Abstract
Objective
To evaluate lesion location after pediatric cerebellar tumor resection in relation to the development of severe cognitive and affective disturbances, or cerebellar cognitive affective syndrome (CCAS).
Methods
The postsurgical lesion location of 195 pediatric patients with cerebellar tumors was mapped onto a template brain. Individuals with CCAS were matched to 2 participants without CCAS by sex, age, and lesion volume. Lesion analyses included both a hypothesis-driven evaluation of the cerebellar outflow pathway (deep nuclei and superior cerebellar peduncles) and data-driven multivariate lesion symptom mapping. Lesion-associated networks were evaluated by comparing connectivity patterns between the lesion location of cases with and those without CCAS with resting-state functional connectivity MRI data from large normative adult and pediatric cohorts.
Results
CCAS was present in 48 of 195 participants (24.6%) and was strongly associated with cerebellar outflow tract lesions (p < 0.0001). Lesion symptom mapping also highlighted the cerebellar outflow pathway, with peak findings in the fastigial nuclei extending into the inferior vermis. Lesion network mapping revealed that the cerebellar region most associated with CCAS was functionally connected to the thalamic mediodorsal nucleus, among other sites, and that higher connectivity between lesion location and the mediodorsal nucleus predicts CCAS occurrence (p < 0.01). A secondary analysis of 27 participants with mutism revealed similar localization of lesions and lesion-associated networks.
Conclusion
Lesions of the cerebellar outflow pathway and inferior vermis are associated with major cognitive and affective disturbances after pediatric cerebellar tumor resection, and disrupted communication between the cerebellum and the thalamic mediodorsal nucleus may be important.
Childhood brain tumors commonly arise from the cerebellum.1 About 25% of children undergoing cerebellar tumor resection develop severe cognitive and affective symptoms, which may include emotional lability, executive dysfunction, and language impairment, among others.1–3 These symptoms have been labeled posterior fossa syndrome,1 cerebellar cognitive affective syndrome (CCAS),4–7 and, when mutism or severely diminished speech is present, cerebellar mutism syndrome (CMS).8 These postoperative symptoms are a risk factor for poorer long-term cognitive outcomes,9 and as survival rates increase, there is greater emphasis on optimizing long-term outcomes.
The pathogenesis of these symptoms is poorly understood. Studies have highlighted increased risk when lesions involve the superior cerebellar peduncles, dentate nuclei, brainstem, and vermis,5,10–20 possibly by disrupting cerebrocerebellar communication.18,19,21 Communication between the cerebellar and cerebral cortices relies on Purkinje cells of the cerebellar cortex projecting to deep cerebellar nuclei, which project via the superior cerebellar peduncles to the thalamus and then cerebral cortex. An anatomic bottleneck exists at the deep nuclei and superior cerebellar peduncles, where small lesions can disrupt vast cerebrocerebellar processing networks.
Here, we evaluate the role of lesion location in the development of postoperative severe cognitive and affective symptoms (CCAS), with a secondary analysis of mutism (CMS). We use an a priori region-of-interest (ROI) analysis of the cerebellar outflow pathway (deep nuclei and superior cerebellar peduncles) and data-driven multivariate lesion symptom mapping. Finally, we use normative functional connectivity data to evaluate the networks derived from the cerebellar region most associated with deficits to infer the disrupted cerebrocerebellar networks.22
Methods
Patient acquisition, demographics, and outcome classification
This was a retrospective analysis of 231 pediatric patients who underwent cerebellar tumor resection between 1988 and 2017 at the University of Iowa Hospitals and Clinics (n = 79) and Massachusetts General Hospital (n = 152). We included patients who underwent cerebellar tumor resection, had a postsurgical MRI scan, and had behavioral data relating to surgical outcome. Participants were excluded if they were >18 years of age at the time of diagnosis, if they had significant lesion involvement outside the cerebellum, or if lesion borders were poorly delineated. The development of severe postsurgical cognitive and affective sequelae was determined through a review of medical records by individuals blinded to lesion location. Patients with severe cognitive or affective symptoms after surgery that could not be attributed to other causes, such as developing executive dysfunction, mutism, irritability, and emotion dysregulation, were classified first more broadly as having CCAS, with a secondary analysis focusing on a subgroup of CCAS patients with mutism (CMS). This analysis of CMS included only patients with definite mutism, not individuals with diminished speech. Additional descriptive data available for analysis included handedness, lesion laterality, tumor type and size, resection cavity volume, presurgical hydrocephalus, total vs subtotal tumor resection, and the presence of relapse.
Main analyses (case-control)
Case-control design
Each patient with CCAS (n = 48) was matched to 2 individuals who did not develop CCAS after cerebellar tumor resection (n = 96). Participants were matched on age, sex, and lesion volume.
Analysis of MRIs
Lesion location was assessed from an MRI performed in the postsurgical chronic epoch (≥1 month after surgery) whenever available to limit any distortion or edema that may obscure the lesion borders. Each MRI was performed for clinical indications; thus, MRI scanners, sequences, and time since operation were not homogeneous across the sample. However, each participant included had a well-delineated cerebellar resection bed of sufficient quality to segment the lesion boundaries. A 3D reconstruction of each postsurgical lesion was manually mapped onto template brain (1-mm Montreal Neurological Institute [MNI] 152 template brain, fsl.fmrib.ox.ac.uk/fsl/) by a researcher blinded to the behavioral outcome using FSL software.23 To ensure anatomic accuracy, lesion boundaries were evaluated by a neurologist (A.D.B.) who was blinded to behavioral outcomes.
