Abstract
Background:
Substance use disorders (SUDs) are common in healthcare settings and contribute to poor outcomes, particularly in patients living with HIV. We assessed initiation, engagement, and retention in SUD treatment and pharmacotherapy following an index SUD episode in a national sample of HIV-infected and uninfected patients receiving care in the Department of Veterans Affairs (VA) healthcare system.
Methods:
We used electronic national VA data (years 2000-2015) from 52,995 HIV-infected and 111,229 age-, race-, gender-, and region-matched uninfected patients. We defined index SUD episodes as outpatient visits or inpatient/residential admissions with associated primary or secondary ICD-9 codes for substance use in patients without SUD-related services or pharmacotherapy in the preceding 5 months.
Results:
Overall, 57,428 (35%) patients had at least 1 index SUD episode. HIV-infected patients were more likely than uninfected controls to have at least one index SUD episode (35.7% vs. 34.6%; p < .001). Rates of initiation, engagement, and retention in SUD treatment after the index SUD episode were less than 17% for both groups. In adjusted models, HIV-infected patients were more likely than uninfected patients to be retained in SUD treatment at 6 months (Odds Ratio 1.10; 95% Confidence Interval 1.04-1.16). SUD pharmacotherapy initiation and engagement was uncommon in both HIV-infected and uninfected patients.
Conclusions:
In this national VA sample, initiation of SUD treatment and pharmacotherapy were uncommon for both HIV-infected and uninfected patients. Interventions to improve initiation, engagement, and retention in the full range of services, including SUD pharmacotherapy, is warranted for all patients with SUD in the VA.
Keywords: HIV, substance use disorders, substance use disorder treatment, pharmacotherapy, opioid treatment
1. Introduction
Substance use disorders (SUDs) are common among HIV-infected patients and contribute to poor outcomes.(SAMHSA, 2010;Williams et al., 2016) Among HIV-infected patients, SUDs are associated with decreased adherence to antiretroviral treatment,(Azar et al., 2010; Lucas, 2011; Palepu et al., 2004) increased transmission of HIV to others,(MacArthur et al., 2012; Mathers et al., 2010; Metsch et al., 2008) decreased quality of HIV care, (Korthuis et al., 2012) and increased mortality. (Braithwaite et al., 2007; DeLorenze et al., 2010; Justice et al., 2016; Korthuis et al., 2016;Justice, 2016; Marshall et al., 2017) Although SUD treatment has the potential to mitigate these poor outcomes, it is often not initiated.(Low et al., 2016; Scott-Sheldon et al., 2017; Sordo et al., 2017; Williams et al., 2017b)
Multiple behavioral therapies, including Motivational Enhancement Therapy, Cognitive Behavioral Therapy, and Twelve Step Facilitation, are effective behavioral treatments for SUD in both HIV-infected and uninfected populations. Likewise, pharmacotherapy treatments for some SUDs, such as methadone, buprenorphine, and naltrexone for opioid use disorders and disulfiram, naltrexone, and acamprosate for alcohol use disorders, are all Food and Drug Administration (FDA)-approved treatments that can help initiate abstinence and prevent relapse in patients with SUD. Unfortunately, the “real world” provision of SUD behavioral treatment and pharmacotherapy in eligible patients has been poor. The Institute of Medicine reported that only 10% of US adults with SUD received high quality SUD treatment. (Institute of Medicine, 2006) In HIV-infected patients, for whom SUD pharmacotherapy improves outcomes and decreases infectivity, one would hope for higher SUD treatment rates. One retrospective study from 2013 of individuals in an integrated healthcare system in the western US found, however, that only 15% of HIV-infected patients with SUD received one or more SUD specialty care visits within 12 months of diagnosis. (Satre et al, 2013)
US military Veterans comprise a vital population for the study of SUD and HIV care. (Larney et al, 2015) Over 461,927 (8.3%) of Veterans in care at the Department of Veterans Affairs (VA)had at least one documented VA service episode for SUD in FY2010. (Oliva et al, 2011a) Similarly, the VA is the single largest provider of HIV care in the US, currently caring for approximately 27,000 Veterans living with HIV (of about 44,180 HIV-infected since 1999, counting deaths, according to Veterans Aging Cohort Study [VACS]). As with other populations, Veterans with SUD have poor initiation, engagement, and retention in SUD treatment. A recent RAND report found that only 16% of Veterans with SUD initiated treatment and only 15% engaged in treatment. (Watkins et al., 2011a; Watkins et al., 2011b) Receipt of pharmacotherapy for Veteran patients with opioid use disorder (OUD) also is poor; about 35% of Veterans receive medications for OUD. (Wyse et al., 2018) However, it is not known if use of SUD treatment services differs among HIV-infected and uninfected Veterans with SUD. Understanding such differences is an important first step before identifying predictors of and barriers to SUD treatment and development of delivery interventions.
In this study, we sought to describe the frequency of initiation, engagement, and retention in SUD behavioral treatment and pharmacotherapy following an index SUD episode among HIV -infected and uninfected Veterans receiving care in the VA healthcare system.
2. Methods
2.1. Data source and sample
We used data from January 2000 to October 2015 from VACS, a large national sample of all HIV-infected Veterans in care with matched uninfected controls.(Justice et al., 2006) VACS assembles electronic national VA electronic medical record data from multiple sources, including the Corporate Data Warehouse and Pharmacy Benefits Management databases, to define a national population of all HIV-infected Veterans under care and matched uninfected controls. No supplemental data are collected directly from patients. HIV-infected Veterans are identified with International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes 042-044 (AIDS) and V08 (asymptomatic HIV), and diagnosis related groups (DRG) codes 4888-490. At least 2 HIV-related outpatient diagnostic codes or at least 1 HIV-related inpatient diagnostic code was required for inclusion as HIV-infected. This approach has been validated against the VA Immunology Case Registry. (Fultz et al., 2006) Each HIV-infected Veteran was then matched 1:2 on age, gender, region (Veterans Integrated Systems Network), and fiscal year with 2 Veterans without an HIV-related ICD-9-CM or DRG code. At the time of these analyses through October 2015, the VACS included 52,995 HIV-infected Veterans and 111,229 uninfected Veterans.
The study protocol was approved by the University of Pittsburgh, VA Pittsburgh Healthcare System, and VA Connecticut Healthcare System Institutional Review Boards.
2.2. Definition of index substance use disorder episode
We identified index SUD inpatient or outpatient episodes that represented a potential opportunity to refer an individual for initiation of SUD treatment. Our definition of an index SUD episode was based on work of the Washington Circle Policy Group, (Garnick et al, 2006; Garnick et al, 2007; Garnick et al., 2009; Harris et al, 2007; McCorry et al., 2000) later adopted by the National Committee for Quality Assurance (NCQA), the Healthcare Effectiveness Data and Information Set (HEDIS), and the VA, and used by a RAND Corporation national review of VA mental health and substance use treatment services. (Watkins, 2011b) Following the method employed by RAND, we defined the start of an index SUD episode as: 1) the date of a SUD diagnosis-related admission or transfer to an inpatient/residential mental health bed; or 2) an outpatient encounter where SUD is the primary or secondary diagnosis. Index SUD episodes must follow a break in care (where “break in care” is defined as no inpatient or outpatient encounters with SUD as the primary or secondary diagnosis for 5 or more months where AND no SUD-related medications for 5 or more months). This definition is intended to represent anew opportunity to initiate SUD treatment for a patient. Of note, RAND’s use of “5 or more months” for “break in care” differed from the Washington Circle and NCQA/HEDIS use of “60 days or more” for the same purpose. We conservatively used “5 months or more” to ensure the patient had not recently completed SUD treatment. We used ICD-9-CM codes (Appendix) 291,291.x, 291.xx, 303, 303.x, 303.xx, 305.x, and 305.xx for alcohol diagnoses, ICD-9-CM codes 292, 292.x, 292.xx, 304, 304.xx, 305, and 305.xx for drug and/or alcohol use disorders, and methadone clinic visits or buprenorphine, naltrexone, disulfiram, and acamprosate prescriptions as qualifying pharmacotherapy.
