Significance
As autotrophic organisms, sugar status in plants must be constantly monitored and reacted to in order to maintain sugar homeostatic states crucial for growth regulation, environmental stress tolerance, and productivity. α-Amylase (αAmy) is the key enzyme hydrolyzing starch into sugars and is regulated by sugar levels; it is induced by sugar starvation but repressed by sugar provision. Two MYBs compete for binding to the same αAmy promoter element to regulate this process, with MYBS1 promoting and MYBS2 repressing αAmy expression. Induction of αAmy expression by suppressing MYBS2 enhances stress tolerance and productivity. Phosphorylation of MYBS2 is critical for regulating its sugar-dependent nucleocytoplasmic shuttling and interactions with 14-3-3 proteins, representing a regulatory mechanism for reversible gene expression by sugar status.
Keywords: rice, MYB transcription factor, sugar repression, abiotic stress tolerance, grain weight
Abstract
Autotrophic plants have evolved distinctive mechanisms for maintaining a range of homeostatic states for sugars. The on/off switch of reversible gene expression by sugar starvation/provision represents one of the major mechanisms by which sugar levels are maintained, but the details remain unclear. α-Amylase (αAmy) is the key enzyme for hydrolyzing starch into sugars for plant growth, and it is induced by sugar starvation and repressed by sugar provision. αAmy can also be induced by various other stresses, but the physiological significance is unclear. Here, we reveal that the on/off switch of αAmy expression is regulated by 2 MYB transcription factors competing for the same promoter element. MYBS1 promotes αAmy expression under sugar starvation, whereas MYBS2 represses it. Sugar starvation promotes nuclear import of MYBS1 and nuclear export of MYBS2, whereas sugar provision has the opposite effects. Phosphorylation of MYBS2 at distinct serine residues plays important roles in regulating its sugar-dependent nucleocytoplasmic shuttling and maintenance in cytoplasm by 14-3-3 proteins. Moreover, dehydration, heat, and osmotic stress repress MYBS2 expression, thereby inducing αAmy3. Importantly, activation of αAmy3 and suppression of MYBS2 enhances plant growth, stress tolerance, and total grain weight per plant in rice. Our findings reveal insights into a unique regulatory mechanism for an on/off switch of reversible gene expression in maintaining sugar homeostatic states, which tightly regulates plant growth and development, and also highlight MYBS2 and αAmy3 as potential targets for crop improvement.
In plants, as autotrophic organisms, a range of sugar homeostatic states is crucial for growth regulation, environmental stress tolerance, and productivity, meaning that sugar status must be continuously monitored and elicit an appropriate reaction. An integrated signaling network coordinates sugar status—reflecting sugar production in source tissues to its utilization or storage in sink tissues—that involves cross-talk among sugars, hormones, and environmental cues and that regulates developmental and stress-adaptive processes (1, 2). Nearly all fundamental processes throughout the lifecycle of plants are modulated by sugars. In general, sugar provision up-regulates genes involved in biosynthesis, transport, and storage of reserves as well as cell growth, and it down-regulates those associated with photosynthesis, reserve mobilization, and stress responses, whereas sugar starvation has the opposite effects (3–5).
Upon assimilation in photosynthetic source leaves, newly fixed carbon is utilized for cellular respiration and metabolism, transiently stored in vacuoles as sucrose or in plastids as starch, and transported as sucrose to sink tissues, such as growing tissues (to generate energy) or developing organs (for long-term storage) (1, 6). Despite sugars being of central importance to plant growth, too much of them can be detrimental. For example, ectopic expression of a yeast invertase, which converts sucrose to glucose and fructose in the apoplast, leads to decreased sucrose export and accumulation of carbohydrates in leaves, with subsequent inhibition of photosynthesis, stunted growth, impaired root formation, and necrosis in tobacco leaves (7). Rice and maize mutant lines defective in a tonoplast sucrose transporter, SUT2, accumulate higher concentrations of sugars in leaves but exhibit growth retardation and reduced biomass and grain yield, presumably due to reduced transport of sucrose out of vacuoles in source leaves to sink tissues/organs where sugar is in high demand (8, 9).
Starch, which constitutes ∼75% of cereal grain dry weight (10), acts as the major carbon source for generating energy and metabolites during germination and seedling growth. α-Amylase (αAmy) is the most abundant hydrolase and plays a central role in starch mobilization and, thus, in the rate of seedling growth. Our previous studies in rice revealed that sugar starvation up-regulates αAmy expression by controlling its transcription rate and mRNA stability (3, 11, 12). All αAmy isolated from cereals contain a TATCCA element (the TA box) or variant at positions ∼90 to ∼150 base pairs upstream of the transcription start sites (13). αAmy transcriptional regulation is mediated through a sugar response complex (SRC) in αAmy promoters, in which the TA box is a key cis-acting element (14–16). MYBS1 is a single DNA binding repeat (R1) MYB transcription factor that interacts with the TA box and induces the αAmy promoters under sugar starvation (17, 18). MYBS1 expression and its nuclear import is promoted by sugar starvation, whereas sugar provision has the opposite effects (17, 19).
In cereals, the stored reserves in the endosperm are degraded and mobilized by a battery of enzymes and transporters acting in concert, and gibberellin (GA) is the major hormone that initiates these processes (20). GA activates αAmy promoters through the GA response complex (GARC), in which the adjacent GA response element (GARE) and TA box are key elements that act synergistically (14, 21). The MYBS1–TA box interaction is essential for GARC and SRC functions (14, 17), demonstrating that MYBS1 is an essential node in GA and sugar starvation cross-signaling. In rice and barley, MYBGA is a GA-inducible R2R3 MYB transcriptional factor that binds the GARE and activates αAmy and hydrolase gene promoters in aleurone cells surrounding the starchy endosperm (19, 22). GA antagonizes sugar-mediated repression of αAmy expression by enhancing conuclear transport of MYBGA and MYBS1 and formation of a stable bipartite MYB–DNA complex to activate αAmy and hydrolase gene promoters (19).
The 14-3-3 protein family is a highly conserved group of dimeric proteins that dock onto phosphorylated serine (Ser) and threonine (Thr) residues in their target proteins (23, 24). In plants, target proteins of 14-3-3 proteins are involved in signal transduction and gene regulation of various biological processes, and binding of client proteins by 14-3-3 proteins may lead to alteration in conformation, activity, stability, or intracellular localization (25, 26). That 14-3-3 proteins are involved in sugar regulation has been reported for yeast cells. In Saccharomyces cerevisiae, a 14-3-3 protein (Bmh1) is required for interaction with heat-shock protein HSP70 (Ssb) to recruit a phosphatase (Glc7) that dephosphorylates and inactivates the protein kinase SNF1, a process necessary for glucose repression (27). However, 14-3-3 protein-mediated sugar regulation of gene expression has not been explored in plants.
We previously identified another R1 MYB (MYBS2) that also specifically binds to the TA box of αAmy promoters (17). The function of MYBS2 had been unknown prior to this study. Here, by using αAmy as a biochemical marker, we show that sugar provision and starvation counteract each other by regulating the competition between MYBS1 and MYBS2 for binding to the TA box as a transcriptional activator and repressor of αAmy promoters, respectively. Notably, phosphorylation regulates the sugar-dependent nucleocytoplasmic shuttling of MYBS2 and its cytoplasmic interaction with 14-3-3 proteins, which plays an essential role in regulating the on/off switch of reversible gene expression in response to sugar status. We also observed that manipulation of MYBS2 and αAmy can lead to beneficial effects on plant growth, stress tolerance, and grain productivity, representing a unique approach to crop improvement.
Results
MYBS2 Is a Negative Regulator of Germination and Plant Growth, and Suppresses αAmy Expression.
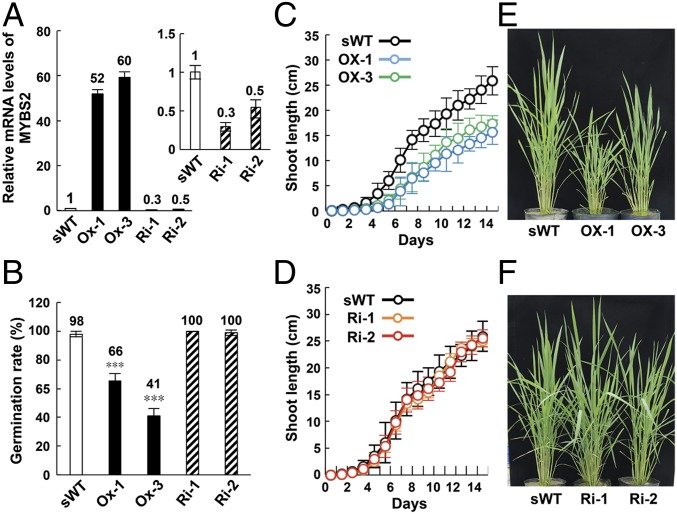
We first investigated the physiological function of MYBS2 in plant growth by gain- and loss-of-function analyses in transgenic rice overexpressing either MYBS2 cDNA or MYBS2 RNA interference (Ri) constructs under the control of the Ubi promoter. Levels of recombinant MYBS2 mRNAs increased by 52- to 60-fold in transgenic seedlings of 2 overexpressing (Ox) lines, whereas endogenous MYBS2 mRNA decreased by 50 to 70% in 2 silencing (Ri) lines, relative to the segregated WT (sWT) (Fig. 1A). We compared the phenotypes of these transgenic lines to sWT and found that germination rates in 2 Ox lines were reduced by 34 to 59%, but were unchanged in the Ri lines (Fig. 1B). Moreover, seedling growth up to 14 d was delayed in Ox lines but remained unchanged in the Ri lines (Fig. 1 C and D). Plant height at 90 d (i.e., directly before heading) was also shorter in Ox lines, but similar to sWT in Ri lines (Fig. 1 E and F). The seed size of Ox lines is not different from that of the sWT.
