Significance
Organic glasses are useful, versatile materials for current and future organic electronic technologies. They are nonequilibrium materials, so a single-component system can be processed in different ways to create a wide range of solids with diverse structural and optical properties. In this work, we use physical vapor deposition to prepare glasses with structure similar to that of an aligned smectic liquid crystal from a molecule that does not have any liquid crystal phases. This shows the potential to create highly structured glasses from a wide range of molecules.
Keywords: organic glasses, physical vapor deposition, liquid crystals
Abstract
We show that glasses with aligned smectic liquid crystal-like order can be produced by physical vapor deposition of a molecule without any equilibrium liquid crystal phases. Smectic-like order in vapor-deposited films was characterized by wide-angle X-ray scattering. A surface equilibration mechanism predicts the highly smectic-like vapor-deposited structure to be a result of significant vertical anchoring at the surface of the equilibrium liquid, and near-edge X-ray absorption fine structure (NEXAFS) spectroscopy orientation analysis confirms this prediction. Understanding of the mechanism enables informed engineering of different levels of smectic order in vapor-deposited glasses to suit various applications. The preparation of a glass with orientational and translational order from a nonliquid crystal opens up an exciting paradigm for accessing extreme anisotropy in glassy solids.
While glasses are sometimes characterized as “randomly packed” and lacking “structure,” in reality, glasses can show a wide range of interesting and useful packing motifs. Because glasses are out-of-equilibrium, there are in principle an essentially infinite number of structurally distinct, mechanically stable glassy solids even for a single-component system. While many of these different glasses will have similar properties, it has been shown that significant variations in properties such as density (1), stress response (2), and thermal stability (3) can be achieved in glasses of single-component systems. Ultimately, these property differences result from different glass structures.
One of the most interesting and useful aspects of structure in glasses is anisotropy. There are many ways to induce anisotropy in glasses, such as thermomechanical processing of polymer (4) and metallic (5) glasses, or physical vapor deposition of organic (6, 7) and inorganic (8) systems. For organic glasses, orientational anisotropy allows modulation of light absorption and emission, and anisotropic packing with nearest neighbors modulates charge mobility. Anisotropic glasses have been successfully used in applications such as organic light-emitting diodes (OLEDs) (9) and are increasingly being explored for other emerging organic electronics, including field-effect transistors (10, 11) and photovoltaics (12). In contrast to the difficulty of growing large single crystals with controlled orientation, macroscopically aligned glasses without grain boundaries can easily be produced (13). Glasses can also readily incorporate guest species without disrupting macroscopic homogeneity (14, 15). Since anisotropic glasses combine the processing advantages of glasses with anisotropy that yields favorable electronic and optical properties, they are promising for future technology such as flexible and printed devices (16).
There has been considerable interest in using liquid crystals to produce highly anisotropic glasses, and several preparation routes have been explored (17). When a liquid crystal is cooled, a frozen version of its structure is formed, unless nucleation into a 3D crystal occurs first. The solid formed by the liquid crystal-cooling route will include strong orientational (18), and possibly translational (19), correlation between neighboring molecules. If the liquid crystal is aligned along a common direction before cooling by using alignment layers or an electromagnetic field (20), the solid that is formed will have strongly anisotropic local and macroscopic structure. Vapor deposition of molecules with liquid-crystalline phases has recently been identified as another method for producing highly anisotropic, macroscopically aligned glasses with both orientational (13, 21) and translational (22, 23) order. Glasses prepared with liquid-crystalline order have enhanced charge mobility (24, 25) compared to their isotropic counterparts.
In this work, we identify a route for producing highly anisotropic glasses with orientational and translational order similar to that of a smectic liquid crystal. In contrast to the examples discussed above, we show that smectic-like glasses can be achieved using a molecule (posaconazole) that does not have any equilibrium liquid crystal phases. We characterize the molecular packing of the glasses by grazing-incidence wide-angle X-ray scattering (GIWAXS) (26, 27). Using near-edge X-ray absorption fine structure (NEXAFS) spectroscopy, we determine the molecular orientation at the free surface of an isotropic glass prepared by cooling the equilibrium liquid. We find that the high level of order at the free surface of posaconazole allows the deposition of glasses with significant smectic-like ordering. With this result, we recognize that even molecules that do not form liquid crystals can have extremely anisotropic locally ordered solid packing arrangements reminiscent of liquid crystal phases. There is every reason to expect that there are many organic molecules with the potential to form glasses with similar liquid-crystalline anisotropy, if the right preparation method can be identified.
