alga Euglena agilis Carter was exposed to the polycyclic aromatic hydrocarbon (PAH) anthracene for 96 h under optimal photosynthetically active radiation (PAR), and responses of growth, photosynthetic pigment production, and photosynthetic efficiency were assessed. Anthracene reduced the growth rate (μ) and levels of chlorophyll a (Chl a), chlorophyll b (Chl b), and total carotenoids. The growth rate was more sensitive than photosynthetic parameters, with a median effective concentration (EC50) of 4.28 mg L−1. Between 5 and 15 mg L−1, anthracene inhibited the maximum quantum yield (Fv/Fm) of photosystem II (PSII) and the maximum photosynthetic electron transport rate through PSII (rETRmax) with EC50 values of 14.88 and 11.8 mg L−1, respectively. At all anthracene concentrations, intracellular reactive oxygen species (ROS) were elevated, indicating increased oxidative stress. Anthracene presumably reduced the PSII efficiency of photochemical energy regulation and altered the photochemistry through intracellular ROS formation. Acute exposure to PAHs may induce severe physiological changes in phytoplankton cells, which may influence vital ecological processes within the aquatic environments. Additionally, growth and Chl a content may serve as sensitive risk assessment parameters of anthracene toxicity in water management since EC50 values for both overlap with anthracene levels (8.3 mg L−1) permitted by the US Environmental Protection Agency (USEPA).
Subject terms: Environmental impact, Photosystem II
Introduction
Polycyclic aromatic hydrocarbons (PAHs) comprise a diverse family of hydrocarbons, each composed of two or more fused benzene rings. They are ubiquitous in terrestrial and aquatic ecosystems and are introduced by natural and man-made processes such as volcanic eruptions, crude oil spills, fossil fuel combustion, oil refining, automobile exhausts and industrial effluents1. PAHs are hydrophobic in nature and their persistence in the environment is a consequence of their low water solubility2. Due to their toxic, carcinogenic, mutagenic and photosensitising effects, especially their ability to generate singlet oxygen and other ROS, PAHs are considered one of the most prevalent groups of aquatic contaminants of high global concern3.
Anthracene is a three-ring, low molecular weight PAH with relatively high water solubility than other toxic PAHs4. Anthracene adversely affects the growth and photosynthesis of natural phytoplankton communities, with a toxicity threshold value less than its aqueous solubility1. Moreover, it is one of the most rapidly modified hydrocarbons and is assumed to be a strong photosensitizer5 that induces intracellular oxidative stress and blockage of the photosynthetic electron transport chain6 through the formation of ROS. Given the wide occurrence and high toxicity to aquatic organisms, anthracene is now on the list of priority pollutants, with a recommended water quality criterion of 8.3 mg L−1 and interim water quality guidelines of 0.012 μg L−1 for the protection of freshwater life set by the Canadian Council of Ministers of the Environment7,8.
Microalgae are an important group of primary producers in aquatic habitats that play a pivotal role in aquatic ecosystems, forming the food and energy base for all organisms, and powering food webs and biogeochemical cycling. They are readily exposed to toxic waterborne contaminants and tend to reach an equilibrium with pollutants rather rapidly because they are small with a proportionally large surface area9. Various studies have reported the toxic effects of PAHs to freshwater algae in terms of growth, photosynthesis, and respiration, with special attention given to interactions with solar and ultraviolet (UV) radiation10–12. However, Brack et al.13 suggested that anthracene toxicity is largely independent of irradiation and the intact compound itself contributes to toxicity. EC50 values reported for anthracene in freshwater microalgae range between 0.024 and 5 mg L−1 (Table 1). Among the various toxicity criteria studied, inhibition of photosynthesis is particularly pertinent as it inevitably results in reduced growth, biomass yield and loss of competitive ecological advantage.
Table 1.
Anthracene toxicity data for freshwater microalgae.
| Tested taxa | Criterion | Test period | Effect (mg L−1) | Reference |
|---|---|---|---|---|
| Chlamydomonas angulosa | Photosynthesis | 3 h | 0.54, EC50 | 42 |
| Chlorella vulgaris | Photosynthesis | 3 h | 0.24, EC50 | |
| Selenastrum capricornutum | Growth | 96 h | >40, EC50 | 63 |
| Pseudokirchneriella subcapitata | Growth | 34 h | 0.037, EC50 | 1 * |
| P. subcapitata | Primary production | 36 h | 0.024, EC50 | |
| Chlorella protothecoides | Growth | 96 h | 0.85, EC50 | 64 |
| Natural Phytoplankton | Fv/Fm | 30 min | <0.2, EC50 | 10 |
| Chlorella vulgaris | Growth | 96 h | 1.27, EC50 | 10 |
| Coenochloris pyrenoidosa | Growth | 96 h | 1.47, EC50 | 10 |
| Scenedesmus subspicatus | Growth, area under curve | 7 d | 1.04, EC50 | 65 |
| S. armatus | Population density | 24 h | 0.25, EC50 | 32 |
| S. vacuolatus | Population density | 24 h | 0.5, EC50 | 11 |
| Scenedesmus spp. | Growth | 24 h | 0.25, significant reduction | 66 |
| Chlamydomonas reinhardtii | Population density | 24 h | 0.28, EC50 | 38 |
| Desmodesmus subspicatus | Growth | 72 h | 0.26, EC50 | 67 |
| Microcystis aeruginosa | Growth | 72 h | >0.06, significant reduction | 68 |
| Anabaena fertilissima | Growth | 8 d | 5.0, EC50 | 62 |
*Under UV-A radiation (12.5 Wm−2).
Currently, most countries have legislation and regulations on accepted values for toxicity derived from bioassays that are applied to regulate agricultural and industrial chemicals, biocides, cosmetics, food additives, medicines and other substances14.
The genus Euglena contains motile, unicellular, photosynthetic eukaryotes found in many aquatic habitats, especially shallow eutrophic water bodies. These flagellates have rapid growth rates and can be easily cultured in the laboratory at low cost, ensuring year-round availability. Euglena spp. are sensitive to physicochemical changes and pollution in the surrounding environment, therefore, are potentially used as model organisms in ecotoxicological studies15,16. Widely used endpoints in bioassays involving Euglena are, growth inhibition17, photosynthesis and respiration18, chlorophyll content19, gene expression20 and motility/orientation21. Despite large quantities of toxicity data from the analysis of pollutants such as metals and herbicides21–23, the effects of PAHs on Euglena remain poorly understood.
The ultimate goal of bioassay tests is to provide representative and incorporative criteria regarding exposure conditions, thereby improving risk assessment and management of water quality. In this respect, multiple, rather than single, endpoint assays may be more reliable for comprehensive risk assessment of toxicants. Such an approach may facilitate insight into the mechanisms of toxicity and provide information on the relative sensitivity of selected parameters to toxicant concentration and/or exposure duration, thereby establishing methods for detecting changes caused by particular phytotoxicants24. In the present study, we investigated the ecotoxicological effects of anthracene on three endpoints of Euglena agilis Carter, including growth, pigmentation, and Chl a fluorescence which were then compared with permitted levels of anthracene in aquatic environments set by the US Environmental Protection Agency (USEPA). Phytoplankton is the main biomass producers in aquatic ecosystems, contributing ca. 50% of the atmospheric carbon dioxide fixation25. Any negative effects of anthracene on the growth and photosynthesis of phytoplankton would be detrimental to entire aquatic ecosystems and food chains.
