Abstract
Background
Peripheral regional anaesthesia techniques are well established for postoperative pain treatment following knee surgery. The adductor canal block (ACB) is a new technique, which can be applied as a single shot or by catheter for continuous regional analgesia.
Objectives
To compare the analgesic efficacy and adverse events of ACB versus other regional analgesic techniques or systemic analgesic treatment for adults undergoing knee surgery.
Search methods
We searched CENTRAL, MEDLINE, and Embase, five other databases, and one trial register on 19 September 2018; we checked references, searched citations, and contacted study authors to identify additional studies.
Selection criteria
We included all randomized controlled trials (RCTs) comparing single or continuous ACB versus other regional analgesic techniques or systemic analgesic treatment. Inclusion was independent of the technique used (landmarks, peripheral nerve stimulator, or ultrasound) and the level of training of providers.
Data collection and analysis
We used Cochrane’s standard methodological procedures. Our primary outcomes were pain intensity at rest and during movement; rate of accidental falls; and rates of opioid‐related adverse events. We used GRADE to assess the quality of evidence for primary outcomes.
Main results
We included 25 RCTs (1688 participants) in this review (23 trials combined within meta‐analyses). In 18 studies, participants underwent total knee arthroplasty (TKA), whereas seven trials investigated patients undergoing arthroscopic knee surgery. We identified 11 studies awaiting classification and 11 ongoing studies.
We investigated the following comparisons.
ACB versus sham treatment
We included eight trials for this comparison. We found no significant differences in postoperative pain intensity at rest (2 hours: standardized mean difference (SMD) ‐0.56, 95% confidence interval (CI) ‐1.20 to 0.07, 4 trials, 208 participants, low‐quality evidence; 24 hours: SMD ‐0.49, 95% CI ‐1.05 to 0.07, 6 trials, 272 participants, low‐quality evidence) or during movement (2 hours: SMD ‐0.59, 95% CI ‐1.5 to 0.33; 3 trials, 160 participants, very low‐quality evidence; 24 hours: SMD 0.03, 95% CI ‐0.26 to 0.32, 4 trials, 184 participants, low‐quality evidence). Furthermore, they noted no evidence of a difference in postoperative nausea between groups (24 hours: risk ratio (RR) 1.91, 95% CI 0.48 to 7.58, 3 trials, 121 participants, low‐quality evidence). One trial reported that no accidental falls occurred 24 hours postoperatively (low‐quality evidence).
ACB versus femoral nerve block
We included 15 RCTs for this comparison. We found no evidence of a difference in postoperative pain intensity at rest (2 hours: SMD ‐0.74, 95% CI ‐1.76 to 0.28, 5 trials, 298 participants, low‐quality evidence; 24 hours: SMD 0.04, 95% CI ‐0.09 to 0.18, 12 trials, 868 participants, high‐quality evidence) or during movement (2 hours: SMD ‐0.47, 95% CI ‐1.86 to 0.93, 2 trials, 88 participants, very low‐quality evidence; 24 hours: SMD 0.56, 95% CI ‐0.00 to 1.12, 9 trials, 576 participants, very low‐quality evidence). They noted no evidence of a difference in postoperative nausea (24 hours: RR 1.22, 95% CI 0.42 to 3.54, 2 trials, 138 participants, low‐quality evidence) and no evidence that the rate of accidental falls during postoperative care was significantly different between groups (24 hours: RR 0.20, 95% CI 0.04 to 1.15, 3 trials, 172 participants, low‐quality evidence).
Authors' conclusions
We are currently uncertain whether patients treated with ACB suffer from lower pain intensity at rest and during movement, fewer opioid‐related adverse events, and fewer accidental falls during postoperative care compared to patients receiving sham treatment. The same holds true for the comparison of ACB versus femoral nerve block focusing on postoperative pain intensity. The overall evidence level was mostly low or very low, so further research might change the conclusion. The 11 studies awaiting classification and the 11 ongoing studies, once assessed, may alter the conclusions of this review.
Plain language summary
Advantages and problems of a specific nerve block in adults undergoing knee surgery
Background
Postoperative pain following knee surgery continues to be a relevant healthcare problem. Combinations of different analgesics are the best way to treat postoperative pain. One way is to block specific nerves (called regional anaesthesia) that are responsible for pain development. For many years, blocking the femoral nerve, which is responsible for sensation (e.g. pain) and movement of the upper leg, was very important. In recent years, blocking only one specific part of this nerve (called adductor canal block), which does not influence movement of the upper leg, has become more interesting.
Review question
We investigated advantages and problems of the adductor canal block compared to sham treatment (patients received saline instead of drugs) and other regional anaesthesia for postoperative pain treatment in adults undergoing knee surgery.
Study characteristics
We included 25 clinical studies in which people are randomly put into one of two or more treatment groups (called 'randomized controlled trials'), with results reported from a total of 1688 participants (929 females, 759 males). Participants were 29 to 72 years old. Eight trials compared participants receiving adductor canal block against patients receiving saline. A total of 15 RCTs compared adductor canal block versus femoral nerve block. The evidence is current to October 2018. No trial was funded by industry.
Key results
We are uncertain whether patients treated with adductor canal block have lower pain intensity at rest or during movement (e.g. walking) compared with those who received only saline. It is unclear whether rates of adverse events after taking opioids (e.g. nausea) or after accidental falls during postoperative care are lower. It is also uncertain whether patients receiving adductor canal block show different postoperative pain intensity at rest and during movement compared to those treated with femoral nerve block. We noted no differences in adverse events after taking opioids and after accidental falls.
Quality of the evidence
We rated the quality of evidence for many outcomes as low or very low. In contrast, we rated pain at rest (at 24 hours) as high‐quality evidence.
Summary of findings
Background
Description of the condition
Knee surgery (e.g. knee arthroplasty, arthroscopic knee surgery) is very commonly performed in western countries (knee replacement: USA 650,000 (2010); Germany 156,000 (2012)). Major goals following knee surgery include providing sufficient postoperative pain treatment to assist early physical therapy and allowing patients to return early to their physical capacity and to be discharged early from the hospital. Patients suffer from moderate to severe postoperative acute pain (Gerbershagen 2013), and if this pain is insufficiently treated, it might become chronic (Althaus 2014; Pogatzki‐Zahn 2012). Recently published data demonstrate that the incidence of chronic pain in adults undergoing total knee replacement is 10% to 34% after three months to five years on follow‐up pain measurement (Beswick 2012), and around 20% of patients describe moderate to severe sleep disturbances and alterations in quality of life one year after surgery (Grosu 2015). Finally, clear evidence suggests that use of regional analgesia, especially in joint arthroplasty surgery (Guay 2017; Guay 2017a), is associated with superior postoperative outcomes (pulmonary compromise, pneumonia, infection, acute renal failure, mechanical ventilation, blood product transfusion) (Memtsoudis 2013), and it might reduce the risk of chronic postsurgical pain (Weinstein 2018).
Description of the intervention
In recent years, adductor canal block through selective block of sensory nerves has become an interesting new option for postoperative pain treatment following knee surgery. The knee is innervated by the femoral nerve (via three vasti branches and the saphenous nerve), the posterior branch of the obturator nerve, and genicular branches of the tibial and common peroneal branches of the sciatic nerve (Bendtsen 2014a). The adductor canal includes the femoral vessels, the saphenous nerve, a nervous branch to the vastus medialis muscle, and sometimes the posterior branch of the obturator nerve (Bendtsen 2014b). The adductor canal is roofed by continuous fascia starting with the vasoadductor membrane distally (Andersen 2015). Adductor canal block, which is performed most often via ultrasound, can be used as a single shot or as continuous nerve block provided through a catheter.
How the intervention might work
Postoperative pain following knee surgery can be managed with systemic analgesics or regional blockade (neuraxial blockade or peripheral nerve blocks). Neuraxial blocks (e.g. epidural catheters) are used less frequently for postoperative pain treatment following knee surgery; distal peripheral nerve blocks (e.g. femoral nerve blocks) are performed more frequently because they involve lower risk for severe adverse events (e.g. epidural bleeding) (Cozowicz 2015). For a long time, femoral nerve block was the gold standard regional analgesic technique for postoperative pain treatment following knee surgery (Chan 2014). However, adductor canal block might be associated with a lower degree of motor blockade than femoral nerve block, and might provide better conditions for early rehabilitation, quicker return to mobility, and less risk for accidental falls during hospital care compared with femoral nerve block (Mariano 2014). It must be mentioned that two other large studies have indicated that appropriate fall prevention strategies should be used for all hospitalized patients, even those not receiving regional blockade (Johnson 2014), and it is not clear whether regional analgesia definitively increases risk for inpatient falls (Memtsoudis 2014). After the femoral vessels have been identified, the saphenous nerve might be blocked typically at two locations: subsartorially, or more distally within the adductor canal. Cadaveric studies have demonstrated that dye is normally spread freely into the adductor canal after a subsartorial injection, so that the primary injection site might not be clinically relevant for clinical efficacy (Cowlishaw 2015; Tubbs 2007). Several cadaveric studies have revealed that a small amount of dye spreads to other nerves as well (e.g. sciatic, femoral), so that possible motor blockade cannot be definitively excluded (Andersen 2015; Cowlishaw 2015; Gautier 2015).
Why it is important to do this review
In patients undergoing knee surgery, femoral nerve block (and epidural catheter for special cases such as bilateral knee arthroplasty) is believed to be the gold standard for acute pain management because it provides better analgesia than is provided by systemic analgesic treatment for adults undergoing knee surgery (Chan 2014). However, this block might be associated with a higher degree of motor blockade, possibly increasing the risk for inpatient falls (Johnson 2013; Wasserstein 2013). As has been mentioned, evidence regarding use of femoral nerve block and risk for inpatient falls is currently inconclusive. Many RCTs published in recent years have compared analgesic efficacy and safety between adductor canal block and other regional analgesic techniques (particularly femoral nerve block). A quantitative systematic review has not been conducted to analyse analgesic efficacy and adverse effects of adductor canal block compared with other regional analgesic techniques or systemic analgesic treatment for patients undergoing knee surgery.
Objectives
To compare the analgesic efficacy and adverse events of adductor canal block versus other regional analgesic techniques or systemic analgesic treatment for adults undergoing knee surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) investigating adductor canal block in comparison with other regional analgesic techniques or systemic analgesic treatment. Cluster RCTs, cross‐over RCTs, and quasi‐RCTs were not included.
Types of participants
We included all adults (≥ 18 years old) undergoing knee surgery, irrespective of sex or type of surgery.
Types of interventions
We included all RCTs comparing single or continuous adductor canal block versus sham treatment (patients received saline instead of local anaesthetics), single or continuous femoral nerve block, or any other regional anaesthetic technique. Inclusion was independent of the technique used (landmarks, peripheral nerve stimulator, or ultrasound) and the level of training of providers.
Types of outcome measures
Primary outcomes
Mean difference in postoperative pain at rest/during movement (2 hours (within the postoperative care unit), 24 hours, 48 hours)
Rates of opioid‐related adverse events (nausea, vomiting, postoperative nausea and vomiting, pruritus, respiratory depression, sedation (2 hours (within the postoperative care unit), 24 hours, 48 hours))
Rate of accidental falls during postoperative care
Secondary outcomes
Cumulative mean morphine requirement (2 hours (within the postoperative care unit), 24 hours, 48 hours)
Degree of quadriceps muscle strength (2 hours (within the postoperative care unit), 24 hours, 48 hours)
Rate of chronic postsurgical pain (after 3 months, 6 months, 1 year)
Rates of block‐related adverse events (accidental vascular puncture, paraesthesia, motor blockade, failed block, neurological impairment)
We applied no restrictions regarding the scales that were used to measure pain and quadriceps muscle strength.
Search methods for identification of studies
Electronic searches
We searched for studies through systematic and sensitive search strategies, as described in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 6 (Higgins 2011). We applied no language, publication year, or publication status restrictions. We searched the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL) (2018, Issue 8), in the Cochrane Library.
MEDLINE (Ovid SP, 1946 to 19 September 2018).
Embase (Ovid SP, 1974 to 19 September 2018).
Web of Science (1945 to 19 September 2018).
We developed a subject‐specific search strategy for MEDLINE and modified it appropriately for the other databases. When appropriate, we used the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials, as described in theCochrane Handbook for Systematic Reviews of Interventions, Chapter 6 (Lefebvre 2011). Search strategies can be found in Appendix 1, Appendix 2, Appendix 3, and Appendix 4. Searches were last run 19 September 2018.
Searching other resources
We checked the bibliographic references and citations of relevant studies and reviews for further references to trials. We searched ClinicalTrials.gov (www.clinicaltrials.gov), along with the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/), for unpublished and ongoing studies; Open Grey for grey literature (http://opengrey.eu/); and Google Scholar for additional trials (25 February 2018). When necessary, we contacted trial authors for additional information. We developed the search strategy in consultation with the Information Specialist.
Data collection and analysis
Three review authors (AS, CMF, SR) independently scanned article titles to exclude irrelevant studies.
Selection of studies
The same three review authors (AS, CMF, SR) identified studies that might be included in this review. We applied no restrictions according to publication type or language. If we encountered disagreements, we consulted a third review author (EPZ) and resolved all differences by discussion. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), as well as a Characteristics of excluded studies table.
Data extraction and management
Four review authors (AS, SR, CMF, SW) independently extracted data using a standardized data extraction form developed by the review authors. If necessary, we tried to receive missing data by contacting the leading authors of relevant articles. At each step of data extraction, we resolved differences by discussion within the group of review authors.
Assessment of risk of bias in included studies
Two review authors (CMF, SR) independently assessed risk of bias of included studies by using the Cochrane tool for assessing risk of bias (Review Manager 2014). Standard components of domains included adequacy of allocation generation (random sequence generation (e.g. computer‐generated table)); allocation concealment (e.g. SNOSE (sequentially numbered opaque sealed envelopes)); blinding of participants, personnel dealing directly with participants, and outcome assessors; completeness of outcome data (e.g. no missing outcome data, description of reasons for missing data); possible selected outcome reporting (reporting of primary outcome data (at least postoperative pain scores)); and any other potential sources of bias (e.g. extreme baseline imbalance). We assessed every component as having 'low risk' of bias, 'high risk' of bias, or 'unclear risk' of bias. Within the current review, we have provided a 'Risk of bias' graph as part of the Characteristics of included studies table and a 'Risk of bias' summary figure, which summarize risk of bias assessments for all included studies. Both responsible review authors resolved disagreements by discussion with a third review author (AS).
Measures of treatment effect
For proportions (dichotomous outcomes), we calculated the risk ratio (RR) with 95% confidence interval (CI), and for continuous data, we estimated the mean difference (MD) with 95% CI. For the outcome 'postoperative pain', we used the standardized mean difference (SMD) as a summary statistic in meta‐analysis because we did not transform results based on a numerical rating scale (NRS) or a visual analogue scale (VAS). For the outcome 'cumulative postoperative morphine consumption', we converted all reported opioids into intravenous morphine equivalents by using an opioid conversion table (http://opioidcalculator.practicalpainmanagement.com/). To estimate the statistical significance of these results, we calculated the 95% CI for each item. Furthermore, we assessed the number needed to treat for an additional beneficial outcome (NNTB) for efficacy outcomes, and the number needed to treat for an additional harmful outcome (NNTH) for adverse events, if enough trials could be pooled (> 4 trials per outcome).
We considered a difference of 10% (increase or decrease) as the minimum clinically relevant difference, but for rare outcomes such as inpatient falls, we assumed that a difference of 1% was clinically relevant. For SMDs, we considered 0.2 a small effect, 0.5 a medium effect, and > 0.8 a large effect (Pace 2011).
The protocol reports a plan to perform a trial sequential analysis (TSA) to calculate the required information size (IS; number of participants needed in a meta‐analysis to detect or reject a certain intervention effect) and the sequential monitoring boundaries (testing for statistical significance before the IS has been reached) for primary dichotomous outcomes (rates of opioid‐related adverse events, rate of accidental falls). Both the IS and the monitoring boundaries provide information relevant to estimation of the level of evidence for the experimental intervention, as cumulative meta‐analyses are at risk of producing type I errors as a result of sparse data and repetitive testing of accumulating data (Brok 2008; Thorlund 2009; Wetterslev 2008; Wetterslev 2009). Given that all dichotomous outcomes of this review included only a small number of participants (< 400 participants) and estimated effects included the line of no effect in all cases, TSA does not provide any new information. We downgraded results for all dichotomous outcomes for imprecision by one level.
For the primary continuous outcome of pain (summary statistic: SMD), we calculated the optimal information size (OIS), which is similar to a sample size calculation for an individual trial, if more than 200 participants were included for that outcome (Brant 2005).
Dealing with missing data
If we identified missing data (patient dropouts, selective outcome reporting), we contacted relevant study authors to request further information. We performed sensitivity analyses focused on the possible influence of these missing data by inputting missing data as 'best case' or 'worst case' scenarios, if these data were rated as relevant. If missing data were randomly distributed between experimental and control groups, we included in the meta‐analysis only data on participants with known results. Finally, we analysed the possible influence of studies with incomplete outcome reporting within a sensitivity analysis. We calculated missing standard deviations from standard errors or CIs, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If data were reported as median values with interquartile ranges, we assumed that the median was very similar to the mean when the distribution of data was symmetrical, and we used the median directly in the meta‐analysis and calculated the standard deviation from the interquartile range in accordance with Higgins 2011. We did not pool asymmetrical data for meta‐analysis.
Assessment of heterogeneity
We assessed clinical and methodological differences within included studies to decide whether studies were sufficiently homogeneous to be combined. Within subgroup analyses, we analysed the influence of clinical heterogeneity according to surgery (total knee replacement vs arthroscopic knee surgery), local anaesthetic dose, continuous versus single shot technique, and location of the adductor canal block (proximal vs distal). According to Higgins 2011, we performed subgroup analyses only if more than 10 trials were included for this outcome. We reported statistical heterogeneity using the I² statistic. We calculated this value for each of the outcomes listed above and assessed the extent of heterogeneity as low (< 25%), moderate (25% to 50%), or high (> 75%), depending on the value of the I² statistic (Higgins 2003).
Assessment of reporting biases
We created funnel plots for outcomes including more than 10 trials by plotting effect estimates of included trials versus their precision (inverse of the standard error of the point estimate). We used these plots only as a guiding technique or to detect possible reporting bias and small‐study effects. If asymmetry was suggested by visual assessment, we investigated by performing exploratory analyses (e.g. Arcsine test for binary data, Egger´s test for continuous data). To adjust for small‐study effects, we used Duval and Tweedie's trim and fill method. We performed all statistical tests for publication bias by using R software (R package: meta, metasens).
Data synthesis
For dichotomous data, we used the Mantel‐Haenszel method, and for continuous data, we used the inverse variance method in Review Manager 2014. We used the fixed‐effect model for meta‐analysis when it was reasonable to assume that studies were estimating the same underlying treatment effect (i.e. when trials were examining the same intervention, and trial populations and methods were judged sufficiently similar). When clinical heterogeneity was sufficient to suggest that underlying treatment effects differed between trials, or when we detected substantial statistical heterogeneity (> 50%), we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. However, after taking into account that study weights were more balanced under the random‐effects than the fixed‐effect model (assigning large studies less relative weight and small studies more relative weight), we reported summary statistics in conjunction with results of a sensitivity analysis (obtained via both models).
Computational problems can occur when no events are observed in one or both groups in an individual study (Cochrane Handbook for Systematic Reviews of Interventions, Section 16.9.2) (Higgins 2011). RevMan ignores zero/zero event trials and uses a constant continuity correction of 0.5 for studies with zero events in one arm. Excluding such trial data potentially creates the risk of inflating the magnitude of the pooled treatment effect. We included zero total event trials to take into account the sample sizes of these studies. To assess the robustness of estimated treatment effects, we will perform alternative non‐fixed zero‐cell corrections that have been explored by Sweeting and colleagues, including a correction proportionate to the reciprocal of the size of the contrasting study arm, which these investigators found preferable to the fixed 0.5 correction when arm sizes were not balanced (Sweeting 2004). We performed different types of continuity corrections using TSA software v0.9 Beta (Thorlund 2011), and we have presented these corrections in a sensitivity analysis.
We reported summary RRs, MDs, and SMDs along with 95% CIs. We considered RRs, with the range of lower and upper bounds of the 95% CI not crossing one, and MDs, respectively, as well as SMDs with the range of lower and upper bounds of the 95% CI not crossing zero, to be statistically significant (P < 0.05).
Subgroup analysis and investigation of heterogeneity
We investigated the influence of clinical and methodological heterogeneity. We performed subgroup analyses to calculate RR, MD, or SMD in conjunction with corresponding CI for each subgroup, if heterogeneity exceeds 50%. We used a random‐effects model Chi² test of heterogeneity to compare subgroups. Additionally, we considered non‐overlapping subgroup CIs as consistent with a statistically significant difference.
We analysed data pertaining to the following subgroups, if available.
Type of surgery (total knee replacement vs arthroscopic knee surgery).
Type of local anaesthetic (long‐ vs short‐lasting vs mixture of local anaesthetics).
Continuous versus single shot regional analgesia.
Location of adductor canal block (proximal vs distal).
Type of anaesthesia technique (general anaesthesia, neuraxial anaesthesia).
Use of perioperative non‐opioid analgesics.
Use of sciatic nerve block.
Sensitivity analysis
We performed sensitivity analyses focused on the following issues.
Influence of study quality, by excluding trials assessed as having high risk of bias for random sequence generation/allocation concealment and blinding.
Influence of incomplete outcome data reporting, by inputting missing participants in 'best case' versus 'worst case' scenarios.
Effect estimate under the fixed‐effect model.
Influence of inclusion of randomized trials with zero events.
'Summary of findings' table and GRADE
We used the GRADE approach to rate the quality of evidence and the grading strength of recommendations in healthcare associated with the following (primary) outcomes in our review (Guyatt 2011a; Guyatt 2011b).
Mean difference in postoperative pain.
Rates of opioid‐related adverse events.
Rate of accidental falls during postoperative care.
