Significance
Endoplasmic reticulum (ER) stress plays an important role in obesity and type 2 diabetes mellitus (T2DM), with controversy in mechanisms and regulatory pathways. We found that chronic low-grade ER stress in lean mice promoted hyperglycemia due to enhanced hepatic gluconeogenesis via ubiquitin-specific peptidase14 (USP14) induction, which, in turn, increased the stability of adenosine 3′,5′-cyclic monophosphate–responsive element binding protein to enhance glucagon action and hepatic gluconeogenesis. Overexpression of USP14 in the liver significantly increased hepatic glucose output. Liver-specific knockdown of USP14 abrogated the effects of ER stress on glucose metabolism, improved hyperglycemia and glucose intolerance in obese mice. Our findings show a mechanism underlying ER stress-induced disruption of glucose homeostasis, and present USP14 as a potential therapeutic target against T2DM.
Keywords: type 2 diabetes, gluconeogenesis, hepatic glucose production, ER stress, USP14
Abstract
Endoplasmic reticulum (ER) stress plays an important role in metabolic diseases like obesity and type 2 diabetes mellitus (T2DM), although the underlying mechanisms and regulatory pathways remain to be elucidated. Here, we induced chronic low-grade ER stress in lean mice to levels similar to those in high-fat diet (HFD)–fed obese mice and found that it promoted hyperglycemia due to enhanced hepatic gluconeogenesis. Mechanistically, sustained ER stress up-regulated the deubiquitinating enzyme ubiquitin-specific peptidase 14 (USP14), which increased the stability and levels of 3′,5′-cyclic monophosphate–responsive element binding (CREB) protein (CBP) to enhance glucagon action and hepatic gluconeogenesis. Exogenous overexpression of USP14 in the liver significantly increased hepatic glucose output. Consistent with this, liver-specific knockdown of USP14 abrogated the effects of ER stress on glucose metabolism, and also improved hyperglycemia and glucose intolerance in obese mice. In conclusion, our findings show a mechanism underlying ER stress-induced disruption of glucose homeostasis, and present USP14 as a potential therapeutic target against T2DM.
The liver is the master regulator of glucose metabolism, and it maintains normal blood glucose levels in response to nutritional and hormonal cues. Increased levels of circulating glucagon during the fasting state stimulate hepatic gluconeogenesis, while the postprandial spike in insulin inhibits gluconeogenesis and activates glycogen synthesis (1). On the other hand, selective insulin resistance and hyperglucagonemia during obesity enhance hepatic gluconeogenesis, resulting in hyperglycemia and glucose intolerance, which is the basis of metabolic diseases like type 2 diabetes mellitus (T2DM) (2, 3). Therefore, a better understanding of the complex regulatory networks governing glucose metabolism in the liver can identify novel therapeutic targets.
The endoplasmic reticulum (ER) is a system of continuous flattened membranes that serve as sites for protein synthesis, folding, and maturation (4, 5). Impaired protein folding and secretion lead to the accumulation of unfolded or misfolded proteins in the lumen, a phenomenon termed ER stress, which triggers the unfolded protein response (UPR) (4, 5). In eukaryotes, at least 3 pathways of UPR signaling have been identified: the inositol-requiring enzyme 1α (IRE1α)/X box binding protein-1 (XBP-1), protein kinase R-like ER kinase (PERK), and activating transcription factor 6 (ATF6) (4, 5). Studies show a strong association between ER stress and impaired glucose homeostasis, indicating its crucial role in the onset and/or progression of T2DM (6–10). For example, multiple ER stress markers are activated in the liver of obese rodents and humans (11–14), and alleviating ER stress by 4-phenyl butyric acid or taurine-conjugated ursodeoxycholic acid normalized hyperglycemia and restored insulin sensitivity in leptin-deficient (ob/ob) mice (15). Based on these findings, several hepatic conditional knockout mouse models lacking UPR pathway genes have been developed to further elucidate the specific role of ER stress in glucose metabolism (16–21).
Although ER stress has emerged as an important player in T2DM, the mechanisms involved remain incompletely understood. In addition, inconsistent results have been reported. For example, XBP-1 is down-regulated in the hepatocytes of obese mice (22–24), and hepatic overexpression of XBP-1 decreased blood glucose levels and improved glucose intolerance and insulin sensitivity (25, 26). However, other studies have reported aberrantly high levels of XBP-1 in obese livers (27, 28). Overexpression of XBP-1 in the liver is associated with hyperglycemia, increased hepatic glucose production, and insulin resistance (27), and the hepatic conditional XBP-1 knockout mice are resistant to fructose-induced hyperglycemia and insulin resistance (17). In addition, induction of acute ER stress in mice using high-dose tunicamycin (HD-Tun) resulted in hypoglycemia and decreased hepatic gluconeogenesis (22, 29), indicating that acute and chronic ER stress may have distinct metabolic consequences. Since obesity is a steady process of weight gain, we hypothesized that it is associated with altered glucose metabolism due to sustained, as opposed to acute, ER stress.
In the current study, we induced low-grade sustained ER stress in lean mice and found that it increased hepatic gluconeogenesis, leading to hyperglycemia. Mechanistically, chronic ER stress up-regulated the deubiquitinating enzyme ubiquitin-specific peptidase 14 (USP14), which stabilized the 3′,5′-cyclic monophosphate–responsive element binding (CREB) protein (CBP) to augment glucagon action, thereby promoting gluconeogenesis.
Results
Sustained ER Stress Disrupts Glucose Homeostasis in Lean Mice.
