Significance
Testing ecological hypotheses of human evolution requires an understanding of the ancient plant and animal communities within which our ancestors lived. Though present-day ecosystems provide the baseline for reconstructing the ecological context of human evolution, the extent to which modern ecosystems are representative of past ones is unknown. Through analyses of a fossil dataset spanning the last 7 Myr, we show that eastern African communities of large-bodied mammalian herbivores differed markedly from those today until ∼700,000 y ago. Because large herbivores are ecosystem engineers and shape biotic communities in ways that impact a wide variety of species, this implies that the vast majority of early human evolution transpired in the context of ecosystems that functioned unlike any known today.
Keywords: functional traits, megaherbivore, non-analog faunas, paleoanthropology, paleoecology
Abstract
Present-day African ecosystems serve as referential models for conceptualizing the environmental context of early hominin evolution, but the degree to which modern ecosystems are representative of those in the past is unclear. A growing body of evidence from eastern Africa’s rich and well-dated late Cenozoic fossil record documents communities of large-bodied mammalian herbivores with ecological structures differing dramatically from those of the present day, implying that modern communities may not be suitable analogs for the ancient ecosystems of hominin evolution. To determine when and why the ecological structure of eastern Africa’s herbivore faunas came to resemble those of the present, here we analyze functional trait changes in a comprehensive dataset of 305 modern and fossil herbivore communities spanning the last ∼7 Myr. We show that nearly all communities prior to ∼700 ka were functionally non-analog, largely due to a greater richness of non-ruminants and megaherbivores (species >1,000 kg). The emergence of functionally modern communities precedes that of taxonomically modern communities by 100,000s of years, and can be attributed to the combined influence of Plio-Pleistocene C4 grassland expansion and pulses of aridity after ∼1 Ma. Given the disproportionate ecological impacts of large-bodied herbivores on factors such as vegetation structure, hydrology, and fire regimes, it follows that the vast majority of early hominin evolution transpired in the context of ecosystems that functioned unlike any today. Identifying how past ecosystems differed compositionally and functionally from those today is key to conceptualizing ancient African environments and testing ecological hypotheses of hominin evolution.
A central goal of human evolutionary studies is to understand how climatically mediated environmental changes over the last ∼7 Myr shaped the anatomical, behavioral, and technological evolution of our early ancestors (1–3). Forging causal links between environmental change and hominin evolution requires detailed reconstructions of the ancient ecosystems within which our ancestors lived, including faunal community composition, the strength and direction of species interactions (e.g., predator–prey relationships), vegetation structure, and the spatial distribution of foods and other vital resources (4, 5). This research focus has fueled the generation and analysis of detailed paleoenvironmental archives across Africa, with key proxies derived from mammalian faunas (6, 7), isotopic geochemistry of fossil herbivore teeth and soil carbonates (8–10), paleobotanical remains (11–13), and multiproxy lacustrine sequences (14, 15). Though we now know considerably more about African paleoclimatic and paleoenvironmental change than we did decades ago (16), translating this knowledge to an understanding of the selective pressures that shaped hominin evolution remains an ongoing challenge (3–5).
One of the primary challenges we face is that our inferences made about the structure and functioning of ancient ecosystems are strongly shaped by those of the present (see refs. 3 and 4 for discussions of other challenges). For example, reconstructions of past climate or vegetation structure are routinely based on present-day relationships between these variables and various proxies, such as mammal species composition, the distribution of herbivore functional traits, or the isotopic composition of soil carbonates (10, 17–20). While reliance on modern analogs is an essential component of paleoecological and paleoenvironmental reconstruction (21), it limits our ability to evaluate how ancient ecosystems might have differed from those today (22).
In conflict with hominin paleoecology’s deep roots in uniformitarian approaches founded on identifying similarities between the past and present (23–25), the existence of ancient ecosystems with no modern analogs is now supported by a growing body of evidence from eastern Africa’s rich and well-dated late Cenozoic fossil record. For example, recent studies have shown that many Plio-Pleistocene herbivore communities were considerably richer in megaherbivores (species >1,000 kg) (26) and were characterized by atypical dietary guild structures (8) relative to their modern counterparts. These important ecological differences may have persisted until recently, as Late Pleistocene faunas are often dominated by extinct herbivore taxa (27, 28), including some with morphologies unique among living and fossil mammals (29). Because large-bodied mammalian herbivores possess key functional traits that influence ecosystems in ways that impact a wide variety of species (30, 31), it follows that the ancient ecosystems of hominin evolution were both compositionally and functionally unlike any in Africa today.
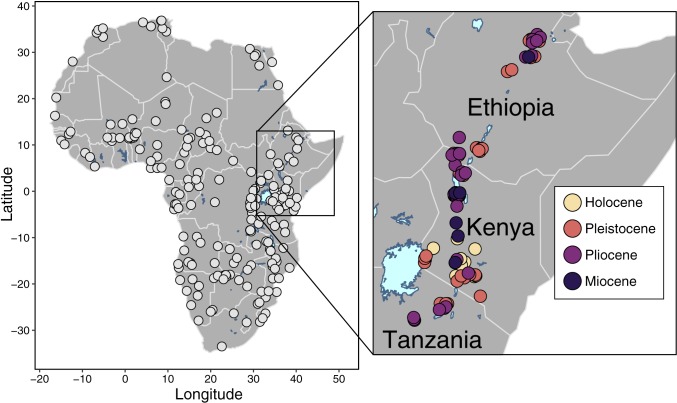
Despite long-standing interest in the emergence of taxonomically “modern” faunas in eastern Africa (27, 32–35), we have little understanding of when or why eastern Africa’s herbivore communities came to resemble those of the present in terms of their functional ecology. With this in mind, here we analyze the functional evolution of eastern African large herbivore communities (orders Artiodactyla, Perissodactyla, and Proboscidea) over the last 7 Myr using a comprehensive database of 101 fossil communities sampling >300 taxa (Fig. 1). We focus on 3 functional traits: body size (size 2: 18 to 80 kg; size 3: 80 to 350 kg; size 4: 350 to 1,000 kg; size 5: >1,000 kg), digestive physiology (ruminant, non-ruminant), and dietary strategy (C3 browser, C3-C4 mixed feeder, C4 grazer). We chose these traits as they represent fundamental biological and ecological aspects of herbivore species and their ability to shape the ecosystems around them (36). We compare the functional structure of our fossil herbivore communities to more than 200 modern communities across Africa (Fig. 1), using the latter as a baseline that allows us to identify non-analog communities in the past and determine when the functional structure of eastern African herbivore communities came to resemble the present day.
