Abstract
Objective:
Cancer and its treatment are known to be important risk factors for sepsis, contributing to an estimated 12% of U.S. sepsis admissions in the 1990s. However, cancer treatment has evolved markedly over the past two decades. We sought to examine how cancer-related-sepsis differs from non-cancer-related sepsis.
Design:
Observational cohort.
Setting:
National Readmissions Database (2013–2014), containing all-payer claims for 49% of US population.
Patients:
1,104,363 sepsis hospitalizations.
Interventions:
We identified sepsis hospitalizations in the U.S. National Readmissions Database using explicit codes for severe sepsis, septic shock, or Dombrovskiy criteria (concomitant codes for infection and organ dysfunction). We classified hospitalizations as cancer-related versus non-cancer-related based on the presence of secondary diagnosis codes for malignancy. We compared characteristics (site of infection, organ dysfunction) and outcomes (in-hospital mortality, 30-day readmissions) of cancer-related versus non-cancer-related sepsis hospitalizations. We also completed subgroup analyses by age, cancer types and specific cancer diagnoses.
Measurements and Main Results:
There were 27,481,517 hospitalizations in NRD 2013–2014, of which 1,104,363 (4.0%) were for sepsis and 4,150,998 (15.1%) were cancer-related. In-hospital mortality in cancer-related-sepsis was 27.9%, versus 19.5% in non-cancer-related sepsis. The median count of organ dysfunctions was indistinguishable, but the rate of specific organ dysfunctions differed by small amounts (e.g., hematologic dysfunction 20.1% in cancer-related vs 16.6% in non-cancer related, p <0.001). Cancer-related sepsis was associated with an adjusted absolute increase in in-hospital mortality ranging from 2.2%−15.2% compared to non-cancer related sepsis. The mortality difference was greatest in younger adults and waned with age. 23.2% of patients discharged from cancer-related-sepsis were re-hospitalized within 30 days, compared to 20.1% in non-cancer-related sepsis, p<0.001.
Conclusions:
In this cohort of over 1 million U.S. sepsis hospitalizations, more than 1 in 5 were cancer-related. The difference in mortality varies substantially across age spectrum and is greatest in younger adults. Readmissions were more common after cancer-related-sepsis.
Keywords: Infection, Neoplasms, Mortality, Biostatistics, Critical Care Outcomes
INTRODUCTION
Sepsis, life-threatening organ dysfunction in response to infection(1), is a leading cause of hospitalization and mortality(2, 3). Cancer and its treatments (e.g. chemotherapy, radiation, surgery, bone marrow transplantation, and blood products) are known to increase risk for sepsis. In the 1990s, cancer was estimated to contribute to 12% of U.S. sepsis admissions(4, 5). Moreover, an estimated 5% of all hospitalizations among cancer patients were due to sepsis (4); in-hospital mortality was 2.5-fold higher in cancer-related versus non-cancer-related sepsis admissions(6–8); and 30% of cancer deaths were associated with sepsis(9).
Over the past two decades, however, cancer treatment has evolved substantially. Cytogenetic testing has allowed for a more personalized approach to cancer treatment(10, 11). Hematopoietic stem cell transplant has become safer and more successful(10). Radiation therapy and chemotherapy options in lymphoma have led to reduced cytoxicity and adverse effects such as congestive heart failure(12). Further developments, including chimeric antigen receptor therapy (CAR-T)(13) and oncolytic virus therapy(14), are reshaping the field of cancer therapies.
Given these advances in treatment over the past 15–20 years, we sought to examine the epidemiology and outcomes of cancer-related sepsis in the current era of cancer treatment. We hypothesized that the proportion of sepsis hospitalizations that are cancer-related would be higher than prior estimates since people are now living longer with cancer. Secondly, we hypothesized that the epidemiology of sepsis (site of infection, organ dysfunctions) would differ between cancer-related and non-cancer-related sepsis hospitalizations, since cancer may predispose to particular types of sepsis. Thirdly, we hypothesized that in-hospital mortality and 30-day readmissions would be higher in cancer-related versus non-cancer-related sepsis.