Fig. 1.
MYBS2 is a negative regulator of germination and plant growth. sWT and transgenic rice lines overexpressing (Ox) or underexpressing (Ri) MYBS2 were used in the experiment. (A) Total RNA were extracted from leaves of 7-d-old seedlings and subjected to qRT-PCR analysis. The Inset shows comparison of MYBS2 mRNA levels between sWT and Ri lines. (B) Transgenic seeds were germinated in −S medium at 28 °C for 5 d, before determining germination rates. Error bars represent SD. Asterisks indicate significant differences (Student’s t test, ***P < 0.001). (C and D) Two-day-old seedlings of sWT and transgenic lines with similar shoot lengths were grown in medium for up to 14 d. Seedling growth was determined by measuring shoot length. (E and F) Plants in C and D were transferred to a greenhouse for continuous growth and the morphology of 90-d-old plants was assessed. n = 30 for all experiments.
To determine whether αAmy expression is regulated by MYBS2, we first cultured embryo calli of MYBS2 Ox and Ri lines in a medium without sugar (−S), and then shifted cells to a medium with sugar (+S). Levels of αAmy3 and aAmy8 mRNAs gradually decreased with time in sWT, and were rapidly reduced in an Ox line, but not significantly altered in an Ri line (SI Appendix, Fig. S1). We also observed that αAmy3 expression in rice seedlings was suppressed in Ox lines (by 40 to 60%), but was activated in RNAi lines (by 5- to 6-fold) (Fig. 2A).
Fig. 2.
MYBS2 represses αAmy expression and promoter activities through the TA box. (A) Seedlings of sWT, MYBS2 (full-length or truncated) Ox and Ri lines were cultured in −S medium for 10 d. Total RNAs were extracted from leaves and used for qRT-PCR analysis using αAmy3-specific primers. (B and C) Rice embryos were cotransfected with effector and reporter plasmids, incubated in −S medium for 24 h, before assaying for luciferase activity. The value for luciferase activity of the reporter construct in the absence of the effector was set to 1×, and all other values were calculated relative to this value. Error bar indicates the SE for 3 replicate experiments. (B) Effector constructs. (C) Luciferase activities of in rice embryos carrying reporter constructs aAmy3-35Smp:Luc and 6xTA-35Smp:Luc in the presence of effector constructs. Asterisks indicate significant differences (Student’s t test, *P < 0.05, **P < 0.01).
MYBS2 Competes against MYBS1 for Binding to the TA Box and Represses αAmy Promoter under Sugar Starvation.
To understand the mechanism of MYBS2-mediated sugar repression of αAmy expression, we assessed the effect of MYBS1, MYBS2, and MYBS2(Ri) (expression driven by the Ubi promoter) (Fig. 2B) on the activity of promoters containing the αAmy3 SRC and 6 tandem repeats of the TA box (6xTA) individually fused to the CaMV35S minimal promoter using a rice embryo transient expression system. Our results showed that the activity of the 2 promoters was enhanced by MYBS1, and was even more significantly enhanced by the MYBS2 Ri construct, but was repressed by MYBS2 in −S medium (Fig. 2C). These gain- and loss-of-function analyses suggest that MYBS2 is a transcriptional repressor and that it offsets the transcriptional transactivation activity of MYBS1 on the TA box of αAmy3 SRC promoters under sugar starvation.
We investigated the effect of different MYBS1:MYBS2 ratios on the activity of the 6xTA promoter using effector and reporter constructs and the rice embryo transient expression system (SI Appendix, Fig. S2A). We found that promoter activity was considerably enhanced upon transfection of a fixed amount of MYBS2 but increasing amounts of MYBS1 in both +S and −S media (SI Appendix, Fig. S2B), and the same was true for fixed amounts of MYBS1 but increasing amounts of MYBS2(Ri) (SI Appendix, Fig. S2C). Together, these data indicate that MYBS1 and MYBS2 compete against each other for binding to the 6xTA promoter.
Nuclear Import of MYBS2 Is Promoted by Sugar Provision.
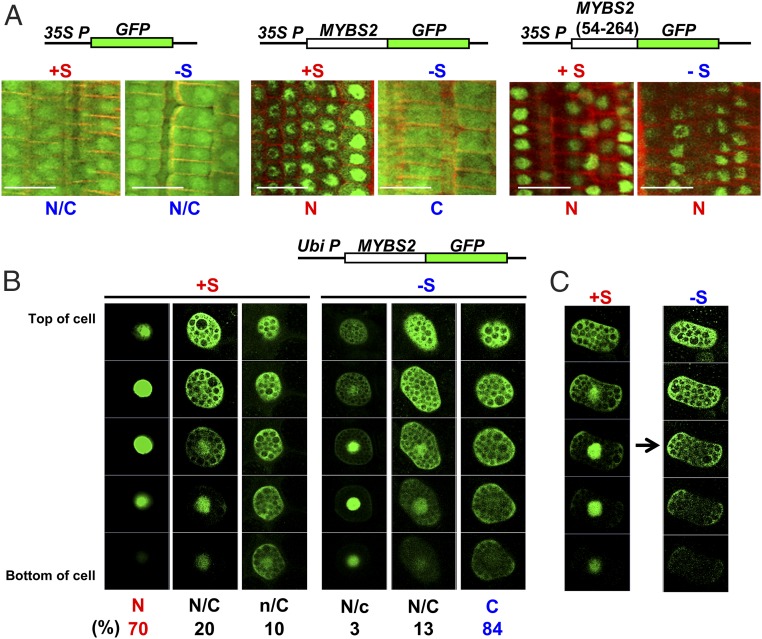
To investigate if MYBS2 function is regulated at the subcellular level, we expressed CaMV35S:MYBS2-GFP and CaMV35S:GFP in transgenic rice and examined images of GFP signal in root tips. GFP by itself localized to both the nucleus and cytoplasm, regardless of whether +S or −S medium was used, whereas MYBS2-GFP was found in the nuclei of root cells incubated in +S medium but in the cytoplasm of those cultured in −S medium (Fig. 3A and SI Appendix, Fig. S3).
Fig. 3.
Nuclear import of MYBS2 is promoted by sugar provision. (A) Roots of 5-d-old transgenic rice seedlings overexpressing CaMV35S:GFP and CaMV35S:MYBS2-GFP and CaMV35S:MYBS2(54-264)-GFP were incubated in +S or −S medium for 24 h under darkness, and the GFP signal in root tips was examined by confocal microscopy. The red-colored cell walls were stained with propidium iodide that is a membrane-impermeable dye for staining the extracellular space and its constituents (including cell walls and secreted polysaccharides). (Scale bar, 20 μm.) (B and C) Barley aleurones were transfected with Ubi:MYBS2-GFP and incubated in +S or −S medium for 24 h. Thirty optical sections, each of 0.9 to 1.1 μm thickness, were prepared for each cell (SI Appendix, Fig. S4), but only 5 regularly spaced sections (sections 4, 10, 16, 22, and 28; from top to bottom of cell) are shown here. “N” and “C” indicate higher GFP signals in the nucleus and cytoplasm, respectively, whereas “n” and “c” represent lower respective signals. Percentage indicates the number of cells with GFP distribution in the nucleus or cytoplasm divided by the total number of cells examined. n > 200. (B) After transfection, barley aleurones were incubated in +S or −S medium for 24 h. (C) After transfection, barley aleurones were incubated in +S medium for 24 h, and transferred (indicated by the arrow) to −S medium for a further 24 h.
We also investigated sugar-mediated regulation of the subcellular localization of MYBS2 based on the spatial dynamics of MYBS2-GFP using a barley aleurone transient expression system (19, 28). As MYBS2-GFP signal was distributed throughout different focal planes of the aleurone cells, we prepared 30 optical sections for each cell (SI Appendix, Fig. S4), but only 5 regularly spaced sections representing each cell are shown in Fig. 3B. MYBS2 was mainly localized (∼70%) in the nucleus in +S medium, but predominantly (∼84%) in the cytoplasm in −S medium (Fig. 3B and SI Appendix, Fig. S4 A and B). Localization of MYBS2-GFP shifted from the nucleus to the cytoplasm when the same cell was transferred from +S to −S medium (Fig. 3C and SI Appendix, Fig. S4C).
Collectively, these data demonstrate that although MYBS2 shuttles between the nucleus and cytoplasm, it is preferentially imported into the nucleus when sugar is provided.
MYBS2 Is a Phosphoprotein.