Results
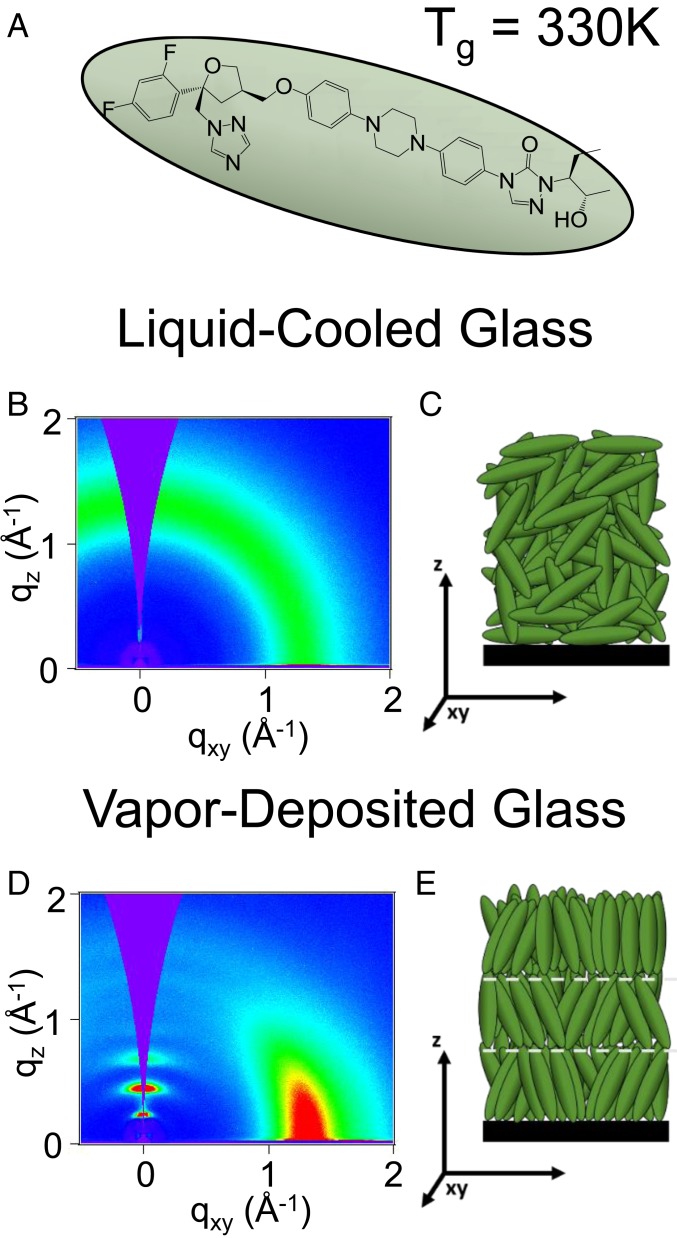
While our main results concern vapor-deposited glasses of posaconazole, the isotropic liquid-cooled glass of posaconazole is an important point of reference. The molecular structure of posaconazole is shown in Fig. 1A. Above its glass-transition temperature, Tg, posaconazole is an isotropic liquid (21). When a glass is formed by cooling the equilibrium liquid to room temperature from 334 K (Tg + 4 K), it inherits the structure of the isotropic equilibrium liquid, as shown in the GIWAXS pattern in Fig. 1B. The sole feature in the X-ray diffraction of the liquid-cooled glass of posaconazole is a broad amorphous peak around q ∼ 1.3 Å−1 that is equally distributed azimuthally. We associate this with the distance of closest lateral approach between neighboring rods (∼0.48 nm). An illustration of the structure of the isotropic liquid-cooled glass is presented in Fig. 1C.
Fig. 1.
X-ray scattering of liquid-cooled and vapor-deposited glasses of posaconazole shows that high levels of smectic-like order are accessible only through vapor deposition. (A) Molecular structure of posaconazole, which has a glass transition at 330 K. (B) GIWAXS of liquid-cooled posaconazole is consistent with an isotropic glass, as illustrated in C. (D) GIWAXS of posaconazole vapor-deposited at 324 K at 0.02 nm s−1 reveals smectic-like translational order and nematic-like orientational order, as illustrated in E.
When vapor deposited, posaconazole produces a glass with significant smectic-like order that is not present in the liquid-cooled glass. By vapor-depositing at a rate of 0.02 nm s−1 on a silicon substrate held at 324 K, a glass with the X-ray scattering pattern shown in Fig. 1D is produced. In contrast to the liquid-cooled glass, the vapor-deposited glass has a high level of translational smectic-like order, which produces the intense peaks at qz values of ∼(0.2, 0.4, and 0.6) Å−1. These 3 peaks resemble those of the equilibrium smectic liquid-crystal phase of itraconazole (28, 29), suggesting that the order in the vapor-deposited glass of posaconazole is smectic-like. Corrected for instrumental broadening, the Scherrer correlation length of the peak at qz ∼ 0.4 Å−1 is 18.4 nm, which is equal to approximately 6 molecular lengths. The vapor-deposited glass also has a high degree of orientational order consistent with smectic-like packing, as evidenced by the localized azimuthal distribution of the broad peak at q ∼ 1.3 Å−1. Rather than being isotropic, the scattering from the lateral packing of neighboring rods is focused in-plane, consistent with the structure illustrated in Fig. 1E. The high degree of orientational order as revealed by X-ray scattering is consistent with previous birefringence and infrared absorption data on vapor-deposited posaconazole (21). The correlation length from this broad peak, corrected for instrumental broadening, is 2.9 nm, which is equal to approximately 6 molecules arranged laterally in-plane; this small correlation length is consistent with the view that the vapor-deposited film is not a 3D crystal. Despite posaconazole having no known nematic or smectic phases, the vapor-deposited glass shows features similar to those of an aligned smectic liquid crystal (22).
To understand the origin of the structure of the vapor-deposited glass, we investigate the equilibrium liquid. A surface equilibration mechanism has been proposed to explain the unique properties of vapor-deposited glasses (1, 6, 8). The surface of an organic glass can be much more mobile than the bulk, with diffusion coefficients that can be up to 8 orders of magnitude larger (30). During deposition, each deposited molecule resides at the free surface for (typically) a few seconds, during which the molecules utilize enhanced surface mobility to equilibrate toward the structure of the (metastable) equilibrium liquid. As the deposition progresses, the partially (or fully) equilibrated molecules become buried in the bulk glass with relaxation times that exceed accessible experimental time scales. While we expect that vapor deposition of posaconazole follows this surface equilibration mechanism, the vapor-deposited structure (Fig. 1 D and E) has a high degree of concurrent orientational and translational order atypical of other nonliquid crystalline systems studied to date (27, 31, 32). Therefore, we infer that posaconazole must have a different surface structure during deposition than other systems studied thus far.