We constructed 'Summary of findings' tables using GRADE software (www.gradepro.org). Through the GRADE approach, we appraised the quality of evidence on the basis of the extent to which one can be confident that the estimate of effect reflects the item assessed. The quality of the body of evidence reflects within‐study risk of bias (methodological quality), indirectness, heterogeneity of the data (inconsistency), imprecision of effect estimates, risk of publication bias, and magnitude of effect.
For risk of bias, we judged the quality of evidence as adequate when most information was derived from studies at low risk of bias; we downgraded the quality by one level when most information was provided by studies at high or unclear risk of bias; and we downgraded the quality by two levels when the proportion of data from studies at high risk of bias was sufficient to affect interpretation of results (sensitivity analysis) (Guyatt 2011c).
For inconsistency, we downgraded the quality of evidence by one level when the I² statistic was 50% or higher without satisfactory explanation (subgroup analysis), and by two levels when the I² statistic was 75% or higher with no explanation (Guyatt 2011c).
We judged the quality of evidence for indirectness as adequate if outcome data were based on direct comparisons of interest, on the population of interest, and on the outcome of interest (not surrogate markers) (Guyatt 2011d).
If the 95% CI excluded a risk ratio of 1.0 or an SMD of 0.0, and the total number of participants exceeded the IS (RR) or OIS (SMD) criterion, precision was adequate (Guyatt 2011e); we did not downgrade if the 95% CI was narrow and included a risk ratio of 1.0 or an SMD of 0.0 (no appreciable difference between treatments), or if the total number of participants exceeded the IS or OIS criterion. We downgraded the quality of evidence for imprecision by one level when the confidence interval around the effect size was large or overlapped an absence of effect and failed to exclude an important benefit or harm, and when the number of participants was smaller than the required information size (IS or OIS), or the monitoring boundaries were not crossed (see TSA). We generally downgraded the evidence by one level if fewer than 400 patients were included for dichotomous outcomes and if 200 patients were included for continuous outcomes.
For publication bias (Guyatt 2011f), we downgraded the quality of evidence by one level if the statistical test for funnel plot asymmetry suggested publication bias, and if the adjustment for small‐study effects as assessed by Duval and Tweedie’s fill and trim analysis changed the conclusion. We downgraded the level of evidence for publication bias by two levels if most trials were small and were industry sponsored.
The GRADE assessment resulted in one of four levels of 'quality'; these expressed our confidence in the estimate of effect (Balshem 2011).
High: further research is very unlikely to change our confidence in the estimate of effect.
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: any estimate of effect is very uncertain.
Results
Description of studies
Results of the search
We identified 846 related articles by searching electronic databases (Figure 1). After reviewing the titles, we selected 55 articles for abstract review, of which we excluded 19 articles and determined that 11 trials were very new trials currently awaiting assessment. Finally, 25 studies including 1688 participants met the inclusion criteria of this review. All studies were RCTs using a parallel group design. One group selected additionally a cross‐over design ‐ Memtsoudis 2015 (see Characteristics of included studies table).
1.
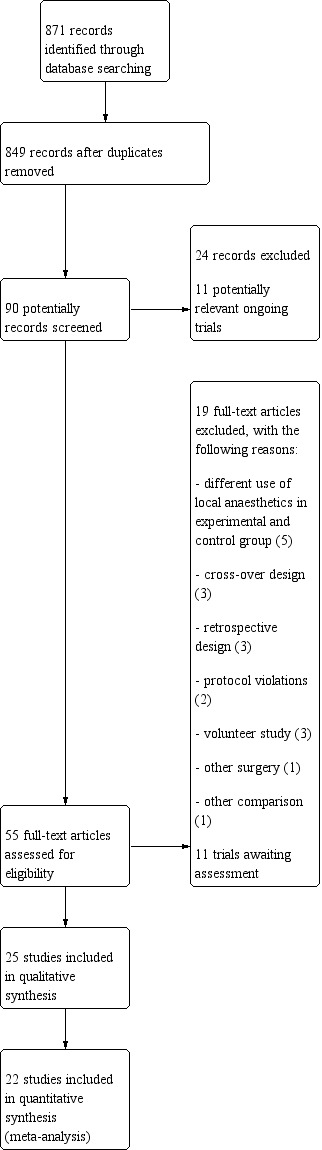
Study flow diagram.
Included studies
We included 25 RCTs. Please see the Characteristics of included studies tables for details.
Support
The RCTs were published between 2008 and 2017. Four RCTs were funded by a charitable organization, and nine by departmental resources. Four studies received no financial support. The remaining trials did not specify the source of funding.
Setting
The 25 included studies were performed in Canada (n = 3), China (n = 3), Egypt (n = 2), Denmark (n = 6), Germany (n = 1), India (n = 1), Iran (n = 1), Korea (n = 1), USA (n = 6), and Turkey (n = 1).
Study population
The number of participants in these studies varied from 30 to 159. Studies included significantly more female adults (females n = 929 vs males n = 759). In most studies, adductor canal block was performed in participants undergoing total knee arthroplasty (TKA) (18/25 studies). Only seven trials used block in patients scheduled for arthroscopic knee surgery (Abdallah 2016; Akkaya 2008; Espelund 2013; Espelund 2014a; Hanson 2013; Messeha 2016; Rahimzadeh 2017). The population undergoing TKA was similar regarding diagnosis and ranged from 42 to 83 years of age. In comparison, the group of participants with arthroscopic knee surgery was significantly younger on average (18 to 65 years).
Intervention
Included studies compared adductor canal block versus femoralis nerve block or placebo. Fifteen trials explored the analgesic efficacy of adductor canal block (ACB) and femoralis nerve block (FNB). Another eight trials compared the analgesic effect of ACB using perineural local anaesthetic (LA) or placebo (saline). Two studies compared ACB versus periarticular infiltration. Finally, one study compared the effect of ACB versus psoas compartment block (Messeha 2016).
Thirteen RCTs used the LA ropivacaine in different concentrations. Most trial authors used 0.5% to 0.75% ropivacaine, with the exception of four studies, which used 0.2% (Sztain 2015; Zhang 2014), 0.25% (Li 2017), or 0.375% ropivacaine (Wiesmann 2016). The other studies infiltrated lidocaine 2% (Machi 2015), levobupivacaine 0.25% (Akkaya 2008), and 0.125% (Rahimzadeh 2017), 0.25% (Macrinici 2017; Memtsoudis 2015; Nader 2016), or 0.5% bupivacaine (Messeha 2016). Four authors added ephedrine to LA (Abdallah 2016; Hanson 2013; Messeha 2016; Nader 2016). Sawhney 2016 used additional ketorolac and morphine in the infiltration solution.
Most trial authors performed the nerve block preoperatively. In five studies, researchers conducted the block procedure postoperatively (Jaeger 2013; Macrinici 2017; Rahimzadeh 2017; Zhang 2014; Zhao 2017). Eleven studies selected continuous postoperative administration of LA: ropivacaine 0.2% to 0.25% (Andersen 2013; Elkassabany 2016; Jaeger 2013; Jaeger 2014; Jenstrup 2012; Shah 2014; Sztain 2015; Wiesmann 2016; Zhang 2014; Zhao 2017) or lidocaine 2% (Machi 2015). The remaining trials used a single injection procedure.
Most trial authors performed an ultrasound‐guided injection nerve block technique. Only one trial author used the combination of nerve stimulation (NS) and ultrasound (Zhang 2014).
For surgical procedures, most participants received general anaesthesia. Eight studies performed spinal anaesthesia (Elkassabany 2016; Hegazy 2015; Jaeger 2013; Jenstrup 2012; Machi 2015; Nader 2016; Sawhney 2016; Shah 2014). Two performed combined spinal‐epidural anaesthesia (Memtsoudis 2015; Zhang 2014). Three trials reported that they additionally provided local infiltration analgesia (LIA) to both groups (Andersen 2013; Nader 2016; Sztain 2015).
Most trials (13 out of 25 studies) reported that an additional multi‐modal analgesic regimen was started preoperatively and was continued postoperatively (Elkassabany 2016; Espelund 2013; Espelund 2014a; Hanson 2013; Hegazy 2015; Jaeger 2014; Koh 2017a; Li 2017; Machi 2015; Macrinici 2017; Nader 2016; Sawhney 2016; Sztain 2015). Opioids were given as rescue analgesics in all studies. Seven out of 25 of the included trials reported the use of a prophylactic drug against postoperative nausea and vomiting (PONV) (Andersen 2013; Elkassabany 2016; Hanson 2013; Memtsoudis 2015; Sawhney 2016; Shah 2014; Wiesmann 2016).
Excluded studies
We excluded 19 studies. The reasons for their exclusion are given in the Characteristics of excluded studies table. We excluded three trials because volunteers were investigated (Jaeger 2013b; Kwofie 2013, Monahan 2016), and we excluded three trials because they performed only retrospective analysis (Grant 2017; Gwam 2017; Seo 2017). One trial investigated hindfoot and ankle surgery instead of knee surgery (Joe 2016). Four RCTs compared ACB within a cross‐over design and were therefore excluded (Espelund 2014bGrevstad 2014Grevstad 2015; Sorensen 2016).
We excluded five studies because they used two different local anaesthetics (Beausang 2016), or they used different volumes of local anaesthetics within study groups (Henshaw 2016Kim 2014Ortiz‐Gomez 2017Sogbein 2017). Some participants were treated differently than described in the protocol (Jaeger 2012). Another trial provided additional treatment that was not part of the original protocol (Hanson 2014).
We excluded Shah 2015 because it compared single versus continuous ACB blockade.
Studies awaiting classification
We have presented 11 studies that are awaiting classification. Please refer to the Characteristics of studies awaiting classification table for details.
Ongoing studies
Within www.clinicaltrials.gov and http://www.who.int/ictrp/en/, 11 ongoing potentially relevant trials are registered and are recruiting patients. Please refer to the Characteristics of ongoing studies table for details.
Risk of bias in included studies
The risk of bias graph and summary can be seen in Figure 2 and Figure 3. The graph displays review authors’ judgements about each risk of bias item presented as percentages across all included RCTs. The risk of bias summary shows review authors’ judgements about each risk of bias item for each included study.
2.
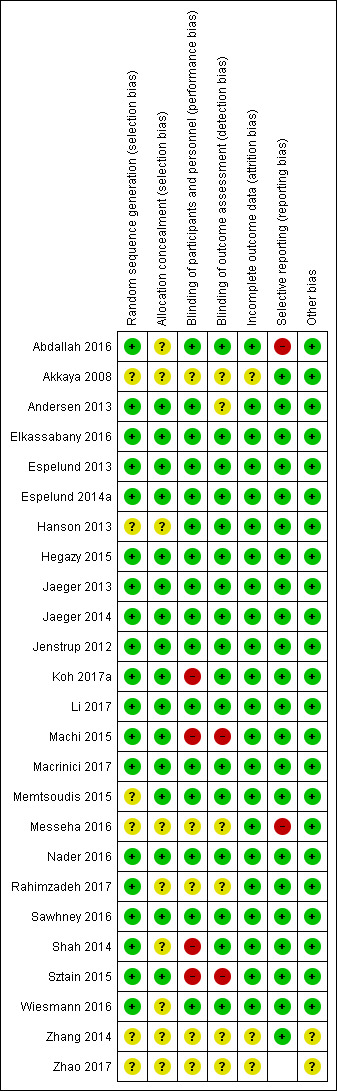
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
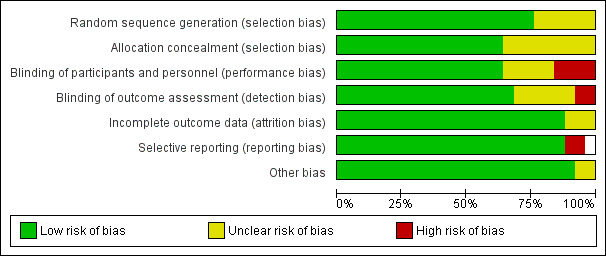
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
We judged six trials as having unclear risk of bias as they did not provide clear information on how the randomization sequence was generated (Akkaya 2008; Hanson 2013; Memtsoudis 2015; Messeha 2016; Zhang 2014; Zhao 2017). We judged all other studies as having low risk of bias due to adequate randomization.
Concealment of allocation
Sixteen of the included studies described allocation concealment; we judged them as having low risk of bias (Andersen 2013; Elkassabany 2016; Espelund 2013; Espelund 2014a; Hegazy 2015; Jaeger 2013; Jaeger 2014; Jenstrup 2012; Koh 2017a; Li 2017; Machi 2015; Macrinici 2017; Memtsoudis 2015; Nader 2016; Sawhney 2016; Sztain 2015). Nine trials did not report the method of allocation concealment, and we judged them as having unclear risk of bias (Abdallah 2016; Akkaya 2008; Hanson 2013; Messeha 2016; Rahimzadeh 2017; Shah 2014; Wiesmann 2016; Zhang 2014; Zhao 2017).
Blinding
Sixteen out of 25 trials were performed as double‐blind studies, with the participant and the provider of the intervention blinded to therapy (Abdallah 2016; Andersen 2013; Elkassabany 2016; Espelund 2013; Espelund 2014a; Hanson 2013; Hegazy 2015; Jaeger 2013; Jaeger 2014; Jenstrup 2012; Li 2017; Macrinici 2017; Memtsoudis 2015; Nader 2016; Sawhney 2016; Wiesmann 2016). We assessed five studies as having unclear risk of bias because blinding was not mentioned (Akkaya 2008; Messeha 2016; Rahimzadeh 2017; Zhang 2014; Zhao 2017). We rated six trials as having unclear risk of bias because they did not describe blinding of outcome assessment (Akkaya 2008; Andersen 2013; Messeha 2016; Rahimzadeh 2017; Zhang 2014; Zhao 2017). We rated two studies as having high risk of bias due to total non‐blinding (Machi 2015; Sztain 2015).
Incomplete outcome data
Three trials did not adequately report all evaluation data (Akkaya 2008; Zhang 2014; Zhao 2017). The remaining trials reported that all participants were included in the analysis; we assessed them as having low risk of bias.
Selective reporting
Two studies did not report all secondary outcomes; we therefore judged them to be at high risk of bias for selective reporting (Abdallah 2016; Messeha 2016). Due to insufficient data sources, we rated one study as having unclear risk of bias (Zhao 2017). We judged all other trials as having low risk of bias because all outcomes were measured and reported in full length, as judged from study reports (methods sections).
Other potential sources of bias
We found no further potential sources of bias in 23 trials. We rated two trials as having unclear risk of bias because data sources were insufficient (Zhang 2014; Zhao 2017).
Effects of interventions
for the main comparison.
| Adductor canal block compared with sham treatment for postoperative pain following knee surgery | ||||||
|
Patient or population: adult participants undergoing knee surgery (arthroscopic knee surgery or total knee replacement) Settings: postoperative care in hospital, Turkey (one trial), Denmark (four trials), USA (one trial) Intervention: adductor canal block Comparison: sham treatment (saline injection) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham treatment | Adductor canal block | |||||
|
Postoperative pain at rest (VAS 0 to 100 mm, NRS 0 to 10) (2 hours) |
Mean postoperative pain at rest (2 hours postoperatively) in the intervention group was 0.56 standard deviations lower (1.2 lower to 0.07 higher) | 208 (4) | ⊕⊕⊝⊝ lowa | Standard deviation of 0.5 represents a moderate effect | ||
|
Postoperative pain at rest (VAS 0 to 100 mm, NRS 0 to 10) (24 hours) |
Mean postoperative pain at rest (24 hours postoperatively) in the intervention group was 0.49 standard deviations lower (1.05 lower to 0.07 higher). | 272 (6) | ⊕⊕⊝⊝ lowa | Standard deviation of 0.5 represents a moderate effect | ||
|
Postoperative pain during movement (VAS 0 to 100 mm, NRS 0 to 10) (2 hours) |
Mean postoperative pain during movement (2 hours postoperatively) in the intervention group was 0.59 standard deviations lower (1.5 lower to 0.33 higher) | 160 (3) | ⊕⊝⊝⊝ very lowb | Standard deviation of 0.5 represents a moderate effect | ||
|
Postoperative pain during movement (VAS 0 to 100 mm, NRS 0 to 10) (24 hours) |
Mean postoperative pain during movement (24 hours postoperatively) in the intervention group was 0.03 standard deviations higher (0.26 lower to 0.32 higher) | 184 (4) | ⊕⊕⊝⊝ lowc | Standard deviation of 0.2 represents a small effect | ||
| Postoperative nausea (24 hours) | Two out of 61 participants in the sham group suffered from nausea | Five out of 60 participants in the adductor canal group suffered from nausea | RR 1.91 (95% CI 0.48 to 7.58) | 121 (3) | ⊕⊕⊝⊝ lowc | |
| Accidental falls during postoperative care (24 hours) | No patient out of 24 participants in the sham group suffered from an accidental fall | No patient out of 24 participants in the adductor canal group suffered from an accidental fall | 48 (1) | ⊕⊕⊝⊝ lowd | Only 1 small trial assessed this outcome | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NRS: numerical rating scale; RR: risk ratio; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels due to inconsistency (unexplained high heterogeneity).
bDowngraded by two levels due to inconsistency (unexplained high heterogeneity) and by one level due to imprecision (failed required information size).
cDowngraded by two levels due to imprecision (failed required information size, large confidence intervals).
dDowngraded by two levels due to imprecision because information is derived from only one small trial.
2.
| Adductor canal block compared with femoral nerve block for postoperative pain following knee surgery | ||||||
|
Patient or population: adult participants undergoing knee surgery (arthroscopic knee surgery or total knee replacement) Settings: postoperative care in hospital, USA (seven trials), China (two trials) Germany (one trial), India (one trial), Iran (one trial), Denmark (one trial) Intervention: adductor canal block Comparison: femoral nerve block | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Femoral nerve block | Adductor canal block | |||||
|
Postoperative pain at rest (VAS 0 to 100 mm, NRS 0 to 10) (2 hours) |
Mean postoperative pain at rest (2 hours postoperatively) in the intervention group was 0.74 standard deviations lower (‐1.76 lower to 0.28 higher) | 298 (5) | ⊕⊕⊝⊝ lowa | Standard deviation of 0.8 represents a large effect | ||
|
Postoperative pain at rest (VAS 0 to 100 mm, NRS 0 to 10) (24 hours) |
Mean postoperative pain at rest (24 hours postoperatively) in the intervention group was 0.04 standard deviations higher (‐0.09 lower to 0.18 higher) | 868 (12) | ⊕⊕⊕⊕ high | Standard deviation of 0.2 represents a small effect | ||
|
Postoperative pain during movement (VAS 0 to 100 mm, NRS 0 to 10) (2 hours) |
Mean postoperative pain during movement (2 hours postoperatively) in the intervention group was 0.47 standard deviations lower (‐1.86 lower to 0.93 higher) | 88 (2) | ⊕⊝⊝⊝ very lowb | Standard deviation of 0.5 represents a moderate effect | ||
|
Postoperative pain during movement (VAS 0 to 100 mm, NRS 0 to 10) (24 hours) |
Mean postoperative pain during movement (24 hours postoperatively) in the intervention group was 0.56 standard deviations higher (‐0.00 lower to 1.12 higher) | 576 (9) | ⊕⊝⊝⊝ very lowb | Standard deviation of 0.5 represents a moderate effect | ||
|
Postoperative nausea (24 hours) |
Five out of 70 participants in the femoral nerve block group suffered from postoperative nausea 24 hours postoperatively | Six out of 68 participants in the adductor canal block group suffered from postoperative nausea 24 hours postoperatively | 138 (2) | ⊕⊕⊝⊝ lowc | ||
|
Accidental falls during postoperative care (24 hours) |
Six out of 84 participants in the femoral nerve block group suffered from an accidental fall | No patient out of 88 participants in the adductor canal block group suffered from an accidental fall | 172 (3) | ⊕⊕⊝⊝ lowc | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NRS: numerical rating scale; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels due to inconsistency (unexplained high heterogeneity).
bDowngraded by two levels due to inconsistency (unexplained high heterogeneity) and by one level due to imprecision (failed required information size).
cDowngraded by two levels due to imprecision (failed required information size, large confidence intervals).
Comparison 1: adductor canal block (ACB) versus sham treatment
Eight trials compared the analgesic effect of ACB using perineural local anaesthetic (LA) or saline (sham treatment) (Akkaya 2008; Andersen 2013; Espelund 2013; Espelund 2014a; Hanson 2013; Jaeger 2014; Jenstrup 2012; Nader 2016) (Table 1). The trial author groups Andersen and Jaeger applied a continuous infusion of local anaesthetics via a catheter (Andersen 2013; Jaeger 2014); the others provided single shot regional anaesthesia.
Primary outcomes
Mean differences in postoperative pain at rest/during movement (2 hours (within the postoperative care unit), 24 hours, 48 hours)
Six included trials investigated postoperative pain intensity at rest and during movement at three different time points (Akkaya 2008; Andersen 2013; Espelund 2013; Espelund 2014a; Hanson 2013; Jaeger 2014). However, only data for pain at rest, respectively, and during movement 2 hours and 24 hours after surgery were sufficient for us to combine them within a meta‐analysis. All results showed no significant differences between adductor canal and placebo groups (pain at rest: 2 hours: standardized mean difference (SMD) ‐0.56, 95% confidence interval (CI) ‐1.20 to 0.07, 4 trials, 208 participants, I² = 79%, Analysis 1.1; 24 hours: SMD ‐0.49, 95% CI ‐1.05 to 0.07, 6 trials, 272 participants, I² = 80%, Analysis 1.2; pain during movement: 2 hours: SMD ‐0.59, 95% CI ‐1.5 to 0.33, 3 trials, 160 participants, I² = 87%, Analysis 1.3 24 hours: SMD 0.03, 95% CI ‐0.26 to 0.32, 4 trials, 184 participants, I² = 0%, Analysis 1.4; Figure 4).
1.1. Analysis.
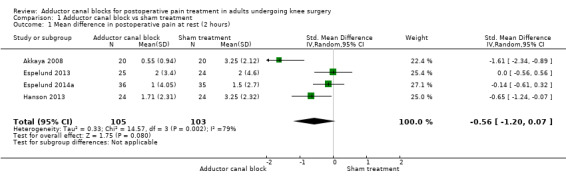
Comparison 1 Adductor canal block vs sham treatment, Outcome 1 Mean difference in postoperative pain at rest (2 hours).