Bioinformatics analysis using the NetPhos 3.1 server (http://www.cbs.dtu.dk/services/NetPhos/) revealed several potential phosphorylation sites in MYBS2. To determine if MYBS2 is phosphorylated, total proteins extracted from the embryo calli of transgenic rice carrying CaMV35S:MYBS2-GFP were subjected to regular and Phos-Tag immunoblot assays using anti-GFP antibodies. Two protein bands were detected in most samples with the Phos-Tag immunoblot assay (SI Appendix, Fig. S5 A and B). To confirm that MYBS2 is phosphorylated, we treated total proteins with λ protein phosphatase prior to Phos-Tag immunoblotting. We detected 2 protein bands with molecular mass of ∼70 kDa in cells cultured in +S and −S media, but their molecular weights shifted to ∼56 kDa (the predicted molecular weights of MYBS2 plus GFP) upon treatment with λ protein phosphatase (SI Appendix, Fig. S5C), indicating that the 2 higher molecular mass proteins were phosphorylated forms of MYBS2. Thus, our analysis reveals that MYBS2 is phosphorylated in both +S and −S media.
Immunoprecipitation (IP) coupled with mass spectrometry can identify phosphorylation sites in a target protein with high sensitivity (29). Accordingly, we extracted total protein from embryo calli of the CaMV35S:MYBS2-GFP transgenic line and subjected it to IP using a GFP-trapping method followed by mass spectrometry-based analysis. We unequivocally identified 2 phosphorylation sites at Ser53 and Ser75 in MYBS2 (SI Appendix, Fig. S6).
Phosphorylation at Ser75 Promotes the Sugar-Dependent Nuclear Localization of MYBS2.
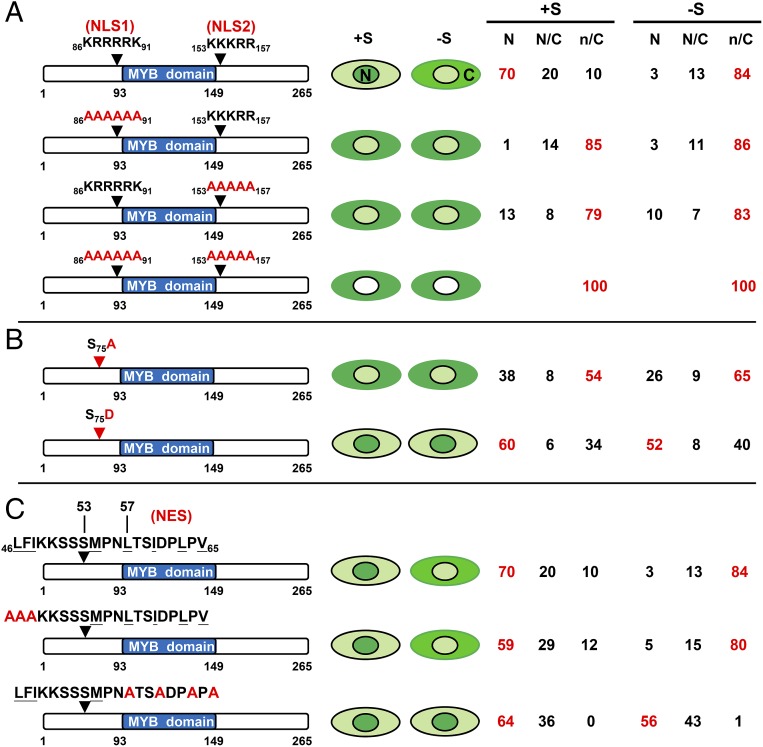
MYBS2 contains 2 putative nuclear localization signals (NLSs) rich in Arg (R) and Lys (K) amino acids—86KRRRRK91 (designated as NLS1) and 153KKKRR157 (NLS2)—flanking the MYB domain (Fig. 4A). To determine if these 2 putative NLSs play a role in regulating nucleocytoplasmic shuttling of MYBS2, we generated constructs with mutations in the 2 putative NLSs by substituting Arg/Lys with Ala (A) and analyzed their activity in barley aleurones. As depicted in Fig. 4A, WT and both single NLS-mutated MYBS2 constructs were primarily distributed in the cytoplasm in −S medium. In +S medium, WT MYBS2 was mainly localized in the nucleus, but both single NLS-mutated MYBS2 constructs were predominantly distributed in the cytoplasm. However, mutation of both NLSs resulted in exclusively cytoplasmic localization of MYBS2, regardless of whether medium included sugar (+S) or lacked it (−S). These results indicate that mutation of either NLS1 or NLS2 impairs the nuclear import of MYBS2 and that mutation of both NLSs completely prevents nuclear import of MYBS2, regardless of the presence or absence of sugar in the medium.
Fig. 4.
MYBS2 contains NLS and NES and phosphorylation at Ser75 regulates its sugar-dependent nuclear localization. (Left) Barley aleurones were transfected with Ubi:MYBS2 (full-length or mutated)-GFP and incubated in +S or −S medium for 24 h. (Center) Dark green indicates higher GFP signal, light green indicates lower GFP signal, and white indicates no GFP signal in the nucleus (N) or cytoplasm (C). (Right) nucleocytoplasmic partitioning (in percent, %) of different forms of MYBS2-GFP. “N” and “C” indicate higher GFP signals in the nucleus and cytoplasm, respectively, whereas “n” and “c” represent lower respective signals. n > 200. (A) Conserved amino acid residues Lys (K) and Arg (R) of the bipartite NLS (NLS1 and NLS2) flanking the MYB domain in MYBS2 were substituted with Ala (A). (B) Ser75 of MYBS2 was substituted with Ala or Asp (D). (C) Conserved hydrophobic amino acid residues (underlined) in NES of MYBS2 were substituted with Ala.
Phosphorylation plays an important role in regulating the nucleocytoplasmic trafficking of cargo proteins, and phosphorylation upstream of the NLS has been shown to enhance the nuclear import of the large tumor antigen of simian-virus 40 (SV40 T-antigen) (30). We investigated if phosphorylation at Ser75 (SI Appendix, Fig. S6B) impacts MYBS2 nuclear import by generating constructs in which Ser75 was substituted with an amino acid that cannot be phosphorylated (Ala) or mimics constitutive phosphorylation (Asp). We found that relatively more MYBS2(S75A)-GFP accumulated in the cytoplasm and more MYBS2(S75D)-GFP accumulated in the nucleus of both +S and −S media (Fig. 4B and SI Appendix, Fig. S7), demonstrating that phosphorylation at Ser75 promotes nuclear import of MYBS2 and that it may be responsible for the nuclear localization of MYBS2 under conditions of sugar provision.
During the course of identifying functional domains of MYBS2, we found that deletion of amino acids 1 to 53 conferred greater repression on 6xTA promoter activity (SI Appendix, Fig. S8 A, Left), resulting in exclusively nuclear localization of MYBS2-GFP in both +S and −S media (SI Appendix, Fig. S8 A, Right). This observation indicates that this N-terminal domain may contain a nuclear export signal (NES) or amino acid sequences required for retention of MYBS2 in the cytoplasm. This supposition was confirmed by MYBS2(54-265)-GFP with a functional NLS1 or NLS2 being exclusively localized in the nucleus, or strictly in the cytoplasm upon both NLSs being mutated, in both +S and −S media (SI Appendix, Fig. S8B). MYBS2(54-265)-GFP was also exclusively localized in the nucleus in transgenic rice roots incubated in both +S and −S media (Fig. 3A). Bioinformatic analysis predicted a putative NES that is located at amino acids 46 to 65 of MYBS2 (Fig. 4C). Mutation of hydrophobic amino acids at the N-terminal end of this putative NES did not affect the nuclear localization of MYBS2 in +S or −S medium, but changes of hydrophobic amino acids at the C-terminal region (amino acids 57 to 65) led to increased nuclear localization of MYBS2 in −S medium (Fig. 4C). This result indicates that amino acids 57 to 65, which match well the canonical NES consensus sequence (Φ-X2-Φ-X2-Φ-X-Φ, Φ = L, I, or V) (31), is indeed the core of a functional NES.
MYBS2 Interacts with Specific 14-3-3 Protein.
To identify proteins that may interact with MYBS2 and regulate its nucleocytoplasmic shuttling, we extracted total proteins from embryo calli of the CaMV35S:MYBS2-GFP and CaMV35S:GFP (as a control) transgenic lines and subjected them to coimmunoprecipitation (co-IP) using a GFP-trapping method followed by linear-trap quadrupole mass spectrometry-based proteomic analysis. MYBS2-interacting proteins were identified from 2 MYBS2-GFP overexpressing transgenic lines, but not from the control, under highly stringent criteria with a false-discovery rate of <1% for peptides identification. Proteins with top Mascot scores >400 are shown in SI Appendix, Table S1. Among them, 7 rice GF14 (14-3-3) proteins were identified as being highly abundant MYBS2-interacting partners. Most of these GF14 proteins exhibited high sequence coverage and a large number of significant unique peptides in datasets from 2 transgenic lines, indicative of relatively high-confidence candidate interacting proteins. Expression of several GF14 is activated by sugar starvation (SI Appendix, Fig. S9).