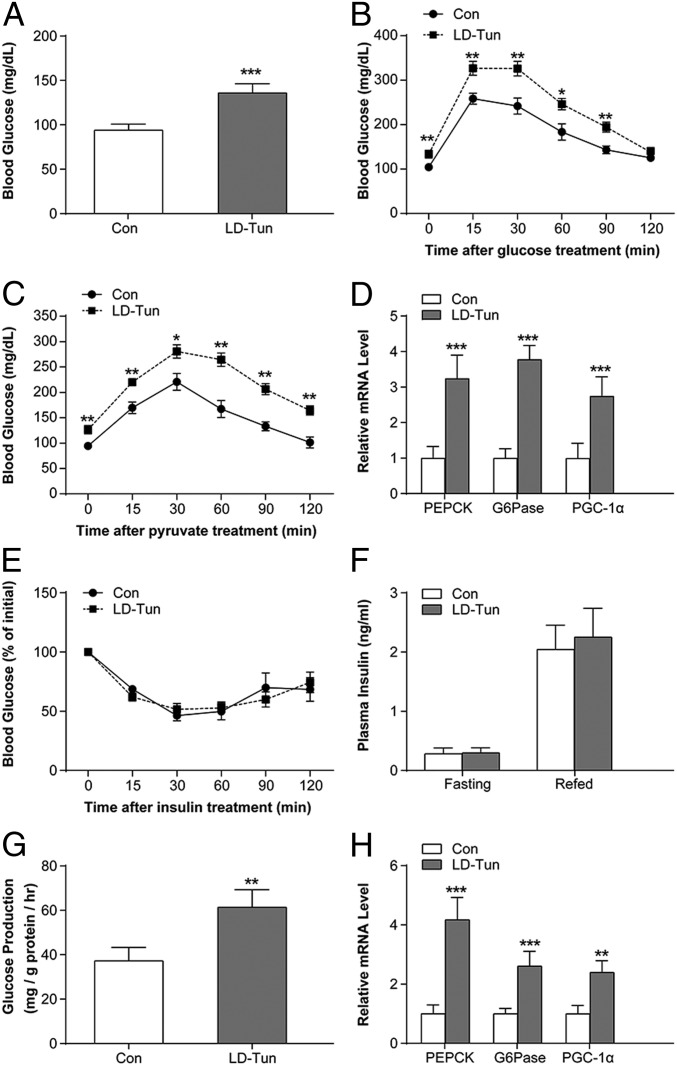
To determine the effects of sustained ER stress on glucose metabolism, lean C57BL/6 mice were given daily intraperitoneal injections of either low-dose tunicamycin (LD-Tun; 0.2 mg/kg) or saline for 14 d. LD-Tun moderately up-regulated the ER stress markers (phosphorylation status of PERK; messenger RNA [mRNA] expression of GRP78, ATF4, and EDEM) in the liver (SI Appendix, Fig. S1 A and B) to levels comparable to those seen in mice fed a high-fat diet for 12 wk (SI Appendix, Fig. S1 C and D). In contrast, acute ER stress induced by HD-Tun (1.0 mg/kg) dramatically increased the expression levels of the above markers (SI Appendix, Fig. S1 E and F). Low-grade ER stress was accompanied by increased blood glucose levels and glucose intolerance (Fig. 1 A and B), without any changes in body weight, body fat percentage, food intake, and energy expenditure (SI Appendix, Fig. S2 A–E). In addition, hepatic glucose production measured in terms of pyruvate tolerance (Fig. 1C) and expression levels of the hepatic gluconeogenic enzymes phosphoenolpyruvate carboxykinase and G6Pase (Fig. 1D) were enhanced by LD-Tun treatment. The PGC-1α gene, which coactivates gluconeogenic transcription factors like HNF4α, FOXO1, and C/EBPs (30, 31), was also up-regulated by LD-Tun (Fig. 1D). However, insulin sensitivity (Fig. 1E), insulin-stimulated AKT phosphorylation (SI Appendix, Fig. S2F), and fasting and postprandial plasma insulin levels were relatively unaffected by LD-Tun (Fig. 1F). In contrast, acute treatment with HD-Tun lowered blood glucose concentrations and suppressed the gluconeogenic genes (SI Appendix, Fig. S2 G and H), which are consistent with previous reports (22, 29).
Fig. 1.
Sustained ER stress induces hyperglycemia and hepatic gluconeogenesis. (A) Blood glucose levels in LD-Tun– or saline-treated (Con) C57BL/6 mice after 14 d. Results of glucose tolerance (B) and pyruvate tolerance (C) tests in the stressed and control mice are shown (n = 8 per group). (D) Relative mRNA expression levels of gluconeogenic genes in the liver of stressed and control mice. PEPCK, phosphoenolpyruvate carboxykinase. (E) Insulin tolerance test in mice receiving LD-Tun or saline. (F) Plasma insulin levels in the above groups. (G) Glucose production in MPHs treated with LD-Tun (0.25 μg/mL) or vehicle control (Con) for 48 h normalized to total protein levels. (H) Relative mRNA expression levels of gluconeogenic genes in the above groups. *P < 0.05, **P < 0.01, ***P < 0.001.
In agreement with the in vivo findings, LD-Tun increased cellular glucose production and up-regulated gluconeogenic genes in mouse primary hepatocytes (MPHs) (Fig. 1 G and H). Similar results were also detected in MPHs incubated with low-dose thapsigargin (LD-Tha), another agent widely used to trigger cellular ER stress (SI Appendix, Fig. S2 I and J). Taken together, our data support the notion that obesity is associated with chronic and low-grade ER stress, which increases hepatic gluconeogenesis and disrupts glucose homeostasis.
USP14 Mediates the Gluconeogenic Effects of Sustained ER Stress.