Materials and Methods
Algal test species and culture conditions
Euglena agilis Carter was cultured in mineral medium (pH 5)26 in 1 L Erlenmeyer flasks at 25 °C under white fluorescent irradiance (PAR; 400–700 nm) of 30 µmol photons m–2 s−1 (FL400, Kum-Ho, Seoul, Korea) on a 16:8 h light:dark (LD) cycle. All experiments were performed using cells at the exponential growth phase.
Test chemicals and exposure
Anthracene (99% purity, CAS No. 120–12–7) was purchased from Sigma Aldrich (Saint Louis, MO, USA) and test solutions at the desired concentrations were prepared by serial dilution from stocks in high-performance liquid chromatography (HPLC)-grade dimethyl sulphoxide (DMSO; Sigma Aldrich). Microplate toxicity tests of 96 h in duration were conducted in 24-well cell culture plates (well diameter = 15.6 mm, growth area = 1.9 cm2; SPL Life Sciences, Gyeonggi-Do, Korea) with a test volume of 2 mL per well. Equal volumes of cell suspension and anthracene stock solutions were mixed to obtain final concentrations of 0.625, 1.25, 2.5, 5, 10 and 15 mg L−1, along with untreated controls. The concentration of the carrier solvent did not exceed 0.2% v/v of the test culture volume. The initial cell density was 10 ± 0.5 × 104 cells mL−1 of suspension. An additional solvent toxicity test (96 h) was conducted with a maximum DMSO concentration of 0.2% v/v. Organisms were exposed to nominal concentrations of anthracene and all treatments were performed in triplicate. The well plates were covered with parafilm to avoid evaporation and mixing of the volatile toxicant.
Measurement of growth rate
Growth rates were determined by measuring the number of cells in each well on the first and final days using a hemocytometer (Marienfeld, Germany). The specific growth rate (μ) was calculated using the following formula:
where N1 and N2 are the number of cells at time t1 (initial) and t2 (final), respectively.
Estimation of photosynthetic pigments
Photosynthetic pigment content was estimated using standard protocols27. Briefly, 1 mL cell suspension was collected from each replicate culture and centrifuged before extraction, and 1 mL of 90% v/v acetone was added followed by vigorous vortexing and centrifugation at 10,000 × g for 5 min at 4 °C. Supernatants were withdrawn and their optical density was measured spectrophotometrically at 470, 664 and 647 nm using an S-3100 UV/Vis spectrophotometer (Scinco, Seoul, Korea). Pigment concentrations are expressed as μg mL−1 of suspension.
Measurement of chlorophyll a (Chl a) fluorescence
Chl a fluorescence was measured using a pulse amplitude modulation (PAM) imaging instrument (Walz, Germany) as a proxy for photosynthetic performance. For measurement of maximum quantum yield (Fv/Fm) and electron transport rate (ETR), samples were kept in the dark for 10–15 min and then subjected to pulsed light emitted by a diode at ~0.15 µmol photons m−2 s−1 to obtain the initial fluorescence yield (Fo), which denotes the fluorescence yield when all PSII reaction centres are open with fully oxidized plastoquinone A(QA). A saturation pulse of ~5000 µmol photons m−2 s−1 emitted by a built-in halogen lamp was then applied to produce the maximum fluorescence yield (Fm), which is induced by a short saturating pulse of actinic light that reduces all QA. The maximum PSII quantum yield (Fv/Fm) was then derived from the equation (Fm − Fo)/Fm.
Rapid light curves were produced using 10 s pulses of actinic light increased stepwise from 0 to 335 (0, 1, 11, 21, 36, 56, 81, 111, 146, 186, 231, 281 and 335) μmol photons m−2 s−1. The relative electron transport rate (rETR) was calculated by multiplying the effective quantum yield (ΦPSII = F′m − [F/F′m], where F′m is the maximum light-acclimated fluorescence yield and F is the light-acclimated fluorescence yield) by photon flux density (PFD) and plotting against PFD. The ETR is relative because the absorbance of light by cells was not measured. Maximum electron transport rate (ETRmax) was derived from the hyperbolic tangent formula rETR = ETRmax ∗ tanh (α/I/ETRmax), adapted from Jassby and Platt28, where α indicates the electron transport rate under light-limited conditions. Alterations in Chl a fluorescence due to changes in non-photochemical quenching (NPQ) and photochemical quenching (qP) were calculated from (Fm − F′m)/F′m and (F′m − Ft)/(F′m − F′o), respectively29.
Measurement of ROS levels
The oxidant-sensing fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; Sigma Aldrich; CAS No: 4091–99–0) was used to detect intracellular ROS generation in E. agilis treated with anthracene. DCFH-DA (5 μM, final concentration) solubilized in ethanol was added to the cell suspension and incubated on a shaker at room temperature in the dark for 1 h30. The fluorescence intensity was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm using a Spectra MAX Gemini EM microplate fluorescence reader (Molecular Devices, CA, USA). The relative production of ROS is represented as relative fluorescence units (RFU).
Statistical analyses
Data are presented as means ± 95% confidence intervals (CI). All parameters were compared across treatments with one-way analysis of variance (ANOVA, n = 3, p < 0.05) using the JMP software (JMP® Pro version 13.1, SAS Institute, USA). Multiple comparison tests based on the least significant difference (LSD) were then carried out to find significant differences (p < 0.05) from controls and between treatments. The effective concentration at which 50% inhibition occurs (EC50) was estimated by the linear interpolation method using ToxCalc 5.0 (Tidepool Science, USA). The coefficient of variation (CV), the standard deviation expressed as a percentage of the mean, was calculated to estimate the precision of test values.
Capsule
Anthracene significantly reduces growth and photosynthesis in the freshwater flagellate Euglena agilis via intracellular ROS generation.
Results and Discussion
Effect of anthracene on cell growth
The carrier solvent used in this study (DMSO) had no significant inhibitory effects on cell growth (ANOVA, df = 6, F = 1.3, P > 0.05) or photosynthetic efficiency (ANOVA, df = 5, F = 0.56, P > 0.05) of E. agilis, even at the maximum concentration of 0.2% (v/v) in the growth medium (Fig. 1). Okumura et al.31 previously demonstrated the suitability of DMSO as a carrier solvent in Euglenoid tests.
Figure 1.
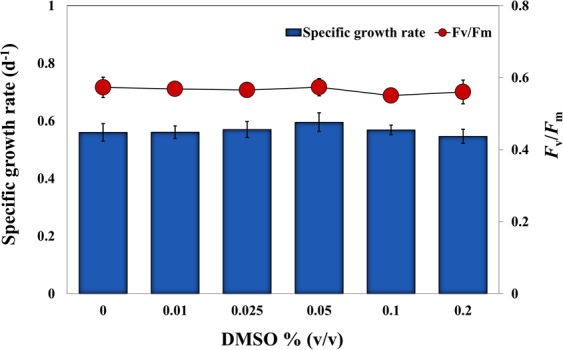
Effect of the carrier solvent DMSO on growth and photosynthesis (Fv/Fm) of E. agilis. A maximum concentration of 2% (v/v) was tested.