2.3. Definition of initiation, engagement, and retention in substance use disorder treatment
To identify SUD treatment services, we used VACS data to identify outpatient “stop codes” (a unit of healthcare utilization location and type of care) and inpatient bed location indicative of SUD treatment services after the index episode. We categorized the codes into outpatient non-mental health non-SUD (restricted to general internal medicine, primary care, and infectious disease clinics), outpatient mental health non-SUD-specific, outpatient mental health SUD-specific, inpatient/residential non-mental health non-SUD, inpatient/residential mental health non-SUD-specific, inpatient/residential mental health SUD-specific, and “any setting” (all-inclusive) (Appendix). Such stop codes are accurate for true SUD behavioral treatment if they are mental health- or SUD-specific but less so if not mental health- or SUD-specific. (Harris et al., 2010b) Only outpatient and inpatient/residential services with anICD-9-CM SUD code as the primary diagnosis qualified as a SUD treatment service.
We then collated the number and timing of all SUD treatment services, including outpatient encounters and number of inpatient/residential days, for the 12 months following the index SUD episode and calculated whether participants met criteria for initiation, engagement, and retention in SUD treatment. We used performance measures developed by the Washington Circle and used by NCQA/HEDIS and the RAND VA analysis to determine whether each index SUD episode was followed by initiation and engagement in SUD treatment. We added measures used by the RAND VA analysis to define retention in SUD treatment at 3, 6, and 12 months. (Table 1). Per the NCQA/HEDIS and RAND measures, we defined SUD treatment initiation as a second SUD service occurring within 14 days of the index SUD episode. We defined outpatient engagement as receipt of 2 SUD services within 30 days of the index SUD episode. We made one modification to the original definition for engagement. The original definition was initiation of SUD treatment within 14 days of the index SUD episode plus 2 additional services within 30 days after initiation. Given our a priori expectation of low likelihood of initiation within 14 days of the index episode, we modified the definition of engagement to 2 SUD treatment services within 30 days after the index SUD episode. Lastly, we defined retention in SUD treatment as follows: 1) 3 month retention as 7 or more SUD specialty visits or SUD-related inpatient/residential admissions with a combined length of stay of 7 or more days within 3 months of the index SUD episode; 2) 6 month retention as 13 or more SUD specialty visits or SUD-related inpatient/residential admissions with a combined length of stay of 13 or more days within 6 months of the index SUD episode; and 3) 12 month retention as 25 or more SUD specialty visits or SUD-related inpatient/residential admissions with a combined length of stay of 25 or more days within 12 months of the index SUD episode.
Table 1.
SUD Treatmen Quality of Care/Performance Indicators.
| Quality/Performance Indicator | Definition |
|---|---|
| Initiation/Engagement | |
| Initiation | Index SUD service followed by a second service within 14 days |
| Outpatient engagement | Received 2+ additional services within 30 days after the index SUD episode |
| Retention: | |
| 3 months | 7+ SUD specialty visits or SUD-related inpatient/residential admission with a combined length of stay of 7+ days |
| 6 months | 13+ SUD specialty visits or SUD-related inpatient/residential admission with a combined length of stay of 13+ days |
| One year | 25+ SUD specialty visits or SUD-related inpatient/residential admission with a combined length of stay of 25+ days |
We assessed receipt of SUD pharmacotherapy for opioid and alcohol use disorders. For opioid use disorder, we defined methadone treatment with visits to the Opioid Agonist Clinic (stop code 523) and buprenorphine treatment using Pharmacy Benefits Management prescription data. For alcohol use disorder, we assessed receipt of prescriptions for naltrexone, acamprosate, and disulfiram, all via the oral route, through Pharmacy Benefits Management data. We defined initiation of SUD pharmacotherapy as medication prescription or methadone clinic visit within 14 days of the index SUD episode and engagement as continued prescription or methadone clinic visits at 30 days after the index SUD episode. We did not assess longer term retention in SUD pharmacotherapy for this analysis.
2.4. Analysis
Our analysis was primarily descriptive, with no a priori hypotheses, and limited to the first index SUD episode for each patient during the 2000 to 2015 study period. We used descriptive and bivariate (t-tests and Chi-square) statistics to compare the following among HIV -infected and uninfected Veterans: demographics, index SUD episodes, substances used, initiation/engagement/retention in SUD treatment by clinical location, and receipt of SUD pharmacotherapy for opioid use disorders and alcohol use disorders. We constructed logistic regression models for the outcomes of SUD treatment initiation, engagement at 30 days, and retention at 6 months, with HIV status as the main independent variable and adjusted for age, race, gender, urban vs. rural residence, hepatitis C status, and psychiatric comorbidity. For assessing trends in SUD pharmacotherapy over time, we partitioned the study period into 3 segments (2000-2004, 2005-2009, and 2010-2015) and tested for trend. All analyses were performed with STATA software. All P values were two-tailed and considered significant at P < 0.05.
3. Results
3.1. Characteristics of patients
The total sample of 164,224 patients included 52,995 (32.2%) HIV-infectedand 111,229 (67.8%) uninfected patients and was primarily male (97%) and racially diverse (40% white, 47% black, 8% Hispanic, 5% other). Of the total sample, 57,428 (35%) had at least one index SUD episodes and there were 199,185 index SUD episodes from January 2000 to October 2015. Compared with uninfected patients, HIV-infected patients were more likely to have an index SUD episode (35.7% vs. 34.6%; p < .001) or index illicit drug episode (29.2% vs. 24.6%; p < .001) but less likely to have an index alcohol episode (25.8% vs. 28.0%; p < .001). The characteristics of the 18,898 HIV-infected and 38,530 uninfected patients with at least 1 index SUD episode are shown in Table 2.
Table 2.
Characteristics of patients with at least 1 index SUD episode during study period, stratified by HIV status.