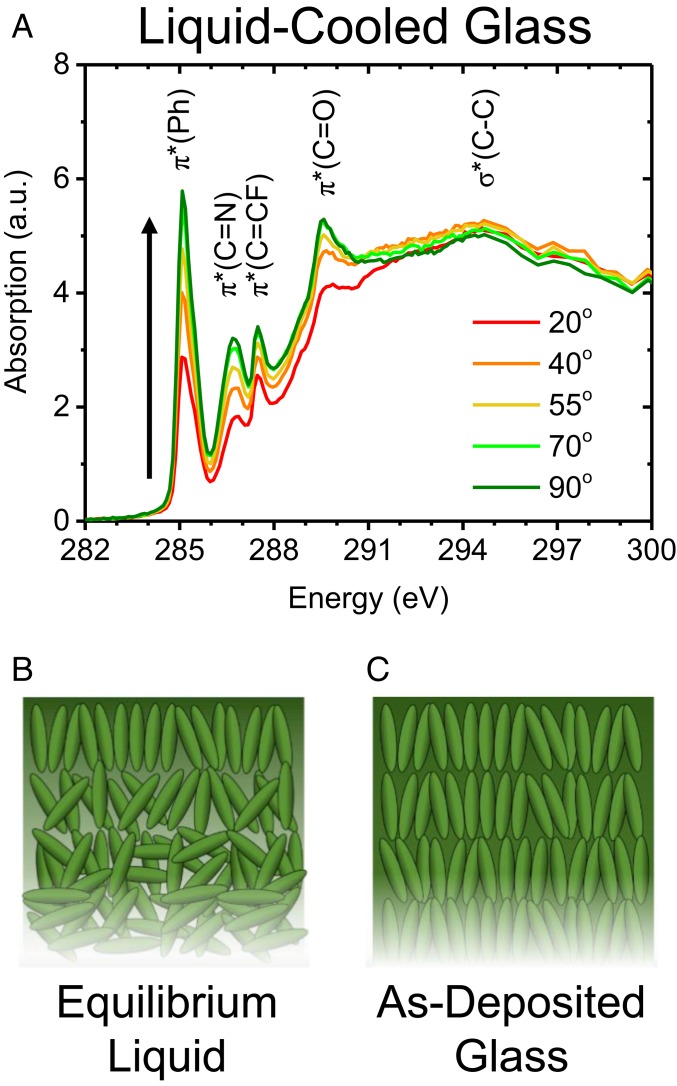
Because the surface equilibration mechanism predicts that the structure of vapor-deposited posaconazole depends upon the surface structure of the equilibrium liquid, we use NEXAFS spectroscopy to study the molecular orientation at the surface. We performed incident angle-dependent NEXAFS (33) on the liquid-cooled glass at the carbon K-edge. We expect that the liquid-cooled glass has a structure that accurately approximates that of the equilibrium liquid just above Tg. Within the energy range shown in Fig. 2A, we note several main features. Peaks around 285.2, 286.7, and 287.5 eV correspond to the 1s → π* transitions for the C – C aromatic phenyl bonds, the C = N bonds in triazole (34), and C = CF bonds in fluorobenzene rings (35, 36), respectively. Our C = CF peak assignment is supported by the absence of this peak system in NEXAFS spectroscopy of itraconazole (SI Appendix), which lacks fluorobenzene rings. The peak at 289.6 eV is assigned to the 1s → π* carbonyl transition (37), while a broad peak around ∼294 eV corresponds to the 1s → σ* carbon–carbon resonance. The sharp transitions at energies below ∼290 eV provide sufficient signal-to-noise to determine incident angle-dependent absorption, so we use these resonances to report the orientation of the central core of posaconazole. Data were collected in partial electron-yield mode with a grid bias of −219 V, and we therefore expect the NEXAFS measurements to probe roughly the top 6 nm of the free surface, which is approximately 2 molecular layers.
Fig. 2.
NEXAFS spectroscopy shows that the molecules at the free surface of the liquid-cooled glass, and therefore the equilibrium liquid of posaconazole, are vertically oriented. (A) NEXAFS at the carbon K-edge for a liquid-cooled posaconazole glass, deposited as an equilibrium isotropic liquid at 334 K and cooled to room temperature at 1 K min−1. a.u., arbitrary units; Ph, phenyl group. (B) Illustration of the proposed surface structure of the posaconazole liquid. The top layer of posaconazole molecules is oriented with an average long axis tilt of ∼33° from the surface normal (SI Appendix). This anisotropy is present only at the surface and gives way to an isotropic bulk revealed by ellipsometry (SI Appendix). (C) When vapor-deposited, the molecular orientation throughout the bulk of the glass resembles that of the surface of the equilibrium liquid.
The molecules at the free surface of posaconazole show strong vertical orientation in contrast to the random orientation in the bulk of the liquid-cooled glass. Fig. 2A shows the incident angle-dependent partial electron yield NEXAFS spectra of the liquid-cooled glass (deposited into the equilibrium liquid at 334 K and subsequently cooled to room temperature at 1 K min−1). Changing the incident angle of p-polarized light alters the angle of the electric field vector relative to the 1s → π* transition dipole vectors. For the 1s → π* resonances, the maximum intensity occurs when p-polarized radiation is at normal incidence to the substrate, and the minimum occurs at lower incidence angles. This is consistent with a 1s → π* transition dipole-vector orientation preferentially parallel to the substrate; our fit to the data provides an angle of 57° between the 1s → π* transition dipole and surface normal.
