Abstract
Global increases in temperatures and urbanization are impacting the epidemiology of mosquito-borne diseases. Urbanization processes create suitable habitats for vector mosquitoes in which there are a reduced number of predators, and human hosts are widely available. We hypothesize that mosquito vector species, especially Aedes aegypti, are locally concentrated primarily in those specific habitats at the neighborhood levels that provide suitable conditions and environmental resources needed for mosquito survival. Determining how mosquito vector species composition and abundance depend on environmental resources across habitats addresses where different types of vector control need to be applied. Therefore, our goal was to analyze and identify the most productive aquatic habitats for mosquitoes in Miami-Dade County, Florida. Immature mosquito surveys were conducted throughout Miami-Dade County from April 2018 to June 2019, totaling 2,488 inspections. Mosquitoes were collected in 76 different types of aquatic habitats scattered throughout 141 neighborhoods located in the urbanized areas of Miami-Dade County. A total of 44,599 immature mosquitoes were collected and Ae. aegypti was the most common and abundant species, comprising 43% of all specimens collected. Aedes aegypti was primarily found in buckets, bromeliads, and flower pots, concentrated in specific neighborhoods. Our results showed that aquatic habitats created by anthropogenic land-use modifications (e.g., ornamental bromeliads, buckets, etc.) were positively correlated with the abundance of Ae. aegypti. This study serves to identify how vector mosquitoes utilize the resources available in urban environments and to determine the exact role of these specific urban features in supporting populations of vector mosquito species. Ultimately, the identification of modifiable urban features will allow the development of targeted mosquito control strategies optimized to preventatively control vector mosquitoes in urban areas.
Subject terms: Biodiversity, Community ecology, Ecological epidemiology, Ecosystem ecology, Urban ecology
Introduction
Global increases in temperatures and urbanization are impacting the epidemiology of mosquito-borne diseases1, resulting in severe outbreaks, even in formerly non-endemic areas2–5. Urbanization consists of altering the natural environment to make it more suitable for human populations and to accommodate both the growth of the local population and people moving from rural areas to cities6,7. Importantly, urbanization processes create suitable habitats for vector mosquitoes in which there are a reduced number of predators, and human hosts are wide available6–9. Public health efforts to control mosquito-borne diseases rely on mosquito control, which can achieve local success but generally is not enough to prevent arbovirus outbreaks.
Miami-Dade County, Florida is at risk for several arbovirus outbreaks including dengue (DENV), West Nile (WNV), chikungunya (CHIKV), Zika (ZIKV), and yellow fever (YFV) viruses that have occurred in past decades10–15. During the 2016 ZIKV outbreak, where there were locally acquired cases16; the virus was introduced to Miami on multiple occasions in different areas17.
Miami has complex environmental and socioeconomic features. Miami is one of the most important gateways to the U.S. due to an increased flow of people coming and going from endemic areas in the Caribbean region and Latin America, substantially increasing the risk of arbovirus introduction. In addition, Miami has the appropriate conditions for mosquitoes year-round, as the tropical monsoon climate is highly conducive for mosquitoes even during the winter18. Miami is also undergoing intense increases in urbanization19,20 that is impacting the population dynamics of vector mosquitoes and subsequently the risk of arbovirus transmission21,22.
Recent findings exposed the unexpected scenario that Aedes (Stegomyia) aegypti (Linnaeus, 1762) are successfully using ornamental bromeliads as larval habitats in Miami-Dade County, Florida21. Furthermore, subsequent studies on construction sites and tire shops in urban areas of Miami-Dade County showed that vector mosquitoes are breeding in high numbers in these areas. Results also showed reduced biodiversity of species in these habitats sheltering almost exclusively Ae. aegypti and Culex (Culex) quinquefasciatus (Say, 1823)20,23. These findings highlight the need to determine how the abundance of immature populations of vector mosquito species at point source locations is related to both features of the local environment and availability of breeding sites, representing vital resources needed by mosquito species for them to exist and propagate in definable urban habitats.
We hypothesize that mosquito vector species, especially Ae. aegypti, are locally concentrated primarily in those specific habitats at the neighborhood levels that provide suitable conditions and environmental resources needed for mosquito survival. Determining how mosquito vector species composition and abundance depend on environmental resources across habitats addresses where different types of vector control need to be applied. Therefore, our goal was to analyze and identify the most productive aquatic habitats for mosquitoes in Miami-Dade County, Florida.
Results
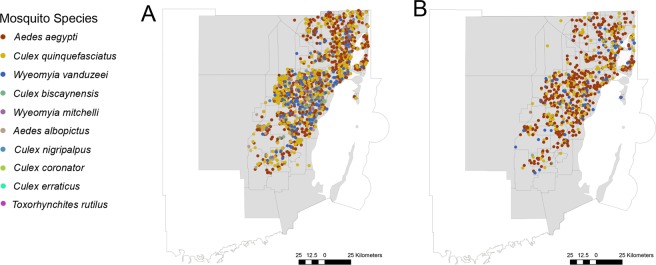
Mosquitoes were collected in 76 different types of aquatic habitats (Supplementary Table 1) scattered throughout 141 neighborhoods located in the urbanized areas of Miami-Dade County. A total of 44,599 immature mosquitoes were collected, from which 19,206 were Ae. aegypti larvae and 2,997 pupae, 325 Aedes (Stegomyia) albopictus (Skuse, 1895) larvae and 65 pupae, 1.736 Culex (Micraedes) biscaynensis (Zavortink & O’Meara, 1999) larvae and 19 pupae, 212 Culex (Culex) coronator (Dyar & Knab, 1906) larvae and 4 pupae, 13 Culex (Melanoconion) erraticus (Dyar & Knab, 1906) larvae, 14,358 Cx. quinquefasciatus larvae and 1,193 pupae, 174 Culex (Culex) nigripalpus (Theobald, 1901) larvae and 3 pupae, 873 Wyeomyia (Wyeomyia) mitchelli (Theobald, 1905) larvae and 129 pupae, 3,054 Wyeomyia (Wyeomyia) vanduzeei (Dyar & Knab, 1906) larvae and 236 pupae, and 2 Toxorhynchites (Lynchiella) rutilus (Dyar and Knab, 1869) larvae (Fig. 1, Table 1, Supplementary Fig. S1).
Figure 1.