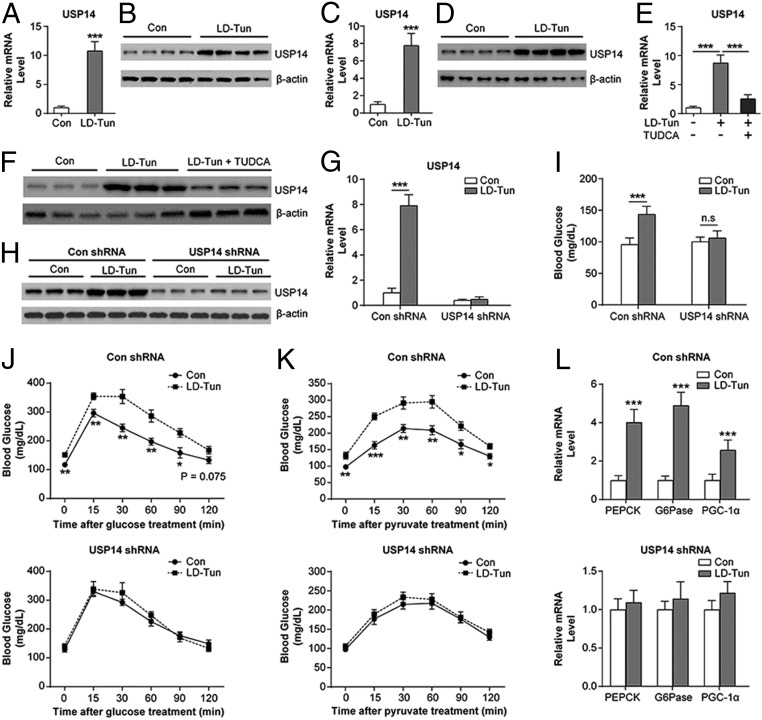
To elucidate the mechanisms underlying the effects of sustained ER stress on glucose metabolism, we compared the transcriptomes of saline- and LD-Tun–treated livers by RNA-sequencing analysis (SI Appendix, Fig. S3A), using P < 0.05 and fold change > 1.5 as the thresholds. Over 1,131 genes were altered in the liver of LD-Tun–treated mice relative to the control mice, of which 792 were up-regulated and 339 were down-regulated. The top 20 up- and down-regulated genes are listed in SI Appendix, Fig. S3 B and C, and include the gene encoding for USP14, which has been shown to regulate hepatic triglyceride metabolism by our recent study (32). In addition, USP14 has been previously identified as a binding partner of IRE1α, and thereby a potential mediator of the UPR (33, 34). Therefore, we hypothesized that USP14 plays a role in ER stress-associated gluconeogenesis. Consistent with our hypothesis, USP14 was significantly up-regulated in the liver of LD-Tun–treated mice (Fig. 2 A and B). The up-regulation of USP14 was also observed in LD-Tun– or LD-Tha–treated MPHs (Fig. 2 C and D and SI Appendix, Fig. S4 A and B). Alleviation of ER stress by the chemical chaperone tauroursodeoxycholic acid, previously shown to protect against ER stress (15, 27), largely attenuated tunicamycin-induced USP14 in MPHs (15, 27) (Fig. 2 E and F). These data demonstrate that activation of ER stress leads to USP14 induction.
Fig. 2.
USP14 is required for the gluconeogenic effects of sustained ER stress. Relative expression of USP14 mRNA (A and C) and protein (B and D) in the liver of LD-Tun–treated and control (Con) mice (A and B) and cultured MPHs (C and D) is shown. (E and F) USP14 mRNA and protein levels in MPHs treated with LD-Tun + tauroursodeoxycholic acid (TUDCA), LD-Tun, or saline. (G and H) USP14 mRNA and protein levels in the liver of stressed and control mice treated with USP14 short hairpin RNA (shRNA) or negative control (n = 6 per group). Blood glucose levels (I) and glucose (J) and pyruvate (K) tolerance in the above groups are shown. n.s., not significant. (L) Relative mRNA levels of gluconeogenic genes in the liver of mice in the above groups. PEPCK, phosphoenolpyruvate carboxykinase. *P < 0.05, **P < 0.01, ***P < 0.001.
To determine whether induction of USP14 is essential for the effects of sustained ER stress on glucose metabolism, we inhibited its function either pharmacologically or genetically. Mice treated with LD-Tun and IU1, a small-molecule inhibitor of USP14 (35, 36), showed partial improvement in glucose levels (SI Appendix, Fig. S5 A–C) as well as attenuation in gluconeogenic gene expression levels (SI Appendix, Fig. S5D). Endogenous USP14 knockdown in lean mice (Fig. 2 G and H) blunted the effects of LD-Tun on glucose homeostasis and gluconeogenesis (Fig. 2 I–L), but did not affect the induction of GRP78, ATF4, and EDEM (SI Appendix, Fig. S5E). Taken together, our data suggest that USP14 mediates the gluconeogenic effects of sustained ER stress.
Sustained ER Stress Up-Regulates USP14 through ATF4.
We next sought to determine if USP14 is a molecular target of sustained ER stress. It has been previously shown that ATF4 is a downstream effector of the ER stress pathway that triggers apoptosis (37). As a result, overexpression of ATF4 in MPHs up-regulated USP14 (SI Appendix, Fig. S6 A and B), whereas a dominant-negative mutated ATF4 (38, 39) attenuated the USP14 levels induced by LD-Tun (SI Appendix, Fig. S6 C and D). Similar results were also observed in MPHs isolated from the hepatic ATF4 knockout mice (SI Appendix, Fig. S6 E and F). To delineate the molecular basis for this regulation, we identified 2 potential ATF4 binding sites (TGCTA) at the proximal promoter region of the USP14 gene (SI Appendix, Fig. S6G), which are conserved in humans and mice (SI Appendix, Fig. S6G). A luciferase reporter assay showed that ATF4 overexpression up-regulated the transcriptional activity of the USP14 promoter when both sites were intact (SI Appendix, Fig. S6H), whereas mutation in either or both sites partially and completely blocked promoter activation by ATF4 (SI Appendix, Fig. S6H). In addition, direct binding of ATF4 to this region was observed, which was further enhanced after LD-Tun treatment in both mouse livers and MPHs (SI Appendix, Fig. S6 I and J). Taken together, these findings suggest that sustained ER stress up-regulates USP14 via ATF4.
