Although stoichiometry is a chemistry term derived from the Greek words stoikhein (element) and metron (measure), the phrase underlies all biological processes. Cell biology depends upon protein–protein interactions, which are defined by stoichiometry and binding affinities. The recent article by Marchetti et al. (1) revisits the stoichiometry of the p75 neurotrophin receptor, which has been the subject of considerable interest and debate. For more than 30 y, this topic generated multiple models and controversies (2–5). Why has the p75 receptor drawn so much attention?
The p75 receptor is highly relevant to the neurotrophic theory which predicted programmed neuronal cell death occurs during development due to a competition for low concentrations of trophic factors such as nerve growth factor (NGF) (6). The ability of p75 to induce cell death or survival, and to participate in high-affinity ligand binding, is consistent with its ability to determine pruning and connectivity between neurons and their targets (7). In addition, p75 has been implicated in neurodegenerative disorders such as Alzheimer’s disease (8).
As the first identified member of the tumor necrosis factor (TNF) receptor superfamily, p75 shares many common features of extracellular cysteine-rich repeats of 40 amino acids and a death domain at the C terminus. These motifs are also found in the Fas, TNFR1, and death receptors, DR3–6 (9). When discovered as an NGF receptor, skepticism arose about the identification of p75, due to the lack of a catalytic domain and a smaller than expected size. The identification of the TrkA tyrosine kinase receptor as another NGF receptor seemed to reconcile this discrepancy, but did not completely resolve the question of stoichiometry or high-affinity binding. The association of p75 with its ligands has been enigmatic, since a receptor monomer, dimer, and trimer have been reported.
The ratio of NGF binding to p75 was apparently solved even before the receptor was identified. From its beginnings, NGF was known to behave as a noncovalent dimer in a complex with accessory proteins (10). Moreover, a number of cross-linking experiments with 125I-NGF in PC12 and melanoma cells seemingly gave rise to higher-weight molecular products that suggested a dimer of p75 is complexed to a dimer of NGF (11, 12). Chemical cross-linking is often used to define ligand–receptor interactions. But it is an approach that is relatively inefficient and gives a variety of cross-linked products, making a definitive assignment problematic.
The dimerization model was solidified by a series of studies showing p75 forms a covalent dimer through a specific disulfide linkage in its transmembrane domain (13, 14). The critical cysteine was mapped to Cys257 in the middle of the bilayer. The p75 neurotrophin receptor participates in a wide range of processes, ranging from apoptosis to changes in synaptic plasticity and neuronal morphology. NGF, BDNF, NT-3, NT-4, and their precursors, proneurotrophins, are all ligands for p75 (4). Interestingly, dimerization occurred in the absence of ligand. Many cell lines, p75-transfected cells, and sympathetic neurons verified the disulfide linkage via the Cys257 residue was responsible for dimerization.
The p75 dimer configuration was also responsible for signaling by p75 through JNK and NFκB activities. This was accompanied by changes in the conformation of p75 from fluorescence resonance energy transfer (FRET) anisotrophy measurements (14). Significantly, mutation to Cys257Ala abolished dimer formation and negated several signaling modules, including caspase-3 and NF-κB activities and interactions with adaptor proteins, such as TRAF6, a NF-κB regulator. These signals are ultimately responsible for mediating neuronal cell death. As support, a knockout mouse lacking the critical Cys residue or the death domain showed greater cell death in the cortex and hippocampus (15).
These in vitro and in vivo studies were confirmed by other independent determinations (16, 17). Expression of the p75 ectodomain in baculovirus allowed for the use of ultracentrifugation sedimentation, X-ray scattering, and mass spectrometry to detect dimers of NGF with p75 (17). The structure of NT-3, a close relative of NGF, bound to the extracellular domain of p75 revealed a 2:2 ratio (18). In the same study, a 2:2 stoichiometry was also observed between p75 and NGF.
Contrary to these results, the recent PNAS paper by Marchetti et al. (1) reports detection of p75 monomers as the predominant form over dimers. Using cell biological approaches of sensitive single-molecule tracking measurements in neuroblastoma cells and primary neurons, an inducible p75 construct containing a peptide tag was followed under different pharmacological conditions. For this study, Marchetti et al. (1) used total internal reflection fluorescence (TIRF) microscopy, FRET, and labeling with Q dots to investigate the outcomes of p75 in these cell types (1). The measurements detected the existence of a p75 monomer by TIRF imaging at high levels (77%), but did not depend upon the availability of Cys257 or the presence of NGF ligands. Under these conditions, a dimer of p75 could be detected at lower levels. Functional consequences on growth cone collapse and apoptosis were tracked by following the monomeric species of p75. Of significance, membrane diffusion of p75 monomers into cholesterol-rich domains favored an apoptotic outcome.