| Baseline Characteristics | Total Sample (n=57428) | HIV-infected (n=18898) | HIV-uninfected (n=38530) | P-value |
|---|---|---|---|---|
| Age, mean years (SD) | 52.9 (8.9) | 52.0 (9.0) | 53.4 (8.8) | < .001 |
| Male Gender | 56296 (98.0) | 18405 (97.4) | 37891 (98.3) | < .001 |
| Race/Ethnicity | ||||
| White | 19761 (34.4) | 6274 (33.2) | 13487 (35.0) | < .001 |
| Black | 31982 (55.7) | 10674 (56.5) | 21308 (55.3) | |
| Hispanic | 4546 (7.9) | 1537 (8.1) | 3009 (7.8) | |
| Other/unknown | 1139 (2.0) | 413 (2.2) | 726 (1.9) | |
| Residence | < .001 | |||
| Urban | 38021 (66.2) | 11853 (62.7) | 26168 (67.9) | |
| Rural | 5909 (10.3) | 1263 (6.7) | 4646 (12.1) | |
| Unknown | 13498 (23.5) | 5782 (30.6) | 7716 (20.0) | |
| Hepatitis C | 5266 (9.2) | 2722 (14.4) | 2544 (6.6) | < .001 |
| Psychiatric Diagnoses | ||||
| Depression | 10396 (18.1) | 3780 (20.0) | 6616 17.2) | < .001 |
| PTSD | 6440 (11.2) | 1579 (8.4) | 4861 (12.6) | < .001 |
| Bipolar Disorder | 3745 (6.5) | 1155 (6.1) | 2590 (6.7) | .005 |
| Schizophrenia | 4047 (7.1) | 886 (4.7) | 3161 8.2) | < .001 |
| Tobacco Smoking | ||||
| Never | 8331 (15.4) | 2711 (15.4) | 5620 (15.3) | < .001 |
| Former | 5572 (10.3) | 1601 (9.1) | 3971 (10.8) | |
| Current | 40365 (74.4) | 13286 (75.5) | 27069 (73.8) | |
| Receiving Antiretroviral Therapy | - | 15062 (79.7) | - | - |
| Baseline CD4 Count, mean (SD) (n=14,595) | - | 457.1 (413.3) | - | - |
| CD4 ≥200 (n=14,595) | - | 11520 (78.9) | - | - |
| HIV Viral Load < 500 (n=14518) | - | 9433 (65.0) | - | - |
Note. Abbreviations: Human Immunodeficiency Virus (HIV), Standard Deviation (SD), Substance Use Disorder (SUD)
Data cell values are n (%) unless otherwise indicated as mean (SD)
P-values reflect comparison among HIV-infected and uninfected columns
3.2. Index SUD episodes
Overall, HIV-infected and uninfected were similar in number of index SUD episodes and substances used (Table 3). Among the 57,428 patients with at least 1 index SUD episode, 31.1% had a single index SUD episode, 17.9% had 2, 12.8% had 3, 9.8% had 4, 7.6% had 5, 6.2% had 6, and 14.5% had 7 or more index SUD episodes during the study period. HIV-infected patients had slightly fewer index SUD episodes (3.2 vs. 3.6; p<0.001) than uninfected patients. Most index SUD episodes occurred in non-mental health/non-SUD outpatient, SUD outpatient, and mental health outpatient settings. HIV-infected patients were less likely than uninfected to have their index SUD episode in a mental health and/or SUD setting (56.8% vs. 60.5%; p <.001). Of those with an index SUD episode, 66.5% had a substance dependence diagnosis (ICD-9 303.x or 304.x) at the index episode with alcohol dependence in 44.1% and drug dependence in 42.6%; 20.2% of patients had both alcohol dependence and drug dependence diagnoses. At the index SUD episode, HIV-infected patients were more likely than uninfected patients to be recognized as having a diagnosis related to opioids, cocaine, and amphetamines, and less likely to have an alcohol-related diagnosis code. Sedative-hypnotics, cannabis, and hallucinogen diagnoses were comparable.
Table 3.
SUD Treatment for first index SUD episode, 2000–2015.
| Total Sample (n=57428) | HIV-infected (n=18898) | HIV-uninfected (n=38530) | P-value | |
|---|---|---|---|---|
| Number of index SUD episodes | <.001 | |||
| 1 | 17867 (31.1) | 6247 (33.1) | 11620 (30.2) | |
| 2 | 10281 (17.9) | 3555 (18.8 | 6726 (17.5) | |
| 3 | 7367 (12.8) | 2438 (12.9) | 4929 (12.8) | |
| 4 | 5644 (9.8) | 1886 (10.0) | 3758 (9.8) | |
| 5 | 4357 (7.6) | 1383 (7.3) | 2974 (7.7) | |
| 6 | 3570 (6.2) | 1073 (5.7) | 2497 (6.5) | |
| 7 or more | 8342 (14.5) | 2316 (12.3) | 6026 (15.6) | |
| Number of index SUD episodes, mean (SD) | 3.5 (2.7) | 3.2 (2.5) | 3.6 (2.8) | <.001 |
| Location of index SUD episode (N=52074) | <.001 | |||
| Non-MH Non-SUD Inpatient | 1051 (2.0) | 418 (2.4) | 633 (1.8) | |
| SUD Inpatient | 253 (0.5) | 94(0.5) | 159 (0.5) | |
| MH non-SUD Inpatient | 4034 (7.8) | 1252 (7.3) | 2782 (8.0) | |
| Emergency Department | 1934 (3.7) | 582 (3.4) | 1352 (3.9) | |
| Non-MH Non-SUD Outpatient | 15782 (30.3) | 5135 (29.8) | 10647 (30.6) | |
| SUD Outpatient | 6316 (12.1) | 2217 (12.9) | 4099 (11.8) | |
| MH non-SUD Outpatient | 15493 (29.8) | 4754 (27.6) | 10739 (30.8) | |
| Other MH Outpatient | 2467 (4.7) | 768 (4.5) | 1699 (4.9) | |
| Nursing Home | 141 (0.3) | 62 (0.4) | 79 (0.2) | |
| Non-MH Inpatient (not otherwise classified) | 4603 (8.8) | 1975 (11.4) | 2628 (7.6) | |
| Substance treated during index SUD episode | ||||
| Alcohol | 37764 (65.8) | 10982 (58.1) | 26782 (69.5) | < .001 |
| Opioids | 7387 (12.9) | 2993 (15.8) | 4394 (11.4) | < .001 |
| Cocaine | 17923 (31.2) | 7069 (37.4) | 10854 (28.2) | <.001 |
| Amphetamine/Stimulant | 2206 (3.8) | 1161 (6.1) | 1045 (2.7) | <.001 |
| Sedative/Hypnotic | 1141 (2.0) | 371 (2.0) | 770 (2.0) | 0.776 |
| Cannabis | 10562 (18.4) | 3520 (18.6) | 7042 (18.3) | 0.310 |
| Opioid plus another drug | 5308 (9.2) | 2225 (11.8) | 3083 (8.0) | <.001 |
| Combined drugs (without opioid) | 28536 (49.7) | 10747 (56.9) | 17789 (46.2) | <.001 |
| Hallucinogen | 206 (0.4) | 77 (0.4) | 129 (0.3) | 0.171 |
Note. Data cell values are n(%) unless otherwise indicated as mean (SD)
Abbreviations: Mental Health (MH); Substance Use Disorder (SUD)
Substances used (%) may total to more than 100% due to multiple substances used over SUD episode
P-values reflect comparison among HIV-infected and uninfected columns
3.3. Initiation, engagement, and retention in SUD treatment
Rates of initiation, engagement, and retention in SUD treatment after the index SUD episode were less than 20% for both HIV-infected and uninfected patients (Table 4). Initiation rates did not differ between HIV-infected and uninfected but engagement and retention rates were slightly better among HIV-infected patients. As expected, most SUD treatment occurred in a SUD-specific clinical setting. Among those who initiated SUD treatment, the mean number of SUD treatment visits following an index SUD episode did not differ between HIV-infected and uninfected (mean [SD]: 23.0 [86.7] vs. 21.2 [75.9]; p=0.992).
Table 4.