We can connect the 1s → π* transition-dipole orientation to the posaconazole long axis orientation by modeling posaconazole as a rod, such that the vectors normal to the conjugated planes of its core equally adopt all possible orientations orthogonal to the long molecular axis. This assumption is supported by our GIWAXS data in Fig. 1D, which has a broad peak at qxy ∼ 1.3 Å−1 that occurs at too large of a d-spacing to be associated with a pi-pi stacking interaction. This allows us to rule out posaconazole orientations where the molecular long axis is lying down with its conjugated plane “edge on” upon the substrate. Strong vertical orientation of the long molecular axis is further supported by the behavior of some of the other resonances in the spectra shown in Fig. 2, particularly the carbon–carbon 1s → σ*, which exhibits an out-of-plane orientation preference consistent with a vertical long axis. Modeling posaconazole as a rod allows an analysis identical to that of a planar orbital with high substrate symmetry (33). Using this approach, we solve for an average angle of ∼33° between the posaconazole long axis and surface normal (SI Appendix). The surface orientation of posaconazole is different from that revealed by simulations of other organic molecules, which tend to exhibit mild perpendicular orientation at the free surface (38, 39). We infer that this highly vertical orientation of the long axis at the free surface is therefore responsible for the high degree of orientational order seen in the vapor-deposited glass. It was previously hypothesized that posaconazole may have a highly oriented surface structure similar to that observed in nematic liquid crystals (21, 40); Fig. 2 confirms that hypothesis. The isotropic bulk structure of the liquid-cooled glass of posaconazole is indicated in Fig. 1B and was further confirmed by ellipsometry (SI Appendix) (21).
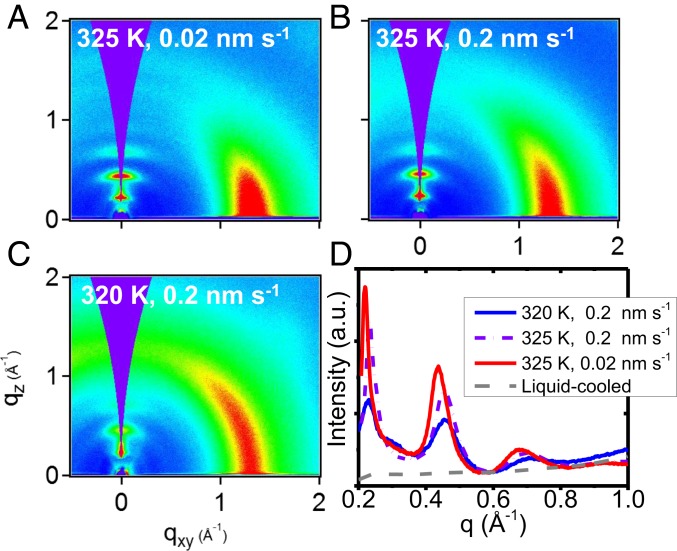
Both the substrate temperature and deposition rate can be changed to manipulate the precise structure of vapor-deposited glasses of posaconazole. GIWAXS of 3 vapor-deposited posaconazole glasses are shown in Fig. 3. Each of the glasses shown has a different layer d-spacing and a different degree of orientational order, as shown in Fig. 3D. With just a single order of magnitude increase in deposition rate at 325 K, the layering peak moves from q = 0.219 to 0.232 Å−1, which corresponds to a 6% decrease in layer d-spacing from 2.87 to 2.71 nm. The orientational order varies greatly from glass to glass, even in the narrow temperature and deposition rate window presented here. Given a wide range of deposition conditions, the structure of posaconazole can be continuously varied to create a range of solids with differing structural and therefore mechanical (2) and optical (41) properties.
Fig. 3.
Vapor deposition can produce a wide range of structures that depend upon both substrate temperature and deposition rate. (A–C) GIWAXS patterns from vapor-deposited glasses of posaconazole, with various degrees of order depending on deposition conditions; 2D patterns show different amounts of translational and orientational order from different combinations of substrate temperature and deposition rate. (D) One-dimensional integrations near qz for 2D patterns in A–C, which show the degree and spacing of smectic-like ordering. A liquid-cooled glass, prepared by cooling the liquid from 334 K, is shown for reference. a.u., arbitrary units.
The effects of changing the substrate temperature and deposition rate can be explained by the surface equilibration mechanism (6, 31), which posits that the structure and mobility at the free surface of the glass during deposition is responsible for the structure of the bulk vapor-deposited glass. Comparing the glasses prepared at 0.2 nm s−1 at 325 and 320 K (Fig. 3 B and C), the glass deposited at a higher substrate temperature has greater orientational order, shown by the increased azimuthal localization of the broad peak at q ∼ 1.3 Å−1 into the near-qxy direction (SI Appendix). At higher substrate temperatures, molecules at the surface can more thoroughly equilibrate toward the equilibrium surface structure before becoming locked into the glassy bulk (30). (A change in the substrate temperature may also change the tilt angle at the equilibrium free surface; in this work, we assume the equilibrium surface orientation to be approximately constant over the range of substrate temperatures shown.) Comparing Fig. 3 A and B, the glass deposited at a lower rate (Fig. 3A) shows a further increase in order as each molecule spends more time at the highly mobile free surface (42, 43). Therefore, given a highly orientationally ordered equilibrium free surface, lower deposition rates and higher substrate temperatures result in glasses with more ordered molecules. The molecular orientation at the free surface derived from NEXAFS provides an approximate upper bound on vertical molecular orientation in the vapor-deposited glass. We may estimate the molecular orientation in the bulk glass using the out-of-plane peak spacing by approximating the molecules as 3.2 nm (44) rigid rods. Assuming that the layer spacing is caused by molecular tilt, the molecules in the glass prepared at the highest substrate temperature and lowest rate (Fig. 3A) have long axes tilted ∼26° from the surface normal, roughly consistent with the orientation determined by NEXAFS. The sharpening of the peaks at q values of ∼(0.2, 0.4, and 0.6) Å−1 for the glass deposited at higher substrate temperature and lower rates indicates a growing coherence length of out-of-plane translational ordering (45).