Lesion characteristics
For descriptive purposes, the lesions were overlapped to display the extent of coverage. To visualize whether differences existed between the CCAS group and all other participants, a lesion subtraction analysis was conducted to highlight areas that were preferentially damaged in the CCAS group. This was done by subtracting the non-CCAS proportional lesion overlap from that of the CCAS group. Regions that survived the subtraction had a higher proportion of damage in the CCAS group (if a voxel was affected in 75% of patients with CCAS and 8% of participants without CCAS, then this voxel would have a difference value of 75% − 8% = 67%).
A priori ROI
To test the hypothesis that patients with CCAS would have a greater disruption in their cerebellar outflow pathway, we created a 3D ROI using the deep nuclei (dentate, interposed [consists of the emboliform and globose], and fastigial nuclei) from the Spatially Unbiased Infra-Tentorial Template cerebellar deep nuclei atlas (diedrichsenlab.org/imaging/propatlas.htm) transformed into MNI space with FSL. This was combined with a previously published probabilistic map of the superior cerebellar peduncles with a 10% threshold applied.24 The final mask was binarized and edited to combine the ROIs into a single contiguous ROI extending from the deep nuclei along the superior cerebellar peduncles to the brainstem; it was created before any hypotheses were tested (figure 1).
Figure 1. Cerebellar outflow pathway lesion load.

The cerebellar outflow pathway is shown in blue on the left extending from the deep cerebellar nuclei along the superior cerebellar peduncles. The entire region of interest was separated into 1-mm oblique coronal slices perpendicular to the angle of the superior cerebellar peduncles (17°), of which 3 examples are shown. A sample lesion is shown in red that overlaps with the cerebellar outflow pathway. The extent the lesion overlap with the outflow pathway is quantified as a proportion of total voxels for each slice. Slice 83 (1) has a damaged proportion value of 0.15; slice 77 (2) has a damaged proportion of 0.73; and slice 71 (3) has a proportion of damage of 0.30. Across all slices, the maximum proportional value served as the cerebellar outflow pathway lesion load, which was used for subsequent analyses. This value would come from slice 77 (2) in the right panel among the slices shown, where the lesion overlaps with 73% of voxels in the outflow pathway for that slice.
Cerebellar outflow pathway lesion load
The extent of lesion involvement of the cross-sectional area of the cerebellar outflow pathway was quantified with methods used previously for quantifying lesion load to the corticospinal tract.25 First, the ROI was divided into individual 1-mm cross-sectional slices perpendicular to the longitudinal angle of the superior cerebellar peduncles (17° superior-posterior from standard coronal MNI152 orientation). Next, the proportion of damage for each slice was calculated as lesioned voxels divided by the total voxels for that slice (figure 1). The maximum proportional value across all slices represented the degree of disruption to the cerebellar outflow pathway and was used for subsequent analyses. The main analysis compared the cerebellar outflow pathway lesion load between the CCAS cohort and the matched comparison group. We also performed secondary analyses of the right and left superior cerebellar peduncles and deep cerebellar nuclei.
Data-driven exploratory analyses (all participants)
Multivariate lesion symptom mapping
Multivariate lesion symptom mapping was performed to identify regions of the cerebellum related to CCAS that were not constrained by a priori hypotheses. This was conducted with sparse canonical correlations for neuroimaging analyses implemented by the LESYMAP package in R (github.com/dorianps/LESYMAP). This technique provides several advantages relative to mass univariate statistics.26 Each voxel is assigned a weight to generate a hypothetical score with maximal correlation with the true behavioral score, applied to regions with a minimum threshold of 5% overlap. A 4-fold cross-validation correlation was used to correlate the predicted and true CCAS scores with a maximum value of 1. With this model, the statistical significance is applied to the overall map as opposed to individual voxels. To ensure that the findings were not limited to a specific approach, we also used a mass univariate approach with nonparametric mapping software available in NiiStat (nitrc.org/projects/niistat/) with a Liebermeister statistical test with a false discovery rate of 5% and lesion volume as a covariate.
Lesion network mapping
We evaluated the functional connectivity network of the site in the cerebellum with the strongest association with CCAS using results from the lesion symptom mapping analysis. This analysis relied on a recently developed method that infers the lesion-associated networks using normative resting-state functional connectivity MRI (rs-fcMRI) data.22,27 A publicly available rs-fcMRI dataset was used that included 98 healthy right-handed participants (age 22 ± 3.2 years, 50 females),28 as used previously for similar analyses.22,27 Processing of the rs-fcMRI data has been described in detail elsewhere.29,30,31
Using the network map derived from this cerebellar region associated with CCAS, we next evaluated the specificity of regional peaks in connectivity. For example, if CCAS-associated lesions have significantly higher connectivity with a network node than non-CCAS lesions, this would support specificity, whereas nonsignificant differences in connectivity strength would suggest nonspecific network effects that may be more general to the cerebellum without discriminating between CCAS and non-CCAS lesions. We identified regions within our lesion network map using the FSL clustering algorithm with a conservative z score threshold ≥10. Ten-millimeter spherical ROIs were placed at local maxima within each cluster. This threshold eliminated any network findings in the cerebral cortex, so a secondary clustering step was performed with a z-score threshold of 8.5 to identify the peak network node within the cerebral cortex. Next, the strength of correlation between these regional clusters and the individual lesion masks was compared between CCAS- and non–CCAS-associated lesions to evaluate for significant between-group differences after correction for multiple comparisons.