These findings recall a previous X-ray crystallographic study that captured a complex of a p75 monomer with a homodimer of NGF (19). In addition to the atomic details, the 2:1 NGF–p75 extracellular domain complex was verified by size exclusion chromatography, light scattering, and calorimetry. Formation of this complex causes an unexpected conformational change in the structure of NGF that excluded the dimerization of a second p75 molecule (19). The resulting asymmetric arrangement provided a mechanism to account for this unusual structure. A follow-up study found that NGF binds to TrkA in a 2:2 stoichiometry, using different nonoverlapping sites on the NGF molecule (20).
Given these studies indicating the potential for dimers and monomers of p75 receptors, a wrinkle in this picture is the presence of p75 oligomers that form trimers. The rationale is from TNF receptor members which predominantly bind their ligands and adaptor proteins as trimers (21, 22). Using stringent nonreducing conditions and high-resolution gel electrophoresis of lysates from PC12 cells, murine brain, and sensory neurons gave rise to monomers and trimers, which were the most abundant species (23). Formation of the p75 trimer appeared to require the key transmembrane cysteine, but did not affect growth cone collapse induced by the proNGF ligand. The presence of a p75 trimer is very curious, as older studies of β-amyloid peptide and the rabies virus glycoprotein postulated that p75 bound to these ligands as a trimer (24, 25).
The recent article by Marchetti et al. revisits the stoichiometry of the p75 neurotrophin receptor, which has been the subject of considerable interest and debate.
How can these divergent results be reconciled? Cell type specificity is marked by its lipid composition, glycosylation profile, trafficking, and attendant adaptor and chaperone proteins. In addition, p75 associates with many cell surface proteins—Nogo receptor, LINGO, and leucine-rich repeat proteins, along with Trk receptors and their interactors. These contacts likely affect receptor stoichiometry, conformation, and signaling. Conformation changes can alter the consequences of p75 receptor localization (14) and ligand binding (19). A lesson can be learned from different ligands of the EGF receptor, which give rise to asymmetric alterations in receptor conformation resulting in weak or strong kinetics of signaling (26). In fact, an X-ray crystal analysis of proNGF ligand plus p75 gave a 2:2 stoichiometry, suggesting alternative complexes can exist between similar molecules (27).
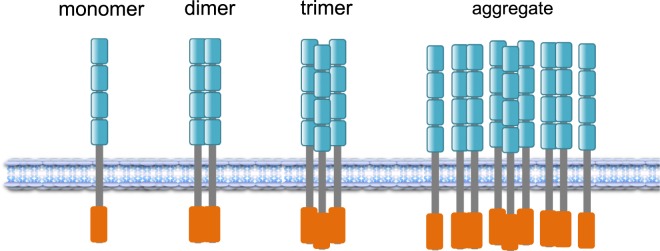
Another variable is the level of expression. In particular, p75 is abundantly up-regulated in many cell types after injury and disease (28). As such, it behaves like a cytokine or a TNF family member, which are often triggered by inflammation or damage (28). Biochemical studies showed that p75 and TrkA receptors are in very high-molecular-weight complexes after overexpression (29). Size exclusion chromatography detected complexes ranging from 300 to 800 kDa. Hence any model depicting the stoichiometry of p75 has to consider not only monomer, dimer, and trimers, but also perhaps higher-order aggregates, especially in cases after neural injury (Fig. 1).
Fig. 1.
Models of p75 neurotrophin receptor configuration. Four cysteine-rich domains represent the ligand-binding site (in cyan). A death domain sequence is located in the C terminus (in red). In addition to monomers, dimers, and trimers, large oligomeric complexes containing p75 have been observed (29). Image courtesy of Khalil Saadipour (New York University Langone Medical Center, New York, NY).
In addition to these observations, p75 is a focal point to address many cellular questions of substance, which are overlooked in this commentary. The questions embody the mechanism of high-affinity binding; whether there is a heterocomplex with Trk receptors, the other gorilla in the room; consequences of γ-secretase cleavage of p75; and the contribution of p75 to neurodegeneration. Stoichiometric analysis stands to provide insights to all of these questions. The different receptor configurations imply that alternative signaling platforms are possible. As p75 levels affect plasticity and many neuropsychiatric disorders, the different structural views of p75 monomers and its oligomers will also provide a basis of therapeutic drug design in the future.
Acknowledgments
Research by M.V.C. was supported by National Institute of Neurological Disorders and Stroke (NS21072) and National Institute of Aging (AG025970).
Footnotes
The author declares no competing interest.
See companion article on page 21563.