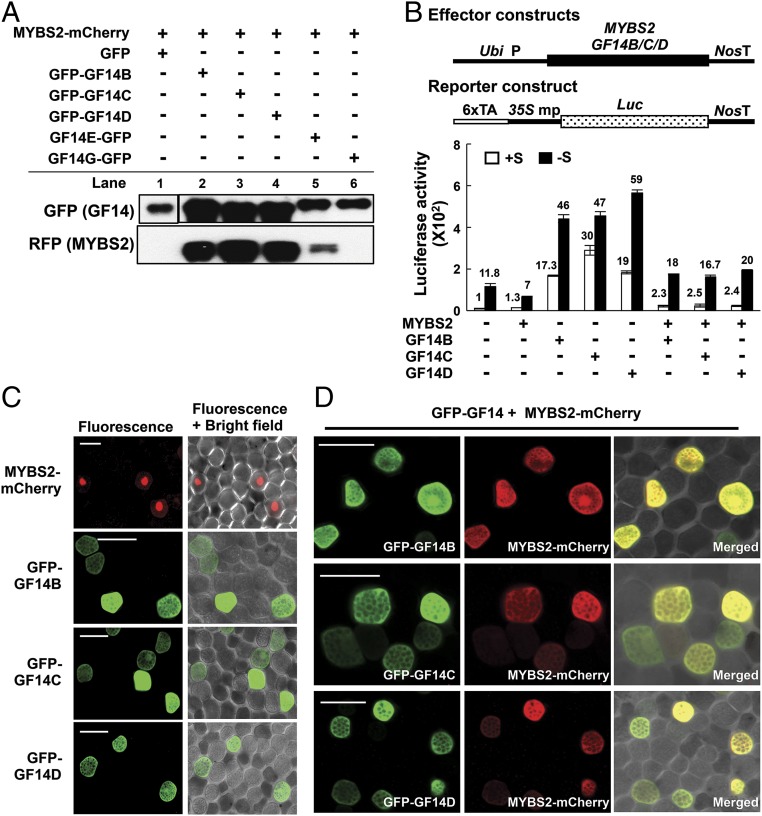
We selected 4 of the GF14 candidate proteins with high Mascot scores (GF14B, GF14C, GF14D, and GF14E) as representatives for interaction with MYBS2 and 1 with a lower score (GF14G) for further analysis. For in vivo interaction assays, we cotransfected rice embryo calli with Ubi:MYBS2-mCherry and individual Ubi:GFP-GF14, Ubi:GF14-GFP or Ubi:GFP (as a negative control) constructs. Total proteins were extracted and the MYBS2-GF14 protein complex was isolated using the GFP-trapping method before conducting immunoblot analysis using anti-GFP and anti-RFP antibodies. The anti-GFP antibodies could detect the GFP− control and all GFP-tagged GF14 proteins, whereas the anti-RFP antibodies could only detect MYBS2-mCherry in the elution of GFP-tagged GF14B, GF14C, GF14D, and GF14E, but not GF14G or the GFP-negative control (Fig. 5A). Similar results were obtained with the in vitro interaction assays (SI Appendix, Fig. S10).
Fig. 5.
MYBS2 specifically interacts with certain 14-3-3 proteins (GF14). (A) In vivo co-IP and immunoblot analysis: Rice embryo calli were cotransfected with Ubi:MYBS2-mCherry and Ubi:GFP-GF14 or Ubi:GFP (negative control) constructs by particle bombardment and incubated in +S medium for 16 h. Total proteins were extracted and GFP/GFP-fused proteins were isolated using GFP trap. Anti-GFP and anti-RFP antibodies were then used to detect coprecipitated GFP-GF14 or GF14-GFP and MYBS2-mCherry proteins, respectively, by means of immunoblotting. (B) Rice embryo calli were cotransfected with the effectors Ubi:MYBS2 and Ubi:GF14 and the reporter 6xTA-35Smp:Luc, then incubated in +S or −S medium for 24 h, before assaying for luciferase activity. Values for luciferase activity of the reporter construct in the absence of the effector were set to 1× and other values were calculated relative to this value. (C) Barley aleurones were cotransfected with Ubi:MYBS2-mCherry or Ubi:GFP-GF14 alone, incubated in +S medium for 24 h, and then examined under confocal microscopy. (Scale bars, 50 µm.) (D) Barley aleurones were cotransfected with Ubi:MYBS2-mCherry and Ubi:GFP-GF14, incubated in +S medium for 24 h, and then examined under confocal microscopy. (Scale bars, 50 µm.)
Sugar-Mediated Repression of the αAmy3 Promoter Is Released by Cytoplasmic 14-3-3 Proteins Restricting MYBS2.
We further investigated the role of 14-3-3 proteins in regulating MYBS2 function. Rice embryo calli were cotransfected with the effectors Ubi:MYBS2 and Ubi:GF14 and the reporter 6xTA-35S mp:Luc. Individually, GF14B, GF14C, and GF14D significantly enhanced 6xTA promoter activity in both +S and −S medium, whereas cotransfection of GF14 with MYBS2 only partially did so (Fig. 5B). Since MYBS2 is a phosphoprotein, we further investigated if 14-3-3 proteins regulate the subcellular localization of MYBS2 in response to sugar levels. Barley aleurones were transfected with Ubi:MYBS2-mCherry or Ubi:GFP-GF14 alone and incubated in +S or −S medium. MYBS2-mCherry was localized in the nucleus in +S medium, whereas 3 GFP-GF14s were found in the cytoplasm regardless of whether +S or −S medium was employed (Fig. 5C and SI Appendix, Fig. S11). We then cotransfected Ubi:MYBS2-mCherry and individual Ubi:GFP-GF14 into barley aleurones and incubated them in +S medium. Interestingly, MYBS2-mCherry became mostly localized in the cytoplasm upon interacting with individual GFP-GF14 proteins (Fig. 5D), demonstrating that retention of MYBS2 in the cytoplasm through interaction with 14-3-3 proteins is responsible for relieving sugar repression of the TA box under sugar starvation.
Phosphorylation of MYBS2 at Ser53 Is Necessary for Interaction with 14-3-3 Proteins in the Cytoplasm under Sugar Starvation.
MYBS2 contains a putative 14-3-3 binding domain 49KKSSSMP55 (consensus sequence underlined) that matches well with the consensus 14-3-3 binding motif (R/K)XX(pS/pT)XP or (R/K)XXX(pS/pT)XP (32, 33). The Ser53 residue in the consensus 14-3-3 binding motif of MYBS2 (Fig. 6A) was predicted as a phosphorylation site based on our mass spectrometry analysis (SI Appendix, Fig. S6A). To investigate whether phosphorylation in the consensus 14-3-3 binding motif regulates its interaction with MYBS2, we substituted each Ser residue in the Ser51-53 amino acid cluster to Ala. Single or triple Ser mutation variants of MYBS2—MYBS2(S51A), MYBS2(S52A), MYBS2(S53A), and MYBS2(S51-53A)—were fused to mCherry, and rice embryo calli were cotransfected with Ubi:GFP-GF14D (a representative of GF14) and Ubi:MYBS2 (WT or mutant). The MYBS2-GF14D protein complex was isolated from total cell extract using the GFP-trapping method and subjected to immunoblot analysis using anti-GFP and anti-RFP antibodies. The anti-GFP antibodies could detect GFP-GF14D present in all protein complex samples, but the anti-RFP antibodies could only detect MYBS2-mCherry in protein samples containing WT MYBS2, MYBS2(S51A), or MYBS2(S52A), but not MYBS2(S53A) or MYBS2(S51-53A) (Fig. 6B). This analysis demonstrates that phosphorylation at Ser53 is necessary for the interaction between MYBS2 and 14-3-3 proteins.
Fig. 6.
Phosphorylation of MYBS2 at Ser53 is necessary for interactions with 14-3-3 (GF14) proteins in the cytoplasm. (A) Consensus 14-3-3 binding motif (underlined) in MYBS2. Potential phosphorylation residues Ser51-53 (red font) in MYBS2 were identified by LC/MA/MS (SI Appendix, Fig. S6A). (B) Rice embryo calli were cotransfected with Ubi:MYBS2 (point mutated)-mCherry and Ubi:GFP-GF14D constructs and incubated in +S medium for 24 h. Total proteins were extracted and GFP-GF14D was isolated using GFP trap. Anti-GFP and anti-RFP antibodies were then used to detect coprecipitated GF14 and MYBS2 proteins, respectively, by means of immunoblotting. (C–F) Barley aleurones were transfected with point-mutated MYBS2 alone or with 14-3-3 protein (GF14D), then incubated in +S (C and D) or −S (E and F) medium for 24 h, before being examined under confocal microscopy. (Scale bars, 50 µm.) “N” and “C” indicate higher GFP or mCherry signals in the nucleus and cytoplasm, respectively, whereas “n” represents lower signals in the nucleus. (C and E) Barley aleurones were transfected with Ubi:MYBS2(S51A)-GFP, Ubi:MYBS2(S52A)-GFP or Ubi:MYBS2(S53A)-GFP. (D and F) Barely aleurones were cotransfected with Ubi:MYBS2(mutant)-mCherry and Ubi:GFP-GF14D.