1.2. Analysis.
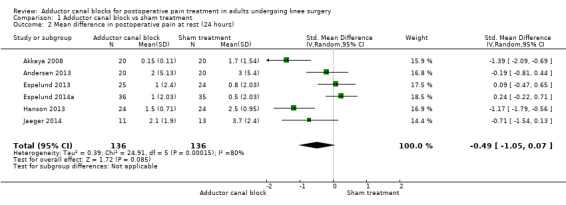
Comparison 1 Adductor canal block vs sham treatment, Outcome 2 Mean difference in postoperative pain at rest (24 hours).
1.3. Analysis.
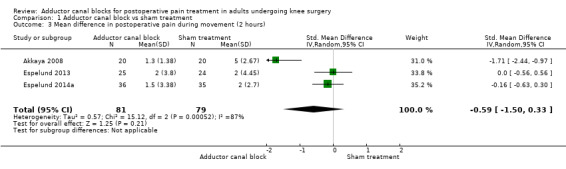
Comparison 1 Adductor canal block vs sham treatment, Outcome 3 Mean difference in postoperative pain during movement (2 hours).
1.4. Analysis.
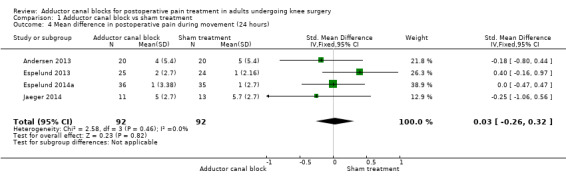
Comparison 1 Adductor canal block vs sham treatment, Outcome 4 Mean difference in postoperative pain during movement (24 hours).
4.
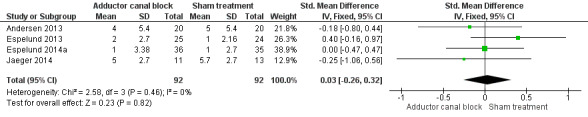
Forest plot of comparison: 1 Adductor canal block vs sham treatment, outcome: 1.4 Mean difference in postoperative pain during movement (24 hours).
Only one study provided data for the outcome postoperative pain at rest/during movement 48 hours after surgery (Andersen 2013): trial authors reported no significant differences between groups (P = 1.0; P = 0.44). Due to the small number of trials (< 10 trials), high heterogeneity observed for the analyses ‐ Analysis 1.1Analysis 1.2Analysis 1.3 ‐ could not be explored within subgroup analyses.
Sensitivity analyses focusing on the influence of study quality or of missing participants were not possible because trials with high risk of bias were not included for these outcomes and no trials reported dropouts. The sensitivity analysis focusing on the influence of using the fixed‐effect model showed lower SMD but significant differences for the outcomes pain at rest 2 hours postop (SMD ‐0.45, 95% CI ‐0.73 to ‐0.17, P = 0.002); pain at rest 24 hours postop (SMD ‐0.37, 95% CI ‐0.61 to ‐0.11, P = 0.004); and pain during movement 2 hours postop (SMD ‐0.41, 95% CI ‐0.73 to ‐0.08, P = 0.01). There were no differences between random‐effects and fixed‐effect models for the outcome pain during movement 24 hours postop. We prepared no funnel plot because fewer than 10 trials were included for all outcomes. Finally, we calculated the optimal information size (OIS) for the outcomes pain at rest 2 hours and pain at rest 24 hours; results showed that the number of necessary participants was reached (postoperative pain at rest 2 hours: 48 participants in each sample; postoperative pain at rest 24 hours: 15 participants in each sample).
Using the GRADE approach, we downgraded the level of evidence for the outcomes postoperative pain at rest (2 hours, 24 hours) by two levels due to inconsistency (unexplained high heterogeneity) (low‐quality evidence), and we downgraded the outcome postoperative pain during movement (2 hours) by two levels due to inconsistency (unexplained high heterogeneity), and by one level due to imprecision (failed required information size) (very low‐quality evidence).
The outcome postoperative pain during movement 24 hours was rated as low‐quality evidence due to imprecision (failed required information size, large confidence interval). Due to missing meta‐analyses, evidence for the outcomes postoperative pain at rest, respectively, and during movement (48 hours) was rated as very low quality.
Rate of opioid‐related adverse events (nausea, vomiting, postoperative nausea and vomiting, respiratory depression, pruritus, sedation (2 hours (within the postoperative care unit), 24 hours, 48 hours))
Five studies reported data about opioid‐related adverse events (Akkaya 2008; Espelund 2013; Hanson 2013; Jaeger 2014; Jenstrup 2012). We combined data for nausea (2 hours, 24 hours), vomiting (2 hours, 24 hours), PONV (24 hours), and sedation (2 hours, 24 hours). For all other outcomes, no data were available. Meta‐analyses for mentioned opioid‐related adverse events did not show any significant differences between participants receiving adductor canal block and those given placebo (nausea 2 hours: risk ratio (RR) 1.75, 95% CI 0.56 to 5.49, 2 trials, 79 participants, I² = 0%, Analysis 1.5: nausea 24 hours: RR 1.91, 95% CI 0.48 to 7.58, 3 trials, 121 participants, I² = 0%, Analysis 1.6; vomiting 24 hours: RR 1.18, 95% CI 0.56 to 2.47, 2 trials, 79 participants, I² = 0%, Analysis 1.7; postoperative nausea and vomiting (PONV) 24 hours: RR 0.54, 95% CI 0.29 to 1.02, 2 trials, 111 participants, I² = 39%, Analysis 1.8; sedation 2 hours: RR 0.51, 95% CI 0.17 to 1.52, 2 trials, 91 participants, I² = 64%, Analysis 1.9; sedation 24 hours: RR 0.78, 95% CI 0.20 to 3.07, 2 trials, 73 participants, I² = 67%, Analysis 1.10). Only one trial including 59 participants reported the outcome vomiting 2 hours after surgery, but no participants suffered from this event (Espelund 2013). Due to the small number of included trials for this comparison, moderate heterogeneity of the outcomes sedation 2 hours (Analysis 1.9), sedation 24 hours, could not be further explored (Analysis 1.10). We rated no included trials reporting data for these outcomes as having high risk of bias. The sensitivity analysis focusing on the influence of using the fixed‐effect model showed higher RRs for the outcome sedation (2 hours, 24 hours), but these failed to show significance (2 hours: RR 0.7, 95% CI 0.49 to 1.0, P = 0.05; 24 hours: RR 1.03, 95% CI 0.53 to 2.00, P = 0.93). No included trial reported dropouts or zero events, so no sensitivity analyses were performed. We prepared no funnel plot because fewer than 10 trials were included for all outcomes. We did not perform a trial sequential analysis (TSA) because included participants were too few (< 400 participants).
1.5. Analysis.
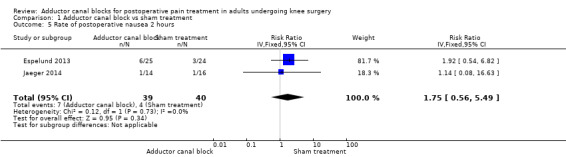
Comparison 1 Adductor canal block vs sham treatment, Outcome 5 Rate of postoperative nausea 2 hours.
1.6. Analysis.
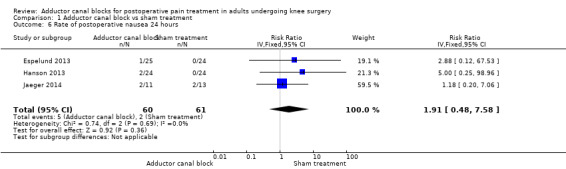
Comparison 1 Adductor canal block vs sham treatment, Outcome 6 Rate of postoperative nausea 24 hours.
1.7. Analysis.
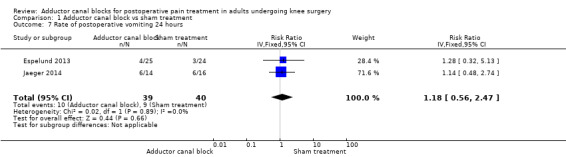
Comparison 1 Adductor canal block vs sham treatment, Outcome 7 Rate of postoperative vomiting 24 hours.
1.8. Analysis.
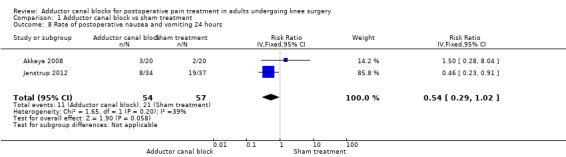
Comparison 1 Adductor canal block vs sham treatment, Outcome 8 Rate of postoperative nausea and vomiting 24 hours.
1.9. Analysis.
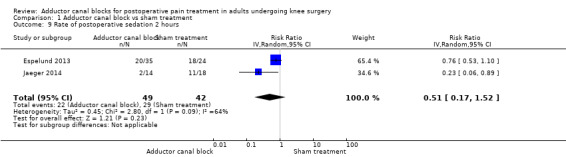
Comparison 1 Adductor canal block vs sham treatment, Outcome 9 Rate of postoperative sedation 2 hours.
1.10. Analysis.
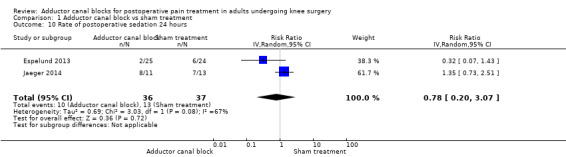
Comparison 1 Adductor canal block vs sham treatment, Outcome 10 Rate of postoperative sedation 24 hours.
Using the GRADE approach, we rated the evidence for vomiting (2 hours) as very low quality due to imprecision (failed required population, large confidence intervals, no meta‐analysis). We downgraded the level of evidence for nausea (2 hours, 24 hours), vomiting (24 hours), and PONV (24 hours) from high to low quality due to imprecision (failed required population, large confidence intervals), whereas we downgraded the level of evidence for sedation (2 hours, 24 hours) to very low quality due to inconsistency (unexplained heterogeneity) and imprecision (failed required population, large confidence intervals).
Rate of accidental falls during postoperative care
Only one study (48 participants) reported on this outcome (Hanson 2013). However, no participants suffered from an accidental fall 24 hours postoperatively. No additional analyses could be performed.
We judged the GRADE level as very low quality due to imprecision (failed required population, no meta‐analysis).
Secondary outcomes
Cumulative mean morphine requirement (2 hours (within the postoperative care unit), 24 hours, 48 hours)
Five studies (232 participants) reported the cumulative mean morphine requirement at 24 hours (Akkaya 2008; Espelund 2013; Hanson 2013; Jaeger 2014; Jenstrup 2012).
The cumulative morphine requirement at 2 hours after surgery was reported in one trial (Jenstrup 2012).
No trial reported the cumulative morphine requirement at 48 hours after surgery.
The meta‐analysis revealed a significantly lower morphine requirement 24 hours postop in participants treated with ACB compared to placebo (mean difference (MD) ‐15.88 mg, 95% CI ‐30.87 to ‐0.89, 5 trials, 232 participants, I² = 80%, Analysis 1.11; Figure 4).
1.11. Analysis.
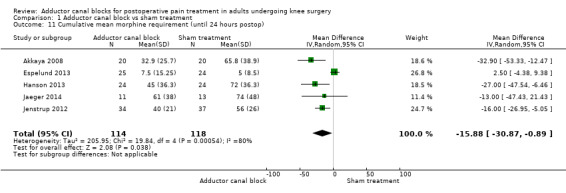
Comparison 1 Adductor canal block vs sham treatment, Outcome 11 Cumulative mean morphine requirement (until 24 hours postop).
Jenstrup 2012 reported a mean difference of morphine requirement of 2 mg 2 hours postop (95% CI ‐5.08 to 1.08) between treatment and control groups including 71 participants, which failed significance.
Degree of quadriceps muscle strength (2 hours (within the postoperative care unit), 24 hours, 48 hours)
Only Jaeger 2013 reported measurement of voluntary isometric contraction (MVIC) for muscle strength of the quadriceps muscle and adductor muscle group with a dynamometer. These researchers showed significantly better contraction of the quadriceps muscle in the group of participants treated with ACB (Table 3).
1. Degree of quadriceps muscle strength (2 hours (within the postoperative care unit), 24 hours, 48 hours).
| Reference | Quadriceps MVIC | Adductor MVIC | Quadriceps strength scale MMT | Adductor strength scale MMT | Duration of SLR | Modified Bromage Scale |
| Abdallah 2016 | # within 60 minutes |
|||||
| Elkassabany 2016 | # within first 24 hours postoperative |
|||||
| Jaeger 2013 | # at 24 hours postoperative |
‐ at 24 hours postoperative |
||||
| Koh 2017a | ‐ at 1 week postoperative |
# within first 24 hours postoperative |
# within first 24 hours postoperative |
|||
| Li 2017 | # within first 12 hours postoperative |
‐ within first 72 hours postoperative |
||||
| Macrinici 2017 | # within first 24 hours postoperative |
|||||
| Memtsoudis 2015 | ‐ within first 48 hours postoperative |
|||||
| Rahimzadeh 2017 | ‐ within first 24 hours postoperative |
|||||
| Wiesmann 2016 | # at 24 hours postoperative |
|||||
| Zhang 2014 | # within first 48 hours postoperative |
|||||
| Zhao 2017 | ‐ within 48 hours postoperative |
MMT=manual muscle testing for quadriceps or adductor strength; MVIC =Measurement of voluntary isometric contraction; SLR =straight leg raising; # =favours experimental group, ‐= no difference between experimental or control groups.
Rate of chronic postsurgical pain (after 3 months, 6 months, 1 year)
No included trials reported data on chronic postsurgical pain.
Rates of block‐related adverse events (accidental vascular puncture, paraesthesia, motor blockade, failed block, neurological impairment)
Only two included trials (89 participants) reported the number of participants with failed block (Analysis 1.12). No participant suffered from failed block. No other block‐related adverse events were mentioned.
1.12. Analysis.
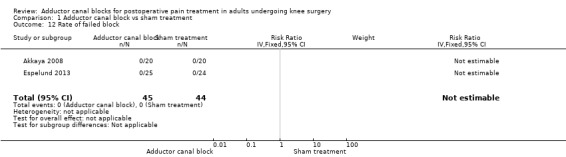
Comparison 1 Adductor canal block vs sham treatment, Outcome 12 Rate of failed block.
Comparison 2: adductor canal block versus femoral nerve block
Fifteen trials explored the analgesic efficacy of ACB and FNB (Abdallah 2016Elkassabany 2016; Hegazy 2015; Jaeger 2013; Koh 2017a; Li 2017; Machi 2015; Macrinici 2017; Memtsoudis 2015; Rahimzadeh 2017; Shah 2014; Sztain 2015; Wiesmann 2016; Zhang 2014; Zhao 2017) (Table 2). Eight groups used catheters (Elkassabany 2016; Jaeger 2013; Machi 2015; Shah 2014; Sztain 2015; Wiesmann 2016; Zhang 2014; Zhao 2017); the others applied single shot ACB and FNB.
Primary outcomes
Mean differences in postoperative pain at rest/during movement (1 hour (within the postoperative care unit), 24 hours, 48 hours)
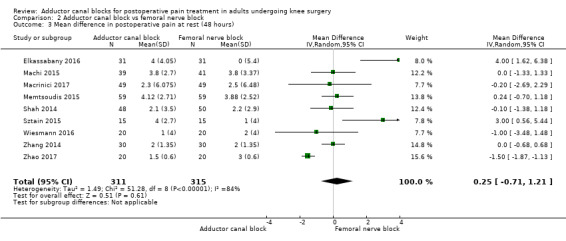
Thirteen included trials investigated postoperative pain intensity at rest and during movement at three different time points (Abdallah 2016; Andersen 2013; Elkassabany 2016; Jaeger 2013; Machi 2015; Macrinici 2017; Memtsoudis 2015; Rahimzadeh 2017; Shah 2014; Sztain 2015; Wiesmann 2016; Zhang 2014; Zhao 2017). Meta‐analyses could be performed for all time points and showed no significant differences between adductor canal and femoral nerve block groups (pain at rest: 2 hours: SMD ‐0.74, 95% CI ‐1.76 to 0.28, 5 trials, 298 participants, I² = 93%, Analysis 2.1; 24 hours: SMD 0.04, 95% CI ‐0.09 to 0.18, 12 trials, 868 participants, I² = 42%, Analysis 2.2; Figure 5; 48 hours: SMD 0.25, 95% CI ‐0.71 to 1.21, 9 trials, 626 participants, I² = 84%, Analysis 2.3; pain during movement: 2 hours: SMD ‐0.47, 95% CI ‐1.86 to 0.93, 2 trials, 88 participants, I² = 90%, Analysis 2.4; 24 hours: SMD 0.56, 95% CI ‐0.00 to 1.12, 9 trials, 576 participants, I² = 89%, Analysis 2.5; 48 hours: SMD 0.07, 95% CI ‐0.1 to 0.24, 8 trials, 528 participants, I² = 0%, Analysis 2.6; Figure 6).
2.1. Analysis.
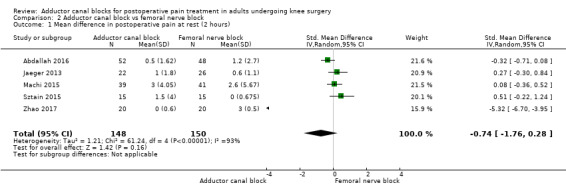
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 1 Mean difference in postoperative pain at rest (2 hours).
2.2. Analysis.
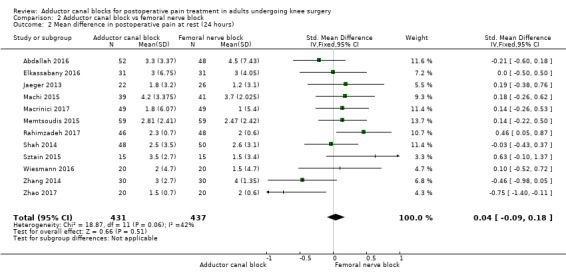
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 2 Mean difference in postoperative pain at rest (24 hours).
5.
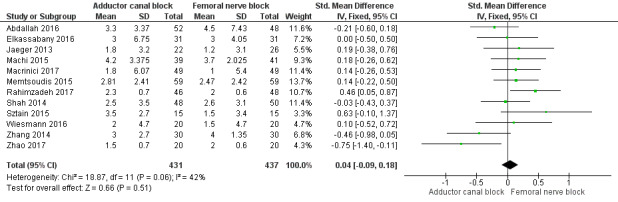
Forest plot of comparison: 2 Adductor canal block vs femoral nerve block, outcome: 2.2 Mean difference in postoperative pain at rest (24 hours).
2.3. Analysis.
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 3 Mean difference in postoperative pain at rest (48 hours).
2.4. Analysis.
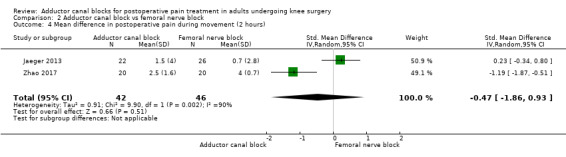
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 4 Mean difference in postoperative pain during movement (2 hours).
2.5. Analysis.
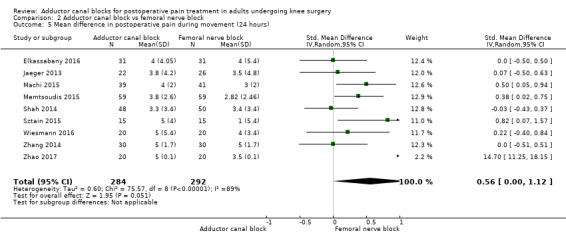
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 5 Mean difference in postoperative pain during movement (24 hours).
2.6. Analysis.
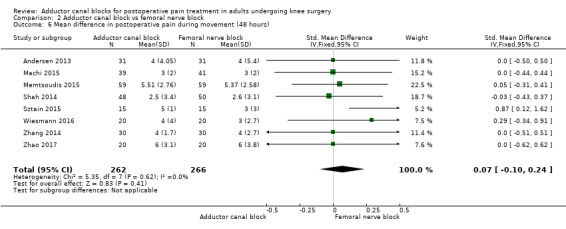
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 6 Mean difference in postoperative pain during movement (48 hours).
6.
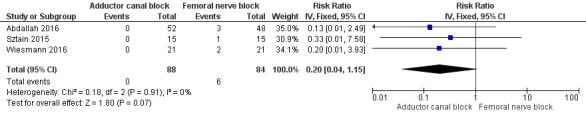
Forest plot of comparison: 2 Adductor canal block vs femoral nerve block, outcome: 2.8 Rate of accidental falls during postoperative care 24 hours.
Available data were insufficient for us to perform a subgroup analysis for the heterogeneous outcomes pain at rest (Analysis 2.1Analysis 2.3), respectively, and pain during movement (Analysis 2.4Analysis 2.6). Sensitivity analyses focusing on the influence of study quality excluding trials with high risk of bias showed again no significant differences (pain at rest: 2 hours: SMD ‐2.49, 95% CI ‐7.97 to 2.99, P = 0.37, Jaeger 2013; Zhao 2017 24 hours: SMD 0.05, 95% CI ‐0.11 to 0.22, P = 0.52, Elkassabany 2016; Jaeger 2013; Macrinici 2017; Memtsoudis 2015; Rahimzadeh 2017; Sztain 2015; Wiesmann 2016; Zhang 2014; Zhao 2017 48 hours: SMD 0.05, 95% CI ‐1.13 to 1.23, P = 0.94, Elkassabany 2016; Macrinici 2017; Memtsoudis 2015; Wiesmann 2016; Zhang 2014; Zhao 2017; pain during movement: 24 hours: SMD 0.886, 95% CI ‐0.02 to 1.78, P = 0.06, Elkassabany 2016; Jaeger 2013; Memtsoudis 2015; Wiesmann 2016; Zhang 2014; Zhao 2017, 48 hours: SMD 0.05, 95% CI ‐0.16 to 0.27, P = 0.62, Andersen 2013; Memtsoudis 2015; Wiesmann 2016; Zhang 2014; Zhao 2017). We did not perform an analysis focusing on the influence of missing data because all data were reported within the trials. The sensitivity analysis focusing on the influence of using the fixed‐effect model showed a significant difference only for the outcomes pain at rest 48 hours postop (SMD ‐0.78, 95% CI ‐1.06 to ‐0.5, P < 0.001) and pain during movement 24 hours postop (SMD 0.25, 95% CI 0.08 to 0.42, P = 0.004). There were no differences between random‐effects and fixed‐effect models for the other outcomes. We prepared a funnel plot only for the outcome pain at rest 24 hours, but this showed no asymmetry. Finally, we calculated the OIS for the outcomes pain at rest 2 hours, 24 hours, 48 hours, respectively, and pain during movement 24 hours, 48 hours. Results showed that the number of necessary participants was reached for all outcomes, with the exception of pain during movement 24 hours.