Most of the toxicity data available for the effects of anthracene on freshwater microalgae are based on growth inhibition (Table 1). Growth is an important endpoint parameter that reflects the overall vitality of a population under the tested conditions. Addition of anthracene to the culture medium resulted in a concentration-dependent inhibition of the specific growth rate of E. agilis (Fig. 2). Compared with controls, the final day cell densities were significantly lower at all tested concentrations, and μ was significantly reduced from 0.53 for control cells to 0.12 at the highest anthracene dose (ANOVA, df = 6, F = 198.82, P < 0.001). The EC50 value for growth was 4.28 mg L−1 (Table 2), which is greater than the values previously reported for several freshwater microalgae (Table 1). At nominal concentrations exceeding 0.05 mg L−1, anthracene significantly inhibits the growth of freshwater phytoplankton32. For example, the growth of Selenastrum capricornutum was extremely sensitive to anthracene, aggravated by UV radiation, with a 22 h EC50 value of 3.9–37.4 μg L−1 33. Our results suggest that anthracene itself is potentially toxic to freshwater primary producers, and could add synergistic effects with other stressors such as UV radiation32.
Figure 2.
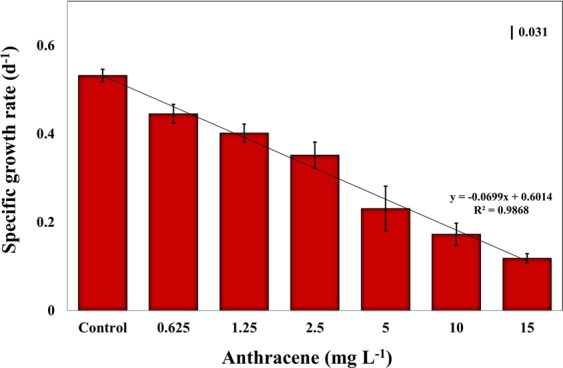
Effect of 96 h anthracene exposure on the specific growth rate (days−1) of E. agilis. Mean and 95% confidence intervals (CI) are shown (n = 3). Vertical bar indicates LSD, least significant difference.
Table 2.
NOEC, LOEC and EC values (mg L−1) plus CI and p-values for inhibition of E. agilis exposed to anthracene for 96 h.
| Test Criterion | NOEC | LOEC | Mean EC10, 95% CI and CV (%) | Mean EC50, 95% CI and CV (%) | p-value |
|---|---|---|---|---|---|
| Growth rate (μ) | <0.625 | 0.625 |
0.38 (0.3–0.55) 10.10 |
4.28 (3.58–5.14) 6.63 |
<0.05 |
| Chl a | <0.625 | 0.625 |
0.31 (0.12–0.22) 8.46 |
5.59 (2.84–7.98) 11.77 |
<0.05 |
| Chl b | <0.625 | 0.625 |
0.34 (0.16–0.63) 16.56 |
8.14 (5.52–10.73) 7.99 |
<0.05 |
| Carotenoids | <0.625 | 0.625 |
0.41 (0.34–0.54) 6.35 |
>15 | <0.05 |
| Fv/Fm | 2.5 | 5 |
3.18 (0.93–4.93) 14.88 |
13.74 (11.90–15.65) 3.41 |
<0.05 |
| rETRmax | 2.5 | 5 |
2.16 (0.89–5.52) 15.57 |
11.80 (8.92–14.68) 7.43 |
<0.05 |
*NOEC, no observed effect concentration; LOEC, lowest observed effect concentration; CI, confidence interval; CV, coefficient of variation.
*Mean and 95% CI are shown (n = 3).
Growth inhibition due to PAH exposure in microalgae and higher aquatic plants has been previously reported34, and the extent of growth inhibition depends on the species studied, the chemicals tested and the duration of exposure. Reduction in growth can result from an accumulation of anthracene within the lipid fraction of cells and subsequent changes in membrane properties35. PAH accumulation in membranes can cause an expansion of the membrane surface area, inhibition of primary ion pumps, and an increase in proton permeability, leading to dissipation of the electrical potential and pH gradient, which ultimately results in inhibition of cellular growth36. Additionally, a reduction in photosynthesis can lead to impaired growth, since these are highly interrelated phenomena, each being a function of the utilization of energy from light and nutrients. Even moderate changes in the function of the photosynthetic apparatus can lead to a marked reduction in energy production within chloroplasts34.
Effect of anthracene on pigment content
Euglena contains both Chl a and b as light-harvesting pigments, along with the carotenoids, diadinoxanthin, and diatoxanthin37. Despite studies on the effect of anthracene on growth and photosynthesis in algae, limited information is available on their interference with photosynthetic pigment production. Anthracene did not affect chlorophyll biosynthesis in Chlamydomonas reinhardtii strain cw92 at concentrations up to 1 mg L−1 38, or in three Desmodesmus spp. up to 0.25 mg L−1 37. However, in the present study, anthracene (>0.625 mg L−1) had a pronounced effect on photosynthetic pigments content in E. agilis. The most abundant pigment in E. agilis was Chl a (7.14 μg mL−1) followed by carotenoids (1.72 μg mL−1) and Chl b (1.25 μg mL−1). At the lowest test concentration (0.625 mg L−1), there were significant reductions in Chl a, Chl b and total carotenoids of up to 20%, 16%, and 17%, respectively, while at the highest concentration, reductions of 58%, 64%, and 49% were observed (Fig. 3). The adverse effect on pigment content was concentration-dependent, with 96 h EC50 values of 5.59 mg L−1, 8.14 mg L−1 and >15 mg L−1 for Chl a (ANOVA, df = 6, F = 334.54, P < 0.05), Chl b (ANOVA, df = 6, F = 40.05, P < 0.05) and total carotenoids (ANOVA, df = 6, F = 130.11, P < 0.05), respectively (Table 2).
Figure 3.
