Significance
To date, wastewater-based epidemiology has focused on reporting drug and pharmaceutical consumption patterns by analyzing domestic wastewater. Here we explore the relationships between chemicals in wastewater and social, demographic, and economic parameters of the respective populations. We show the extent to which consumption of chemicals such as opioids and illicit drugs are associated with sociodemographics. We also examine chemicals that reflect individuals’ consumption of food components in wastewater and show that disparities in diet are associated with educational level. Our study shows that chemicals in wastewater reflect the social, demographic, and economic properties of the respective populations and highlights the potential value of wastewater in studying the sociodemographic determinants of population health.
Keywords: socioeconomics, food, drugs, public health, wastewater
Abstract
Wastewater is a potential treasure trove of chemicals that reflects population behavior and health status. Wastewater-based epidemiology has been employed to determine population-scale consumption of chemicals, particularly illicit drugs, across different communities and over time. However, the sociodemographic or socioeconomic correlates of chemical consumption and exposure are unclear. This study explores the relationships between catchment specific sociodemographic parameters and biomarkers in wastewater generated by the respective catchments. Domestic wastewater influent samples taken during the 2016 Australian census week were analyzed for a range of diet, drug, pharmaceutical, and lifestyle biomarkers. We present both linear and rank-order (i.e., Pearson and Spearman) correlations between loads of 42 biomarkers and census-derived metrics, index of relative socioeconomic advantage and disadvantage (IRSAD), median age, and 40 socioeconomic index for area (SEIFA) descriptors. Biomarkers of caffeine, citrus, and dietary fiber consumption had strong positive correlations with IRSAD, while tramadol, atenolol, and pregabalin had strong negative correlation with IRSAD. As expected, atenolol and hydrochlorothiazide correlated positively with median age. We also found specific SEIFA descriptors such as occupation and educational attainment correlating with each biomarker. Our study demonstrates that wastewater-based epidemiology can be used to study sociodemographic influences and disparities in chemical consumption.
Wastewater-based epidemiology (WBE) is a method of systematically sampling and analyzing chemical residues in wastewater influent to measure a population’s consumption of or exposure to chemicals. Per-capita consumption of specific chemicals can be estimated by normalizing biomarker concentrations in wastewater to the volume of wastewater and size of the contributing population (1). Chemicals commonly measured by WBE include illicit drugs such as cocaine and methamphetamine (2), licit drugs such as tobacco and caffeine (3), pharmaceuticals (4), and personal-care products such as ultraviolet filters (5). Exposure to chemicals such as endocrine disruptors (6) or flame retardants (7) can also be measured, as can hydrophobic chemicals which partition to particulate matter (8). There are also theoretical prospects of measuring biomarkers of diet (9, 10).
Most WBE studies have compared chemical loads over time, or between different communities, or some combination of the two. Analysis of temporal trends can monitor population chemical consumption or exposure over time, such as changes in drug use in response to government interventions such as plain cigarette packaging (11) or cannabis legalization (12). Spatial analyses can provide insights into differences in population lifestyles or behavior, such as the prevalence of illicit drug consumption in different countries (2).
WBE studies corroborate or validate their findings by comparing wastewater results with independent measures of consumption or exposure. In the case of chemical consumption, these measures may consist of survey estimates of self-reported drug consumption, illicit drug seizure rates, or pharmaceutical sales volumes. In the case of exposures, they may include variations in environmental pollen (13) or temperature (14). In this way, WBE has been used to identify relationships between chemical consumption and environmental phenomena (15).
Two recent studies have explored the contribution of the social determinants of health to WBE findings. In a temporal study in Athens, major financial and healthcare sector difficulties between 2010 and 2014 coincided with increased use of benzodiazepines, antidepressants, and illicit drugs and decreased use of antibiotics and nonsteroidal antiinflammatory drugs (NSAIDs). The study illustrated how a severe socioeconomic downturn was accompanied by changes in proxy biomarkers of population mental health (16). A study on different catchments serving the city of Beijing showed that antibiotics and other pharmaceutical and personal-care product (PPCP) loads correlated with population density and housing indices, showing that PPCP consumption volume in Beijing was associated with socioeconomic measures at a population level (17). These first WBE socioeconomic studies used broad, generic indicators of socioeconomic status (SES), which result in highly specific case studies rather than more generalized trends.
The use of validated and widely accepted socioeconomic metrics, such as those used by government and sociodemographic researchers, can improve the quality, interpretability, and transdisciplinary relevance of WBE studies. The Australian Bureau of Statistics, for example, uses national census data to calculate various socioeconomic indexes for areas (SEIFA) descriptors. These are measures of income, education, employment, housing, and more. Selections of SEIFA descriptors have been used to generate broad indicators of community-level SES such as the index of relative socioeconomic advantage and disadvantage (IRSAD) (18). IRSAD has been used in various peer-reviewed studies in medical (19), environmental (20), public health (21), and behavioral (22) disciplines.
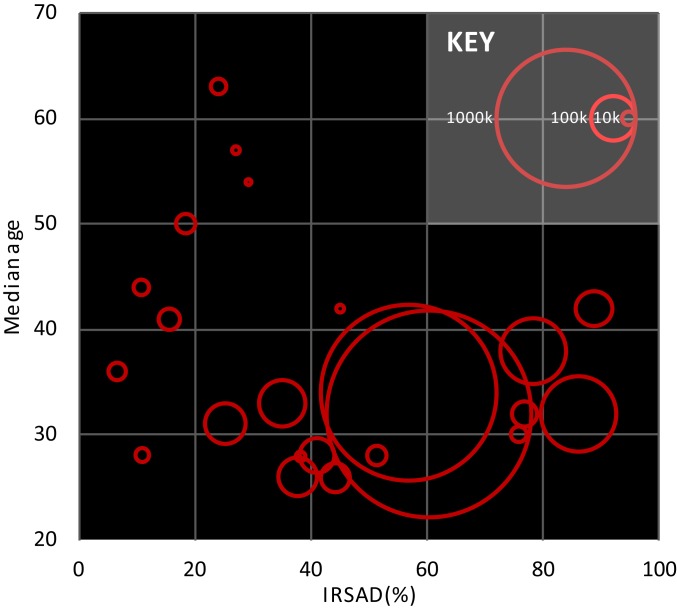
The present study examined relationships between WBE biomarkers and the population median age, average IRSAD, and 40 SEIFA descriptors (Table 1) for 22 wastewater treatment plant (WWTP) catchments in Australia (Fig. 1). Specifically, the study examined whether sociodemographic measures correlated with consumption of food components, PPCPs, and licit and illicit drugs by analyzing wastewater influent samples collected during the week of the 2016 national census. Per-capita loads of each biomarker were calculated for each WWTP catchment. Using data collected from the same census and georeferencing software, the median age (henceforth age), IRSAD, and SEIFA descriptors were calculated for the population served by each WWTP catchment. We used this to study the correlations between per-capita loads of WBE biomarkers and IRSAD, age, and SEIFA descriptors.
Table 1.