USP14 Stimulates Hepatic Gluconeogenesis and Glucose Output.
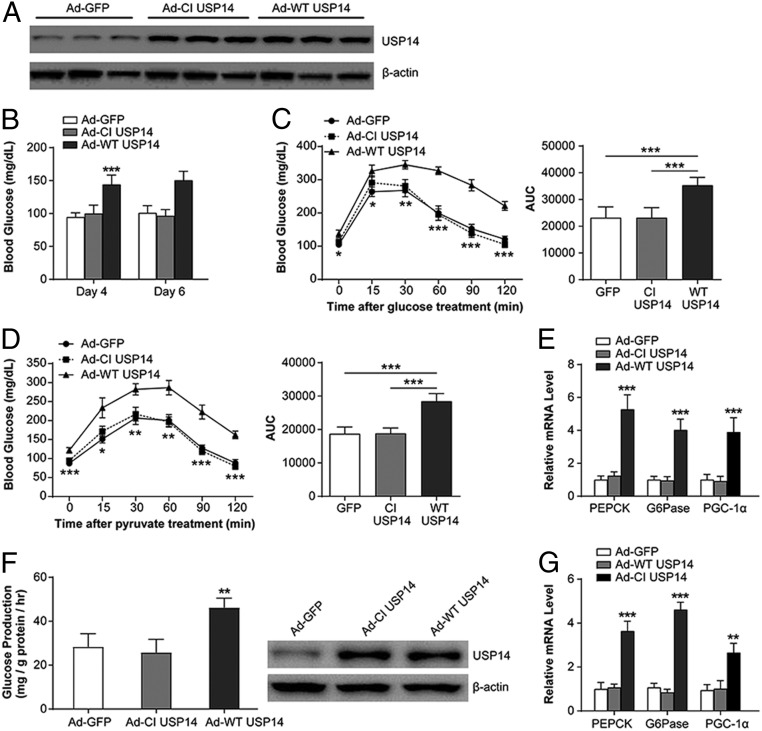
To determine whether USP14 is sufficient to alter hepatic glucose metabolism, lean mice were injected with adenoviruses expressing either the wild-type USP14 (Ad-WT USP14) or its catalytically inactive form (Ad-CI USP14) (40) (Fig. 3A). Overexpression of exogenous WT USP14 in the liver significantly increased blood glucose levels on days 4 and 6 after infection, compared with the Ad-GFP– and Ad-CI USP14–infected mice (Fig. 3B). In addition, mice infected with Ad-WT USP14 demonstrated worse glucose intolerance and enhanced hepatic glucose output, as shown by the glucose and pyruvate tolerance tests, respectively, on days 9 and 12 after infection (Fig. 3 C and D). Consistent with this, gluconeogenic genes were also induced in mice overexpressing WT USP14 (Fig. 3E). Enhanced glucose production and gluconeogenic gene expression were also observed in MPHs overexpressing Ad-WT USP14 (Fig. 3 F and G). Therefore, USP14 has a direct effect on hepatic glucose metabolism.
Fig. 3.
Overexpression of hepatic USP14 causes hyperglycemia and promotes gluconeogenesis. (A) USP14 protein levels in C57BL/6 mice infected with Ad-GFP, Ad-CI USP14, and Ad-WT USP14. (B) Blood glucose levels in the above groups on days 4 and 6 after adenoviral infection. (C and D) Glucose and pyruvate tolerance in the above groups. AUC, area under the curve. (E) Relative mRNA levels of gluconeogenic genes in the above groups. PEPCK, phosphoenolpyruvate carboxykinase. (F) Glucose levels in MPHs transfected with Ad-GFP, Ad-CI USP14, or Ad-WT USP14. (G) Relative mRNA levels of gluconeogenic genes in the above groups (n = 6 per group). *P < 0.05, **P < 0.01, ***P < 0.001.
Inhibition of Hepatic USP14 Improves Glucose Metabolism in Obese Mice.