METHODS
Study Population and Cohort Identification
The National Readmissions Database (NRD), produced by the Healthcare Cost and Utilization Project (HCUP), contains all-payer hospital discharges from 22 US states, or roughly 50% of all hospitalizations in the United States(15). Since NRD is a publicly available de-identified dataset, this study did not require approval by the institutional review board. In the NRD (January 1,2013 to December 31, 2014), we identified sepsis hospitalizations by (1) explicit International Classification of Diseases, 9th version, Clinical Modification (ICD-9-CM) diagnosis codes for severe sepsis (995.92) or septic shock (785.52); or (2) Dombrovskiy criteria, concurrent diagnostic codes for bacteremia or sepsis and acute organ dysfunction(16). We selected this method because it has a higher positive predictive value than other claims-based methods(5, 17, 18), however does not require hospitals to code for severe sepsis or septic shock(16, 17). Hospitalizations among infants were excluded due to the low rate of malignancy in this population(19). Age was analyzed as a continuous variable as well as by age groups: 1–14, 15–17, 18–25, 26–44, 45–64, 65–79, 80–85, and ≥85 years.
Hospitalization Characteristics
We classified sepsis hospitalizations as cancer-related versus non-cancer-related based on evidence of cancer in secondary hospitalization diagnoses. Specifically, we identified and grouped cancer diagnoses using HCUP Clinical Classification Software (CCS) groupings for neoplasms (11–43), and further sub-divided into solid, hematologic, and other (i.e. not specified) malignancies(20). We did not have information regarding stage or treatment method for malignancy. We classified site of infection as respiratory, genitourinary, gastrointestinal, skin/soft tissue, joint/bone, central nervous system (CNS), cardiovascular, and other/unknown based the highest ranking diagnosis code indicating an infectious site (Supplemental Table 1), as in prior work(21). Acute organ dysfunctions were identified using Dombrovskiy criteria(16) (Supplemental Table 1). For adult hospitalizations, we measured individual comorbidities and total burden of comorbid disease using the Deyo implementation of the weighted Charlson Comorbidity Index (CCI)(22–24). Malignancy was excluded from the calculation of the CCI given it was also the exposure. For pediatric hospitalizations, we measured comorbidities using the Pediatric Complex Chronic Conditions Classification System (Supplemental Table 2) version 2(25).
Outcomes and Statistical Analysis
We examined differences in patient and hospitalization characteristics (age, sex, co-morbidities, payer type, site of infection, number and types of organ dysfunction) and outcomes (length of stay, in-hospital mortality, 30-day all-cause re-hospitalization, and 30-day readmission for another episode of sepsis) between cancer-related and non-cancer-related sepsis hospitalizations. For readmissions, we limited the analysis to index hospitalizations with a January-November discharge to allow for full 30-day follow-up in the dataset. Furthermore, we limited to hospitalizations in the patient’s state of residency, as hospitalizations in other states would be more likely to be followed by a readmission not captured in the NRD.
To compare characteristics of cancer-related and non-cancer-related sepsis hospitalizations, we used t-tests, Mann-Whitney or Kruskal-Wallis for continuous variables, and chi-square or fisher-exact tests for categorical variables. To examine the association of cancer status with mortality across age, we used several multivariable modeling strategies. First, we fit logistic regression models stratified by relevant age groups, reporting both odds ratios (ORs) and absolute differences in predicted probabilities of death after adjusting for age, gender, payer type, income quartile, comorbidities, and site of infection. Next, we used modified Poisson regression to report risk ratios (RRs) and absolute risk differences (RDs)(26–28), and modeled across age flexibly using restricted cubic splines(29). We modeled the relationship between cancer-related sepsis and age separately for pediatric (ages 1–17) and adult (ages 18+) hospitalizations, given the differing methods for comorbidity measurement in these populations.
We completed subgroup analyses by cancer type (solid tumor, hematologic tumor, and other/not specified) and by specific cancer diagnoses (e.g. pancreatic cancer, lung cancer, breast cancer) since sepsis characteristics and outcomes may differ by tumor type(30, 31). Lastly, as a “negative control”, we performed analyses of sepsis hospitalizations with versus without hypothyroidism and dementia.
All analyses were performed with Stata MP 15 (College Station, TX). Given the size of the dataset and multiple comparisons, we set statistical significance at p<0.001(32). In alignment with NRD data use agreement, cells with numbers 1–11 are reported as ≤11.
RESULTS
Cohort Demographics and Characteristics
There were 27,481,517 hospitalizations in NRD 2013–2014, of which 1,104,363 (4.0%) were for sepsis and 4,150,998 (15.1%) were cancer-related (i.e., had any diagnostic code indicating malignancy). 234,641 cancer-related hospitalizations (5.7%) were for sepsis. Among patients ages 1–14, 15–17, 18–25, 26–44, 45–64, 65–79, 80–85, and >85 years, cancer-related hospitalizations accounted for 20.05%, 14.2%, 7.5%, 10.1%, 19.5%, 25.7%, 23.6%, and 19.5% of sepsis hospitalizations, respectively.
