Significance
Electronic-cigarette smoke (ECS) is designed to deliver nicotine, and its use is gaining popularity. Previously, we found that ECS induces DNA damage and inhibits DNA repair in the mouse lungs and bladder urothelium. Nicotine induces the same types of DNA adducts and has a similar effect on DNA repair inhibition in human cells. Nicotine also enhances human cells’ mutation and tumorigenic transformation susceptibility. Our current results show that ECS-exposed mice developed lung adenocarcinoma and bladder urothelial hyperplasia, indicating that ECS is a lung carcinogen and a potential bladder carcinogen in mice. While it is well established that tobacco smoke poses a huge threat to human health, the threat ECS poses to humans is not yet known and warrants in-depth investigation.
Keywords: electronic-cigarette, lung cancer, bladder hyperplasia, DNA damage, DNA repair
Abstract
Electronic-cigarettes (E-cigs) are marketed as a safe alternative to tobacco to deliver the stimulant nicotine, and their use is gaining in popularity, particularly among the younger population. We recently showed that mice exposed to short-term (12 wk) E-cig smoke (ECS) sustained extensive DNA damage in lungs, heart, and bladder mucosa and diminished DNA repair in lungs. Nicotine and its nitrosation product, nicotine-derived nitrosamine ketone, cause the same deleterious effects in human lung epithelial and bladder urothelial cells. These findings raise the possibility that ECS is a lung and bladder carcinogen in addition to nicotine. Given the fact that E-cig use has become popular in the past decade, epidemiological data on the relationship between ECS and human cancer may not be known for a decade to come. In this study, the carcinogenicity of ECS was tested in mice. We found that mice exposed to ECS for 54 wk developed lung adenocarcinomas (9 of 40 mice, 22.5%) and bladder urothelial hyperplasia (23 of 40 mice, 57.5%). These lesions were extremely rare in mice exposed to vehicle control or filtered air. Current observations that ECS induces lung adenocarcinomas and bladder urothelial hyperplasia, combined with our previous findings that ECS induces DNA damage in the lungs and bladder and inhibits DNA repair in lung tissues, implicate ECS as a lung and potential bladder carcinogen in mice. While it is well established that tobacco smoke poses a huge threat to human health, whether ECS poses any threat to humans is not yet known and warrants careful investigation.
Tobacco smoke (TS) is a traditional way of delivering nicotine, which is a powerful central nervous system stimulant that provides smokers with instantaneous gratification and leads to long-term tolerance and addiction (1). Unfortunately, in addition to nicotine, TS contains numerous carcinogens generated during tobacco curing and burning (2, 3). For the past few decades, TS has been the leading cause of human cancers (4). In fact, up to 85% of lung cancers and 50% of bladder cancers can be linked to TS (4). Electronic-cigarettes (E-cigs) are an invention designed to deliver nicotine in aerosols via the controlled heating of an organic solution containing nicotine (5). This process avoids tobacco leaves and burning, and generates only aerosols composed of nicotine and the relatively harmless solvents isopropylene glycol and vegetable glycerin. Because of this, E-cigs are promoted as delivering a TS “high” without the known adverse effects, and E-cigs have been adopted as a safe replacement for conventional cigarettes. E-cigs are widely used as a gateway for TS cessation and have even been applied as a therapeutic alternative for reducing TS-related respiratory diseases (5, 6).
It is well established that during the curing and burning of tobacco, nicotine can be transformed into nitrosamines via nitrosation, and that many of these nitrosamines, such as nicotine-derived nitrosamine ketone (NNK) and nitrosonornicotine (NNN), are potent human and animal carcinogens (2, 3, 7). Hence, measuring nitrosamine levels in body fluids has become a gold standard for assessing the potential carcinogenic effect of TS (7, 8). This method has been adapted to address the potential carcinogenic effects of E-cig smoke (ECS) (9). It has been found that the level of 4-(methylnitrosoamino)-4-(3-pyidyl)-1-butanol (NNAL), an NNK derivative, in the urine and saliva of E-cig smokers is only 5% of the levels found in comparable tobacco smokers (9). This has led to the assumption that nicotine nitrosation does not take place in ECS and that only a minute quantity of nitrosamines is present in ECS (9). This finding has supported the recommendation from public health experts, including Public Health England, that E-cigs are 95% safer than conventional cigarettes (10), and has prompted many epidemiologists to speculate that switching from TS to ECS could save millions of lives (11).