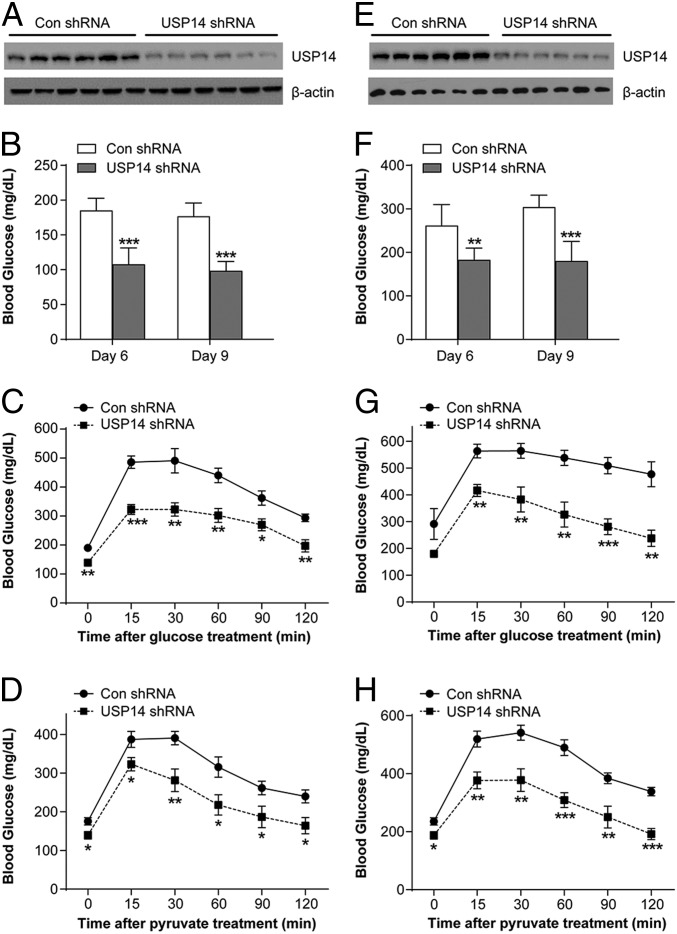
The results demonstrating that obesity is associated with low-grade ER stress (SI Appendix, Fig. S1 A–D) indicated that USP14 is a suitable target for controlling obesity-related hyperglycemia. Indeed, high-fat diet (HFD)–fed obese mice treated with IU1 for 14 d showed lower blood glucose levels and improved glucose tolerance (SI Appendix, Fig. S7 A and B), along with reduced hepatic glucose production and decreased gluconeogenic genes (SI Appendix, Fig. S7 C and D). Similar results were obtained after USP14 knockdown using its adenoviral short hairpin RNA (Fig. 4 A–D). Depletion of hepatic USP14 also improved glucose homeostasis and reduced gluconeogenesis in leptin-deficient ob/ob mice (Fig. 4 E–H). Taken together, USP14 can be successfully targeted to improve glucose homeostasis during obesity.
Fig. 4.
Hepatic USP14 knockdown improves glucose metabolism in obese mice. (A) Mice were fed a high-fat diet for 12 wk and then infected with adenoviral short hairpin RNA (shRNA) USP14 or negative control (Con). USP14 protein levels were determined by Western blots. (B) Blood glucose levels in the above groups on days 6 and 9 after infection. (C and D) Glucose and pyruvate tolerance in the above groups. (E) USP14 protein levels in ob/ob mice infected with adenoviral shRNA USP14 or Con shRNA. (F) Blood glucose levels in the above groups on days 6 and 9 after infection. (G and H) Glucose and pyruvate tolerance in the above groups (n = 6 per group). *P < 0.05, **P < 0.01, ***P < 0.001.
USP14 Increases Hepatic Gluconeogenesis by Augmenting Glucagon Action.
To determine the mechanistic basis of USP14 action, we analyzed its effects on glucagon and insulin, which maintain glucose homeostasis in the fasted and fed states, respectively. In the streptozotocin-induced diabetic mice lacking insulin activity (SI Appendix, Fig. S8A), hepatic knockdown of USP14 significantly alleviated hyperglycemia (SI Appendix, Fig. S8B) and down-regulated the gluconeogenic genes (SI Appendix, Fig. S8C). These results indicate that insulin likely does not mediate the regulation of gluconeogenesis by USP14. In contrast, glucagon tolerance tests showed that blood glucose levels were significantly higher after glucagon injection in the USP14-overexpressing mice (SI Appendix, Fig. S9A). In addition, USP14 overexpression and knockdown in the MPHs enhanced and impaired glucagon-stimulated glucose production, respectively (SI Appendix, Fig. S9 B and C), as well as the expression of gluconeogenic genes (SI Appendix, Fig. S9 D and E). Based on the results so far, we next examined if sustained ER stress also enhanced glucagon action in a USP14-dependent manner. Glucagon tolerance tests revealed a higher glucagon-stimulated increase in blood glucose levels in LD-Tun–treated mice compared with the control mice (SI Appendix, Fig. S9F). Consistent with this, LD-Tun significantly increased both basal and glucagon-stimulated glucose production in MPHs, which was attenuated by USP14 knockdown (SI Appendix, Fig. S9G). Taken together, USP14 promotes gluconeogenesis and hepatic glucose production by augmenting glucagon action.
USP14 Increases CBP Stability.