Effect of age on connectivity
We also evaluated whether the spatial distribution of connectivity at this cerebellar site was similar using normative pediatric functional connectivity data and whether connectivity strength varies as a function of age. To generate a normative pediatric functional connectivity dataset, we used the publicly available preprocessed controls (≤18 years old) from the Autism Brain Imaging Data Exchange Preprocessed Connectome Project.32 The Connectome Computation System pipeline described there is most similar to the pipeline used above; however, the software implementation differed. Data from 402 typically developing controls 6 to 18 years of age were used to generate a voxel-wise map of connectivity derived from the cerebellum region most highly associated with CCAS. The ROI-ROI pair-wise correlations between the cerebellar ROI and specific other ROIs such as the mediodorsal region of the thalamus were also obtained to examine correlation with age.
Secondary analyses
Cerebellar mutism syndrome
A secondary analysis evaluated lesion localization and lesion network mapping as it relates to the presence of mutism (CMS), which was seen in a subset of 27 patients with CCAS.
Motor testing
A subset of participants had motor performance assessed with the Purdue Pegboard.33 We included these data as a test of the validity of the brain-behavior relationships derived from this dataset, because there is a well-established anatomic relationship of motor function with the ipsilateral cerebellum. We evaluated Purdue Pegboard scores relative to cerebellar outflow lesion load using Pearson correlation.
Standard protocol approvals, registrations, and patient consents
This study was approved by the institutional review boards at the University of Iowa Hospitals and Clinics and Massachusetts General Hospital. Patient consent was waived because this study was conducted retrospectively.
Data availability
Data are available on request.
Results
Participants' demographics and lesion characteristics
One hundred ninety-five of 231 participants met the inclusion criteria of this study. Excluded individuals had significant lesion burden outside the cerebellum (n = 15), indistinct lesion boundaries (n = 14), or age >18 years (n = 7). The final 195 participants ranged from 3 months to 18 years of age (mean ± SD 6.8 ± 4.2 years, median 6.2 years), with 43% being female. Medulloblastoma was diagnosed in 124 (63.6%) participants, followed by ependymoma (n = 39, 20%), astrocytoma/glioma (n = 23, 11.8%) and other/unclassified tumors (n = 9, 4.6%).
Variables associated with CCAS
CCAS was diagnosed in 48 participants (24.6%); 20 (41.7%) were female. We evaluated whether CCAS was preferentially seen in association with any demographic or descriptive variables using χ2 testing unless otherwise indicated. The rate of CCAS was higher in participants with bilateral lesions crossing the midline relative to unilateral lesions (47 of 155 with bilateral lesions had CCAS vs 1 of 40 with a unilateral left-sided lesion, p < 0.0001). A higher proportion of patients with medulloblastoma developed CCAS relative to other tumor types (40 of 124 vs 8 of 71, p = 0.0010). Other variables that were evaluated for a subset of participants with recorded data and did not appear significantly different in the CCAS vs non-CCAS groups included the following: resection cavity volume (mean ± SD 11,226.02 ± 12,801.15 vs 8,736.89 ± 12,121.58 mm3, respectively, p = 0.2246, independent-samples t test), the presence of presurgical hydrocephalus (43 of 46 with CCAS vs 98 of 114 without CCAS, p = 0.2800), handedness (CCAS in 4 of 14 left-handed, 1 of 14 ambidextrous, and 30 of 100 right-handed participants, p = 1.0), presurgical tumor volume (34.38 ± 19.95 vs 32.43 ± 26.71 cm3, p = 0.7409, independent-samples t test, n = 106, CCAS = 24), extent of resection (gross total [30 of 44 with CCAS vs 93 of 133 without CCAS, p = 0.8515], partial [5 of 44 with CCAS vs 21 of 133 without CCAS, p = 0.6249], near-total resection [9 of 44 with CCAS vs 19 of 133 without CCAS, p = 0.3456]), and tumor relapse (4 of 37 with CCAS vs 30 of 123 without CCAS, p = 0.1074).
Case-control demographics
Each of the 48 patients with CCAS was matched to 2 individuals without CCAS (n = 96, total of 144 for case-control analysis) by age, sex, and lesion volume. The age range for the CCAS and non-CCAS groups was 1 to 15 and 1 to 16 years, respectively. The mean ± SD lesion volume of patients with CCAS was 11,226.02 ± 12,801.15 mm3 compared to 9,303.08 ± 13,193.26 mm3 for those without CCAS. After matching, there were no significant group differences in age, sex, or lesion volume (p = 0.9502, 1.0, and 0.4065, respectively).
Lesion characteristics and subtraction
Lesion overlaps for all participants, those with CCAS, and those without CCAS are displayed in figure 2, A–C for descriptive purposes. Figure 2D shows the proportional subtraction of the non-CCAS lesion overlap from the CCAS lesion overlap such that any remaining voxels were damaged at a proportionally higher rate in the CCAS group. This analysis highlighted a peak in the vermal lobule IX extending to the adjacent fastigial, interposed, and medial dentate nuclei.
Figure 2. Lesion overlap maps.
(A) Lesion overlap of all participants had a peak of 110 of 195 at the vermal lobule IX, Montreal Neurological Institute (MNI) coordinate 0, −52, −38. (B) Patients with cerebellar cognitive affective syndrome (CCAS) had a peak lesion overlap of 39 of 48 at vermal lobule IX (MNI coordinate −1, −51, −39), and (C) participants without CCAS had a peak overlap of 71 or 147 also at vermal lobule IX (MNI coordinate 0, −52, −38). (D) A proportional subtraction, or difference map, of non-CCAS lesions subtracted from CCAS-associated lesions showed a regional peak involving vermis lobule IX (MNI coordinate −4, −53, −31) and involving the adjacent fastigial, interposed, and medial dentate nuclei (coordinates −3, −52, −28; −5, −59, −32; and −12, −53, −33, respectively).