Initiation, Engagement, and Retention in SUD Treatment for first index SUD episode, 2000–2015.
| Total Sample (n=57428) | HIV-infected (n=18898) | HIV-uninfected (n=38530) | P-value | |
|---|---|---|---|---|
| Initiation | ||||
| Initiated treatment after index episode, any setting | 9223 (16.1) | 3208 (17.0) | 6015 (15.6) | <.001 |
| Initiated treatment after index episode, outpatient SUD setting | 6617 (11.5) | 2364 (12.5) | 4253 (11.0) | <.001 |
| Initiated treatment after index episode, outpatient or inpatient/residential SUD setting | 6768 (11.8) | 2416 (12.8) | 4352 (11.3) | <.001 |
| Initiated pharmacotherapy within 14 days after index episode* | 792 (2.7) (n=29694) | 276 (3.0) (n=9221) | 516 (2.5) (n=20473) | 0.019 |
| Engagement | ||||
| Engaged in treatment after index episode, any setting | 6053 (10.5) | 2177 (11.5) | 3876 (10.1) | <.001 |
| Engaged in treatment after index episode, outpatient SUD setting | 5847 (10.2) | 2101 (11.1) | 3746 (9.7) | <.001 |
| Engaged in treatment after index episode, outpatient or inpatient/residential SUD setting | 8413 (14.7) | 3028 (16.0) | 5385 (14.0) | <.001 |
| Engaged pharmacotherapy within 30 days of index episode* | 1057 (3.6) (n=29694) | 368 (4.0) (n=9221) | 689 (3.4) (n=20473) | 0.007 |
| Retention | ||||
| Retained in treatment after index episode, any setting | ||||
| 3 months | 9209 (16.0) | 3177 (16.8) | 6032 (15.7) | <.001 |
| 6 months | 7591 (13.2) | 2600 (13.8) | 4991 (13.0) | 0.007 |
| 1 year | 5502 (9.6) | 1868 (9.9) | 3634 (9.4) | 0.083 |
| Retained in treatment after index episode, outpatient or inpatient/residential SUD-specific settings | ||||
| 3 months | 7591 (13.2) | 2660 (14.1) | 4931(12.8) | <.001 |
| 6 months | 6371 (11.1) | 2222 (11.8) | 4149 (10.8) | <.001 |
| 1 year | 4488 (7.8) | 1539 (8.1) | 2949 (7.7) | 0.040 |
Note. Data cell values are n(%)
P-values reflect comparison among HIV-infected and uninfected columns
The denominators for the pharmacotherapy initiation and engagement variables are restricted to patients with alcohol and opioid use disorders.
In adjusted logistic regression models, HIV-infected patients had greater odds of initiating SUD treatment within 14 days (Odds Ratio 1.05; 95% Confidence Interval 1.00-1.10; p=.052) and of retention in SUD treatment at 6 months (Odds Ratio 1.10; 95% Confidence Interval 1.04-1.16; p=.001 ) following an index SUD episode, compared with uninfected patients. (Table 5) Compared with White patients, Black patients had greater odds of initiation, engagement, and retention in SUD treatment whereas, compared with urban patients, rural patients had lower odds of the 3 outcomes.
Table 5.
Logistic regression estimates on outcomes of SUD treatment initiation, engagement (30 days), and retention (6 months) in any location.
| Characteristic | SUD Treatment Outcome | ||
|---|---|---|---|
| Initiation | Engagement (30 days) | Retention (6 months) | |
| Adjusted Odds Ratio (95% Confidence Interval) | |||
| HIV infection (ref. uninfected) | 1.05 (1.00-1.10) | .99 (.93-1.05) | 1.10 (1.04-1.16) |
| Age | .969 (.966-.971) | .965 (.962-.968) | .967 (.965-.970) |
| Female (ref. male) | .86 (.73-1.02) | .84 (.69-1.02) | .81 (.68-.98) |
| Race/ethnicity (ref. white) | |||
| Black | 1.42 (1.35-1.50) | 1.56 (1.46-1.65) | 1.58 (1.49-1.67) |
| Hispanic | .95 (.86-1.04) | .85 (.75-.96) | 1.02 (.92-1.13) |
| Other | .80 (.66-.96) | .82 (.65-1.04) | .68 (.54-.86) |
| Rural residence (ref. urban residence) | |||
| Rural 1 | .75 (.69-.82) | .72 (.65-.80) | .68 (.62-.75) |
| Unknown | .79 (.75-.83) | .80 (.75-.85) | .74 (.70-.79) |
| Hepatitis C infected (ref. uninfected) | 1.58 (1.50-1.65) | 1.62 (1.53-1.72) | 1.84 (1.75-1.94) |
| Psychiatric comorbidity (ref. no psych. comorbidity) | .97 (.92-1.01) | .89 (.84-.95) | 1.15 (1.09-1.21) |
Note. Bolded data cells indicate p-value < .05
3.4. Trends in SUD pharmacotherapy
Rates of initiation of SUD pharmacotherapy for opioid use disorders and alcohol use disorders were low for all patients (Table 4 and Figures 1a, 1b, 2a, 2b). HIV-infected and uninfected patients with opioid use disorder did not differ in receipt of methadone. While other SUD pharmacotherapy was uncommon for all patients, HIV -infected patients were less likely than uninfected to receive any non-methadone pharmacotherapy (1.6% vs. 2.3%; p < .001). Compared with uninfected, HIV-infected with opioid SUD alone were less likely to receive buprenorphine (1.9% vs. 3.3%, p < .001); those with alcohol SUD were less likely to receive acamprosate (1.6% vs. 2.3%, p < .001) but as likely to receive naltrexone (0.5% vs. 0.4%, p = .18). As shown in the Figures, methadone receipt decreased from 2000 to 2015 in both HIV -infected and uninfected whereas buprenorphine and naltrexone receipt tended to increase in both groups over that time. Among those with alcohol use disorder, HIV-infected were less likely than uninfected to receive naltrexone during the most recent (2009-2015) time segment. Acamprosate use increased initially in both HIV-infected and uninfected before settling at a low rate, just above 1%.
Fig. 1.
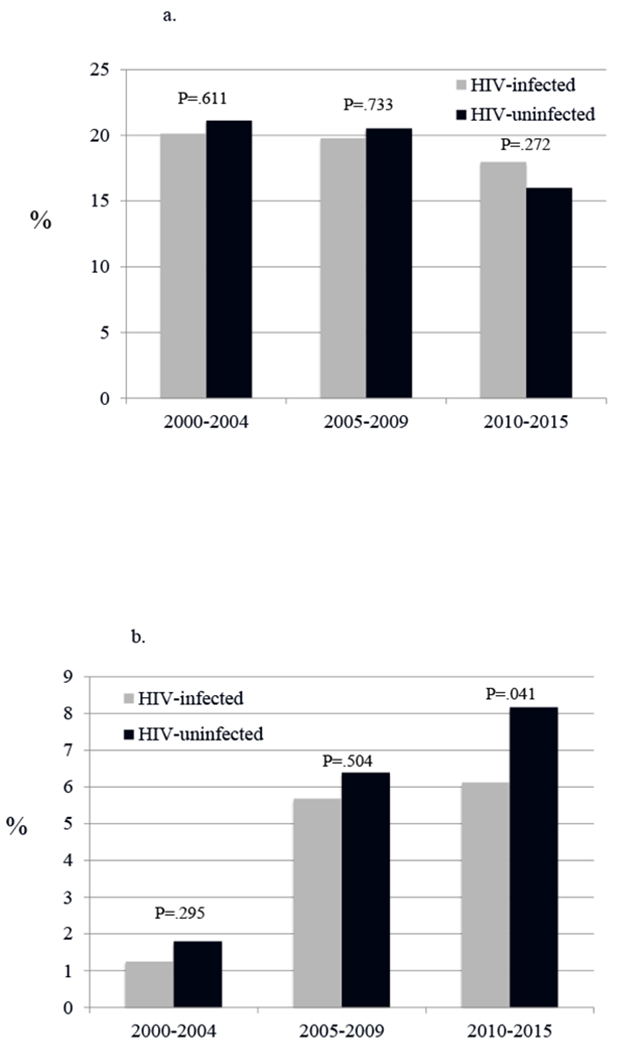
Trends over time in opioid agonist therapy following a new clinical episode for opioid use disorder, by HIV infection status.
a. Trends in methadone initiation
*Test for trend is statistically significant for uninfected (P=.002), but not for HIV-infected
b. Trends in burprenorphine initiation
*Test for trend is statistically significant for both HIV-infected and uninfected (both P<.001)
Fig. 2.