SEIFA descriptors used in the present study
| Descriptors | IRSAD weighting | Definition |
| ATSCHOOL | NA | People aged 15 y and over who are still attending secondary school |
| ATUNI | 0.36 | People aged 15 y and over at a university or other tertiary institution |
| CERTIFICATE | −0.36 | People aged 15 y and over whose highest level of education is a certificate III or IV qualification (intermediate or advanced vocational training) |
| DEGREE | NA | People aged 15 y and over whose highest level of education is a bachelor degree or higher |
| DIPLOMA | 0.5 | People aged 15 y and over whose highest level of education is an advanced diploma or diploma |
| NOEDU | −0.34 | People aged 15 y and over who have no educational attainment |
| NOYEAR12ORHIGHER | −0.85 | People aged 15 y and over whose highest level of education is year (grade) 11 or lower |
| CHILDJOBLESS | −0.76 | Families with children under 15 y of age and jobless parents |
| DISABILTYU70 | −0.69 | People aged under 70 y who need assistance with core activities |
| ENGLISHPOOR | NA | People who do not speak English well or at all |
| FEWBED | NA | Classifiable occupied private dwellings with one or no bedrooms |
| HIGHBED | 0.44 | Occupied private dwellings with 4 or more bedrooms |
| GROUP | NA | Occupied private dwellings that are group occupied private dwellings |
| LONE | NA | Occupied private dwellings that are lone person occupied private dwellings |
| HIGHCAR | NA | Occupied private dwellings with 3 or more cars |
| NOCAR | −0.33 | Occupied private dwellings with no cars |
| HIGHMORTGAGE | 0.72 | Occupied private dwellings paying more than $2,800 per mo in mortgage |
| HIGHRENT | 0.47 | Occupied private dwellings paying more than $470 per wk in rent |
| LOWRENT | −0.64 | Occupied private dwellings paying less than $215 per wk in rent (excluding $0 per wk) |
| INC_LOW | −0.89 | People with stated annual household equalized income between $1 and $25,999 (approximately first and second deciles) |
| INC_HIGH | 0.83 | People with stated annual household equalized income greater than $78,000 (approximately ninth and tenth deciles) |
| NONET | −0.78 | Occupied private dwellings with no internet connection |
| OCC_DRIVERS | −0.62 | Employed people classified as machinery operators and drivers |
| OCC_LABOR | −0.79 | Employed people classified as laborers |
| OCC_MANAGER | 0.47 | Employed people classified as managers |
| OCC_PROF | 0.71 | Employed people classified as professionals |
| OCC_SKILL5 | NA | Employed people working in a Skill Level 5 occupation (commensurate with compulsory secondary education, vocational Certificate I, or a short period of on-the-job training) |
| OCC_SKILL4 | NA | Employed people working in a Skill Level 4 occupation (commensurate with vocational Certificate II or III or at least 1 y of relevant experience) |
| OCC_SKILL2 | NA | Employed people working in a Skill Level 2 occupation (commensurate with AQF Associate Degree, Advanced Diploma or Diploma, or at least 3 y of relevant experience) |
| OCC_SKILL1 | NA | Employed people working in a Skill Level 1 occupation (commensurate with bachelor degree or higher qualification, or at least 5 y of relevant experience) |
| ONEPARENT | −0.65 | Families that are one-parent families with dependent offspring only |
| OWNING | NA | Occupied private dwellings owning the dwelling they occupy without a mortgage |
| MORTGAGE | NA | Occupied private dwellings owning the dwelling they occupy with a mortgage |
| SEPDIVORCED | −0.6 | People aged 15 y and over who are separated or divorced |
| UNEMPLOYED | −0.66 | People (in the labor force) who are unemployed |
| UNEMPLOYED1 | NA | People aged 15 y and over who are unemployed |
| UNINCORP | NA | Owner of an unincorporated enterprise |
| OCC_SERVICE_L | −0.54 | Employed people classified as Low Skill Community and Personal Service Workers |
| OCC_SALES_L | −0.32 | Employed people classified as Low Skill Sales |
| OVERCROWD | −0.33 | Occupied private dwellings requiring one or more extra bedrooms (based on Canadian National Occupancy Standard) |
NA: not applicable. Details regarding variable composition can be found elsewhere (18).
Fig. 1.
IRSAD and median age of catchments featured in this study. Each catchment is depicted by a circle whose area represents its population size.
Results and Discussion
The present paper focuses on 42 biomarkers which were measured in wastewater above the limit of quantification with >80% frequency (SI Appendix, Table S1). The concentrations of these biomarkers in wastewater are shown in SI Appendix, Fig. S1. We report correlations between per-capita normalized loads (henceforth loads) of these substances with catchment age, catchment IRSAD, and SEIFA descriptors using Pearson (23) and Spearman rank-order correlation (24) to capture both linear and nonlinear correlations. Using the confusion matrix (25), a false positive detection rate of 0.01% corresponded to |R| = 0.35 (SI Appendix, Fig. S2), and we applied a more conservative significance threshold of |R| = 0.5.
Vitamins.
We measured biomarkers of vitamins B3, E, and B6 in wastewater to assess population dietary vitamin intake. Both N-methyl-2-pyridone-5-carboxamide (2PY) and N-methyl-4-pyridone-3-carboxamide (4PY) are formed from consumption of nicotinamide, a major B3 vitamer (26, 27). α-Carboxyethyl hydrochroman (αCEHC) is formed from the metabolism of α-tocopherol (28, 29), the predominant form of vitamin E in dietary sources (30). Urinary 4-pyridoxic acid accounts for 40 to 60% of dietary vitamin B6 intake (31) and is a marker of short-term vitamin B6 intake (27, 32, 33).
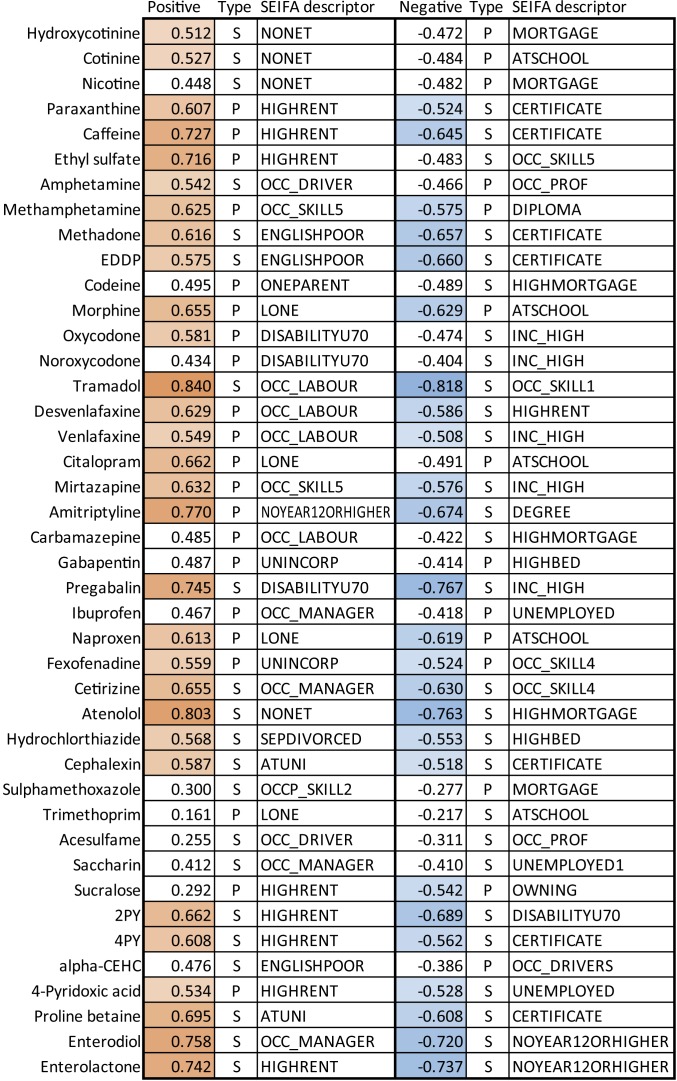
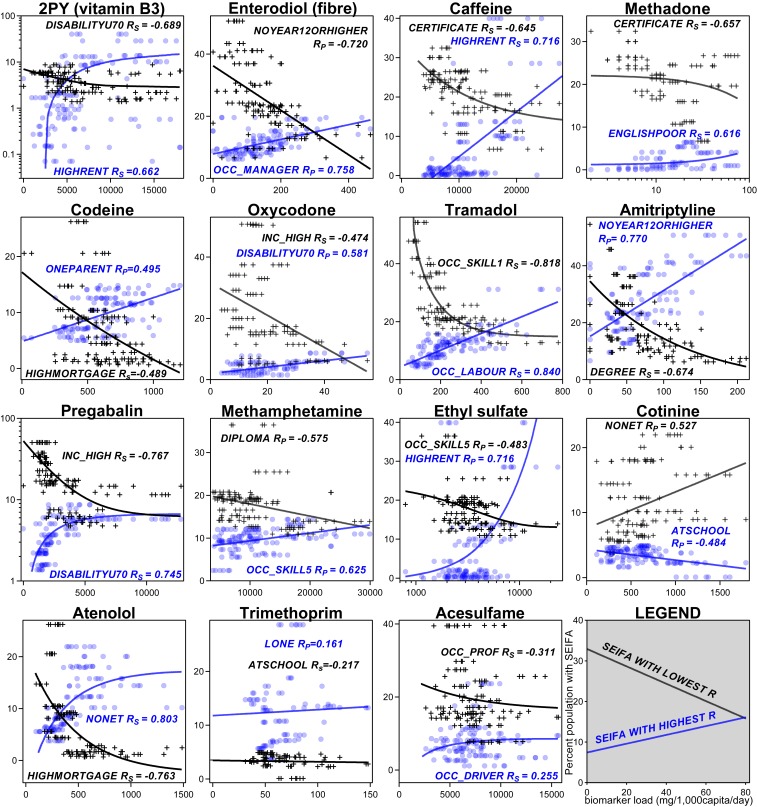
We found significant linear correlations between all 4 vitamin biomarkers, particularly between the vitamin B metabolites 2PY, 4PY, and 4-pyridoxic acid (Rs = 0.555 to 0.895; SI Appendix, Fig. S3). This suggests that their relative intake is largely homogeneous within populations. The vitamin markers had insignificant correlation with age, but 2PY (and to a lesser extent 4PY and 4-pyridoxic acid) correlated significantly with IRSAD (RS = 0.611; Fig. 2). αCEHC did not reach significant correlations with any SEIFA descriptors (see Fig. 4). In contrast, all B vitamin biomarkers were most correlated with HIGHRENT (R = 0.534 to 0.662, private dwellings paying >$470 per wk in rent) and had negative correlation with descriptors of lower SES such as DISABILITYU70 (people under 70 y of age with a disability) or UNEMPLOYED (people in labor force and unemployed; Figs. 3 and 4). This agrees with the literature, as socioeconomically disadvantaged groups are less likely to meet nutritional guidelines for micro- and macronutrients (34, 35) than advantaged groups (36). Insignificant correlations between αCEHC and sociodemographic measures may be due to the ubiquity of vitamin E in a range of easily accessible foods such as oils (37, 38). Interestingly, some vitamin biomarkers correlated significantly with ENGLISHPOOR (RS = 0.427 to 0.608; Fig. 3 and SI Appendix, Fig. S6). This agrees with the trend of higher fruit and vegetable intake among Australian residents born outside of English-speaking countries (39).