Using the GRADE approach, we downgraded the level of evidence for the outcomes postoperative pain at rest (2 hours, 48 hours) by two levels due to inconsistency (unexplained high heterogeneity) (low‐quality evidence). We downgraded postoperative pain during movement (2 hours) to very low‐quality evidence due to inconsistency (unexplained high heterogeneity) and imprecision (large confidence interval). We rated the outcome postoperative pain during movement (24 hours) as very low‐quality evidence due to inconsistency (unexplained high heterogeneity) and imprecision (failed required optimal information size). We rated the outcomes postoperative pain at rest (24 hours) and postoperative pain during movement (48 hours) as high‐quality evidence.
Rates of opioid‐related adverse events (nausea, vomiting, postoperative nausea and vomiting, pruritus, sedation (2 hours (within the postoperative care unit), 24 hours, 48 hours))
Only five included trials reported opioid‐related adverse events (Abdallah 2016; Hegazy 2015; Li 2017; Shah 2014; Zhao 2017). However, meta‐analyses could be performed only for the outcomes nausea (24 hours) and PONV (24 hours). Both analyses revealed no significant differences between participants treated with ACB or FNB (nausea 24 hours: RR 1.22, 95% CI 0.42 to 3.54, 2 trials, 138 participants, I² = 0%, Analysis 2.7; PONV 24 hours: RR 0.68, 95% CI 0.44 to 1.04, 2 trials, 151 participants, I² = 0%, Analysis 2.10). Shah and colleagues reported no significant differences between groups regarding risk for vomiting 24 hours (Shah 2014), respectively, nor Abdallah and colleagues regarding risk for PONV (2 hours) (Abdallah 2016). Hegazy reported no participants with respiratory depression in any group (Hegazy 2015). All other opioid‐related adverse events were not mentioned.
2.7. Analysis.
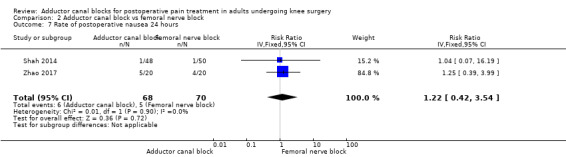
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 7 Rate of postoperative nausea 24 hours.
2.10. Analysis.
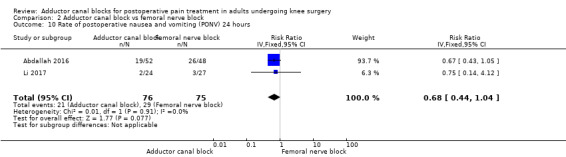
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 10 Rate of postoperative nausea and vomiting (PONV) 24 hours.
Due to limited data, there was no need to perform sensitivity analyses focusing on the influence of study quality or zero events. No dropouts were reported. We prepared no funnel plot because fewer than 10 trials were included for all outcomes. We did not perform a TSA because groups included fewer than 400 participants.
Using the GRADE approach, we rated the evidence for nausea (24 hours) and PONV (24 hours) as low quality due to imprecision (failed required population, large confidence intervals). We downgraded the level of evidence for vomiting (2 hours, 24 hours, 48 hours), nausea (2 hours, 48 hours), PONV (2 hours, 48 hours), sedation, respiratory depression, and urinary retention from high to very low quality due to imprecision (failed required population) and limited data.
Rate of accidental falls during postoperative care
Accidental falls were mentioned within four included trials (Abdallah 2016; Jaeger 2013; Sztain 2015; Wiesmann 2016). After 24 and 48 hours, there were no differences in risk for an accidental fall between groups (24 hours: RR 0.20, 95% CI 0.04 to 1.15, 3 trials, 172 participants, I² = 0%, Analysis 2.11; Figure 6; 48 hours: RR 0.27, 95% CI 0.01 to 6.11, 2 trials, 75 participants, Analysis 2.12). Due to limited data, no additional analyses could be performed.
2.11. Analysis.
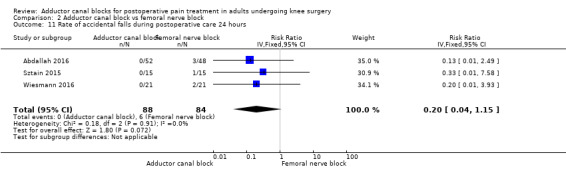
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 11 Rate of accidental falls during postoperative care 24 hours.
2.12. Analysis.
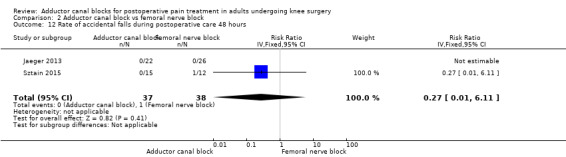
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 12 Rate of accidental falls during postoperative care 48 hours.
We therefore judged that the GRADE level for both outcomes was low due to imprecision (failed required population, large confidence intervals).
Secondary outcomes
Cumulative mean morphine requirement (2 hours (within the postoperative care unit), 24 hours, 48 hours)
Eight trials reported the cumulative morphine requirement at three different assessments among participants treated with ACB or FNB (Abdallah 2016; Elkassabany 2016; Hegazy 2015; Jaeger 2013; Machi 2015; Macrinici 2017; Sztain 2015; Wiesmann 2016). At all time points, there were no significant differences between groups (2 hours: MD 1.0 mg, 95% CI ‐0.79 to 2.79, 5 trials, 305 participants, I² = 0%, Analysis 2.13; 24 hours: MD ‐1.03 mg, 95% CI ‐3.48 to 1.41, 6 trials, 418 participants, I² = 0%, Analysis 2.14). Only one trial including 80 participants reported that there were again no differences in the cumulative morphine requirement between patients treated with ACB or FNB (P = 1.0) (Machi 2015).
2.13. Analysis.
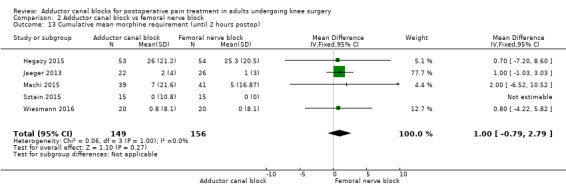
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 13 Cumulative mean morphine requirement (until 2 hours postop).
2.14. Analysis.
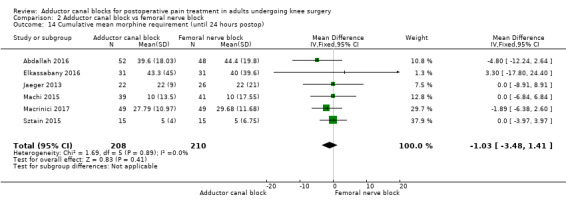
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 14 Cumulative mean morphine requirement (until 24 hours postop).
Degree of quadriceps muscle strength (2 hours (within the postoperative care unit), 24 hours, 48 hours)
Eleven trials reported data focusing on the degree of quadriceps muscle strength at three different time points (Abdallah 2016; Elkassabany 2016; Jaeger 2013; Koh 2017a; Li 2017; Macrinici 2017; Memtsoudis 2015; Rahimzadeh 2017; Wiesmann 2016; Zhang 2014; Zhao 2017). Due to large heterogeneity, we decided to analyse this outcome qualitatively. The data are presented in Table 3. Abdallah 2016 reported that one hour after surgery, measurement of voluntary isometric contraction for muscle strength with a dynamometer showed significantly better results among participants treated with ACB versus FNB. Six trials showed significantly greater quadriceps muscle strength following ACB compared to FNB at 24 hours after surgery (Elkassabany 2016; Jaeger 2013; Koh 2017a; Li 2017; Macrinici 2017; Wiesmann 2016). In contrast, only Ramizadeh and colleagues showed no differences at the same time point (Rahimzadeh 2017). However, at 48 hours postop, three studies reported no significant difference in quadriceps muscle strength (Memtsoudis 2015; Wiesmann 2016; Zhao 2017), whereas only Zhang 2014 still showed a significant difference in quadriceps muscle strength, between ACB and FNB.
Rate of chronic postsurgical pain (after 3 months, 6 months, 1 year)
None of the included trials reported data focusing on this outcome.
Rates of block‐related adverse events (accidental vascular puncture, paraesthesia, motor blockade, failed block, neurological impairment)
Only five included trials reported block‐related adverse events. The most common adverse event was failed block, but there was no significant difference between blocks (RR 1.46, 95% CI 0.16 to 12.99, 3 trials, 281 participants, I² = 31%, Analysis 2.15). Four trials mentioned that there were no participants with a block‐related neurological impairment (385 participants; Analysis 2.16). Additionally, only Elkassabany and colleagues mentioned that there was no participant suffering from an accidental vascular puncture during placement (Elkassabany 2016).
2.15. Analysis.
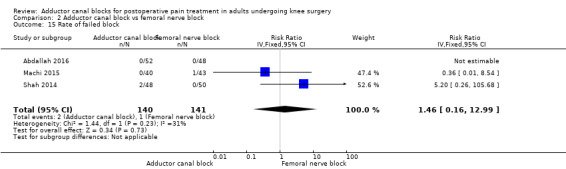
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 15 Rate of failed block.
2.16. Analysis.
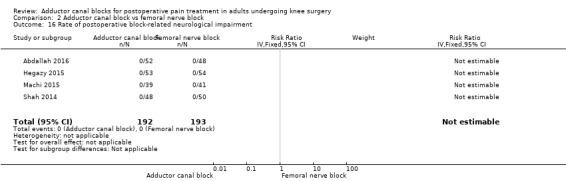
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 16 Rate of postoperative block‐related neurological impairment.
Comparison 3: adductor canal block versus periarticular infiltration
Two trials investigated efficacy and adverse events with ACB and periarticular infiltration (Nader 2016; Sawhney 2016). Due to limited data, no meta‐analyses could be performed. Therefore the results were described qualitatively. Nader 2016 compared participants undergoing TKA treated with periarticular infiltration and ACB versus periarticular infiltration alone. There were no significant differences in postoperative pain during movement at 30 hours after surgery, but participants receiving ACB together with periarticular infiltration required significantly less opioid 36 hours after surgery. However there was no significant difference in risk for nausea or vomiting 36 hours after surgery. Sawhney 2016 investigated also participants undergoing TKA treated with periarticular infiltration and ACB versus periarticular infiltration alone. They showed that participants treated with ACB in combination with periarticular infiltration reported significantly less pain during movement than participants treated with periarticular infiltration alone at 24 hours and 48 hours after surgery. However, there were no differences in the rates of opioid‐related adverse events of nausea, vomiting, and pruritus.
Comparision 4: adductor canal block versus psoas compartment block
Only Messeha 2016, which included 90 participants, compared the analgesic efficacy of ACB versus psoas compartment block. Trial authors demonstrated that participants treated with psoas compartment block showed significantly lower pain scores at rest until 2 hours after surgery compared to those treated with ACB. However, significance was failed 24 hours after surgery. No other relevant outcomes were reported.
Discussion
Summary of main results
This systematic review included 25 randomized controlled trials (RCTs) (1688 participants) comparing adductor canal block (ACB) versus sham treatment, femoral nerve block (FNB), or other regional anaesthetic techniques in adults undergoing knee surgery. Compared to sham treatment, patients treated with ACB reported no significant differences in postoperative pain intensity at rest and during movement. Furthermore, there was no significant difference in the risk ratio (RR) of the opioid‐related adverse events nausea, vomiting, postoperative nausea and vomiting (PONV), and sedation. No other opioid‐related adverse events were mentioned. Only one trial reported the outcome accidental falls during postoperative care, and no patient was suffering from this. Cumulative morphine consumption was significantly less in patients treated with ACB compared to sham treatment. The rate of chronic postsurgical pain was not reported. Only one trial showed that patients with ACB had significantly better quadriceps motor function after surgery. Block‐related adverse events were only poorly reported. Only two included trials reported that no patient suffered from failed block.
The comparison ACB versus FNB showed again no significant differences in postoperative pain intensity at rest and during movement. There was no significant difference in the opioid‐related adverse events nausea and PONV. For other opioid‐related adverse events, no meta‐analyses could be performed due to lack of data. The rate for accidental falls during postoperative care was not significantly different between groups. Furthermore, the cumulative morphine requirement was not significantly different. However, a qualitative analysis clearly revealed that more studies reported significantly greater quadriceps muscle strength following ACB compared to sham treatment or FNB at 24 hours after surgery, but this difference failed significance at 48 hours after surgery as reported by more studies comparing ACB versus FNB. Again, block‐related adverse events were only poorly reported, and meta‐analyses could be performed only for the rate of failed block, which showed no significant difference. No patient suffered from a neurological impairment as mentioned by four included trials. Other block‐related adverse events were not reported.
No meta‐analyses could be performed for the comparison ACB versus periarticular infiltration. One trial showed no significant differences in postoperative pain during movement at 30 hours after surgery, but patients receiving ACB together with periarticular infiltration required significantly less opioid 36 hours after surgery. However, there was no significant difference in the risk for nausea or vomiting 36 hours after surgery. In contrast, another trial showed that patients treated with ACB combined with periarticular infiltration reported significantly less pain at 24 hours and 48 hours after surgery, but there were no differences in rates of the opioid‐related adverse events nausea, vomiting, and pruritus. No other relevant outcomes were investigated.
Again no meta‐analyses could be performed for the comparison ACB versus psoas compartment block. One trial reported that patients treated with psoas compartment block showed significantly lower pain scores at rest until two hours after surgery compared to those treated with ACB. However, significance was failed 24 hours after surgery. No other relevant outcomes were reported.
Overall completeness and applicability of evidence
This systematic review finally included 25 trials including 1688 participants. However, the sample size for each trial was small, which increases the risk for heterogeneity and limits external validation. We excluded trials using different volumes, types, and concentrations of local anaesthetic (LA) because these facts might influence the duration of analgesia and sensory blockade. Due to the fact that patients undergoing surgery regularly report the highest postoperative pain intensity at the first postoperative day, we decided to investigate clinically relevant postoperative pain outcomes at three different time points. The comparison ACB versus sham treatment was investigated by only eight included trials, so that the calculated minimum number of patients for all primary outcomes failed. Pain intensity at rest and during movement could be analysed only within meta‐analyses for the first time points (2 hours, 24 hours), whereas data were limited for the last time point. In contrast, 13 trials compared ACB versus FNB, so that the required information size was reached for all pain intensity outcomes apart from the outcome pain intensity during movement (24 hours). It is interesting to note that although five out of eight trials (investigating the comparison ACB vs sham treatment), respectively, and eight out of 13 trials (comparing ACB vs FNB) reported cumulative opioid consumption, no trial reported all relevant opioid‐related adverse events, which are clinically more relevant for the patient, compared to cumulative opioid consumption. Most data could be combined for nausea, vomiting, or PONV within both comparisons, but again not for all investigated time points. Another clinically relevant outcome ‐ especially after knee surgery ‐ is the rate of accidental falls during perioperative care. However, this was only poorly reported, and no meta‐analysis could be performed for the comparison with sham treatment. In contrast, study data could be combined for this outcome at two time points if ACB was compared with FNB, but the results failed significance. The degree of quadriceps muscle strength is an outcome that is reported by many trials focusing on efficacy and safety following ACB in knee surgery. However, many different muscle tests were performed, and currently there is no international standard for reporting functional recovery following knee surgery (total knee arthroplasty (TKA), arthroscopic knee surgery). Therefore, we decided to report this outcome qualitatively, but only one trial reported data for the comparison ACB versus sham treatment. Because ACB blocks only sensory nerve compared to femoral nerve block, the degree of quadriceps muscle strength was reported by 11 out of 13 included trials. However, most trials focused only on the first 24 hours following surgery, so that fewer trials were available for the last time point. Another reason for this might be the fact that more trials (14 out of 25 trials) performed single shot block with a shorter duration of blockade (< 24 hours). Although chronic postsurgical pain is another clinically relevant outcome for patients following knee surgery (Beswick 2012), no included studies reported this outcome. The observation period within the included trials focused only on early recovery following surgery. If regional anaesthetic techniques are investigated within trials, block‐related adverse events (e.g. accidental vascular puncture) are important outcomes. Unfortunately, again no included trials reported all relevant adverse events. Data could be combined only for the rate of failed block for the comparisons ACB versus sham treatment, respectively, and FNB. For comparisons with other regional anaesthetic techniques, no meta‐analyses could be performed for any primary or secondary outcome.
To conclude, although we planned to investigate several clinically relevant postoperative pain outcomes at several relevant time points, included trials reported only data with a specific focus (e.g. functional recovery, postoperative morphine consumption) and missed other important pain‐ and block‐related outcomes.
Quality of the evidence
The most important limitation of the present review is the small number of trials per outcome, so that many planned subgroup analyses exploring heterogeneity could not be performed. Accordingly, the evidence for many primary outcomes was downgraded by two levels due to inconsistency (unexplained heterogeneity). Furthermore, trial sequential analysis (TSA) for dichotomous outcomes, respectively, and optimal information size (OIS) for continuous outcomes could be performed for only one primary outcome for the comparison "adductor canal block versus femoral nerve block". Therefore, imprecision (failed required population, large confidence intervals) was another major problem for our results.
To conclude, we rated only two primary outcomes (postoperative pain at rest (24 hours) and during movement (48 hours) in patients treated with ACB or FNB) as high‐quality evidence, whereas we rated a large number of outcomes as low‐ or very low‐quality evidence with large risk that further research might change conclusions in the future.
Potential biases in the review process
We made two major changes to the protocol (Schnabel 2016), which might have biased the results of the present review: TSA (for dichotomous primary outcomes), respectively, and OIS (for continuous primary outcomes) were performed only if more than 400, respectively, and 200 participants were included to reduce the number of useless analyses (see Differences between protocol and review). Furthermore, we decided to perform subgroup analyses to explore heterogeneity only if more than 10 trials were included for this outcome. Accordingly, we considered these aspects within the GRADE ratings by downgrading if needed. We decided to analyse many clinically primary outcomes at different time points, which might have made it difficult to get an easy view of the evidence from included trials. Unfortunately, these relevant outcomes were only seldom reported. As mentioned above, most trials focused only on specific topics (e.g. functional recovery) and missed several other important postoperative pain‐ and block‐related outcomes. Therefore, there is an urgent need to define relevant core outcome domains for postoperative acute pain (Boric 2017; Cooper 2016; Hussain 2018; Puljak 2018), which should be reported by all future trials investigating interventions for postoperative pain treatment.
Agreements and disagreements with other studies or reviews
Within two years, five meta‐analyses focusing on a comparable topic were published (Dong 2016; Gao 2017; Kuang 2017; Wang 2017; Xing 2017). Four meta‐analyses focused on the comparison of ACB versus FNB (Dong 2016; Gao 2017; Kuang 2017; Wang 2017), and another meta‐analysis investigated ACB combined with periarticular infiltration versus periarticular infiltration alone (Xing 2017). Our results were almost comparable to those focusing on the comparison with FNB (no significant differences in pain intensity at rest and during movement, cumulative opioid consumption, and opioid‐related adverse events). However, one meta‐analysis reported significantly lower risk of accidental falls in the ACB group, which contrasts with our results (Wang 2017). These review authors did not differentiate between time points, in contrast to us. Another earlier meta‐analysis including eight RCTs reported no significant differences in the degree of quadriceps muscle strength (Dong 2016). The meta‐analysis focusing on the comparison ACB combined with periarticular infiltration versus periarticular infiltration alone pooled four included trials and demonstrated significantly reduced postoperative pain intensity (after 0, 1, 2 days), lower opioid consumption (after 0, 1, 2 days), and lower rates of nausea, respectively, and vomiting (after 0, 1, 2 days) (Xing 2017). Four meta‐analyses also used the GRADE approach in assessing evidence (Gao 2017; Kuang 2017; Wang 2017; Xing 2017), but no trial investigated the required information size. Therefore, the evidence level of many outcomes within our review was lower compared to those published in other reviews. Furthermore, we clearly stated that we performed subgroup analyses only if more than 10 trials were included. Therefore many necessary analyses exploring possible reasons for heterogeneity could not be performed, which also lowered the evidence level of outcomes in our review.
Authors' conclusions
Implications for practice.
We are currently uncertain whether patients treated with ACB suffer from lower pain intensity at rest and during movement, fewer opioid‐related adverse events, and fewer accidental falls during postoperative care compared to those given sham treatment. The same holds true for the comparison of ACB versus FNB focusing on postoperative pain intensity. Nevertheless, due to lack of data, for most primary outcomes the required information size has not been reached (apart from pain intensity for the comparison ACB vs FNB), so that the quality of evidence for many outcomes is low or very low. Additionally, due to lack of data, we were not able to perform subgroup analyses ‐ especially focusing on the influence of surgery or continuous versus single shot regional analgesia ‐,which might have influenced the results. The latter two aspects are very important because arthroscopic knee surgery is less painful than knee joint replacement. Furthermore, if no additives (such as dexamethasone (Pehora 2017), buprenorphine (Schnabel 2017)) are used in single shot regional anaesthesia, nerve catheters offer significantly longer analgesia. Further research is therefore required to clarify the role of ACB for postoperative pain treatment following knee surgery.
The 11 studies under Studies awaiting classification and the 11 Ongoing studies, once assessed, may alter the conclusions of this review.