We attribute the translational order in the vapor-deposited glasses to 2 possible origins. Translational order at the free surface of the liquid may persist beyond the top layer of molecules, as indicated by Fig. 2B. The NEXAFS measurements cannot provide direct evidence for this translational order, but such order has been observed at the equilibrium free surface of other nonliquid crystalline systems (31, 46). Deposition would naturally trap this layered structure into the glass. Alternately, it is possible that a layered glass might be formed solely on the basis of a strong tendency for vertical alignment in the topmost liquid layer. In this case, equilibration during deposition would try to create a top layer of vertically oriented molecules. Once complete coverage was achieved, subsequently deposited molecules might begin to create new layers, while leaving those below undisturbed. Molecular dynamics computer simulations of the vapor-deposition process might succeed in distinguishing between these 2 explanations (38, 47).
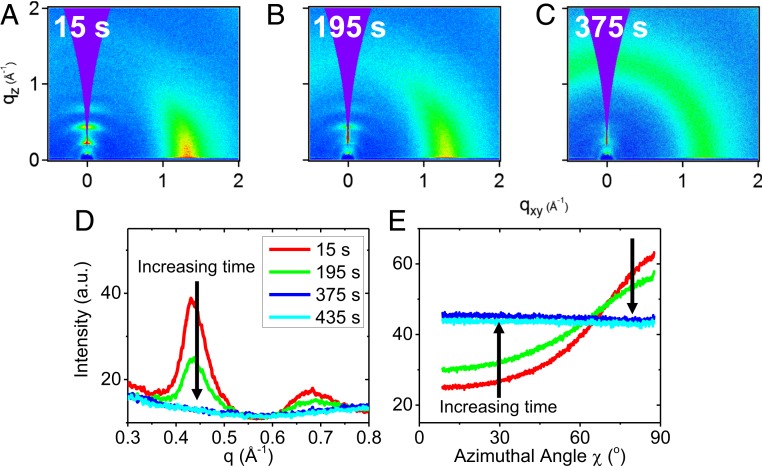
Despite having highly organized molecular packing, vapor-deposited solids of posaconazole are not crystalline, as shown by their evolution during annealing. We prepared a posaconazole glass at a rate of 0.02 nm s−1 on a substrate held at 325 K and then annealed it isothermally at 335 K (Tg + 5 K). The complete experiment is detailed in Materials and Methods. As shown in Fig. 4, the highly ordered vapor-deposited glass transforms irreversibly into the isotropic liquid when held slightly above Tg (and far below the crystal melting point of 443 K). Fig. 4 shows the loss of translational and orientational order requires less than 400 s. In contrast, if 3D crystals were responsible for the order in the vapor-deposited materials, we would observe sharpening of the peaks during annealing as the crystals grew in size; no sharp peaks are observed in the 2D patterns in Fig. 4 A through C. When the sample shown in Fig. 4 was cooled at 1 K min−1 after annealing, the glass remained isotropic, indicating an irreversible transformation. The transformation from ordered glass to isotropic liquid shown in Fig. 4 takes roughly 300 times as long as the transformation from ordinary glass to isotropic liquid (44); this result is similar to what has been observed for other PVD glasses (21, 48).
Fig. 4.
Vapor-deposited posaconazole glasses irreversibly transform into the isotropic supercooled liquid when held well below the crystal melting point. (A–C) GIWAXS from 30-s exposures of a posaconazole glass vapor-deposited at 325 K and 0.02 nm s−1 during annealing at 335 K. (D) Integrations near qz show the decay of smectic-like order during annealing. (E) Radial integrations of the broad peak around q ∼ 1.3 Å−1 show the decay of orientational nematic order during annealing. a.u., arbitrary units.
Discussion
The striking order shown by vapor-deposited glasses of posaconazole (Figs. 1 and 3), characteristic of an aligned smectic liquid crystal, is remarkable in light of the absence of equilibrium liquid crystal phases for this molecule. These glasses are much more highly structured than may previously have been expected for glasses prepared from nonmesogens. Our work highlights the ability of vapor deposition to produce highly anisotropic nonequilibrium packing arrangements that cannot at present be attained with other methods. We anticipate that this work will extend the range of systems from which highly anisotropic glasses can be prepared.
It will be important to refine strategies for the identification of additional nonmesogens that, similar to posaconazole, can produce highly ordered glasses. The amphiphilic nature of posaconazole may be responsible for its vertical orientation at the free surface of the liquid. In recent simulations of vapor-deposited glasses of amphiphilic fluorinated molecules, the fluorinated tails segregate to the surface (49); it is plausible that posaconazole behaves similarly during deposition, and this provides a guide in selecting additional systems for study. Our investigation of posaconazole was motivated by its structural similarity with itraconazole, a system known to form smectic liquid crystals. This suggests that it would be fruitful to investigate additional sets of structurally similar molecules that contain both mesogens and nonmesogens. More generally, molecular dynamics computer simulations have proven useful for understanding the surface structure of liquids (31, 39, 50), and these could be employed to screen additional candidates. It would be particularly interesting to identify candidates with disk-like cores that might result in glasses with columnar order.
We anticipate that these glasses with high, tunable levels of orientational and translational order may be useful for new applications in organic electronics. The nonequilibrium nature of these materials means that for a single molecular system, precise properties of the glass can be “dialed in” by changing the rate and substrate temperature during deposition. The surface equilibration mechanism can be used to predict the correct deposition parameters for the desired application. Liquid-crystalline order has been shown to produce enhanced charge mobility (24, 25) and unique optoelectronic properties (16, 24, 51, 52). We anticipate that the solids prepared by the route described here combine the advantages of anisotropic glasses produced from liquid crystals with the compositional flexibility and macroscopic homogeneity of liquid-cooled glasses, making them important materials for the next generation of devices.