Fig. 1.
The geographic distribution of the modern (continental map) and fossil (Inset) larger herbivore communities.
Results
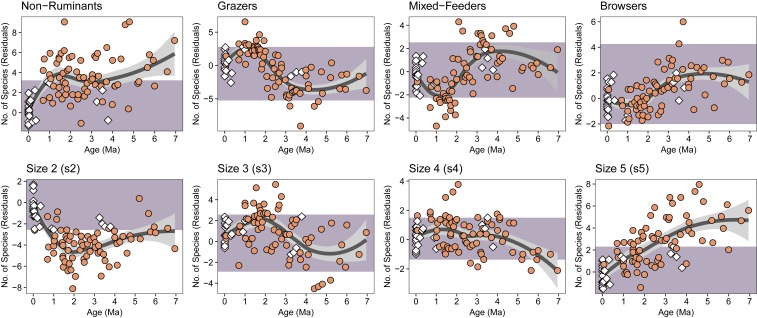
In trait-by-trait comparisons, we find that the functional structure of fossil herbivore communities differed significantly from those today (Fig. 2). For example, in terms of digestive physiology, modern African herbivore communities are overwhelmingly comprised of ruminants, with most species belonging to Bovidae (Dataset S1). Non-ruminant herbivores (Elephantidae, Equidae, Hippopotamidae, Rhinocerotidae, Suidae) are far less common, with at most 8 species occurring in sympatry. In contrast, the eastern African fossil record documents communities that were substantially richer in non-ruminants (Dataset S2). Following methods established elsewhere to account for biases in the fossil record (e.g., differential sampling and time averaging) (26), residual analysis indicates that many fossil communities (n = 37) sample a greater number of non-ruminants than observed in any community today. A sharp decline in non-ruminants beginning ∼1 Ma places all fossil communities within the modern range of variation after ∼700 ka (Fig. 2).
Fig. 2.
Temporal trends in the functional trait composition of eastern African large herbivore communities over the last 7 Myr. Purple shading represents the modern range of variation for each trait. White diamonds indicate fossil communities that fall within the modern range of variation for all 8 functional traits. Orange circles indicate fossil communities that are non-analog; they fall outside the modern range of variation for one or more of the functional traits illustrated here. Dark gray lines represent LOESS regressions with a smoothing factor of 0.75; 95% confidence limits in light gray (see SI Appendix for further information).
Likewise, body size distributions render many fossil communities functionally non-analog, with the most important differences observed among size 2 (18 to 80 kg) and size 5 (>1,000 kg) taxa. Size 2 herbivores are poorly represented over much of the last 7 Myr, such that most fossil communities fall well below the modern range of variation (Fig. 2). A steady increase in the richness of size 2 herbivores beginning ∼2 Ma places fossil communities within the modern range of variation after ∼700 ka. With respect to size 3 (80 to 350 kg) and size 4 (350 to 1,000 kg) herbivores, most fossil communities fall within the modern range of variation, though the interval between 3 and 1 Ma includes substantially more size 3 species, and to a lesser extent size 4 species, than is observed today. Lastly, fossil communities include many more size 5 (megaherbivore) species than their modern counterparts (see also ref. 26). A steady, long-term decline beginning ∼4.5 Ma eventually places fossil communities consistently within the range of modern variation by ∼700 ka.
Dietary structure plays a secondary role in driving differences between fossil and modern herbivore communities (Fig. 2). Grazers are occasionally underrepresented between ∼5 and 3 Ma, but a gradual increase in richness beginning ∼3 Ma culminates in a larger number of grazers than is observed today between ∼2 and 1 Ma, with younger fossil communities (<1 Ma) falling within the modern range of variation. Mixed feeders show the inverse trend. Though there are subtle changes in the representation of browsers through time, including a gradual decline beginning ∼3.5 Ma, all but 3 fossil communities fall within the modern range of variation.