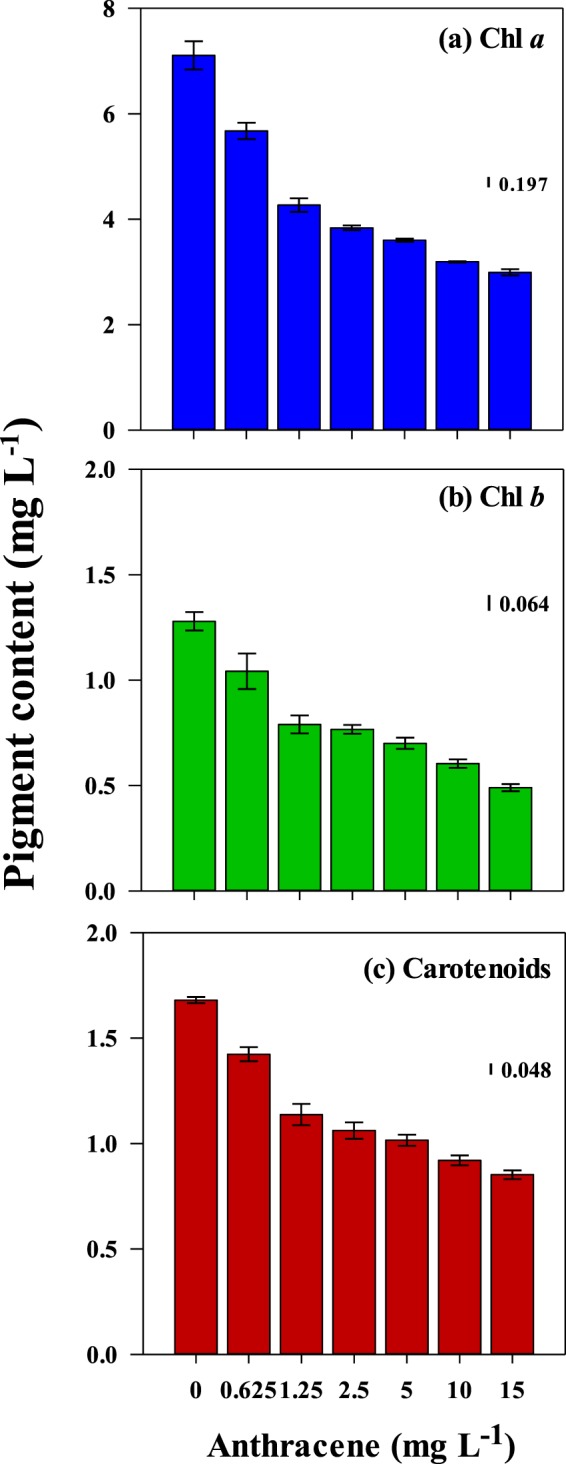
Effect of exposure to anthracene for 96 h on photosynthetic pigmentscontent in E. agilis; (a) Chl a, (b) Chl b, and (c) total carotenoids. Mean and 95% confidence intervals (CI) are shown (n = 3). Vertical bars indicate LSD, least significant difference.
The molecular mechanism of the reduction in pigment levels may involve the accumulation of lipophilic anthracene in thylakoid membranes39, resulting in conformational changes in their structure and composition. In general, reduced pigmentation under chemical stress results from inhibition of enzymes related to chlorophyll synthesis, degradation of chlorophyll and DNA damage40, or accelerated degradation of pigments due to increased ROS formation at various positions in the photosynthetic electron transport chain. Moreover, carotenoids prevent photo-oxidative destruction of chlorophylls41 and, therefore, a reduction in carotenoids could have additional serious consequences on chlorophyll molecules. The simultaneous reduction in all three photosynthetic pigments suggests that the major target of anthracene toxicity is the thylakoid compartment of chloroplasts. These results also indicate that in addition to causing a severe reduction in growth, anthracene exposure may reduce photosynthetic performance via the destruction of pigments responsible for harvesting available photons.
Inhibition of photosynthesis
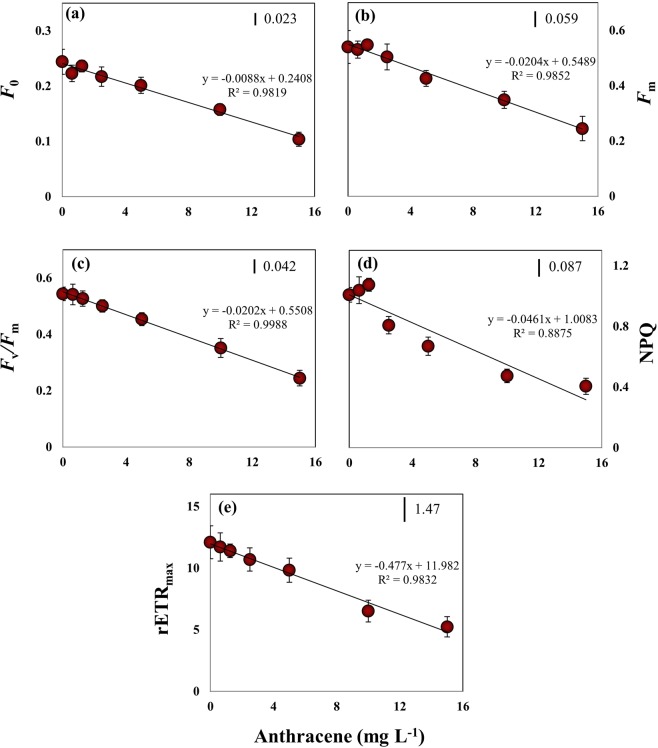
Anthracene is a strong inhibitor of phytoplankton photosynthesis in vivo32,35,38,42. We conducted in vivo Chl a fluorescence measurements as an intriguing tool to reveal the toxic effects of anthracene on the photosynthetic machinery of E. agilis (Fig. 4). The quantum yield and quantum efficiency parameters are indicators of the efficiency of solar energy absorption, which decreases under chemical stress, implying that stress negatively impacts photon absorption and conversion of solar energy during photosynthesis43. We found that at higher anthracene concentrations (>5 mg L−1), there were significant reductions in dark fluorescence (F0; ANOVA, df = 6, F = 40.90, P < 0.05), which reflects emission by excited Chl a molecules in the antennae structure of PSII, and in maximal fluorescence (Fm; ANOVA, df = 6, F = 33.54, P < 0.05), which can be attributed to severe loss of pigments and/or inactivation of PSII reaction centres. It is evident that at higher anthracene doses, reduction in the number of cells and pigment levels resulted in an overall decline in the light-harvesting by E. agilis. Moreover, no significant variation (ANOVA, df = 3, F = 2.01, P > 0.05) in F0 was observed between 0–2.5 mg L–1 anthracene, despite significant reductions in the concentration of Chl a (Fig. 3a), suggesting that pigment molecules associated with PSII reaction centres are less affected. Instead, anthracene may pose a more serious threat to the pigment pool of PSI. This interpretation is supported by the findings of Huang et al.44, who suggested that PSI is the primary site of action of anthracene. However, Chl a fluorescence measurements in plants and algae have suggested inhibition of the cytochrome-b6/f complex and/or photo-oxidative damage to PSII as additional modes of anthracene toxicity6,38.
Figure 4.
Effect of exposure to anthracene for 96 h on Chl a fluorescence parameters of E. agilis. (a) Minimum fluorescence (Fo). (b) Maximum fluorescence (Fm). (c) Maximum quantum yield of PSII (Fv/Fm), (d) Non-photochemical quenching (NPQ), (e) Maximum relative electron transport rate (rETRmax). Mean and 95% confidence intervals (CI) are shown (n = 3). Vertical bars indicate LSD, least significant difference.
Fv/Fm, an estimate of the photochemical conversion efficiency of PSII in the dark, has been widely used to assess the acute toxicity of aromatic hydrocarbons in freshwater plants and phytoplankton6,35. An Fv/Fm value of ~0.55 relative units (RU) was recorded in our control E. agilis population, comparable to the value reported previously for Euglena gracilis21. Fv/Fm did not significantly vary up to 1.25 mg L−1 anthracene (Fig. 4c). However, at higher concentrations, Fv/Fm was declined (ANOVA, df = 6, F = 63.18, P < 0.05) by 17% (5 mg L−1), 36% (10 mg L−1), and 55% (15 mg L−1) with an EC50 of 13.74 mg L−1 (Table 2). Toxicity of anthracene on Fv/Fm in microalgae taxa has not been reported previously, so direct comparison of the sensitivity of E. agilis with other species is not possible. Nevertheless, in the macrophyte Lemna gibba, Fv/Fm appeared to be a more sensitive biomarker of anthracene toxicity, with a 4 h EC50 value of 2 mg L−1 44.