Materials and Methods
Posaconazole VETRANAL analytical standard was used as-received from Sigma Aldrich. Samples were prepared in a custom-built vacuum chamber at a base pressure of 10−6 torr. The source to substrate distance was 11 cm (21). Samples prepared for GIWAXS ranged in thickness from 300 to 450 nm, with the exception of those shown in Fig. 1, which are 1 μm thick. Previous work has shown that, above 100 nm, bulk properties are independent of film thickness (6, 21). Samples prepared for NEXAFS were slightly thinner (∼70 nm), to avoid sample-charging effects, which can introduce artifacts (53). All samples for both techniques were prepared on identical 1-inch undoped silicon <1 0 0> wafers with 2-nm native oxide (Virginia Semiconductor).
GIWAXS was performed at beamline 11-3 at the Stanford synchrotron radiation lightsource. Scans were acquired at an incidence angle of θin = 0.14°, which is above the critical angle for posaconazole. The X-ray energy is 12.7 keV, with a sample-to-detector distance of 315 mm. When calculating instrumental broadening, a 25-mm spot size was assumed to account for a grazing-incidence footprint on a 25-mm wafer. Scans were performed in a helium atmosphere. As-deposited and liquid-cooled scattering patterns were collected using exposure times of 120 s. Data were reduced using the WAXStools plugin (54) in the Nika 2D SAS package for Igor Pro (55, 56). Smectic-like order integrations (Figs. 3D and 4E) were taken near qz, rather than directly along qz, since scattering from directly along qz is unavailable from grazing-incidence geometry (57). Scans were integrated between azimuthal angle χ = 4° to 8° and χ = −4° to −8°, where χ = 0° is along qz. For each scan, the 2 integrations were summed and normalized by the average film thickness determined by spectroscopic ellipsometry (M-2000U; J.A. Woollam).
NEXAFS data were collected using the Soft X-ray Spectroscopy beamline at the Australian synchrotron (AS). Scans were performed using linearly polarized X-rays and 5 different sample tilt angles, such that the electric field vector was aligned at θin = 90°, 70°, 55°, 40°, and 20° relative to the sample surface normal. X-ray absorption was monitored using the channeltron detector operating in partial electron yield mode with a bias of −219 V. Scans were recorded over an energy range of 230 to 430 eV. The data were corrected and analyzed using the Quick AS NEXAFS Tool (QANT) (58). Each scan was corrected for energy offset by normalizing to the highly oriented pyrolyzed graphite internal standard. The absorption values were corrected using both the gold-mesh instantaneous flux monitor and a photodiode secondary reference. After corrections, each scan was normalized using the empirical step method described by Gann et al. (58); the preedge intensity values were scaled to 0 by subtracting the average absorption between 275 to 280 eV, and the postedge intensity values were scaled to 1. The normalized scans were analyzed using the multipeak fit function in QANT. The data between 275 to 390 eV were well-fit by the sum of an “edge” baseline and 9 Gaussian peaks. The peak positions and widths, determined by fitting the θin = 55° dataset, were held constant, while the peak heights were fitted for each sample. The fit results are provided in the SI Appendix.
To prepare liquid-cooled glasses, 2 methods were used. For the liquid-cooled glass used for GIWAXS, shown in Fig. 1B, a 1,100-nm-thick glass of posaconazole deposited at 329 K at 0.2 nm s−1 was held at 334 K in an He atmosphere for 10 min. It was then cooled at a rate of 1 K min−1 to 328 K and then brought to room temperature at ambient conditions. For NEXAFS, the liquid-cooled glass was prepared by vapor-depositing directly at 334 K in vacuum. After deposition, the liquid was immediately cooled to 320 K at a rate of 1 K min−1, after which it was brought to room temperature at 5 K min−1. Bulk isotropy of the glass was confirmed using spectroscopic ellipsometry (SI Appendix).
For in situ annealing measurements shown in Fig. 4A, 310-nm-thick sample deposited at 325 K and 0.2 nm s−1 (shown in Fig. 3A) was brought from room temperature to 335 K. For Fig. 4, we took the starting time for the experiment as the time when the sample reached 335 K; the film was above Tg for no more than 10 s before this time. Images were collected at θin = 0.14° for 30-s exposures. Annealing times reported are the midpoint of each measurement. Several spots were used to avoid beam damage (SI Appendix). After annealing, the sample was cooled to room temperature at ambient conditions, and all 5 spots were remeasured to ensure consistency between spots. Smectic-like order integrations (Fig. 4E) were performed as described above. Orientational order measurements (Fig. 4F) were integrated with respect to azimuthal angle χ from q = 1.1 to 1.7 Å−1.
Supplementary Material
Acknowledgments
This research was primarily supported by NSF through the University of Wisconsin Materials Research Science and Engineering Center (Grant DMR-1720415). J.L.T. acknowledges support from the National Research Council Research Associateship Programs. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract DE-AC02-76SF00515. NEXAFS was undertaken on the Soft X-ray Spectroscopy beamline at the Australian Synchrotron, part of the Australian Nuclear Science and Technology Organisation. We thank Tim Dunn and Chris Tassone for assistance on Beamline 11-3. Certain commercial equipment, instruments, or materials (or suppliers, software, etc.) are identified in this paper to foster understanding. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 21341.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1908445116/-/DCSupplemental.