References
- 1.Marchetti L., et al. , Fast-diffusing p75NTR monomers support apoptosis and growth cone collapse by neurotrophin ligands. Proc. Natl. Acad. Sci. U.S.A. 116, 21563–21572 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bothwell M., Functional interactions of neurotrophins and neurotrophin receptors. Annu. Rev. Neurosci. 18, 223–253 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Barker P. A., p75NTR is positively promiscuous: Novel partners and new insights. Neuron 42, 529–533 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Hempstead B. L., Deciphering proneurotrophin actions. Handb. Exp. Pharmacol. 220, 17–32 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Barker P. A., A p75(NTR) pivoting paradigm propels perspicacity. Neuron 62, 3–5 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Barde Y. A., Trophic factors and neuronal survival. Neuron 2, 1525–1534 (1989). [DOI] [PubMed] [Google Scholar]
- 7.Lee K. F., Davies A. M., Jaenisch R., p75-deficient embryonic dorsal root sensory and neonatal sympathetic neurons display a decreased sensitivity to NGF. Development 120, 1027–1033 (1994). [DOI] [PubMed] [Google Scholar]
- 8.Murphy M., et al. , Reduction of p75 neurotrophin receptor ameliorates the cognitive deficits in a model of Alzheimer’s disease. Neurobiol. Aging 36, 740–752 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Locksley R. M., Killeen N., Lenardo M. J., The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 104, 487–501 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Bradshaw R. A., et al. , Nerve growth factor: Structure/function relationships. Protein Sci. 3, 1901–1913 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grob P. M., Berlot C. H., Bothwell M. A., Affinity labeling and partial purification of nerve growth factor receptors from rat pheochromocytoma and human melanoma cells. Proc. Natl. Acad. Sci. U.S.A. 80, 6819–6823 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puma P., Buxser S. E., Watson L., Kelleher D. J., Johnson G. L., Purification of the receptor for nerve growth factor from A875 melanoma cells by affinity chromatography. J. Biol. Chem. 258, 3370–3375 (1983). [PubMed] [Google Scholar]
- 13.Vilar M., et al. , Ligand-independent signaling by disulfide-crosslinked dimers of the p75 neurotrophin receptor. J. Cell Sci. 122, 3351–3357 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilar M., et al. , Activation of the p75 neurotrophin receptor through conformational rearrangement of disulphide-linked receptor dimers. Neuron 62, 72–83 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka K., Kelly C. E., Goh K. Y., Lim K. B., Ibáñez C. F., Death domain signaling by disulfide-linked dimers of the p75 neurotrophin receptor mediates neuronal death in the CNS. J. Neurosci. 36, 5587–5595 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sykes A. M., et al. , The effects of transmembrane sequence and dimerization on cleavage of the p75 neurotrophin receptor by γ-secretase. J. Biol. Chem. 287, 43810–43824 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aurikko J. P., et al. , Characterization of symmetric complexes of nerve growth factor and the ectodomain of the pan-neurotrophin receptor, p75NTR. J. Biol. Chem. 280, 33453–33460 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong Y., Cao P., Yu H. J., Jiang T., Crystal structure of the neurotrophin-3 and p75NTR symmetrical complex. Nature 454, 789–793 (2008). [DOI] [PubMed] [Google Scholar]
- 19.He X. L., Garcia K. C., Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science 304, 870–875 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Wehrman T., et al. , Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron 53, 25–38 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Chan F. K., et al. , A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 288, 2351–2354 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Chan F. K., Three is better than one: Pre-ligand receptor assembly in the regulation of TNF receptor signaling. Cytokine 37, 101–107 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anastasia A., Barker P. A., Chao M. V., Hempstead B. L., Detection of p75NTR trimers: Implications for receptor stoichiometry and activation. J. Neurosci. 35, 11911–11920 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaar M., et al. , Amyloid beta binds trimers as well as monomers of the 75-kDa neurotrophin receptor and activates receptor signaling. J. Biol. Chem. 277, 7720–7725 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Langevin C., Jaaro H., Bressanelli S., Fainzilber M., Tuffereau C., Rabies virus glycoprotein (RVG) is a trimeric ligand for the N-terminal cysteine-rich domain of the mammalian p75 neurotrophin receptor. J. Biol. Chem. 277, 37655–37662 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Freed D. M., et al. , EFGR ligands differentially stabilize receptor dimers to specify signaling kinetics. Cell 171, 683–695.e18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng, et al. , Molecular and structural insight into proNGF engagement of p75NTR and sortilin. J Mol Biol 396, 967–984 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibáñez C. F., Simi A., p75 neurotrophin receptor signaling in nervous system injury and degeneration: Paradox and opportunity. Trends Neurosci. 35, 431–440 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Jung K.-M., et al. , Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor. J. Biol. Chem. 278, 42161–42169 (2003). [DOI] [PubMed] [Google Scholar]