Implications for research.
Due to limited data, several relevant outcomes (opioid‐related adverse events, block‐related adverse events, accidental falls during postoperative care) were only poorly reported but should be studied in the future. This was surprising because many more included trials reported cumulative opioid consumption. We showed a significantly lower morphine requirement 24 hours postop in participants treated with ACB compared to placebo. However, this did not influence the risk for opioid‐related adverse events possibly due to lack of data. Furthermore, if opioid‐related adverse events were reported, most trial authors whose results might have been affected by PONV prophylaxis and general anaesthesia applied by many included trials focused only on nausea, vomiting, and PONV. Additionally, block‐related adverse events are important critical outcomes following all regional anaesthetic techniques, but almost no data were provided by the included trials. Although many trials focused on the degree of quadriceps muscle strength assessed by different tests, only a few have reported the number of patients suffering from an accidental fall during postoperative care, which is a serious complication following knee surgery and possibly regional anaesthesia. Therefore, one major advantage of using ACB instead of femoral nerve or psoas compartment block might be conservation of motor function and possibly reduced risk for accidental falls. Furthermore due to limited data, we were not able to perform interesting subgroup analyses (e.g. proximal vs distal adductor canal blocks (Sztain 2018)), and we were not able to perform meta‐analyses focusing on the comparisons of ACB versus psoas compartment block or ACB versus periarticular infiltration. Future trials are needed to clarify these comparisons. In conclusion, future trials investigating the efficacy and safety of ACB versus sham treatment or other regional anaesthetic techniques should focus on patient‐relevant outcomes including opioid‐related adverse events or block‐related adverse events (e.g. accidental falls) rather than on opioid consumption or degree of quadriceps muscle strength.
Acknowledgements
We would like to thank Stephan Kettner (Content Editor); Marialena Trivella (Statistical Editor); and Luis Muñoz, Nelson Chua, and Joanne Guay (Peer Reviewers) for help and editorial advice provided during preparation of the protocol for the systematic review (Schnabel 2016).
Appendices
Appendix 1. MEDLINE (via PubMed) search strategy
((((randomized controlled trial [pt] OR controlled clinical trial [pt] OR random* [tiab] OR placebo [tiab] OR drug therapy [sh] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))) AND ((knee AND (arthroplas* OR surgery OR surgical OR replac* OR arthrosco*)) OR ((Postoperat* or post operat*) AND (pain OR recovery)) OR postoperative pain[MeSH Terms])) AND (((adduct* OR saphenous*)))
Appendix 2. Embase (Ovid SP) search strategy
1 exp knee surgery/ or (knee and (arthroplas* or surg* or replac* or arthrosco* or operat*)).ti,ab,hw. or postoperative pain/ or postoperative care/ or postoperative complication/dt, pc, rh or pain/pc or ((post operat* or postoperat*) adj6 (pain* or recovery)).ti,ab,hw. 2 adductor canal block/ or (adduct* or saphenous*).ti,ab,hw. 3 ((crossover procedure or double blind procedure or single blind procedure).sh. or (crossover* or cross over*).ti,ab. or placebo*.ti,ab,sh. or (doubl* adj blind*).ti,ab. or (controlled adj3 (study or design or trial)).ti,ab. or allocat*.ti,ab. or trial*.ti,ab. or randomized controlled trial.sh. or random*.ti,ab.) not ((exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)) 4 1 and 2 and 3
Appendix 3. CENTRAL (the Cochrane Library) search strategy
#1 ((adduct* OR saphenous*)) #2 (knee* AND (arthroplas* OR surg* OR replac* OR arthrosco*)) OR ((postoperat* or post operat*) AND (pain OR recovery)) #3 MeSH descriptor: [Pain, Postoperative] explode all trees #4 MeSH descriptor: [Postoperative Care] explode all trees #5 MeSH descriptor: [Postoperative Complications] explode all trees #6 #2 or #3 or #4 or #5 #7 #1 and #6 in Trials
Appendix 4. Web of Science search strategy
# 1 TS=(knee and (arthroplas* or surg* or replac* or arthrosco* or operat*)) OR TS=((“post operative” or postoperative) NEAR/6 (pain* or recover*)
# 2 TS=(adduct* or saphenous*)
# 3 TS=clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=(controlled NEAR (trial* or stud*)) OR TS=follow‐up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=((single or double or triple or treble) or (mask* or blind*)) OR TS=multicenter
#4 #3 AND #2 AND #1
Data and analyses
Comparison 1. Adductor canal block vs sham treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean difference in postoperative pain at rest (2 hours) | 4 | 208 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.20, 0.07] |
| 2 Mean difference in postoperative pain at rest (24 hours) | 6 | 272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.05, 0.07] |
| 3 Mean difference in postoperative pain during movement (2 hours) | 3 | 160 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.50, 0.33] |
| 4 Mean difference in postoperative pain during movement (24 hours) | 4 | 184 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.26, 0.32] |
| 5 Rate of postoperative nausea 2 hours | 2 | 79 | Risk Ratio (IV, Fixed, 95% CI) | 1.75 [0.56, 5.49] |
| 6 Rate of postoperative nausea 24 hours | 3 | 121 | Risk Ratio (IV, Fixed, 95% CI) | 1.91 [0.48, 7.58] |
| 7 Rate of postoperative vomiting 24 hours | 2 | 79 | Risk Ratio (IV, Fixed, 95% CI) | 1.18 [0.56, 2.47] |
| 8 Rate of postoperative nausea and vomiting 24 hours | 2 | 111 | Risk Ratio (IV, Fixed, 95% CI) | 0.54 [0.29, 1.02] |
| 9 Rate of postoperative sedation 2 hours | 2 | 91 | Risk Ratio (IV, Random, 95% CI) | 0.51 [0.17, 1.52] |
| 10 Rate of postoperative sedation 24 hours | 2 | 73 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.20, 3.07] |
| 11 Cumulative mean morphine requirement (until 24 hours postop) | 5 | 232 | Mean Difference (IV, Random, 95% CI) | ‐15.88 [‐30.87, ‐0.89] |
| 12 Rate of failed block | 2 | 89 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Adductor canal block vs femoral nerve block.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean difference in postoperative pain at rest (2 hours) | 5 | 298 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.76, 0.28] |
| 2 Mean difference in postoperative pain at rest (24 hours) | 12 | 868 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.09, 0.18] |
| 3 Mean difference in postoperative pain at rest (48 hours) | 9 | 626 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.71, 1.21] |
| 4 Mean difference in postoperative pain during movement (2 hours) | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.86, 0.93] |
| 5 Mean difference in postoperative pain during movement (24 hours) | 9 | 576 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [‐0.00, 1.12] |
| 6 Mean difference in postoperative pain during movement (48 hours) | 8 | 528 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.10, 0.24] |
| 7 Rate of postoperative nausea 24 hours | 2 | 138 | Risk Ratio (IV, Fixed, 95% CI) | 1.22 [0.42, 3.54] |
| 8 Rate of accidental falls during postoperative care 24 hours | 3 | 172 | Risk Ratio (IV, Fixed, 95% CI) | 0.20 [0.04, 1.15] |
| 9 Rate of accidental falls during postoperative care 48 hours | 2 | 75 | Risk Ratio (IV, Fixed, 95% CI) | 0.27 [0.01, 6.11] |
| 10 Rate of postoperative nausea and vomiting (PONV) 24 hours | 2 | 151 | Risk Ratio (IV, Fixed, 95% CI) | 0.68 [0.44, 1.04] |
| 11 Rate of accidental falls during postoperative care 24 hours | 3 | 172 | Risk Ratio (IV, Fixed, 95% CI) | 0.20 [0.04, 1.15] |
| 12 Rate of accidental falls during postoperative care 48 hours | 2 | 75 | Risk Ratio (IV, Fixed, 95% CI) | 0.27 [0.01, 6.11] |
| 13 Cumulative mean morphine requirement (until 2 hours postop) | 5 | 305 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐0.79, 2.79] |
| 14 Cumulative mean morphine requirement (until 24 hours postop) | 6 | 418 | Mean Difference (IV, Fixed, 95% CI) | ‐1.03 [‐3.48, 1.41] |
| 15 Rate of failed block | 3 | 281 | Risk Ratio (IV, Fixed, 95% CI) | 1.46 [0.16, 12.99] |
| 16 Rate of postoperative block‐related neurological impairment | 4 | 385 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.8. Analysis.
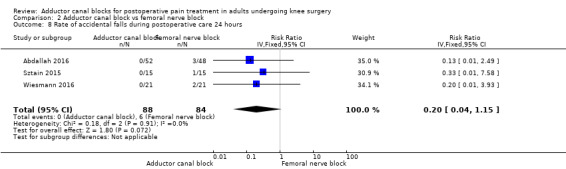
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 8 Rate of accidental falls during postoperative care 24 hours.
2.9. Analysis.
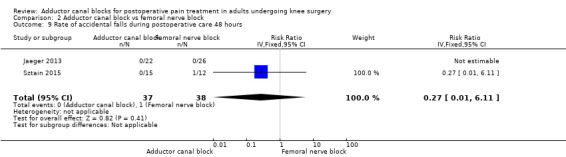
Comparison 2 Adductor canal block vs femoral nerve block, Outcome 9 Rate of accidental falls during postoperative care 48 hours.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdallah 2016.
| Methods | RCT Parallel design |
|
| Participants | 100 patients (ACB: 31.6 years (28.9 to 34.3), 38 males; FNB: 33.3 years (30.7 to 35.9), 26 males), elective unilateral anterior cruciate ligament reconstruction, general anaesthesia | |
| Interventions | Single shot Preoperative Study period: May 2013 to March 2015 ACB: 20 mL R 0.5% with epinephrine (52) FNB: 20 mL R 0.5% with epinephrine (48) |
|
| Outcomes |
|
|
| Notes | Country: USA Conflict of interest: no conflicts of interest among primary researchers Funding: Merit Award Program, Department of Anesthesia, University of Toronto, Canada, University Health Network Innovation Fund Plan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “an investigator with no further involvement in the study generated a list of random numbers in varying block sizes by using an online computer randomization service (www.Randomization.com). The unique randomization code was used to randomize consenting study participants at a 1:1 ratio with no restrictions for either of the 2 study groups: ACB group or FNB group“ |
| Allocation concealment (selection bias) | Unclear risk | Quote: “the results of the allocation were concealed in sealed opaque envelopes and kept with the research coordinator. On the day of surgery, the research coordinator handed an envelope to the attending anesthesiologist or a directly supervised regional anesthesia fellow in the block procedure room immediately before administering the study block to the participant” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “the staff anesthesiologist or fellow performing the block had no further role in the study., […] patient and assessor‐blinded, […] blinded PACU nursing staff“ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ”[…] all outcome data were collected by a blinded research coordinator” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | High risk | Quote: "only minimal secondary outcome data were missing" |
| Other bias | Low risk | No apparent bias |
Akkaya 2008.
| Methods | RCT Parallel design |
|
| Participants | 40 patients (ACB: 43.6 ± 9.51, 9 males; placebo: 47.9 ± 12.22, 11 males), all patients had menial meniscectomy, general anaesthesia | |
| Interventions | Single shot Preoperative Study period: unclear ACB: 10 mL LB 0.5% (20) Placebo: 1 mL saline (20) |
|
| Outcomes |
|
|
| Notes | Country: Turkey Conflict of interest: unclear Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were randomized into 2 groups […]“ |
| Allocation concealment (selection bias) | Unclear risk | Quote: “after written consent from each patient, an envelope was drawn and the patient was allocated into a group” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: n/a |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: n/a |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Patient evaluation was not adequately described |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Andersen 2013.
| Methods | RCT Parallel design |
|
| Participants | 40 patients (ACB: 69 years (54 to 75), 9 males, placebo: 66 years (44 to 74), 10 males), scheduled for unilateral TKA, SPA, and single‐dose LIA | |
| Interventions | Continuous Postoperative Study period: March 2011 to January 2012 ACB: 15 mL R 0.75% (20) Placebo: 15 mL saline (20) |
|
| Outcomes |
|
|
| Notes | Country: Denmark Conflict of interest: no conflicts of interest Funding: BK Medical, Herlev, Denmark provided ultrasound machines Study authors were contacted for further data, but we received no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “computerized random‐number tables [...] were used to randomize the patients” |
| Allocation concealment (selection bias) | Low risk | Quote: “[…] sealed opaque envelopes were used to randomize the patients” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “[…] all other investigators as well as the patients were blinded“ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “[…] all other investigators as well as the patients were blinded“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Elkassabany 2016.
| Methods | RCT Parallel design | |
| Participants | 62 patients (ACB: 63 ± 8 years, 9 males; FNB: 65 ± 8 years, 12 males), scheduled for primary unilateral TKA, ACB | |
| Interventions | Continuous
Preoperative Study period: unclear ACB: 20 mL R 0.5% bolus and 8 mL/h 0.2% R after surgery (31) FNB: 20 mL R 0.5% bolus and 8 mL/h 0.2% R after surgery (31) |
|
| Outcomes |
|
|
| Notes | Country: USA Conflict of interest: no conflicts of interest among primary authors Funding: Education and Development funds, Department of Anesthesiology and Critical Care, University of Pennsylvania |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “a computer‐generated randomization table was used for patient allocation to 1 of the 2 study groups: the FNB group or the ACB group. Randomization was performed in blocks of 10 patients” |
| Allocation concealment (selection bias) | Low risk | Quote: “patients’ assignments were written in a sealed envelope that was opened only after patient consent for the study” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “the dressing over the catheter was made wide enough to conceal the difference of the catheter location between FNB and ACB groups. The nurses on the floor, the research coordinator, and the physical therapist were all blinded to the nature of patient assignment. All PT measurements were performed by the same physical therapist who was blinded to the nature of group assignment“ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “all PT measurements were performed by the same physical therapist who was blinded to the nature of group assignment. The questionnaires were administered by the same research assistant who was blinded to the group assignments“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Espelund 2013.
| Methods | RCT Parallel design | |
| Participants | 49 patients (ACB: 28 ± 11 years, 17 males; placebo: 33 ± 7 years, 21 males), arthroscopic reconstruction of ACL, general anaesthesia | |
| Interventions | Single
Postoperative Study period: June 2010 to March 2012 ACB: 30 mL R 0.75% (25) Placebo: 30 mL saline 0.9% (24) |
|
| Outcomes |
|
|
| Notes | Country: Denmark Conflict of interest: no conflicts of interest Funding: Department of Anaesthesiology, University of Copenhagen, Glostrup Hospital, Capital Region of Denmark |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “fifty identical packages containing either ropivacaine 7.5 mg/ml or 0.9% saline (control group) were labelled with name of the project and numbered according to a computer‐generated block randomisation list prepared by the pharmacy in five blocks, each containing 10 numbers” |
| Allocation concealment (selection bias) | Low risk | Quote: “fifty identical packages ... were labelled with name of the project and numbered according to a computer‐generated block randomisation list prepared by the pharmacy in five blocks, each containing 10 numbers. Data from the patients were registered according to the randomisation number. Each package was opened and the medicine prepared in a syringe by a nurse not involved in the study or postoperative care of the patient. All medications administered ‘in hospital’ were given to the patient and registered by one of the investigators" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “each package was opened and the medicine prepared in a syringe by a nurse not involved in the study or postoperative care of the patient. All medications administered ‘in hospital’ were given to the patient and registered by one of the investigators. No investigator person treating or nursing the patients was aware of group assignment until all patients had been included and data collection was completed“ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "no investigator […] was aware of group assignment until all patients had been included and data collection was completed. Data were collected by an investigator consulting the patients directly in hospital and afterwards by telephone“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported Quote: "one patient in the intervention group had to stop mobilising at 8 h postoperatively due to intraarticular bleeding and pain" |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Espelund 2014a.
| Methods | RCT Parallel design | |
| Participants | 81 patients (ACB: 46 ± 14 years, 16 males; placebo: 43 ± 14 years, 19 males), minor arthroscopic knee surgery, general anaesthesia | |
| Interventions | Single
Postoperative Study period: November 2010 to August 2011 ACB: 30 mL R 0.75% (36) Placebo: 30 mL saline 0.9% (35) |
|
| Outcomes |
|
|
| Notes | Country: Denmark Conflict of interest: no conflicts of interest Funding: Department of Anaesthesiology, University of Copenhagen, Glostrup Hospital, Capital Region of Denmark Study authors were contacted for further data, but we received no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “seventy‐two identical packages containing either ropivacaine 7.5 mg/ml or isotonic saline (placebo) were prepared by the hospital pharmacy and labelled with project and randomisation ID, according to a computer‐generated block randomisation list, the blocks containing 9 × 8 numbers“ |
| Allocation concealment (selection bias) | Low risk | Quote: ”seventy‐two identical packages containing … were prepared by the hospital pharmacy and labelled with project and randomisation ID ... Data from the included patients were registered according to the related randomisation number. Each package containing study medicine was opened and the medicine prepared in a neutral syringe" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "each package containing study medicine was opened and the medicine prepared in a neutral syringe. This was done by a nurse who was not otherwise involved in the study or in the postoperative care of the patient. All medications administered ‘in hospital’ were administered and registered by one of the investigators. No investigator or person treating or nursing the patients was aware of group assignment until all patients had been included and data‐handling was completed” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "no investigator or person treating or nursing the patients was aware of group assignment until all patients had been included and data‐handling was completed” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Hanson 2013.
| Methods | RCT Parallel design |
|
| Participants | 48 patients (ACB: 54 ± 11 years, 20 males; placebo: 51 ±11 years, 17 males), scheduled for knee arthroscopy and primary unilateral medial meniscectomy, general anaesthesia | |
| Interventions | Single Preoperative Study period: June 2011 to June 2012 ACB: 15 mL R 0.5% with 1:400,000 E (24) Placebo: 2 mL saline solution (24) |
|
| Outcomes |
|
|
| Notes | Country: USA Conflict of interest: no conflicts of interest Funding: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were assigned by pre‐randomized sealed envelopes to receive an ultrasound‐guided saphenous nerve block at the adductor canal (n = 25) or a sham block (n = 25)” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “patients were assigned by pre‐randomized sealed envelopes to receive an ultrasound‐guided saphenous nerve block at the adductor canal (n = 25) or a sham block (n = 25)” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blinded"... “All anesthesia, surgical, and nursing personnel caring for the patient intraoperatively were blinded to the randomization of the subjects. ... Nurses blinded to the randomization of the study patients recorded the patients’ pain scores on an 11‐point numerical rating scale (NRS) ...” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “data were collected on the day of surgery and via telephone conversation by a blinded investigator 24 hr after the surgical procedure“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Hegazy 2015.
| Methods | RCT Parallel design | |
| Participants | 107 patients (ACB: 62 ± 11 years 26 males; FNB: 63 ± 12 years, 25 males), elective unilateral primary TKA, SPA | |
| Interventions | Single
Preoperative Study period: June 2013 to March 2014 ACB: 20 mL R 0.5% (53) FNB: 20 mL R 0.5% (54) |
|
| Outcomes |
|
|
| Notes | Country: Egypt Conflict of interest: no conflicts of interest Funding: unclear Study authors were contacted for further data, but we received no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “they were assigned to either the ACB group or the FNB group (1:1 allocation, parallel trial design), on the basis of a computer‐generated randomization list created by independent researcher“ |
| Allocation concealment (selection bias) | Low risk | Quote: “group assignment was concealed by opaque envelopes that were opened only after enrolment” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “the two anesthesiologists performing the block were expert in ultrasound‐guided nerve blocks and aware of the treatment but not involved in any other aspect of the study including data collection, but both patients and research assistant were blinded to the group assignment and the type of the block” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “a research assistant recorded the patient’s demographic data (age, sex, height, weight, BMI, ASA physical status classification) preoperatively. In addition, all patients were tutored in the numeric rate scale (NRS) for pain score assessment, as well as trained in the timed up and go (TUG) test and in the use of intravenous patient‐controlled analgesia“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Jaeger 2013.
| Methods | RCT Parallel design |
|
| Participants | 48 patients (ACB: 70 ± 8 years, 5 males; FNB: 66 ± 9 years, 14 males), scheduled for TKA, SPA | |
| Interventions | Continuous
Postoperative Study period: November 2011 to November 2012 ACB: 30 mL R 0.5%, infusion of R 0.2% 8 mL/h during the next 24 hours (22) FNB: 30 mL R 0.5%, infusion of R 0.2% 8 mL/h during the next 24 hours (26) |
|
| Outcomes |
|
|
| Notes | Country: Denmark Conflict of interest: no conflicts of interest Funding: Award of the European Society of Regional Anaesthesia and Pain Therapy Research Grant Study authors were contacted for further data, and they responded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was based on a computer‐generated block randomization list (each block containing 10 numbers), in a 1:1 ratio" |
| Allocation concealment (selection bias) | Low risk | Quote: "upon inclusion in the study, subjects received the treatment assigned according to the randomization list, in consecutively numbered, sealed, opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “all subjects, outcome assessors, and clinical personnel were blinded to the intervention except for the investigators performing the blocks. These investigators were not involved in data collection or in handling the data. Care was taken to assure blinding of the subject and other clinical personnel. During block performance, the patient was shielded from other patients and staff, and the patient’s view of the injection site was blocked by blankets. Each subject received both the assigned treatment catheter and a sham catheter to facilitate blinding of the patient and staff” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “all subjects, outcome assessors, and clinical personnel were blinded ...” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Jaeger 2014.