Cerebellar outflow pathway lesion load
The CCAS group had significantly greater damage to the cerebellar outflow pathway than the matched comparison group. This was true when looking at the total bilateral cerebellar outflow pathway (p < 0.0001) and at subcomponents of the cerebellar outflow pathway individually, including right and left side analyzed separately (p = 0.0022 and p < 0.0001, respectively), superior cerebellar peduncles without deep nuclei (p < 0.0001), deep nuclei without superior cerebellar peduncles (p < 0.0001), right and left superior cerebellar peduncles (both with p < 0.0001), and right and left deep nuclei (p = 0.0002 and p < 0.0001, respectively).
Risk of CCAS stratified by cerebellar outflow pathway lesion load
For descriptive purposes, we measured the rate of CCAS as it relates to the extent of cerebellar outflow pathway lesion load. While 25% is a commonly reported rate of severe postoperative cognitive and affective symptoms,11,18,21 figure 3 shows that the risk is minimal when the cerebellar outflow pathway is spared and closer to 50% (double the standard risk) when the lesion involves 75% to 100% of the cerebellar outflow pathway.
Figure 3. Risk of CCAS stratified by cerebellar outflow pathway lesion load.
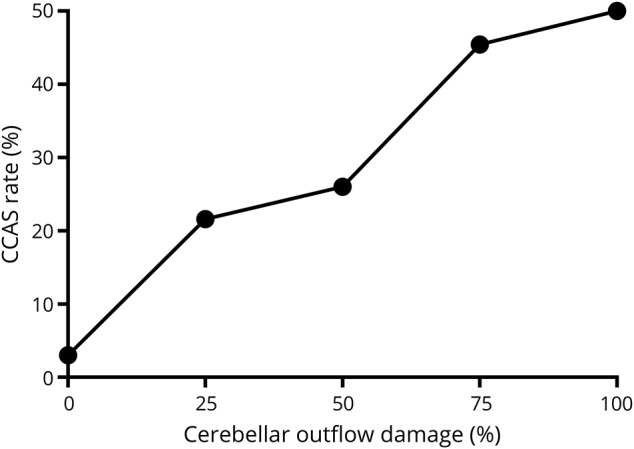
This figure demonstrates that cerebellar cognitive affective syndrome(CCAS) rates are higher as the proportion of cerebellar outflow damage increases. The number of participants with CCAS compared to those without CCAS was as follows: no cerebellar outflow damage CCAS = 1, no CCAS = 32; 0% to 25% damage CCAS = 16, no CCAS = 58; 25% to 50% damage CCAS = 13, no CCAS = 37; 50% to 75% damage CCAS = 10, no CCAS = 12; and 75% to 100% damage CCAS = 8, no CCAS = 8.
Lesion symptom mapping
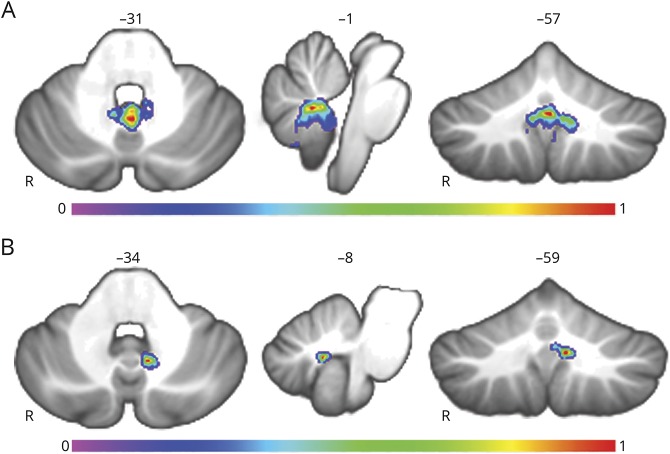
Multivariate lesion symptom mapping demonstrated that lesion location was a significant predictor of CCAS, with the overall map being statistically significant with a cross-validation r of 0.3409 (p < 8 × 10−7). The region maximally associated with CCAS development was located along the cerebellar outflow pathway in the region of the deep nuclei (fastigial [3, −55, −30], extending laterally to include the interposed [−7, −59, −34] and medial dentate [14, −53, −38]) and extending superiorly into the superior cerebellar peduncles. This region extended beyond the cerebellar outflow pathway to involve the inferior vermis (vermal lobules IX [−1, −57, −31], VIIIA [−1, −58, −31], VIIIB [−6, −60, −35], and X [−1, −51, −31], in decreasing order of strength), as shown in figure 4A and with a higher threshold in figure 4B. These findings were not limited to a single statistical approach because the same regions survived a false discovery rate correction of 5% (p < 0.05) using a mass univariate approach, with peaks at the vermal lobule IX and VIIIA (−4, −52, −34 and −2, −59, −31, respectively) extending to the fastigial and interposed nuclei (3, −55, −30 and −6, −59, −34, respectively).
Figure 4. Lesion symptom mapping results.
(A) Results of the lesion symptom mapping analysis of all 195 participants. Warmer voxels have a higher association with the development of cerebellar cognitive affective syndrome with the overall map being statistically significant (p < 0.0001). (B) The same map with a higher statistical threshold highlights the strongest findings in the fastigial and interposed nuclei of the cerebellum and vermal lobule IX with peak Montreal Neurological Institute coordinates: 3, −55, −30; −7, −59, −34; and −1, −57, −31, respectively.