Map displaying the distribution of immature mosquitoes collected in Miami-Dade County, Florida for (A) larvae and (B) Pupae. Each color represents a mosquito species. Urban areas are displayed in gray. The figure was produced using ArcGIS 10.2 (Esri, Redlands, CA), using freely available layers from the Miami-Dade County’s Open Data Hub— https://gis-mdc.opendata.arcgis.com/.
Table 1.
Immature mosquito species collected in Miami-Dade County from April 2018 to June 2019.
| Neighborhood | Number of Inspections | Aedes aegypti | Aedes albopictus | Culex biscaynensis | Culex coronator | Culex erraticus | Culex quinquefasciatus | Culex nigripalpus | Wyeomyia mitchelli | Wyeomyia vanduzeei | Toxorhynchites rutilus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | P | L | P | L | P | L | P | L | P | L | P | L | P | L | P | L | P | L | P | ||
| Auburdale | 6 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 109 | 0 | 0 | 0 | 0 | 0 | 6 | 3 | 0 | 0 |
| Aventura | 7 | 16 | 9 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 118 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Bal Harbor | 2 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bay Harbor Island | 6 | 50 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 46 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bay Shore | 5 | 15 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Bay Village | 2 | 50 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 44 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bird Drive Basin | 59 | 178 | 25 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 910 | 15 | 4 | 0 | 0 | 0 | 30 | 3 | 0 | 0 |
| Biscayne Park | 11 | 84 | 17 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 46 | 0 | 0 | 0 | 0 | 0 | 80 | 0 | 0 | 0 |
| Biscayne Point | 4 | 11 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blue Lagoon | 5 | 21 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Brickell | 5 | 12 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Brownsville | 24 | 157 | 49 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 130 | 38 | 1 | 0 | 0 | 0 | 0 | 7 | 0 | 0 |
| Buena Vista | 23 | 264 | 39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 309 | 18 | 0 | 0 | 0 | 0 | 28 | 1 | 0 | 0 |
| Bunche Park | 5 | 99 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| C-9 Basin Area | 1 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Calusa | 25 | 76 | 34 | 0 | 0 | 19 | 0 | 2 | 0 | 0 | 0 | 316 | 6 | 0 | 0 | 0 | 0 | 82 | 2 | 0 | 0 |
| Carol City | 43 | 373 | 56 | 4 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 380 | 2 | 0 | 0 | 7 | 2 | 14 | 2 | 0 | 0 |
| Catalina Lakes | 21 | 136 | 18 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 24 | 19 | 0 | 0 | 22 | 0 | 15 | 1 | 0 | 0 |
| Central Downtown | 6 | 10 | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 13 | 4 | 0 | 0 | 1 | 0 | 19 | 0 | 0 | 0 |
| Central Gables | 6 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 29 | 0 | 0 | 0 |
| Civic Center | 33 | 192 | 25 | 2 | 0 | 0 | 0 | 1 | 0 | 13 | 0 | 322 | 46 | 0 | 0 | 0 | 1 | 13 | 0 | 0 | 0 |
| Coastal Wetland | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coral Terrace North | 8 | 17 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 329 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Coral Terrace South | 23 | 200 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 78 | 0 | 0 | 0 | 4 | 35 | 8 | 1 | 0 | 0 |
| Country Club Of Miami | 7 | 30 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 37 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cutler | 88 | 331 | 80 | 5 | 0 | 209 | 0 | 0 | 1 | 0 | 0 | 229 | 63 | 0 | 0 | 9 | 0 | 271 | 5 | 0 | 0 |
| Cutler Ridge | 22 | 1386 | 25 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 60 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| Dadeland | 9 | 208 | 22 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 43 | 0 | 0 | 0 | 1 | 0 | 9 | 0 | 0 | 0 |
| Doral Area | 11 | 80 | 28 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 53 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Douglas Park | 6 | 28 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| East Goulds | 44 | 321 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 234 | 25 | 0 | 0 | 1 | 0 | 23 | 12 | 0 | 0 |
| East Homestead | 6 | 15 | 6 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| East Kendall | 59 | 334 | 52 | 3 | 1 | 230 | 1 | 1 | 0 | 0 | 0 | 134 | 36 | 5 | 0 | 104 | 1 | 108 | 6 | 0 | 0 |
| East Liberty City | 23 | 323 | 13 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 132 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| East Naranja | 13 | 187 | 10 | 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 80 | 2 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| East South Miami | 4 | 121 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 23 | 0 | 0 | 0 |
| East South Miami City | 4 | 4 | 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| East Turnpike Area | 2 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 49 | 0 | 0 | 0 |
| Eastern Shores | 20 | 168 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 3 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 |
| El Portal | 4 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 6 | 0 | 23 | 10 | 0 | 0 |
| Flagler Westside | 16 | 92 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 42 | 5 | 9 | 0 | 7 | 0 | 3 | 0 | 0 | 0 |
| Flamingo | 7 | 5 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 17 | 0 | 0 | 0 | 0 | 62 | 0 | 0 | 0 |
| Florida City | 10 | 111 | 8 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 34 | 0 | 6 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| Gables Bayfront | 12 | 54 | 34 | 0 | 0 | 65 | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 14 | 11 | 0 | 0 |
| Golden Glades | 27 | 45 | 41 | 109 | 0 | 0 | 0 | 50 | 0 | 0 | 0 | 94 | 3 | 0 | 0 | 125 | 0 | 53 | 1 | 0 | 0 |
| Granada | 12 | 266 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 134 | 8 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Grapeland | 10 | 255 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hammocks | 56 | 231 | 70 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 294 | 11 | 32 | 0 | 0 | 0 | 24 | 3 | 0 | 0 |
| Hialeah - Area 1 | 5 | 31 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 75 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hialeah - Area 2 | 11 | 31 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 28 | 0 | 0 | 0 |