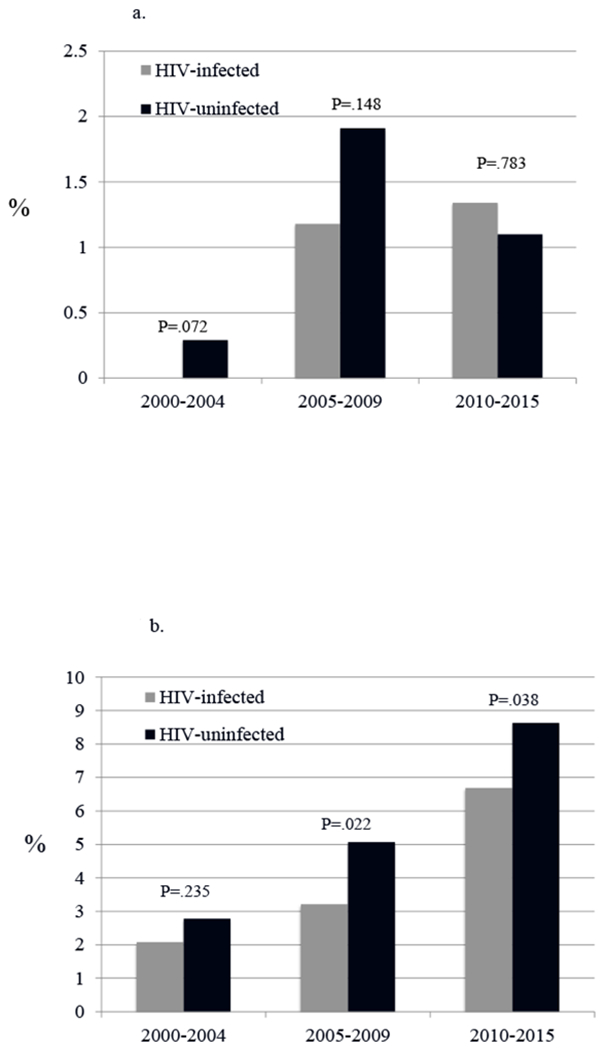
Trends over time in alcohol pharmacotherapy agonist therapy following a new clinical episode for alcohol use disorder, by HIV infection status.
a. Trends in acamprosate use
*Test for trend is statistically significant for both HIV-infected (P<.001) and uninfected (P<.01)
b. Trends in oral naltrexone use
*Test for trend is statistically significant for both HIV-infected and uninfected (P<.001 for both)
4. Discussion
In this large national sample, we found that HIV-infected Veterans are more likely than uninfected controls to have an index SUD episode and to meet criteria for opioid, cocaine, and amphetamine use disorders. Among those with an index SUD episode, initiation, engagement, and retention in behavioral SUD treatment and use of pharmacotherapy was low for both HIV -infected and uninfected Veterans. Among those who initiated behavioral SUD treatment, there appeared to be a substantial number of treatment encounters (mean > 20) but with large variation. In adjusted models, HIV-infected patients were more likely to be retained in SUD treatment at 6 months. Prescriptions of buprenorphine for opioid use disorder and naltrexone for alcohol use disorder slowly increased over time in both HIV-infected and uninfected. Although we found some statistically significant differences in SUD treatment between HIV-infected and uninfected Veterans, the differences were small and likely reflected the large sample size rather than clinical importance.
Our findings of low initiation, engagement, and retention rates for behavioral SUD treatment are similar to the rates found in RAND’s capstone evaluation of the VA’s mental health and SUD treatment program, an evaluation that was not stratified by HIV status, and in other studies using non-VA, non-HIV settings. (Watkins et al., 2011a; Watkins et al., 2011; SAMSHA, 2017) Although not tested directly in our analysis, our findings suggest that barriers to initiating SUD treatment may not differ substantially between HIV-infected and uninfected Veterans with SUD and that improvements in initiation, engagement, and retention in SUD treatment services and pharmacotherapy are necessary for all patients with SUD, regardless of HIV status. Our findings of higher odds of initiation, engagement, and retention in SUD treatment among Black patients may be related to urban residence and greater access to services. Our finding of lower odds of initiation, engagement, and retention in SUD treatment among patients with rural residence is consistent with other recent analyses of VA data and supports the need for interventions to increase SUD treatment in rural areas. (Bensley et al., 2019)
Our findings of marginally significant higher odds of SUD treatment initiation and significantly higher odds of SUD treatment retention at 6 months among HIV-infected patients, compared with uninfected patients, are counter to some prior findings in the VA. For example, in an analysis of national VA data, a positive screen for unhealthy alcohol use was followed by a brief motivational intervention in 57% of HIV-infected and 74% of uninfected Veterans. (Williams et al., 2017b) In this latter study, alcohol use disorder was more common in HIV-infected (49%) than in uninfected (32%). Since those with alcohol use disorder are more likely to need SUD treatment, this disparity in the prevalence of alcohol use disorder might explain why the proportion of HIV-infected and uninfected receiving SUD treatment is similar in our analysis, despite potentially lower exposure to brief intervention.
Our finding of low, but increasing, rates of SUD pharmacotherapy is consistent with prior work done in the general VA population with SUD.( Gordon et al., 2007; Harris et al., 2012; Harris et al., 2013; Oliva et al., 2012; Oliva et al., 2013) For opioid use disorder, methadone treatment has decreased as office-based opioid agonist therapy with buprenorphine has increased, albeit at rates slower than hoped for given the FY 2009 VA national mandate to increase access to buprenorphine.(Oliva et al., 2013) We found decreasing methadone receipt and increasing buprenorphine receipt over time among the HIV - infected, mirroring the observation in uninfected Veterans. (Oliva et al., 2013; Wyse et al., 2018) Prior studies identified predictors of buprenorphine treatment within the VA system, including the following: male sex, age 56 or older, absence of a mental health diagnosis, presence of buprenorphine services at VA facility, and specialty SUD treatment services available on weekends.(Oliva et al., 2012; Oliva et al., 2011b) In other work, we found that Black race, Hepatitis C infection, and alcohol related diagnosis were associated with higher odds of initiating any opioid agonist treatment whereas HIV infection, psychiatric diagnosis and rural residence were associated with lower odds of initiation. (Wyse et al., 2019) Predictive factors were similar regardless of HIV status. These findings underscore the need to improve access to opioid use disorder pharmacotherapy for all patients and that special emphasis should be given to rural areas.