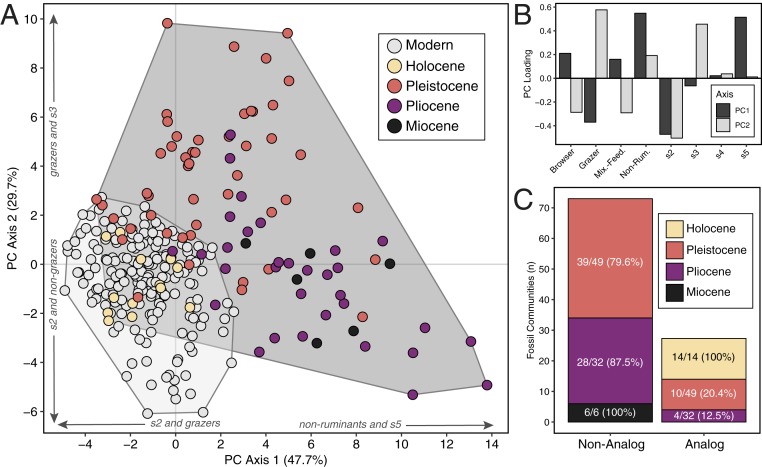
Synthesizing the residual analyses discussed above, Fig. 3A presents the first 2 axes of a principal component analysis (PCA) of trait residuals for all functional categories in both the modern and fossil datasets. Collectively, these axes account for ∼77% of the variation in the functional composition of herbivore communities (PC1: 47.7% of variance; PC2: 29.7% of variance). PC1 separates communities dominated by size 5 and non-ruminant taxa (positive loadings) from those dominated by size 2 and grazing taxa (negative loadings) (Fig. 3B). PC2 separates communities rich in size 3 and grazing taxa (positive loadings) from those rich in size 2 taxa and mixed feeders or browsers (negative loadings) (Fig. 3B). Compared to the present, fossil communities occupy a considerably larger amount of functional trait space (Fig. 3A), with most pre-Holocene communities falling outside of the range of community structures documented today (100% of Miocene, 87.5% of Pliocene, and 79.6% of Pleistocene communities have no modern analogs; Fig. 3C).
Fig. 3.
Comparison of the functional composition of modern and fossil large herbivore communities. (A) The first 2 axes of a PCA of richness-corrected functional trait residuals for modern and fossil communities; the light gray hull encloses the modern range of community variation, and the dark gray hull encloses the fossil range of variation. (B) PC1 and PC2 loadings for all functional traits. (C) Stacked barplot of the number of non-analog versus analog fossil herbivore communities grouped by their geological epoch. Fossil communities are considered non-analog if the residual for any given trait falls outside the modern range of variation (Fig. 2).
Discussion
For much of the last 7 Myr, the interval encompassing the entirety of hominin evolution, fossil eastern African herbivore communities were characterized by functional ecological structures unlike any observed in Africa today. We emphasize that our comparison of eastern African fossil communities to modern communities from across the continent renders our analysis conservative (i.e., it is more difficult to identify non-analog fossil communities), because the considerably larger spatial scale of the modern sites encompasses a massive range of environmental and functional ecological variation (e.g., relative to an eastern African modern dataset). Building on the large body of research concerned with non-analog species associations (i.e., co-occurrence of taxa that are presently allopatric) in late Quaternary contexts (37), we refer to such communities as functionally non-analog. A handful of Pliocene faunas (Moiti Member, Koobi Fora Fm.; Kaiyumung Member, Nachukui Fm.; South Turkwell; Member A, Shungura Fm.) cannot be shown to differ from present-day African communities (Fig. 2), though they are not well sampled (their richness ranges from 6 to 12 versus a Pliocene mean of 19; Dataset S2), limiting our power to detect ecological differences relative to the present. Only after ∼700 ka do communities routinely fall within the modern range of variation, but even then there are exceptions, including the late Middle Pleistocene faunas from Member I (∼200 ka) of the Kibish Formation in Ethiopia (38, 39) and the Late Pleistocene faunas (between ∼100 ka and 35 ka) from Karungu in Kenya (40). These exceptions aside, the emergence of functionally modern communities shortly after ∼700 ka considerably predates the emergence of taxonomically modern communities (Fig. 4E), which did not occur until the onset of the Holocene (27, 28). This decoupling implies that functional modernity is not due to the replacement of archaic lineages with extant taxa but is instead the outcome of long-term ecological changes that altered the functional composition of eastern African faunal communities and ecosystems.
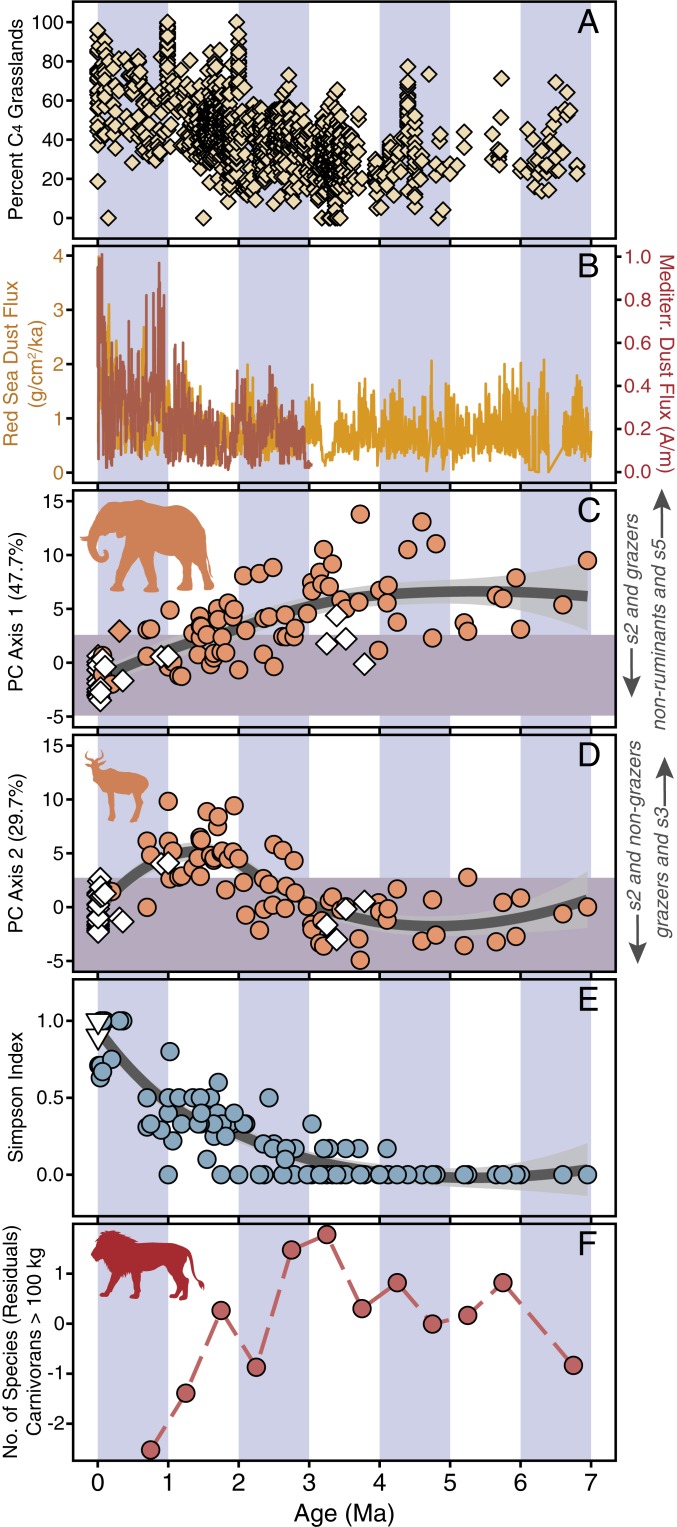
Fig. 4.