| Hialeah - Area 3 | 7 | 33 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 17 | 0 | 0 | 1 | 0 | 5 | 0 | 0 | 0 |
| Hialeah - Area 4 | 3 | 18 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 |
| Hialeah - Area 5 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hialeah - Area 6 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hialeah - Area 7 | 13 | 79 | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 151 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hialeah Gardens | 5 | 36 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 0 |
| Homestead | 18 | 56 | 29 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 327 | 3 | 0 | 0 | 0 | 2 | 30 | 0 | 0 | 0 |
| Homestead Base | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Homestead Lakes | 7 | 21 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 31 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Horse Country | 7 | 7 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 32 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Interama | 1 | 11 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ives Estate | 15 | 175 | 16 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 168 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kendale Lakes | 71 | 430 | 103 | 0 | 1 | 34 | 0 | 0 | 0 | 0 | 0 | 289 | 54 | 0 | 0 | 10 | 0 | 20 | 4 | 0 | 0 |
| Kendall | 120 | 804 | 160 | 8 | 0 | 438 | 0 | 0 | 0 | 0 | 0 | 309 | 23 | 4 | 0 | 87 | 0 | 201 | 9 | 0 | 0 |
| Kendall North | 15 | 36 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 108 | 0 | 0 | 0 | 0 | 2 | 12 | 1 | 0 | 0 |
| Key Biscayne - Bay Area | 11 | 148 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 4 | 0 | 0 | 0 | 0 | 5 | 0 | 0 |
| Keystone Islands | 15 | 122 | 22 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 60 | 0 | 21 | 0 | 28 | 0 | 1 | 5 | 0 | 0 |
| La Gorce | 3 | 152 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leisure City Area | 34 | 239 | 14 | 1 | 0 | 25 | 1 | 0 | 0 | 0 | 0 | 85 | 3 | 0 | 0 | 11 | 0 | 74 | 0 | 0 | 0 |
| Little Havana | 11 | 67 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 340 | 0 | 0 | 0 | 2 | 0 | 10 | 0 | 0 | 0 |
| Little River | 9 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 115 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Management Area - 1 | 9 | 87 | 5 | 2 | 0 | 0 | 0 | 42 | 0 | 0 | 0 | 315 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Marbella Park | 7 | 34 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 93 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Metro-Lindgren | 33 | 205 | 51 | 0 | 0 | 3 | 0 | 16 | 0 | 0 | 0 | 232 | 22 | 0 | 0 | 28 | 0 | 21 | 2 | 0 | 0 |
| Miami Industrial | 7 | 22 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| Miami Lakes | 18 | 176 | 16 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 103 | 11 | 0 | 0 | 0 | 0 | 4 | 12 | 0 | 0 |
| Miami Shores | 10 | 87 | 12 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 17 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| Miami Springs - Area 1 | 16 | 22 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 66 | 47 | 0 | 0 | 3 | 0 | 20 | 2 | 0 | 0 |
| Miami Springs - Area 2 | 26 | 93 | 36 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 29 | 6 | 7 | 0 | 14 | 0 | 3 | 2 | 0 | 0 |
| Miami Springs - Area 3 | 25 | 168 | 53 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 2 | 0 | 0 | 2 | 0 | 36 | 1 | 0 | 0 |
| Naranja | 15 | 82 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 39 | 8 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 0 |
| Nautilus | 3 | 59 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Norland | 18 | 266 | 27 | 0 | 0 | 3 | 0 | 30 | 0 | 0 | 0 | 32 | 0 | 0 | 0 | 3 | 0 | 6 | 0 | 0 | 0 |
| Normandy Isle | 29 | 275 | 8 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 119 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| North Bayfront | 17 | 72 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 324 | 9 | 0 | 0 | 14 | 0 | 13 | 2 | 0 | 0 |
| North Gables | 8 | 50 | 1 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 4 | 26 | 0 | 0 | 7 | 0 | 0 | 4 | 0 | 0 |
| North Grove | 25 | 66 | 92 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 219 | 26 | 6 | 0 | 3 | 6 | 114 | 0 | 0 | 0 |
| North Hialeah Gardens | 5 | 30 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| North Opalocka | 6 | 99 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 27 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| North Palm Springs | 5 | 47 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| North Redlands | 104 | 1103 | 118 | 35 | 0 | 120 | 0 | 25 | 1 | 0 | 0 | 818 | 52 | 9 | 0 | 50 | 42 | 25 | 20 | 2 | 0 |
| North Shore | 4 | 69 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 49 | 0 | 0 | 0 | 12 | 0 | 0 | 2 | 0 | 0 |
| Oceanpoint | 6 | 52 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ojus | 24 | 77 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 145 | 0 | 0 | 0 | 0 | 13 | 69 | 0 | 0 | 0 |
| Olympia Heights | 6 | 35 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Omni - Boulevard | 5 | 43 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 |
| Opalocka City | 12 | 218 | 26 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 43 | 0 | 1 | 0 | 40 | 0 | 1 | 11 | 0 | 0 |
| Overtown | 3 | 4 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 |
| Perrine | 35 | 153 | 30 | 0 | 0 | 73 | 0 | 0 | 0 | 0 | 0 | 257 | 0 | 0 | 1 | 1 | 0 | 106 | 4 | 0 | 0 |
| Richmond | 23 | 292 | 47 | 0 | 3 | 38 | 0 | 2 | 0 | 0 | 0 | 131 | 4 | 0 | 0 | 2 | 1 | 76 | 0 | 0 | 0 |
| Saga Bay | 18 | 147 | 11 | 31 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 121 | 3 | 0 | 0 | 39 | 0 | 17 | 0 | 0 | 0 |
| Scott Lake | 16 | 120 | 111 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 204 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Shenandoah | 28 | 214 | 23 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 87 | 5 | 0 | 0 | 3 | 0 | 95 | 2 | 0 | 0 |
| South Gables | 5 | 20 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| South Golden Glades | 18 | 122 | 42 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 76 | 4 | 0 | 0 | 0 | 0 | 37 | 1 | 0 | 0 |
| South Grove | 18 | 222 | 33 | 0 | 0 | 19 | 0 | 0 | 0 | 0 | 0 | 8 | 9 | 0 | 0 | 15 | 0 | 51 | 7 | 0 | 0 |