Likely as a result of this reasoning, the popularity of E-cig smoking is rising rapidly. Currently 3.2% of adults in the United States and 3.6 million junior-high and high-school students have embraced E-cig smoking (10). Given the widespread use of E-cigs, their health effects—particularly their carcinogenicity—deserve careful scrutiny (10). Assessing the safety of E-cigs must examine 3 critical issues. First, is the level of nitrosamines in the E-cig smokers’ urine, saliva, or blood representative of the carcinogenic effects of ECS? Second, while it is established that TS contains substantial amounts of nitrosamines from nicotine nitrosation during tobacco curing and burning, it is unknown if inhaled nicotine in ECS can be nitrosated and transformed into nitrosamines. In light of the findings that human cells have ample cytochrome p450 enzymes that are able to metabolize nitrosamines rapidly into DNA-damaging products (7, 8), we are confronted with the third and the most important question: Can nitrosamine level in the urine, saliva, and blood represent the extent of nitrosation of inhaled ECS nicotine in vivo?
These questions led us to assess the effects of ECS and nicotine by determining the DNA damage induced by ECS in different organs rather than measuring NNK, NNN, and NNAL in the blood and urine of a mouse model (12). We previously observed that ECS induces mutagenic DNA adducts (cyclic 1,N2-γ-hydroxy-propano-deoxyguanosine [γ-OH-PdG] and O6-methyl-dG) in the lungs, heart, and bladder mucosa and inhibits DNA repair in the lungs in a mouse model (12). We also found that nicotine and NNK both induce the same DNA adducts, impair DNA repair functions, and enhance cell mutational and tumorigenic transformation susceptibility in human lung and bladder epithelial cells (12). Based on these observations, we propose that ECS, as well as nicotine, may induce lung and bladder cancer (12). In this study, we examined the tumorigenicity of ECS in mice.
Methods
ECS Exposure.
A total of 85 male FVB/N mice (6 to 8 wk old; The Jackson Laboratory) were randomly placed into 3 groups. One group (n = 45) was exposed to ECS generated from e-juice (nicotine [36 mg/mL] dissolved in vehicle [Veh; isopolypropylene glycol and vegetable glycerin at a 1:1 ratio]). We maintained the particulate matter concentration in the chamber at 130 mg/m3 and the aerosol nicotine concentration at 0.196 mg/m3 (SI Appendix, Table S1). The second group (n = 20) was exposed to Veh. Aerosols for both groups were generated using an automated 3-port E-cig aerosol generator (e∼Aerosols) set at a constant voltage (1.9 A, 4.0 V) (SI Appendix, Table S1), the same as is done in commercial E-cigs (12, 13). Mice were subjected to whole-body exposure. The exposure conditions were the same as previously described (12). Mice were exposed for 4 h per day and 5 d per week for 54 wk. The third group (n = 20) remained housed in the animal room, exposed to the ambient filtered air (FA). During the 54-wk period, 3 ECS mice were found dead and 2 ECS mice had to be killed because of inactiveness. No lung tumor was observed in these 5 mice, and 1 was found to have a large intestinal polyp. One Veh-exposed mouse was found dead, and 1 was killed due to a paralyzed leg. Two FA-exposed mice were also found dead. No lung tumor was observed in these 2 Veh and 2 FA mice. At the end of the 54-wk exposure, 40 ECS-exposed, 18 Veh-exposed, and 18 FA-exposed mice survived. The average body weights among these 3 groups were similar (FA group, 34.4 ± 5.84 g; Veh group, 34.0 ± 2.78 g; and ECS group, 35.1 ± 2.99 g; ECS vs. FA, P = 0.67; ECS vs. Veh, P = 0.1998), and all mice appeared healthy. These mice were killed to examine tumor formation in different organs.