Lesion network mapping
We next examined which brain areas are functionally connected with the peak finding from the lesion symptom mapping result in the cerebellum (figure 4B). Regions with strong functional connectivity to this site in the cerebellum include the parvocellular division of the mediodorsal nucleus (−1, −10, 10) extending into the magnocellular division, along with anterodorsal and anteroventral nuclei, according to the Morel thalamic atlas (figure 5A).34,35 Other regional peaks were seen at the anterior cingulate cortex (−3, 41, 3), along with other subcortical regions in the brainstem (9, −35, −31) extending to the red nucleus (5, −24, −9) and far inferior claustrum extending toward the right and left temporal stem near the tail of the caudate (29, −21, −4 and −25, −20, −6, respectively). To evaluate whether these network findings were specific to lesions causing CCAS, we compared the strength of connectivity between peak clusters and each CCAS and non-CCAS lesion location. The peak clusters were represented by 10-mm spheres at the peak coordinates reported above. This analysis showed that functional connectivity between the lesion locations and the mediodorsal nucleus of the thalamus, right temporal stem, and right red nucleus was higher in cases with CCAS (figure 5B), whereas lesion connectivity with the anterior cingulate and left temporal stem was not significantly different between the 2 groups after correction for multiple comparisons.
Figure 5. Lesion-associated networks derived from adult data.
(A) Network of regions functionally connected to the cerebellar region associated with cerebellar cognitive affective syndrome (CCAS) is shown. This network was derived from seed-based functional connectivity from a large normative dataset of healthy adults using the result shown in figure 4B as the seed region. Regions with positively correlated oscillatory patterns are shown in warm colors, and negatively correlated networks did not reach significance at this threshold. Peak coordinates for the parvocellular division of the mediodorsal nucleus of the thalamus, left temporal stem, right temporal stem, and right red nucleus are as follows: (−1, −10, 10), (−25, −20, −6), (29, −21,-4), and (5, −24, −9), respectively. (B) Fisher r-to-z transformed scores show significantly higher functional connectivity between lesion sites causing CCAS compared to those not associated with CCAS at the mediodorsal (MD) nucleus of the thalamus (parvocellular), right temporal stem, and right red nucleus with values of p = 0.0029, 0.0012, and 0.0080, respectively. Other sites of the network had higher correlation with CCAS lesions but did not reach statistical significance after correction for multiple compressions (left temporal stem and anterior cingulate cortex with p = 0.0180 and 0.1790, respectively). Error bars represent SEM.
Next, we evaluated the connectivity of the cerebellar ROI in a normative pediatric functional connectivity dataset. The spatial correlation pattern was similar to the adult findings with a regional peak also present in the parvocellular division of the mediodorsal nucleus (−3, −16, 8; figure 6) that extends into the adjacent magnocellular division and includes anteromedial and anteroventral nuclei, along with subcortical regions in the brainstem (8, −34, −32) extending to the red nucleus (5, −19, −12) and the right temporal stem (25, −26, −4). There was a weak positive correlation between age and functional connectivity between the cerebellum ROI and the mediodorsal nucleus of the thalamus (r = 0.1097, p = 0.0278).
Figure 6. Lesion-associated network derived from pediatric data.

Spatial distribution of the network derived from the cerebellum region most associated with developing cerebellar cognitive affective syndrome is shown. Results are similar to those from the adult dataset (figure 5A), including regional peaks in the mediodorsal nucleus of the thalamus (parvocellular) and right red nucleus at Montreal Neurological Institute coordinates (−3, −16, 8) and (5, −19, −12), respectively.
Cerebellar mutism syndrome
As a secondary analysis, we further divided the 48 patients with CCAS into those with and without mutism (CMS vs CCAS without mutism). There were no patients with mutism who were not already classified as having CCAS in the main analysis. Both groups had significantly higher bilateral cerebellar outflow pathway lesion load relative to their matched non-CCAS comparison group (CMS p = 0.0111 and CCAS without mutism p = 0.0024). There was no difference in cerebellar outflow pathway lesion load between the CMS and CCAS without mutism groups (p = 0.8664). Multivariate lesion symptom mapping of CMS was similar to that of the broader classification of CCAS shown in figure 4, with regional peaks in lobule IX of the vermis at (−1, −57, −31), where 24 of 27 patients with mutism had lesions, along with the fastigial nuclei and lobule X of the vermis (r = 0.2429, p = 0.0012; figure 7A). The CCAS without mutism group had a peak finding deviated to the left of the midline in the region of the interposed nucleus at (−8, −59, −34) and extending to the medial dentate nucleus and inferior vermis (vermal lobule IX; r = 0.2568, p = 0.0008; figure 7B). The same network analyses were repeated for each group with results very similar to those shown in figure 5A with a regional peak in the mediodorsal nucleus (parvocellular) at (2, −10, 10) for the CMS group and more lateralized to the right parvocellular division of the mediodorsal nucleus (2, −16, 8) for the CCAS without mutism group.
Figure 7. Cerebellar mutism lesion symptom mapping.
(A) Multivariate lesion symptom mapping results of cerebellar mutism syndrome (CMS) are shown. The overall map is similar to that of figure 4A with a regional peak in vermal lobule IX (−1, −57, −31) extending to the fastigial nuclei and vermal lobule X. (B) The same analysis as in panel (A) in the cohort with cerebellar cognitive affective syndrome without mutism has a peak finding in the region of the interposed nucleus and extending to the medial dentate nucleus (−8, −59, −34).
