Significance
Finding a suitable oviposition site is a challenging task for a gravid female moth. At the same time, it is of paramount importance considering the limited capability of most caterpillars to relocate to alternative host plants. The hawkmoth, Manduca sexta, oviposits on solanaceous plants. Larvae hatching on a plant that is already attacked by conspecific caterpillars face food competition. Here, we show that feces from conspecific caterpillars are sufficient to deter a female M. sexta from ovipositing on a plant. Furthermore, we not only identify the responsible compound in the feces but also localize the population of sensory neurons that governs the female’s avoidance. Hence, our work increases the understanding of how animals cope with a competitive environment.
Keywords: Manduca sexta, Ir8a, CRISPR-Cas9, insect olfaction, insect–plant interactions
Abstract
Finding a suitable oviposition site is a challenging task for a gravid female moth. At the same time, it is of paramount importance considering the limited capability of most caterpillars to relocate to alternative host plants. The hawkmoth, Manduca sexta (Sphingidae), oviposits on solanaceous plants. Larvae hatching on a plant that is already attacked by conspecific caterpillars can face food competition, as well as an increased exposure to predators and induced plant defenses. Here, we show that feces from conspecific caterpillars are sufficient to deter a female M. sexta from ovipositing on a plant and that this deterrence is based on the feces-emitted carboxylic acids 3-methylpentanoic acid and hexanoic acid. Using a combination of genome editing (CRISPR-Cas9), electrophysiological recordings, calcium imaging, and behavioral analyses, we demonstrate that ionotropic receptor 8a (IR8a) is essential for acid-mediated feces avoidance in ovipositing hawkmoths.
For insects, finding appropriate sites for oviposition is a challenging task, and the decision of a gravid female will have clear consequences for the fitness of its progeny. Due to fragility and limited mobility, the offspring face many threats: limited food availability, intra- and interspecific competition (1), predation (2), and attack by parasitoids (3). Therefore, gravid females must carefully examine the environment prior to selecting the oviposition site. For this, they utilize visual (4, 5), gustatory (6, 7), mechanosensory (8), and olfactory (9, 10) cues. Among these modalities, olfaction plays a pivotal role in an insect’s life, as it provides information not only about oviposition sites but also about other biologically relevant resources such as food and mating partners (11).
Insects rely on a sophisticated olfactory system to detect volatile chemicals in the environment. Several protein families are involved, with odorant receptors (ORs) and ionotropic receptors (IRs), 2 types of ligand-gated ion channels, being the key detecting elements (12–14). On the surface of the antenna, the main olfactory organ, numerous hair-like structures (sensilla) contain olfactory sensory neurons (OSNs), which represent the basic units of sensory reception. Sensilla involved in olfaction occur in 3 morphological types: basiconic, trichoid, and coeloconic. In the vinegar fly, Drosophila melanogaster (12, 15), as well as in other investigated insect species (16–18), ORs are expressed in the dendritic membrane of OSNs housed in basiconic and trichoid sensilla, whereas IRs are expressed by OSNs housed in coeloconic sensilla. ORs are extremely divergent and different insect species express from 10 OR genes in head lice (19) to more than 300 in ants (20). The OR type expressed in an OSN dictates the odorant specificity of the neuron (21). ORs are coexpressed together with the conserved odorant receptor-coreceptor (Orco), which is essential for dendritic localization of ORs and OR-dependent odorant detection (13, 22). IRs usually are less divergent (23), and at least 2 IR coreceptors, ionotropic receptor 8a (IR8a) and IR25a, form ligand-gated ion channels with other odorant-tuned IRs (12, 24). The different receptor types, however, differ not only in their local expression but also in their response profiles. While most ORs are broadly tuned to alcohols, aldehydes, aromatics, esters, or terpenes (21), IRs primarily respond to a restricted subset of odors, including mainly acids and amines (25). At least in Drosophila and Aedes aegypti, IR8a is required for acid detection (26, 27). IR25a, on the other hand, seems to be coexpressed with IRs responding to amines (28) and is also involved in the detection of temperature (29), humidity (30), and salt (31).
The tobacco hawkmoth Manduca sexta (Lepidoptera: Sphingidae) is an established model for insect olfaction (16) and odor-guided behavior (32). The recent identification of 73 OR genes and 21 olfactory IR genes and their expression patterns in male and female moths (16) and the establishment of the CRISPR-Cas 9 technique in M. sexta (33) have made the species an even more powerful model for olfactory neuroethology. The larvae of these moths feed on various plants of the family Solanaceae, including coyote tobacco (Nicotiana attenuata) and jimson weed (Datura wrightii) (Fig. 1A). It was reported that a single M. sexta caterpillar consumes 1 to 10 tobacco plants before pupation (1), resulting in complete defoliation of the plants and accumulation of feces under the plant. Therefore, it is crucial for M. sexta females to find a suitable host plant that is not already occupied by a conspecific larva.
Fig. 1.