We noted that the reduction in Fv/Fm at >2.5 mg L−1 anthracene was accompanied by a significant loss of NPQ (Fig. 4d). Although values were not statistically significant, NPQ tended to increase up to 1.25 mg L−1 and then decreased significantly thereafter (ANOVA, df = 3, F = 44.74, P < 0.05). This decline in Chl a fluorescence quenching can be attributed to impairment of electron transport downstream from PSII and an elevated reduction of the PQ pool45. NPQ is produced through the generation of an H+ electrochemical gradient across the thylakoid membranes46 and is an indicator of absorbed energy that is dissipated through heat loss and other non-photochemical mechanisms. We assume that a severe reduction in the photosynthetic process at high anthracene levels likely reduces the magnitude of the pH gradient, thereby affecting NPQ values, consistent with the view of González–Moreno et al.47 who reported similar observations on fluorescence quenching in E. gracilis under salt stress. Severe reduction in Fv/Fm of E. gracilis exposed to NaCl had resulted in a diminution of the pH gradient across the thylakoid membranes and subsequently, up to 95% reduction in NPQ47.
In the present study, anthracene reduced the ETR across PSII at higher concentrations (Fig. 4e). rETR is an empirical estimate of the rate of the flow of electrons through the electron transport chain. rETRmax was not significantly affected up to 1.25 mg L−1 anthracene, whereas 5, 10 and 15 mg L−1 doses resulted in 18%, 46%, and 56% reductions, respectively, with an EC50 value of 11.8 mg L−1 (Table 2). Thus, the threshold value of anthracene for a significant reduction in ETR was higher than that for growth inhibition. In aquatic plants, PAHs inhibit photosynthetic electron transport at concentrations below which growth and CO2 fixation are inhibited44. For PAHs in general, the target of their toxicity to photosynthesis is the electron transport downstream from PSII, specifically at cytochrome-b6f. Inhibition of electron transport blocks reoxidation of the reduced plastoquinone pool (PQH2) and the absorbed energy cannot be used in photochemistry6. A probable consequence of inhibition of the electron transport chain at PSII is the transfer of energy from triplet chlorophylls to oxygen, forming singlet oxygen species, which induces oxidative damage of cells48. The generation of free radicals and subsequent intracellular oxidative stress is a prominent mechanism of anthracene toxicity in freshwater phytoplankton38.
When DCFH-DA fluorescence emission in anthracene exposed E. agilis cells was measured, a significant increase was observed at all dosages (ANOVA, df = 6, F = 81.11, P < 0.05), indicating a rise in intracellular ROS levels (Fig. 5). ROS level at 2.5 mg L−1 anthracene was almost double than that in the controls. The subsequent reduction in fluorescence at high anthracene (>5 mg L−1) can be attributed to reduced cell growth and diminished enzyme activities, although values were still significantly higher (p < 0.5) than in controls. The major site of ROS production in photosynthetic organisms is the disrupted electron transport chain across PSII49. We report here, for the first time in freshwater microalga taxa, the significant elevation of intracellular ROS levels under anthracene stress. In Euglena spp., ROS play a significant role in metal toxicity45, UV damage and defense mechanisms50. However, ROS, generated by chemical stressors, trigger adverse effects through multifaceted actions inside the cell. They attack thylakoid lipids and initiate peroxyl radical chain reactions, eventually destroying membranes and pigment-protein complexes45. Moreover, in chloroplasts, ROS cause lipid peroxidation, which results in the disruption of photosynthetic pigments, and the inactivation and degradation of RuBisCo and other components of the Calvin cycle51. Our results correspond to Babu et al.52, who found that 1,2-dihydroanthraquinone, a photoproduct of anthracene, inhibited photosynthetic electron transport, leading to the overproduction of O2− and subsequent oxidation of proteins, membranes, and pigments in Lemna gibba.
Figure 5.
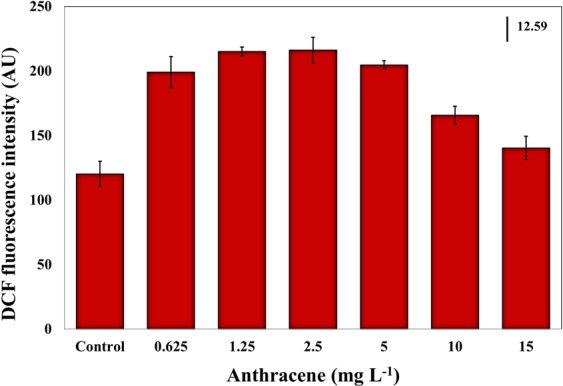
Effect of exposure to anthracene for 96 h on DCFH-DA fluorescence intensity. The excitation wavelength was 485 nm and the emission wavelength was 530 nm. Mean and 95% confidence intervals (CI) are shown (n = 3). Vertical bar indicates LSD, least significant difference.
DCFH-DA is more suitable to estimate total ROS production rather than as a probe for a particular type of ROS53. The superoxide anion radicals (O2−) produced in the electron transport chain is a precursor for many other ROS species. They are rapidly converted to hydrogen peroxide (H2O2) and subsequently to hydroxy radicals (OH•) by enzymatic reactions54. DCFH-DA does not directly react with O2− but can be oxidized to highly fluorescent DCF by H2O2 and OH• radicals55. Thus, DCFH-DA probing of ROS revealed the overall cellular redox status of E. agilis under anthracene stress. Higher ROS also reflect the inefficiency of both photochemical pathways and protective regulatory mechanisms to process the excitation energy at PSII. Our data suggest that ROS generation and consequential oxidative stress play a pivotal role in acute anthracene toxicity in the model organism, E. agilis. We detected significant ROS levels under optimal PAR irradiation, where photo-modification of the parent compound is less likely. Under high oxidative damage, Euglena relies on the activation of antioxidant enzymes such as ascorbate peroxidase (APX) and glutathione peroxidase (GPX)56, and biosynthesis of antioxidant metabolites such as reduced glutathione (GSH) and its derivatives57. Furthermore, some canonical metabolites act as indicators of oxidative damage, such as malondialdehyde (MDA)58. Thus, antioxidant/oxidant responses upon anthracene exposure may represent a promising area for further investigation.
We further analyzed the three photochemical quantum yields of PSII measured by imaging PAM to describe the response of PSII photochemistry to anthracene (Fig. 6). Y(II) represents the fraction of excitation energy converted photochemically at PSII. The remaining fraction, 1–Y(II), is the sum of the yields of regulated dissipation, referred to as Y(NPQ), and unregulated dissipation, indicated by Y(NO)59. As stated before, anthracene reduced the Fv/Fm in a dose-dependent manner, and the photon energy requirements for a complete reduction of QA decrease drastically. This explains the gradual reduction in Y(II) with increasing anthracene doses and a corresponding increase in Y(NO) (Fig. 6). Y(NO) denotes the excess energy, the fraction of absorbed energy used for the generation of free radicals (ROS) via an apparent catalytic transfer of electrons occurred from the reduced PQ pool to O2. The higher quantum yield of non-regulated non-photochemical energy loss of PSII (Y(NO)) is a significant stress response, suggesting potential damage to the photosynthetic apparatus exerted by anthracene. Moreover, the declining Y(NPQ) is an indicator of the failure in regulated non-photochemical quenching mechanisms to process the excess energy at PSII. These PSII quantum yield parameters collectively indicates the reduced efficiency of photochemical energy regulation imposed by anthracene exposure.