To further investigate whether phosphorylation at Ser53 is necessary for sequestering MYBS2 in the cytoplasm under sugar starvation, we transfected barley aleurones with Ubi:MYBS2(mutant)-GFP constructs. The MYBS2-mCherry protein had already been shown to localize in the nucleus in +S medium (Fig. 5C). We used GFP as the reporter here to demonstrate that the subcellular localization of mutant MYBS2 is not affected by fusion with different reporters. In +S medium, MYBS2(mutant)-GFP alone localized in the nucleus (Fig. 6C). We then cotransfected barley aleurones with Ubi:MYBS2(mutant)-mCherry and Ubi:GFP-GF14D constructs. In +S medium, GF14D localized in the cytoplasm, as did MYBS2(S51A) and MYBS2(S52A), whereas MYBS2(S53A) remained in the nucleus (Fig. 6D). In −S medium, a large proportion of MYBS2(S51A) and MYBS2(S52A) became localized in the cytoplasm, probably due to sequestration by endogenous GF14 proteins, but MYBS2(S53A) remained in the nucleus (Fig. 6E). We then cotransfected barley aleurones with Ubi:MYBS2(mutant)-mCherry and Ubi:GFP-GF14D and incubated in −S medium, and found that GF14D, MYBS2(S51A) and MYBS2(S52A) were all localized in the cytoplasm, whereas MYBS2(S53A) remained in the nucleus (Fig. 6F). We also performed a control transfection experiment to show that the MYBS2(mutant)-mCherry signal was not a bleed-over due to strong GFP signal (SI Appendix, Fig. S12). Together, these findings demonstrate that phosphorylation of MYBS2 at Ser53 is necessary for this protein to be retained in the cytoplasm by 14-3-3 proteins under sugar starvation.
Both MYBS1 and MYBS2 Are Present in the Nucleus Early in the Transition from Normal Metabolic to Sugar-Depleted Conditions.
Nuclear export of MYBS1 occurs concomitantly with nuclear import of MYBS2 under conditions of sugar provision (19) (Fig. 3). To determine whether MYBS1 and MYBS2 can both be present in the nucleus and compete for binding to the TA box in αAmy promoters during the transition from normal metabolic (high sugar level) to sugar-depleted (low sugar level) conditions, we investigated the kinetics of nucleus-cytoplasm shuttling of MYBS1 and MYBS2. We cotransfected barley aleurones with Ubi:MYBS1-mCherry and Ubi:MYBS2-GFP. Transfected cells were incubated in −S or +S media for 16 h, and then transferred to +S or −S media for another 20 h, respectively (SI Appendix, Fig. S13 A and C). A shift in fluorescence intensity between the nucleus and cytoplasm could be detected for both MYBSs upon transferring cells between the +S and −S conditions (SI Appendix, Fig. S13 B and D). Although increase in nuclear export of MYBS1 occurred, MYBS2 was rapidly imported into the nucleus upon transferring cells from −S to +S conditions (SI Appendix, Fig. S13B). In contrast, although MYBS1 was rapidly imported to the nucleus and MYBS2 was exported to the cytoplasm upon transferring cells from +S to −S conditions, MYBS1 and MYBS2 could still be colocalized in the nucleus prior to transferring to −S conditions (SI Appendix, Fig. S13D). These results indicate that relatively high levels of MYBS1 are colocalized with MYBS2 in the nucleus during the transition between high sugar level and sugar-depleted conditions.
Reduced MYBS2 Expression Up-Regulates αAmy under Abiotic Stress, and Ectopic Expression of αAmy3 Enhances Abiotic Stress Tolerance.
Since αAmy is activated by various biotic and abiotic stresses such as water stress, viral/bacterial infection, wounding, heat, or abscisic acid (ABA) in different plant species (34–37), we investigated whether MYBS2 regulates αAmy3 expression in response to abiotic stresses. We found that expression of MYBS2 in rice seedlings was suppressed by dehydration (air drying; by 70%) and heat (42 °C; by 80%), with concomitant dramatic activation of αAmy3 expression under conditions of dehydration (by 95-fold) and heat stress (by 2,478-fold) (Fig. 7A).
Fig. 7.
Reduced MYBS2 expression up-regulates αAmy3 that enhances abiotic stress tolerance in rice. (A and B) Total RNAs were extracted from seedlings for qRT-PCR analysis using αAmy3-, αAmy7- and MYBS2-specific primers. The value of mRNA level in the nontreated control (CK) was set to 1×, and all other values were calculated relative to this value. (A) Ten-day-old seedlings of sWT rice were treated with the indicated abiotic stress. (B) Seeds of sWT, MYBS2 (full-length or truncated) Ox and Ri lines were germinated in medium with or without 400 mM sorbitol (S) for 8 d. n = 60. (C and D) Shoot length of seedlings in experiment (B) was determined and plant morphology was photographed on day 8. (E–I) Transgenic rice overexpressing Ubi:αAmy3 or αAmy3:αAmy3 were used in the experiment. Seeds were germinated in medium without sorbitol (CK) or with 400 mM sorbitol (S). (E) Total RNA were extracted from 8-d-old seedlings for qRT-PCR analysis using aAmy3-specific primers. (F and H) Germination rates were determined daily up to day 5. (G and I) Shoot length was determined and plant morphology was photographed at day 8. n = 30. Error bars represent SD. Asterisks indicate significant differences (Student’s t test, **P < 0.01, ***P < 0.001).
To determine whether suppression of MYBS2 and activation of αAmy is correlated with stress tolerance, we subjected MYBS2 Ox and Ri lines to osmotic stress treatment (i.e., sortibol treatment to mimic osmotic stress). We found that aAmy3 and aAmy7 expression in both control and sorbitol-treated seedlings was reduced in MYBS2-Ox and MYBS2(54-265)-Ox lines but increased in Ri lines (Fig. 7B). Seedling shoot length of MYBS2-Ox lines was shorter than that of the sWT under both control and sorbitol-treated conditions (Fig. 7 C and D), and significantly greater than that of the sWT in Ri lines under sorbitol-treated conditions (Fig. 7D). These data indicate that reduced MYBS2 expression not only up-regulates aAmy expression, particularly under osmotic stress, but it also enhances osmotic stress tolerance in rice.
We also generated transgenic rice carrying Ubi:αAmy3 or αAmy3:αAmy3, and first showed that accumulation of αAmy3 was significantly increased in transgenic lines under sorbitol treatment (Fig. 7E). The significantly greater expression of αAmy3 driven by the Ubi promoter under sorbitol treatment could be due to enhanced stability of αAmy3 mRNA, a posttranscriptional regulation similar to sugar starvation (3, 11, 12). The germination rate and seedling shoot length of αAmy3-Ox lines were similar to sWT without sorbitol treatment (Fig. 7 F and G) but they were significantly greater than sWT under sorbitol treatment (Fig. 7 H and I), indicating that ectopic expression of αAmy3 confers osmotic stress tolerance on rice.
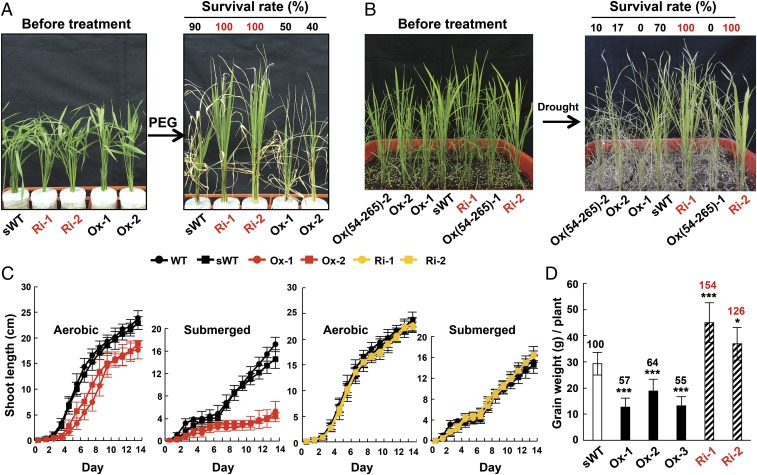
To further investigate whether down-regulation of MYBS2 improves plant stress tolerance, we subjected the MYBS2 Ox and Ri lines to various abiotic stress treatments. Using PEG treatment to mimic osmotic stress, we observed that the survival rate of the Ri lines (100%) was twice that of the Ox lines (40 to 50%) and higher than that for the sWT (90%) (Fig. 8A). Upon drought treatment in soil, the survival rate of the Ri lines (100%) was significantly greater than that of the sWT line (70%), and dramatically greater than that of the MYBS2-Ox lines (0–17%) and MYBS2(54-265)-Ox lines (0–10%) (Fig. 8B). Deletion of the N-terminal amino acid residues 1 to 53 caused exclusively nuclear localization of MYBS2 (Fig. 3A and SI Appendix, Fig. S8B), which is consistent with the low expression of αAmy3 in rice seedlings (Figs. 2A and 7B) and the reduced survival rate of seedlings under drought stress (Fig. 8B). The growth of seedlings was delayed slightly under aerobic conditions but significantly under submerged conditions in the Ox lines relative to the sWT. However, the performance of the Ri lines was similar to the sWT (Fig. 8C). These studies demonstrated that reduced MYBS2 expression or enhanced αAmy3 expression promotes osmotic and drought stress tolerance in rice. Although overexpression of MYBS2 results in lower tolerance to submergence, reduced MYBS2 expression does not enhance submergence tolerance.
Fig. 8.
Reduced MYBS2 expression enhances osmotic and drought stress tolerance and total grain weight per plant in rice. Segregated (sWT), MYBS2-Ox, MYBS2(54-265)-Ox, and MYBS2-Ri lines were used in these experiments. (A) Ten-day-old seedlings were treated with 15% PEG for 7 d and 20% PEG for another 16 d before determining the survival rate. n = 10. (B) Ten-day-old seedlings were transferred to soil with regular water for 3 wk, before cessation of watering for the next 21 d, and then the survival rate was determined. n = 10. (C) Seeds were germinated in medium, and shoots grown under aerobic or submerged condition for 14 d, shoot length was then determined. n = 30. (D) Total grain weight per rice plant grown in irrigated field. The value of total grain weight per plant in the sWT was set to 100%, and all other values were calculated relative to this value. n = 12. Significance levels with the t test: *P < 0.05, ***P < 0.001.
