Abstract
Objective
To develop a disease-specific severity index for adults with autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) (DSI-ARSACS) that considers the 3 components (pyramidal, cerebellar, neuropathic) of the disease, and to document its content validity, internal consistency, and construct validity.
Methods
The Beta DSI-ARSACS (17 items) was developed based on literature review and expert inputs and then administered to 26 participants. Items reduction was based on Cronbach α and desirable criteria. Performance measures were administered to assess the construct validity of the final version of the DSI-ARSACS.
Results
The final DSI-ARSACS have 8 items that can be easily performed during usual medical follow-up. The mean score was 19.6 ± 8.1 (range 6.0–35.5) and the Cronbach α was 0.912. The DSI-ARSACS score increased with disease stage and age (p ≤ 0.001) and was closely correlated with other measures assessing similar construct (9-Hole Peg Test, 10-Meter Walk Test, Scale for the Assessment and Rating of Ataxia, Berg Balance Scale, Barthel Index) (rs = 0.75–0.95, p < 0.01). A moderate but not significant correlation was found with the 6-Minute Walk test (rs = −0.611, p = 0.108).
Conclusions
The DSI-ARSACS is a valid measure of disease severity for the adult ARSACS population that is able to distinguish between patients with different clinical profiles. Further documentation of metrologic properties is necessary, but these first results are promising.
Autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS), first described in 1978,1 is the most common inherited recessive ataxias in the province of Quebec (Canada)2,3 and the most frequent recessive ataxia worldwide after Friedreich ataxia.4,5 It is an early-onset ataxia characterized by symptoms from 3 components: pyramidal (e.g., spasticity), cerebellar (e.g., ataxia), and neuropathic (e.g., distal amyotrophy), each of them being expressed at different levels from one individual to another. Consequently, a high variability is shown among patients with ARSACS, regardless of their age.6 Patients become regular wheelchair users around 40 year old (ranging from 17 to 58 years).6,7 To date, no curative treatment is available for ARSACS. With the advancement of knowledge on molecular pathogenesis, therapeutic trials could be available soon. A validated neurologic assessment tool is necessary to assess ARSACS-specific symptoms and their severity. There are a number of generic ataxia rating scales, like the International Cooperative Ataxia Rating Scale8 and the Scale for the Assessment and Rating of Ataxia (SARA),9 but none includes the 3 components of ARSACS, and they are thus not completely suitable for this population. A disease-specific scale might better document the overall disease severity and the natural history of the disease and help patient recruitment based on level of impairment in each component.
The objectives of this study were to (1) develop a disease-specific severity index for ARSACS (DSI-ARSACS) and (2) document its internal consistency and construct validity among an adult population.
Methods
Participants
Participants were recruited in 2015 using a stratified random sampling strategy according to age (18–59 years) and sex among a subset of 175 people with ARSACS listed at the Neuromuscular Clinic of the Centre Intégré Universitaire de Santé et de Services Sociaux du Saguenay Lac-St-Jean (CIUSSS-SLJS) (Quebec, Canada). Inclusion criteria were (1) 18 years or older, (2) diagnosis of ARSACS confirmed by DNA analysis, and (3) able to provide informed consent. Patients with other diseases causing functional limitations, having a Baclofen intrathecal pump, or being pregnant were excluded.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Ethics Review Board of the CIUSS-SLSJ, and written informed consent was obtained from each participant.
Data collection and instruments
This study was part of a larger data collection where participants were seen over 3 half-day sessions, 2 weeks apart. Beta version of the DSI-ARSACS was administered at the second visit only. The preliminary version contains 17 items addressing the pyramidal, cerebellar, and neuropathic features of the disease and the total score ranges from 0 to 74, a higher score indicating a higher level of severity. A questionnaire was also completed by all participants to obtain data about their age, sex, mobility level, and walking aids. Data about disease duration were not collected since this approximately corresponds to age, the diagnosis usually being made at gait initiation in our cohort. To validate the final version of the DSI-ARSACS, several tests were performed during the 3 visits at the clinic.