Temporal trends in the functional and taxonomic evolution of eastern Africa’s large herbivore communities relative to climatic and environmental proxies. (A) The percentage of C4 vegetation inferred from soil carbonate δ13C of eastern African paleosol carbonates; %C4 data from Faith et al. (26) using δ13C data compiled by Levin (16). (B) Dust flux proxy for aridity from the Mediterranean (Ocean Drilling Program [ODP] Site 967; red line) (69) and the Arabian Sea (ODP 721/722; yellow line) (2, 70). (C) Fossil PC1 scores through time (Fig. 3). (D) Fossil PC2 scores through time (Fig. 3). (E) The evolution of taxonomic modernity as reflected by the highest pairwise Simpson similarity index value observed between a fossil assemblage and each modern community (0 = no taxonomic overlap; 1 = complete taxonomic overlap). Orange circles in C–E indicate non-analog fossil communities, and white diamonds or triangles indicate analog communities or those lacking extinct taxa, respectively. Dark gray lines in C–E indicate LOESS regression (smoothing factor = 0.75); 95% confidence limits in light gray. (F) Changes in large carnivoran richness across 0.5-Myr bins. As a control for differential sampling of the fossil record, values represent residuals derived from an ordinary least-squares regression modeling the relationship between the number of fossil sites and the number of taxa in a given bin (see SI Appendix for full details and SI Appendix, Table S3 for raw data).
Drivers of Community Change.
One of the most substantial and well-documented changes to eastern African ecosystems since the late Miocene is the expansion of C4 grasslands (16), shown in Fig. 4A using stable carbon isotope records (δ13C) from soil carbonates. Many of the functional changes in fossil herbivore communities, synthesized here in a plot of PC1 scores through time (Fig. 4C), track the δ13C soil record and can reasonably be interpreted as a response to grassland expansion. Turning to individual traits, prior to ∼1 Ma, dietary structure closely tracks the percentage of C4 biomass inferred from the δ13C composition of soil carbonates in eastern Africa, with grazers increasing and browsers and mixed feeders declining as grasses cover a greater proportion of landscapes (Fig. 2). These trends are consistent with herbivore tooth enamel δ13C data spanning 4 to 1 Ma from the Turkana Basin (8). In addition, the long-term decline of megaherbivores—which our analyses show to be one of the key variables distinguishing fossil communities from modern ones (Figs. 2 and 3B)—inversely tracks the expansion of C4 grasslands. Megaherbivores favoring C3 browse were preferentially affected, strongly implying a cause–effect relationship (26).
The consequences of grassland expansion and megaherbivore decline likely translated to important shifts in the functional structure of the eastern African carnivore guild. Today, the largest African carnivorans (>100 kg) capable of taking megaherbivore prey (41) are represented only by lions (Panthera leo). However, a far greater diversity of large carnivorans existed during the Plio-Pleistocene (42), with eastern African fossil assemblages often documenting the co-occurrence of 2 to 4 species. This includes lions and their ancestors, as well as giant hyenas (e.g., Crocuta eturono and Pachycrocuta brevirostris), sabertooth felids (Dinofelis and Homotherium), and highly carnivorous bears (Agriotherium) (43). Large carnivoran richness steadily declines after the ∼3.0- to 3.5-Ma interval, broadly tracking the expansion of C4 grasses and the associated loss of megaherbivores (Fig. 4F). Previous work has documented a robust association between the richness of large carnivorans and megaherbivores, implying that diverse megaherbivore communities facilitated coexistence among large predators, likely because juvenile megaherbivores were important prey (41). Loss of tree or bush cover is also likely to have been detrimental to ambush predators (41), including some sabertooths (44). The coupled relationships between grassland expansion, megaherbivore decline, and large carnivoran extinctions strongly suggests that long-term bottom-up ecosystem change played a key role in the emergence of functional modernity in both the large carnivore and herbivore guilds.