M. sexta oviposition on N. attenuata and D. wrightii is affected by larval feces. (A) M. sexta host plants coyote tobacco (N. attenuata, Left) and jimson weed (D. wrightii, Right). (B) Schematic drawing of the wind tunnel assay. (C) Oviposition index of mated females toward feces of M. sexta caterpillars reared on N. attenuata and D. wrightii. Oviposition index = (number of eggs on plant with feces − number of eggs on plant without feces)/total egg number. (D) GC/MS profile of headspace of feces from a M. sexta caterpillar reared on N. attenuata. IS, internal standard. (E) GC/MS profile of headspace of feces from a M. sexta caterpillar reared on D. wrightii. (F, Left) Oviposition index of gravid females to carboxylic acids (at a dilution of 10−2) emitted from M. sexta caterpillar feces. (F, Right) Oviposition index of gravid females to various doses of 3-methylpentanoic acid and hexanoic acid. Deviation of the index against 0 was tested with the Wilcoxon signed-rank test (n = 17 to 20). Orange boxes depict P < 0.05. Boxplots depict median and upper and lower quartiles. Whiskers depict quartiles ± 1.5× the IQR. All data were included in the statistical analysis.
Volatiles emitted from larval feces have been shown to act as kairomones and attract parasitoids and predators (2, 3, 34, 35). Therefore, the smell of larval feces not only indicates the occupancy of the host plant, and the resulting potential for intraspecific competition, but also an increased susceptibility to parasitization and predation. Hence, female moths should avoid sites that are already occupied by conspecific larvae and could do so by, for example, detecting chemical cues emanating from larval feces. In several insect species, female oviposition has been found to be deterred by conspecific larval feces (36). Thus, larval feces alone are sufficient to signal potential competition to the female. However, the molecular and cellular mechanisms by which female insects avoid feces remain unknown.
Here, we investigate whether the oviposition of M. sexta is deterred by feces from its larvae. We first show that M. sexta females, like other insects, display oviposition aversion toward conspecific caterpillar feces stemming from different host plants. Next, we identify specific carboxylic acids emitted from the feces as key compounds that confer oviposition aversion. By performing electrophysiological recordings, calcium imaging, and behavioral analyses with mutant moths that lack either Orco or one of the IR coreceptors, Ir8a or Ir25a, we demonstrate that IR8a is essential for acid-mediated feces avoidance during oviposition.
Results and Discussion
Feces of Caterpillars Fed on N. attenuata Repel Oviposition.
To test whether gravid females of M. sexta avoid ovipositing in the presence of feces, we tested their behavior in a 2-choice assay in a wind tunnel. The moths were allowed to oviposit for 3 min either on an undamaged N. attenuata plant that was equipped with 10 g of larval feces (from caterpillars that had fed on other N. attenuata plants) or on an undamaged control plant (Fig. 1B). In these experiments, the moths laid, on average, 14.3 ± 1.7 eggs (mean ± SEM) during the 3-min test on both plants. The allocation of eggs depended on the presence of feces, with the moths laying significantly fewer eggs on the plant with caterpillar feces in comparison to the control plant (Fig. 1C). A similar preference was observed when moths were given a choice between a plant with feces and a control plant without feces in a steady-air tent (SI Appendix, Fig. S1), confirming that feces avoidance is consistent in different behavioral paradigms. We conclude that even in the absence of plant damage, caterpillar feces induce oviposition avoidance in M. sexta. Former studies suggested that ovipositing M. sexta females mainly use plant- and larva-derived odors to avoid competition (37, 38). In our study, where the amount of feces was higher (but still ecologically reasonable, as we used feces that were produced by a single larva during 1 night), feces alone were sufficient to induce oviposition avoidance. Females tested in our experiments were raised on an artificial diet and had no prior experience with the plants or the feces. We therefore conclude that the feces-induced oviposition avoidance is innate.
3-Methylpentanoic Acid and Hexanoic Acid Govern Oviposition Avoidance to Larval Feces.
To identify the active compound responsible for feces avoidance, we raised M. sexta caterpillars on N. attenuata plants and afterward collected the headspace of the resulting feces using a solid-phase microextraction (SPME) fiber. Gas chromatography/mass spectrometry (GC/MS) analysis revealed similar results as in a previous study (2), with 3-methylpentanoic acid being the most abundant compound, followed by 2 other branched aliphatic acids: 3-methylbutanoic acid and 4-methylpentanoic acid (Fig. 1D).