References
- 1.Swallen S. F., et al. , Organic glasses with exceptional thermodynamic and kinetic stability. Science 315, 353–356 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Tangpatjaroen C., Bagchi K., Martínez R. A., Grierson D., Szlufarska I., Mechanical properties of structure-tunable, vapor-deposited TPD glass. J. Phys. Chem. C 122, 27775–27781 (2018). [Google Scholar]
- 3.Rodríguez-Tinoco C., Gonzalez-Silveira M., Ràfols-Ribé J., Lopeandía A. F., Rodríguez-Viejo J., Transformation kinetics of vapor-deposited thin film organic glasses: The role of stability and molecular packing anisotropy. Phys. Chem. Chem. Phys. 17, 31195–31201 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Botto P. A., Duckett R. A., Ward I. M., The yield and thermoelastic properties of oriented poly(methyl methacrylate). Polymer 28, 257–262 (1987). [Google Scholar]
- 5.Sun Y. H., et al. , Flow-induced elastic anisotropy of metallic glasses. Acta Mater. 112, 132–140 (2016). [Google Scholar]
- 6.Dalal S. S., Walters D. M., Lyubimov I., de Pablo J. J., Ediger M. D., Tunable molecular orientation and elevated thermal stability of vapor-deposited organic semiconductors. Proc. Natl. Acad. Sci. U.S.A. 112, 4227–4232 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokoyama D., Molecular orientation in small-molecule organic light-emitting diodes. J. Mater. Chem. 21, 19187–19202 (2011). [Google Scholar]
- 8.Hellman F., Surface‐induced ordering: A model for vapor‐deposition growth of amorphous materials. Appl. Phys. Lett. 64, 1947–1949 (1994). [Google Scholar]
- 9.Ràfols-Ribé J., et al. , High-performance organic light-emitting diodes comprising ultrastable glass layers. Sci. Adv. 4, eaar8332 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuda T., et al. , Carrier transport properties of monodisperse glassy-nematic oligofluorenes in organic field-effect transistors. Chem. Mater. 17, 264–268 (2005). [Google Scholar]
- 11.Sirringhaus H., et al. , Mobility enhancement in conjugated polymer field-effect transistors through chain alignment in a liquid-crystalline phase. Appl. Phys. Lett. 77, 406–408 (2000). [Google Scholar]
- 12.Menke S. M., Holmes R. J., Energy-cascade organic photovoltaic devices incorporating a host-guest architecture. ACS Appl. Mater. Interfaces 7, 2912–2918 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Gómez J., et al. , Vapor deposition of a smectic liquid crystal: Highly anisotropic, homogeneous glasses with tunable molecular orientation. Soft Matter 12, 2942–2947 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama D., Sakaguchi A., Suzuki M., Adachi C., Horizontal orientation of linear-shaped organic molecules having bulky substituents in neat and doped vacuum-deposited amorphous films. Org. Electron. 10, 127–137 (2009). [Google Scholar]
- 15.Jiang J., Walters D. M., Zhou D., Ediger M. D., Substrate temperature controls molecular orientation in two-component vapor-deposited glasses. Soft Matter 12, 3265–3270 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Magliulo M., et al. , Printable and flexible electronics: From TFTs to bioelectronic devices. J. Mater. Chem. C 3, 12347–12363 (2015). [Google Scholar]
- 17.Suga H., Seki S., Thermodynamic investigation on glassy states of pure simple compounds. J. Non-Cryst. Solids 16, 171–194 (1974). [Google Scholar]
- 18.Sorai M., Seki S., Glassy liquid crystal of the nematic phase of N-(o-Hydroxy-p-methoxybenzylidene)-p-butylaniline. Bull. Chem. Soc. Jpn. 44, 2887 (1971). [Google Scholar]
- 19.Tsuji K., Sorai M., Seki S., New finding of glassy liquid crystal–A non-equilibrium state of cholesteryl hydrogen phthalate. Bull. Chem. Soc. Jpn. 44, 1452 (1971). [Google Scholar]
- 20.Jerome B., Surface effects and anchoring in liquid crystals. Rep. Prog. Phys. 54, 391–451 (1991). [Google Scholar]
- 21.Gómez J., et al. , Nematic-like stable glasses without equilibrium liquid crystal phases. J. Chem. Phys. 146, 054503 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Gujral A., et al. , Highly organized smectic-like packing in vapor-deposited glasses of a liquid crystal. Chem. Mater. 29, 849–858 (2017). [Google Scholar]
- 23.Gujral A., et al. , Vapor-deposited glasses with long-range columnar liquid crystalline order. Chem. Mater. 29, 9110–9119 (2017). [Google Scholar]
- 24.Funahashi M., Hanna J. I., High carrier mobility up to 0.1 cm2 V–1 s–1 at ambient temperatures in thiophene-based smectic liquid crystals. Adv. Mater. 17, 594–598 (2005). [Google Scholar]
- 25.Eccher J., et al. , Thermal evaporation versus spin-coating: Electrical performance in columnar liquid crystal OLEDs. ACS Appl. Mater. Interfaces 7, 16374–16381 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Rivnay J., Mannsfeld S. C. B., Miller C. E., Salleo A., Toney M. F., Quantitative determination of organic semiconductor microstructure from the molecular to device scale. Chem. Rev. 112, 5488–5519 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Gujral A., O’Hara K. A., Toney M. F., Chabinyc M. L., Ediger M. D., Structural characterization of vapor-deposited glasses of an organic hole transport material with X-ray scattering. Chem. Mater. 27, 3341–3348 (2015). [Google Scholar]
- 28.Benmore C. J., et al. , A SAXS-WAXS study of the endothermic transitions in amorphous or supercooled liquid itraconazole. Thermochim. Acta 644, 1–5 (2016). [Google Scholar]