Sepsis hospitalizations had a median age of 70 years, were majority Medicare beneficiaries (746,078, 67.6%), with multiple co-morbidities (median CCI 3, IQR 1–5), modest acute organ dysfunction (median 1, IQR 1–2), and a median length of stay of 8 days (IQR 4–14). The most common sites of infection were respiratory (37.7%), genitourinary (26.5%), and gastrointestinal (10.5%). In-hospital mortality occurred in 235,210 (21.3%).
Epidemiology of Cancer-Related vs. Non-Cancer-Related Sepsis Hospitalizations
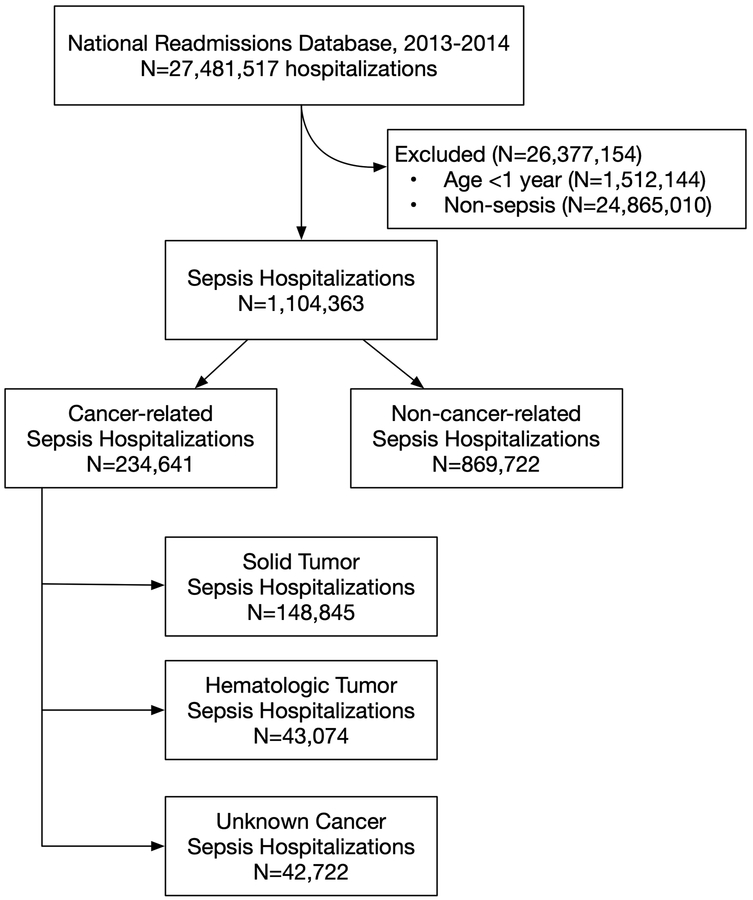
Of the 1,104,363 sepsis hospitalizations, 234,641 (21.2%; 95%CI: 21.1%−21.3%) were cancer-related, and 869,722 (78.8%; 95%CI 78.7%−78.8%) were non-cancer-related (Table 1). Of the 234,641 cancer-related sepsis hospitalizations, 63.4% were solid tumor, 18.4% were hematologic, and 18.2% unknown or non-specified tumor type (Figure 1).
Table 1.
Sepsis Hospitalization Demographics
| Characteristics | Non-cancer-related sepsis hospitalizations (N=869,722) | Cancer-related sepsis hospitalizations (N=234,641) | p |
|---|---|---|---|
| Patient Characteristics | |||
| Age (years), median (IQR) | 69 (56–81) | 71 (62–81) | <0.001 |
| Charlson Comorbidity Index (revised) | |||
| median, IQR | 3 (1–5) | 0 (2–4) | <0.001 |
| Pediatric Chronic Conditions (revised) | |||
| median, IQR | 1 (0–2) | 1 (1–2) | 0.25 |
| Gender | |||
| Male | 436,000 (50.1%) | 130,729 (55.7%) | <0.001 |
| Hospitalization Characteristics | |||
| Organ dysfunction count | |||
| median (IQR) | 1 (1–2) | 1 (1–2) | 0.63 |
| Individual Organ Dysfunctions, N (%) | |||
| Renal | 529,748 (60.9%) | 137,481 (58.6%) | <0.001 |
| Cardiovascular | 396,849 (45.6%) | 110,559 (47.1%) | <0.001 |
| Pulmonary | 338,155 (38.9%) | 87,726 (37.4%) | <0.001 |
| Hematologic | 144,393 (16.6%) | 47,136 (20.1%) | <0.001 |
| Liver | 54,872 (6.3%) | 12,417 (5.3%) | <0.001 |
| Neurologic | 52,482 (6.0%) | 10,624 (4.5%) | <0.001 |
| None | 44,478 (5.1%) | 11,174 (4.8%) | <0.001 |
| Site of Infection, N(%) | <0.001 | ||
| Respiratory | 328,419 (37.8%) | 88,554 (37.7%) | |
| Genitourinary | 236,453 (27.2%) | 56,550 (24.1%) | |
| Gastrointestinal | 86,216 (9.9%) | 29,608 (12.6%) | |
| Skin/soft tissue | 66,894 (7.7%) | 10,890 (4.6%) | |
| Bacteremia/Fungemia | 16,195 (1.9%) | 6,425 (2.7%) | |
| Joint/bone | 17,305 (2.0%) | 1,760 (0.8%) | |
| Central Nervous System | 6,750 (0.8%) | 1,233 (0.5%) | |
| Endocarditis/Myocarditis | 1,965 (0.2%) | 356 (0.2%) | |
| Missing or Unknown | 109,525 (12.6%) | 39,265 (16.7%) | |
| Hospital Length of Stay (days), Alive | |||
| median (IQR) | 8 (4–14) | 8 (4–14) | <0.001 |
| Hospital Length of Stay (days), Deceased | |||
| median (IQR) | 6 (2–14) | 6 (2–14) | <0.001 |