| Methods | RCT Parallel design | |
| Participants | 30 patients (ACB: 65 years (50 to 78) 8 males; placebo 67 years (42 to 83), 8 males), scheduled for revision TKA, general anaesthesia | |
| Interventions | Continuous
Postoperative Study period: August 2010 to March 2013 ACB: 30 mL R 0.75%, after 6 hours 15 mL bolus and 8 mL/h R 0.2% (14) (2nd analysis: 11) Placebo: 30 mL saline, after 6 hours 15 mL bolus and 8 mL/h saline (16) (2nd analysis: 13) |
|
| Outcomes |
|
|
| Notes | Country: Denmark Conflict of interest: no conflicts of interest Funding: no funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “the pharmacy prepared the study medication in identical prepacked boxes, consecutively numbered according to a computer generated block randomization list (1:1 ratio, blocks of 10)” |
| Allocation concealment (selection bias) | Low risk | Quote: “the pharmacy prepared the study medication in identical prepacked boxes... Subjects were assigned consecutive numbers upon inclusion to the study and received the study medication from the corresponding boxes“ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “a research fellow neither involved in the study nor in the care of the patient administered the study medication in unlabeled syringes for injection and unmarked drug bags for infusion, before handing it over to the investigators. Ropivacaine and saline are identical in appearance. All investigators, staff and patients were blinded to the treatment groups. The randomization key was first broken after all patients were enrolled, data computed and statistical analyses performed“ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “all investigators, staff and patients were blinded to the treatment groups. The randomization key was first broken after all patients were enrolled, data computed and statistical analyses performed“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Jenstrup 2012.
| Methods | RCT Parallel design | |
| Participants | 71 patients (ACB: 67 ± 7 years, 18 males; placebo: 67 ± 9 years, 19 males), scheduled for primary TKA, SPA | |
| Interventions | Continuous
Postoperative Study period: August 2010 to March 2011 ACB: 30 mL R 0.75%, 15 mL boluses R 0.75% after 6 hours, 12 hours, 18 hours, 24 hours postoperatively (34) Placebo: 30 mL saline, 15 mL boluses saline after 6 hours, 12 hours, 18 hours, 24 hours postoperatively (37) |
|
| Outcomes |
|
|
| Notes | Country: Denmark Conflict of interest: no conflicts of interest Funding: institutional and departmental funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the study medication was prepared by the pharmacy in identical glass containers and pre‐packed in boxes, one for each patient. These were consecutively numbered according to a computer generated block randomization list, performed by the pharmacy in a 1:1 ratio, each block containing 10 numbers, except for the last block, which only contained five numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: “upon inclusion into the study the participants were assigned consecutive numbers and received the study medication in the corresponding boxes“ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “all investigators, staff, and patients were blinded to the treatment groups. The randomization key was first broken once enrollment of all patients was completed and data computed“ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “all investigators, staff, and patients were blinded to the treatment groups. The randomization key was first broken once enrollment of all patients was completed and data computed“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Koh 2017a.
| Methods | RCT Parallel design |
|
| Participants | 50 patients (mean age 66.9 years; 49 females, 1 male) | |
| Interventions | Single Postoperative Study period: July 2015 to April 2016 ACB: 10 mL R 0.75% + fentanyl PCA FNB: 10 mL R 0.75% + fentanyl PCA |
|
| Outcomes |
|
|
| Notes | Country: South Korea Conflict of interest: no conflicts of interest Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “… computer‐generated randomization table, permuted in to blocks of 4 and 6….” |
| Allocation concealment (selection bias) | Low risk | Quote: “….allocation was assigned ….by a scrub nurse who was not involved…”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: …”independent investigator and patients were unaware…until data analyses were completed…” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: …”independent investigator and patients were unaware…until data analyses were completed…” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | No apparent bias |
| Other bias | Low risk | No apparent bias |
Li 2017.
| Methods | RCT Parallel design | |
| Participants | 77 patients (ACB: 62.3 ± 6.5 years, 11 males; FNB: 61.4 ± 6.8 years, 13 males; MIA: 62.6 ± 7.3 years, 14 males), unilateral primary total knee arthroplasty for osteoarthritis, ACB | |
| Interventions | Single Preoperative Study period: unclear ACB: 20 mL R 0.5% (24) FNB: 20 mL R 0.5% (27) Multi‐site infiltration: 30 mL R 0.25% + 0.1 mg E periarticular + joint capsule; 20 mL R 0,25% + 0.1 mg E joint, 20 mL mix subcutaneous tissue (26) |
|
| Outcomes |
|
|
| Notes | Country: China Conflict of interest: unclear Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “a computerized random number generator was used" |
| Allocation concealment (selection bias) | Low risk | Quote: ”numbers were stored in opaque sealed envelopes. The patient was asked to select one envelope on the morning of surgery" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “this trial was blind to the patients, surgery, and statisticians“ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “this trial was blind to the patients, surgery, and statisticians“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Machi 2015.
| Methods | RCT Parallel design | |
| Participants | 80 patients (ACB: 67 ± 8 years, 16 males; FNB: 66 ± 7 years, 14 males), scheduled for unilateral, tricompartment knee arthroplasty, SPA, or general anaesthesia | |
| Interventions | Continuous
Preoperative Study period: unclear ACB: 30 mL lidocaine 2%, 3 day R 0.2% at a rate of 6 mL/h, 4 mL bolus (39) FNB: 30 mL lidocaine 2%, 3 day R 0.2% at a rate of 6 mL/h, 4 mL bolus (41) |
|
| Outcomes |
|
|
| Notes | Country: USA Conflict of interest: no conflicts of interest Funding: University California Academic Senate, Summit Medical, Teleflex Medical |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomization lists were created by Investigational Drug Service personnel using a computer‐generated randomization table in blocks of four, with a 1:1 ratio, stratified by both treatment center and surgeon“ |
| Allocation concealment (selection bias) | Low risk | Quote: “treatment allocation was concealed using consecutively numbered, sealed, opaque envelopes that were opened only after confirmation by ultrasound that either insertion site would be acceptable" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “neither study participants nor investigators were masked to treatment group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “last, subjects and investigators were not masked to treatment group. Although it is unlikely that subjects had a predisposition toward one insertion site versus another, outcome assessors (nursing staff, physical therapists, and investigators) may have had preconceived bias toward one of the two treatments. In addition, caretaker bias may have been subconsciously transferred to patients, and therefore biased the results“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Macrinici 2017.
| Methods | RCT Parallel design | |
| Participants | 93 analysed patients (ACB: 67 ± 8 years, 19 males; FNB: 67 ± 8 years, 18 males), set to undergo TKA, multi‐modal analgesic regimen, and LIA | |
| Interventions | Single Postoperative Study period: unclear ACB: 30 mL of LA (100 mg bupivacaine + epinephrine) (46, 6 months, n =40) FNB: 30 mL of LA (100 mg bupivacaine + epinephrine) (47, 6 months, n = 42) |
|
| Outcomes |
|
|
| Notes | Country: USA Conflict of interest: no conflicts of interest Funding: PSJMC, Medical Staff Office, Joliet and American Associates of Illinois Study authors were contacted for further data; they responded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “the randomization lists were generated by SAS PROC PLAN using a randomized block design with a fixed block size of 4. A random‐number seed was supplied to the program to start the random‐number generator used by SAS. Limited unblinded pharmacy personnel assigned patients to interventions" |
| Allocation concealment (selection bias) | Low risk | Quote: “the pharmacy was instructed to label each syringe with the following information: randomization number, subject number, and treatment location. The syringes were labeled with only the treatment location and not the actual treatment assignment, so the site staff who were performing the injections remained blinded to the treatment“ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “the design was double‐blind with limited pharmacy personnel having the patient identifiers with the interventions assigned: the anesthesiologists, surgeons, patients, and physical therapists had no knowledge of which nerve block procedures had the local anesthetic medication or normal saline administered to the patient“ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “the trained physical therapist team at PSJMC performed all the evaluations. ... the anesthesiologists, surgeons, patients, and physical therapists had no knowledge of which nerve block procedures had the local anesthetic medication or normal saline administered to the patient“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Memtsoudis 2015.
| Methods | RCT Parallel and cross‐over design | |
| Participants | 59 patients (age: 64.41 ± 7.36 years, 26 males), scheduled for bilateral TKA, SPA, and epidural catheter | |
| Interventions | Single
Preoperative Study period: April 2012 to September 2013 ACB + FNB: 15 mL B 0.25%, 30 mL B 0.25% (30) left leg saphenous, right leg femoral FNB + ACB: 30 mL B 0.25%, 15 mL B 0.25% (29) right leg saphenous, left leg femoral |
|
| Outcomes |
|
|
| Notes | Country: USA Conflict of interest: no conflicts of interest Funding: Hospital for Special Surgery, Department of Anesthesiology, New York, Anna‐Maria and Stephen Kellen Physician‐Scientist Career Development Award, New York |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “the two extremities were randomised to receive either US guided subsartorial” |
| Allocation concealment (selection bias) | Low risk | Quote: “[…] using blinded envelopes prepared by a independent research assistant and only visible to the attending anaesthesiologist assigned" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “patients, surgeons, physical therapists and research assistants performing the follow‐up were blinded to the randomisation. Subsequently, blocks were performed as randomised using a sterile technique" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “[...] research assistants performing the follow‐up were blinded to the randomisation. All study data were collected and managed by using REDCap electronic data capture tools through the Clinical and Translational Science Center at Weill Cornell Medical College" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Messeha 2016.
| Methods | RCT Parallel design | |
| Participants | 90 patients (ACB: 40.3 ± 12.9 years, 27 males; PCB: 42.5 ± 14.2 years, 29 males), elective laparoscopic knee surgeries, sciatic nerve block | |
| Interventions | Single
Preoperative Study period: 9 months ACB: 25 mL B 0.5% with adrenaline 1:400,000 and sciatic nerve block (45) PCB: 25 mL B 0.5% with adrenaline 1:400,000 and sciatic nerve block (45) |
|
| Outcomes |
|
|
| Notes | Country: Egypt Conflict of interest: no conflicts of interest Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “[…] patients were randomly divided into two equal groups (45 patients in each group)” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “[…] using closed envelope techniques” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | High risk | Not all (secondary) outcomes were reported |
| Other bias | Low risk | No apparent bias |
Nader 2016.
| Methods | RCT Parallel design | |
| Participants | 40 patients (ACB: 68 years (62 to 73), 5 males; placebo: 67 years (59 to 72), 7 males), elective TKA, SPA, and LIA | |
| Interventions | Single
Preoperative Study period: September 2014 to October 2015 ACB: 10 mL B 0.25% with E 1:300,000 (20) Placebo: 10 mL saline (20) |
|
| Outcomes |
|
|
| Notes | Country: USA Conflict of interest: no conflicts of interest Funding: institutional and departmental resources |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “subjects were randomized into 2 groups using a computerized random number generator” |
| Allocation concealment (selection bias) | Low risk | Quote: “[…] a sequentially numbered sealed opaque envelope was opened by a study investigator not involved in care of the patient. The same investigator prepared the study medication and labeled it as 'study drug'" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “the surgeons, the anesthesiologist involved in patient care during the surgery, the research personnel involved in patient evaluation, as well as the patient were blinded to the study arm“ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “study personnel unaware of group allocation made postoperative follow‐up assessments. ... Patients were evaluated at the surgeon's office at 3 weeks after surgery by the same investigator" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Rahimzadeh 2017.
| Methods | RCT Parallel design | |
| Participants | 92 patients (ACB: 35.3 ± 15.8 years, 32 males; FNB: 37.5 ± 15.2 years, 29 males), undergoing arthroscopic knee surgery, general anaesthesia | |
| Interventions | Single
Postoperative Study period: March 2014 to June 2015 ACB: 12 mL B 0.125% (46) FNB: 12 mL B 0.125% (46) |
|
| Outcomes |
|
|
| Notes | Country: Iran Conflict of interest: no conflicts of interest Funding: no funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “[…] randomised into two groups using the block randomisation method based on block of 4” |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Sawhney 2016.
| Methods | RCT Parallel design | |
| Participants | 159 (145 follow‐up) patients (ACB + PI: 68.3 ± 9.7 years, 21 males; ACB + PI sham: 66.4 ± 9.6 years, 20 males; ACB sham + PI: 67.6 ± 9.4 years, 18 males), patients scheduled for primary TKA, SPA | |
| Interventions | Single shot
Preoperative Study period: May 2013 to February 2014 ACB + PI (periarticular infiltration): 30 mL R 0.5% and 110 mL saline solution consisting of 300 mg R, 10 mg morphine, and 30 mg ketorolac (50) ACB + PI sham: 30 mL R 0.5% (46) ACB sham + PI: 110 mL saline solution consisting of 300 mg R, 10 mg morphine, and 30 mg ketorolac (49) |
|
| Outcomes |
|
|
| Notes | Country: Canada Conflict of interest: no conflicts of interest Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “after consent was obtained, participants’ baseline demographic information was collected and randomly assigned to 1 of the 3 groups via a web‐based computerized block randomization service (randomize.net)“ |
| Allocation concealment (selection bias) | Low risk | Quote: “a blinded 110‐mL PI solution bag was prepared by the pharmacy and delivered to the operating room (OR)” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “all participants, orthopedic surgeons, members of the acute pain service, and outcome assessors were blinded to the group allocation. The surgical team was blinded to the contents of the study bag” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “[…] outcome assessors were blinded to the group allocation” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Shah 2014.
| Methods | RCT Parallel design | |
| Participants | 98 patients (ACB: 68.31 ± 7.56 years, 13 males; FNB: 65.94 ± 7.22 years, 14 males), elective unilateral primary TKA, SPA | |
| Interventions | Continuous Postoperative Study period: July 2013 to January 2014 ACB: 30 mL R 0.75%, bolus injection of R 0.25% every 4 hours until POD 2 (48) FNB: 30 mL R 0.75%, bolus injection of R 0.25% every 4 hours until POD 2 (50) |
|
| Outcomes |
|
|
| Notes | Country: India Conflict of interest: no conflicts of interest Funding: unclear Study authors were contacted for further data; they responded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “100 patients undergoing unilateral TKA were enrolled and randomized into two groups, (1) continuous adductor canal block (CACB) group and (2) continuous femoral nerve block (CFNB) group, using a computer generated randomization table with a permutation block of six" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “the patients and a clinical investigator, who prospectively collected all clinical information, were unaware of the group identities until the final data analysis” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “the patients and a clinical investigator, who prospectively collected all clinical information, were unaware of the group identities until the final data analysis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Sztain 2015.
| Methods | RCT Parallel design | |
| Participants | 30 patients (ACB: 70 ± 10 years, 8 males; FNB: 68 ± 12 years, 8 males), undergoing unilateral, unicompartment knee arthroplasty, LIA | |
| Interventions | Continuous
Preoperative Study period: unclear ACB: R 0.2% infusion for 2 days, basal rate 6 mL/h; 4 mL bolus; 30 minute lockout (15) FNB: R 0.2% infusion for 2 days, basal rate 6 mL/h; 4 mL bolus; 30 minute lockout (15) |
|
| Outcomes | Pain at rest on NRS (2 hours, 24 hours, 48 hours) Pain during movement on NRS (2 hours, 24 hours, 48 hours) Degree of quadriceps muscle strength (2 hours, 24 hours, 48 hours) Cumulative opioid consumption (24 hours, 48 hours) Rate of accidental falls |
|
| Notes | Country: USA Conflict of interest: no conflicts of interest Funding: University California Academic Senate, Summit Medical, Teleflex Medical |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Investigational Drug Service personnel used a computer to create randomization lists with a 1:1 ratio, in blocks of 4, stratified by both surgeon and treatment center" |
| Allocation concealment (selection bias) | Low risk | Quote: “[…] the subject was randomized using numbered, sealed, opaque envelopes to receive either a femoral or an adductor canal perineural catheter“ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded Quote: “outcome assessors (nursing staff, physical therapists, and investigators) may have had preconceived bias toward 1 of the 2 treatments" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “outcome assessors (nursing staff, physical therapists, and investigators) may have had preconceived bias toward 1 of the 2 treatments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Wiesmann 2016.
| Methods | RCT Parallel design | |
| Participants | 42 patients (ACB; 72 years (59 to 75), 9 males; FNB: 66 years (62 to 74), 9 males), elective unilateral TKA, ASNB | |
| Interventions | Continuous
Preoperative Study period: May 2013 to November 2014 ACB: 15 mL R 0.375%, R 0.2% infusion basal rate 6 mL/h, 6 mL bolus, 30 minute lockout (21) FNB: 15 mL R 0.375%, R 0.2% infusion basal rate 6 mL/h, 6 mL bolus, 30 minute lockout (21) |
|
| Outcomes |
|
|
| Notes | Country: Germany Conflict of interest: unclear Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “[…] the block‐random allocation sequence was generated on http://www.sealedenvelope.com“ |
| Allocation concealment (selection bias) | Unclear risk | Quote: “sealed envelope randomisation was used" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “[…] patients were not informed as to their group. ... Insertion sites were occluded using a sterile draping technique covering both possible catheter insertion sites to maintain double blinding. .... Staff performing the mobilisation tests and documenting the data were also unaware of the randomisation“ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “staff performing the mobilisation tests and documenting the data were also unaware of the randomisation. ... Only two trained study assistants assessed patients’ baseline parameters and test results to reduce potential bias factors" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were evaluated; no data were missing |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Low risk | No apparent bias |
Zhang 2014.
| Methods | RCT Parallel design |
|
| Participants | 60 patients (ACB: 63.7 ± 5.8 years, 6 males; FNB: 61.9 ± 6.7 years, 8 males), scheduled for total knee replacement, combined spinal‐epidural anaesthesia | |
| Interventions | Continuous
Postoperative Study period: unclear ACB: R 0.2% infusion basal rate 5 mL/h (30) FNB: R 0.2% infusion basal rate 5 mL/h (30) |
|
| Outcomes |
|
|
| Notes | Country: China Conflict of interest: unclear Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... randomly divided into a femoral group and an adductor group..." |
| Allocation concealment (selection bias) | Unclear risk | Quote: n/a |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: n/a |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: n/a |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient source |
| Selective reporting (reporting bias) | Low risk | Obviously, all primary and secondary outcome data were reported |
| Other bias | Unclear risk | Insufficient source |
Zhao 2017.
| Methods | RCT Parallel design |
|
| Participants | 40 patients (7 males, 63.8 ± 10.1), severe knee ostarthritis undergoing unilateral knee arthoplasty | |
| Interventions | Continuous
Postoperative Study period: April 2016 to September 2016 ACB: (20) FNB: (20) |
|
| Outcomes |
|
|
| Notes | Country: China Conflict of interest: unclear Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...all the patients were randomized ... " |
| Allocation concealment (selection bias) | Unclear risk | Quote: n/a |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: n/a |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: n/a |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient source |
| Other bias | Unclear risk | Insufficient source |
ACB: adductor canal block.
ACL: anterior cruciate ligament.
B: bupivacaine.
CFNB: continuous femoral nerve block.
E: epinephrine.
FNB: femoral nerve block.
LA: local anaesthetic.
LB: levobupivacaine.
LIA: local infiltration analgesia.
MMT: manual muscle testing.
MVIC: maximal voluntary isometric contraction.
NRS: numerical rating scale.
PACU: postoperative anaesthesia care unit.
PCA: patient‐controlled analgesia.
PCB: psoas compartment block.
PI: periarticular infiltration.
POD: postoperative day.
R: ropivacaine.
RCT: randomized controlled trial.
SPA: spinal analgesia.
TKA: total knee arthroplasty.
VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beausang 2016 | Different use of LA in ACB and control groups |
| Espelund 2014b | Cross‐over design |
| Grant 2017 | Retrospective analysis |
| Grevstad 2014 | Cross‐over design |
| Grevstad 2015 | Cross‐over design |
| Gwam 2017 | Retrospective analysis |
| Hanson 2014 | Some participants were treated differently than was described in the protocol with additional infiltrations |
| Henshaw 2016 | Different amount of LA |
| Jaeger 2012 | Some participants were treated differently than was described in the protocol |
| Jaeger 2013b | Volunteers |
| Joe 2016 | Investigated hindfoot and ankle surgery instead of knee surgery |
| Kim 2014 | Different amount of LA |
| Kwofie 2013 | Volunteers were investigated |
| Monahan 2016 | Volunteers were investigated |
| Ortiz‐Gomez 2017 | Different amount of LA |
| Seo 2017 | Retrospective analysis |
| Shah 2015 | Comparison: single vs continuous ACB |
| Sogbein 2017 | Comparison: ACB vs periarticular infiltration; different amount of LA |
| Sorensen 2016 | Cross‐over design |
ACB: adductor canal block.
LA: local anaesthetic.
Characteristics of studies awaiting assessment [ordered by study ID]
Biswas 2018.
| Methods | RCT Parallel design |
| Participants | 201 participants |
| Interventions |
|
| Outcomes |
|
| Notes | Declaration of interests: none Funding: no information regarding funding |
Chisholm 2014.
| Methods | RCT Parallel design |
| Participants | 80 participants |
| Interventions |
|
| Outcomes |
|
| Notes | Declaration of interests: none Funding: no information within the article |
Grosso 2018.
| Methods | RCT Parallel design |
| Participants | 155 participants |
| Interventions |
|
| Outcomes |
|
| Notes | Declaration of interests: none Funding: Orthopaedic Research and Education Foundation (OREF) Grant 16‐023 |
Kampitak 2018.
| Methods | RCT Parallel design |
| Participants | 60 participants |
| Interventions | Single‐injection adductor canal block Local infiltration analgesia |
| Outcomes |
|
| Notes | Declaration of interests: none Funding: Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University, Grant number RA58/047 |
Kayupov 2018.
| Methods | RCT Parallel design |
| Participants | 145 participants |
| Interventions |
|
| Outcomes |
|
| Notes | Declaration of interests: none Funding: departmental funding |
Lenz 2018.
| Methods | RCT Parallel design |
| Participants | 82 participants |
| Interventions |
|
| Outcomes |
|
| Notes | Declaration of interests: none Funding: departmental funding |
Leung 2018.
| Methods | RCT Parallel design |
| Participants | 165 participants |
| Interventions | Continuous adductor canal block Sham catheter |
| Outcomes |
|
| Notes | Declaration of interests: none Funding: The Kovler Family Foundation and The Barnett Family Trust |
Lim 2018.
| Methods | RCT Parallel design |
| Participants | 30 participants |
| Interventions | Adductor canal block Femoral nerve block |
| Outcomes |
|
| Notes | Declaration of interests: none Funding: departmental funding |
Rousseau‐Saine 2018.
| Methods | RCT Parallel design |
| Participants | 63 participants |
| Interventions |
|
| Outcomes |
|
| Notes | Declaration of interests: none Funding: departmental |
Runner 2018.
| Methods | RCT Parallel design |
| Participants | 102 participants |
| Interventions |
|
| Outcomes |
|
| Notes | Declaration of interests: senior author JWX is a consultant for Arthrex, Mye‐Eye, Linvatec, and VisionScope and has received educational support from Linvatec Funding: none |
Tong 2018.
| Methods | RCT Parallel design |
| Participants | 40 participants |
| Interventions |
|
| Outcomes |
|
| Notes | Declaration of interests: none Funding: departmental |
CSE: combined spinal‐epidural.