Several functional traits analyzed here indicate substantial shifts in community composition after ∼1 Ma that cannot be explained by grassland expansion (Fig. 2), a trend exemplified by the change in PC2 scores through time (Fig. 4D). Despite continued increases in C4 biomass, grazers sharply decline at this time and are replaced by mixed feeders, representing an abrupt reversal of a >2 Myr-long trend (Fig. 2). This reversal also corresponds to the major decline of non-ruminants (Fig. 2), whose elevated richness from 7 to 1 Ma plays the biggest role in rendering fossil communities functionally non-analog (Fig. 3B). Thus, with respect to the functional traits considered here, the transition at ∼1 Ma represents one of the most significant episodes of faunal change in eastern Africa since the late Miocene.
We propose that this change is the result of high-amplitude climate fluctuations related to the establishment of 100 kyr glacial–interglacial cycles during the mid-Pleistocene revolution (2, 45). Dust flux records from marine cores tracking eastern Africa’s precipitation history indicate pulses of aridity after ∼1 Ma that were both more severe and more frequent than those of preceding intervals (2, 45) (Fig. 4B). Such pulses of aridity would be particularly detrimental to non-ruminants and grazers. First, both functional groups are highly dependent on surface water (46), a trait that enhances extinction risk in the face of reduced water availability (47). The likelihood of extinction is further enhanced because non-ruminants are at a competitive disadvantage when resources are limited, which is expected under arid conditions, because they require more forage to extract the same amount of energy compared to their ruminant counterparts (48–50). Likewise, grazers typically suffer disproportionately high mortality during prolonged dry phases because the shallow-rooting grasses on which they feed are drought intolerant (47, 51–53). Perhaps more important is that the rarity of grasses in arid environments (e.g., the Horn of Africa) typically favors low-biomass communities dominated by mixed feeders or browsers (e.g., the bovid tribe Antilopini) to the exclusion of grazers (e.g., the bovid tribe Alcelaphini) (46), implying that pulses of aridity are likely to be associated with habitat loss for grazing species. Thus, we propose that the outcome of repeated pulses of aridity is a selective winnowing of these groups to the benefit of ruminants and mixed feeders over the last ∼1 Myr, resulting in the emergence of eastern African herbivore communities resembling those of the present day in terms of functional ecology.
The Ecological Context of Hominin Evolution.
Our results indicate that the vast majority of hominin evolution in eastern Africa transpired in the context of faunal communities unlike any known today. This poses a potential challenge for reconstructing African paleoenvironments, particularly with respect to some techniques that use relationships between faunas and environments in modern settings to reconstruct the past (17–20, 54). Our observations leave little doubt that the present is not a sufficient analog for much of the past, and strongly imply that the environments of hominin evolution were markedly different from those of today. Thus, by framing the environmental context of hominin evolution in terms of the present, we are likely overlooking unique and important aspects of the environments in which our ancestors lived.
Perhaps the most significant consequence of recognizing functionally non-analog herbivore communities in the past concerns their effects on vegetation structure and ecosystem processes. Through both direct (e.g., consumptive) and indirect effects (e.g., altering of fire regimes), herbivores are a key determinant of vegetation structure (30, 55, 56). There are strong reasons to believe that fossil non-analog communities altered the dynamic relationships between herbivory, climate, fire, and vegetation structure known in modern ecosystems. For example, because non-ruminants have considerably larger forage requirements than ruminants, their prominence in communities prior to ∼700 ka implies a greater role of herbivory in shaping habitat structure and fire regimes. Moreover, considering the disproportionate impacts of megaherbivores today (30, 31), their exceptional richness from the late Miocene through the Early Pleistocene, which implies increased megaherbivore biomass (26), was likely associated with enhanced nutrient cycling, reduced fire frequencies and extent, and more heterogeneous vegetation mosaics (57–60). The latter could account (in part) for the prominence of “mosaic” habitat reconstructions of eastern African hominin environments (summarized in ref. 5), which has been proposed recently in light of Kanapoi’s (∼4.2 to 4.1 Ma) high proboscidean diversity (61). Finally, by altering vegetation structure and fire regimes in ways that are not observed in the present day, functionally non-analog communities can also account for the observation that paleoaridity estimates in eastern Africa vary independently of vegetation proxies and aspects of mammalian community structure (e.g., dental ecometrics) that are known to be robust indicators of climate today (9). Taken as a whole, such observations imply that future studies of ancient herbivory regimes and reconstructions of their relationships to both vegetation communities and higher trophic levels are essential for understanding the environmental context of our evolution.
Conclusions
Our analyses show that the emergence of functionally modern herbivore communities in eastern Africa is a geologically recent phenomenon occurring within the last ∼700,000 y, which we link to the combined effects of C4 grassland expansion coupled with enhanced climate variability and pulses of aridity after ∼1 Ma. Past communities contained a considerably larger amount of functional diversity than those today, with the most important differences concerning the exceptional richness of megaherbivores and non-ruminants, followed to a lesser extent by differences in dietary structure. These observations indicate that the vast majority of hominin evolution in eastern Africa transpired in the context of ecosystems lacking modern analogs. Given what we know of herbivore influences on present-day ecosystems, the contrast in functional trait structure between past and present communities implies differences in vegetation structure, fire regimes, nutrient cycling, and other ecological processes. In light of such differences, it follows that in the effort to understand how ancient environments mediated the behavioral and biological evolution of hominins, we must consider the ways in which the ancient ecosystems within which our ancestors lived differed from those of the present.