Motor testing
Purdue Pegboard right-hand test scores were significantly correlated with right cerebellar outflow pathway lesion load (higher lesion load corresponds to lower performance, n = 67, r = −0.4114, p = 0.0005), and the same was true of the left cerebellum and the left-hand scores (n = 67, r = −0.5160, p < 0.0001). Correlations were significantly lower when the laterality was reversed, consistent with known laterality of cerebellar motor control (left cerebellum outflow with right hand r = 0.0173, p = 0.8895; right cerebellum outflow with left hand r = −0.2322, p = 0.0586; both ipsilateral r values were significantly higher than the crossed laterality r, p < 0.05). This finding was also present when we limited the analysis to right-hand–dominant patients (n = 59, right outflow lesion load correlates with right-hand performance, r = −0.4066, p = 0.0014), and left outflow lesion load correlated with left-hand performance (r = −0.5959, p < 0.0001), while the inverse laterality was significantly weaker for the right-hand performance and the left outflow lesion load (r = −0.0189 and p = 0.8871) and for the left-hand performance and the right outflow lesion load (r = −0.2906 and p = 0.0256). A multivariate lesion symptom mapping analysis of motor performance of Purdue Pegboard scores across the 67 participants did not reach significance.
Brainstem analysis
Although patients with significant brain injury outside the cerebellum were excluded from the main analysis, we checked whether individuals excluded due to significant brainstem injury might have higher rates of CCAS, as proposed elsewhere.11,12 Of the 15 individuals excluded for lesions outside the cerebellum, 12 had significant brainstem involvement, and 1 of these had CCAS. While not conclusive, this is consistent with localization of CCAS to the cerebellum and not brainstem in most cases.
Discussion
This study, to the best of our knowledge, is the largest lesion mapping study of cognitive and affective symptoms that accompany pediatric cerebellar tumor resection to date. Our results show a strong relationship between damage to the cerebellar outflow pathway and the development of these symptoms. This finding is consistent with prior studies that have implicated lesion involvement of these regions.13,14,16,20,21 Our large sample size allowed us to demonstrate that the key neuroanatomic correlate appears to be a pathway as opposed to a specific site within the pathway. Furthermore, both the deep nuclei and the superior cerebellar peduncles appear to be important, indicating that future studies may benefit from calculating lesion load across this pathway as opposed to focusing exclusively on individual regions.
Our data-driven lesion symptom mapping results highlighted the fastigial nuclei as the peak finding, along with significant results in the adjacent interposed and medial dentate nuclei, superior cerebellar peduncles, and vermis (lobules IX and X). The fastigial nuclei findings were present for the entire CCAS group and the subset with mutism. The fastigial nuclei have been implicated in cerebellar mutism before and are thought to have a major role in limbic processing, relative to the dentate nuclei, which are more implicated in executive function and cognition.36–38
Our findings are consistent with the notion that postsurgical cognitive and affective impairments are driven by disrupted cerebrocerebellar connectivity. Our lesion network mapping highlights the mediodorsal nucleus of the thalamus as a target that is functionally connected to the cerebellar region most relevant for these symptoms. This is consistent with direct anatomic projections of the fastigial and dentate nuclei to the thalamic mediodorsal nucleus.39,40 The mediodorsal nucleus projects to the anterior cingulate, medial and lateral prefrontal, and orbitofrontal cortices. Several studies have demonstrated that direct pathology of the mediodorsal nucleus can lead to a wide range of cognitive, emotional, and behavioral deficits, as well as impairments in attention, working, and autobiographical memory.41,42 To the best of our knowledge, the mediodorsal nucleus of the thalamus has not been invoked in prior studies of CCAS or CMS. However, prior studies have demonstrated that patients with CCAS have hypometabolism in the cortical regions to which the mediodorsal nucleus projects, including the anterior cingulate and prefrontal cortex, which could be viewed as indirect support for deafferentation of the mediodorsal nucleus.9,15,18
Our findings also highlight the possibility that among patients with CCAS there may be some finer localization related to the presence or absence of mutism. A discrete region in the cerebellar midline involving vermis lobules IX and X and the fastigial nuclei was lesioned in almost every patient displaying mutism, whereas patients with CCAS without mutism were more likely to have lesions just to the left of the midline involving the interposed and medial dentate nuclei and left superior cerebellar peduncle.
Our results indicate that medulloblastoma is a risk factor for CCAS development compared to other tumor types and that this could be due to the location of these tumors or the more aggressive resection with this tumor type.15,19,21,43,44 Similarly, our results support bilateral lesions that cross the midline as a risk for CCAS, while only a single subject with a unilateral left-lateralized lesion had CCAS.10,14,19,20 We did not find a significant association between some variables previously associated with CCAS development, including handedness, presurgical hydrocephalus, tumor relapse, tumor volume, and total vs subtotal tumor resection.12,19,43–45
There are several limitations of this study. First, the cognitive and affective symptoms we evaluated were based on a retrospective evaluation of clinical records. There are active efforts underway to develop formal criteria for describing and measuring postsurgical cognitive and affective symptoms after pediatric tumor resection, but there is not yet widespread agreement or adoption among clinicians.1,4–6,8,45 With prospective data collection on a continuous scale, it may be possible to localize motor, cognitive, and affective symptoms with finer detail than was possible here. Moreover, CCAS likely exists on a continuum, while our classification was binary. As a result, participants with milder symptoms may have been classified as not having CCAS. However, these false negatives would serve only to reduce the statistical differences seen here. This study was also conducted retrospectively, with the majority of imaging obtained in the chronic postsurgical time frame, even if symptoms had resolved at the time of the scan. The decision to use scans from the chronic phase allowed a better view of the resection cavity without distortion and edema present in the acute postsurgical period. However, we acknowledge that transient postsurgical changes such as edema and hydrocephalus could have contributed to the symptoms of CCAS and are not accounted for by the current study design.17 Lastly, the focus of this study was on lesion location and did not consider details of the surgical approach or the postsurgical treatment plan, which may also affect the incidence of CCAS. Future prospective designs that follow up symptoms and imaging longitudinally may reveal further insights into the neural basis of CCAS and identify additional risk factors not evident with the current design.