Histopathology.
The mice were killed at the end of 54 wk of exposure in accordance with New York University Institutional Animal Care and Use Committee protocols IA17-00048 and 170313-01. The lungs, heart, liver, kidneys, intestine, pancreas, brain, spleen, and bladder were harvested and examined with the naked eye for tumor formation. All organs were immediately fixed in and stored in a 10% formalin solution until section preparation. Slides of lung and bladder samples were prepared and stained with hematoxylin and eosin (H & E) at the Histology Core, New York University Langone Medical Center. In addition to H & E staining, bladder tissue slides were stained with antibodies for the proliferation markers MCM-2 and PCNA and the basal cell marker KRT5 at the New York University Urology Histology Core. All slides were examined independently by 3 pathologists.
Statistical Analysis.
GraphPad Prism 7.0 and 1-way ANOVA with the least significant difference (LSD) post hoc test were used for statistical analysis of lung adenocarcinoma and bladder urothelium hyperplasia formation, respectively, in the 3 groups (ECS, Veh, and FA) of mice.
Results
ECS Induces Lung Adenocarcinoma.
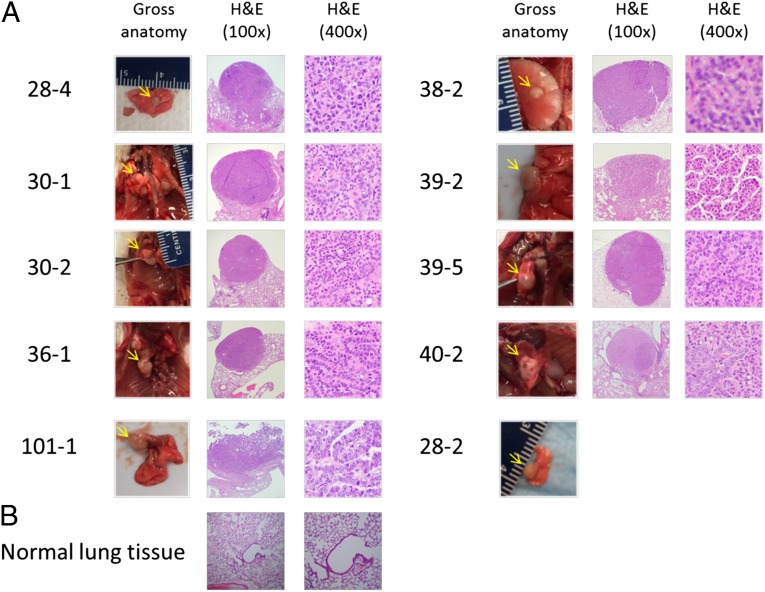
Because it takes over 2 decades for tobacco smokers to develop lung and bladder cancer, and because TS is also related to other human cancers, we examined the tumor formation in different organs after 54 wk of exposure (4, 14, 15). An examination of the gross anatomy of the mice revealed tumor-like growth in the skin, abdominal cavity, intestines, and lungs. A summary of tumor formation observed in all experimental mice is presented in Table 1. These tumor-like tissues were further examined microscopically. The results show that 9 of 40 (22.5%) mice exposed to ECS developed lung tumors. All lung tumors, subjected to histological examination by 3 pathologists, were identified as adenocarcinomas (Fig. 1). Of these 9 lung tumor-bearing mice, 8 had a single lung adenocarcinoma and 1 formed multiple ipsilateral lung adenocarcinomas (Fig. 1). None of the mice exposed to Veh developed lung tumors. Only 1 of 18 (5.6%) mice exposed to FA had 1 adenocarcinoma formed in the lung. The statistical analyses of lung adenocarcinoma occurrence in ECS-, Veh-, and FA-exposed mice are presented in Tables 2 and 3 and SI Appendix, Table S2 A–E. The results show that the higher lung adenocarcinoma incidence in ECS-exposed versus Veh-exposed mice (P = 0.0454), versus the combination of Veh- and FA-exposed mice (P = 0.0154), and versus Veh- and FA-exposed mice (P = 0.0352) is statistically significant.