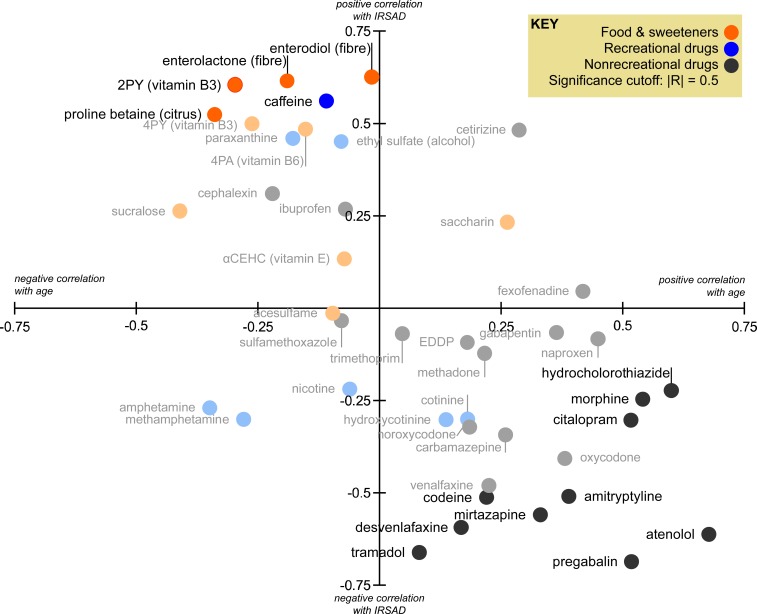
Fig. 2.
Correlations (R, Pearson or Spearman) of biomarker with catchment median age and IRSAD. Each biomarker is plotted using the highest |R| value. Biomarkers without significant correlations are faded. Amphetamine and methamphetamine are illicit drugs. R values are provided numerically in SI Appendix, Figs. S3 and S4. 4PA, 4-pyridoxic acid.
Fig. 4.
Linear or first-order correlation coefficients (R) of normalized biomarker loads with SEIFA descriptors, whose definitions are supplied in Table 1. Correlation type is designated P (Pearson) or S (Spearman). The significance cutoff was |R| = 0.5.
Fig. 3.
Correlations of wastewater biomarker loads with catchment-specific SEIFA descriptors, whose definitions are provided in Table 1. For each biomarker, SEIFA descriptors with the greatest positive (blue) and negative (black) R values are plotted. Linear or first-order regressions are shown for illustration purposes. SEIFA descriptor definitions are provided in Table 1.
Fiber and Citrus.
We measured the lignan consumption biomarkers enterodiol and enterolactone and the citrus consumption biomarker proline betaine. Small and medium cohort studies have shown dose-dependent relationships between citrus consumption and urinary proline betaine excretion (40–42). Enterodiol and enterolactone form in the gut from the metabolism of the lignans matairesinol and secoisolariciresinol, which is abundant in whole grains, legumes, seeds, and fruits (43, 44). Both are urinary biomarkers of dietary fiber and polyphenol consumption from fruit and grains (45, 46) and are inversely associated with body mass index (47). We considered proline betaine, enterodiol, and enterolactone to be indicators of a healthy diet.
In our dataset, no food biomarker correlated with age, but all correlated significantly with IRSAD (Fig. 2). Correlation between the sister biomarkers enterodiol and enterolactone was strong (RS = 0.886; SI Appendix, Fig. S4). Of all biomarkers in our study, enterodiol had the highest correlations with IRSAD (RP = 0.626, RS = 0.612; Fig. 2). Its greatest SEIFA correlates were OCC_MANAGER (people employed as managers, RP = 0.758) and NOYEAR12ORHIGHER (age >14 y and highest educational attainment year/grade 11 or lower, RS = −0.720; Fig. 3). Likewise, proline betaine was correlated with SEIFA descriptors related to education, specifically ATUNI (age >14 y and attending university or other tertiary institution, RS = 0.695) and CERTIFICATE (RS = −0.608; Fig. 4). Socioeconomically disadvantaged groups (including those in Australia) are less likely to purchase or consume grains, fruit, vegetables, and other foods high in fiber than socioeconomically advantaged groups (37–39, 48). Our results agree with these and suggest education and occupation as important factors in diet disparity.
Caffeine.
Dietary caffeine intake derives primarily from coffee and tea in adult populations and soft drinks among adolescents (49). As the caffeine content of coffee (especially espresso) is generally severalfold greater than that for tea or soft drinks (49), we consider coffee consumption to be the major source of caffeine and its metabolite paraxanthine in wastewater. As expected, caffeine and paraxanthine showed strong cross-correlations, and their correlations with other biomarkers were very similar (SI Appendix, Figs. S3 and S4). Both caffeine markers correlated significantly with the markers of vitamin, citrus, and fiber consumption (RS = 0.645 to 0.960; SI Appendix, Fig. S3). This may suggest a population-level association between consumption of caffeine and a diet rich in vitamins, citrus, and fiber, perhaps reflecting different food choices among populations with higher caffeine intake. Caffeine loads had significant linear correlation with IRSAD (Fig. 2). Additionally, caffeine and paraxanthine loads increased with HIGHRENT (RP = 0.727, RP = 0.606) and decreased with CERTIFICATE (age >14 y and highest level of education is an intermediate to advanced level of vocational training, Rs = −0.659, Rs = −0.532; Figs. 3 and 4). This suggests that caffeine consumption is associated with aspects of financial capability and educational attainment.
Our results agree with an Australian dietary survey conducted in 2011 to 2012 (n = 6,232). Habitual consumers of espresso or ground coffee lived in households with higher SES and consumed more dietary caffeine than those who consumed mix coffee or instant coffee, or those not consuming coffee (50). Espresso and ground coffee consumers were also more likely to have a bachelor’s degree or higher than other groups (50), which is analogous to our findings with the SEIFA descriptors. Our findings also support cohort studies which find higher caffeine consumption among higher SES adolescents (51) and adults (52). Although this trend may be reversed for specific demographics at risk for caffeine abuse (53), our results suggest the prevailing population-scale trend is a mild correlation between caffeine consumption and IRSAD. We suggest that increased caffeine consumption in socioeconomically advantaged groups may reflect 1) greater financial freedom to indulge in caffeinated beverages (i.e., coffee) and/or 2) cultural institutionalization of regular coffee drinking among advantaged and/or educated populations.
Opioids.