For alcohol use disorders, we observed an initial increase and leveling off over time of naltrexone use in the HIV-infected group and steadily increasing rates of naltrexone use in the uninfected group. The reason for the leveling of naltrexone use in HIV -infected is not clear, but is potentially related to prescriber concern about adverse effects (e.g., liver toxicity) or to increased prevalence of pain syndromes among HIV-infected patients that may require opioid treatment (a contraindication for naltrexone treatment). Acamprosate use was very low overall and perhaps reflected its more complicated dosing (3 times daily) and conflicting findings from randomized controlled trials (Anton et al., 2006) and meta-analyses (Jonas et al., 2014; Palpacuer et al., 2018) regarding benefit for alcohol use disorder. Prior studies have identified predictors of alcohol use disorder pharmacotherapy within the VA system, including female gender, white race, age 54 years or less, more severe alcohol use disorder, receipt of specialty SUD care, and specialty SUD treatment available on evenings and weekends. (Harris et al., 2010; Harris, 2012; Williams et al., 2017a) Predictors of alcohol pharmacotherapy may differ in the HIV- infected population. We may expect to see increased alcohol pharmacotherapy in the VA system in the future as initiatives to promote such treatment are ongoing and interest in other agents (e.g., topirimate, gabapentin, varenicline) is growing.(Hagedorn et al., 2016; Harris et al., 2016; Oliva et al., 2014)
Our study has several limitations. First, the sample is limited to Veterans in care within the VA healthcare system, which may limit the generalizability of our findings. However, the findings still constitute an important contribution, as the VA is the largest provider of HIV care in the US. Second, the analysis focused on data only through October 2015, given restrictions of the dataset, and may therefore not reflect recent trends. Given historical analyses, our expectation is that behavioral SUD treatment has remained at a low prevalence but that rates of buprenorphine and alcohol pharmacotherapy have continued to increase. Third, our analysis does not include services outside of the VA system (e.g., those covered by Medicare, Medicaid, or community SUD treatment services). This has the potential for some differential bias between HIV-infected and uninfected as we expect HIV-infected Veterans to be more likely to receive most of their care through the VA. Fourth, the measures of initiation, engagement, and retention are indicators of facility performance much more than of actual SUD quality of care. (Harris et al., 2009) Therefore, our data are limited in their assessment of the quality of behavioral SUD treatment provided. Fifth, our definition of retention includes inpatient and residential care which may indicate the patient had not been participating in outpatient SUD treatment. Because treatment visits may have been concentrated early in any of the time periods (3, 6, or 12 months) assessed for retention, the retention measure may not reflect SUD treatment continuously through the time period. However, we felt it was important to adhere to the retention definition used by the RAND capstone evaluation for ease of result comparison. Sixth, our analyses rely on electronic administrative and clinical data, which may contain errors and missing data. Lastly, we did not assess use of extended-release naltrexone pharmacotherapy for opioid use disorder since it was not widely available in the VA during the study timeframe.
In summary, our study of national VA data indicates a large gap in behavioral SUD treatment and SUD pharmacotherapy for both HIV-infected and uninfected Veterans with SUD. Future research should focus on determining the comparative impact of different types of SUD treatment and pharmacotherapy on the quality of HIV care and HIV outcomes, and on developing interventions to improve access to and delivery of the full range of SUD services, including behavioral therapy and SUD pharmacotherapy. The findings of several ongoing trials in delivery of novel SUD treatment services in HIV clinics will be invaluable for informing efforts designed to improve SUD care in HIV-infected individuals. (Hagedorn et al., 2016; Hagedorn et al., 2018; Harris et al., 2017) For example, early results from the STEP trial found substantial uptake (40% vs. 8%) in pharmacotherapy for alcohol use disorder among HIV-infected patients who received integrated addiction physician management compared to treatment as usual. (Edelman et al., 2016; Edelman et al., 2017) Implementation of innovative approaches such as these may be necessary to improve SUD care and outcomes for HIV-infected and uninfected patients with SUD.
Highlights.
Substance use disorders are common in HIV-infected patients but are often untreated
Less than 17% of HIV-infected patients initiated substance use disorder treatment
Low rates of treatment and pharmacotherapy were similar for HIV -infected and uninfected
Substance use disorder pharmacotherapy is increasing but remains underutilized
Acknowledgements:
We thank Ethan Lennox of the Center for Research on Health Care, University of Pittsburgh, for editing and administrative assistance.
Role of Funding Source: Supported by R01 AA022886 to Dr. Kevin L. Kraemer and U01 AA020790 to Dr. Amy Justice. The VACS is a cooperative agreement with the National Institute of Alcohol Abuse and Alcoholism (VACS Scientific Collaborator: Dr. Kendall Bryant, co-author).
Appendix.
ICD-9-CM Codes, Clinic Stop Codes, and Inpatient/Residential Codes
| ICD-9-CM Codes |
|---|
| (291) Alcoholic psychoses |
| (291.0) Alcohol withdrawal delirium |
| (291.1) Alcohol-induced persisting amnestic disorder |
| (291.2) Alcohol-induced persisting dementia |
| (291.3) Alcohol-induced psychotic disorder with hallucinations |
| (291.4) Idiosyncratic alcohol intoxication |
| (291.5) Alcohol-induced psychotic disorder with delusions |
| (291.8) Alcohol Psychosis NEC |
| (291.9) Alcohol Mental Disor NOS |
| (292) Drug psychoses |
| (292.0) Drug withdrawal |
| (292.1) Drug-induced psychotic disorders |
| (292.11) Drug-induced psychotic disorder with delusions |
| (292.12) Drug-induced psychotic disorder with hallucinations |
| (292.2) Pathological drug intoxication |
| (292.8) Other specified drug-induced mental disorders |
| (292.81) Drug-induced persisting delirium |
| (292.82) Drug-induced persisting dementia |
| (292.83) Drug-induced persisting amnestic disorder |
| (292.84) Drug-induced mood disorder |
| (292.85) Drug-induced sleep disorders |
| (292.89) Other specified drug-induced mental disorders |
| (292.9) Unspecified drug-induced mental disorder |
| (303) Alcohol dependence syndrome |
| (303.0) Alcohol intoxication, acute, unspec. |
| (303.9) Other and unspecified alcohol dependence |
| (303.91) Alcoh Dep NEC/NOS-Contin (Chronic) |
| (303.92) Alcoh Dep NEC/NOS-Episodic |
| (303.93) Alcoh Dep NEC/NOS-Remiss |
| (304) Drug dependence |
| (304.0) Opioid type dependence |
| (304.1) Sedative, hypnotic or anxiolytic dependence |
| (304.2) Cocaine dependence |
| (304.3) Cannabis dependence |
| (304.4) Amphetamine and other psychostimulant dependence |
| (304.5) Hallucinogen dependence |
| (304.6) Other specified drug dependence |
| (304.7) Combinations of opioid type drug with any other drug dependence |
| (304.8) Combinations of drug dependence excluding opioid type drug |
| (304.9) Unspecified drug dependence |
| (305) Nondependent abuse of drugs |
| (305.0) Nondependent alcohol abuse |
| (305.01) Alcohol Abuse-Continuous |
| (305.02) Alcohol Abuse-Episodic |
| (305.03) Alcohol Abuse-In remission |
| (305.1) Nondependent tobacco use disorder |
| (305.2) Nondependent cannabis abuse |
| (305.3) Nondependent hallucinogen abuse |
| (305.4) Nondependent sedative, hypnotic or anxiolytic abuse |
| (305.5) Nondependent opioid abuse |
| (305.6) Nondependent cocaine abuse |
| (305.7) Nondependent amphetamine or related acting sympathomimetic abuse |
| (305.8) Nondependent antidepress ant type abuse |