Upper extremity functions
Fine dexterity was assessed with the 9-Hole Peg Test (9HPT),10 which presents excellent intrarater and interrater reliability.11
Mobility
Balance was assessed with the Berg Balance Scale (BBS)12 (validated in ARSACS13); walking speed was assessed with the 10-Meter Walk Test (10MWT)14 at comfortable speed and maximum speed; walking endurance was assessed using the 6-Minute Walk Test (6MWT).15,16 The 10MWT and 6MWT both have excellent interrater reliability in ARSACS.13
Disease severity scale
The SARA was used.9 Even if 2 items of the final disease-specific severity index are similar to those of the SARA, it was decided to administer the SARA as it is a recognized scale to assess severity of cerebellar ataxia in other ataxias. In addition, the 2 items are not assessed and scored in the same way.
Participation was assessed using the Barthel Index17 and quality of life using the 12-Item Short-Form Health Survey (SF-12), v2.18
Development of the scale
The Beta DSI-ARSACS was developed according to the method proposed by Streiner and Norman.19 The planning phase includes defining the population, instrument objectives, and desirable criteria, as well as a literature review of the concept under study. The development team included 5 ARSACS experts: 2 neurologists, an occupational therapist, and 2 physical therapists. The construction phase includes the selection of the sources of items and their selection. The sources of items were based on items present in available scales based on a systematic review of the literature with the following key words: (rating scale) combined with (ataxia or spastic* or pyramidal or neuropathic) (not pharmacologic treatment), using language (English, French), species (human research), and date restrictions (until April 2012). The following databases were consulted: PUBMED, CINAHL, AMED, ACCESS MEDICINE, SCOPUS, and ACADEMIC SEARCH COMPLETE. An extraction table was constructed including each component (e.g., ataxia) and potential items from existing scales (e.g., finger-to-nose test from the SARA). The selection of items for the Beta version was based on a consultation of experts using the Delphi method (2 rounds) and pretest among 60 patients during their medical follow-up. During the process, the following desirable criteria oriented the development of the scale: (1) it should rate motor functional impairments and their progression, and should therefore not include neurologic signs without functional implications; (2) it should focus on the main ARSACS signs; less common features should be recorded in an inventory of signs and symptoms; (3) no technical equipment should be necessary to administer the scale, and items should be based on standard neurologic examination and allow the standardization of testing and rating procedures; and (4) the administration time should be short enough to be done during clinical follow-up. Impairments that are stable (e.g., nystagmus) were not retained in the Beta version of the disease-specific severity index since they would not be able to capture the progression of disease severity over time.
Validation
The construct validity of the final DSI-ARSACS was assessed using hypothesis testing according to COSMIN methodology,20 and hypotheses were formulated a priori by the research team.
Convergent validity
The total score of the DSI-ARSACS should be higher when age and the SARA and 9HPT total score increase. The DSI-ARSACS score should be lower when the BBS, Barthel Index, 10MWT, 6MWT, and the physical component of the SF-12 scores increase.