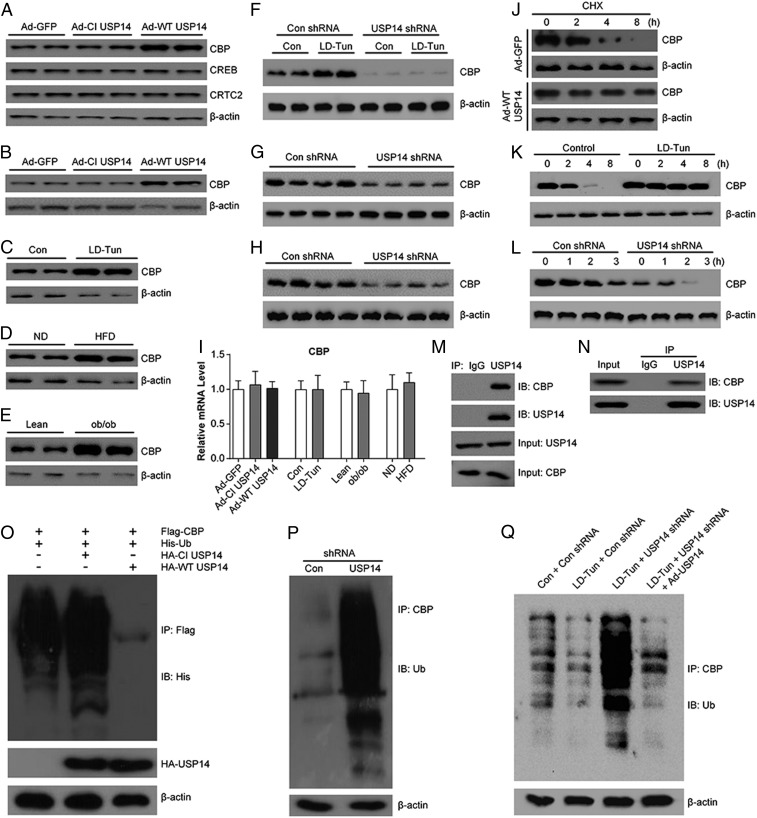
The fact that the catalytic function of USP14 is essential for its gluconeogenic roles suggests that USP14 may promote glucagon action by stabilizing the latter’s target proteins. Circulating glucagon increases the intracellular adenosine 3′,5′-cyclic monophosphate (cAMP) levels, which triggers cAMP-dependent protein kinase A to phosphorylate the CREB protein in the hepatocytes (41). The CREB protein binds to the CRE site at the promoter regions of gluconeogenic genes, and activates their transcription through recruiting the 2 coactivators: CREB-regulated transcription coactivator 2 (CRTC2) and CBP (41). USP14 overexpression significantly increased CBP levels in the liver and MPHs (Fig. 5 A and B), without affecting those of CREB and CRTC2 (Fig. 5A). CBP levels were also higher in livers of LD-Tun–treated mice and obese mice compared with the corresponding control mice (Fig. 5 C–E). The increased protein levels of CBP in these mice was substantially attenuated by knockdown of USP14 (Fig. 5 F–H). In contrast, mRNA levels of CBP were not affected (Fig. 5I), indicating that USP14 regulates CBP stability at the posttranslational level. In line with these observations, the half-life of CBP was increased in USP14-overexpressing or LD-Tun–treated MPHs (Fig. 5 J and K), while knocking down USP14 accelerated the degradation of CBP (Fig. 5L). Furthermore, a direct physical interaction between USP14 and CBP was confirmed by coimmunoprecipitation in MPHs and mouse livers (Fig. 5 M and N). To test whether this interaction could suppress CBP ubiquitination, Flag-tagged CBP, His-tagged ubiquitin, and hemagglutinin-tagged CI USP14 or WT USP14 plasmids were cotransfected into HEK293T cells. As a result, ladders with higher molecular weight were detected in anti-Flag immunoprecipitates using anti-His antibodies (Fig. 5O), indicating that CBP is polyubiquitinated. Coexpression of WT USP14, not CI USP14, significantly inhibited ubiquitin conjugation to CBP (Fig. 5O). Conversely, knockdown of endogenous USP14 dramatically enhanced polyubiquitination of endogenous CBP in MPHs (Fig. 5P). In addition, LD-Tun treatment reduced the ubiquitination of CBP in MPHs (Fig. 5Q, lane 2 vs. lane 1). In contrast, knockdown of endogenous USP14 expression dramatically increased the ubiquitination of CBP, even in the presence of LD-Tun (Fig. 5Q, lane 3 vs. lane 2), which was reversed by additional overexpression of USP14 (Fig. 5Q, lane 4 vs. lane 3). Therefore, our results indicate that USP14 is required for the deubiquitination of CBP induced by LD-Tun. Collectively, these data indicate that USP14 binds to CBP and prevents its ubiquitination-mediated degradation.
Fig. 5.
USP14 increases CBP stability. (A) CBP, CRTC2, and CREB protein levels in the liver of mice infected with Ad-GFP, Ad-CI USP14, or Ad-WT USP14. CBP levels in the MPHs transfected with Ad-GFP, Ad-CI USP14, or Ad-WT USP14 (B); liver of mice treated with LD-Tun or saline (C); and liver of HFD-fed obese (D) and ob/ob (E) mice are shown. Con, control; ND, not determined. (F) CBP levels in mice as indicated. shRNA, short hairpin RNA. (G and H) CBP levels in HFD-fed obese and ob/ob mice infected with Con shRNA or USP14 shRNA. (I) Relative CBP mRNA levels in the liver of mice as indicated (n = 6 per group). (J) CBP levels in control and USP14-overexpressing MPHs. Cells were treated with 25 μg/mL cycloheximide (CHX) for the indicated time. (K) CBP levels in control or LD-Tun–treated MPHs. Cells were treated with 25 μg/mL CHX for the indicated time. (L) CBP levels in Con shRNA- or USP14 shRNA-infected MPHs. Interaction between USP14 and CBP in HEK293T cells (M) and MPHs (N) is shown. IB, immunoblot; IP, immunoprecipitation. (O) Ubiquitination of CBP in HEK293T cells. Cell were cotransfected with Flag-tagged CBP, His-tagged ubiquitin, and hemagglutinin (HA)-tagged CI USP14 or WT USP14 plasmids and immunoprecipitated with Flag antibodies. (P) Ubiquitination of CBP in MPHs. Cells were transfected with Con shRNA or USP14 shRNA and incubated with MG132, and the cell lysates were immunoprecipitated with anti-CBP antibody and probed using antiubiquitin antibody. (Q) Ubiquitination of CBP in MPHs as indicated.