Materials and Methods
Our database of 204 modern African herbivore communities (Dataset S1) comes from datasets of protected areas (national parks, game reserves) compiled by Kamilar et al. (62) and Rowan et al. (63). Functional trait attributes (body size class, diet, digestive physiology) of extant species were based on Kingdon et al. (64) and other expert references (e.g., refs. 65 and 66). Data for fossil herbivore communities (Dataset S2) and functional trait attributes come from Faith et al. (26). Size classes broadly correspond to a widely used scheme for African mammals (67), with assignments for fossil taxa based on published body mass estimates, descriptions provided in taxonomic diagnoses, the size of living or fossil relatives, and expert opinion. Size class 1 taxa (<18 kg) were excluded from all analyses due to potential taphonomic biases against recovery of smaller herbivore taxa in the fossil record (68). Dietary assignments for fossil taxa are based on published δ13C data, dental mesowear and microwear, or the diets of living and fossil relatives. Digestive physiology (ruminant versus non-ruminant) assignments are based on phylogeny (e.g., all Bovidae are considered ruminants). See SI Appendix for full details concerning functional trait assignments.
To control for time averaging, sampling variability, and taphonomic differences between fossil communities, we examine the absolute number of herbivore taxa within a given functional type relative to overall community richness; see full discussion in SI Appendix. We generated ordinary least-squares linear regressions to model the richness of a given functional type as a function of overall community richness in the modern sample (SI Appendix), with residual analysis used to establish the range of variation in communities today. We then calculated residuals for the fossil data relative to these linear regressions; fossil residuals falling outside the range of modern variation are considered functionally non-analog. These residuals are tightly correlated with those obtained using linear regressions for the fossil data only, implying that our calculation of residuals relative to the modern regressions has not obscured long-term temporal trends in functional trait composition (SI Appendix). Temporal trends are illustrated using locally estimated scatterplot smoothing (LOESS), with a smoothing factor of 0.75. The PCA of modern and fossil herbivore communities was conducted on the variance–covariance matrix derived from all (untransformed) functional trait residuals.
To illustrate the evolution of taxonomic modernity, we calculated taxonomic similarity between a given fossil assemblage and each of the modern communities using Simpson’s similarity index, with the highest value for any pairwise comparison taken as the best modern analog (plotted in Fig. 4E). The index is calculated as M/Nmin, where M is the number of shared species and Nmin corresponds to the number of species present in the assemblage with the fewest species. A Simpson’s similarity index of 0 means no taxonomic overlap, whereas 1 denotes complete taxonomic overlap. This index is well suited for incomplete data (e.g., fossil assemblages that do not sample all taxa in a paleocommunity), because it treats 2 assemblages as identical if one is a subset of the other. Only fossil taxa with species-level assignments were included in this analysis. Fossil taxa identified using the open nomenclature designation “cf.” were treated as belonging to the species in question (e.g., fossil Hippopotamus cf. amphibius is the same taxon as modern H. amphibius). Taxa identified using “aff.” were treated as not belonging to the species in question, with the understanding that aff. is typically used to designate a species closely related to, but distinct from, a known taxon (e.g., fossil H. aff. amphibius is distinct from modern H. amphibius).
Supplementary Material
Acknowledgments
We thank the editor and the anonymous reviewers for their valuable feedback on a previous version of this manuscript. J.R. is supported by a Darwin Postdoctoral Fellowship at the University of Massachusetts Amherst.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909284116/-/DCSupplemental.
References
- 1.Potts R., Environmental hypotheses of hominin evolution. Am. J. Phys. Anthropol. 41 (suppl. 27), 93–136 (1998). [DOI] [PubMed] [Google Scholar]
- 2.deMenocal P. B., African climate change and faunal evolution during the Pliocene-Pleistocene. Earth Planet. Sci. Lett. 220, 3–24 (2004). [Google Scholar]
- 3.Behrensmeyer A. K., Climate change and human evolution. Science 311, 476–478 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Marean C. W., et al. , A new research strategy for integrating studies of paleoclimate, paleoenvironment, and paleoanthropology. Evol. Anthropol. 24, 62–72 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Kingston J. D., Shifting adaptive landscapes: Progress and challenges in reconstructing early hominid environments. Am. J. Phys. Anthropol. 50 (suppl. 45), 20–58 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Reed K. E., Spencer L. M., Rector A. L., “Faunal approaches in early hominin paleoecology” in Early Hominin Paleoecology, Sponheimer M., Lee-Thorp J. A., Reed K. E., Ungar P. S., Eds. (University Press of Colorado, Boulder, CO, 2013), pp. 3–34. [Google Scholar]
- 7.Bobe R., Alemseged Z., Behrensmeyer A. K., Eds., Hominin Environments in the East African Pliocene: An Assessment of the Faunal Evidence (Springer, Dordrecht, 2007). [Google Scholar]