Discriminant validity (known-group validity)
(1) The DSI-ARSACS score will be different between participants in each age group (≤29; 30–39; ≥40 years); the score should be higher among older participants. (2) The DSI-ARSACS score will be different between participants at a different mobility disease stage (first walking difficulty, use of a walking aid, wheelchair); the score should be higher among participants with more severe stage of the disease. (3) The DSI-ARSACS should not be able to distinguish between men and women. In addition, floor and ceiling effects were documented. The proportion of participants getting the maximum (ceiling effect) and the minimum (floor effect) possible scores must be less than 15%21 and skewness statistics should range from −1 to +1.22
Statistical analysis
Participants' characteristics are presented as the mean and SD for continuous variables and frequency and percentage for categorical variables. The normality of the distribution was determined using the Kolmogorov-Smirnov statistic (a nonsignificant result [p > 0.05] indicates a normal distribution). The skewness and kurtosis values were determined to obtain information concerning the scores' distribution. The skewness value provides information about the symmetry of the distribution; a value close to 0 indicates normal distribution, a positive value suggests that more individuals obtained low score (clustered to the left of the graph), conversely for a negative value.23 Kurtosis value indicates how much the distribution is “peaked”; a negative value indicates that the distribution is relatively flat (many individuals in the extremes) while a positive value suggests that the distribution is peaked (clustered in the center).23 The internal consistency was also determined using the Cronbach α coefficient, which determines if items of a scale measure the same construct of if they are redundant. An α coefficient around 0.90 indicates that the scale is reliable.24 To assess the construct validity (convergent and discriminant), the DSI-ARSACS total score was correlated with disease duration and the scores of the 9HPT, grip and pinch strength, BBS, 10MWT, 6MWT, SARA, and Barthel Index using the Spearman ρ coefficient. The known-group validity was assessed using the Mann-Whitney U Test for sex, age, and disease stage groups. For disease stages and age groups, post hoc analyses were made to find out which groups were statistically different from one another by doing repeated Mann-Whitney U tests. To control for type 1 error, Bonferroni adjustment was applied to the α value; significance was determined with a revised α level of 0.017. Nonparametric analyses were conducted due to the sample size (lower than 30). Data were analyzed using IBM SPSS Statistics for Windows, version 24.0 (IBM, Armonk, NY).
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
A total of 26 participants completed the DSI-ARSACS and the other outcome measures and were thus included in this study. Characteristics of the participants are presented in table 1. The mean age was 40 years and 53.8% were men. Ten participants were able to perform the 10MWT and 8 were able to perform the 6MWT. Twenty-four participants (92.3%) are homozygous for the 8844delT mutation, and 2 are compound heterozygotes for the 7504C>T or the 814C>T and the common 8844 delT mutations.
Table 1.
Characteristics of the sample (n = 26)
Disease-specific severity index reduction
The Beta version of the disease-specific severity index included 17 items for a maximum score of 74 (indicating maximal severity). Cronbach α was initially 0.933. By removing 4 items (foot deformities, tear a sheet, Achilles tendon retraction, and knee flexors muscle tone), Cronbach α reached 0.945. Five other items have been removed: fast alternating hand movements, standing position, sitting balance eyes open and closed, and LEMOCOT (lower limb coordination). Reasons to remove these items were high correlation with other items, poor reliability, material needed to perform the test, and lack of clinical relevance. The final Cronbach α of the 8-item DSI-ARSACS is 0.912 (table 2).
Table 2.
Final item selectiona
Validity of the DSI-ARSACS
The final DSI-ARSACS total score can range from 0 to 38. None of the 26 patients obtained the minimum or the maximum score, and the mean score is 19.6 ± 8.1 (ranging from 6.0 to 35.5). Scores were normally distributed (Kolmogorov-Smirnov p = 0.200), the skewness statistic is −0.219, and kurtosis is −0.755 (figure 1).
Figure 1. Distribution of the disease-specific severity index for autosomal recessive spastic ataxia of Charlevoix-Saguenay total score.