| In-Hospital Mortality | 169,791 (19.5%) | 65,419 (27.9%) | <0.001 |
Definitions of site of infection and acute organ dysfunctions are presented in Supplemental Table 1.
Payer Type demographics are presented in Supplemental Table 6.
IQR, interquartile range; SD, standard deviation
Figure 1: Study Flow.
Identification methods for sepsis and cancer are presented in Supplemental Table 1
Compared to non-cancer-related sepsis hospitalizations, cancer-related sepsis hospitalizations were older (median age 71 vs 69 years, p <0.001), more likely to have private insurance (17.8% vs 14.1%, p< 0.001), and had fewer non-cancer comorbidities (median revised CCI of 3 vs 0, p<0.001) (Table 1, Supplemental Tables 3 and 4). The burden of organ dysfunction was indistinguishable between cancer-related and non-cancer-related sepsis (median 1 organ dysfunction for both groups, p=0.63). However, rates of individual organ dysfunctions differed by small magnitudes (1% to 4% per individual organ dysfunction) (Table 1). For example, cancer-related sepsis hospitalizations were more likely to have hematologic dysfunction (20.1% versus 16.6%, p<0.001), but less likely to have pulmonary (37.4% versus 38.9%, p<0.001) or renal dysfunction (58.6% versus 60.9%, p<0.001). Site of infection also differed, with cancer-related sepsis hospitalizations having a higher rate of gastrointestinal infection (12.6% versus 9.9%), bacteremia/fungemia (2.7% versus 1.9%), and unknown/missing site (16.7% versus 12.6%) (Table 1), p<0.001 for each.
Outcomes of Cancer-Related vs. Non-Cancer-Related Sepsis Hospitalizations
Compared to non-cancer-related sepsis, cancer-related-sepsis hospitalizations had higher in-hospital mortality (27.9% versus 19.5%, p<0.001). Mortality was consistently higher in cancer-related sepsis, across subgroups defined by site of infection and burden of acute organ dysfunction. (Supplemental Tables 5 and 6). Among the subset of patients for whom readmissions could be reliably measured (January-November live discharges in state of residency), rates of 30-day readmission were higher after cancer-related versus non-cancer-related sepsis hospitalizations (23.2% versus 20.1%, p<0.001). In the subset of patients aged ≥65 years, rates of readmission were likewise higher after cancer-related sepsis (21.3% versus 20.0%, p<0.001). The median time from discharge to readmission was 12 days (IQR 6–20), and did not differ between cancer-related versus non-cancer-related sepsis. Rates of 30-day readmission for recurrent sepsis were also higher in cancer-related versus non-cancer-related sepsis (6.2% versus 5.4%, p<0.001), but similar among patients aged ≥65 years (5.9% versus 5.8%, p=0.16).