To investigate the impact of these compounds on M. sexta oviposition, we pipetted 10 μL of one of the compounds (diluted 10−2 in mineral oil) onto a filter paper and attached this filter paper 2 cm upwind of a detached N. attenuata leaf before presenting this leaf to a mated female in the wind tunnel. When compared with a control leaf, where the attached filter paper just contained the solvent, only 3-methylpentanoic acid elicited significant avoidance (Fig. 1 F, Left). To address the behavioral sensitivity of M. sexta toward 3-methylpentanoic acid, we further performed the wind tunnel test with lower amounts of the compound and identified the behavioral threshold to be between 10−4 and 10−3 dilutions (i.e., between 9.3 μg and 93 μg) (Fig. 1 F, Right), which corresponds well to the reported amount of 30 μg of acids in 1 g of M. sexta feces (2). In addition, we have collected volatiles from the feces of Spodoptera littoralis larvae (i.e., another common Nicotiana herbivore) that were raised on N. attenuata. We found the same acids as in the feces from M. sexta larvae (SI Appendix, Fig. S2), indicating that M. sexta is able to detect and avoid feces not only from conspecific but also from allospecific potential competitors. We conclude that 3-methylpentanoic acid is the major compound governing feces avoidance of ovipositing females in the context of N. attenuata.
Having shown that feces from caterpillars reduce the attraction of N. attenuata plants to ovipositing females, we asked whether this also holds true for the relationship between M. sexta and its other main host plant, D. wrightii. We now let female moths choose to oviposit either on a D. wrightii leaf that was equipped with feces (from caterpillars raised on D. wrightii) or on a control leaf without feces. Again, females preferred to oviposit on the control leaf (Fig. 1C), suggesting that also at the host plant, D. wrightii, feces induce oviposition avoidance in M. sexta females.
To identify the active compounds responsible for feces avoidance in D. wrightii, we raised M. sexta caterpillars on D. wrightii plants and then collected and analyzed the volatiles as before. The chemical profile of the feces was dominated by hexanoic acid this time, and accompanied by 2 other minor compounds, heptanoic acid and pentanoic acid (Fig. 1E). Again, when an ovipositing female had to choose between a D. wrightii leaf that was equipped with one of the 3 acids and a control leaf, only leaves with hexanoic acid (10 μL at a 10−2 dilution) were avoided (Fig. 1 F, Left). This avoidance could still be observed even when we reduced the amount of hexanoic acid 10-fold (Fig. 1 F, Right). We conclude that hexanoic acid is the major compound governing feces avoidance of ovipositing females in the context of D. wrightii. When performing choice experiments in the wind tunnel with additional aliphatic acids and the 2 host plants, we found that only 6-carbon aliphatic acids elicited avoidance (SI Appendix, Fig. S3).
Both the Odorant Coreceptor Orco and IR8a Participate in Acid Sensing.
To determine which olfactory pathway is governing the detection of 3-methylpentanoic acid and hexanoic acid, we performed electroantennography (EAG) measurements on wild-type (WT) moths and on odorant coreceptor heterozygous (Orco+/−) and homozygous (Orco−/−) moths that were recently generated in our laboratory using CRISPR-Cas9 genome editing (33). While WT moths and Orco+/− moths exhibited robust EAG responses to the acids, Orco−/− moths showed reduced responses (SI Appendix, Fig. S4). However, clear EAG responses to the acids remained, indicating that the IR pathway is also involved in acid detection.
To address whether the remaining response to acids in Orco−/− moths were indeed resulting from activation of the IR pathway, we generated 2 IR mutant lines, Ir8a−/− and Ir25a−/−, again using CRISPR-Cas9 genome editing. The resulting Ir8a−/− mutant contained a 339-base pair (bp) deletion (93 bp at exon2, 170 bp at intron2, and 76 bp at exon3), while the Ir25a−/− mutant contained a 154-bp deletion (154 bp at exon2) in the genome. As both deletions resulted in frameshifts and the occurrence of premature stop codons (SI Appendix, Fig. S5A), we expected both mutations to result in nonfunctional ionotropic coreceptors. We found no difference regarding pupal weight and length in either Ir8a−/− or Ir25a−/− mutants, when compared with the heterozygous controls (SI Appendix, Fig. S5B). Furthermore, in EAG experiments, both mutants exhibited normal responses to the OR-detected pheromone bombykal (SI Appendix, Fig. S5C), suggesting the absence of relevant off-target effects.
However, when performing EAG experiments with Ir8a−/− and Ir25a−/− moths, only Ir8a−/− moths exhibited significantly reduced responses to both behaviorally active acids when compared with WT moths, while the acid responses in Ir25a−/− moths remained unaffected (Fig. 2A).
Fig. 2.