Figure 6.
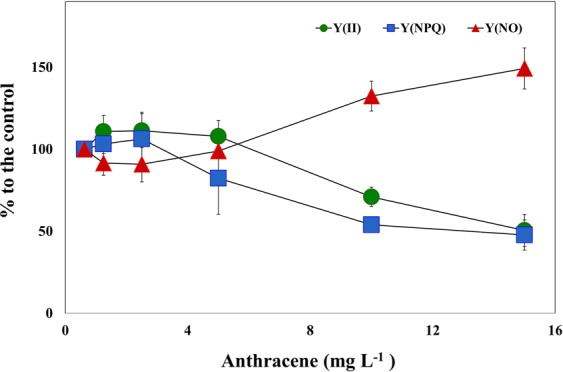
Effect of exposure to anthracene on overall energy conversion at PSII in terms of three quantum yields; (i) photochemical quantum yield of photosystem II, Y(II); (ii) quantum yield of non-photochemical fluorescence quenching due to downregulation of the light harvesting function, Y(NPQ); (iii) quantum yield of non-photochemical fluorescence quenching other than that caused by down-regulation of the light harvesting function, Y(NO). Values are given as % relative to untreated controls.
On the downside of our methodology, anthracene has a higher n-octanol/water partition coefficient (KOW) of 4.5460 and, therefore, multi-well plate assay is likely to underestimate the toxicity potential, because a loss of exposure concentration due to lipophilicity is expected for hydrophobic compounds with KOW > 461. We controlled the evaporative loss of the toxicant by sealing the well plates, however, loss in nominal concentration due to physicochemical properties of the tested PAH may have adversely affected the toxicity thresholds reported here. Nevertheless, our data confirmed the mode of anthracene toxicity in E. agilis through Chl a fluorescence technique and the role of ROS in the overall toxicity response.
Conclusions
Microalgae play a pivotal role in primary production in aquatic ecosystems, hence microalgal ingestion in polluted water bodies is a major route by which toxic chemicals can enter the food chain. The results of the present study confirm that anthracene exerts phytotoxic effects on E. agilis by disrupting growth, pigmentation and photosynthesis. Any severe reduction in these parameters will be followed by a loss of ecological competence and diminished survival of the entire E. agilis population, which could have a devastating impact on associated food chains.
Five principal conclusions derived from this study are:
The addition of anthracene resulted in a concentration-dependent reduction in cellular growth which appears to be highly related to a reduction in photosynthesis.
Anthracene had a pronounced negative effect on photosynthetic pigment content and a simultaneous reduction in all three photosynthetic pigments suggests that the major target of anthracene toxicity is the thylakoid compartment of chloroplasts. These results also indicate that in addition to causing a severe reduction in growth, anthracene exposure may reduce photosynthetic performance via the destruction of pigments responsible for harvesting available photons.
Toxicity of anthracene on Y(II), rETRmax and non-photochemical quenching parameters in microalgae taxa has for the first time been reported in the current study. The PSII quantum yield parameters collectively indicate the reduced efficiency of photochemical energy regulation, impairment of electron transport downstream from PSII, and an elevated reduction of the PQ pool imposed by anthracene exposure.
There was a significant increase in DCFH-DA fluorescence emission in anthracene exposed E. agilis cells, indicating a rise in the intracellular ROS levels. A probable source of generation of ROS would be an inhibition of the electron transport chain at PSII which would have transferred energy from triplet chlorophylls to oxygen, forming singlet oxygen species. A corresponding increase in Y(NO) with increasing anthracene also confirmed that the fraction of absorbed energy might have been used for the generation of free radicals (ROS) via an apparent catalytic transfer of electrons that occurred from the reduced PQ pool to O2.
Growth and Chl a content of E. agilis may serve as sensitive risk assessment parameters of anthracene toxicity in water management since EC50 values for both overlap with anthracene levels (8.3 mg L−1) permitted by the US Environmental Protection Agency (USEPA).
Acknowledgements
We acknowledge Professor Taejun Han at the Ghent University Global Campus and Incheon National University and Professor Murray T. Brown at Plymouth University for their constructive and valuable guidance.
Author contributions
S.K. designed and performed the experiment and wrote the draft manuscript. J.P contributed to construction of tables and figures, performance of statistics, making the final revisions, and writing the responses to the reviewers. The paper was reviewed and approved by both authors prior to submission for peer review.
Data availability
Data can be obtained by contacting the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gala WR, Giesy JP. Photo–induced toxicity of anthracene to the green alga, Selenastrum capricornutum. Archives of Environmental Contamination and Toxicology. 1992;23:316–323. doi: 10.1007/BF00216240. [DOI] [Google Scholar]
- 2.Chaudhry, G. R. Biological degradation and bioremediation of toxic chemicals. Dioscorides Press, Portland, OR, USA (1994).
- 3.Behera BK, et al. Polycyclic Aromatic Hydrocarbons (PAHs) in inland aquatic ecosystems: Perils and remedies through biosensors and bioremediation. Environmental Pollution. 2018;241:212–233. doi: 10.1016/j.envpol.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Andersson TA, et al. Solubility of Acenaphthene, Anthracene, and Pyrene in Water At 50 °C to 300 °C. Journal of Chemical & Engineering Data. 2005;50:1177–1183. doi: 10.1021/je0495886. [DOI] [Google Scholar]
- 5.Krylov SN, et al. Mechanistic quantitative structure–activity relationship model for the photoinduced toxicity of polycyclic aromatic hydrocarbons: I Physical model based on chemical kinetics in a two–compartment system. Environmental Toxicology and Chemistry. 1997;16:2283–2295. [Google Scholar]
- 6.Mallakin A, et al. Sites of toxicity of specific photooxidation products of anthracene to higher plants: inhibition of photosynthetic activity and electron transport in Lemna gibba L G–3 (duckweed) Environmental Toxicology. 2002;17:462–471. doi: 10.1002/tox.10080. [DOI] [PubMed] [Google Scholar]
- 7.USEPA (U.S. Environmental Protection Agency), National recommended water quality criteria for priority pollutants. Office of Water, USEPA, Washington, DC (2009).
- 8.Canadian Council of Ministers of the Environment, Canadian water quality guidelines for the protection of aquatic life: Polycyclic aromatic hydrocarbons (PAHs). In: Canadian environmental quality guidelines, Canadian Council of Ministers of the Environment, Winnipeg (1999).