Difference in Outcomes by Age Group
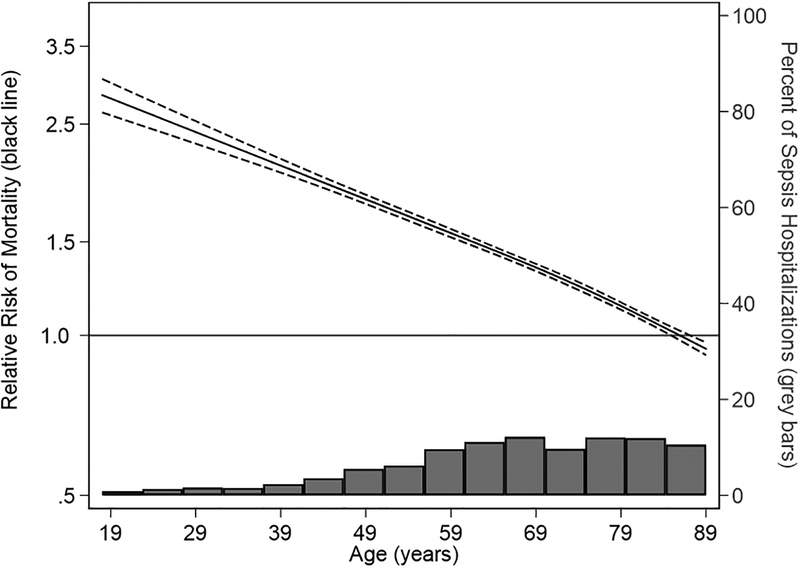
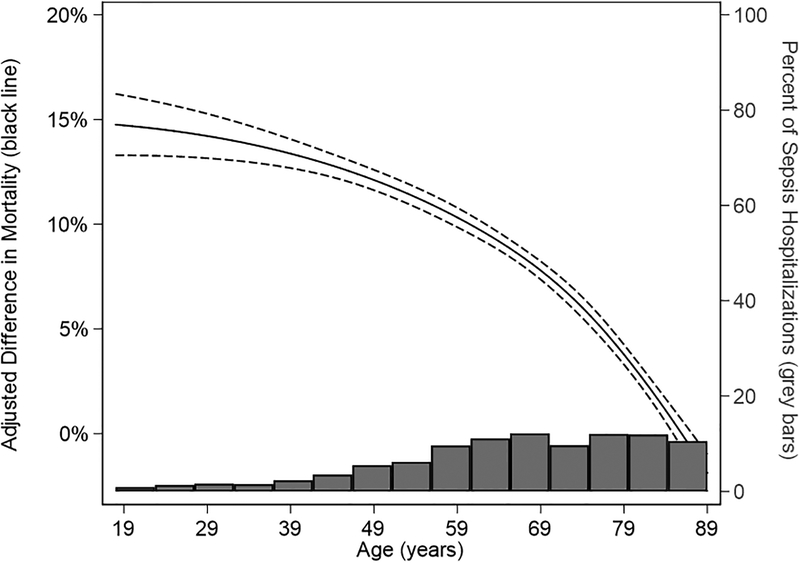
After adjusting for age, gender, payer type, non-cancer comorbidities, income and site of infection, odds of in-hospital mortality with cancer-related sepsis remained higher in both pediatric (adjusted OR 1.7; 95% CI: 1.4–2.2) and adult cohorts (adjusted OR 1.6; 95%CI: 1.5–1.6). Among adults, the adjusted absolute increase in in-hospital mortality was largest in younger adults and declined with increasing age until there was no difference in in-hospital mortality among cancer-related versus non-cancer related sepsis hospitalization at age 85 years and older: 18–25 years (adjusted OR 3.9; 95% CI: 3.3–4.5); 26–44 years (adjusted OR 3.2; 95% CI: 3.0–3.4); 45–64 years (adjusted OR 2.2; 95% CI: 2.1–2.2); 65–79 years (adjusted OR 1.6; 95% CI: 1.5–1.6); 80–85 years (adjusted OR 1.2; 95% CI: 1.2–1.3); >85 years (adjusted OR 1.0; 95% CI: 1.0–1.1) (Table 2). This corresponded to an adjusted mortality difference of 5.3%, 15.2%, 14.9%, 12.3%, 7.9%, 2.2%, and 0.1%, respectively (Table 2). Results of the Poisson regression modeling adjusted relative risk (Figure 2, Supplemental Figure 1) and risk difference (Figure 3, Supplemental Figure 2) showed similar reduction in risk difference with advancing age.
Table 2.