Detection and processing of feces-emitted odors. (A) EAG responses (in microvolts ± SEM; the response to solvent was subtracted) of M. sexta antennae isolated from WT, Orco−/− (Orco mutant), Orco+/− (Orco heterozygous), Ir8a−/− (Ir8a mutant), Ir8a+/− (Ir8a heterozygous), Ir25a−/− (Ir25a mutant), and Ir25a+/− (Ir25a heterozygous). EAG responses to 3-methylpentanoic acid and hexanoic acid are shown. Bars with same letter are not significantly different from each other. ANOVA; n = 20 to 25 females per genotype. (B) Percentage of coeloconic sensilla responding to the 2 behaviorally active acids in different genotypes. Bars with same letter are not significantly different from each other. Fisher's exact test with Bonferroni–Holms correction for multiple comparisons; n = 29 to 32 sensilla. (C) Heat map representation of SSR responses of coeloconic sensilla from different moth genotypes. (D) Schematic of 23 putative olfactory glomeruli at the dorsal surface of the right antennal lobe. The schematic was created for each individual moth based on the activation patterns of 19 diagnostic odorants (45), and numbers identify glomeruli that were most strongly activated by the tested acids (16) or that showed acid-specific activation (22 and 23). (E) Examples of calcium imaging recordings in WT, IR25a−/−, and IR8a−/− female moths after stimulation with the 2 behaviorally active acids. The increase of fluorescence is color-coded (scale) and superimposed onto the view of the antennal lobe; circles indicate positions of glomeruli 16 (black outline), 22 (brown), and 23 (blue). max, maximum. (F) Bars show the mean response of a glomerulus (after subtraction of the solvent response) to an odorant. Error bars indicate SD. Bars with the same letter are not significantly different from each other (ANOVA; n = 4 to 6 females per genotype).
IR8a Pathway Is Essential for Detecting and Avoiding Acids from Caterpillar Feces.
We next asked which sensillum type is involved in the detection of the acids in caterpillar feces. According to the well-studied Drosophila species (12, 21, 39, 40), IR-expressing OSNs are mainly housed in coeloconic sensilla. Furthermore, in M. sexta, previous single-sensillum recordings (SSRs) from trichoid and basiconic sensilla showed little to no response to acids (41, 42). We therefore hypothesized that coeloconic sensilla of M. sexta house IR-expressing OSNs that are involved in acid detection. In contrast to the antenna of female D. melanogaster, which contains only 54 coeloconic sensilla (40), the antenna of female M. sexta carries about 3,600 (41). This makes the identification and recording from identified individual coeloconic sensilla almost impossible. We therefore recorded from 28 coeloconic sensilla from the middle part of the antenna, which should cover a wide range of functional types, and stimulated them with a set of 52 odorants from different chemical classes (SI Appendix, Fig. S6). Consistent with previous studies in D. melanogaster (40) and Bombyx mori (43), OSNs housed in coeloconic sensilla in WT M. sexta were mainly activated by acids and amines. The 2 behaviorally active acids activated mainly OSNs in nonoverlapping groups of coeloconic sensilla. The number of coeloconic sensilla responding to hexanoic acid was about 2-fold higher than the number responding to 3-methylpentanoic acid, and the intensity of responses to hexanoic acid was stronger.
We found reduced numbers of coeloconic sensilla responding to 3-methylpentanoic acid and hexanoic acid in Ir8a−/− moths, when compared with the other 3 genotypes (Fig. 2 B and C; representative recording traces are shown in SI Appendix, Fig. S7). Interestingly, increased numbers of coeloconic sensilla exhibited enhanced responses to acids in Ir25a−/− moths, whereas the responses to amines were almost abolished. The enhanced responses in Ir25a−/− moths toward acids could be due to more energy being available to OSNs responding to acids, as these OSNs no longer have to compete with amine-tuned OSNs in the same sensillum. Such a phenomenon has been reported for gustatory receptors, where sensory neurons in the same sensillum have been shown to interact, exhibiting competition, inhibition, or activation (44). In conclusion, our results show that IR8a, but neither Orco nor IR25a, is required for acid detection in OSNs of coeloconic sensilla.
We conclude that IR8a is involved in the detection of the key compounds governing feces avoidance. We next asked where in the antennal lobe (i.e., the first olfactory processing center of the moth’s brain) this IR-related acid detection becomes processed. A recently published functional analysis of the moth’s antennal lobe (45) revealed 3 glomeruli that strongly responded to acids. In another study (33), activation of 2 of these glomeruli was not affected by knocking out Orco, supporting that these 2 glomeruli become innervated by IR-expressing OSNs. When performing calcium imaging experiments with moths that lacked either a functional IR25a or IR8a, the responses to acids in Ir25a mutants were unaffected compared with control animals (Fig. 2 E and F). However, when testing Ir8a mutants, we observed a slightly reduced response to hexanoic acid and a significantly reduced response to 3-methylpentanoic acid (Fig. 2 E and F) in only those 2 glomeruli that were independent of Orco in the former study. Together with the EAG results, we conclude that both Orco and IR8a, but not IR25a, are involved in acid sensing and that IR8a-expressing OSNs involved in the detection target a subset of glomeruli on the medial surface of the antennal lobe.