| (305.9) Other drug abuse-Unspecified |
| Clinical Stop Codes |
| Outpatient Codes |
| Non-Mental Health, Non-SUD specific (restricted to Primary Care) |
| (301) General Internal Medicine |
| (310) Infectious Disease |
| (323) Primary Care |
| Mental Health non-SUD specific (restricted to those with at least 10,000 SUD episodes or integrated care (534)) |
| (502) Mental Health - Individual |
| (505) Day treatment - Individual |
| (506) Day hospital - Individual |
| (509) Psychiatry - Individual |
| (510) Psychology - Individual |
| (512) Mental Health Consultation |
| (522) HUD/VA Shared Housing – Individual (homeless program) |
| (527) Mental Health Telephone |
| (529) HCHV/Homeless Chronically Mentally Ill |
| (532) Psychosocial Rehab – Individual (deactivated 10/10/2012) |
| (534) Mental Health Integrated Care (activated 2008) |
| (535) Mental Health Vocational Assistance - Individual |
| (539) Mental Health Integrated Care Group (activated 2010) |
| (540) PSTD Clinical Team |
| (550) Mental Health Group |
| (552) Mental Health Intensive Case Management, Individual |
| (553) Day Treatment - Group |
| (554) Day Hospital - Group |
| (558) Psychology - Group |
| (559) Psychosocial Rehab – Group (deactivated 10/10/2012) |
| (561) PCT – Post Traumatic Stress - Group |
| (573) Mental Health Incentive Therapy Face-to-Face |
| (574) Mental Health Compensated Work Therapy/Transitional Work Experience |
| (575) Mental Health Vocational Assistance Group |
| (727) Residential Rehab Treatment Program Aftercare – Group (deactivated 2009) |
| Mental Health SUD-specific |
| (513) Substance Use Disorder – Individual |
| (514) Substance Use Disorder – Home Visit |
| (519) Substance Use Disorder/PTSD Teams |
| (523) Opioid Substitution |
| (545) Telephone – Substance Use Disorder |
| (547) Intensive Substance Use Disorder – Group |
| (548) Intensive Substance Use Disorder – Individual |
| (560) Substance Use Disorder - Group |
| Inpatient/Residential Codes |
| Non-Mental Health, Non SUD-specific |
| Codes (1, 1D, 1E, 1F, 1G, 1H, 1J, 1N, 2 - 24, 31- 32, 34 – 36, 40 – 41, 48 – 63, 65, 78, 82 – 83, 97). Omit 30 (Pediatrics) and the nursing home codes |
| Mental Health non-SUD specific |
| (1K) Psychiatry Residential Rehab Program |
| (1L) PTSD Residential Rehab Program |
| (25) Psychiatry Domiciliary (deactivated 6/1/2010) |
| (26) Psychiatry Domiciliary PTSD (deactivated 6/1/2010) |
| (28) Homeless Compensated Work Therapy (CWT)/Transitional Residential (RT) (deactivated 10/1/2009) |
| (33) GEM Psychiatry |
| (37) Domiciliary Homeless |
| (38) PTSD CWT/TR (deactivated 10/1/2009) |
| (39) General CWT/TR |
| (70) Acute Psychiatry, < 45 days (inactive code) |
| (71) Long Term Psychiatry, > 45 days (inactive code) |
| (75) Halfway House (deactivated 7/1/2006) |
| (76) Psychiatric Mentally Infirm (deactivated 7/1/2006)) |
| (77) PRRTP (psych resid rehab treatment program, I think) (deactivated 7/1/2007) |
| (79) Specialty Inpatient PTSD Unit |
| (85) Domiciliary |
| (88) Domiciliary PTSD |
| (89) STAR I, II, & III |
| (91) Eval/BRF Treatment PTSD Unit |
| (92) Psychiatry – General Intermediate |
| (93) High Intensity General Psychiatry Inpatient |
| (94) Psychiatric Observation |
| Mental Health SUD-speciflc |
| (1M or SAS 111) Substance Abuse Residential Program (Domiciliary) |
| (27) Substance Abuse Residential Rehab (code discontinued 6/1/2010) |
| (29) Substance Abuse Compensated Work Therapy/Transitional Residential (code discontinued 10/1/2009) |
| (72) Alcohol Dependence Treatment Unit |
| (73) Drug Dependence Treatment Unit |
| (74) Substance Abuse Treatment Unit |
| (84) Substance Abuse Intermediate Care (code discontinued 7/1/2006) |
| (86) Domiciliary Substance Abuse |
| (90) Substance Abuse STAR I, II, & III (code discontinued 7/1/2006) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflict declared.
References
- Anton RF, et al. , 2006. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE Study: a randomized controlled trial. JAMA. 295(17), 2003–2017. DOI: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Azar MM, et al. , 2010. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend. 112(3), 178–193. DOI: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensley KM, et al. , 2019. Differences in receipt of alcohol-related care across rurality among VA patients living with HIV with unhealth alcohol use. J Rural Health. doi: 10.1111/jrh.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, et al. , 2007. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 19(4), 459–466. DOI: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenze GN, et al. , 2010. Mortality after diagnosis of psychiatric disorders and co-occurring substance use disorders among HIV -infected patients. AIDS Patient Care STDS. 24(11), 705–712. DOI: 10.1089/apc.2010.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman EJ, et al. , 2016. Implementation of integrated stepped care for unhealthy alcohol use in HIV clinics. Addict. Sci. Clin. Pract 11(1), 1 DOI: 10.1186/s13722-015-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman EJ, et al. , 2017. The starting treatment for ethanol in primary care trials (step trials): protocol for three parallel multi-site stepped care effectiveness studies for unhealthy alcohol use in HIV -positive patients. Contemp. Clin. Trials 52, 80–90. DOI: 10.1016/j.cct.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz SL, et al. , 2006. Development and verification of a “virtual” cohort using the national VA health information system. Med. Care 44(8 Suppl 2), S25–30. DOI: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- Garnick DW, et al. , 2006. Performance measures for alcohol and other drug services. Alcohol Res. Health 29(1), 19–26. [PMC free article] [PubMed] [Google Scholar]
- Garnick DW, et al. , 2007. Are Washington Circle performance measures associated with decreased criminal activity following treatment? J. Subst. Abuse Treat 33(4), 341–352. DOI: 10.1016/j.jsat.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnick DW, et al. , 2009. Adapting Washington Circle performance measures for public sector substance abuse treatment systems. J. Subst. Abuse. Treat 36(3), 265–277. DOI: 10.1016/j.jsat.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ, et al. , 2007. Implementation of buprenorphine in the Veterans Health Administration: results of the first 3 years. Drug Alcohol Depend. 90(2–3), 292–296. DOI: 10.1016/j.drugalcdep.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Hagedorn HJ, et al. , 2016. Enhancing access to alcohol use disorder pharmacotherapy and treatment in primary care settings: ADAPT-PC. Implement. Sci 11(1), 64 DOI: 10.1186/s13012-016-0431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn H, et al. , 2018. Advancing pharmacological treatments for opioid use disorder (ADaPT- OUD): protocol for testing a novel strategy to improve implementation of medication-assisted treatment for veterans with opioid use disorders in low-performing facilities. Addict Sci Clin Pract. 13(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AH, et al. , 2007. Veterans Affairs facility performance on Washington Circle indicators and casemix-adjusted effectiveness. J. Subst. Abuse Treat 33(4), 333–339. DOI: 10.1016/j.jsat.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Harris AH, et al. , 2009. HEDIS initiation and engagement quality measures of substance use disorder care: impact of setting and health care specialty. Popul. Health Manag 12(4), 191–196. DOI: 10.1089/pop.2008.0028. [DOI] [PubMed] [Google Scholar]
- Harris AH, et al. , 2010a. Pharmacotherapy of alcohol use disorders in the veterans health administration. Psychiatr. Serv 61(4), 392–398. DOI: 10.1176/ps.2010.61.4.392. [DOI] [PubMed] [Google Scholar]
- Harris AH, et al. , 2010b. Are VHA administrative location codes valid indicators of specialty substance use disorder treatment? J. Rehabil. Res. Dev 47(8), 699–708. [DOI] [PubMed] [Google Scholar]
- Harris AH, et al. , 2012. Pharmacotherapy of alcohol use disorders by the Veterans Health Administration: patterns of receipt and persistence. Psychiatr. Serv 63(7), 679–685. DOI: 10.1176/appi.ps.201000553. [DOI] [PubMed] [Google Scholar]