We also found that the total grain weight per plant was reduced in 3 MYBS2 Ox lines, yet increased in 2 MYBS2 Ri lines, in greenhouse and field conditions (SI Appendix, Fig. S14). The increase in grain weight per plant in the Ri-1 line is consistently greater than that in the Ri-2 line, which correlates with the lower MYBS2 mRNA level in the Ri-1 line than in the Ri-2 line (Fig. 1A). In a recent field test with T4 generation seeds, grain weights per plant were reduced by 36 to 45% for the Ox lines, but increased by 26 to 54% in the Ri lines, relative to the sWT (Fig. 8D). Together, these findings demonstrate that MYBS2 is a negative regulator of abiotic stress tolerance and seed development.
Discussion
Mechanisms Underlying Reciprocal Regulation of αAmy by MYBS1 and MYBS2 in Relation to Plant Growth and Stress Tolerance.
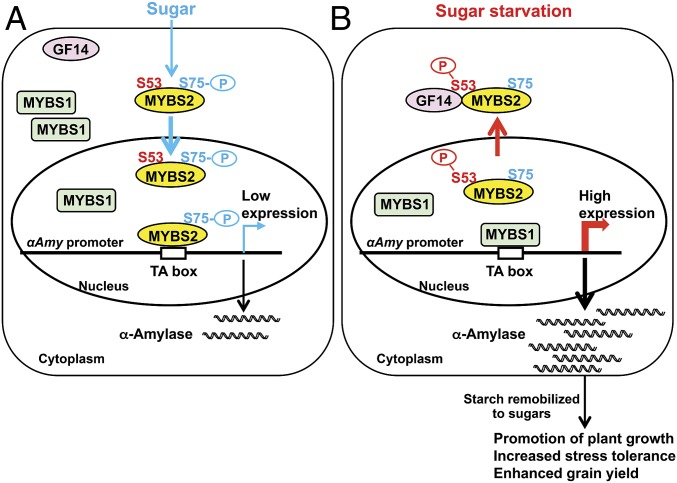
Our investigations have not only demonstrated that reversible sugar homeostatic states play important roles in plant growth and development, but we have also deciphered the molecular mechanism by which sugar levels switch on and off gene expression. As illustrated in Fig. 9, when sugar is supplied, Ser75-phosphorylated MYBS2 is imported into the nucleus to prevent MYBS1 from activation of αAmy. Under sugar starvation, Ser53-phosphorylated MYBS2 is exported from the nucleus and interacts with 14-3-3 proteins in the cytoplasm, leading to de-repression of αAmy. The sugar-mediated nucleocytoplasmic shuttling and competition between MYBS2 and MYBS1 for reversible gene expression constitutes an essential regulatory mechanism of sugar status on plant growth, stress tolerance and, ultimately, productivity. This regulatory system confers adaptive plasticity and secures optimal carbon supplies for plant growth under an ever-changing environment, and highlights MYBS2 and αAmy3 as targets for breeding stress-tolerant crops. Moreover, the significance of this work is beyond plant biology as reversible regulation of gene expression is a key to any organism in need to maintaining any sugar homeostatic states.
Fig. 9.
Proposed mechanism underlying reciprocal regulation of αAmy by MYBS1 and MYBS2 during sugar provision (A) and sugar starvation (B). Details of the model are described in the text.
Sugar homeostatic states represent a balance between demand for sugars and their production, and in plants, sugar homeostasis appears to cover a far broader range than in humans. In rice, hexose concentrations are increased from 30 to 60 mM in the embryo/seedling and from 80 to 500 mM in the endosperm within 7 d after germination (38). Together, MYBS1 and MYBGA are responsible for the rapid increase in sugar concentrations in the endosperm after germination (19). Sugars are transported from the endosperm to the embryo to support seedling growth, reflecting sugar status dynamics between the endosperm and the developing seedlings. While sugar concentration is increasing after germination, MYBS2 expression is also enhanced up to day 6 after germination (SI Appendix, Fig. S15A), when a balance between the action of MYBS1 and MYBS2 is likely achieved and sugar concentrations in the embryo/seedling stabilize by day 7 (38). As we have shown in this work, the balance between MYBS1 (to enhance αAmy expression) and MYBS2 (to lower αAmy expression) does not follow an all-or-none pattern, but reflects a more gradual shift in cellular localizations (SI Appendix, Fig. S13). Thus, MYBS1 and MYBS2 work together to maintain sugar levels within an acceptable range during rice seed germination and seedling growth in rice.
The On/Off Switching of αAmy Expression by Sugar Is Regulated by Nucleocytoplasmic Shuttling of 2 MYBSs That Compete for the Same Promoter cis-Element.
Although MYBS1 and MYBS2 shuttle between the nucleus and cytoplasm due to the presence of both NLS and NES, their respective high and low sugar-regulated nuclear export seems to follow different kinetics. The +S condition-induced nuclear export of MYBS1 is a relatively slow process (SI Appendix, Fig. S13B). This scenario is further supported by other cytoplasm-nucleus shuttling experiments. Under the +S condition for 24 h, 51% of MYBS1 is still present in the nucleus, yet 90% of MYBS2 has already been imported into the nucleus (19) (SI Appendix, Table S2). In contrast, under the −S condition for 24 h, 90% of MYBS1 has already been imported into the nucleus, whereas only 16% of MYBS2 is present in the nucleus, probably due to being efficiently trapped by cytoplasmic 14-3-3 proteins (SI Appendix, Table S2). Since MYBS1 does not exit the nucleus quickly, there are occasions when both MYBS1 and MYBS2 are in the nucleus soon (within hours) after shifting from −S to +S conditions, by which time competition for binding to the TA box in the aAmy promoter comes into play under normal metabolic (high sugar level) conditions. Therefore, our studies appear to support the model proposed in Fig. 9.
Here we show that MYBS2 is preferentially expressed in tissues (such as root hairs, companion cells and vascular bundles) where sugars are transported (SI Appendix, Fig. S15 B–E), and its expression is up-regulated by sugar transcriptionally and posttranscriptionally (SI Appendix, Figs. S9, S15B, and S16). Our previous study shows that MYBS2 represses the TA box promoter activity but it may weakly activate the αAmy3 SRC promoter activity (17). In the present study, our gain- and loss-of-function analyses indicate that MYBS2 competes against MYBS1 for binding to the TA box to repress αAmy promoters. Through transient expression assays, we found that αAmy3 SRC and the 6xTA box promoters were suppressed (by 50%) by MYBS2 overexpression, and activated (by 2.2- to 3.7-fold) by MYBS2(Ri) (Fig. 2C). These results are consistent with the outcome of our transgenic stable expression assays in which αAmy3 mRNA accumulation was suppressed (by 40 to 60%) by MYBS2 overexpression and activated (by 5.4- to 5.7-fold) by MYBS2(Ri) (Fig. 2A). Our studies consistently showed that MYBS1 is a much stronger activator than MYBS2 (17). Even if MYBS2 has a weak activator activity, it behaves like a repressor when competing with MYBS1. Since reduced expression of MYBS2 enhances plant growth, seed development and stress tolerance, plants would appear to be subject to sugar repression most of the time during their life cycle.
Phosphorylation of MYBS2 Plays Critical Roles in Regulating Its Sugar-Dependent Nucleocytoplasmic Shuttling and Interaction with 14-3-3 Proteins.
We found that sugar-dependent regulation of nucleocytoplasmic shuttling of MYBS2 is the reverse of that of MYBS1. The NLS and NES are directly involved in the sugar-dependent subcellular targeting of MYBS1 (19), whereas they do not seem to be directly involved in the sugar-dependent subcellular localization of MYBS2 (Fig. 4 and SI Appendix, Fig. S8B). Instead, we show that phosphorylated Ser75 upstream of NLS1 promotes the sugar-dependent nuclear localization of MYBS2 (Fig. 4B); this is a scenario somewhat similar to the phosphorylation at a Ser residue upstream of the NLS of the SV40 T-antigen to facilitate recognition of the NLS by importin α1 (an essential adaptor protein for the nuclear import of cargo proteins) that subsequently interacts with the nuclear pore complex and passes through the channel (30).
Our studies support the model that sugar-mediated repression of the TA box promoter is caused by MYBS2 (localized in the nucleus when sugar is supplied) preventing MYBS1 from binding to the TA box (Fig. 9A). Regulation of nucleocytoplasmic shuttling of a transcription factor by a 14-3-3 protein has previously been demonstrated in mammalian cells. The carbohydrate-response element-binding protein (ChREBP) is a transcriptional activator of genes encoding enzymes that convert excess carbohydrates to fat storage in the liver. Under glucose starvation, ChREBP is exported to the cytoplasm where it is sequestered by a 14-3-3 protein, leading to inhibition of the expression of enzymes necessary for glycolysis and lipogenesis (39). In our study, MYBS2 is a transcriptional repressor and its nuclear export and retention by 14-3-3 proteins in the cytoplasm are important for activation of αAmy under sugar starvation.