- 9.Wang XS, et al. Modeling the bioconcentration of hydrophonic organic chemicals in aquatic organisms. Chemosphere. 1996;32:1783–1793. doi: 10.1016/0045-6535(96)00083-5. [DOI] [Google Scholar]
- 10.Marwood AC, et al. Intact and photomodified polycyclic aromatic hydrocarbons inhibit photo– synthesis in natural assemblages of Lake Erie phytoplankton exposed to solar radiation. Ecotoxicology and Environmental Safety. 1999;44:322–327. doi: 10.1006/eesa.1999.1840. [DOI] [PubMed] [Google Scholar]
- 11.Grote M, et al. Modeling photoinduced algal toxicity of polycyclic aromatic hydrocarbons. Environmental Science & Technology. 2005;39:4141–4149. doi: 10.1021/es048310v. [DOI] [PubMed] [Google Scholar]
- 12.Bi X, et al. Effect of anthracene (ANT) on growth, microcystin (MC) production and expression of MC synthetase (mcy) genes in Microcystis aeruginosa. Water, Air, & Soil Pollution. 2016;227:1–8. doi: 10.1007/s11270-016-2956-2. [DOI] [Google Scholar]
- 13.Brack W, et al. Identification of toxic products of anthracene photomodification in simulated sunlight. Environmental Toxicology and Chemistry. 2003;22:2228–2237. doi: 10.1897/02-450. [DOI] [PubMed] [Google Scholar]
- 14.Erzinger, G. S. & Häder, D.-P. 4-Regulations, political and societal aspects, toxicity limits, Bioassays. Elsevier, 51–67 (2018).
- 15.Aronsson KA, Ekelund NGA. Biological eff ects of wood ash application to forest and aquatic ecosystems. Journal of Environmental Quality. 2004;33:1595–1605. doi: 10.2134/jeq2004.1595. [DOI] [PubMed] [Google Scholar]
- 16.Engel F, et al. Comparative toxicity of physiological and biochemical parameters in Euglena gracilis to short-term exposure to potassium sorbate. Ecotoxicology. 2015;24:153–162. doi: 10.1007/s10646-014-1367-7. [DOI] [PubMed] [Google Scholar]
- 17.Aronsson KA, Ekelund NGA. Effects on motile factors and cell growth of Euglena gracilis after exposure to wood ash solution; assessment of toxicity, nutrient availability and pH–dependency. Water, Air, & Soil Pollution. 2005;162:353–368. doi: 10.1007/s11270-005-7250-7. [DOI] [Google Scholar]
- 18.De Filippis LF, et al. The effect of sublethal concentrations of zinc, cadmium and mercury on Euglena. Archives of Microbiology. 1981;128:407–411. doi: 10.1007/BF00405922. [DOI] [Google Scholar]
- 19.Navarro L, et al. Comparison of physiological changes in Euglena gracilis during exposure to heavy metals of heterotrophic and autotrophic cells. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology. 1997;116:265–272. [Google Scholar]
- 20.Dos Santos FVV, et al. Gene expression patterns in Euglena gracilis: insights into the cellular response to environmental stress. Gene. 2007;389:136–145. doi: 10.1016/j.gene.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed H, Häder D-P. A fast algal bioassay for assessment of copper toxicity in water using Euglena gracilis. Journal of Applied Phycology. 2010;22:785–792. doi: 10.1007/s10811-010-9520-z. [DOI] [Google Scholar]
- 22.Pettersson M, Ekelund NGA. Effects of the herbicides Roundup and Avans on Euglena gracilis. Archives of Environmental Contamination and Toxicology. 2006;50:175–181. doi: 10.1007/s00244-004-0042-z. [DOI] [PubMed] [Google Scholar]
- 23.Li M, et al. Genotoxicity of organic pollutants in source of drinking on microalga Euglena gracilis. Ecotoxicology. 2009;18:669–676. doi: 10.1007/s10646-009-0343-0. [DOI] [PubMed] [Google Scholar]
- 24.Nestler H, et al. Multiple-endpoint assay provides a detailed mechanistic view of responses to herbicide exposure in Chlamydomonas reinhardtii. Aquatic toxicology. 2012;110:214–224. doi: 10.1016/j.aquatox.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Sebastian C, et al. Effects of solar and artificial ultraviolet-radiation on pigment composition and photosynthesis in three Prorocentrum strains. Journal of Experimental Marine Biology and Ecology. 1994;182:251–263. doi: 10.1016/0022-0981(94)90055-8. [DOI] [Google Scholar]
- 26.Checcucci A, et al. Action spectra for photo–accumulation of green and colorless Euglena: evidence for identification of receptor pigments. Photochemistry and Photobiology. 1976;23:51–54. doi: 10.1111/j.1751-1097.1976.tb06770.x. [DOI] [PubMed] [Google Scholar]
- 27.Jeffrey SW, Humphrey GF. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Plant Physiology and Biochemistry. 1975;167:191–194. [Google Scholar]
- 28.Jassby AD, Platt T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnology and oceanography. 1976;21:540–547. doi: 10.4319/lo.1976.21.4.0540. [DOI] [Google Scholar]
- 29.Maxwell K, Johnson GN. Chlorophyll fluorescence—a practical guide. Journal of experimental botany. 2000;51:659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 30.Rastogi RP, et al. Detection of reactive oxygen species (ROS) by the oxidant–sensing probe 2′,7′–dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochemical and Biophysical Research Communications. 2010;397:603–607. doi: 10.1016/j.bbrc.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Okumura Y, et al. Influence of organic solvents on the growth of marine microalgae. Archives of Environmental Contamination and Toxicology. 2001;41:123–128. doi: 10.1007/s002440010229. [DOI] [PubMed] [Google Scholar]
- 32.Aksmann A, Tukaj Z. The effect of anthracene and phenanthrene on the growth, photosynthesis and SOD activity of green alga Scenedesmus armatus depend on the PAR irradiance and CO2 level. Archives of Environmental Contamination and Toxicology. 2004;47:177–184. doi: 10.1007/s00244-004-2297-9. [DOI] [PubMed] [Google Scholar]
- 33.Gala WR, Giesy JP. Using the carotenoid biosynthesis inhibiting herbicide, Fluridone, to investigate the ability of carotenoid pigments to protect algae from the photoinduced toxicity of anthracene. Aquatic Toxicology. 1993;27:61–70. doi: 10.1016/0166-445X(93)90047-5. [DOI] [Google Scholar]
- 34.Marwood CA, et al. Chlorophyll fluorescence as a bioindicator of effects on growth in aquatic macrophytes from mixtures of polycyclic aromatic hydrocarbons. Environmental Toxicology and Chemistry. 2001;20:890–898. doi: 10.1002/etc.5620200425. [DOI] [PubMed] [Google Scholar]
- 35.Pokora W, Tukaj Z. The combined effect of anthracene and cadmium on photosynthetic activity of three Desmodesmus (Chlorophyta) species. Ecotoxicology and Environmental Safety. 2010;73:1207–1213. doi: 10.1016/j.ecoenv.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Sikkema J, et al. Effects of the membrane action of Tetralin on the functional and structural properties of artificial and bacterial membranes. Journal of Bacteriology. 1992;174:2986–2992. doi: 10.1128/jb.174.9.2986-2992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hager A, Stransky H. Das Carotenoidmuster und die Verbreitung des lichtinduzierten Xanthophyllcyclus in verschiedenen Algenklassen. Archives of Microbiology. 1970;73:77–89. [PubMed] [Google Scholar]