We analyzed the opioids methadone and its metabolite 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), codeine, morphine, oxycodone and its metabolite noroxycodone, and tramadol. Codeine was the only opioid available over the counter (OTC) during the time of sampling. Loads of codeine, oxycodone, and noroxycodone were significantly correlated with each other (RS = 0.516 to 0.724; SI Appendix, Fig. S4). This reflects catchment-level polydrug use and the coexcretion of oxycodone and noroxycodone following oxycodone consumption (54, 55). Similarly, methadone and EDDP correlated (RS =0.948) as expected (SI Appendix, Fig. S3). Morphine was the only opioid significantly correlating with age (RS = 0.543; Fig. 2), which matches its primary use in chronic pain which increases with age (56, 57). It should be noted that morphine residues can also be present from the metabolism of other opioids such as codeine and heroin (58). Significant correlations with IRSAD were only seen for tramadol (RS = −0.701) and codeine (RS = 0.539; Fig. 2), suggesting that a broad SES indicator does not explain methadone, morphine, and oxycodone consumption at a population scale. However, strength of correlations was higher with specific socioeconomic descriptors. SEIFA correlates of all opioids studied showed moderate to strong correlations with descriptors of socioeconomic disadvantage, and strong inverse correlations with SEIFA measures of socioeconomic advantage (Fig. 4). As illustrated in Fig. 3, methadone loads were slightly lower in catchments with fewer ENGLISHPOOR (individuals with poor English-speaking ability) and more residents with limited educational attainment. Codeine loads were lower in catchments with fewer HIGHMORTGAGE (private dwellings paying more than $2,800 per mo in mortgage) and were generally higher in catchments with more ONEPARENT (one-parent families with dependent offspring only). Oxycodone loads increased with the proportion of DISABILITYU70 and decreased with increasing household income. Tramadol loads were lowest in populations with greater percentage of skilled workers or a low percentage of laborers. Overall, these results are in line with the demographic correlates of opioid use. Opioid users are more likely to be taking other opioids (54) and have other health and lifestyle complications (59). In Australia, laborers and groups with limited education are more likely to use opioids (60). Likewise, in other countries opioid use is associated with limited educational achievement, lower household income, and other substance abuse behavior (61, 62), and these match our findings regarding the SEIFA variables. However, methadone, codeine, (nor)oxycodone, and tramadol use were not associated with catchment age, which can be considered a proxy for algesia.
Antidepressants.
We measured the antidepressants citalopram, desvenlafaxine, its parent compound venlafaxine, mirtazapine, and amitriptyline, all prescription pharmaceuticals with little difference in price during the time of sampling. We considered antidepressants as a proxy for psychological distress. All antidepressants tested had no significant association with age (SI Appendix, Figs. S3 and S4). All antidepressants tested except citalopram had significant inverse correlations with IRSAD (RS = −0.529 to −0.701; Fig. 2). Interestingly, these antidepressants seemed to diverge in terms of SEIFA correlates. Des/venlafaxine had strongest positive correlations with OCC_LABOR (people employed as laborers, RP = 0.629 and 0.549, respectively; Fig. 4) and significant correlations with similar educational and occupation descriptors (SI Appendix, Figs. S6 and S7). Amitriptyline was most influenced by educational descriptors; NOYEAR12ORHIGHER (RP =0.770) and DEGREE (age >14 y and highest educational attainment a bachelor degree or higher, RS = −0.674; Fig. 2). In contrast, citalopram had stronger correlations with social descriptors such as LONE (private dwellings with one resident), SEPDIVORCED (age >14 y and separated or divorced), and NONET (occupied private dwellings with no internet) than with education and occupation descriptors (Fig. 4 and SI Appendix, Figs. S6 and S7), hinting at a difference in demographics between des/venlafaxine, amitriptyline, and citalopram consumers. In Australia between 2003 and 2005 there were significant associations between antidepressant use and lower SES among those aged ≥30 y but a statistically insignificant trend in those aged 20 to 29 y and a reverse association in those under 19 y (63). The lack of a consistent trend encompassing all age groups may explain why correlations were not significant when correlated with IRSAD but significant with SEIFA descriptors.
We found significant correlations between antidepressants and methadone, codeine, morphine, tramadol, and pregabalin (SI Appendix, Figs. S3 and S4). This may reflect individual and/or population-scale polydrug use; patients prescribed opioids are also often prescribed antidepressants (64), and Australian pregabalin users often coingest opioids (65).
Anticonvulsants.
We measured the anticonvulsants carbamazepine, gabapentin, and pregabalin. Carbamazepine and gabapentin had insignificant correlation with increasing age, and carbamazepine also had insignificant correlations with decreasing IRSAD (SI Appendix, Figs. S3 and S4). Pregabalin was significantly correlated with increasing age (RP = 0.510) and decreasing IRSAD (RS = −0.731; Fig. 2). Pregabalin correlated significantly with opioids and antidepressants, and this was true to a lesser extent for carbamazepine and gabapentin (SI Appendix, Figs. S3 and S4). These findings are expected as anticonvulsants are also used to treat neuropathic pain (66). The different anticonvulsants seem to be associated with age, IRSAD, and other biomarkers of distress to differing extents (SI Appendix, Figs. S3 and S4), suggesting consumption of each is driven by different sociodemographic drivers. Fig. 3 shows pregabalin loads were lowest in catchments with greater proportion of the population with INC_HIGH (household income in highest 2 deciles, RS = −0.767) and in catchments with fewer DISABILITYU70 (RS = 0.745). The second most positive and negative SEIFA correlates of pregabalin were INC_LOW (household income in lowest 2 deciles, RS = 0.688) and HIGHMORTGAGE (RS = −0.748), highlighting income as an important factor for pregabalin use (SI Appendix, Figs. S6 and S7).
Pregabalin and gabapentin abuse is of increasing public health concern (67); pregabalin use in particular has also been linked to increased suicide risk in Australia. The links between lower SES and higher pregabalin use, as well as its correlation with opioids, antidepressants, and atenolol are in agreement with trends observed in Greece during a worsening social and financial crisis (16). Therefore, opioids, antidepressants, anticonvulsants, and atenolol may be considered proxies of socioeconomic distress.
Illicit Drugs.
Methamphetamine is an illicit drug, and around 4% of a dose of methamphetamine is excreted as amphetamine (68). This seems to be reflected in the significant correlations between the 2 biomarkers (SI Appendix, Figs. S3 and S4). Amphetamine can be sourced from both illicit and legal sources, although licit usage is negligible at a population scale (SI Appendix, Supplementary Information Text 1). Amphetamine and methamphetamine had insignificant correlations with IRSAD and age (Fig. 2) but both had moderate inverse correlations with SEIFA descriptors of socioeconomic disadvantage (Fig. 4). For example, methamphetamine consumption was relatively lower in catchments with fewer OCC_SKILL5 (low-skilled workers, RP = 0.625; Fig. 3). Conversely, catchments with more than 15% of the population with DIPLOMA (highest educational attainment is a diploma) had relatively low methamphetamine consumption (Fig. 3). The influence of SES on methamphetamine use is multifaceted and difficult to segregate by broad socioeconomic indices such as IRSAD and age. However, the SEIFA descriptors best correlating with their use are similar to demographic characteristics of known meth/amphetamine users (69, 70).
Alcohol.
Ethyl sulfate, a biomarker of ethanol (alcohol) consumption (71), was not correlated with age (RP = −0.081) and had insignificant correlation with IRSAD (RP = 0.449; Fig. 2). This result agrees with an Australian dietary recall study (n = 9,345) conducted in 2011 to 2012 which found insignificant difference between alcohol intake among SES quintiles and most age groups (39). However, ethyl sulfate was significantly correlated with HIGHRENT (RP = 0.716; Figs. 2 and 4) and similar indicators of socioeconomic advantage such as INC_HIGH (RP = 0.601) and OCC_MANAGER (RP = 0.537; SI Appendix, Figs. S6), indicating higher alcohol consumption in these sociodemographically advantaged groups.
Tobacco.
The tobacco consumption markers hydroxycotinine, cotinine, and nicotine all strongly correlated with each other (SI Appendix, Figs. S3 and S4). This is expected, as hydroxycotinine and cotinine are metabolites of nicotine and their excretion is relatively consistent between individuals in urine (72). No tobacco biomarkers significantly associated with catchment age or IRSAD (Fig. 2). However, with the exclusion of an outlier catchment, nicotine had significant negative correlations with IRSAD (RP = −0.504; SI Appendix, Supplementary Information Text 2). This suggests that correlation between IRSAD and tobacco consumption is not robust at a population scale. The Australian National Drug Strategy Household Survey of 2016 found smoking prevalence in the lowest and highest IRSAD quintiles to be 20.0% and 9.3%, respectively (73). Such a trend may have been difficult to detect in free-living populations as they are not strictly segregated by SES. Figs. 3 and 4 show hydroxycotinine and cotinine correlated significantly with NONET (RS = 0.512 to 0.527). Inverse correlations with MORTGAGE (dwellings occupied with a mortgage) or ATSCHOOL (individuals aged 15 y or older attending secondary school) were insignificant (RS = −0.472 to −0.484). This suggests that tobacco use may be higher among the socially isolated, and lower among the middle to upper-middle class. These findings agree with studies in Australian and other populations that found inverse correlations between nicotine use and various proxies of SES (21, 74).