Adjusted In-hospital Cancer-related Sepsis Mortality by Age Group
| Age Group | Odds Ratio (95% CI) | Adjusted Difference in Probability (95% CI) |
|---|---|---|
| 1–14 years | 1.6 (1.2–2.2) | 5.1% (1.9%–8.1%) |
| 15–17 years | 1.8 (1.1–3.0) | 4.9% (0.3%–9.6%) |
| 18–25 years** | 3.9 (3.3–4.5) | 15.2% (12.8%–17.5%) |
| 26–44 years** | 3.2 (3.0–3.4) | 14.9% (14.0%–15.9%) |
| 45–64 years** | 2.2 (2.1–2.2) | 12.3% (11.9%–12.7%) |
| 65–79 years** | 1.6 (1.5–1.6) | 7.9% (7.6%–8.2%) |
| 80–85 years** | 1.2 (1.2–1.3) | 3.9% (3.4%–4.5%) |
| >85 years** | 1.0 (1.0–1.1) | 0.1% (0.0%–1.0%) |
adjusted for age, gender, payer type, income quartile, site of infection, and comorbidities (neuromuscular, metabolic, cardiovascular, respiratory, renal, gastrointestinal, hematologic, transplant, and technology dependent
adjusted for age, gender, payer type, income quartile, site of infection, and comorbidities (complicated diabetes, severe liver disease, cerebrovascular disease, myocardial infarction, congestive heart failure, chronic kidney disease, and chronic respiratory disease)
Figure 2: Cancer-Related versus Non-Cancer-Related Sepsis Relative Risk of Mortality.
Solid black line indicates relative risk of mortality. Dashed lines indicate 95% confidence limits. Grey bars indicate the distribution of sepsis hospitalizations by age.
Adjusted for: gender, payer type, income quartile, site of infection, comorbidities (CHF, MI, complicated diabetes, severe liver disease, cerebrovascular disease, hemiplegia, chronic kidney disease, and chronic respiratory disease)
Figure 3: Cancer-Related versus Non-Cancer-Related Sepsis Mortality Risk Difference.
Solid black line indicates risk difference in mortality. Dashed lines indicate 95% confidence limits. Grey bars indicate the distribution of sepsis hospitalizations by age.
Adjusted for: gender, payer type, income quartile, site of infection, comorbidities (CHF, MI, complicated diabetes, severe liver disease, cerebrovascular disease, hemiplegia, chronic kidney disease, and chronic respiratory disease)
Difference in Epidemiology and Outcomes by Tumor Type
Hematologic and other (unclassified) tumor types had a greater burden of acute organ dysfunctions (median 2 versus 1, p<0.001); a higher proportion of cardiovascular failure (51.1% versus 44.8%, p<0.001); a higher proportion with respiratory site of infection (42.4% versus 36.3%, p<0.001); and a longer length of stay (median 9 days versus 7 days, p<0.001) (Supplemental Table 7).
In-hospital mortality was higher for other tumor types (37.1%, OR 1.8) and hematologic tumor type (30.6%, OR 1.4) compared to the solid tumor group (24.5%) (Supplemental Table 8). These differences in in-hospital mortality persisted after adjustment for age, payer type, length of stay, gender, site of infection, and comorbidities (Supplemental Table 9). When examining more granular tumor sub-types, in-hospital mortality was greatest for neoplasms of unknown site (42.4%, OR 2.7), secondary malignancies (37.9%, OR 2.4), lung cancer (37.1%, OR 2.2), and leukemias (33.1%, OR 1.8) (Supplemental Table 10).
Difference in Sepsis Epidemiology and Outcomes in “Negative Control” Populations
In-hospital mortality and 30-day readmissions were each indistinguishable between sepsis hospitalizations with and without dementia (21.9% versus 21.3%, and 20.0% versus 20.7%, respectively, p>0.001 for each). Though in-hospital mortality differed in sepsis hospitalizations with and without hypothyroidism, the magnitude of difference was small (19.7% versus 21.6%, p<0.001). Rates of 30-day readmissions were indistinguishable (20.7% versus 20.7%, p>0.001).
DISCUSSION
In this recent all-payer sample of more than 1 million US sepsis hospitalizations, we found that one in five sepsis hospitalizations was associated with malignancy, an increase from the one in eight reported in earlier cohorts from the 1990s(5). In-hospital mortality was higher in cancer-related sepsis hospitalizations (27.9% vs 19.5% in non-cancer-related sepsis), even after adjustment for age, payer type, length of stay, gender, site of infection, and comorbidities. Cancer-related sepsis was consistently associated with excess mortality in subgroup analyses by site of infection and individual organ dysfunctions. However, the adjusted difference in mortality varied substantially across the age spectrum. Overall, the magnitude of difference in mortality between cancer-related and non-cancer-related sepsis hospitalization was smaller than in prior studies from the 1990s(4, 7, 8).
There are many plausible explanations for differential outcomes between cancer-related and non-cancer-related sepsis including the cancer itself, cancer treatment and resulting immune suppression, critical care provider bias (i.e., less willingness to accept to ICU), and differing goals of care. Furthermore, the mortality difference between cancer-related and non-cancer-related sepsis may be declining over time as a result of changes in any of these factors. For example, as cancer survival has improved over time(33), patients and clinicians may be less likely to pursue comfort care in cancer-related sepsis. Given the growing awareness of sepsis (particularly in the setting of cancer), cancer patients may present earlier after onset of infectious symptoms. Likewise, clinicians may recognize sepsis more readily and initiate treatment sooner in this high risk population(34).