- 29.Teerakapibal R., Huang C., Gujral A., Ediger M. D., Yu L., Organic glasses with tunable liquid-crystalline order. Phys. Rev. Lett. 120, 055502 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Yu L., Surface mobility of molecular glasses and its importance in physical stability. Adv. Drug Deliv. Rev. 100, 3–9 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Bagchi K., et al. , Origin of anisotropic molecular packing in vapor-deposited Alq3 glasses. J. Phys. Chem. Lett. 10, 164–170 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Liu T., et al. , Birefringent stable glass with predominantly isotropic molecular orientation. Phys. Rev. Lett. 119, 095502 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Stöhr J., Outka D. A., Determination of molecular orientations on surfaces from the angular dependence of near-edge x-ray-absorption fine-structure spectra. Phys. Rev. B Condens. Matter 36, 7891–7905 (1987). [DOI] [PubMed] [Google Scholar]
- 34.Ehlert C., Holzweber M., Lippitz A., Unger W. E. S., Saalfrank P., A detailed assignment of NEXAFS resonances of imidazolium based ionic liquids. Phys. Chem. Chem. Phys. 18, 8654–8661 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Hitchcock A. P., et al. , Inner shell excitation and ionization of the monohalobenzenes. J. Electron Spectrosc. Relat. Phenom. 13, 345–360 (1978). [Google Scholar]
- 36.Cooney R. R., Urquhart S. G., Chemical trends in the near-edge X-ray absorption fine structure of monosubstituted and para-bisubstituted benzenes. J. Phys. Chem. B 108, 18185–18191 (2004). [Google Scholar]
- 37.Urquhart S. G., Ade H., Trends in the carbonyl core (C 1S, O 1S) → π*C=O transition in the near-edge X-ray absorption fine structure spectra of organic molecules. J. Phys. Chem. B 106, 8531–8538 (2002). [Google Scholar]
- 38.Lyubimov I., et al. , Orientational anisotropy in simulated vapor-deposited molecular glasses. J. Chem. Phys. 143, 094502 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Walters D. M., Antony L., de Pablo J. J., Ediger M. D., Influence of molecular shape on the thermal stability and molecular orientation of vapor-deposited organic semiconductors. J. Phys. Chem. Lett. 8, 3380–3386 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Pershan P. S., Braslau A., Weiss A. H., Als-Nielsen J., Smectic layering at the free surface of liquid crystals in the nematic phase: X-ray reflectivity. Phys. Rev. A Gen. Phys. 35, 4800–4813 (1987). [DOI] [PubMed] [Google Scholar]
- 41.Zola R. S., et al. , Dynamic control of light direction enabled by stimuli-responsive liquid crystal gratings. Adv. Mater. 31, e1806172 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Kearns K. L., et al. , Hiking down the energy landscape: Progress toward the kauzmann temperature via vapor deposition. J. Phys. Chem. B 112, 4934–4942 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Kearns K. L., Krzyskowski P., Devereaux Z., Using deposition rate to increase the thermal and kinetic stability of vapor-deposited hole transport layer glasses via a simple sublimation apparatus. J. Chem. Phys. 146, 203328 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Adrjanowicz K., et al. , Molecular dynamics of the supercooled pharmaceutical agent posaconazole studied via differential scanning calorimetry and dielectric and mechanical spectroscopies. Mol. Pharm. 10, 3934–3945 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Leadbetter A. J., Norris E. K., Distribution functions in three liquid crystals from X-ray diffraction measurements. Mol. Phys. 38, 669–686 (1979). [Google Scholar]
- 46.Regan M. J., et al. , Surface layering in liquid gallium: An X-ray reflectivity study. Phys. Rev. Lett. 75, 2498–2501 (1995). [DOI] [PubMed] [Google Scholar]
- 47.Youn Y., et al. , All-atom simulation of molecular orientation in vapor-deposited organic light-emitting diodes. J. Mater. Chem. C 6, 1015–1022 (2018). [Google Scholar]
- 48.Chen Z., Sepúlveda A., Ediger M. D., Richert R., Dynamics of glass-forming liquids. XVI. Observation of ultrastable glass transformation via dielectric spectroscopy. J. Chem. Phys. 138, 12A519 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Moore A. R., et al. , Effects of microstructure formation on the stability of vapor-deposited glasses. Proc. Natl. Acad. Sci. U.S.A. 116, 5937–5942 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sadati M., et al. , Molecular structure of canonical liquid crystal interfaces. J. Am. Chem. Soc. 139, 3841–3850 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Philp Chen H.-M., Ou J. J., Chen S. H., “Glassy liquid crystals as self-organized films for robust optoelectronic devices” in Nanoscience with Liquid Crystals, Li Q., Ed. (Springer, New York, NY, 2014), pp. 179–208. [Google Scholar]
- 52.Eccher J., Faria G. C., Bock H., von Seggern H., Bechtold I. H., Order induced charge carrier mobility enhancement in columnar liquid crystal diodes. ACS Appl. Mater. Interfaces 5, 11935–11943 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Zimmermann U., et al. , NEXAFS and ARUP spectroscopy of an organic single crystal: α-perylene. Mol. Cryst. Liq. Crys. A 339, 231–259 (2000). [Google Scholar]
- 54.Oosterhout S. D., et al. , Mixing behavior in small molecule:fullerene organic photovoltaics. Chem. Mater. 29, 3062–3069 (2017). [Google Scholar]
- 55.Ilavsky J., Nika: Software for two-dimensional data reduction. J. Appl. Cryst. 45, 324–328 (2012). [Google Scholar]
- 56.Zhang F., et al. , Glassy carbon as an absolute intensity calibration standard for small-angle scattering. Metall. Mater. Trans., A Phys. Metall. Mater. Sci. 41, 1151–1158 (2010). [Google Scholar]
- 57.Mannsfeld S. C. B., Virkar A., Reese C., Toney M. F., Bao Z., Precise structure of pentacene monolayers on amorphous silicon oxide and relation to charge transport. Adv. Mater. 21, 2294–2298 (2009). [Google Scholar]
- 58.Gann E., McNeill C. R., Tadich A., Cowie B. C. C., Thomsen L., Quick AS NEXAFS tool (QANT): A program for NEXAFS loading and analysis developed at the Australian synchrotron. J. Synchrotron Radiat. 23, 374–380 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.