| South Miami Heights | 41 | 254 | 17 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 405 | 29 | 0 | 0 | 2 | 0 | 65 | 1 | 0 | 0 |
| South Naranja | 6 | 38 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 |
| South North Miami Beach | 19 | 90 | 19 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 217 | 38 | 0 | 0 | 0 | 0 | 143 | 0 | 0 | 0 |
| Sunny Isles | 5 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 52 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sunset East | 15 | 191 | 37 | 6 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 146 | 4 | 0 | 0 | 62 | 0 | 0 | 0 | 0 | 0 |
| Sunset Islands | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Sunset West | 42 | 469 | 25 | 8 | 5 | 99 | 0 | 0 | 0 | 0 | 0 | 129 | 38 | 3 | 0 | 11 | 2 | 101 | 4 | 0 | 0 |
| Surfside | 6 | 38 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 8 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| Sweetwater | 8 | 48 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 71 | 10 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Tamiami | 44 | 259 | 42 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 234 | 14 | 0 | 0 | 11 | 0 | 36 | 3 | 0 | 0 |
| Tamiami - Lindgren | 41 | 225 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 211 | 45 | 0 | 0 | 0 | 0 | 17 | 2 | 0 | 0 |
| Transitional Area | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| University | 15 | 161 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 102 | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 |
| Venetian Islands | 44 | 664 | 94 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 184 | 4 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| West Ave | 2 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 |
| West Cutler Area | 13 | 17 | 14 | 0 | 0 | 18 | 0 | 0 | 0 | 0 | 0 | 149 | 15 | 0 | 0 | 0 | 0 | 16 | 0 | 0 | 0 |
| West Flagler | 20 | 179 | 78 | 2 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 82 | 35 | 0 | 0 | 0 | 0 | 22 | 0 | 0 | 0 |
| West Goulds | 4 | 19 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 7 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | 0 |
| West Homestead | 11 | 83 | 13 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 138 | 4 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| West Kendall | 19 | 38 | 7 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 58 | 9 | 0 | 0 | 8 | 0 | 85 | 0 | 0 | 0 |
| West Lake Lucerne | 5 | 23 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 21 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 |
| West Little River | 26 | 408 | 26 | 30 | 0 | 12 | 1 | 0 | 0 | 0 | 0 | 42 | 6 | 0 | 0 | 0 | 1 | 25 | 2 | 0 | 0 |
| West Miami | 4 | 72 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| West Miami Lakes | 38 | 233 | 20 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 92 | 2 | 15 | 0 | 21 | 0 | 103 | 1 | 0 | 0 |
| West Miami Shores | 22 | 93 | 10 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 79 | 4 | 0 | 0 | 26 | 0 | 59 | 4 | 0 | 0 |
| West North Miami | 11 | 100 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 194 | 6 | 0 | 0 | 15 | 0 | 37 | 0 | 0 | 0 |
| West Quail Roost | 23 | 260 | 54 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 204 | 6 | 0 | 0 | 10 | 0 | 0 | 3 | 0 | 0 |
| West South Miami | 13 | 117 | 13 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 18 | 7 | 6 | 0 | 4 | 0 | 1 | 3 | 0 | 0 |
| West South Miami City | 13 | 27 | 1 | 0 | 1 | 97 | 0 | 0 | 0 | 0 | 0 | 60 | 2 | 0 | 0 | 13 | 0 | 43 | 1 | 0 | 0 |
| West Sweetwater | 22 | 57 | 5 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 68 | 21 | 0 | 0 | 0 | 0 | 21 | 1 | 0 | 0 |
| West Tamiami | 18 | 116 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 52 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Westchester | 68 | 365 | 135 | 0 | 0 | 130 | 0 | 1 | 0 | 0 | 0 | 153 | 15 | 0 | 1 | 19 | 0 | 48 | 0 | 0 | 0 |
| Westview | 14 | 63 | 7 | 1 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 221 | 2 | 3 | 0 | 0 | 1 | 0 | 4 | 0 | 0 |
| Westwood Lakes | 21 | 68 | 3 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 115 | 6 | 3 | 0 | 1 | 0 | 37 | 0 | 0 | 0 |
| Wynwood | 33 | 421 | 28 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 170 | 42 | 0 | 1 | 3 | 0 | 16 | 2 | 0 | 0 |
| Total | 2,488 | 19206 | 2997 | 325 | 65 | 1736 | 19 | 212 | 4 | 13 | 0 | 14358 | 1193 | 174 | 3 | 873 | 129 | 3054 | 236 | 2 | 0 |
L = larvae; P = pupae.
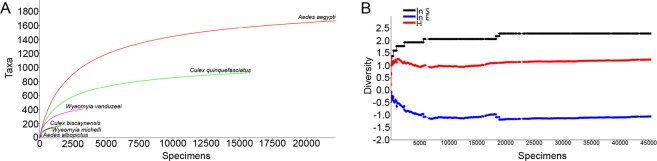
Based on the totality of collected mosquitoes, the individual rarefaction curves resulted in moderately high asymptotic curves for Ae. aegypti and Cx. quinquefasciatus with high degree of confidence for predicting the expected presence of those species for smaller samples. The cumulative SHE profiles indices reached stability after a short period of initial variation and yielded relatively low values for the Ln S, Ln E and H. These results are indicating an uneven mosquito assembly with low diversity and reduced richness of species in the urbanized areas of Miami (Fig. 2).
Figure 2.
Biodiversity indices for the immature mosquitoes collected in Miami-Dade County, Florida from April 2018 to June 2019. (A) Individual rarefaction curves (Y-axis = number of species; X-axis = number of specimens); (B) Plots of cumulative SHE profiles (ln S, H and ln E). (Y-axis = diversity values for log abundance, Shannon index and log evenness; X-axis = number of specimens).
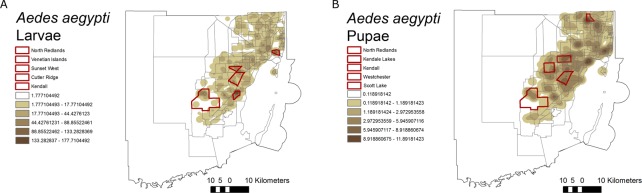
Aedes aegypti was the most abundant and widespread mosquito species in Miami-Dade County. From the 141 neighborhoods surveyed in this study, Ae. aegypti larvae were found in 138 neighborhoods and pupae in 127 neighborhoods. However, Ae. aegypti were more concentrated in specific neighborhoods, and only in six were more than 500 specimens collected: Cutler Ridge, 1,386 larvae and 25 pupae; North Redlands, 1,103 larvae and 118 pupae; Kendall, 804 larvae and 160 pupae; Venetian Islands 664 larvae and 94 pupae; and Kendale Lakes, 430 larvae and 103 pupae (Fig. 3).
Figure 3.
Heat map based on the relative abundance of Aedes aegypti larvae (A) and pupae (B) in Miami-Dade County, Florida. Highlighted in red are the neighborhoods with the highest abundance of Ae. aegypti. The figure was produced using ArcGIS 10.2 (Esri, Redlands, CA), using freely available layers from the Miami-Dade County’s Open Data Hub— https://gis-mdc.opendata.arcgis.com/.