Nonsteroidal Antiinflammatory Drugs.
We studied the OTC NSAIDs ibuprofen and naproxen, which differ in their duration of efficacy. The relatively short-acting ibuprofen is used to treat acute pain, whereas naproxen is longer-acting and better suited to treating chronic pain (75). Insignificant correlation (RP = 0.425) between ibuprofen and naproxen (SI Appendix, Figs. S3) suggests limited relationship between the use of these NSAIDs. Ibuprofen loads were not correlated with age or IRSAD (Fig. 2), and correlations with SEIFA descriptors were also insignificant (Fig. 4), suggesting relatively uniform use across the sociodemographic spectrum. In contrast, naproxen approached significant correlation with age (RP = 0.425) but not IRSAD (Fig. 2 and SI Appendix, Figs. S3) and was inversely correlated with ATSCHOOL (RP = −0.619, people aged 15 y and over attending secondary school; Fig. 4), suggesting lower use in populations with younger people. Overall, our results suggest that naproxen use is slightly lower in younger catchments, which agrees with its application for chronic pain (75).
Antihistamines.
Fexofenadine and cetirizine are both OTC antihistamines used to treat allergy symptoms and can be considered biomarkers of population histamine burden in a WBE context (76). Significant correlations between the antihistamines and hydroxycotinine and cotinine (SI Appendix, Figs. S3 and S4) may reflect the greater prevalence of asthma among individuals exposed to tobacco smoke (77, 78). Neither antihistamine was associated with age or IRSAD in our dataset (|R| = 0.05 to 0.384; Fig. 2). This is expected as general socioeconomic indices are not a consistent correlate of allergic disease incidence despite higher allergic disease incidence in urbanized populations (77, 79, 80). Rather, environmental factors such as pollen, dust, and dampness may be more important drivers of allergic disease (77, 81).
Antihypertensives.
Atenolol was significantly correlated with increasing age (RP = 0.660) and decreasing IRSAD (RS = −0.657; Fig. 2). This is anticipated since atenolol is a commonly prescribed (82) beta blocker for hypertension (83). Atenolol loads decreased with HIGHMORTGAGE (RS = −0.763) and increased with NONET (RS = 0.803; Fig. 3). This confirms the expectation of a higher prevalence of hypertension in older and socioeconomically disadvantaged individuals (84, 85). Atenolol also had significant correlations with several opioids and antidepressants (SI Appendix, Figs. S3 and S4). This is consistent with greater use of analgesics among older individuals and supports multiple cohort studies which show depression increases the risk of hypertension (86). Hydrochlorothiazide is an OTC diuretic often used as a first-line treatment for hypertension. Like atenolol, hydrochlorothiazide correlated with age, but it contrasts with atenolol in its lack of significant correlation with IRSAD (Fig. 2) and lower correlations with SEIFA descriptors (Fig. 4). This may reflect its use as a first-line of treatment and therefore a more uniform use throughout different sociodemographic groups.
Antibiotics.
We measured markers of the antibiotics cephalexin (a cephalosporin and broad-spectrum antibiotic), sulfamethoxazole (a sulfonamide), and trimethoprim. Significant correlation between sulfamethoxazole and trimethoprim (SI Appendix, Figs. S3 and S4) reflects the fact that the 2 drugs are commonly prescribed in combination for bacterial infections. No antibiotics had significant correaltion with age or IRSAD (|R| = 0.006 to 0.280; Fig. 2). In particular, sulfamethoxazole and trimethoprim had insignificant correlation with SEIFA variables (|R| =0.161 to 0.300; Figs. 3 and 4). Our results suggest that sociodemographic factors have no major bearing on antibiotic use (particularly sulfamethoxazole and trimethoprim) at a population scale. The literature on antibiotic use and sociodemographics is mixed; European studies have shown SES may drive usage (87, 88), including use of cephalosporins, sulfonamides, and trimethoprim (89). However, other studies in Europe and the United States highlight physician density and remuneration as major predictors of antibiotic use (88, 90).
Sweeteners.
Artificial sweeteners are found in beverages, condiments, breads, cereals, dairy products, and pharmaceuticals (91). We measured levels of acesulfame, saccharin, and sucralose. The artificial sweeteners had insignificant correlations with age or IRSAD (Fig. 2), with the strongest correlation being between sucralose and age (RS = −0.445). Acesulfame in particular had negligible correlations with age (RP = −0.164) and IRSAD (RS = −0.065). Similarly, correlations between acesulfame and SEIFA descriptors were insignificant (Figs. 3 and 4). Of the artificial sweeteners, only sucralose reached significant correlation with a SEIFA descriptor, OWNING (dwellings owned without a mortgage, RP = 0.538; Fig. 4). Our results suggest mildly lower saccharin consumption among populations which are older but not socioeconomically disadvantaged. Results regarding acesulfame support an earlier WBE study that found per-capita loads to have a strong linear relationship (R2 = 0.995) with catchment population size of 10 WWTPs spread out over 4 Australian states and territories (92).
Assumptions and Limitations.
This study has certain methodological limitations. As the study deals with correlations between sociodemographic outcomes and biomarkers, we assume that the presence of a biomarker of consumption in the wastewater solely reflects consumption by individuals. This is reasonable for biomarkers such as antidepressants. It may be less accurate for illicit drugs, which potentially may be discarded via the wastewater stream, and for proline betaine, an osmolyte in citrus flesh, which may enter the sewer system via food scraps or industrial processing of citrus. However, the association of proline betaine with IRSAD (Fig. 2) and ATUNI (Fig. 4) implies higher loads from metropolitan catchments less likely to have wastewater input from food processing industries. This suggests that industrial processing were not a major issue in our study.
In-sewer transformation can affect some biomarkers. This means that the load that enters the sewer system may differ from the load sampled (93). In planning this study, we avoided measuring biomarkers known to transform rapidly in sewer systems such as paracetamol (94). The extent of in-sewer transformation is catchment-specific and is not known for all chemicals tested in this study, in some cases due to the novel nature of their application.
The loads of a biomarker in wastewater do not give insight into the prevalence of its excretion in the population sampled. For example, if different catchments have the same load of a biomarker, we cannot determine whether these communities have the same number of people exposed at the same level or a fewer number of people exposed at a higher level. This is a limitation of the method but it is an advantage from an ethical viewpoint because it ensures the anonymity of individuals in the catchment area. Hall et al. (95) provide suggestions on how WBE studies should be reported to avoid any adverse impacts on residents of wastewater catchment areas.
Our wastewater samples came from 21.1% of the Australian population. Our study was limited to catchments from where samples were available and so is not a randomly selected subset of the Australian population. Nevertheless, the selected locations ensured that the proportion of the population in urban catchments as compared to rural catchments in our dataset approximated to the proportions in the overall Australian population.
The census-derived data used to calculate median age, IRSAD, and SEIFA for the catchment areas were made available in georeferenced mesh blocks. When the boundary of a WWTP intersected mesh blocks, the proportion of area inside the WWTP catchment was used to scale the mesh-block calculation. This calculation assumed a uniform distribution of population inside each mesh block, which is unlikely in most cases. Nevertheless, these uncertainties are expected to be minimal as each mesh block contains a relatively small number of people (96).
In terms of the correlation analysis, we used Pearson linear correlation (i.e., parametric) and Spearman rank-order correlation (i.e., nonparametric). The parametric approach is much more sensitive to trends in the data but assumes a normal distribution for the data. On the other hand, nonparametric methods (i.e., Spearman correlation) are less sensitive and do not assume any data distribution. We combined the 2 methods rather than transforming the data and using the parametric approach. Additionally, our approach can only capture the direct bivariate correlation between different descriptors; more sophisticated multivariate methods would be able to isolate the associations with a combination of descriptors. Finally, the correlation coefficient threshold for statistical significance was defined using the confusion matrix approach (i.e., bootstrapping). This method only provides a probability of false detection for each correlation coefficient value, which has its own limitations (97).