Discussion
Although it is widely appreciated that ER stress plays an important role in the control of glucose metabolism, the molecular basis remains unclear. Unlike acute ER stress, which induces hypoglycemia and disrupts gluconeogenesis (22, 29), we found that sustained ER stress promotes hyperglycemia and enhances hepatic glucose production, indicating different mechanisms at play in both phenomena. Sustained ER stress up-regulated USP14 in the liver, whereas hepatic knockdown of USP14 attenuated ER stress-induced hyperglycemia in both lean and obese mice. Consistent with this, hepatocyte-specific overexpression of USP14 increased hyperglycemia and glucose intolerance even in the healthy mice. In addition, we discovered a function of USP14 in regulating the glucagon response; while hepatic knockdown of USP14 decreased gluconeogenesis and impaired the glucagon response, USP14 overexpression had the opposite effect. The hypothesized model of USP14-mediated metabolic control is depicted in SI Appendix, Fig. S10.
USP14 increased the amount of CBP without affecting its mRNA levels. Based on the known deubiquitinating functions of USP14, we hypothesize that it binds with CBP to remove the polyubiquitin chains and prevent proteasomal degradation. The resulting stabilization and accumulation of CBP increases the expression of gluconeogenic genes, leading to enhanced hepatic gluconeogenesis. The CREB/CBP/CRTC2 network is crucial to glucose metabolism, although its regulatory mechanisms have not been completely elucidated. Montminy and coworkers (42, 43) found that CRTC2 is dephosphorylated during fasting and is phosphorylated and degraded in the postprandial stages. Sheng et al. (44) showed that high levels of the nuclear factor κB-inducing kinase during obesity phosphorylate CREB and increase its stability. In addition, CBP is phosphorylated at serine 436 by insulin signaling and is a target of the antidiabetic drug metformin (45, 46). Mice expressing the S436A mutant of CBP displayed hyperglycemia and glucose intolerance during the fasting state (45). Therefore, identification of novel partners for the CREB/CBP/CRTC2 pathway may advance our understanding of the architecture of this crucial metabolic regulatory network. However, the cross-talk between deubiquitylation and phosphorylation of CBP in the context of sustained ER stress or obesity was not determined in our study.
Previous studies have demonstrated that the transcriptional coactivator P300 is also regulated by stress pathways and maintains basal hepatic gluconeogenesis (47). In addition, Cao et al. (27) demonstrated that P300 activity could be activated by ER stress and impairs insulin signaling, as well as promoting hepatic glucose production and hyperglycemia through acetylation of IRS1/2. Moreover, Bricambert et al. (48) showed that P300 overexpression disrupts liver glucose homeostasis and leads to the development of glucose intolerance and insulin resistance through acetylation of ChREBP. Here, our results showed that protein levels of P300 were not altered in C57BL/6 mice with USP14 overexpression or HFD-fed obese mice with USP14 knockdown (SI Appendix, Fig. S11 A and B), suggesting that the effects of USP14 on gluconeogenesis are independent of P300. However, we do not exclude the possibility that USP14 may regulate glucose metabolism by other mechanisms, in addition to increasing CBP stability.
The protein turnover and stability in eukaryotic cells is maintained by the ubiquitination/deubiquitination cascade (49). Our recent study found that aberrantly high levels of USP14 in the liver of obese mice and humans promote hepatosteatosis and hypertriglyceridemia by stabilizing fatty acid synthase (FASN) (32), which might increase the risk of glucose intolerance and T2DM (50). However, we found that FASN knockdown in the MPHs did not affect the gluconeogenic role of USP14 (SI Appendix, Fig. S12 A and B), indicating a bifurcation between USP14-mediated hepatic gluconeogenesis and triglyceride metabolism. In addition, studies have shown a direct interaction between IRE1α and USP14 in neurons and macrophages (33, 34), and Mao et al. (18) showed that hepatic IRE1α responds to glucagon signaling to regulate gluconeogenesis. However, knocking down IRE1α in the MPHs did not affect USP14-driven gluconeogenesis (SI Appendix, Fig. S12 C and D), indicating that the gluconeogenic effects of USP14 are independent of IRE1α. Since FASN, IRE1α, and CBP are localized in the cytoplasm and nucleus, we hypothesize that the metabolic function of USP14 depends on its subcellular localization, which may be determined by distinct upstream signaling pathways.
In conclusion, our results establish that sustained ER stress promotes hyperglycemia and alters glucose homeostasis through induction of USP14, which, in turn, increases hepatic gluconeogenesis by enhancing glucagon action via stabilizing CBP. USP14 might therefore be a novel therapeutic target for the treatment of hyperglycemia and T2DM.
Materials and Methods
All animal protocols were reviewed and approved by the Animal Care Committee of Zhongshan Hospital, Fudan University. Extended materials and methods are described in SI Appendix. All values are shown as mean ± SEM. Groups were compared using a 2-tailed Student’s t test, and P values less than 0.05 were considered statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001).
Supplementary Material
Acknowledgments
This work was supported by the National Key Research and Development Program of China (Grant 2016YFC1304801), National Natural Science Foundation of China (Grants 31530033, 81773018, 81500617, and 81600372), Science and Technology Commission of Shanghai Municipality (Grant 16JC1404100), and Shanghai Municipal Health Bureau (Grant 20184Y0284).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The RNA-sequencing data have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE135372).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1907288116/-/DCSupplemental.