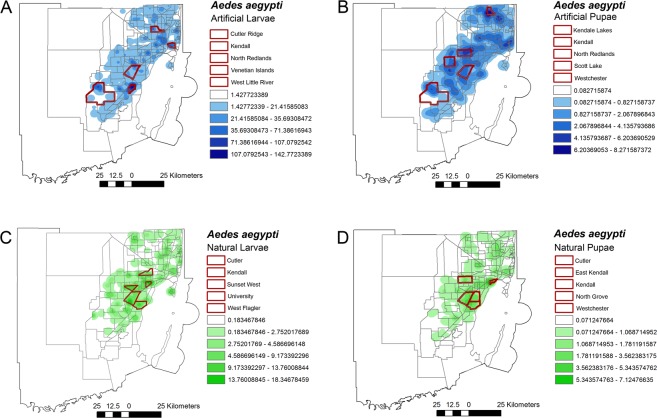
Immature forms of Ae. aegypti were more abundantly found in artificial breeding sites than natural. A total of 15,701 larvae and 2,044 pupae were collected in artificial aquatic habitats while only 2,703 larvae and 850 pupae were collected in natural habitats. Interestingly, the most productive neighborhoods differed according to natural and artificial habitats, but in Kendall a high abundance of Ae. aegypti was shown in both natural and artificial habitats (Fig. 4).
Figure 4.
Heat map based on the relative abundance of Aedes aegypti breeding in natural and artificial habitats in Miami-Dade County, Florida. (A) Larvae and (B) pupae collected in artificial breeding habitats, and (C) Larvae and (D) pupae collected in natural breeding habitats. Highlighted in red are the neighborhoods with the highest abundance of Ae. aegypti. The figure was produced using ArcGIS 10.2 (Esri, Redlands, CA), using freely available layers from the Miami-Dade County’s Open Data Hub— https://gis-mdc.opendata.arcgis.com/.
The most productive aquatic habitats for Ae. aegypti in Miami-Dade County during this study were buckets, bromeliads, and flower pots, representing approximately 38% of all Ae. aegypti collected. The ten most productive breeding sites were responsible for approximately 67% of collected Ae. aegypti (Table 2).
Table 2.
Most productive breeding sites for Aedes aegypti in Miami-Dade County, Florida.
| Breeding Habitats | Larvae | Pupae | Total |
|---|---|---|---|
| Bucket | 2,804 | 335 | 3139 |
| Bromeliad | 2,206 | 701 | 2907 |
| Flower Pot | 2,203 | 294 | 2497 |
| Tire | 1,901 | 165 | 2066 |
| Fountain | 1,050 | 141 | 1191 |
| Plastic Container | 1,015 | 77 | 1092 |
| Storm Drain | 401 | 273 | 674 |
| Planter | 508 | 74 | 582 |
| Bird bath | 353 | 48 | 401 |
| Pot | 298 | 60 | 358 |
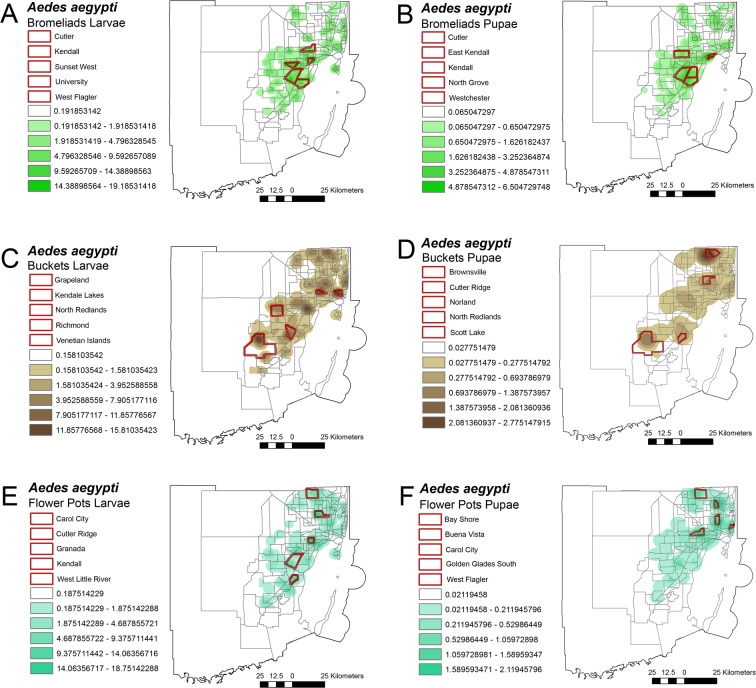
The three aquatic habitats in which Ae. aegypti was most abundantly found are common throughout Miami-Dade County. Bromeliads were responsible for supporting the development of Ae. aegypti in urban areas of Miami. These plants are common in highly urbanized areas and have been correlated with the production of Ae. aegypti larvae and pupae21. The relative abundance of larvae and pupae was moderately dissimilar regarding to point source location being more abundant in different neighborhoods (Fig. 5A,B).
Figure 5.
Most productive Aedes aegypti breeding habitats. (A) larvae and (B) pupae collected in bromeliads; (C) Larvae and (D) pupae collected in buckets and (E) larvae and (F) pupae collected in flower pots. The figure was produced using ArcGIS 10.2 (Esri, Redlands, CA), using freely available layers from the Miami-Dade County’s Open Data Hub— https://gis-mdc.opendata.arcgis.com/.
The geospatial analysis revealed that buckets were found to be present in most neighborhoods directly overlapping with the relative abundance of immature Ae. aegypti. However, apart from the North Redlands the neighborhoods with higher number of larvae (Fig. 5C) were not the ones with the most pupae (Fig. 5D). Flower pots were the third most productive Ae. aegypti aquatic habitat, and apart from Kendall, were not correlated with the presence of bromeliads. Flower pots were found in highly urbanized areas such as Granada and as well as suburban areas such as Cutler Ridge (Fig. 5E,F).