There is a strong relationship between disruption of the cerebellar outflow pathway and the development of CCAS in pediatric patients undergoing cerebellar tumor resection. Disruption of cerebrocerebellar connectivity with the mediodorsal nucleus may mediate the symptoms and represents a testable hypothesis for further study. Future studies will need to evaluate remote changes in these regions while patients are experiencing symptoms to further explore the network basis of CCAS. A better understanding of the networks underlying CCAS could lead to new strategies for targeted neuromodulation to augment recovery.46 Future work should also focus on whether lesion involvement of the cerebellar outflow pathway may be used prospectively to predict surgical outcomes, with the eventual goal of using this information to inform personalized prognosis or even to tailor surgical approaches to avoid these regions when possible.44 Finally, this finding adds to a growing literature highlighting the importance of the cerebellum in the development of cognition, and it may have relevance for the anatomic underpinnings of developmental disorders with cognitive and affective symptoms in which the cerebellum is implicated.47–50
Acknowledgment
The authors thank Demi Eble and Elizabeth Weyman for assistance in organizing clinical data, Mark Bowren for assistance with lesion symptom mapping, and Louis Soussand for assistance with the pediatric functional connectivity data.
Glossary
- CCAS
cerebellar cognitive affective syndrome
- CMS
cerebellar mutism syndrome
- MNI
Montreal Neurological Institute
- ROI
region of interest
- rs-fcMRI
resting-state functional connectivity MRI
Appendix. Authors

Footnotes
Editorial, page 693
Study funding
This study was funded by 4K12HD027748-24, NIH–National Institute of Neurological Disorders and Stroke Child Neurology Career Development Program 1K12NS098482-01, Aiming for a Cure, and the Roy J. Carver Charitable Trust. A.L.C. was supported by T32MH112510.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Lanier JC, Abrams AN. Posterior fossa syndrome: review of the behavioral and emotional aspects in pediatric cancer patients. Cancer 2017;123:551–559. [DOI] [PubMed] [Google Scholar]
- 2.Wisoff JH, Epstein FJ. Pseudobulbar palsy after posterior fossa operation in children. Neurosurgery 1984;15:707–709. [DOI] [PubMed] [Google Scholar]
- 3.Rekate HL, Grubb RL, Aram DM, Hahn JF, Ratcheson RA. Muteness of cerebellar origin. Arch Neurol Am Med Assoc 1985;42:697–698. [DOI] [PubMed] [Google Scholar]
- 4.Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain 2018;141:248–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain 2000;123:1041–1050. [DOI] [PubMed] [Google Scholar]
- 6.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain 1998;121:561–579. [DOI] [PubMed] [Google Scholar]
- 7.Manto M, Mariën P. Schmahmann's syndrome: identification of the third cornerstone of clinical ataxiology. Cerebellum Ataxias 2015;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gudrunardottir T, Morgan AT, Lux AL, et al. Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Childs Nerv Syst 2016;32:1195–1203. [DOI] [PubMed] [Google Scholar]
- 9.De Smet HJ, Baillieux H, Wackenier P, et al. Long-term cognitive deficits following posterior fossa tumor resection: a neuropsychological and functional neuroimaging follow-up study. Neuropsychology 2009;23:694–704. [DOI] [PubMed] [Google Scholar]
- 10.Puget S, Boddaert N, Viguier D, et al. Injuries to inferior vermis and dentate nuclei predict poor neurological and neuropsychological outcome in children with malignant posterior fossa tumors. Cancer 2009;115:1338–1347. [DOI] [PubMed] [Google Scholar]
- 11.Robertson PL, Muraszko KM, Holmes EJ, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology Group. J Neurosurg Pediatr 2006;105:444–451. [DOI] [PubMed] [Google Scholar]
- 12.Pols SYCV, van Veelen MLC, Aarsen FK, Gonzalez Candel A, Catsman-Berrevoets CE. Risk factors for development of postoperative cerebellar mutism syndrome in children after medulloblastoma surgery. J Neurosurg Pediatr 2017;20:35–41. [DOI] [PubMed] [Google Scholar]
- 13.Ojemann JG, Partridge SC, Poliakov AV, et al. Diffusion tensor imaging of the superior cerebellar peduncle identifies patients with posterior fossa syndrome. Childs Nerv Syst 2013;29:2071–2077. [DOI] [PubMed] [Google Scholar]
- 14.Avula S, Kumar R, Pizer B, et al. Diffusion abnormalities on intraoperative magnetic resonance imaging as an early predictor for the risk of posterior fossa syndrome. Neuro Oncol 2015;17:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soelva V, Hernáiz Driever P, Abbushi A, et al. Fronto-cerebellar fiber tractography in pediatric patients following posterior fossa tumor surgery. Childs Nerv Syst 2013;29:597–607. [DOI] [PubMed] [Google Scholar]
- 16.Morris EB, Phillips NS, Laningham FH, et al. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain 2009;132:3087–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C. Mutism and pseudobulbar symptoms after resection of posterior-fossa tumors in children: incidence and pathophysiology. Neurosurgery 1995;37:885–893. [DOI] [PubMed] [Google Scholar]
- 18.Catsman-Berrevoets CE, Aarsen FK. The spectrum of neurobehavioural deficits in the posterior fossa syndrome in children after cerebellar tumour surgery. Cortex 2010;46:933–946. [DOI] [PubMed] [Google Scholar]
- 19.Law N, Greenberg M, Bouffet E, et al. Clinical and neuroanatomical predictors of cerebellar mutism syndrome. Neuro Oncol 2012;14:1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusano Y, Tanaka Y, Takasuna H, et al. Transient cerebellar mutism caused by bilateral damage to the dentate nuclei after the second posterior fossa surgery. J Neurosurg 2006;104:329–331. [DOI] [PubMed] [Google Scholar]