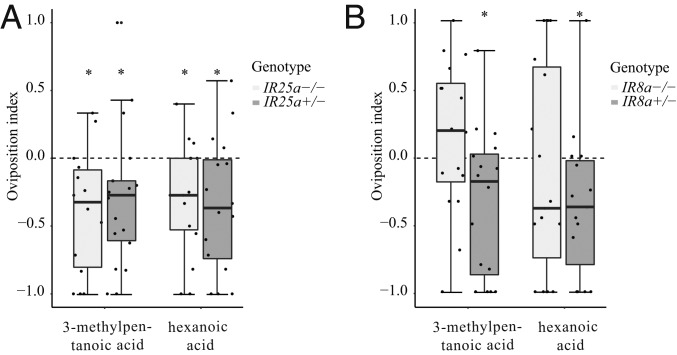
Next, we asked whether any of the 3 coreceptors governs the behavioral avoidance toward acids in ovipositing M. sexta. Unfortunately, the oviposition rates of Orco−/− moths were too low to draw any conclusions regarding the involvement of Orco in oviposition avoidance, which is in accordance with the findings of a former study, where knocking out the Orco receptor resulted in significantly reduced oviposition behavior in M. sexta (33). Interestingly, however, mutation of Ir25a did not affect oviposition behavior (Fig. 3A), while Ir8a−/− moths were no longer repelled by the tested acids (Fig. 3B). We conclude that ovipositing females rely on IR8a for avoidance of acids from caterpillar feces.
Fig. 3.
IR8a is necessary for acid avoidance of ovipositing M. sexta females. A 2-choice assay shows the oviposition indexes of the homozygous and heterozygous (as a control) Ir25a (A) and Ir8a (B) mutants for the feces-emitted compounds 3-methylpentanoic acid and hexanoic acid (details on choice assay are provided in Methods and Fig. 1). Deviation of the index against 0 was tested with the Wilcoxon signed-rank test (n = 14 to 19). *P < 0.05.
Several studies have shown that larval feces and odors deter female oviposition (36). Feces-emitted acids play a crucial role in oviposition avoidance in moth species like Ostrinia species (46) and Helicoverpa armigera (47). Moreover, it was shown that female parasitoid wasps, Cotesia glomerata, use acids emitted by host larvae as cues to locate their host (48), and M. sexta caterpillar feces-emitted acids play a major role in attracting predators like ants (2). Finally, one of the acids we identified in the caterpillar feces (hexanoic acid) has been shown to induce plant defenses against herbivores (49). Obviously, carboxylic acids are potent signals for a gravid female to realize that, at a given plant, the female’s offspring might face conspecific competitors, parasitoids, and predators, as well as an already induced plant defense. Therefore, our finding that ovipositing M. sexta females, like other moths, avoid emitted acids from larval feces is not unexpected. However, the neural and molecular mechanisms, as well as the exact chemistry underlying this behavior, remained elusive. In this study, we only show that M. sexta displays oviposition aversion toward caterpillar feces but also find that only the major volatile compounds (C6 carboxylic acids) emitted are aversive for gravid females. As these acids are present not only in feces of M. sexta larvae but also in feces of S. littoralis larvae (i.e., another herbivore from N. attenuata plants), feces avoidance might provide the ovipositing M. sexta female with the opportunity to avoid not only conspecific but also allospecific competition. By testing mutant moths in which we knocked out different olfactory coreceptors, we show that the coreceptors IR8a and Orco, but not IR25a, participate in the detection of larval feces and that at least IR8a is necessary for feces avoidance.
It was reported that M. sexta lay significantly fewer eggs on plants that were damaged by herbivores (1). From an evolutionary perspective, the plant might tag caterpillars with a distinctive (acid) odor that provides spatial and temporal information about feeding larvae to predators (2). Our data suggest that the ovipositing females can recognize these odors and avoid them to optimize the survival chance of their offspring, which adds another layer of regulation to host choice in M. sexta.
Methods
Insect Rearing and Plant Material.
All animals were reared at the Max Planck Institute for Chemical Ecology, as already described (16). Briefly, eggs were collected from female M. sexta moths, which could freely oviposit on D. wrightii plants. Larvae used in the experiments were reared on an artificial diet, under a 16:8-h light/dark photoperiod, with a relative humidity of 40% at 26 °C. Naive females were mated the second night after emergence and tested during the subsequent night. M. sexta feces were collected daily from fourth- to fifth-instar caterpillars that were raised on either N. attenuata or D. wrightii.
All plants were grown in a greenhouse, as described (50). Plants used for experiments were not yet flowering. Approximately 7 d before being used, plants were transferred to a climate chamber with the same settings as the moth flight cage (16:8-h light/dark photoperiod with a relative humidity of 40% at 26 °C).
Chemical Analysis.