Contrary to prior studies(7, 8), the burden of acute organ dysfunction was similar between cancer-related and non-cancer related sepsis, although there were small differences in the rates of individual organ dysfunctions. Likewise, while the distribution of infection sites differed, the magnitude of difference was small. Thus, consistent with prior studies(31), site of infection and burden of organ dysfunction did not explain the differences in in-hospital mortality between cancer-related and non-cancer-related sepsis. As expected, in-hospital mortality was higher in hematologic and undifferentiated tumor types compared to solid tumors. These differences may relate to differences in the underlying malignancy, stage as presentation, or treatment. Future studies with granular detail on cancer stage and treatment are needed to better understand this association.
Interestingly, the magnitude of difference in mortality between cancer-related and non-cancer-related sepsis varied markedly by age. The greatest difference occurred in young adults—where the absolute adjusted difference in mortality was approximately 15%. The gap in mortality declined with increasing age, ultimately becoming indistinguishable among patients greater than 85 years. Aging itself is associated with immunosenescence, which may be comparable to the impact of cancer and its treatments on immune function (35, 36). Thus, mortality may become indistinguishable in older patients because immune function is similarly impaired in cancer-related sepsis and non-cancer-related sepsis.
More than one in five sepsis hospitalizations was followed by a 30-day readmission. Rates of all-cause and sepsis-specific readmissions were both higher after cancer-related sepsis. However, sepsis-specific readmissions were similar among patients ≥65 years, lending further support to the hypothesis that difference in immune function among patients with cancer-related vs non-cancer-related sepsis may be less pronounced in older patients. Rates of readmissions among negative control populations (with/without dementia and hypothyroidism) were indistinguishable.
This study should be interpreted in the context of several limitations. First, we identified both sepsis and cancer using diagnosis codes, which may result in misclassification in both directions. However, in recent years, the positive predictive value of claims-based and electronic health record-based identification of sepsis (compared to a gold standard of physician adjudication by chart review) have been similar(37). Furthermore, we required that patients have a diagnosis code of bacteremia, sepsis, severe sepsis, or septic shock—which results in greater positive predictive value than methods including all infection codes. Second, we did not have data on cancer stage or treatment. Thus, we are unable to disentangle the impact of cancer and its treatment. Furthermore, differences in mortality by cancer subtype may be confounded by differences in cancer stage at presentation. Third, differences in hematologic failure rates could be due to chemotherapy-induced myelosuppression, rather than different manifestations of sepsis in the setting of cancer. Fourth, we did not have data on treatment limitations, discharge to hospice care, or post-hospital deaths, and it is possible that a greater proportion of cancer-related sepsis patients had treatment limitations, were discharged to hospice, or died in the immediate post-hospital setting. With such a large dataset, there is the possibility of false discovery (type 1 errors). To mitigate this concern, we selected a conservative threshold for statistical significance (p<0.001). Furthermore, we completed a “negative control” analysis, examining differences in sepsis hospitalizations with versus without hypothyroidism, and with versus without dementia. Mortality among negative sepsis controls, with versus without, was either small (hypothyroidism) or indistinguishable (dementia), suggesting that the excess mortality of cancer-related sepsis is not merely a result of false discovery with a large dataset.
CONCLUSION
More than 1 in 5 sepsis hospitalizations occurs in patients diagnosed with cancer. In-hospital mortality in cancer-related-sepsis is 28%, versus 20% in non-cancer-related sepsis. However, the difference in mortality varies substantially across the age spectrum—ranging from 15% absolute difference in young adults to minimal difference in the oldest patients.