Table 1.
Tumor-like growth found in different organs of mice exposed to FA, Veh, and ECS*
| No. of mice with tumor | |||
| Treatment (n = total mice receiving treatment) | |||
| Organs | FA (n = 18) | Veh (n = 18) | ECS (n = 40) |
| Lung† | 1 | 0 | 9‡ |
| Bladder§ | 0 | 0 | 0 |
| Intestine¶ | 1 | 1 | 1 |
| Abdominal Cavity# | 0 | 1 | 2 |
| Skin‖ | 0 | 1 | 1 |
| Liver, heart, brain, spleen, and kidney | 0 | 0 | 0 |
Exposure conditions are described in the main text.
A single lung adenocarcinoma tumor was found in 8 ECS-exposed mice and 1 FA-exposed mouse. Multiple lung adenocarcinomas were found in 1 ECS-exposed mouse (Fig. 1).
Eight of 9 lung tumors were further identified by H & E staining and examined microscopically (Fig. 1). One lung tumor was inadvertently misplaced during the sample preparation.
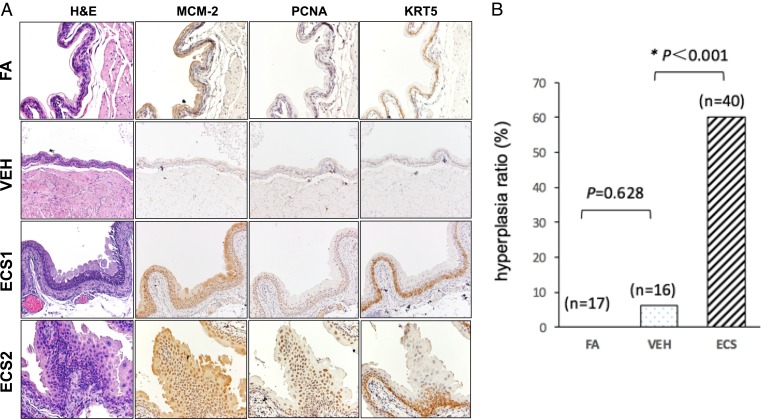
Hyperplasia was found in Veh-exposed (1 of 16) and ECS-exposed (23 of 40) mice. H & E and immunohistological staining results are presented in Fig. 2.
Adenomatous polyps with high-grade dysplasia were found in 1 FA-exposed mouse, 1 Veh-exposed mouse, and 1 ECS-exposed mouse.
A single cystic salivary benign tumor was found in 1 Veh-exposed mouse and 1 ECS-exposed mouse. One benign epidermal inclusion cyst was found in 1 ECS-exposed mouse.
The tumor-like growth in the skin of the Veh-exposed mouse was necrotic tissue and cannot be further characterized; in the ECS-exposed mouse, it was identified as skin with muscle and a small piece of bone and was negative for tumor.
Fig. 1.
ECS exposure induces lung tumor formation in mice. Mice were exposed to FA (n = 20) and aerosols generated by Veh (isopropylene glycol and vegetable glycerin at a 1:1 ratio, n = 20) and ECS (36 mg/mL nicotine in Veh, n = 45) for 4 h per day and 5 d per week for 54 wk as described in the main text. Surviving mice at the end of exposure are as follows: FA-exposed (n = 18), Veh-exposed (n = 18), and ECS-exposed (n = 40). All mice dying before the 54-wk exposure time were lung tumor-free. (A) Lung tumor tissues. Gross anatomy photographs (Left) of ECS-induced lung adenocarcinoma tissues (28-2, 28-4, 30-1, 30-2, 36-1, 38-2, 39-2, 39-5, 40-2) and a lung adenocarcinoma from an FA-exposed mouse (101-1), and histological slides of H & E staining of these lung adenocarcinomas (Center and Right, 100× and 400× magnification, respectively) are presented. (B) Normal lung tissue (Left, 100× magnification; Right, 400× magnification). Notes: (1) Veh exposure does not induce lung tumor. (2) Only a gross anatomy photograph of the lung tumor of ECS-exposed mouse 28-2 is shown.