- 38.Aksmann A, Tukaj Z. Intact anthracene inhibits photosynthesis in algal cells: a fluorescence induction study on Chlamydomonas reinhardtii cw92 strain. Chemosphere. 2008;74:26–32. doi: 10.1016/j.chemosphere.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 39.Duxbury CL, et al. Effects of simulated solar radiation on the bioaccumulation of polycyclic aromatic hydrocarbons by the duckweed Lemna gibba. Environmental Toxicology and Chemistry. 1997;16:1739–1748. doi: 10.1002/etc.5620160824. [DOI] [Google Scholar]
- 40.Garcia DC, et al. Physiological responses of Euglena gracilis to copper stress. Quimica Nova. 2011;34:1211–1214. doi: 10.1590/S0100-40422011000700020. [DOI] [Google Scholar]
- 41.Middleton EM, Teramura AH. The role of flavonol glycosides and carotenoids in protecting soybean from UV–B damage. Plant Physiology. 1993;103:741–752. doi: 10.1104/pp.103.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutchinson TC, et al. The correlation of the toxicity to algae of hydrocarbons and halogenated hydrocarbons with their physical–chemical properties. Environmental Science and Pollution Research. 1980;16:577–586. [Google Scholar]
- 43.Sayyad-Amin P, et al. Changes in photosynthetic pigments and chlorophyll-a fluorescence attributes of sweet-forage and grain sorghum cultivars under salt stress. Journal of Biological Physics. 2016;42:601–620. doi: 10.1007/s10867-016-9428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang X-D, et al. Mechanisms of photoinduced toxicity of photomodified anthracene to plants: inhibition of photosynthesis in the aquatic higher plant Lemna gibba (duckweed) Environmental Toxicology and Chemistry. 1997;16:1707–1715. [Google Scholar]
- 45.Rocchetta I, Küpper H. Chromium– and copper–induced inhibition of photosynthesis in Euglena gracilis analysed on the single–cell level by fluorescence kinetic microscopy. New Phytologist. 2009;182:405–420. doi: 10.1111/j.1469-8137.2009.02768.x. [DOI] [PubMed] [Google Scholar]
- 46.Briantais JM, et al. A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochimica et Biophysica Acta. 1979;548:128–138. doi: 10.1016/0005-2728(79)90193-2. [DOI] [PubMed] [Google Scholar]
- 47.González–Moreno S, et al. Multiple effects of salinity on photosynthesis of the protist Euglena gracilis. Physiologia Plantarum. 1997;101:777–786. doi: 10.1111/j.1399-3054.1997.tb01063.x. [DOI] [Google Scholar]
- 48.Krieger–Liszkay A. Singlet oxygen production in photosynthesis. Journal of Experimental Botany. 2004;56:337–346. doi: 10.1093/jxb/erh237. [DOI] [PubMed] [Google Scholar]
- 49.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 50.Kottuparambil S, et al. UV–B affects photosynthesis, ROS production and motility of the freshwater flagellate. Euglena agilis Carter, Aquatic Toxicology. 2012;122–123:206–213. doi: 10.1016/j.aquatox.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Pätsikkä E, et al. Increase in quantum yield of photoinhibition contributes to copper toxicity in vivo. Plant Physiology. 1998;117:619–627. doi: 10.1104/pp.117.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babu TS, et al. Synergistic effects of a photooxidized polycyclic aromatic hydrocarbon and copper on photosynthesis and plant growth: evidence that in vivo formation of reactive oxygen species is a mechanism of copper toxicity. Environmental Toxicology and Chemistry. 2001;20:1351–1358. doi: 10.1002/etc.5620200626. [DOI] [PubMed] [Google Scholar]
- 53.Pavelescu, L. On reactive oxygen species measurement in living systems. Journal of medicine and life8(Spec Issue), 38 (2015). [PMC free article] [PubMed]
- 54.Wang J, et al. Generation of reactive oxygen species in cyanobacteria and green algae induced by allelochemicals of submerged macrophytes. Chemosphere. 2011;85:977–982. doi: 10.1016/j.chemosphere.2011.06.076. [DOI] [PubMed] [Google Scholar]
- 55.He YY, Häder DP. Reactive oxygen species and UV-B: effect on cyanobacteria. Photochemical & Photobiological Sciences. 2002;1:729–736. doi: 10.1039/B110365M. [DOI] [PubMed] [Google Scholar]
- 56.Sánchez-Thomas R, et al. Accumulation of zinc protects against cadmium stress in photosynthetic Euglena gracilis. Environmental and Experimental Botany. 2016;131:19–31. doi: 10.1016/j.envexpbot.2016.06.009. [DOI] [Google Scholar]
- 57.Garcia-Garcia JD, et al. Bio-recovery of non-essential heavy metals by intra-and extracellular mechanisms in free-living microorganisms. Biotechnology advances. 2016;34:859–873. doi: 10.1016/j.biotechadv.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Rocchetta I, et al. Effect of chromium on the fatty acid composition of two strains of Euglena gracilis. Environmental pollution. 2006;141:353–358. doi: 10.1016/j.envpol.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 59.Klughammer C, Schreiber U. Saturation pulse method for assessment of energy conversion in PSI. PAM Application Notes. 2008;1:11–14. [Google Scholar]
- 60.Lu GN, et al. Estimation of n-octanol/water partition coefficients of polycyclic aromatic hydrocarbons by quantum chemical descriptors. Open Chemistry. 2008;6:310–318. doi: 10.2478/s11532-008-0010-y. [DOI] [Google Scholar]
- 61.OECD, O. Guidance Document on Acute Oral Toxicity Testing. (2000).
- 62.Patel, J. G., Kumar, J. I. N., Kumar, R. N. & Khan, S. R. Biodegradation Capability and Enzymatic Variation of Potentially Hazardous Polycyclic Aromatic Hydrocarbons—Anthracene and Pyrene by. Polycyclic Aromatic Compounds 36(1), 72–87 (2015).
- 63.Cody T.E., Radike M.J., Warshawsky D. The phototoxicity of benzo[a]pyrene in the green alga Selenastrum capricornutum. Environmental Research. 1984;35(1):122–132. doi: 10.1016/0013-9351(84)90119-1. [DOI] [PubMed] [Google Scholar]
- 64.Yan, X. et al. Study on the toxicological response of Chlo rella to anthracene under different nutrient conditions. Journal of Wuhan University (Natural Science Edition) 45(6), 845–848 (1999).
- 65.Djomo J.E., Dauta A., Ferrier V., Narbonne J.F., Monkiedje A., Njine T., Garrigues P. Toxic effects of some major polyaromatic hydrocarbons found in crude oil and aquatic sediments on Scenedesmus subspicatus. Water Research. 2004;38(7):1817–1821. doi: 10.1016/j.watres.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 66.Tukaj, Z. & Pokora, W. Individual and combined effect of anthracene, cadmium, and chloridazone on growth and activity of SOD izoformes in three Scenedesmus species. Ecotoxicology and Environmental Safety65(3), 323–331 (2006). [DOI] [PubMed]
- 67.Baścik-Remisiewicz Agnieszka, Aksmann Anna, Żak Adam, Kowalska Maja, Tukaj Zbigniew. Toxicity of Cadmium, Anthracene, and Their Mixture to Desmodesmus subspicatus Estimated by Algal Growth-Inhibition ISO Standard Test. Archives of Environmental Contamination and Toxicology. 2010;60(4):610–617. doi: 10.1007/s00244-010-9585-3. [DOI] [PubMed] [Google Scholar]
- 68.Bi, X. et al. Effect of Anthracene (ANT) on growth, microcystin (MC) production and expression of MC synthetase (mcy) genes in Microcystis aeruginosa. 10.1038/s41598-019-51451-y Water, Air, & Soil Pollution 227(8) (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be obtained by contacting the corresponding author.