- 8.Cerling T. E., et al. , Dietary changes of large herbivores in the Turkana Basin, Kenya from 4 to 1 Ma. Proc. Natl. Acad. Sci. U.S.A. 112, 11467–11472 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumenthal S. A., et al. , Aridity and hominin environments. Proc. Natl. Acad. Sci. U.S.A. 114, 7331–7336 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerling T. E., et al. , Woody cover and hominin environments in the past 6 million years. Nature 476, 51–56 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Bonnefille R., Cenozoic vegetation, climate changes and hominid evolution in tropical Africa. Global Planet. Change 72, 390–411 (2010). [Google Scholar]
- 12.Bonnefille R., Potts R., Chalié F., Jolly D., Peyron O., High-resolution vegetation and climate change associated with Pliocene Australopithecus afarensis. Proc. Natl. Acad. Sci. U.S.A. 101, 12125–12129 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uno K. T., Polissar P. J., Jackson K. E., deMenocal P. B., Neogene biomarker record of vegetation change in eastern Africa. Proc. Natl. Acad. Sci. U.S.A. 113, 6355–6363 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campisano C. J., et al. , The hominin sites and paleolakes drilling project: High-resolution paleoclimate records from the East African rift system and their implications for understanding the environmental context of hominin evolution. Paleoanthropology 2017, 1–43 (2017). [Google Scholar]
- 15.Owen R. B., et al. , Progressive aridification in East Africa over the last half million years and implications for human evolution. Proc. Natl. Acad. Sci. U.S.A. 115, 11174–11179 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin N. E., Environment and climate of early human evolution. Annu. Rev. Earth Planet. Sci. 43, 405–429 (2015). [Google Scholar]
- 17.Fortelius M., et al. , An ecometric analysis of the fossil mammal record of the Turkana Basin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150232 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed K. E., Paleoecological patterns at the hadar hominin site, Afar regional state, Ethiopia. J. Hum. Evol. 54, 743–768 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Alemseged Z., An integrated approach to taphonomy and faunal change in the Shungura formation (Ethiopia) and its implication for hominid evolution. J. Hum. Evol. 44, 451–478 (2003). [DOI] [PubMed] [Google Scholar]
- 20.de Ruiter D. J., Sponheimer M., Lee-Thorp J. A., Indications of habitat association of Australopithecus robustus in the Bloubank Valley, South Africa. J. Hum. Evol. 55, 1015–1030 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Faith J. T., Lyman R. L., Paleozoology and Paleoenvironments: Fundamentals, Assumptions, Techniques (Cambridge University Press, Cambridge, 2019). [Google Scholar]
- 22.Behrensmeyer A. K., Bobe R., Alemseged Z., “Approaches to the analysis of faunal change during the East African Pliocene” in Hominin environments in the East African Pliocene, Bobe R., Alemseged Z., Behrensmeyer A. K., Eds. (Springer, 2007), pp. 1–24. [Google Scholar]
- 23.Andrews P., Lord J. M., Nesbit Evans E. M., Patterns of ecological diversity in fossil and mammalian faunas. Biol. J. Linn. Soc. Lond. 11, 177–205 (1979). [Google Scholar]
- 24.Reed K. E., Early hominid evolution and ecological change through the African Plio-Pleistocene. J. Hum. Evol. 32, 289–322 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Vrba E. S., “The significance of bovid remains as an indicator of environment and predation patterns” in Fossils in the Making, Behrensmeyer A. K., Hill A. P., Eds. (University of Chicago Press, Chicago, 1980), pp. 247–272. [Google Scholar]
- 26.Faith J. T., Rowan J., Du A., Koch P. L., Plio-Pleistocene decline of African megaherbivores: No evidence for ancient hominin impacts. Science 362, 938–941 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Marean C. W., Gifford-Gonzalez D., Late Quaternary extinct ungulates of East Africa and palaeoenvironmental implications. Nature 350, 418–420 (1991). [Google Scholar]
- 28.Faith J. T., Late Pleistocene and Holocene mammal extinctions on continental Africa. Earth Sci. Rev. 128, 105–121 (2014). [Google Scholar]
- 29.O’Brien H. D., et al. , Unexpected convergent evolution of nasal domes between Pleistocene bovids and cretacrous hadrosaur dinosaurs. Curr. Biol. 26, 503–508 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Ripple W. J., et al. , Collapse of the world’s largest herbivores. Sci. Adv. 1, e1400103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owen-Smith R. N., Megaherbivores: The influence of very large body size on ecology (Cambridge University Press, Cambridge, 1988). [Google Scholar]
- 32.Werdelin L., Lewis M. E., Plio-Pleistocene Carnivora of eastern Africa: Species richness and turnover patterns. Zool. J. Linn. Soc. 144, 121–144 (2005). [Google Scholar]
- 33.Potts R., Deino A., Mid-Pleistocene change in large mammal faunas of East Africa. Quat. Res. 43, 106–113 (1995). [Google Scholar]
- 34.Faith J. T., et al. , New perspectives on middle Pleistocene change in the large mammal faunas of East Africa: Damaliscus hypsodon sp. nov. (Mammalia, Artiodactyla) from Lainyamok, Kenya. Palaeogeogr. Palaeoclimatol. Palaeoecol. 361-362, 84–93 (2012). [Google Scholar]
- 35.Potts R., Variability selection in hominid evolution. Evol. Anthropol. 7, 81–96 (1998). [Google Scholar]
- 36.Malhi Y., et al. , Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. U.S.A. 113, 838–846 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham R. W., “Quaternary mammal communities: Relevance of the individualistic response and non-analogue faunas” in Paleobiogeography: Generating New Insights into the Coevolution of the Earth and Its Biota, Lieberman B. S., Stigall A. L., Eds. (Paleontological Society Papers, Boulder, CO, 2005), vol. 11, pp. 141–157. [Google Scholar]
- 38.Rowan J., Faith J. T., Gebru Y., Fleagle J. G., Taxonomy and paleoecology of fossil Bovidae (Mammalia, Artiodactyla) from the Kibish Formation, southern Ethiopia: Implications for dietary change, biogeography, and the structure of living bovid faunas of East Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 420, 210–222 (2015). [Google Scholar]