Table 2.
Lung adenocarcinoma incidence in ECS-, Veh-, and FA-exposed mice
| Exposure | Mice with tumor* | Mice without tumor* | No. of dead mice before final killing† | Total | Tumor incidence, % |
| FA | 1 | 17 | 2 | 20 | 5.6 |
| Veh | 0 | 18 | 2 | 20 | 0 |
| ECS | 9 | 31 | 5 | 45 | 22.5 |
Surviving mice with tumor or tumor-free up to 54 wk.
All dead mice were lung tumor-free.
Table 3.
Statistical analysis of lung adenocarcinoma incidence in mice exposed to ECS, Veh, and FA*
| Comparison | P value† | Relative risk‡ | 95% CI§ |
| ECS vs. FA | 0.1498 | 4.05 | 0.7733 to 24.14 |
| ECS vs. Veh | 0.0454 | Infinity | 1.22 to infinity |
| ECS vs. (FA + Veh) | 0.0154 | 8.1 | 1.445 to 48.43 |
| ECS vs. FA vs. Veh | 0.0352 | Unpredictable | Unpredictable |
P value, relative risk, and 95% confidence interval (CI) were calculated according to the factors of “mice with tumor” and “mice without tumor” using GraphPad Prism 7.0 software.
Statistic considerations and calculations are presented in SI Appendix, Table S2 A–E.
If we count 8 mice with lung adenocarcinoma (by eliminating the 1 mouse [28-2] that had a lung tumor; however, the tumor was not examined microscopically) for further statistic consideration, then the P values are as follows: ECS vs. FA: 0.2467, ECS vs. Veh: 0.0463, ECS vs. (FA + Veh): 0.0295, and ECS vs. FA vs. Veh: 0.054.
ECS Induces Bladder Urothelial Hyperplasia.
Although no visible tumors were detected in the urinary bladders of any of the experimental groups, hyperplastic changes to the bladder urothelium were evident in mice exposed to ECS upon histological examination (Fig. 2). These lesions were either simple or nodular hyperplasia, characterized by a significant increase of urothelial layers (5 to 8 layers compared with 3 layers in the control groups), expansion of Krt5-positive basal urothelial cells, and a distinct elevation of the cell proliferation markers MCM-2 and PCNA (16, 17). Overall, 23 of 40 (57.5%) ECS-exposed mice, 1 of 16 (6.3%) Veh-exposed mice, and none of 17 (0%) FA-exposed mice developed urothelial hyperplasia (P < 0.001) (Fig. 2). Notably, the frequency of urothelial hyperplasia is slightly higher in mice with lung tumors (6 of 9, 67%) than in mice without lung tumors (18 of 31, 58%), although the difference is not statistically significant (P = 0.64).
Fig. 2.
ECS exposure induces bladder urothelial hyperplasia in mice. Bladder tissues were harvested from the same mice exposed to ECS, Veh, and FA for 54 wk as described in Fig. 1. The tissue slides were prepared for histology examination and stained by H & E or antibodies for proliferation markers MCM-2 and PCNA and basal cell marker KRT5 (200× magnification). (A) Typical staining result of bladder tissues of mice exposed to FA, Veh, and ECS. (B) Histogram presentation of bladder urothelial hyperplasia in mice exposed to FA (n = 17), Veh (n = 16), and ECS (n = 40). Notes: (1) While we were able to examine bladder tissue samples from all 40 ECS-exposed mice, during sample preparation, 1 bladder from FA-exposed mice and 2 from Veh-exposed mice were inadvertently destroyed. (2) The simple (ECS1 mouse) and nodular (ECS2 mouse) hyperplasia had markedly thickened urothelial layers and strong expression of MCM-2, PCNA, and KRT5 (with the latter indicating expansion of basal cells), compared with FA- and VEH-exposed mice, which had very thin urothelial layers with low expression of the proliferation markers.