Conclusion
This WBE study examined the relationship between WBE biomarkers and sociodemographic descriptors. In general, vitamin, citrus, and fiber consumption was associated with IRSAD and educational descriptors, highlighting socioeconomic, educational, and occupational status as important factors in diet quality. Conversely, lower IRSAD was associated with higher loads of tramadol, desvenlafaxine, mirtazapine, pregabalin, and atenolol. This was consistent with higher pharmaceutical use in low SES groups. Increasing population age was associated with morphine, citalopram, atenolol, and hydrochlorothiazide, which may be expected from their increase use among the elderly population. Although WBE has primarily been used for measuring drug consumption, our results demonstrate that it can be used to identify sociodemographic patterns or disparities which associate with consumption of specific chemicals or food components.
Materials and Methods
Wastewater samples analyzed in this study were collected from Australian WWTPs as 7 consecutive daily samples taken around the time of the 2016 Australian census (9 August 2016) (96). Each sample was sampled flow- or time-proportionally. The WWTP catchments were carefully selected to maintain a ratio of urban and rural catchments representative of the greater Australian population (Fig. 1). Twenty-two WWTPs from 6 states and territories were sampled that incorporated 21.1% of the Australian population. Catchment populations ranged from 1,000 to 2.5 million (approximated for anonymity). Five to seven consecutive 24-h composite samples, one of which was collected on census day, were collected from the influent of each WWTP. Each flow- or time-proportional sample was adjusted to pH 2 with 2 M HCl and frozen immediately after sampling. Samples were shipped frozen to the laboratory on ice and stored at −20 °C until analysis. Each sample was filtered with regenerated cellulose filters (0.2 μm; Agilent). One-milliliter aliquots were spiked with internal standard to 10 μg/L for analysis. Detailed sampling procedures are outlined elsewhere (96).
By sampling wastewater at the time of the census, wastewater samples could be matched with sociodemographic data collected in the census. Catchment populations were determined by overlaying georeferenced WWTP catchment maps onto mesh blocks. Details of this methodology can be found elsewhere (96). In brief, the population counts were summed within each catchment area and allocated on a proportional-area basis for mesh-block units which intersected the catchment boundary. When the boundary of a WWTP intersected mesh blocks, the proportion of area inside the WWTP catchment was used to scale the mesh-block calculation. This calculation assumed uniform distribution of population inside each mesh block. Nevertheless, uncertainties arising from these artifacts are expected to be minimal as 99.9% of mesh blocks contain <500 people (96).
IRSAD is devised by the Australian Bureau of Statistics using a selection of SEIFA descriptors to summarize the economic and social conditions of people and households in the area by using descriptors that reflect relative socioeconomic advantage and disadvantage. A high IRSAD score indicates socioeconomic advantage, and a low IRSAD score indicates socioeconomic disadvantage. A full list of the SEIFA descriptors including the loadings of descriptors used to form IRSAD can be found in Table 1. The technical paper describing the construction of the IRSAD is available online (18). In our study, average IRSAD was calculated specifically for each catchment in the same way as age, but using Statistical Area level 1 (SA1) resolution (the second-smallest census unit, typical population 200 to 800 people per unit). SEIFA descriptors were calculated likewise using the SA1 resolution.
SI Appendix, Table S1 lists the Chemical Abstracts Service number and standard manufacturer of each biomarker. All standards were of analytical grade. Reagents used for the analysis were analytical-grade methanol from Merck Pty Ltd and acetic acid from Sigma; HCl was from Merck Pty Ltd. Water was purified using a MilliQ system (0.22-µm-filtered, 18.2 MΩ/cm; Millipore).
Biomarkers were quantified by isotope dilution or internal standards in 2 parallel liquid chromatography tandem mass spectrometry methods. Method A was used to measure mostly illicit and licit drugs and is documented elsewhere (98). Briefly, a Shimadzu Nexera UHPLC system housing a Phenomenex Kinetex 2.6 μm Biphenyl (100 Å, 50- × 2.1-mm) column was connected to an AB SCIEX QTRAP5500 operating in positive and negative ionization modes. Chromatographic and transition details are provided in SI Appendix, Table S2. The remaining biomarkers were analyzed using method B, where a Shimadzu Nexera UHPLC system with a Kinetex 2.6 μm Biphenyl (100 Å, 50- × 2.1-mm) column was connected to a Sciex 6500+ QTRAP mass spectrometer. For both methods, an 8-point calibration series was used to quantify chemicals using isotope dilution methods. A typical run featured blanks and a QAQC sample every 6 to 8 samples. Performance and validation details for method B are provided in SI Appendix, Tables S3–S5.
Biomarker loads in each sample were converted to milligrams per 24 h per 1,000 people using census-derived populations for each WWTP catchment. We did not perform any other data pretreatment in order to minimize the potential of false correlations caused by data pretreatment processes.
Supplementary Material
Acknowledgments
We thank the numerous wastewater treatment plant and Queensland Alliance for Environmental Health Science personnel involved in sampling wastewater from each catchment and Dr. Katherine Langford for proofreading and helpful comments. The Queensland Alliance for Environmental Health Sciences, The University of Queensland, gratefully acknowledges the financial support of the Queensland Health. This project was supported by an Australian Research Council Linkage Project (LP150100364), a Linkage Infrastructure, Equipment and Facilities grant (LE110100032), and a University of Queensland Major Equipment and Infrastructure grant.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. B.W.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910242116/-/DCSupplemental.
References
- 1.Zuccato E., Chiabrando C., Castiglioni S., Bagnati R., Fanelli R., Estimating community drug abuse by wastewater analysis. Environ. Health Perspect. 116, 1027–1032 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas K. V., et al. , Comparing illicit drug use in 19 European cities through sewage analysis. Sci. Total Environ. 432, 432–439 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Baz-Lomba J. A., et al. , Comparison of pharmaceutical, illicit drug, alcohol, nicotine and caffeine levels in wastewater with sale, seizure and consumption data for 8 European cities. BMC Public Health 16, 1035 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golovko O., Kumar V., Fedorova G., Randak T., Grabic R., Seasonal changes in antibiotics, antidepressants/psychiatric drugs, antihistamines and lipid regulators in a wastewater treatment plant. Chemosphere 111, 418–426 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Wang W., Kannan K., Mass loading and emission of benzophenone-3 (BP-3) and its derivatives in wastewater treatment plants in New York State, USA. Sci. Total Environ. 579, 1316–1322 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Kasprzyk-Hordern B., Dinsdale R. M., Guwy A. J., The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 42, 3498–3518 (2008). [DOI] [PubMed] [Google Scholar]
- 7.O’Brien J. W., et al. , Wastewater analysis of Census day samples to investigate per capita input of organophosphorus flame retardants and plasticizers into wastewater. Chemosphere 138, 328–334 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Baker D. R., Kasprzyk-Hordern B., Multi-residue determination of the sorption of illicit drugs and pharmaceuticals to wastewater suspended particulate matter using pressurised liquid extraction, solid phase extraction and liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 1218, 7901–7913 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Thomas K. V., Reid M. J., What else can the analysis of sewage for urinary biomarkers reveal about communities? Environ. Sci. Technol. 45, 7611–7612 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Venkatesan A. K., Chen J., Driver E., Gushgari A., Halden R. U., “Assessing the potential to monitor plant-based diet trends in communities using a wastewater-based epidemiology approach” in Wastewater-Based Epidemiology: Estimation of Community Consumption of Drugs and Diets, Subedi B., Ed. (American Chemical Society, 2019), vol. 1319, pp. 187–198. [Google Scholar]
- 11.Mackie R. S., et al. , Trends in nicotine consumption between 2010 and 2017 in an Australian city using the wastewater-based epidemiology approach. Environ. Int. 125, 184–190 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Burgard D. A., et al. , Using wastewater-based analysis to monitor the effects of legalized retail sales on cannabis consumption in Washington State, USA. Addiction 114, 1582–1590 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harman C., Reid M., Thomas K. V., In situ calibration of a passive sampling device for selected illicit drugs and their metabolites in wastewater, and subsequent year-long assessment of community drug usage. Environ. Sci. Technol. 45, 5676–5682 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Phung D., et al. , Can wastewater-based epidemiology be used to evaluate the health impact of temperature?–An exploratory study in an Australian population. Environ. Res. 156, 113–119 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Daughton C. G., Monitoring wastewater for assessing community health: Sewage Chemical-Information Mining (SCIM). Sci. Total Environ. 619–620, 748–764 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomaidis N. S., et al. , Reflection of socioeconomic changes in wastewater: Licit and illicit drug use patterns. Environ. Sci. Technol. 50, 10065–10072 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., et al. , Wastewater-based epidemiology in Beijing, China: Prevalence of antibiotic use in flu season and association of pharmaceuticals and personal care products with socioeconomic characteristics. Environ. Int. 125, 152–160 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Australian Bureau of Statistics , “Census of population and housing: Socio-economic indexes for areas (SEIFA), Australia, 2016” (Australian Bureau of Statistics, Commonwealth of Australia, 2018).