We identified the volatiles of caterpillars feces using SPME coupled with GC/MS. One gram of feces from caterpillars raised on either D. wrightii or N. attenuata was put into a 500-mL plastic container. A circular filter paper (12-mm diameter, Whatman; Sigma–Aldrich) loaded with 10 μL of diluted bromodecane (1:104 in hexane) was used as an internal standard. Through a hole in the lid of the container, a SPME fiber (50 μm of divinylbenzene/carboxen/polydimethylsiloxane coating; Supelco) was exposed to the container headspace for 30 min at room temperature without agitation, and then introduced into the injector inlet for 2 min at 250 °C in split-less mode. The compounds adsorbed on the fiber were then analyzed by GC/MS (GC: Agilent 6890, Agilent; MS: 5975C MS, Agilent). After fiber insertion, the column temperature was maintained at 40 °C for 2 min and then increased to 260 °C at 15 °C⋅min−1, followed by a final stage of 5 min at 260 °C. Compounds were identified by comparing mass spectra against synthetic standards and NIST 2.0 library matches. All of the synthetic odorants that were tested and confirmed were purchased from Sigma–Aldrich (https://www.sigmaaldrich.com/).
Behavioral Experiments in the Wind Tunnel.
To investigate the behavioral significance of M. sexta feces from caterpillars that had fed on N. attenuata, we performed 2 choice tests in a transparent wind tunnel (220 × 90 × 90 cm3) at 25 °C, 70% relative humidity, 0.3-lux illumination, and a wind speed of 40 cm⋅s−1. Two nonflowering N. attenuata plants of similar size were placed at the upwind end of the wind tunnel. An empty Petri dish (control) or a Petri dish loaded with 10 g of freshly collected feces (treatment) was placed at the base of the plant. A single fifth-instar larva produces about 10 g of feces per day. As described before (45), mated female moths were released at the downwind side of the wind tunnel and were allowed to oviposit on both plants for 3 min. Afterward, the number of eggs on both plants was counted, and the eggs were gently removed after each test. Moths were tested only once, and plants were exchanged after 2 tests. The positions of the treatment and control plants within the wind tunnel were swapped after every second moth. The oviposition indexes were calculated as (T−C)/(T+C), where T is the number of eggs on the treatment site and C is the number of eggs on the control site.
To test the effect of M. sexta feces from caterpillars that were raised on D. wrightii, we conducted a similar 2-choice test in the wind tunnel. Due to the large size of Datura plants, we trimmed plants 7 d before the experiments in such a way that 2 leaves of similar size remained in opposite directions. An empty Petri dish (control) or a Petri dish loaded with 10 g of freshly collected feces (treatment) was placed 10 cm beneath the leaves. Again, mated female moths were allowed to oviposit on both control and treatment leaves, and the resulting eggs and oviposition indexes were calculated afterward.
To determine the functional significance of different volatiles emitted by the feces, we conducted 2-choice tests in the wind tunnel. This time, 2 freshly detached leaves of similar size were presented to the gravid female. Each leaf was attached to the tip of one of 2 upright acrylic glass poles (40 cm high and placed at the upwind end of the wind tunnel with a distance of 40 cm between them). Beneath each leaf, we attached a square filter paper (2 × 2 cm2) loaded with 10 μL of diluted odorant (1:102) or the solvent mineral oil alone. Moths, leaves, and filter papers were tested only once. Experiments were conducted with leaves from both N. attenuata and D. wrightii.
CRISPR-Cas9–Based Genome Editing.
To determine which coreceptor is involved in the acid detection and acid-driven oviposition avoidance, we used olfactory receptor-coreceptor (Orco) mutant moths (33), and generated Ir8a and Ir25a, 2 mutant lines. The M. sexta genome v.1.0 (Mansexv1.0) fasta file and the GFF3 file were submitted to the CHOPCHOP (http://chopchop.cbu.uib.no) database for CRISPR-Cas9 target selection sites. The OGS2.0 gene names (i.e., Msex2.10447-RB, isoform 1; Msex2.02645-RA, isoform 1) were used to select the target site. The single guide RNA (sgRNA) and CRISPR-associated protein 9 (Cas9) were synthesized by Integrated DNA Technologies (https://www.idtdna.com/pages/products/crispr-genome-editing/alt-r-crispr-cas9-system). The microinjection and genotyping were carried out according to previously established procedures (33). After the mutant lines were established, mutations were reconfirmed by Sanger sequencing.
Electrophysiology.
To investigate the antennal responses to feces-emitted carboxylic acids, we performed EAG recordings. We therefore clipped the antenna of a 3-d-old female moth directly above the antenna basis and before the third last flagellum. Antenna preparation, stimuli delivery, and data acquisition and analysis were carried out according to previously established procedures (50). Odorants for EAG analyses were selected based on compounds identified in the headspace of caterpillar feces as well as structurally similar chemicals. Ten microliters of diluted odor (1:102) or solvent alone was pipetted onto circular filter paper (12-mm diameter) and placed into a glass pipette. In addition, we performed SSRs from coeloconic sensilla as described previously (42). Coeloconic sensilla were identified by their characteristic morphology. A total of 29 to 32 coeloconic sensilla were recorded in each genotype. Responses were quantified by counting all spikes recorded from an individual sensillum due to difficulties in reliably distinguishing spikes from individual neurons (25, 40). The response was calculated as the difference in spike number observed 0.5 s before and after the stimulus onset. A heat map was generated in Excel. Calcium imaging experiments were conducted as described previously (45). Chemical Abstracts Service (CAS) numbers for odorants are listed in SI Appendix, Table S1.