Based on our IP-coupled mass spectrometry analysis, MYBS2 possesses 1 phosphorylation site at Ser53 that resides within the consensus 14-3-3 protein-binding motif 49KKSSSMP55 (consensus sequence underlined). We found that phosphorylation at Ser53 alone is necessary for the interaction of MYBS2 with 14-3-3 proteins to retain MYBS2 in the cytoplasm under sugar starvation (Figs. 6 and 9B). This result is consistent with the predicted phosphorylation site in the consensus 14-3-3 protein-binding motif (R/K)XX(pS/pT)XP or (R/K)XXX(pS/pT)XP (32, 33). MYBS2(S51A)-GFP and MYBS2(S52A)-GFP with normal Ser53 are still exported to the cytoplasm, whereas MYBS2(S53A)-GFP without Ser53 is retained in the nucleus (Fig. 6E), indicating that phosphorylation of Ser53 is necessary for the localization of MYBS2 in the cytoplasm under sugar starvation.
MYBS2 possesses functional NLSs and NES and may shuttle dynamically between the nucleus and cytoplasm. However, 2 different mechanisms may be responsible for the preferential nuclear localization of MYBS2 when sugar is supplied and cytoplasmic localization of MYBS2 under sugar starvation. First, when sugar is supplied, lower 14-3-3 protein expression (SI Appendix, Fig. S9) in the cytoplasm facilitates the import of Ser75-phosphorylated MYBS2 to the nucleus (Fig. 9A). Second, higher 14-3-3 protein expression (SI Appendix, Fig. S9) in the cytoplasm under sugar starvation means greater opportunity for interaction with Ser53-phosphorylated MYBS2 exported from the nucleus and retention in the cytoplasm (Fig. 9B). This notion is supported by the observation that the localization of MYBS2 shifts from the cytoplasm to the nucleus when the NES loses functionality under sugar starvation (Fig. 4C). However, the other possibility that additional unidentified factors interact with and sequester the MYBS2 and 14-3-3 protein complex in the cytoplasm when sugar is scarce cannot be completely ruled out.
Eight 14-3-3 genes have been identified in rice, and expression of the 14-3-3 gene family is differentially or coordinately regulated by various abiotic stresses and hormones (40, 41), indicating that 14-3-3 proteins may act in a complex network to regulate gene expression. Increased levels of 14-3-3 proteins may sequester MYBS2, preventing it from entering the nucleus during certain plant growth stages or under abiotic stress conditions. This assumption is supported by our previous study showing that GF14C is activated by both sugar starvation and drought stress, and that ectopic overexpression of GF14C confers drought tolerance on transgenic rice seedlings (42). In the present study, we have demonstrated that several GF14s interact with and restrict MYBS2 in the cytoplasm, allowing MYBS1 to activate the TA box-containing promoters of αAmy in response to low sugar levels or abiotic stress (Fig. 9B).
Suppression of MYBS2 and Activation of αAmy3 Improves Plant Growth, Abiotic Stress Tolerance, and Total Grain Weight per Plant in Rice.
Amylolytic breakdown of stored starch in seeds during germination, or of transitory starch accumulated in the chloroplasts of photosynthetic tissues at night, is a central biochemical event for providing reducing sugars as carbon sources during growth of both dicot and monocot plants. In the endosperm of cereal grains and the cotyledon of most dicots, such as legumes, αAmy is induced by GA and suppressed by ABA upon germination (43, 44). However, in vegetative tissues of both monocots and dicots, αAmy can be induced by various biotic and abiotic stresses, such as by water stress in barley leaves (34), virus infection in tobacco leaves (35), wounding in mung bean cotyledons (36), heat, prolonged darkness, and senescence in pea leaves (45, 46), and ABA, heat, and bacterial pathogens in Arabidopsis leaves (37). The physiological significance of stress-induced αAmy in vegetative tissues was recently addressed in Arabidopsis. Impaired mobilization of starch to sugars in leaves of αAmy- and βAmy-defective Arabidopsis mutants reduces carbon export to roots, which dampens sugar osmolyte accumulation and reduces root growth during osmotic stress, and this osmotic stress-induced starch hydrolysis is mediated by an ABA-dependent pathway (47).
Stress-induced accumulation of osmolytes lowers the water potential of cells and promotes water retention in the plant—a process known as “osmotic adjustment”—thereby maintaining cell turgor for plant growth and survival under stress conditions (48, 49). However, sugars can also stabilize proteins and cell structures (50), and they provide protection against oxidation by removing excess reactive oxygen species (51). Hence, adjustment of sugar production, storage, and utilization in response to changing environments may affect plant fitness, survival, and biomass production.
The TA box is present in the promoters of up to 1,300 genes expressed during rice germination (19, 52), and is also a highly conserved motif involved in the up-regulation of promoters of at least 20 genes under sugar starvation in rice suspension cells (53). MYBS2 competes with MYBS1 for binding to the TA box, therefore it is very likely that MYBS2 has multiple target genes. In this study, we show that expression of MYBS2 is suppressed whereas that of αAmy3 and aAmy7 is induced by dehydration, heat, and osmotic stress in rice seedlings (Fig. 7 A and B), indicating that aAmy genes could be 1of key intermediaries in the MYBS2-dependent negative regulation of tolerance to abiotic stress (osmotic, drought, and submergence) (Figs. 7 and 8). First, MYBS2 overexpression suppresses, whereas MYBS2 knockdown enhances, αAmy3 expression in seedlings grown under normal conditions (Fig. 2A) and osmotic stress conditions (Fig. 7 B–D). Second, αAmy3 overexpression promotes the germination rate and seedling growth of rice under osmotic stress (Fig. 7 H and I). Sugar production via αAmy-mediated starch degradation offers a mechanism for osmotic adjustment under abiotic stress conditions. Our studies demonstrated that αAmy3 is necessary and sufficient for promoting osmotic and drought tolerance in rice.
Other mechanisms may also regulate the antistress process, such as active starch degradation by αAmy meeting the high demand for sugars in cells to fulfill energy and carbon requirements for protection against stresses. This mechanism is important for submergence tolerance of rice seedlings (Fig. 8C). Reduced MYBS2 expression does not enhance submergence tolerance of seedlings, indicating that overexpression of αAmy3 is not sufficient for enhancing submergence tolerance. However, this enzyme is essential for submergence tolerance as indicated by the reduced seedling growth in MYBS2-Ox lines under submergence.
We previously showed that CIPK15, a calcineurin B-like–interacting protein kinase, regulates the expression of MYBS1 and αAmy necessary for germination and seedling growth under submergence (54). Here, we determined the transcript levels of MYBS2 in cipk15 knockout mutant seedlings (54) grown in air or in water. We observed that for WT seedlings grown in water, the MYBS2 mRNA level decreased by 34%. In contrast, for the cipk15 mutants grown in water, their level of MYBS2 mRNA was similar to that of WT (SI Appendix, Fig. S17). Our studies suggest that CIPK15 up-regulates MYBS1 and down-regulates MYBS2 during submergence.
In wheat and rice, mild soil drying or ABA treatment can trigger whole-plant senescence, leading to accelerated remobilization of carbon from stems, and senescing leaves to grains (55, 56). We have shown here that total grain weight per plant is also increased by reduced expression of MYBS2, whereas MYBS2 overexpression decreases it (Fig. 8D), likely due to enhanced carbon remobilization to grains by elevated αAmy expression in rice stems and senescing leaves during grain filling stages when soils normally undergo mild drought stress.
In summary, our studies decipher the regulatory mechanism controlling an on/off switch of reversible sugar signaling and gene expression at the molecular and cellular levels. Our investigation reveals a potential approach to manipulating MYBS2 and αAmy at the whole-plant level for improvement of plant growth, stress tolerance and grain productivity.
Materials and Methods
Details about plant materials, primers, plasmids, plasmid construction, rice transformation, GUS activity assay, rice embryo and barley aleurone transient expression assays, real-time quantitative RT-PCR analysis, subcellular localization in rice root and barley aleurone, antibodies and immunoblot analysis, phosphorylation analysis and phosphor-peptide mapping, IP, and field trials are described in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. John O’Brien for critical review of this manuscript; Dr. Shuen-Fang Lo for technical assistance in field tests; and the Academia Sinica Common Mass Spectrometry Facilities for proteomics and protein modification analysis. This work was supported by Ministry of Science and Technology Grants MOST 104-2321-B-001-054, MOST 105-2321-B-001-035, MOST 105-2321-B-001-003, and MOST 106-2321-B-001-046 (to S.-M.Y.), and MOST 106-2311-B-008-003-MY3 (to C.-A.L.), and in part by the Advanced Plant Biotechnology Center from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904818116/-/DCSupplemental.