Discussion
Nicotine carcinogenicity in animal models has been controversial owing to a large number of conflicting results (18–20). While different tumor types, including leiomyosarcoma, were observed in animals treated with nicotine via drinking water and subcutaneous injection (19, 20), many of these results were criticized for their experimental shortcomings and were deemed to be inadequate evidence for an association between nicotine exposure and its effect on carcinogenesis (19). On the other hand, rats exposed to stream air-vaporized nicotine via inhalation for 2 y showed no significant different tumor formation, including lung tumors (21). However, this particular study was also criticized for lacking necessary bioassays and the small number of experimental animals (22 exposed versus 6 control) (19). Despite of all these inconclusive results, the prevailing thinking remains that nicotine is noncarcinogenic (18). In contrast to the results showing that stream air-vaporized nicotine is not lung carcinogenic in rats (21), our results showed that E-cig nicotine induces lung adenocarcinoma in mice. The sources of this discrepancy are unclear. It has been found that the aerosol size of ECS is smaller than the aerosols generated in TS (22). It is likely that the small size of E-cig aerosol allows the ECS nicotine in it to penetrate deeply into lung tissues, inducing DNA damage in bronchioloalveolar cells, whereas the stream air vapors are mainly deposited in the upper aerodigestive linings and tissues, which are rich in antioxidants such as glutathione, glutathione peroxidase, and superoxide dismutase and can effectively neutralize the metabolites of nitrosamines.
We believe that our results support the conclusion that γ-OH-PdG and O6-methyl-dG, the DNA damage induced by metabolites of nicotine nitrosation products, are likely the major causes for lung as well as bladder carcinogenesis in mice (12, 23, 24). Although no bladder cancers/urothelial carcinomas have been observed, flat and/or papillary urothelial hyperplasia with increased mitotic activity was observed in some of the ECS-exposed mice (SI Appendix, Fig. S1). It should be noted that we found the levels of ECS-induced γ-OH-PdG and O6-methyl-dG in bladder mucosa were only one-fourth and one-fifth of the amount found in the lung tissues, respectively, in mice (12). These results raise the possibility that a longer exposure and/or higher doses of ECS are needed in order for the bladder mucosa to accumulate a sufficient level of γ-OH-PdG– and O6-methyl-dG–induced mutations that could trigger bladder tumorigenesis compared with lung carcinogenesis. We previously observed that mice with increased susceptibility to ECS-induced DNA adduct formation in the lungs are also more susceptible to ECS-induced DNA damage in the bladder (12). In the present study, mice more susceptible to ECS-induced lung tumorigenesis were not more prone to developing urothelial hyperplasia, suggesting that ECS-induced lung tumorigenesis and urothelial hyperplasia are divergent events.
In summary, we showed that ECS exposure of mice induces lung cancer and bladder urothelial hyperplasia. These observations, combined with our previous findings (12) that ECS induces γ-OH-PdG and O6-methyl-dG adducts in the lungs and bladder urothelium and inhibits DNA repair in lung tissues in mice, and that nicotine and NNK induce the same types of DNA adducts and DNA repair inhibition effect and sensitize mutational and tumorigenic cell transformation susceptibility in the human lung epithelial and urothelial cells, indicate that ECS, as well as nicotine and NNK, is a lung carcinogen and a potential bladder carcinogen in mice. It should be noted that TS is a most dangerous environmental agent to which humans are commonly exposed and that ECS may or may not pose any danger to humans. The public should not equate the risk of ECS with that of TS. Our data simply suggest, on the basis of experimental data in model systems, that this issue warrants in-depth study in the future.