- 19.González-Chica D. A., Vanlint S., Hoon E., Stocks N., Epidemiology of arthritis, chronic back pain, gout, osteoporosis, spondyloarthropathies and rheumatoid arthritis among 1.5 million patients in Australian general practice: NPS MedicineWise MedicineInsight dataset. BMC Musculoskelet. Disord. 19, 20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kendal D., Williams N. S. G., Williams K. J. H., Drivers of diversity and tree cover in gardens, parks and streetscapes in an Australian city. Urban For. Urban Green. 11, 257–265 (2012). [Google Scholar]
- 21.Wood L. J., Pereira G., Middleton N., Foster S., Socioeconomic area disparities in tobacco retail outlet density: A Western Australian analysis. Med. J. Aust. 198, 489–491 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Gamble A. L., et al. , Adolescent sleep patterns and night-time technology use: Results of the Australian Broadcasting Corporation’s Big Sleep Survey. PLoS One 9, e111700 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin L. I., A concordance correlation coefficient to evaluate reproducibility. Biometrics 45, 255–268 (1989). [PubMed] [Google Scholar]
- 24.Spearman C., The proof and measurement of association between two things. Am. J. Psychol. 15, 72–101 (1904). [PubMed] [Google Scholar]
- 25.Kohavi R., “A study of cross-validation and bootstrap for accuracy estimation and model selection” in Proceedings of the 14th International Joint Conference on Artificial Intelligence (Morgan Kaufmann, San Francisco, 1995), vol. 2, pp. 1137–1145. [Google Scholar]
- 26.Tsuji T., Fukuwatari T., Sasaki S., Shibata K., Twenty-four-hour urinary water-soluble vitamin levels correlate with their intakes in free-living Japanese schoolchildren. Public Health Nutr. 14, 327–333 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Shibata K., Hirose J., Fukuwatari T., Relationship between urinary concentrations of nine water-soluble vitamins and their vitamin intakes in Japanese adult males. Nutr. Metab. Insights 7, 61–75 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodge J. K., Traber M. G., Elsner A., Brigelius-Flohé R., A rapid method for the extraction and determination of vitamin E metabolites in human urine. J. Lipid Res. 41, 148–154 (2000). [PubMed] [Google Scholar]
- 29.Lodge J. K., Ridlington J., Leonard S., Vaule H., Traber M. G., α- and γ-tocotrienols are metabolized to carboxyethyl-hydroxychroman derivatives and excreted in human urine. Lipids 36, 43–48 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Maras J. E., et al. , Intake of α-tocopherol is limited among US adults. J. Am. Diet. Assoc. 104, 567–575 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Leklem J. E., Vitamin B-6: A status report. J. Nutr. 120 (suppl. 11), 1503–1507 (1990). [DOI] [PubMed] [Google Scholar]
- 32.Schuster K., Bailey L. B., Cerda J. J., Gregory J. F. 3rd, Urinary 4-pyridoxic acid excretion in 24-hour versus random urine samples as a measurement of vitamin B6 status in humans. Am. J. Clin. Nutr. 39, 466–470 (1984). [DOI] [PubMed] [Google Scholar]
- 33.Tsuji T., Fukuwatari T., Sasaki S., Shibata K., Urinary excretion of vitamin B1, B2, B6, niacin, pantothenic acid, folate, and vitamin C correlates with dietary intakes of free-living elderly, female Japanese. Nutr. Res. 30, 171–178 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal A., Monsivais P., Drewnowski A., Nutrient intakes linked to better health outcomes are associated with higher diet costs in the US. PLoS One 7, e37533 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose D., Economic determinants and dietary consequences of food insecurity in the United States. J. Nutr. 129 (suppl. 2S), 517S–520S (1999). [DOI] [PubMed] [Google Scholar]
- 36.Mullie P., Clarys P., Hulens M., Vansant G., Dietary patterns and socioeconomic position. Eur. J. Clin. Nutr. 64, 231–238 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Giskes K., Avendaňo M., Brug J., Kunst A. E., A systematic review of studies on socioeconomic inequalities in dietary intakes associated with weight gain and overweight/obesity conducted among European adults. Obes. Rev. 11, 413–429 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Turrell G., Hewitt B., Patterson C., Oldenburg B., Gould T., Socioeconomic differences in food purchasing behaviour and suggested implications for diet-related health promotion. J. Hum. Nutr. Diet. 15, 355–364 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Grech A., et al. , Socio-demographic determinants of diet quality in Australian adults using the validated healthy eating index for Australian adults (HEIFA-2013). Healthcare (Basel) 5, E7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lang R., et al. , High-throughput quantitation of proline betaine in foods and suitability as a valid biomarker for citrus consumption. J. Agric. Food Chem. 65, 1613–1619 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Lloyd A. J., Beckmann M., Favé G., Mathers J. C., Draper J., Proline betaine and its biotransformation products in fasting urine samples are potential biomarkers of habitual citrus fruit consumption. Br. J. Nutr. 106, 812–824 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Gibbons H., et al. , Demonstration of the utility of biomarkers for dietary intake assessment; proline betaine as an example. Mol. Nutr. Food Res. 61, 1700037 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Mazur W. M., Uehara M., Wähälä K., Adlercreutz H., Phyto-oestrogen content of berries, and plasma concentrations and urinary excretion of enterolactone after a single strawberry-meal in human subjects. Br. J. Nutr. 83, 381–387 (2000). [PubMed] [Google Scholar]
- 44.Lampe J. W., Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J. Nutr. 133 (suppl. 3), 956S–964S (2003). [DOI] [PubMed] [Google Scholar]
- 45.Lampe J. W., et al. , Urinary isoflavonoid and lignan excretion on a Western diet: Relation to soy, vegetable, and fruit intake. Cancer Epidemiol. Biomarkers Prev. 8, 699–707 (1999). [PubMed] [Google Scholar]
- 46.Mennen L. I., et al. , Urinary excretion of 13 dietary flavonoids and phenolic acids in free-living healthy subjects–Variability and possible use as biomarkers of polyphenol intake. Eur. J. Clin. Nutr. 62, 519–525 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Wu X., et al. , Correlations of urinary phytoestrogen excretion with lifestyle factors and dietary intakes among middle-aged and elderly Chinese women. Int. J. Mol. Epidemiol. Genet. 3, 18–29 (2012). [PMC free article] [PubMed] [Google Scholar]
- 48.James W. P. T., Nelson M., Ralph A., Leather S., Socioeconomic determinants of health. The contribution of nutrition to inequalities in health. BMJ 314, 1545–1549 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heckman M. A., Weil J., Gonzalez de Mejia E., Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 75, R77–R87 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Wong T. H. T., Sui Z., Rangan A., Louie J. C. Y., Discrepancy in socioeconomic status does not fully explain the variation in diet quality between consumers of different coffee types. Eur. J. Nutr. 57, 2123–2131 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Walker L. R., Abraham A. A., Tercyak K. P., Adolescent caffeine use, ADHD, and cigarette smoking. Child. Health Care 39, 73–90 (2010). [Google Scholar]
- 52.Santos I. S., Victora C. G., Huttly S., Carvalhal J. B., Caffeine intake and low birth weight: A population-based case-control study. Am. J. Epidemiol. 147, 620–627 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Grewen K., et al. , Stable pessimistic attributions interact with socioeconomic status to influence blood pressure and vulnerability to hypertension. J. Womens Health Gend. Based Med. 9, 905–915 (2000). [DOI] [PubMed] [Google Scholar]
- 54.White A. G., et al. , Economic impact of opioid abuse, dependence, and misuse. Am. J. Pharm. Benefits 3, e59–e70 (2011). [Google Scholar]
- 55.Lalovic B., et al. , Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: Role of circulating active metabolites. Clin. Pharmacol. Ther. 79, 461–479 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Parsells Kelly J., et al. , Prevalence and characteristics of opioid use in the US adult population. Pain 138, 507–513 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Roxburgh A., Bruno R., Larance B., Burns L., Prescription of opioid analgesics and related harms in Australia. Med. J. Aust. 195, 280–284 (2011). [DOI] [PubMed] [Google Scholar]