- 21.Miller NG, Reddick WE, Kocak M, et al. Cerebellocerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors. Am J Neuroradiol 2010;31:288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boes AD, Prasad S, Liu H, et al. Network localization of neurological symptoms from focal brain lesions. Brain 2015;138:3061–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:S208–S219. [DOI] [PubMed] [Google Scholar]
- 24.van Baarsen KM, Kleinnijenhuis M, Jbabdi S, Sotiropoulos SN, Grotenhuis JA, van Cappellen van Walsum AM. A probabilistic atlas of the cerebellar white matter. Neuroimage 2016;124:724–732. [DOI] [PubMed] [Google Scholar]
- 25.Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 2010;41:910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pustina D, Avants B, Faseyitan OK, Medaglia JD, Coslett HB. Improved accuracy of lesion to symptom mapping with multivariate sparse canonical correlations. Neuropsychologia 2017;115:154–166. [DOI] [PubMed] [Google Scholar]
- 27.Fischer DB, Boes AD, Demertzi A, et al. A human brain network derived from coma-causing brainstem lesions. Neurology 2016;87:2427–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckner RL, Roffman JL, Smoller JW. Brain Genomics Superstruct Project (GSP) [online]. Harvard Dataverse; 2014. Available at: dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/25833. Accessed May 11, 2018. [Google Scholar]
- 29.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. From the cover: the human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005;102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 2010;103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry 2012;72:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cameron C, Yassine B, Carlton C, et al. The Neuro Bureau Preprocessing Initiative: open sharing of preprocessed neuroimaging data and derivatives. Front Neuroinform 2013; 7. Available at: https://www.frontiersin.org/10.3389/conf.fninf.2013.09.00041/event_abstract. [Google Scholar]
- 33.Tiffin J, Asher E. The Purdue Pegboard: norms and studies of reliability and validity. J Appl Psychol 1948;32:234–247. [DOI] [PubMed] [Google Scholar]
- 34.Krauth A, Blanc R, Poveda A, Jeanmonod D, Morel A, Székely G. A mean three-dimensional atlas of the human thalamus: generation from multiple histological data. Neuroimage 2010;49:2053–2062. [DOI] [PubMed] [Google Scholar]
- 35.Jakab A, Blanc R, Berényi EL, Székely G. Generation of individualized thalamus target maps by using statistical shape models and thalamocortical tractography. Am J Neuroradiol 2012;33:2110–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmahmann JD. The cerebellum and cognition. Neurosci Lett 2019;688:62–75. [DOI] [PubMed] [Google Scholar]
- 37.Zhang XY, Wang JJ, Zhu JN. Cerebellar fastigial nucleus: from anatomic construction to physiological functions. Cerebellum Ataxias 2016;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabatas S, Yilmaz C, Altinors N, Yildiz O, Agaoglu B. Cerebellar mutism syndrome and its relation to cerebellar cognitive and affective function: review of the literature. Ann Indian Acad Neurol 2010;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelzer EA, Melzer C, Timmermann L, von Cramon DY, Tittgemeyer M. Basal ganglia and cerebellar interconnectivity within the human thalamus. Brain Struct Funct 2017;222:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moers-Hornikx VMP, Sesia T, Basar K, et al. Cerebellar nuclei are involved in impulsive behaviour. Behav Brain Res 2009;203:256–263. [DOI] [PubMed] [Google Scholar]
- 41.Benarroch EE, Golden EC, Graff-Radford J, Jones DT. Mediodorsal nucleus and its multiple cognitive functions. Neurology 2016;87:2161–2168. [DOI] [PubMed] [Google Scholar]
- 42.Schmahmann JD. Vascular syndromes of the thalamus. Stroke 2003;34:2264–2278. [DOI] [PubMed] [Google Scholar]
- 43.Catsman-Berrevoets CE, Van Dongen HR, Mulder PGH, Paz Y Geuze D, Paquier PF, Lequin MH. Tumour type and size are high risk factors for the syndrome of “cerebellar” mutism and subsequent dysarthria. J Neurol Neurosurg Psychiatry 1999;67:755–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson RC, Bouffet E, Leonard JR, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol 2016;17:484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Dongen HR, Catsman-Berrevoets CE, van Mourik M. The syndrome of “cerebellar” mutism and subsequent dysarthria. Neurology 1994;44:2040–2046. [DOI] [PubMed] [Google Scholar]
- 46.Boes AD, Kelly MS, Trapp NT, Stern AP, Press DZ, Pascual-Leone A. Noninvasive brain stimulation: challenges and opportunities for a new clinical specialty. J Neuropsychiatry Clin Neurosci 2018;30:173–179. [DOI] [PubMed] [Google Scholar]
- 47.Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev 2010;20:236–260. [DOI] [PubMed] [Google Scholar]
- 48.Wang SSH, Kloth AD, Badura A. The cerebellum, sensitive periods, and autism. Neuron 2014;83:518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen G, Courchesne E. Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: an fMRI study of autism. Am J Psychiatry 2003;160:262–273. [DOI] [PubMed] [Google Scholar]
- 50.Limperopoulos C, Chilingaryan G, Guizard N, Robertson RL, Du Plessis AJ. Cerebellar injury in the premature infant is associated with impaired growth of specific cerebral regions. Pediatr Res 2010;68:145–150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request.