Construct validity
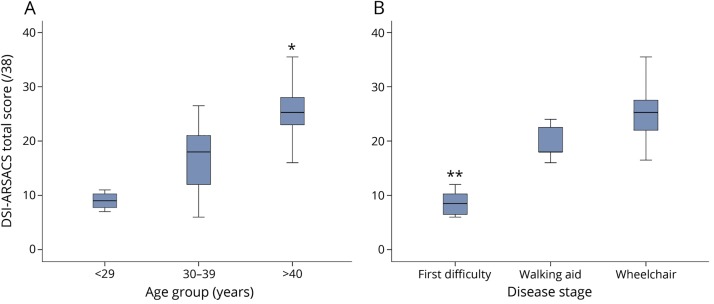
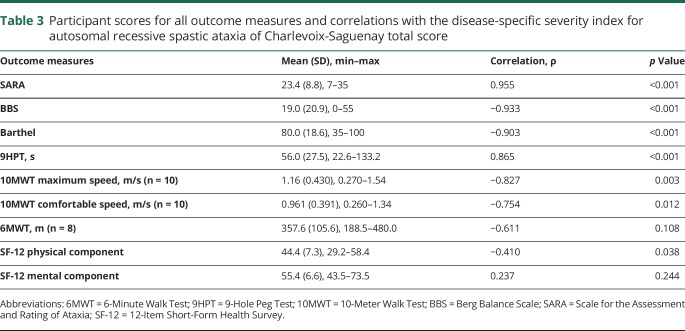
As expected, the total score increases with age and disease stages (p ≤ 0.001). Post hoc analysis demonstrated a difference between the <29 years and >40 years groups (p = 0.001), and between the 30–39 years and the >40 years groups (p = 0.002), but no difference was seen between the 2 younger groups (figure 2). For disease stages (figure 2), there is a difference between the less severe disease stage (first walking difficulty) and the 2 others (p < 0.003).
Figure 2. Disease-specific severity index for autosomal recessive spastic ataxia of Charlevoix-Saguenay (DSI-ARSACS) total score distribution.
DSI-ARSACS total score distribution according to (A) age groups and (B) disease stages. * Post hoc analyses showed a significant difference between the >40 years group and the 2 others (p ≤ 0.002) and ** between the first difficulty group and the 2 others (p < 0.003).
The construct validity was also determined by correlating the DSI-ARSACS total score with 7 other outcome measures (table 3). Results showed that all a priori hypotheses are confirmed, except for the 6MWT. The correlation of −0.611 found between the 6MWT and the DSI-ARSACS was not significant.
Table 3.
Participant scores for all outcome measures and correlations with the disease-specific severity index for autosomal recessive spastic ataxia of Charlevoix-Saguenay total score
Discussion
ARSACS results in manifestations than can be classified into 3 main components according to the neurologic system impaired: pyramidal, cerebellar, and neuropathic. It is not possible to capture these 3 components by using existing scales. The DSI-ARSACS was specifically developed to address the condition of patients with ARSACS. The final version of the scale has been deposited in the SAVOIRS UdeS repository with the complete administration guide that includes detailed instructions for all items. Note that permission from the first author (C.G.) is required for any use of the scale.
The final index includes 8 items reflecting the 3 main components of the disease and matches the desirable criteria. Impairments of cerebellar origin are predominant in ARSACS and affect several functions, which explain the number of items in the disease-specific severity index for this component. Despite the small number of participants included in this study, scores are normally distributed; skewness and kurtosis values indicate that although many participants obtained a score in the extremes (negative kurtosis value, flattened distribution), scores are not clustered in one side of the distribution. Selected participants were therefore representative of a wide range of disease severity level, which increases our confidence in the results. Results confirm the construct validity of the DSI-ARSACS according to its capacity to distinguish between patients based on the disease stage or disease duration, and the significant correlations with outcome measures reflecting the main activity limitations. A strong correlation was found with the 6MWT but did not reach statistical significance. This can probably be explained by the small number of participants (n = 8) who were able to perform the test. Also, as no item addresses the psychological aspects of the disease, the lack of correlation between the mental component of the SF-12 and the DSI-ARSACS score was expected.
The final item selection has been made based on criteria stated above but it is important to note that even if some of them have been removed, they can be interesting for the follow-up of patients with ARSACS. Among them is the LEMOCOT,25 which is a measure of lower limb coordination. In addition to being easy and requiring simple material, its validity and reliability has been demonstrated in the ARSACS population.26
The number of participants is small for a validation study. The next step will thus be to conduct a multicenter study to increase the sample size to further document the validity of the DSI-ARSACS. Since we have designed this tool keeping future clinical trials in mind, it could be used in 2 ways: first for initial patient classification to help case selection and second to measure the efficacy of the treatment. For this, the reliability and responsiveness of the DSI-ARSACS will have to be assessed, to determine its capacity to detect changes over time, and to chart the progression of the disease.