Supplementary Material
Acknowledgments
We thank K. Galdane, E. Halzack, A. Chu, M.-w. Weng, and S. H. Park for technical assistance, and Drs. J. Goldberg, J.-S. Hwang, and M.-w. Weng for statistical analysis. Research was supported by NIH Grants, RO1190678, 1PO1CA165980, P30CA16087, and ES00260.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1911321116/-/DCSupplemental.
References
- 1.Benowitz N. L., Nicotine addiction. N. Engl. J. Med. 362, 2295–2303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hecht S. S., Hoffmann D., Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis 9, 875–884 (1988). [DOI] [PubMed] [Google Scholar]
- 3.Hecht S. S., Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 91, 1194–1210 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Howlader N., et al., Eds., SEER Cancer Statistics Review, 1975-2014 (National Cancer Institute, Bethesda, MD, 2016). https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER website, April 2017. Accessed 2 April 2018.
- 5.Grana R., Benowitz N., Glantz S. A., E-cigarettes: A scientific review. Circulation 129, 1972–1986 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajek P., et al. , A randomized trial of E-cigarettes versus nicotine-replacement therapy. N. Engl. J. Med. 380, 629–637 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Hecht S. S., DNA adduct formation from tobacco-specific N-nitrosamines. Mutat. Res. 424, 127–142 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Hecht S. S., Carmella S. G., Foiles P. G., Murphy S. E., Peterson L. A., Tobacco-specific nitrosamine adducts: Studies in laboratory animals and humans. Environ. Health Perspect. 99, 57–63 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahab L., et al. , Nicotine, carcinogen, and toxin exposure in long-term E-cigarette and nicotine replacement therapy users: A cross-sectional study. Ann. Intern. Med. 166, 390–400 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glantz S. A., Bareham D. W., E-cigarettes: Use, effects on smoking, risks, and policy implications. Annu. Rev. Public Health 39, 215–235 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy D. T., et al. , Potential deaths averted in USA by replacing cigarettes with e-cigarettes. Tob. Control 27, 18–25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H. W., et al. , E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc. Natl. Acad. Sci. U.S.A. 115, E1560–E1569 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J., Pyrgiotakis G., Demokritou P., Development and characterization of electronic-cigarette exposure generation system (Ecig-EGS) for the physico-chemical and toxicological assessment of electronic cigarette emissions. Inhal. Toxicol. 28, 658–669 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services , The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General (US Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA, 2006). [Google Scholar]
- 15.US Department of Health and Human Services , The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General (Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA, 2014). [Google Scholar]
- 16.Van Batavia J., et al. , Bladder cancers arise from distinct urothelial sub-populations. Nat. Cell Biol. 16, 982–991, 1–5 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Saeb-Parsy K., et al. , Diagnosis of bladder cancer by immunocytochemical detection of minichromosome maintenance protein-2 in cells retrieved from urine. Br. J. Cancer 107, 1384–1391 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haussmann H. J., Fariss M. W., Comprehensive review of epidemiological and animal studies on the potential carcinogenic effects of nicotine per se. Crit. Rev. Toxicol. 46, 701–734 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanner T., Grimsrud T. K., Nicotine: Carcinogenicity and effects on response to cancer treatment–A review. Front. Oncol. 5, 196 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grando S. A., Connections of nicotine to cancer. Nat. Rev. Cancer 14, 419–429 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Waldum H. L., et al. , Long-term effects of inhaled nicotine. Life Sci. 58, 1339–1346 (1996). [DOI] [PubMed] [Google Scholar]
- 22.Floyd E. L., Queimado L., Wang J., Regens J. L., Johnson D. L., Electronic cigarette power affects count concentration and particle size distribution of vaping aerosol. PLoS One 13, e0210147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H. W., et al. , Cigarette side-stream smoke lung and bladder carcinogenesis: Inducing mutagenic acrolein-DNA adducts, inhibiting DNA repair and enhancing anchorage-independent-growth cell transformation. Oncotarget 6, 33226–33236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weng M. W., et al. , Aldehydes are the predominant forces inducing DNA damage and inhibiting DNA repair in tobacco smoke carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 115, E6152–E6161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.