References
- 1.Rines A. K., Sharabi K., Tavares C. D., Puigserver P., Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat. Rev. Drug Discov. 15, 786–804 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen M. C., Vatner D. F., Shulman G. I., Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 13, 572–587 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Könner A. C., Brüning J. C., Selective insulin and leptin resistance in metabolic disorders. Cell Metab. 16, 144–152 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Hetz C., Chevet E., Harding H. P., Targeting the unfolded protein response in disease. Nat. Rev. Drug Discov. 12, 703–719 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Xu C., Bailly-Maitre B., Reed J. C., Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Invest. 115, 2656–2664 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotamisligil G. S., Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cnop M., Foufelle F., Velloso L. A., Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 18, 59–68 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Flamment M., Hajduch E., Ferré P., Foufelle F., New insights into ER stress-induced insulin resistance. Trends Endocrinol. Metab. 23, 381–390 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Fu S., Watkins S. M., Hotamisligil G. S., The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 15, 623–634 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Yilmaz E., Endoplasmic reticulum stress and obesity. Adv. Exp. Med. Biol. 960, 261–276 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Ozcan U., et al. , Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Puri P., et al. , Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134, 568–576 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Gregor M. F., et al. , Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58, 693–700 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henkel A. S., Unfolded protein response sensors in hepatic lipid metabolism and nonalcoholic fatty liver disease. Semin. Liver Dis. 38, 320–332 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Ozcan U., et al. , Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji C., et al. , Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology 54, 229–239 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jurczak M. J., et al. , Dissociation of inositol-requiring enzyme (IRE1α)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J. Biol. Chem. 287, 2558–2567 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao T., et al. , PKA phosphorylation couples hepatic inositol-requiring enzyme 1alpha to glucagon signaling in glucose metabolism. Proc. Natl. Acad. Sci. U.S.A. 108, 15852–15857 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao M., et al. , Hepatic IRE1α regulates fasting-induced metabolic adaptive programs through the XBP1s-PPARα axis signalling. Nat. Commun. 5, 3528 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Li K., et al. , MicroRNA-214 suppresses gluconeogenesis by targeting activating transcriptional factor 4. J. Biol. Chem. 290, 8185–8195 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun X., et al. , Hepatic conditional knockout of ATF6 exacerbates liver metabolic damage by repressing autophage through MTOR pathway. Biochem. Biophys. Res. Commun. 505, 45–50 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Vera L., Fischer W. H., Montminy M., The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature 460, 534–537 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S. W., et al. , The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat. Med. 16, 429–437 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J., et al. , Inflammation improves glucose homeostasis through IKKβ-XBP1s interaction. Cell 167, 1052–1066 e18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y., et al. , Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat. Med. 17, 356–365 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng Y., et al. , The Xbp1s/GalE axis links ER stress to postprandial hepatic metabolism. J. Clin. Invest. 123, 455–468 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao J., et al. , Endotoxemia-mediated activation of acetyltransferase P300 impairs insulin signaling in obesity. Nat. Commun. 8, 131 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Loeffelholz C., et al. , Increased lipogenesis in spite of upregulated hepatic 5′AMP-activated protein kinase in human non-alcoholic fatty liver. Hepatol. Res. 47, 890–901 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Feng B., et al. , Endoplasmic reticulum stress inducer tunicamycin alters hepatic energy homeostasis in mice. Int. J. Mol. Sci. 18, E1710 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dominy J. E. Jr, Lee Y., Gerhart-Hines Z., Puigserver P., Nutrient-dependent regulation of PGC-1alpha’s acetylation state and metabolic function through the enzymatic activities of Sirt1/GCN5. Biochim. Biophys. Acta 1804, 1676–1683 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Besseiche A., Riveline J. P., Gautier J. F., Bréant B., Blondeau B., Metabolic roles of PGC-1α and its implications for type 2 diabetes. Diabetes Metab. 41, 347–357 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Liu B., et al. , Proteome-wide analysis of USP14 substrates revealed its role in hepatosteatosis via stabilization of FASN. Nat. Commun. 9, 4770 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagai A., et al. , USP14 inhibits ER-associated degradation via interaction with IRE1alpha. Biochem. Biophys. Res. Commun. 379, 995–1000 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Hyrskyluoto A., et al. , Ubiquitin-specific protease-14 reduces cellular aggregates and protects against mutant huntingtin-induced cell degeneration: Involvement of the proteasome and ER stress-activated kinase IRE1α. Hum. Mol. Genet. 23, 5928–5939 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Kiprowska M. J., et al. , Neurotoxic mechanisms by which the USP14 inhibitor IU1 depletes ubiquitinated proteins and Tau in rat cerebral cortical neurons: Relevance to Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 1157–1170 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Min J. W., Lü L., Freeling J. L., Martin D. S., Wang H., USP14 inhibitor attenuates cerebral ischemia/reperfusion-induced neuronal injury in mice. J. Neurochem. 140, 826–833 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sano R., Reed J. C., ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 1833, 3460–3470 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He C. H., et al. , Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J. Biol. Chem. 276, 20858–20865 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q., et al. , Central activating transcription factor 4 (ATF4) regulates hepatic insulin resistance in mice via S6K1 signaling and the vagus nerve. Diabetes 62, 2230–2239 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walters B. J., et al. , A catalytic independent function of the deubiquitinating enzyme USP14 regulates hippocampal synaptic short-term plasticity and vesicle number. J. Physiol. 592, 571–586 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altarejos J. Y., Montminy M., CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 12, 141–151 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koo S. H., et al. , The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1111 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Dentin R., et al. , Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature 449, 366–369 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Sheng L., et al. , NF-κB–inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nat. Med. 18, 943–949 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou X. Y., et al. , Insulin regulation of hepatic gluconeogenesis through phosphorylation of CREB-binding protein. Nat. Med. 10, 633–637 (2004). [DOI] [PubMed] [Google Scholar]
- 46.He L., et al. , Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 137, 635–646 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He L., et al. , Transcriptional co-activator p300 maintains basal hepatic gluconeogenesis. J. Biol. Chem. 287, 32069–32077 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bricambert J., et al. , Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J. Clin. Invest. 120, 4316–4331 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He M., et al. , The emerging role of deubiquitinating enzymes in genomic integrity, diseases, and therapeutics. Cell Biosci. 6, 62 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tilg H., Moschen A. R., Roden M., NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 14, 32–42 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.