Supplementary Material
Acknowledgements:
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. government. This work was supported by grants K08 GM115859 [HCP] and K12 HL138039 [JPD] from the National Institutes of Health.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, et al. : The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NKJ, et al. : Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med 2016; 193:259–272 [DOI] [PubMed] [Google Scholar]
- 3.Liu V, Escobar GJ, Greene JD, et al. : Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014; 312:90–92 [DOI] [PubMed] [Google Scholar]
- 4.Williams MD, Braun LA, Cooper LM, et al. : Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care 2004; 8:R291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus DC, Linde-Zwirble WT, Lidicker J, et al. : Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 6.Martin GS, Mannino DM, Eaton S, et al. : The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348:1546–1554 [DOI] [PubMed] [Google Scholar]
- 7.Torres VBL, Azevedo LCP, Silva UVA, et al. : Sepsis-Associated Outcomes in Critically Ill Patients with Malignancies. Ann Am Thorac Soc 2015; 12:1185–1192 [DOI] [PubMed] [Google Scholar]
- 8.Rosolem MM, Rabello LSCF, Lisboa T, et al. : Critically ill patients with cancer and sepsis: clinical course and prognostic factors. J Crit Care 2012; 27:301–307 [DOI] [PubMed] [Google Scholar]
- 9.Danai PA, Moss M, Mannino DM, et al. : The epidemiology of sepsis in patients with malignancy. Chest 2006; 129:1432–1440 [DOI] [PubMed] [Google Scholar]
- 10.Dombret H, Gardin C: An update of current treatments for adult acute myeloid leukemia. Blood 2016; 127:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang K-L, Mashl RJ, Wu Y, et al. : Pathogenic Germline Variants in 10,389 Adult Cancers. Cell 2018; 173:355–370.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michot J-M, Lazarovici J, Ghez D, et al. : Challenges and perspectives in the immunotherapy of Hodgkin lymphoma. Eur J Cancer 2017; 85:67–77 [DOI] [PubMed] [Google Scholar]
- 13.Knochelmann HM, Smith AS, Dwyer CJ, et al. : CAR T Cells in Solid Tumors: Blueprints for Building Effective Therapies. Front Immunol 2018; 9:1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosewell Shaw A, Suzuki M: Oncolytic Viruses Partner With T-Cell Therapy for Solid Tumor Treatment. Front Immunol 2018; 9:2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhabra N, Gupta A, Chibber R, et al. : Outcomes and mortality in parturient and non-parturient patients with peripartum cardiomyopathy: A national readmission database study. Pregnancy Hypertens 2017; 10:143–148 [DOI] [PubMed] [Google Scholar]
- 16.Dombrovskiy VY, Martin AA, Sunderram J, et al. : Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 2007; 35:1244–1250 [DOI] [PubMed] [Google Scholar]
- 17.Iwashyna TJ, Odden A, Rohde J, et al. : Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care 2014; 52:e39–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jolley RJ, Sawka KJ, Yergens DW, et al. : Validity of administrative data in recording sepsis: a systematic review. Crit Care 2015; 19:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orbach D, Sarnacki S, Brisse HJ, et al. : Neonatal cancer. Lancet Oncol 2013; 14:e609–620 [DOI] [PubMed] [Google Scholar]
- 20.Healthcare Cost and Utilization Project: HCUP National Readmissions Database (NRD) [Internet]. 2014; Available from: https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp
- 21.Prescott HC, Osterholzer JJ, Langa KM, et al. : Late mortality after sepsis: propensity matched cohort study. BMJ 2016; 353:i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–619 [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383 [DOI] [PubMed] [Google Scholar]
- 24.Gagne JJ, Glynn RJ, Avorn J, et al. : A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 2011; 64:749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feudtner C, Feinstein JA, Zhong W, et al. : Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014; 14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams R: Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata Journal 2012; 12:308–331 [Google Scholar]
- 27.Rabe-Hesketh S, Skrondal A, Pickles, Andrew: Reliable estimation of generalized linear mixed models using adaptive quadrature. The Stata Journal 2002; 1–21 [Google Scholar]
- 28.Zou G: A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–706 [DOI] [PubMed] [Google Scholar]
- 29.Harrell F: Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. 1st ed Springer-Verlag; New York; 2001. [Google Scholar]
- 30.Liu Z, Mahale P, Engels EA: Sepsis and Risk of Cancer Among Elderly Adults in the United States. Clin Infect Dis 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taccone FS, Artigas AA, Sprung CL, et al. : Characteristics and outcomes of cancer patients in European ICUs. Crit Care 2009; 13:R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ioannidis JPA: The Proposal to Lower P Value Thresholds to .005. JAMA 2018; 319:1429–1430 [DOI] [PubMed] [Google Scholar]
- 33.Jemal A, Ward EM, Johnson CJ, et al. : Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J Natl Cancer Inst 2017; 109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larché J, Azoulay E, Fieux F, et al. : Improved survival of critically ill cancer patients with septic shock. Intensive Care Med 2003; 29:1688–1695 [DOI] [PubMed] [Google Scholar]
- 35.Frasca D, Blomberg BB: Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 2016; 17:7–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuentes E, Fuentes M, Alarcón M, et al. : Immune System Dysfunction in the Elderly. An Acad Bras Cienc 2017; 89:285–299 [DOI] [PubMed] [Google Scholar]
- 37.Rhee C, Dantes R, Epstein L, et al. : Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009–2014. JAMA 2017; 318:1241–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.