Discussion
Modifications of the natural environment alter the interactions between vector, host, and pathogen, which ultimately affects the epidemiology of vector-borne diseases24. These diseases are dependent on the natural environment, and environmental changes such as climate change, urbanization, and loss of biodiversity increase the risk of arbovirus transmission for the human population6,18,25–29. Aedes aegypti is the primary vector of DENV, ZIKV, and CHIKV and is well adapted to the urban environment of Miami-Dade, being present year-round18. Previous studies showed that this species is able to thrive in extreme urban environments such as construction sites and tire shops with limited sugar sources and few host species other than humans20,23. Our results show that mosquito vector species can be found in a wide range of aquatic habitats in the urban environments of Miami-Dade County. In these urban settings, practically any object that can hold water, from a deflated basketball to a Jet Ski or a storm drain, is a potential breeding site for vector mosquitoes.
Immature mosquitoes are widely distributed across Miami-Dade County and Ae. aegypti was by far the most common and abundant species, comprising 55.8% of all specimens collected during the timeframe of the study. The remaining seven species collected represent a much smaller proportion of the overall mosquito makeup of Miami-Dade County. Furthermore, larvae from the eight different species that were found, but only Cx. quinquefasciatus and Ae. aegypti were commonly found in the form of pupae, indicating that these species are more widespread in urban aquatic habitats than the remaining species found in this study.
No Cx. nigripalpus pupae were found in urban aquatic habitats indicating that this species may not be able to utilize these habitats successfully. Our findings are in agreement with previous findings in which immature Cx. nigripalpus specimens were not found breeding in aquatic habitats in urban environments in Miami20,21,23. Furthermore, adult Cx. nigripalpus are commonly collected in the edge of the incorporated areas of Miami but are rarely found in urban areas18. Therefore, immature Cx. nigripalpus collected in this study may be the result of specimens migrating from rural areas to urban areas but were unable to survive the harsh conditions of urban habitats.
Aedes aegypti was found in relatively high numbers throughout Miami-Dade County successfully breeding in aquatic habitats in diverse urban environments. However, it was primarily found in certain types of breeding habitats, responsible for supporting the development of Ae. aegypti, concentrated in the specific neighborhoods of Cutler Ridge, North Redlands, Kendall, Wynwood, and the Venetian Islands. The three most productive breeding sites for Ae. aegypti, in terms of numbers of immature mosquitoes produces, including buckets, bromeliads, and flower pots. Our results showed a clear correlation between the availability of breeding sites and the abundance of Ae. aegypti in these top five neighborhoods.
Not surprisingly, similar hotspots were discovered for both larvae and pupae, and these areas are where targeted mosquito control efforts should be most heavily implemented. Among these aquatic habitats responsible for driving the relative abundance of vector mosquitoes, special attention should be given to ornamental bromeliads. They have become an important breeding site for Ae. aegypti representing a challenge for vector mosquito control strategies in urban environments21,30.
Aedes aegypti’s opportunistic behavior allows it to utilize a wide range of breeding sites, both within the natural and artificial realm. It is clear from our larval surveillance data that more immatures were collected in artificial aquatic habitats than natural habitats, yet there are clear differences between the top five neighborhoods for natural and artificial habitats. For larvae found in artificial habitats, the highest densities of immature mosquitoes were found in North Redlands, Wynwood, Venetian Islands, Kendall, and Richmond Heights. However, larvae discovered in natural breeding sites were concentrated (albeit at lower abundances) in southeastern Miami-Dade County in the neighborhoods of Kendall, Cutler, Sunset West, University, and Richmond Heights.
Understanding the most productive breeding sites for Ae. aegypti, and other mosquito vector species, and where they are located within the county, is a powerful tool for targeted mosquito control. The number of immature mosquitoes produced per breeding site could be a useful tool in determining priorities in public health outreach and mosquito control efforts. It is far more desirable to control larvae than adults, and mosquito control practices should not solely prioritize adult control over larval control in order to achieve maximum effectiveness on mosquito control31,32.
However, it is important to understand that neighborhoods that produce mosquitoes from one specific breeding site may not produce many mosquitoes from other breeding sites, and human behavior is a large driver of this phenomenon. For example, it is evident that buckets played a strong role in immature Ae. aegypti abundance in the North Redlands, yet flower pots did not. North Redlands is an unincorporated agricultural area with a small human population, so it is logical that there is a high density of buckets contributing to the large abundance of immature Ae. aegypti mosquitoes, and very few flower pots being utilized as a breeding site.
In terms of bromeliads as a breeding site, it is evident that they play a crucial role in Kendall and the surrounding areas, possibly due to their ornamental nature in private gardens and the accompanying large human population in South Miami-Dade County. Accordingly, bromeliads do not contribute as strongly to North Redlands. This area’s small human population correlates to a lower density of bromeliads in the area, and therefore a minimal correlation between this breeding site and Ae. aegypti abundance. Understanding the connections between the locations of breeding sites in relation to human behavior is key to the development of more effective guided mosquito control strategies.
While Ae. aegypti is widespread throughout the county, its most productive breeding sites are modifiable and easily removed or avoided in urban environments. Buckets and containers can be dumped or turned over, and citizens can be educated on ornamental bromeliads as a potential breeding site. Education and outreach regarding these modifiable urban features could prove a valuable tool to control mosquito populations.
Due to the ability to thrive in urban areas, Ae. aegypti is increasing its presence and abundance worldwide33. The degradation of natural habitats positions the global human population at an overall increased risk for preventable outbreaks, particularly in urban areas, through increasingly severe outbreaks and the emergence of outbreaks in previously non-endemic areas4,17,34. Spread over an area of more than 6 thousand km2 and with more than 3 million residents, Miami-Dade is the most populous and third-largest county in Florida35. Miami’s large and ever-growing population, combined with its aforementioned proximity to endemic areas and appropriate climate for mosquito production year-round, positions the area in a unique situation for a high risk of vector-borne disease transmission and emergence18.
This study serves as a cornerstone for future studies that are needed to identify how vector mosquitoes utilize the resources available in urban environments and to determine the exact role of these specific urban features in supporting populations of vector mosquito species. Ultimately, the identification of modifiable urban features that will lead to the reduction of aquatic habitats for vector mosquitoes will allow the development of targeted mosquito control strategies optimized to preventatively control vector mosquitoes in urban areas.
Methods
Study area
Immature mosquito surveys were conducted in Miami-Dade County, Florida from April 2018 to June 2019, totaling 2,488 inspections. Surveys were requested by citizen complaints through 311 calls, automatically triggering the dispatch of a Mosquito Control inspector to actively search for potential mosquito aquatic habitats within a 50-meter radius from the original point-source location (Fig. 1, Supplementary Fig. S2). The 311 calls represent specific locations where residents deemed they had a serious mosquito problem and needed assistance from the County. Such 311 calls are normal for the State of Florida counties, but the information from site inspections is generally not used to direct mosquito control activities36. In this study, we had inspectors do larval searches from observed breeding habitats.