IQR: interquartile ratio.
POD: postoperative day.
RCT: randomized controlled trial.
VAS: visual analogue scale.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800018463.
| Trial name or title | Efficacy of different lower extremity nerve block combined with general anaesthesia in knee arthroscopic surgery |
| Methods | RCT Parallel design |
| Participants | Unclear |
| Interventions |
|
| Outcomes |
|
| Starting date | September 2018 |
| Contact information | liu711029@hotmail.com |
| Notes | Declaration of interests: unclear Funding: unclear |
NCT02071433.
| Trial name or title | Analgesic efficacy of saphenous nerve blockade for outpatient knee anterior cruciate ligament surgery |
| Methods | RCT Parallel design |
| Participants | 58 participants |
| Interventions |
|
| Outcomes |
|
| Starting date | March 2014 |
| Contact information | w.tenhoope@amc.uva.nl |
| Notes | Declaration of interests: unclear Funding: unclear |
NCT02276495.
| Trial name or title | Does single injection adductor canal block improve postoperative analgesia in patients receiving periarticular local anesthesia injections for total knee arthroplasty? |
| Methods | RCT Parallel design |
| Participants | 90 participants |
| Interventions |
|
| Outcomes |
|
| Starting date | October 2014 |
| Contact information | canalesc@uci.edu |
| Notes | Declaration of interests: unclear Funding: unclear |
NCT02419261.
| Trial name or title | Assessment of sensory and motor blockade of the adductor canal blockade performed for surgery of arthroscopic anterior cruciate ligament repair |
| Methods | RCT Paralell design |
| Participants | 40 participants |
| Interventions |
|
| Outcomes |
|
| Starting date | October 2014 |
| Contact information | jplecoq@chu.ulg.ac.be |
| Notes | Declaration of interests: unclear Funding: unclear |
NCT02863120.
| Trial name or title | Patient outcomes with periarticular liposomal bupivacaine injection vs adductor canal block after primary total knee arthroplasty |
| Methods | RCT Parallel design |
| Participants | 250 participants |
| Interventions |
|
| Outcomes |
|
| Starting date | February /2016 |
| Contact information | atorres@txortho.com |
| Notes | Declaration of interests: unclear Funding: unclear |
NCT03033589.
| Trial name or title | Femoral nerve block versus adductor canal nerve block for peri‐operative analgesia following anterior cruciate ligament reconstruction: evaluation of post‐operative pain and strength |
| Methods | RCT Parallel design |
| Participants | 80 participants |
| Interventions |
|
| Outcomes |
|
| Starting date | May 2016 |
| Contact information | jlynch6@hfhs.org |
| Notes | Conflict of interests: unclear Funding: unclear |
NCT03188809.
| Trial name or title | Adductor canal block versus femoral nerve block with repeated bolus doses: postoperative analgesia and functional outcomes after total knee arthroplasty |
| Methods | RCT Parallel design |
| Participants | 42 participants |
| Interventions |
|
| Outcomes |
|
| Starting date | April 2017 |
| Contact information | kurtbeyogluseda@gmail.com |
| Notes | Declaration of interests: unclear Funding: unclear |
NCT03205540.
| Trial name or title | Epidural analgesia vs adductor canal block in bilateral TKA |
| Methods | RCT Parallel design |
| Participants | 70 participants |
| Interventions |
|
| Outcomes |
|
| Starting date | August 2017 |
| Contact information | stangwiwat@yahoo.com |
| Notes | Declaration of interests: unclear Funding: unclear |
NCT03208478.
| Trial name or title | A prospective comparison of pain and quality of recovery in patients undergoing anterior cruciate ligament reconstruction with adductor canal or femoral perineural infusions |
| Methods | RCT Parallel design |
| Participants | 50 participants |
| Interventions |
|
| Outcomes |
|
| Starting date | June 2018 |
| Contact information | hornj@stanford.edu |
| Notes | Conflict of interests: unclear Funding: unclear |
NCT03518450.
| Trial name or title | Early mobilization and postoperative analgesia after total knee arthroplasty, a prospective comparative study: adductor canal block vs femoral nerve block vs apex femoral triangle block |
| Methods | RCT Parallel design |
| Participants | 126 participants |
| Interventions |
|
| Outcomes |
|
| Starting date | April 2018 |
| Contact information | csalvadores@vhebron.net |
| Notes | Declaration of interests: unclear Funding: unclear |
NCT03620136.
| Trial name or title | Comparison of two techniques of locoregional analgesia in total knee prosthesis surgery: block to the adductor channel versus peri‐articular local infiltrations |
| Methods | RCT Parallel design |
| Participants | 120 participants |
| Interventions |
|
| Outcomes |
|
| Starting date | February 2018 |
| Contact information | caroline.macabeo@chu‐lyon.fr |
| Notes | Declaration of interests: unclear Funding: unclear |
ACB: adductor canal block.
APS‐POQ‐R: Revised American Pain Society Patient Outcome Questionnaire.
CPM: continuous passive motion machine.
CST: 30‐second Chair Stand Test.
FNB: femoral nerve block.
IKDC: International Knee Documentation Committee score.
KOOS: Knee Injury and Osteoarthritis Outcome score.
LOS: length of stay.
MVIC: maximum voluntary isometric contraction.
NRS: numerical rating scale.
OBAS: Overall Benefit of Analgesia score.
OME: oral morphine equivalent.
PACU: postoperative anaesthesia care unit.
PADSS: Post‐Anaesthetic Discharge Scoring System.
PMS: 10‐Point Mobility Scale.
POD: postoperative day.
RCT: randomized controlled trial.
ROM: range of motion.
SF‐12: Short Form 12.
TKA: total knee arthroplasty.
TUG: timed up and go.
VAS: visual analogue scale.
Differences between protocol and review
We made the following changes to the published protocol (Schnabel 2016).
We performed TSA only for dichotomous outcomes and OIS for continuous primary outcomes, if more than 400 and 200 participants were included in the analysis, respectively, or if the 95% CI of the estimated effects did not cross the line of no effect. The quality of evidence for outcomes with small sample sizes was downgraded due to imprecision.
As recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we performed subgroup analyses only if more than 10 trials were included for this outcome.
We added the comparator “or any other regional anaesthetic technique” to the 'Types of interventions' section.
Contributions of authors
Alexander Schnabel (AS), Sylvia Reichl (SR), Christine Meyer‐Frießem (CMF), Stephanie Weibel (SW), Peter Kranke (PK), Peter Zahn (PZ), Esther Pogatzki‐Zahn (EPZ).
Conceiving the review: AS.
Co‐ordinating the review: AS.
Undertaking manual searches: AS, CMF, SW.
Screening search results: AS, CMF, SR.
Organizing retrieval of papers: AS, CMF.
Screening retrieved papers against inclusion criteria: AS, CMF, SR.
Appraising quality of papers: CMF, SR.
Abstracting data from papers: AS, CMF, SW, SR.
Writing to authors of papers for additional information: AS.
Providing additional data about papers: AS.
Obtaining and screening data on unpublished studies: AS.
Managing data for the review: AS, CMF.
Entering data into Review Manager (Review Manager 2014): AS, CMF, SW.
Analysing RevMan statistical data: AS, CMF, SW.
Performing other statistical analyses not using RevMan: SW.
Interpreting data: AS, PK, PZ, EPZ.
Making statistical inferences: AS, PK, SW, EPZ.
Writing the review: AS, PZ, EPZ, SR, CMF.
Securing funding for the review: AS
Performing previous work that served as the foundation of the present study: AS
Serving as guarantor for the review (one review author): AS.
Taking responsibility for reading and checking the review before submission: AS.
Sources of support
Internal sources
No sources of support supplied
External sources
New source of support, Other.
Declarations of interest
Alexander Schnabel: none known.
Sylvia U Reichl: none known.
Stephanie Weibel is an academic researcher. She has received personal payments for consultancies and lecture fees from Genelux Corporation, San Diego, USA (ended March 2014). Genelux Corporation does not produce any products for the intervention of interest in this review.
Christine Meyer‐Frießem has no conflicts of interest regarding the topic of this review. She received a sponsorship award for young pain scientists in May 2012 from Janssen‐Cilag GmbH (Eur. 5.000). Further, she received payments for two clinical lectures from OrionPharma in June 2013 (Eur 300) and from the Grünenthal Group in September 2013 (Eur 1000), all unrelated to the current review and relationships in the past. To her knowledge, the product portfolio of these companies has no direct connection to any topic or aim of the current meta‐analysis.
Peter K Zahn: none known.
Peter Kranke has no conflicts of interest regarding the topic of this review. He has received lecture fees (from FreseniusKabi, MSD, Ratiopharm, Covidien) and has provided consultancy (to MSD, FreseniusKabi, Ratiopharm, Covidien) on topics not related to the current review. He has been involved in the conduct of phase II and phase III clinical trials not related to the current review.
Esther Pogatzki‐Zahn received financial support from Mundipharma GmbH and Grunenthal for research activities, advisory and lecture fees from Grünenthal, MSD Sharp & DOHME GmbH, Mundipharma GmbH, Mundipharma International, Janssen‐Cilag GmbH, TEVA, Fresenius Kabi and AcelRx. She receives scientific support from the DFG, the BMBF, and the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 777500. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. Funding was not related to the present research.
New
References
References to studies included in this review
Abdallah 2016 {published data only}
- Abdallah FW, Whelan DB, Chan VW, Prasad GA, Endersby RV, Theodoropolous J, et al. Adductor canal block provides noninferior analgesia and superior quadriceps strength compared with femoral nerve block in anterior cruciate ligament reconstruction. Anesthesiology 2016;124(5):1053‐64. [PUBMED: 26938989] [DOI] [PubMed] [Google Scholar]
Akkaya 2008 {published data only}
- Akkaya T, Ersan O, Ozkan D, Sahiner Y, Akin M, Gumus H, et al. Saphenous nerve block is an effective regional technique for post‐meniscectomy pain. Knee Surgery, Sports Traumatology, Arthroscopy 2008;16(9):855‐8. [PUBMED: 18574578] [DOI] [PubMed] [Google Scholar]
Andersen 2013 {published data only}
- Andersen HL, Gyrn J, Moller L, Christensen B, Zaric D. Continuous saphenous nerve block as supplement to single‐dose local infiltration analgesia for postoperative pain management after total knee arthroplasty. Regional Anesthesia and Pain Medicine 2013;38(2):106‐11. [PUBMED: 23222363] [DOI] [PubMed] [Google Scholar]
Elkassabany 2016 {published data only}
- Elkassabany NM, Antosh S, Ahmed M, Nelson C, Israelite C, Badiola I, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block: a double‐blinded randomized controlled study. Anesthesia and Analgesia 2016;122(5):1696‐703. [PUBMED: 27007076] [DOI] [PubMed] [Google Scholar]
Espelund 2013 {published data only}
- Espelund M, Fomsgaard JS, Haraszuk J, Mathiesen O, Dahl JB. Analgesic efficacy of ultrasound‐guided adductor canal blockade after arthroscopic anterior cruciate ligament reconstruction: a randomised controlled trial. European Journal of Anaesthesiology 2013;30(7):422‐8. [PUBMED: 23549123] [DOI] [PubMed] [Google Scholar]
Espelund 2014a {published data only}
- Espelund M, Fomsgaard JS, Haraszuk J, Dahl JB, Mathiesen O. The efficacy of adductor canal blockade after minor arthroscopic knee surgery ‐ a randomised controlled trial. Acta Anaesthesiologica Scandinavica 2014;58(3):273‐80. [PUBMED: 24205802] [DOI] [PubMed] [Google Scholar]
Hanson 2013 {published data only}
- Hanson NA, Derby RE, Auyong DB, Salinas FV, Delucca C, Nagy R, et al. Ultrasound‐guided adductor canal block for arthroscopic medial meniscectomy: a randomized, double‐blind trial. Canadian Journal of Anaesthesia = Journal Canadien d'Anesthesie 2013;60(9):874‐80. [PUBMED: 23820968] [DOI] [PubMed] [Google Scholar]
Hegazy 2015 {published data only}
- Hegazy NA, Sultan SS. Comparison between effects of adductor canal block and femoral nerve block on early postoperative course in total knee arthroplasty: a prospective double‐blind, randomized controlled study. Ain‐Shams Journal of Anesthesiology 2015;8(1):124‐8. [Google Scholar]
Jaeger 2013 {published data only}
- Jaeger P, Zaric D, Fomsgaard JS, Hilsted KL, Bjerregaard J, Gyrn J, et al. Adductor canal block versus femoral nerve block for analgesia after total knee arthroplasty: a randomized, double‐blind study. Regional Anesthesia and Pain Medicine 2013;38(6):526‐32. [PUBMED: 24121608] [DOI] [PubMed] [Google Scholar]
Jaeger 2014 {published data only}
- Jaeger P, Koscielniak‐Nielsen ZJ, Schroder HM, Mathiesen O, Henningsen MH, Lund J, et al. Adductor canal block for postoperative pain treatment after revision knee arthroplasty: a blinded, randomized, placebo‐controlled study. PLoS one 2014;9(11):e111951. [PUBMED: 25386752] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jenstrup 2012 {published data only}
- Jenstrup MT, Jaeger P, Lund J, Fomsgaard JS, Bache S, Mathiesen O, et al. Effects of adductor‐canal‐blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta Anaesthesiologica Scandinavica 2012;56(3):357‐64. [PUBMED: 22221014] [DOI] [PubMed] [Google Scholar]
Koh 2017a {published data only}
- Koh HJ, Koh IJ, Kim MS, Choi KY, Jo HU, In Y. Does patient perception differ following adductor canal block and femoral nerve block in total knee arthroplasty? A simultaneous bilateral randomized study. Journal of Arthroplasty 2017;32(6):1856‐61. [DOI: ; PUBMED: 28215966] [DOI] [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li D, Tan Z, Kang P, Shen B, Pei F. Effects of multi‐site infiltration analgesia on pain management and early rehabilitation compared with femoral nerve or adductor canal block for patients undergoing total knee arthroplasty: a prospective randomized controlled trial. International Orthopaedics 2017;41(1):75‐83. [DOI: 10.1007/s00264-016-3278-0; PUBMED: 27557955] [DOI] [PubMed] [Google Scholar]
Machi 2015 {published data only}
- Machi AT, Sztain JF, Kormylo NJ, Madison SJ, Abramson WB, Monahan AM, et al. Discharge readiness after tricompartment knee arthroplasty: adductor canal versus femoral continuous nerve blocks ‐ a dual‐center, randomized trial. Anesthesiology 2015;123(2):444‐56. [PUBMED: 26079800] [DOI] [PubMed] [Google Scholar]
Macrinici 2017 {published data only}
- Macrinici GI, Murphy C, Christman L, Drescher M, Hughes B, Macrinici V, et al. Prospective, double‐blind, randomized study to evaluate single‐injection adductor canal nerve block versus femoral nerve block: postoperative functional outcomes after total knee arthroplasty. Regional Anesthesia and Pain Medicine 2017;42(1):10‐6. [PUBMED: 27811526] [DOI] [PubMed] [Google Scholar]
Memtsoudis 2015 {published data only}
- Memtsoudis SG, Yoo D, Stundner O, Danninger T, Ma Y, Poultsides L, et al. Subsartorial adductor canal vs femoral nerve block for analgesia after total knee replacement. International Orthopaedics 2015;39(4):673‐80. [PUBMED: 25297681] [DOI] [PubMed] [Google Scholar]
Messeha 2016 {published data only}
- Messeha MM. Real‐time ultrasound‐guided comparison of adductor canal block and psoas compartment block combined with sciatic nerve block in laparoscopic knee surgeries. Anesthesia, Essays and Researches 2016;10(2):305‐11. [PUBMED: 27212766] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nader 2016 {published data only}
- Nader A, Kendall MC, Manning DW, Beal M, Rahangdale R, Dekker R, et al. Single‐dose adductor canal block with local infiltrative analgesia compared with local infiltrate analgesia after total knee arthroplasty: a randomized, double‐blind, placebo‐controlled trial. Regional Anesthesia and Pain Medicine 2016;41(6):678‐84. [PUBMED: 27776098] [DOI] [PubMed] [Google Scholar]
Rahimzadeh 2017 {published data only}
- Rahimzadeh P, Faiz HR, Imani F, Hobika GG, Abbasi A, Nader ND. Relieving pain after arthroscopic knee surgery: ultrasound‐guided femoral nerve block or adductor canal block?. Turkish Journal of Anaesthesiology and Reanimation 2017;45(4):218‐24. [PUBMED: 28868169] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sawhney 2016 {published data only}
- Sawhney M, Mehdian H, Kashin B, Ip G, Bent M, Choy J, et al. Pain after unilateral total knee arthroplasty: a prospective randomized controlled trial examining the analgesic effectiveness of a combined adductor canal peripheral nerve block with periarticular infiltration versus adductor canal nerve block alone versus periarticular infiltration alone. Anesthesia and Analgesia 2016;122(6):2040‐6. [PUBMED: 27028771] [DOI] [PubMed] [Google Scholar]
Shah 2014 {published data only}
- Shah NA, Jain NP. Is continuous adductor canal block better than continuous femoral nerve block after total knee arthroplasty? Effect on ambulation ability, early functional recovery and pain control: a randomized controlled trial. Journal of Arthroplasty 2014;29(11):2224‐9. [PUBMED: 25041873] [DOI] [PubMed] [Google Scholar]
Sztain 2015 {published data only}
- Sztain JF, Machi AT, Kormylo NJ, Abramson WB, Madison SJ, Monahan AM, et al. Continuous adductor canal versus continuous femoral nerve blocks: relative effects on discharge readiness following unicompartment knee arthroplasty. Regional Anesthesia and Pain Medicine 2015;40(5):559‐67. [PUBMED: 26115189] [DOI] [PubMed] [Google Scholar]
Wiesmann 2016 {published data only}
- Wiesmann T, Piechowiak K, Duderstadt S, Haupt D, Schmitt J, Eschbach D, et al. Continuous adductor canal block versus continuous femoral nerve block after total knee arthroplasty for mobilisation capability and pain treatment: a randomised and blinded clinical trial. Archives of Orthopaedic and Trauma Surgery 2016;136(3):397‐406. [PUBMED: 26754752] [DOI] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang W, Hu Y, Tao Y, Liu X, Wang G. Ultrasound‐guided continuous adductor canal block for analgesia after total knee replacement. Chinese Medical Journal 2014;127(23):4077‐81. [PUBMED: 25430452] [PubMed] [Google Scholar]
Zhao 2017 {published data only}
- Zhao MW, Wang N, Zeng L, Li M, Zhao ZK, Zhang H, et al. [Comparision for clinical efficiency of continuous adductor canal block and femoral nerve block in total knee arthroplasty]. Beijing da Xue Xue Bao. Yi Xue Ban = Journal of Peking University. Health Sciences 2017;49(1):142‐7. [PUBMED: 28203021] [PubMed] [Google Scholar]
References to studies excluded from this review
Beausang 2016 {published data only}
- Beausang DH, Pozek JP, Chen AF, Hozack WJ, Kaufmann MW, Torjman MC, et al. A randomized controlled trial comparing adductor canal catheter and intraarticular catheter after primary total knee arthroplasty. Journal of Arthroplasty 2016;31(9 Suppl):298‐301. [PUBMED: 27067467] [DOI] [PubMed] [Google Scholar]
Espelund 2014b {published data only}
- Espelund M, Grevstad U, Jaeger P, Holmich P, Kjeldsen L, Mathiesen O, et al. Adductor canal blockade for moderate to severe pain after arthroscopic knee surgery: a randomized controlled trial. Acta Anaesthesiologica Scandinavica 2014;58(10):1220‐7. [PUBMED: 25307707] [DOI] [PubMed] [Google Scholar]
Grant 2017 {published data only}
- Grant AE, Schwenk ES, Torjman MC, Hillesheim R, Chen AF. Postoperative analgesia in patients undergoing primary or revision knee arthroplasty with adductor canal block. Anesthesiology and Pain Medicine 2017;7(3):e46695. [PUBMED: 28824869] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grevstad 2014 {published data only}
- Grevstad U, Mathiesen O, Lind T, Dahl JB. Effect of adductor canal block on pain in patients with severe pain after total knee arthroplasty: a randomized study with individual patient analysis. British Journal of Anaesthesia 2014;112(5):912‐9. [PUBMED: 24401802] [DOI] [PubMed] [Google Scholar]
Grevstad 2015 {published data only}
- Grevstad U, Mathiesen O, Valentiner LS, Jaeger P, Hilsted KL, Dahl JB. Effect of adductor canal block versus femoral nerve block on quadriceps strength, mobilization, and pain after total knee arthroplasty: a randomized, blinded study. Regional Anesthesia and Pain Medicine 2015;40(1):3‐10. [PUBMED: 25376972] [DOI] [PubMed] [Google Scholar]
Gwam 2017 {published data only}
- Gwam CU, Mistry JB, Khlopas A, Chughtai M, Thomas M, Mont MA, et al. Does addition of multimodal periarticular analgesia to adductor canal block improve lengths of stay, pain, discharge status, and opioid use after total knee arthroplasty?. Journal of Arthroplasty 2017;32(5):1470‐3. [PUBMED: 28063774] [DOI] [PubMed] [Google Scholar]
Hanson 2014 {published data only}
- Hanson NA, Allen CJ, Hostetter LS, Nagy R, Derby RE, Slee AE, et al. Continuous ultrasound‐guided adductor canal block for total knee arthroplasty: a randomized, double‐blind trial. Anesthesia and Analgesia 2014;118(6):1370‐7. [PUBMED: 24842182] [DOI] [PubMed] [Google Scholar]
Henshaw 2016 {published data only}