- 39.Assefa Z., Yirga S., Reed K. E., The large-mammal fauna from the Kibish Formation. J. Hum. Evol. 55, 501–512 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Faith J. T., et al. , Paleoenvironmental context of the Middle Stone Age record from Karungu, Lake Victoria Basin, Kenya, and its implications for human and faunal dispersals in East Africa. J. Hum. Evol. 83, 28–45 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Van Valkenburgh B., Hayward M. W., Ripple W. J., Meloro C., Roth V. L., The impact of large terrestrial carnivores on Pleistocene ecosystems. Proc. Natl. Acad. Sci. U.S.A. 113, 862–867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis M. E., Werdelin L., “Patterns of change in the Plio-Pleistocene carnivorans of eastern Africa” in Hominin Environments in the East African Pliocene, Bobe R., Alemseged Z., Behrensmeyer A. K., Eds. (Springer, Dordrecht, 2007), pp. 77–105. [Google Scholar]
- 43.Werdelin L., Peigné S., “Carnivora” in Cenozoic Mammals of Africa, Werdelin L., Sanders W. J., Eds. (University of California Press, Berkeley, 2010), pp. 603–657. [Google Scholar]
- 44.Lewis M. E., Carnivoran paleoguilds of Africa: Implications for hominid food procurement strategies. J. Hum. Evol. 32, 257–288 (1997). [DOI] [PubMed] [Google Scholar]
- 45.Maslin M. A., et al. , East African climate pulses and early human evolution. Quat. Sci. Rev. 101, 1–17 (2014). [Google Scholar]
- 46.Hempson G. P., Archibald S., Bond W. J., A continent-wide assessment of the form and intensity of large mammal herbivory in Africa. Science 350, 1056–1061 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Duncan C., Chauvenet A. L., McRae L. M., Pettorelli N., Predicting the future impact of droughts on ungulate populations in arid and semi-arid environments. PLoS One 7, e51490 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Illius A. W., Gordon I. J., Modelling the nutritional ecology of ungulate herbivores: Evolution of body size and competitive interactions. Oecologia 89, 428–434 (1992). [DOI] [PubMed] [Google Scholar]
- 49.Janis C., The evolutionary strategy of the Equidae and the origins of rumen and cecal digestion. Evolution 30, 757–774 (1976). [DOI] [PubMed] [Google Scholar]
- 50.Van Soest P. J., Nutritional Ecology of the Ruminant (Cornell University Press, Ithaca, 1994). [Google Scholar]
- 51.Hillman J. C., Hillman A. K. K., Mortality of wildlife in Nairobi national park, during the drought of 1973-1974. Afr. J. Ecol. 15, 1–18 (1977). [Google Scholar]
- 52.Walker B. H., Emslie R. H., Owen-Smith R. N., Scholes R. J., To cull or not to cull: Lessons from a southern african drought. J. Appl. Ecol. 24, 381–401 (1987). [Google Scholar]
- 53.Abraham J. O., Hempson G. P., Staver A. C., Drought-response strategies of savanna herbivores. Ecol. Evol. 9, 7047–7056 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Louys J., Meloro C., Elton S., Ditchfield P., Bishop L. C., Analytical framework for reconstructing heterogeneous environmental variables from mammal community structure. J. Hum. Evol. 78, 1–11 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Sankaran M., et al. , Determinants of woody cover in African savannas. Nature 438, 846–849 (2005). [DOI] [PubMed] [Google Scholar]
- 56.Hempson G. P., et al. , Ecology of grazing lawns in Africa. Biol. Rev. Camb. Philos. Soc. 90, 979–994 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Gill J. L., Ecological impacts of the late Quaternary megaherbivore extinctions. New Phytol. 201, 1163–1169 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Johnson C. N., Ecological consequences of late Quaternary extinctions of megafauna. Proc. Biol. Sci. 276, 2509–2519 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doughty C. E., Wolf A., Malhi Y., The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia. Nat. Geosci. 6, 761–764 (2013). [Google Scholar]
- 60.Doughty C. E., et al. , Global nutrient transport in a world of giants. Proc. Natl. Acad. Sci. U.S.A. 113, 868–873 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanders W. J., Proboscidea from Kanapoi, Kenya. J. Hum. Evol., 10.1016/j.jhevol.2018.10.013 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Kamilar J. M., Beaudrot L., Reed K. E., Climate and species richness predict the phylogenetic structure of African mammal communities. PLoS One 10, e0121808 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rowan J., Kamilar J. M., Beaudrot L., Reed K. E., Strong influence of palaeoclimate on the structure of modern African mammal communities. Proc. Biol. Sci. 283, 20161207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kingdon J., Happold D., Butynski T., Hoffman M., Kalina J., Eds., Mammals of Africa (Bloomsbury Publishing, London: ), vols. I–VI, 2013). [Google Scholar]
- 65.Skinner J. D., Chimimba C. T., The Mammals of the Southern African Subregion (Cambridge University Press, Cambridge, 2005). [Google Scholar]
- 66.Estes R. D., The Behavior Guide to African Mammals (University of California Press, Los Angeles, 1991). [Google Scholar]
- 67.Brain C. K., The Hunters or the Hunted? An Introduction to African Cave Taphonomy (University of Chicago Press, Chicago, 1981). [Google Scholar]
- 68.Behrensmeyer A. K., Western D., Dechant Boaz D. E., New perspectives in vertebrate paleoecology from a recent bone assemblage. Paleobiology 5, 12–21 (1979). [Google Scholar]
- 69.Larrasoaña J. C., Roberts A. P., Rohling E. J., Winklhofer M., Wehausen R., Three million years of monsoon variability over the northern Sahara. Clim. Dyn. 21, 689–698 (2003). [Google Scholar]
- 70.deMenocal P. B., Plio-Pleistocene African climate. Science 270, 53–59 (1995). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.