Collection methods
Immature mosquitoes were collected by inspectors with the aid of manual plastic pumps (turkey basters) and entomological dippers, then stored for transport in plastic containers (100 ml) according to the breeding site where they were collected. All collected immature mosquitoes were transported to the Miami-Dade County Mosquito Control Laboratory. Mosquitoes were identified to species using taxonomic keys based on morphological characters37. Larvae were identified immediately after collection and all pupae were allowed to emerge as adults and then identified.
Since this study posed less than minimal risk to participants and did not involve endangered or protected species the Institutional Review Board at the University of Miami determined that the study was exempt from institutional review board assessment (IRB Protocol Number: 20161212).
Breeding site categorization
Breeding sites were organized into two categories: (i) Category 1 - specific breeding habitat in which the specimens were collected; and (ii) Category 2 - artificial or natural to distinguish between man-made and natural features (Supplementary Table 2)38,39.
Analysis
Biodiversity analyses were performed for all collected mosquitoes using individual rarefaction curves to compare mosquito diversity in samples with different sizes. The individual rarefaction curves were also used to provide an estimation of the number of species in samples with fewer specimens and to evaluate sampling sufficiency. Plots of cumulative profiles of species log abundance (ln S), Shannon index (H) and log evenness (ln E) (SHE) were also calculated for all samples. Samples were successively added to the model in chronologic order to assess variations in mosquito community and composition of species40. Analyses were carried out with 10,000 randomizations without replacement and a 95% confidence interval using Past software (v.3.16)41,42.
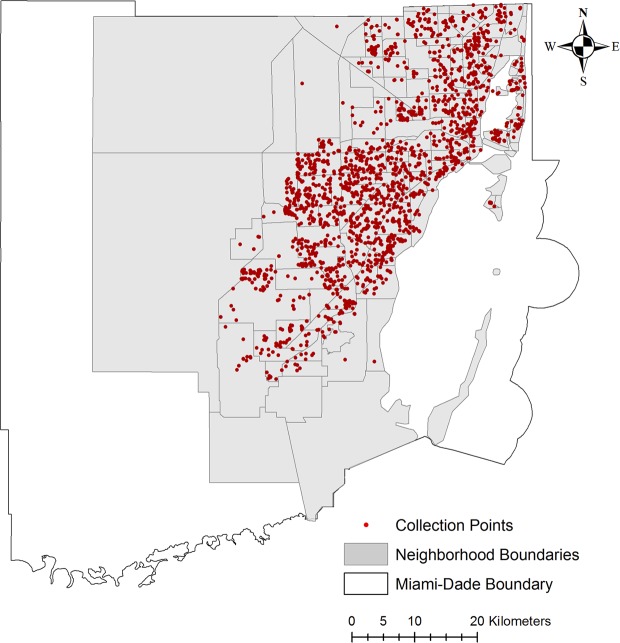
Figures 1, 3–6 were produced using ArcGIS (v.10.2) using maps freely available at www.census.gov and https://gis-mdc.opendata.arcgis.com/. Addresses of breeding sites from survey data were geocoded to map coordinates for consistency and confidentiality.
Figure 6.
Map displaying immature mosquito collection points in Miami-Dade County, Florida. Neighborhoods are displayed in gray and collection points in red. The figure was produced using ArcGIS 10.2 (Esri, Redlands, CA), using freely available layers from the Miami-Dade County’s Open Data Hub— https://gis-mdc.opendata.arcgis.com/.
Supplementary information
Acknowledgements
We would like to thank the staff of Miami-Dade County Mosquito Control Division for their help with field collections. We would also like to thank the residents of Miami-Dade County that graciously allowed us to enter their properties for surveys. This research was supported by CDC (https://www.cdc.gov/) grant 1U01CK000510–03: Southeastern Regional Center of Excellence in Vector-Borne Diseases: The Gateway Program. CDC had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-51787-5.
References
- 1.Rosenberg R, et al. Vital Signs: Trends in reported vector-borne disease cases — United States and Territories, 2004–2016. Morb. Mortal. Wkly. Rep. 2018;67:496–501. doi: 10.15585/mmwr.mm6717e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poletti P, et al. Transmission potential of chikungunya virus and control measures: The case of Italy. PLoS One. 2011;6:e18860. doi: 10.1371/journal.pone.0018860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould EA, Gallian P, De Lamballerie X, Charrel RN. First cases of autochthonous dengue fever and chikungunya fever in France: From bad dream to reality! Clin. Microbiol. Infect. 2010;16:1702–1704. doi: 10.1111/j.1469-0691.2010.03386.x. [DOI] [PubMed] [Google Scholar]
- 4.Gjenero-Margan I, et al. Autochthonous dengue fever in Croatia, August-September 2010. Euro Surveill. 2011;16:1–4. [PubMed] [Google Scholar]
- 5.Gould E, Pettersson J, Higgs S, Charrel R, de Lamballerie X. Emerging arboviruses: Why today? One Heal. 2017;4:1–13. doi: 10.1016/j.onehlt.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson MTJ, Munshi-South J. Evolution of life in urban environments. Science. 2017;358:eaam8327. doi: 10.1126/science.aam8327. [DOI] [PubMed] [Google Scholar]
- 7.Reba M, Reitsma F, Seto KC. Spatializing 6,000 years of global urbanization from 3700 BC to AD 2000. Sci. Data. 2016;3:1–16. doi: 10.1038/sdata.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia P, et al. How does the dengue vector mosquito Aedes albopictus respond to global warming? Parasit. Vectors. 2017;10:140. doi: 10.1186/s13071-017-2071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadee DD, Martinez R. Acta Tropica Aedes aegypti (L.) in Latin American and Caribbean region: With growing evidence for vector adaptation to climate change? Acta Trop. 2016;156:137–143. doi: 10.1016/j.actatropica.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Danauskas JX, Ehrenkranz NJ, Davies JE, Pond WL. Arboviruses and human disease in South Florida. Am. J. Trop. Med. Hyg. 1966;15:205–210. doi: 10.4269/ajtmh.1966.15.205. [DOI] [PubMed] [Google Scholar]
- 11.Gill J, Stark LM, Clark GG. Dengue surveillance in Florida, 1997–98. Emerg Infect Dis. 2000;6:30–35. doi: 10.3201/eid0601.000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rey J. Dengue in Florida (USA) Insects. 2014;5:991–1000. doi: 10.3390/insects5040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitek CJ, Richards SL, Mores CN, Day JF, Lord CC. Arbovirus transmission by Culex nigripalpus in Florida, 2005. J. Med. Entomol. 2008;45:483–93. doi: 10.1093/jmedent/45.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messenger AM, et al. Serological evidence of ongoing transmission of dengue virus in permanent residents of Key West, Florida. Vector Borne Zoonotic Dis. 2014;14:783–787. doi: 10.1089/vbz.2014.1665. [DOI] [PubMed] [Google Scholar]
- 15.Patterson KD. Yellow fever epidemics and mortality in the United States, 1693–1905. Soc. Sci. Med. 1992;34:855–865. doi: 10.1016/0277-9536(92)90255-O. [DOI] [PubMed] [Google Scholar]
- 16.Likos A, et al. Local mosquito-borne transmission of Zika Virus — Miami-Dade and Broward Counties, Florida, June–August 2016. Morb. Mortal. Wkly. Rep. 2016;65:1032–1038. doi: 10.15585/mmwr.mm6538e1. [DOI] [PubMed] [Google Scholar]
- 17.Grubaugh ND, et al. Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature. 2017;546:401–405. doi: 10.1038/nature22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilke ABB, et al. Community composition and year-round abundance of vector species of mosquitoes make Miami-Dade County, Florida a receptive gateway for arbovirus entry to the United States. Sci. Rep. 2019;9:8732. doi: 10.1038/s41598-019-45337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miami-Dade County Building Permits. Available at, http://www.miamidade.gov/permits/.