- Henshaw DS, Jaffe JD, Reynolds JW, Dobson S, Russell GB, Weller RS. An evaluation of ultrasound‐guided adductor canal blockade for postoperative analgesia after medial unicondylar knee arthroplasty. Anesthesia and Analgesia 2016;122(4):1192‐201. [PUBMED: 26771270] [DOI] [PubMed] [Google Scholar]
Jaeger 2012 {published data only}
- Jaeger P, Grevstad U, Henningsen MH, Gottschau B, Mathiesen O, Dahl JB. Effect of adductor‐canal‐blockade on established, severe post‐operative pain after total knee arthroplasty: a randomised study. Acta Anaesthesiologica Scandinavica 2012;56(8):1013‐9. [PUBMED: 22834681 ] [DOI] [PubMed] [Google Scholar]
Jaeger 2013b {published data only}
- Jaeger P, Nielsen ZJ, Henningsen MH, Hilsted KL, Mathiesen O, Dahl JB. Adductor canal block versus femoral nerve block and quadriceps strength: a randomized, double‐blind, placebo‐controlled, crossover study in healthy volunteers. Anesthesiology 2013;118(2):409‐15. [PUBMED: 23241723] [DOI] [PubMed] [Google Scholar]
Joe 2016 {published data only}
- Joe HB, Choo HS, Yoon JS, Oh SE, Cho JH, Park YU. Adductor canal block versus femoral nerve block combined with sciatic nerve block as an anesthetic technique for hindfoot and ankle surgery: a prospective, randomized noninferiority trial. Medicine 2016;95(52):e5758. [PUBMED: 28033291] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2014 {published data only}
- Kim DH, Lin Y, Goytizolo EA, Kahn RL, Maalouf DB, Manohar A, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesiology 2014;120(3):540‐50. [PUBMED: 24401769] [DOI] [PubMed] [Google Scholar]
Kwofie 2013 {published data only}
- Kwofie MK, Shastri UD, Gadsden JC, Sinha SK, Abrams JH, Xu D, et al. The effects of ultrasound‐guided adductor canal block versus femoral nerve block on quadriceps strength and fall risk: a blinded, randomized trial of volunteers. Regional Anesthesia and Pain Medicine 2013;38(4):321‐5. [PUBMED: 23788068] [DOI] [PubMed] [Google Scholar]
Monahan 2016 {published data only}
- Monahan AM, Sztain JF, Khatibi B, Furnish TJ, Jaeger P, Sessler DI, et al. Continuous adductor canal blocks: does varying local anesthetic delivery method (automatic repeated bolus doses versus continuous basal infusion) influence cutaneous analgesia and quadriceps femoris strength? A randomized, double‐masked, controlled, split‐body volunteer study. Anesthesia and Analgesia 2016;122(5):1681‐8. [PUBMED: 26863502] [DOI] [PubMed] [Google Scholar]
Ortiz‐Gomez 2017 {published data only}
- Ortiz‐Gomez JR, Pereperez‐Candel M, Vazquez‐Torres JM, Rodriguez‐Del Rio JM, Torron‐Abad B, Fornet‐Ruiz I, et al. Postoperative analgesia for elective total knee arthroplasty under subarachnoid anesthesia with opioids: comparison between epidural, femoral block and adductor canal block techniques (with and without perineural adjuvants). A prospective, randomized, clinical trial. Minerva Anestesiologica 2017;83(1):50‐8. [PUBMED: 27792212] [DOI] [PubMed] [Google Scholar]
Seo 2017 {published data only}
- Seo SS, Kim OG, Seo JH, Kim DH, Kim YG, Park BY. Comparison of the effect of continuous femoral nerve block and adductor canal block after primary total knee arthroplasty. Clinics in Orthopedic Surgery 2017;9(3):303‐9. [PUBMED: 28861197] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2015 {published data only}
- Shah N, Jain N, Panchal K. Adductor canal blockade following total knee arthroplasty‐continuous or single shot technique? Role in postoperative analgesia, ambulation ability and early functional recovery: a randomized controlled trial. Journal of Arthroplasty 2015;30(8):1476‐81. [PUBMED: 25824025 ] [DOI] [PubMed] [Google Scholar]
Sogbein 2017 {published data only}
- Sogbein OA, Sondekoppam RV, Bryant D, Johnston DF, Vasarhelyi EM, MacDonald S, et al. Ultrasound‐guided motor‐sparing knee blocks for postoperative analgesia following total knee arthroplasty: a randomized blinded study. Journal of Bone and Joint Surgery. American volume 2017;99(15):1274‐81. [PUBMED: 28763413] [DOI] [PubMed] [Google Scholar]
Sorensen 2016 {published data only}
- Sorensen JK, Jaeger P, Dahl JB, Gottschau B, Stephensen SL, Grevstad U. The isolated effect of adductor canal block on quadriceps femoris muscle strength after total knee arthroplasty: a triple‐blinded, randomized, placebo‐controlled trial with individual patient analysis. Anesthesia and Analgesia 2016;122(2):553‐8. [PUBMED: 26649909] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Biswas 2018 {published and unpublished data}
- Biswas A, Perlas A, Ghosh M, Chin K, Niazi A, Pandher B, et al. Relative contributions of adductor canal block and intrathecal morphine to analgesia and functional recovery after total knee arthroplasty: a randomized controlled trial. Regional Anesthesia and Pain Medicine 2018;43(2):154‐60. [PUBMED: 29315129] [DOI] [PubMed] [Google Scholar]
Chisholm 2014 {published data only}
- Chisholm MF, Bang H, Maalouf DB, Marcello D, Lotano MA, Marx RG, et al. Postoperative analgesia with saphenous block appears equivalent to femoral nerve block in ACL reconstruction. Musculoskeletal Journal of Hospital for Special Surgery 2014;10(3):245‐51. [PUBMED: 4171445] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grosso 2018 {published data only}
- Grosso MJ, Murtaugh T, Lakra A, Brown AR, Maniker RB, Cooper HJ, et al. Adductor canal block compared with periarticular bupivacaine injection for total knee arthroplasty: a prospective randomized trial. Journal of Bone and Joint Surgery 2018;100(13):1141‐6. [PUBMED: 29975272] [DOI] [PubMed] [Google Scholar]
Kampitak 2018 {published data only}
- Kampitak W, Tanavalee A, Ngarmukos S, Amarase C, Songthamwat B, Boonshua A. Comparison of adductor canal block versus local infiltration analgesia on postoperative pain and functional outcome after total knee arthroplasty: a randomized controlled trial. Malaysian Orthopaedic Journal 2018;12(1):7‐14. [PUBMED: 29725506] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kayupov 2018 {published data only}
- Kayupov E, Okroj K, Young AC, Moric M, Luchetti TJ, Zisman G, et al. Continuous adductor canal blocks provide superior ambulation and pain control compared to epidural analgesia for primary knee arthroplasty: a randomized, controlled trial. Journal of Arthroplasty 2018;33(4):1040‐4. [PUBMED: 29233569] [DOI] [PubMed] [Google Scholar]
Lenz 2018 {published data only}
- Lenz K, Jensen K, Tanggaard K, Vazin M, Bendtsen TF, Chan V, et al. Comparing low volume saphenous‐obturator block with placebo and femoral‐obturator block for anterior cruciate ligament reconstruction. Minerva Anestesiologica 2018;84(2):168‐77. [PUBMED: 28749093] [DOI] [PubMed] [Google Scholar]
Leung 2018 {published data only}
- Leung P, Dickerson DM, Denduluri SK, Mohammed MK, Lu M, Anitescu M, et al. Postoperative continuous adductor canal block for total knee arthroplasty improves pain and functional recovery: a randomized controlled clinical trial. Journal of Clinical Anesthesia 2018;49:46‐52. [PUBMED: 29890381] [DOI] [PubMed] [Google Scholar]
Lim 2018 {published data only}
- Lim YC, Quek HYK, Phoo WHJ, Mah CL, Tan S. A randomised, controlled trial comparing adductor canal block and femoral nerve block for knee arthroplasty. Singapore Medical Journal 2018:Epub ahead of print. [PUBMED: 30009316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rousseau‐Saine 2018 {published data only}
- Rousseau‐Saine N, Williams SR, Girard F, Hébert LJ, Robin F, Duchesne L, et al. The effect of adductor canal block on knee extensor muscle strength 6 weeks after total knee arthroplasty: a randomized, controlled trial. Anesthesia Analgesia 2018;126(3):1019‐27. [PUBMED: 28799964] [DOI] [PubMed] [Google Scholar]
Runner 2018 {published data only}
- Runner RP, Boden SA, Godfrey WS, Premkumar A, Samady H, Gottschalk MB, et al. Quadriceps strength deficits after a femoral nerve block versus adductor canal block for anterior cruciate ligament reconstruction: a prospective, single‐blinded, randomized trial. Orthopedic Journal of Sports Medicine 2018;6(9):2325967118797990. [PUBMED: 30276220] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tong 2018 {published data only}
- Tong QJ, Lim YC, Tham HM. Comparing adductor canal block with local infiltration analgesia in total knee arthroplasty: a prospective, blinded and randomized clinical trial. Journal of Clinical Anesthesia 2018;46:39‐43. [PUBMED: 29414612] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR1800018463 {unpublished data only}
- ChiCTR1800018463. Efficacy of different lower extremity nerve block combined with general anaesthesia in knee arthroscopic surgery. chictr.org.cn/com/25/showprojen.aspx?proj=31192 (first received 20 September 2018).
NCT02071433 {unpublished data only}
- NCT02071433. Analgesic efficacy of saphenous nerve blockade for outpatient knee anterior cruciate ligament surgery. clinicaltrials.gov/ct2/show/NCT02071433 (first received 25 February 2014).
NCT02276495 {unpublished data only}
- NCT02276495. Does single injection adductor canal block improve postoperative analgesia in patients receiving periarticular local anesthesia injections for total knee arthroplasty?. clinicaltrials.gov/ct2/show/NCT02276495 (first received 28 October 2014).
NCT02419261 {unpublished data only}
- NCT02419261. Assessment of sensory and motor blockade of the adductor canal blockade performed for surgery of arthroscopic anterior cruciate ligament repair. clinicaltrials.gov/ct2/show/NCT02419261 (first received 17 April 2015).
NCT02863120 {unpublished data only}
- NCT02863120. Patient outcomes with periarticular liposomal bupivacaine injection vs adductor canal block after primary total knee arthroplasty. clinicaltrials.gov/ct2/show/NCT02863120 (first received 13 October 2017).
NCT03033589 {unpublished data only}
- NCT03033589. Femoral nerve block versus adductor canal nerve block for peri‐operative analgesia following anterior cruciate ligament reconstruction: evaluation of post‐operative pain and strength. clinicaltrials.gov/ct2/show/NCT03033589 (first received 27 January 2017). [Google Scholar]
NCT03188809 {unpublished data only}
- NCT03188809. Adductor canal block versus femoral nerve block with repeated bolus doses: postoperative analgesia and functional outcomes after total knee arthroplasty. clinicaltrials.gov/ct2/show/NCT03188809 (first received 15 June 2017).
NCT03205540 {unpublished data only}
- NCT03205540. Comparison efficacy of analgesic techniques: continuous epidural analgesia versus bilateral single‐shot adductor canal blocks in patients undergoing bilateral total knee arthroplasty. clinicaltrials.gov/ct2/show/NCT03205540 (first received 2 July 2017).
NCT03208478 {unpublished data only}
- NCT03208478. Pain control for anterior cruciate ligament reconstruction patients with adductor canal or femoral perineural infusions. clinicaltrials.gov/ct2/show/NCT03208478 (first received 5 July 2017).
NCT03518450 {unpublished data only}
- NCT03518450. Femoral triangle block: early mobilization and postoperative analgesia after total knee arthroplasty. clinicaltrials.gov/ct2/show/NCT03518450 (first received 8 May 2018).
NCT03620136 {unpublished data only}
- NCT03620136. Comparison of two techniques of locoregional analgesia in total knee prosthesis surgery: block to the adductor channel versus peri‐articular local infiltrations (SALIA). clinicaltrials.gov/ct2/show/NCT03620136 (first received 8 August 2018).
Additional references
Althaus 2014
- Althaus A, Arranz Becker O, Neugebauer E. Distinguishing between pain intensity and pain resolution: using acute post‐surgical pain trajectories to predict chronic post‐surgical pain. European Journal of Pain 2014;18(4):513‐21. [PUBMED: 23983024] [DOI] [PubMed] [Google Scholar]
Andersen 2015
- Andersen HL, Andersen SL, Tranum‐Jensen J. The spread of injectate during saphenous nerve block at the adductor canal: a cadaver study. Acta Anaesthesiologica Scandinavica 2015;59(2):238‐45. [PUBMED: 25496028] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Bendtsen 2014a
Bendtsen 2014b
Beswick 2012
- Beswick AD, Wylde V, Gooberman‐Hill R, Blom A, Dieppe P. What proportion of patients report long‐term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012;2(1):e000435. [PUBMED: 22357571] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boric 2017
- Boric K, Dosenovic S, Jelicic Kadic A, Boric M, Jeric M, Puljak L. Efficacy and safety outcomes in systematic reviews of interventions for postoperative pain in children: comparison against the recommended core outcome set. Pain Medicine 2018;19(11):2316‐21. [DOI: 10.1093/pm/pnx255; PUBMED: 29045726] [DOI] [PubMed] [Google Scholar]
Brant 2005
- Brant R. Power and Sample Size Calculators: Comparing Means for Two Independent Samples. https://www.stat.ubc.ca/˜rollin/stats/ssize/n2.html (accessed 2019).
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [PUBMED: 18411040] [DOI] [PubMed] [Google Scholar]
Chan 2014
- Chan EY, Fransen M, Parker DA, Assam PN, Chua N. Femoral nerve blocks for acute postoperative pain after knee replacement surgery. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD009941.pub2; PUBMED: 24825360] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2016
- Cooper SA, Desjardins PJ, Turk DC, Dworkin RH, Katz NP, Kehlet H, et al. Research design considerations for single‐dose analgesic clinical trials in acute pain: IMMPACT recommendations. Pain 2016;157(2):288‐301. [PUBMED: 26683233] [DOI] [PubMed] [Google Scholar]
Cowlishaw 2015
Cozowicz 2015
- Cozowicz C, Poeran J, Memtsoudis SG. Epidemiology, trends, and disparities in regional anaesthesia for orthopaedic surgery. British Journal of Anaesthesia 2015;115(Suppl 2):ii57‐67. [PUBMED: 26658202] [DOI] [PubMed] [Google Scholar]
Dong 2016
- Dong CC, Dong SL, He FC. Comparison of adductor canal block and femoral nerve block for postoperative pain in total knee arthroplasty: a systematic review and meta‐analysis. Medicine 2016;95(12):e2983. [PUBMED: 27015172] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gao 2017
- Gao F, Ma J, Sun W, Guo W, Li Z, Wang W. Adductor canal block versus femoral nerve block for analgesia after total knee arthroplasty: a systematic review and meta‐analysis. Clinical Journal of Pain 2017;33(4):356‐68. [PUBMED: 27322397] [DOI] [PubMed] [Google Scholar]
Gautier 2015
Gerbershagen 2013
- Gerbershagen HJ, Aduckathil S, Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology 2013;118(4):934‐44. [PUBMED: 23392233] [DOI] [PubMed] [Google Scholar]
Grosu 2015
- Grosu I, Thienpont E, Kock M, Scholtes JL, Lavand'homme P. Dynamic view of postoperative pain and quality of life evolution after total knee arthroplasty ‐ a prospective observational study. Minerva Anestesiologica 2016;82(3):274‐83. [PUBMED: 26184701] [PubMed] [Google Scholar]
Guay 2017
- Guay J, Parker MJ, Griffiths R, Kopp S. Peripheral nerve blocks for hip fractures. Cochrane Database of Systematic Reviews 2017;5:CD001159. [PUBMED: 28494088] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guay 2017a
- Guay J, Johnson RL, Kopp S. Nerve blocks or no nerve blocks for pain control after elective hip replacement (arthroplasty) surgery in adults. Cochrane Database of Systematic Reviews 2017;10:CD011608. [PUBMED: 29087547] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [PUBMED: 21185693] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical research ed) 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hussain 2018
- Hussain N, Shastri U, McCartney CJ, Gilron I, Fillingim RB, Clarke H, et al. Should thoracic paravertebral blocks be used to prevent chronic post‐surgical pain following breast cancer surgery? A systematic analysis of evidence in light of IMMPACT recommendations. Pain 2018;159(10):1955‐71. [DOI: 10.1097/j.pain.000000000000129; PUBMED: 29794879] [DOI] [PubMed] [Google Scholar]
Johnson 2013
- Johnson RL, Kopp SL, Hebl JR, Erwin PJ, Mantilla CB. Falls and major orthopaedic surgery with peripheral nerve blockade: a systematic review and meta‐analysis. British Journal of Anaesthesia 2013;110(4):518‐28. [PUBMED: 23440367] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2014
- Johnson RL, Duncan CM, Ahn KS, Schroeder DR, Horlocker TT, Kopp SL. Fall‐prevention strategies and patient characteristics that impact fall rates after total knee arthroplasty. Anesthesia and Analgesia 2014;119(5):1113‐8. [PUBMED: 25211392] [DOI] [PubMed] [Google Scholar]
Kuang 2017
- Kuang MJ, Ma JX, Fu L, He WW, Zhao J, Ma XL. Is adductor canal block better than femoral nerve block in primary total knee arthroplasty? A GRADE analysis of the evidence through a systematic review and meta‐analysis. Journal of Arthroplasty 2017;32(10):3238‐48.e3. [PUBMED: 28606458] [DOI] [PubMed] [Google Scholar]
Mariano 2014
Memtsoudis 2013
- Memtsoudis SG, Sun X, Chiu YL, Stundner O, Liu SS, Banerjee S, et al. Perioperative comparative effectiveness of anesthetic technique in orthopedic patients. Anesthesiology 2013;118(5):1046‐58. [PUBMED: 23612126] [DOI] [PMC free article] [PubMed] [Google Scholar]
Memtsoudis 2014
- Memtsoudis SG, Danninger T, Rasul R, Poeran J, Gerner P, Stundner O, et al. Inpatient falls after total knee arthroplasty: the role of anesthesia type and peripheral nerve blocks. Anesthesiology 2014;120(3):551‐63. [PUBMED: 24534855] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Pace 2011
- Pace NL. Research methods for meta‐analyses. Best Practice & Research. Clinical Anaesthesiology 2011;25(4):523‐33. [PUBMED: 22099918] [DOI] [PubMed] [Google Scholar]
Pehora 2017
- Pehora C, Pearson AM, Kaushal A, Crawford MW, Johnston B. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database of Systematic Reviews 2017;11:CD011770. [PUBMED: 29121400] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pogatzki‐Zahn 2012
- Pogatzki‐Zahn EM, Schnabel A, Zahn PK. Room for improvement: unmet needs in postoperative pain management. Expert Review of Neurotherapeutics 2012;12(5):587‐600. [PUBMED: 22550987] [DOI] [PubMed] [Google Scholar]
Puljak 2018
- Puljak L, Dosenovic S, Boric K. Importance of consistent outcomes in randomized controlled trials and systematic reviews about anesthesiology and pain. Pain Management 2018;8(4):251‐3. [PUBMED: 29898641] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schnabel 2017
- Schnabel A, Reichl SU, Zahn PK, Pogatzki‐Zahn EM, Meyer‐Friessem CH. Efficacy and safety of buprenorphine in peripheral nerve blocks: a meta‐analysis of randomised controlled trials. European Journal of Anaesthesiology 2017;34(9):576‐86. [PUBMED: 28763315] [DOI] [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [PUBMED: 15116347] [DOI] [PubMed] [Google Scholar]
Sztain 2018
- Sztain JF, Khatibi B, Monahan AM, Said ET, Abramson WB, Gabriel RA, et al. Proximal versus distal continuous adductor canal blocks: does varying perineural catheter location influence analgesia? A randomized, subject‐masked, controlled clinical trial. Anesthesia and Analgesia 2018;127(1):240‐6. [PUBMED: 29750695] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [PUBMED: 18824467] [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA). Copenhagen Trial Unit, Centre for Clinical Intervention Research 2011:1‐115. [Google Scholar]
Tubbs 2007
- Tubbs RS, Loukas M, Shoja MM, Apaydin N, Oakes WJ, Salter EG. Anatomy and potential clinical significance of the vastoadductor membrane. Surgical and Radiologic Anatomy 2007;29(7):569‐73. [PUBMED: 17618402] [DOI] [PubMed] [Google Scholar]
Wang 2017
- Wang D, Yang Y, Li Q, Tang SL, Zeng WN, Xu J, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta‐analysis of randomized controlled trials. Scientific Reports 2017;7:40721. [PUBMED: 28079176] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wasserstein 2013
- Wasserstein D, Farlinger C, Brull R, Mahomed N, Gandhi R. Advanced age, obesity and continuous femoral nerve blockade are independent risk factors for inpatient falls after primary total knee arthroplasty. Journal of Arthroplasty 2013;28(7):1121‐4. [PUBMED: 23265274] [DOI] [PubMed] [Google Scholar]
Weinstein 2018
- Weinstein EJ, Levene JL, Cohen MS, Andreae DA, Chao JY, Johnson M, et al. Local anaesthetics and regional anaesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database of Systematic Reviews 2018;6:CD007105. [PUBMED: 29926477] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [PUBMED: 20042080] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xing 2017
- Xing Q, Dai W, Zhao D, Wu J, Huang C, Zhao Y. Adductor canal block with local infiltrative analgesia compared with local infiltrate analgesia for pain control after total knee arthroplasty: a meta‐analysis of randomized controlled trials. Medicine 2017;96(38):e8103. [PUBMED: 28930857] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Schnabel 2016
- Schnabel A, Reichl SU, Weibel S, Meyer‐Frießem C, Zahn PK, Kranke P, et al. Adductor canal blocks for postoperative pain treatment in adults undergoing knee surgery. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD012262] [DOI] [PMC free article] [PubMed] [Google Scholar]