References
- 1.Ruan Y. L., Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 65, 33–67 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Yu S. M., Lo S. F., Ho T. D., Source-sink communication: Regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci. 20, 844–857 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Sheu J.-J., Jan S.-P., Lee H.-T., Yu S.-M., Control of transcription and mRNA turnover as mechanisms of metabolic repression of alpha-amylase gene expression. Plant J. 5, 655–664 (1994). [Google Scholar]
- 4.Koch K. E., Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 509–540 (1996). [DOI] [PubMed] [Google Scholar]
- 5.Yu S. M., Cellular and genetic responses of plants to sugar starvation. Plant Physiol. 121, 687–693 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bihmidine S., Hunter C. T. 3rd, Johns C. E., Koch K. E., Braun D. M., Regulation of assimilate import into sink organs: Update on molecular drivers of sink strength. Front. Plant Sci. 4, 177 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Schaewen A., Stitt M., Schmidt R., Sonnewald U., Willmitzer L., Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J. 9, 3033–3044 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eom J. S., et al. , Impaired function of the tonoplast-localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiol. 157, 109–119 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leach K. A., Tran T. M., Slewinski T. L., Meeley R. B., Braun D. M., Sucrose transporter2 contributes to maize growth, development, and crop yield. J. Integr. Plant Biol. 59, 390–408 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Kennedy B. M., “Nutritional quality of rice endosperm” in Rice: Production and Utilization, Luh B. S., Ed. (AVI Publishing Co., Westport, CT, 1980), Chap. 11, pp. 439–469. [Google Scholar]
- 11.Chan M. T., Yu S. M., The 3′ untranslated region of a rice alpha-amylase gene functions as a sugar-dependent mRNA stability determinant. Proc. Natl. Acad. Sci. U.S.A. 95, 6543–6547 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheu J.-J., Yu T.-S., Tong W.-F., Yu S.-M., Carbohydrate starvation stimulates differential expression of rice alpha-amylase genes that is modulated through complicated transcriptional and posttranscriptional processes. J. Biol. Chem. 271, 26998–27004 (1996). [DOI] [PubMed] [Google Scholar]
- 13.Yu S. M., “Regulation of alpha-amylase gene expression” in Molecular Biology of Rice, Shimamoto K., Ed. (Springer, Tokyo, 1999), pp. 161–178. [Google Scholar]
- 14.Chen P. W., Chiang C. M., Tseng T. H., Yu S. M., Interaction between rice MYBGA and the gibberellin response element controls tissue-specific sugar sensitivity of alpha-amylase genes. Plant Cell 18, 2326–2340 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen P.-W., et al. , Rice alpha-amylase transcriptional enhancers direct multiple mode regulation of promoters in transgenic rice. J. Biol. Chem. 277, 13641–13649 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Lu C. A., Lim E. K., Yu S. M., Sugar response sequence in the promoter of a rice alpha-amylase gene serves as a transcriptional enhancer. J. Biol. Chem. 273, 10120–10131 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Lu C. A., Ho T. H., Ho S. L., Yu S. M., Three novel MYB proteins with one DNA binding repeat mediate sugar and hormone regulation of alpha-amylase gene expression. Plant Cell 14, 1963–1980 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu C. A., et al. , The SnRK1A protein kinase plays a key role in sugar signaling during germination and seedling growth of rice. Plant Cell 19, 2484–2499 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong Y. F., et al. , Convergent starvation signals and hormone crosstalk in regulating nutrient mobilization upon germination in cereals. Plant Cell 24, 2857–2873 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fincher G. G., Molecular and cellular biology associated with endosperm mobilisation in germinating cereal grains. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 305–346 (1989). [Google Scholar]
- 21.Lanahan M. B., Ho T. H., Rogers S. W., Rogers J. C., A gibberellin response complex in cereal alpha-amylase gene promoters. Plant Cell 4, 203–211 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gubler F., Kalla R., Roberts J. K., Jacobsen J. V., Gibberellin-regulated expression of a myb gene in barley aleurone cells: Evidence for Myb transactivation of a high-pI alpha-amylase gene promoter. Plant Cell 7, 1879–1891 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madeira F., et al. , 14-3-3-Pred: Improved methods to predict 14-3-3-binding phosphopeptides. Bioinformatics 31, 2276–2283 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison D. K., The 14-3-3 proteins: Integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 19, 16–23 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gökirmak T., Paul A. L., Ferl R. J., Plant phosphopeptide-binding proteins as signaling mediators. Curr. Opin. Plant Biol. 13, 527–532 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Denison F. C., Paul A. L., Zupanska A. K., Ferl R. J., 14-3-3 proteins in plant physiology. Semin. Cell Dev. Biol. 22, 720–727 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Hübscher V., et al. , The Hsp70 homolog Ssb and the 14-3-3 protein Bmh1 jointly regulate transcription of glucose repressed genes in Saccharomyces cerevisiae. Nucleic Acids Res. 44, 5629–5645 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin C. R., et al. , SnRK1A-interacting negative regulators modulate the nutrient starvation signaling sensor SnRK1 in source-sink communication in cereal seedlings under abiotic stress. Plant Cell 26, 808–827 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breitkopf S. B., Asara J. M., Determining in vivo phosphorylation sites using mass spectrometry Curr. Protoc. Mol. Biol.Chap. 18:Unit 18, 19.1–19.27. [DOI] [PMC free article] [PubMed]
- 30.Nardozzi J. D., Lott K., Cingolani G., Phosphorylation meets nuclear import: A review. Cell Commun. Signal. 8, 32 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosugi S., Hasebe M., Tomita M., Yanagawa H., Nuclear export signal consensus sequences defined using a localization-based yeast selection system. Traffic 9, 2053–2062 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Muslin A. J., Tanner J. W., Allen P. M., Shaw A. S., Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell 84, 889–897 (1996). [DOI] [PubMed] [Google Scholar]
- 33.Yaffe M. B., et al. , The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91, 961–971 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Jacobsen J. V., Hanson A. D., Chandler P. C., Water stress enhances expression of an alpha-amylase gene in barley leaves. Plant Physiol. 80, 350–359 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heitz T., Geoffroy P., Fritig B., Legrand M., Two apoplastic alpha-amylases are induced in tobacco by virus infection. Plant Physiol. 97, 651–656 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koizuka N., Tanaka Y., Morohashi Y., Expression of alpha-amylase in response to wounding in mung bean. Planta 195, 530–534 (1995). [Google Scholar]
- 37.Doyle E. A., Lane A. M., Sides J. M., Mudgett M. B., Monroe J. D., An alpha-amylase (At4g25000) in Arabidopsis leaves is secreted and induced by biotic and abiotic stress. Plant Cell Environ. 30, 388–398 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Yu S. M., et al. , Sugars act as signal molecules and osmotica to regulate the expression of alpha-amylase genes and metabolic activities in germinating cereal grains. Plant Mol. Biol. 30, 1277–1289 (1996). [DOI] [PubMed] [Google Scholar]
- 39.Ge Q., et al. , Importin-alpha protein binding to a nuclear localization signal of carbohydrate response element-binding protein (ChREBP). J. Biol. Chem. 286, 28119–28127 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yashvardhini N., Bhattacharya S., Chaudhuri S., Sengupta D. N., Molecular characterization of the 14-3-3 gene family in rice and its expression studies under abiotic stress. Planta 247, 229–253 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Yao Y., Du Y., Jiang L., Liu J. Y., Molecular analysis and expression patterns of the 14-3-3 gene family from Oryza sativa. J. Biochem. Mol. Biol. 40, 349–357 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Ho S. L., et al. , Sugar starvation- and GA-inducible calcium-dependent protein kinase 1 feedback regulates GA biosynthesis and activates a 14-3-3 protein to confer drought tolerance in rice seedlings. Plant Mol. Biol. 81, 347–361 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Jacobsen J. V., Gubler F., Chandler P. M., “Gibberellin action in germinated cereal grains” in Plant Hormones: Physiology, Biochemistry, and Molecular Biology, Davies P. J., Ed. (Kluwer Academic Publishers, Dordrecht, 1995), pp. 246–271. [Google Scholar]
- 44.Taneyama M., Yamauchi D., Minamikawa T., Synthesis and turnover of alpha-amylase in cotyledons of germinating Vigna-Mungo seeds—Effects of exogenously applied end-products and plant hormones. Plant Cell Physiol. 36, 139–146 (1995). [Google Scholar]
- 45.Commuri P. D., Duke S. H., Apoplastic alpha-amylase in pea is enhanced by heat stress. Plant Cell Physiol. 38, 625–630 (1997). [Google Scholar]
- 46.Saeed M., Duke S. H., Amylases in pea tissues with reduced chloroplast density and/or function. Plant Physiol. 94, 1813–1819 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thalmann M., et al. , Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant Cell 28, 1860–1878 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartels D., Sunkar R., Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 24, 23–58 (2005). [Google Scholar]
- 49.Krasensky J., Jonak C., Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 63, 1593–1608 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoekstra F. A., Golovina E. A., Buitink J., Mechanisms of plant desiccation tolerance. Trends Plant Sci. 6, 431–438 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Miller G., Suzuki N., Ciftci-Yilmaz S., Mittler R., Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 33, 453–467 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Tsuji H., et al. , GAMYB controls different sets of genes and is differentially regulated by microRNA in aleurone cells and anthers. Plant J. 47, 427–444 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Wang H. J., et al. , Transcriptomic adaptations in rice suspension cells under sucrose starvation. Plant Mol. Biol. 63, 441–463 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Lee K. W., et al. , Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Sci. Signal. 2, ra61 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Yang J. C., Zhang J. H., Wang Z. Q., Zhu Q. S., Liu L. J., Involvement of abscisic acid and cytokinins in the senescence and remobilization of carbon reserves in wheat subjected to water stress during grain filling. Plant Cell Environ. 26, 1621–1631 (2003). [Google Scholar]
- 56.Yang J., Zhang J., Grain filling of cereals under soil drying. New Phytol. 169, 223–236 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.