Statistics and Figure Preparation.
The sample size of behavioral experiments was determined based on a previous study (45). Data were analyzed and plotted using RStudio (version 1.1.414), R (version 3.4.2; The R Project for Statistical Computing), and GraphPad InStat 3 (https://www.graphpad.com/scientific-software/instat/), while figures were organized and prepared using Adobe Illustrator CS5. The Wilks–Shapiro test was used to determine the normality of each dataset. Normally distributed data were assessed using t tests. Nonnormally distributed data were analyzed using the Wilcoxon signed-rank test, with the null hypothesis that the median of sampled values differs from 0. For the boxplots, the whiskers were calculated as follows: the upper whisker equals the third quartile plus 1.5× the interquartile range (IQR), and the lower whisker equals the first quartile minus 1.5× the IQR. All data were included in the statistical analysis.
Supplementary Material
Acknowledgments
We thank the glasshouse team of the Max Planck Institute for Chemical Ecology for plant cultivation; Sascha Bucks for rearing M. sexta; David Heckel for providing Spodoptera littoralis; and Vajiheh Jafari, Dima Ward, Syed Ali, Komail Raza for help with the wind tunnel experiments. We also thank Kerstin Weniger for her technical support. This work was supported by the Max Planck Society (B.S.H.) and the Alexander von Humboldt Foundation (J.Z.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913485116/-/DCSupplemental.
References
- 1.Kessler A., Baldwin I. T., Defensive function of herbivore-induced plant volatile emissions in nature. Science 291, 2141–2144 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Weinhold A., Baldwin I. T., Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc. Natl. Acad. Sci. U.S.A. 108, 7855–7859 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loke W. H., Ashley T. R., Potential uses of kairomones for behavioral manipulation of Cotesia marginiventris (Cresson). J. Chem. Ecol. 10, 1377–1384 (1984). [DOI] [PubMed] [Google Scholar]
- 4.Kelber A., Ovipositing butterflies use a red receptor to see green. J. Exp. Biol. 202, 2619–2630 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Barreto O., et al. , Biological parameters of Argyrotaenia montezumae (Lepidoptera: Tortricidae) and influence of the oviposition substrate color on fecundity. Ann. Entomol. Soc. Am. 109, 671–677 (2016). [Google Scholar]
- 6.Joseph R. M., Devineni A. V., King I. F. G., Heberlein U., Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 106, 11352–11357 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y., Amrein H., Ionotropic receptors mediate Drosophila oviposition preference through sour gustatory receptor neurons. Curr. Biol. 27, 2741–2750.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroder S., Samietz J., Dorn S., Effect of ambient temperature on mechanosensory host location in two parasitic wasps of different climatic origin. Physiol. Entomol. 31, 299–305 (2006). [Google Scholar]
- 9.Dweck H. K. M., et al. , Olfactory preference for egg laying on citrus substrates in Drosophila. Curr. Biol. 23, 2472–2480 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Molnar B. P., Toth Z., Karpati Z., Synthetic blend of larval frass volatiles repel oviposition in the invasive box tree moth, Cydalima perspectalis. J. Pest Sci. 90, 873–885 (2017). [Google Scholar]
- 11.Hansson B. S., Stensmyr M. C., Evolution of insect olfaction. Neuron 72, 698–711 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Benton R., Vannice K. S., Gomez-Diaz C., Vosshall L. B., Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149–162 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butterwick J. A., et al. , Cryo-EM structure of the insect olfactory receptor Orco. Nature 560, 447–452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benton R., Sachse S., Michnick S. W., Vosshall L. B., Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4, e20 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vosshall L. B., Stocker R. F., Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 30, 505–533 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Koenig C., et al. , A reference gene set for chemosensory receptor genes of Manduca sexta. Insect Biochem. Mol. Biol. 66, 51–63 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Schultze A., Breer H., Krieger J., The blunt trichoid sensillum of female mosquitoes, Anopheles gambiae: Odorant binding protein and receptor types. Int. J. Biol. Sci. 10, 426–437 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo M., et al. , Variant ionotropic receptors are expressed in olfactory sensory neurons of coeloconic sensilla on the antenna of the desert locust (Schistocerca gregaria). Int. J. Biol. Sci. 10, 1–14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkness E. F., et al. , Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle. Proc. Natl. Acad. Sci. U.S.A. 107, 12168–12173 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith C. D., et al. , Draft genome of the globally widespread and invasive Argentine ant (Linepithema humile). Proc. Natl. Acad. Sci. U.S.A. 108, 5673–5678 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallem E. A., Carlson J. R., Coding of odors by a receptor repertoire. Cell 125, 143–160 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Stengl M., Funk N. W., The role of the coreceptor Orco in insect olfactory transduction. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 199, 897–909 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Croset V., et al. , Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 6, e1001064 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abuin L., et al. , Functional architecture of olfactory ionotropic glutamate receptors. Neuron 69, 44–60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silbering A. F., et al. , Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 31, 13357–13375 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ai M., et al. , Ionotropic glutamate receptors IR64a and IR8a form a functional odorant receptor complex in vivo in Drosophila. J. Neurosci. 33, 10741–10749 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raji J. I., et al. , Aedes aegypti mosquitoes detect acidic volatiles found in human odor using the IR8a pathway. Curr. Biol. 29, 1253–1262.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussain A., et al. , Ionotropic chemosensory receptors mediate the taste and smell of polyamines. PLoS Biol. 14, e1002454 (2016). Correction in: PLoS Biol. 14, e1002505 (2016). Correction in: PloS Biol. 16, e1002624 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni L., et al. , The ionotropic receptors IR21a and IR25a mediate cool sensing in Drosophila. eLife 5, e13254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enjin A., et al. , Humidity sensing in Drosophila. Curr. Biol. 26, 1352–1358 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaeger A. H., et al. , A complex peripheral code for salt taste in Drosophila. eLife 7, e37167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haverkamp A., Hansson B. S., Knaden M., Combinatorial codes and labeled lines: How insects use olfactory cues to find and judge food, mates, and oviposition sites in complex environments. Front. Physiol. 9, 49 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fandino R. A., et al. , Mutagenesis of odorant coreceptor Orco fully disrupts foraging but not oviposition behaviors in the hawkmoth Manduca sexta. Proc. Natl. Acad. Sci. U.S.A. 116, 15677–15685 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson J. K., Woods H. A., Protection via parasitism: Datura odors attract parasitoid flies, which inhibit Manduca larvae from feeding and growing but may not help plants. Oecologia 179, 1159–1171 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Grégoire J.-C., et al. , Volatile compounds in the larval frass of Dendroctonus valens and Dendroctonus micans (Coleoptera: Scolytidae) in relation to oviposition by the predator, Rhizophagus grandis (Coleoptera: Rhizophagidae). J. Chem. Ecol. 17, 2003–2019 (1991). [DOI] [PubMed] [Google Scholar]
- 36.Kumari A., Kaushik N., Oviposition deterrents in herbivorous insects and their potential use in integrated pest management. Indian J. Exp. Biol. 54, 163–174 (2016). [PubMed] [Google Scholar]
- 37.Späthe A., et al. , Plant species- and status-specific odorant blends guide oviposition choice in the moth Manduca sexta. Chem. Senses 38, 147–159 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Reisenman C. E., et al. , Species-specific effects of herbivory on the oviposition behavior of the moth Manduca sexta. J. Chem. Ecol. 39, 76–89 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Couto A., Alenius M., Dickson B. J., Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 15, 1535–1547 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Yao C. A., Ignell R., Carlson J. R., Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J. Neurosci. 25, 8359–8367 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shields V. D. C., Hildebrand J. G., Recent advances in insect olfaction, specifically regarding the morphology and sensory physiology of antennal sensilla of the female sphinx moth Manduca sexta. Microsc. Res. Tech. 55, 307–329 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghaninia M., Olsson S. B., Hansson B. S., Physiological organization and topographic mapping of the antennal olfactory sensory neurons in female hawkmoths, Manduca sexta. Chem. Senses 39, 655–671 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Pophof B., Olfactory responses recorded from sensilla coeloconica of the silkmoth Bombyx mori. Physiol. Entomol. 22, 239–248 (1997). [Google Scholar]
- 44.Delventhal R., Carlson J. R., Bitter taste receptors confer diverse functions to neurons. eLife 5, e11181 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bisch-Knaden S., Dahake A., Sachse S., Knaden M., Hansson B. S., Spatial representation of feeding and oviposition odors in the brain of a hawkmoth. Cell Rep. 22, 2482–2492 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Li G., Ishikawa Y., Oviposition deterrents in larval frass of four Ostrinia species fed on an artificial diet. J. Chem. Ecol. 30, 1445–1456 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Xu H., Li G., Liu M., Xing G., Oviposition deterrents in larval frass of the cotton boll worm, Helicoverpa armigera (Lepidoptera: Noctuidae): Chemical identification and electroantennography analysis. J. Insect Physiol. 52, 320–326 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Horikoshi M., et al. , Cotesia glomerata female wasps use fatty acids from plant–herbivore complex in host searching. J. Chem. Ecol. 23, 1505–1515 (1997). [Google Scholar]
- 49.García-Robles I., et al. , Combining hexanoic acid plant priming with Bacillus thuringiensis insecticidal activity against Colorado potato beetle. Int. J. Mol. Sci. 14, 12138–12156 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haverkamp A., Bing J., Badeke E., Hansson B. S., Knaden M., Innate olfactory preferences for flowers matching proboscis length ensure optimal energy gain in a hawkmoth. Nat. Commun. 7, 11644 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.