- 20.Wilke ABB, Vasquez C, Petrie W, Caban-Martinez AJ, Beier JC. Construction sites in Miami-Dade County, Florida are highly favorable environments for vector mosquitoes. PLoS One. 2018;13:e0209625. doi: 10.1371/journal.pone.0209625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilke ABB, Vasquez C, Mauriello PJ, Beier JC. Ornamental bromeliads of Miami-Dade County, Florida are important breeding sites for Aedes aegypti (Diptera: Culicidae) Parasit. Vectors. 2018;11:283. doi: 10.1186/s13071-018-2866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilke ABB, et al. Mosquito adaptation to the extreme habitats of urban construction sites. Trends Parasitol. 2019;35:607–614. doi: 10.1016/j.pt.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Wilke ABB, Vasquez C, Petrie W, Beier JC. Tire shops in Miami-Dade County, Florida are important producers of vector mosquitoes. PLoS One. 2019;14:e0217177. doi: 10.1371/journal.pone.0217177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilke ABB, Beier JC, Benelli G. Complexity of the relationship between global warming and urbanization – an obscure future for predicting increases in vector-borne infectious diseases. Curr. Opin. Insect Sci. 2019;35:1–9. doi: 10.1016/j.cois.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Ceretti-Júnior W, et al. Mosquito faunal survey in a central park of the city of São Paulo, Brazil. J. Am. Mosq. Control Assoc. 2015;31:172–176. doi: 10.2987/14-6457R. [DOI] [PubMed] [Google Scholar]
- 26.Wilke ABB, Wilk-da-Silva R, Marrelli MT. Microgeographic population structuring of Aedes aegypti (Diptera: Culicidae) PLoS One. 2017;12:e0185150. doi: 10.1371/journal.pone.0185150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gubler DJ. Dengue, urbanization and globalization: The unholy trinity of the 21st Century. Trop. Med. Health. 2011;39:S3–S11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zohdy S, Schwartz TS, Oaks JR. The coevolution effect as a driver of spillover. Trends Parasitol. 2019;35:399–408. doi: 10.1016/j.pt.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Rochlin I, Faraji A, Ninivaggi DV, Barker CM, Kilpatrick AM. Anthropogenic impacts on mosquito populations in North America over the past century. Nat. Commun. 2016;7:13604. doi: 10.1038/ncomms13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell JR, Tabachnick WJ. History of domestication and spread of Aedes aegypti - A Review. Mem. Inst. Oswaldo Cruz. 2013;108:11–17. doi: 10.1590/0074-0276130395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiner RC, et al. Quantifying the epidemiological impact of vector control on dengue. PLoS Negl. Trop. Dis. 2016;10:1–11. doi: 10.1371/journal.pntd.0004588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Achee NL, et al. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl. Trop. Dis. 2019;13:e0006822. doi: 10.1371/journal.pntd.0006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraemer MUG, et al. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci. Data. 2015;2:150035. doi: 10.1038/sdata.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vora N. Impact of anthropogenic environmental alterations on vector-borne diseases. Medscape J. Med. 2008;10:238. [PMC free article] [PubMed] [Google Scholar]
- 35.United States Census Bureau. Income and Poverty in the United States 2016. Available at, https://www.census.gov/topics/income-poverty/income.html.
- 36.United States Department of Justice. Building a 311 system: A case study of the Orange County, Florida, Government Service Center. Available at, https://ric-zai-inc.com/Publications/cops-w0447-pub.pdf (2007).
- 37.Darsie, R. F. Jr. & Morris, C. D. Keys to the adult females and fourth-instar larvae of the mosquitoes of Florida (Diptera, Culicidae). 1st ed. Vol. 1. Tech Bull Florida Mosq Cont Assoc (2000).
- 38.Islam S, Haque CE, Hossain S, Rochon K. Role of container type, behavioural, and ecological factors in Aedes pupal production in Dhaka, Bangladesh: An application of zero-inflated negative binomial model. Acta Trop. 2019;193:50–59. doi: 10.1016/j.actatropica.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Troyo A, et al. Seasonal profiles of Aedes aegypti (Diptera: Culicidae) larval habitats in an urban area of Costa Rica with a history of mosquito control. J. Vector Ecol. 2008;33:76–88. doi: 10.3376/1081-1710(2008)33[76:SPOAAD]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buzas MA, Hayek LAC. SHE analysis for biofacies identification. J. Foraminifer. Res. 1998;28:233–239. [Google Scholar]
- 41.Morris EK, et al. Choosing and using diversity indices: Insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 2014;4:3514–3524. doi: 10.1002/ece3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammer Ø, Harper DATT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001;4:9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.