- Harris AH, et al. , 2013. Pharmacotherapy for alcohol dependence: perceived treatment barriers and action strategies among Veterans Health Administration service providers. Psychol. Serv 10(4), 410–419. DOI: 10.1037/a0030949. [DOI] [PubMed] [Google Scholar]
- Harris AH, et al. , 2016. Multifaceted academic detailing program to increase pharmacotherapy for alcohol use disorder: interrupted time series evaluation of effectiveness. Addict. Sci. Clin. Pract 11(1), 15 DOI: 10.1186/s13722-016-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AHS, et al. , 2017. Effects of a multifaceted implementation intervention to increase utilization of pharmacological treatments for alcohol use disorders in the US Veterans Health Administration. J Subst Abuse Treat. 82:107–112. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, 2006. Improving the quality of health care for mental health and substance-use conditions. National Academies Press (US), Washington (DC). [PubMed] [Google Scholar]
- Jonas DE, et al. , 2014. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 311(18), 1889–1900. DOI: 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- Justice AC, et al. , 2006. Veterans Aging Cohort Study (VACS): overview and description. Med. Care 44(8 Suppl 2), S13–24. DOI: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, et al. , 2016. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend. 161, 95–103. DOI: 10.1016/j.drugalcdep.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthuis PT, et al. , 2012. Unhealthy alcohol and illicit drug use are associated with decreased quality of HIV care. J. Acquir. Immune. Defic. Syndr 61(2), 171–178. DOI: 10.1097/QAI.0b013e31826741aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthuis PT, et al. , 2016. Quality ofhiv care and mortality rates in HIV-infected patients. Clin. Infect. Dis 62(2), 233–239. DOI: 10.1093/cid/civ762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larney S, et al. , 2015. Mortality among older adults with opioid use disorders in the Veteran’s Health Administration, 2000-2011. Drug Alcohol Depend. 147, 32–37. DOI: 10.1016/j.drugalcdep.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low AJ, et al. , 2016. Impact of opioid substitution therapy on antiretroviral therapy outcomes: a systematic review and meta-analysis. Clin Infect Dis. 63(8)4094–104. DOI: 10.1093/cid/ciw416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, 2011. Substance abuse, adherence with antiretroviral therapy, and clinical outcomes among HIV-infected individuals. Life Sci. 88(21–22), 948–952. DOI: 10.1016/j.lfs.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur GJ, et al. , 2012. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 345, e5945 DOI: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BDL, et al. , 2017. Long-term alcohol use patterns and HIV disease severity. AIDS. 31(9), 1313–1321. DOI: 10.1097/QAD.0000000000001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers BM, et al. , 2010. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, andnational coverage. Lancet. 375(9719), 1014–1028. DOI: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]
- McCorry F, et al. , 2000. Developing performance measures for alcohol and other drug services in managed care plans: Washington Circle group. Jt. Comm. J. Qual. Improv 26(11), 633–643. [DOI] [PubMed] [Google Scholar]
- Metsch LR, et al. , 2008. HIV transmission risk behaviors among hiv-infected persons who are successfully linked to care. Clin. Infect. Dis 47(4), 577–584. DOI: 10.1086/590153. [DOI] [PubMed] [Google Scholar]
- Oliva EDA; Harris AH; Trafton JA, 2011a. Health services for va patients with substance use disorders: Comparison of utilization in fiscal years 2010, 2009, and 2002 Department of Veterans Affairs PERC, Menlo Park, CA. [Google Scholar]
- Oliva EM, et al. , 2011b. Barriers to use of pharmacotherapy for addiction disorders and how to overcome them. Curr. Psychiatry Rep 13(5), 374–381. DOI: 10.1007/s11920-011-0222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva EM, et al. , 2012. Receipt of opioid agonist treatment in the Veterans Health Administration: facility and patient factors. Drug Alcohol Depend. 122(3), 241–246. DOI: 10.1016/j.drugalcdep.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Oliva EM, et al. , 2013. Trends in opioid agonist therapy in the Veterans Health Administration: is supply keeping up with demand? Am. J. Drug Alcohol Abuse 39(2), 103–107. DOI: 10.3109/00952990.2012.741167. [DOI] [PubMed] [Google Scholar]
- Oliva EM, Harris AH, 2014. If pharmacotherapies for alcohol use disorders are effective, why are they underutilised? Evid. Based Med 19(6), 230–231. DOI: 10.1136/ebmed-2014-110050. [DOI] [PubMed] [Google Scholar]
- Palepu A, et al. , 2004. Uptake and adherence to highly active antiretroviral therapy among hiv-infected people with alcohol and other substance use problems: the impact of substance abuse treatment. Addiction. 99(3), 361–368. DOI: 10.1111/j.1360-0443.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- Palpacuer C, et al. , 2018. Pharmacologically controlled drinking in the treatment of alcohol dependence or alcohol use disorders: a systematic review with direct and network meta-analysis on nalmefene, naltrexone, acamprosate, baclofen and topiramate. Addiction. 113(2), 220–237. DOI: 10.1111/add.13874. [DOI] [PubMed] [Google Scholar]
- Scott-Sheldon LAI, et al. , 2017. Behavioral interventions targeting alcohol use among people living with HIV/AIDS: a systematic review and meta-analysis. AIDSBehav. 21(Suppl 2): 126–143. DOI: 10.1007/sl0461-017-1886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2010. The NSDUH Report: HIV/AIDS and Substance Use. Rockville, MD. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2017. Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17-5044, NSDUH Series H-52). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; Retrieved from https://www.samhsa.gov/data/ [Google Scholar]
- Satre DD, et al. , 2013. Factors associated with treatment initiation for psychiatric and substance use disorders among persons with HIV. Psychiatr. Serv 64(8), 745–753. DOI: 10.1176/appi.ps.201200064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, et al. , 2017. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 357)1550 DOI: 10.1136/bmj.jl550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, et al. , 2011a. Care for veterans with mental and substance use disorders: good performance, but room to improve on many measures. Health Aff (Millwood). 30(11), 2194–2203. DOI: 10.1377/hlthaff.2011.0509. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Pincus HA; Smith B; et al. , 2011b. Veterans Health Administration Mental Health Program Evaluation: Capstone Report. RAND Corporation, Santa Monica, CA. [Google Scholar]
- Williams EC, et al. , 2016. Alcohol use and human immunodeficiency virus (HIV) infection: current knowledge, implications, and future directions. Alcohol Clin. Exp. Res 40(10), 2056–2072. DOI: 10.1111/acer.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, et al. , 2017a. Variation in receipt of pharmacotherapy for alcohol use disorders across racial/ethnic groups: a national study in the U.S. Veterans Health Administration. Drug Alcohol Depend 178, 527–533. DOI: 10.1016/j.drugalcdep.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Williams EC, et al. , 2017b. Among patients with unhealthy alcohol use, those with HIV are less likely than those without to receive evidence-based alcohol-related care: a national VA study. Drug Alcohol Depend. 174 113–120. DOI: 10.1016/j.drugalcdep.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse JJ, et al. , 2018. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. SubstAbus. 39(2):139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse J, et al. , 2019. Predictors of timely opioid agonist treatment initiation among Veterans with and without HIV. Drug Alcohol Depend. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]