- 58.DePriest A., Puet B., Holt A., Roberts A., Cone E. J. F. S. R., Metabolism and disposition of prescription opioids: A review. Forensic Sci. Rev. 27, 115–145 (2015). [PubMed] [Google Scholar]
- 59.White A. G., et al. , Direct costs of opioid abuse in an insured population in the United States. J. Manag. Care Pharm. 11, 469–479 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berecki-Gisolf J., Collie A., McClure R. J., Prescription opioids for occupational injury: Results from workers’ compensation claims records. Pain Med. 15, 1549–1557 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Rigg K. K., Monnat S. M., Urban vs. rural differences in prescription opioid misuse among adults in the United States: Informing region specific drug policies and interventions. Int. J. Drug Policy 26, 484–491 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spiller H., Lorenz D. J., Bailey E. J., Dart R. C., Epidemiological trends in abuse and misuse of prescription opioids. J. Addict. Dis. 28, 130–136 (2009). [DOI] [PubMed] [Google Scholar]
- 63.Page A. N., et al. , Sociodemographic correlates of antidepressant utilisation in Australia. Med. J. Aust. 190, 479–483 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Brands B., Blake J., Sproule B., Gourlay D., Busto U., Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug Alcohol Depend. 73, 199–207 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Cairns R., Schaffer A. L., Ryan N., Pearson S. A., Buckley N. A., Rising pregabalin use and misuse in Australia: Trends in utilization and intentional poisonings. Addiction 114, 1026–1034 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Backonja M.-M., Use of anticonvulsants for treatment of neuropathic pain. Neurology 59 (suppl. 2), S14–S17 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Schifano F., Misuse and abuse of pregabalin and gabapentin: Cause for concern? CNS Drugs 28, 491–496 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Khan U., Nicell J. A., Sewer epidemiology mass balances for assessing the illicit use of methamphetamine, amphetamine and tetrahydrocannabinol. Sci. Total Environ. 421–422, 144–162 (2012). [DOI] [PubMed] [Google Scholar]
- 69.McKetin R., et al. , Impaired physical health among methamphetamine users in comparison with the general population: The role of methamphetamine dependence and opioid use. Drug Alcohol Rev. 27, 482–489 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Hall W., Darke S., Ross M., Wodak A., Patterns of drug use and risk-taking among injecting amphetamine and opioid drug users in Sydney, Australia. Addiction 88, 509–516 (1993). [DOI] [PubMed] [Google Scholar]
- 71.Mastroianni N., Lopez de Alda M., Barcelo D., Analysis of ethyl sulfate in raw wastewater for estimation of alcohol consumption and its correlation with drugs of abuse in the city of Barcelona. J. Chromatogr. A 1360, 93–99 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Jacob P. 3rd, Yu L., Shulgin A. T., Benowitz N. L., Minor tobacco alkaloids as biomarkers for tobacco use: Comparison of users of cigarettes, smokeless tobacco, cigars, and pipes. Am. J. Public Health 89, 731–736 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.AIHW , “National drug Strategy household survey 2016: Detailed findings” in Drug Statistics (Australian Institute of Health and Welfare, Canberra, 2017), p. 168. [Google Scholar]
- 74.Hiscock R., Bauld L., Amos A., Fidler J. A., Munafò M., Socioeconomic status and smoking: A review. Ann. NY Acad. Sci. 1248, 107–123 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Schiff M., Minic M., Comparison of the analgesic efficacy and safety of nonprescription doses of naproxen sodium and Ibuprofen in the treatment of osteoarthritis of the knee. J. Rheumatol. 31, 1373–1383 (2004). [PubMed] [Google Scholar]
- 76.Choi P. M., et al. , Population histamine burden assessed using wastewater-based epidemiology: The association of 1,4-methylimidazole acetic acid and fexofenadine. Environ. Int. 120, 172–180 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Sun Y., Sundell J., Life style and home environment are associated with racial disparities of asthma and allergy in Northeast Texas children. Sci. Total Environ. 409, 4229–4234 (2011). [DOI] [PubMed] [Google Scholar]
- 78.Linneberg A., Nielsen N. H., Menné T., Madsen F., Jørgensen T., Smoking might be a risk factor for contact allergy. J. Allergy Clin. Immunol. 111, 980–984 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Linneberg A., The increase in allergy and extended challenges. Allergy 66 (suppl. 95), 1–3 (2011). [DOI] [PubMed] [Google Scholar]
- 80.Majkowska–Wojciechowska B., et al. , Prevalence of allergy, patterns of allergic sensitization and allergy risk factors in rural and urban children. Allergy 62, 1044–1050 (2007). [DOI] [PubMed] [Google Scholar]
- 81.Brown C. W., Hawkins L., Allergy prevalence and causal factors in the domestic environment: Results of a random population survey in the United Kingdom. Ann. Allergy Asthma Immunol. 83, 240–244 (1999). [DOI] [PubMed] [Google Scholar]
- 82.Pharmaceutical Benefits Scheme , “Expenditure and prescriptions twelve months to 30 June 2017” (Department of Health, Canberra, Australia, 2017).
- 83.DiNicolantonio J. J., et al. , β-Blockers in hypertension, diabetes, heart failure and acute myocardial infarction: A review of the literature. Open Heart 2, e000230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anonymous , Australia’s Health (Australia’s Health Series, Australian Institute of Health and Welfare, Canberra, 2018). [Google Scholar]
- 85.Brummett B. H., et al. , Systolic blood pressure, socioeconomic status, and biobehavioral risk factors in a nationally representative US young adult sample. Hypertension 58, 161–166 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meng L., Chen D., Yang Y., Zheng Y., Hui R., Depression increases the risk of hypertension incidence: A meta-analysis of prospective cohort studies. J. Hypertens 30, 842–851 (2012). [DOI] [PubMed] [Google Scholar]
- 87.Jensen J. N., Bjerrum L., Boel J., Jarløv J. O., Arpi M., Parents’ socioeconomic factors related to high antibiotic prescribing in primary health care among children aged 0-6 years in the Capital Region of Denmark. Scand. J. Prim. Health Care 34, 274–281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Masiero G., Filippini M., Ferech M., Goossens H., Socioeconomic determinants of outpatient antibiotic use in Europe. Int. J. Public Health 55, 469–478 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Covvey J. R., Johnson B. F., Elliott V., Malcolm W., Mullen A. B., An association between socioeconomic deprivation and primary care antibiotic prescribing in Scotland. J. Antimicrob. Chemother. 69, 835–841 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Klein E. Y., et al. , Influence of provider and urgent care density across different socioeconomic strata on outpatient antibiotic prescribing in the USA. J. Antimicrob. Chemother. 70, 1580–1587 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sylvetsky A. C., Rother K. I., Trends in the consumption of low-calorie sweeteners. Physiol. Behav. 164, 446–450 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Brien J. W., et al. , A model to estimate the population contributing to the wastewater using samples collected on census day. Environ. Sci. Technol. 48, 517–525 (2014). [DOI] [PubMed] [Google Scholar]
- 93.van Nuijs A. L. N., et al. , The stability of illicit drugs and metabolites in wastewater, an important issue for sewage epidemiology? J. Hazard. Mater. 239–240, 19–23 (2012). [DOI] [PubMed] [Google Scholar]
- 94.Gao J., et al. , Systematic evaluation of biomarker stability in pilot scale sewer pipes. Water Res. 151, 447–455 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Hall W., et al. , An analysis of ethical issues in using wastewater analysis to monitor illicit drug use. Addiction 107, 1767–1773 (2012). [DOI] [PubMed] [Google Scholar]
- 96.O’Brien J. W., et al. , A national wastewater monitoring program for a better understanding of public health: A case study using the Australian census. Environ. Int. 122, 400–411 (2019). [DOI] [PubMed] [Google Scholar]
- 97.Brereton R. G., Applied Chemometrics for Scientists (John Wiley & Sons, West Sussex, UK, 2007). [Google Scholar]
- 98.O’Brien J. W., et al. , “National wastewater drug monitoring program” (Report 1, The University of Queensland & University of South Australia, Australian Criminal Intelligence Commission, 2017), p. 64.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.