However, results obtained in this study showed strong preliminary validity, and demonstrated the potential of using this new disease-specific scale for the classification and the selection of patients in the context of clinical trials.
Glossary
- 6MWT
6-Minute Walk Test
- 9HPT
9-Hole Peg Test
- 10MWT
10-Meter Walk Test
- ARSACS
autosomal recessive spastic ataxia of Charlevoix-Saguenay
- BBS
Berg Balance Scale
- CIUSSS-SLJS
Centre Intégré Universitaire de Santé et de Services Sociaux du Saguenay Lac-St-Jean
- DSI-ARSACS
disease-specific severity index for autosomal recessive spastic ataxia of Charlevoix-Saguenay
- SARA
Scale for the Assessment and Rating of Ataxia
- SF-12
12-Item Short-Form Health Survey
Appendix. Authors
Study funding
Study funded by the Canadian Institutes of Health Research (Emerging Team Grant TR2-119189), in partnership with Fondation de l’Ataxie Charlevoix-Saguenay and Muscular Dystrophy Canada. C.G. holds a career-grant funding from Fonds de Recherche du Québec-Santé (31011).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Bouchard JP, Barbeau A, Bouchard R, Bouchard RW. Autosomal recessive spastic ataxia of Charlevoix-Saguenay. Can J Neurol Sci 1978;5:61–69. [PubMed] [Google Scholar]
- 2.Gomez CM. ARSACS goes global. Neurology 2004;62:10–11. [DOI] [PubMed] [Google Scholar]
- 3.Bouhlal Y, Amouri R, El Euch-Fayeche G, Hentati F. Autosomal recessive spastic ataxia of Charlevoix-Saguenay: an overview. Parkinson Relat Disord 2011;17:418–422. [DOI] [PubMed] [Google Scholar]
- 4.Thiffault I, Dicaire MJ, Tetreault M, et al. Diversity of ARSACS mutations in French Canadians. Can J Neurol Sci 2013;40:61–66. [DOI] [PubMed] [Google Scholar]
- 5.Synofzik M, Soehn AS, Gburek-Augustat J, et al. Autosomal recessive spastic ataxia of Charlevoix Saguenay (ARSACS): expanding the genetic, clinical and imaging spectrum. Orphanet J Rare Dis 2013;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gagnon C, Brais B, Lessard I, Lavoie C, Côté I, Mathieu J. From motor performance to participation: a quantitative descriptive study in adults with Autosomal recessive spastic ataxia of Charlevoix-Saguenay. Orphanet J Rare Dis 2018;13:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouchard JP. Recessive spastic ataxia of Charlevoix-Saguenay. In: Vinken P, Bruyn G, HL K, de Jong J, eds. Handbook of Clinical Neurology Hereditary Neuropathies and Spinocerebellar Atrophies. Amsterdam: North-Holland Publishing; 1991:451–459. [Google Scholar]
- 8.Trouillas P, Takayanagi T, Hallett M, et al. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome: The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 1997;145:205–211. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz-Hubsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 2006;66:1717–1720. [DOI] [PubMed] [Google Scholar]
- 10.Kellor M, Frost J, Silberberg N, Iversen I, Cummings R. Hand strength and dexterity. Am J Occup Ther 1971;25:77–83. [PubMed] [Google Scholar]
- 11.Gagnon C, Lessard I, Lavoie C, et al. Validity and reliability of outcome measures assessing dexterity, coordination, and upper limbs strength in autosomal recessive spastic ataxia of Charlevoix-Saguenay. Arch Phys Med Rehabil 2018;99:1747–1754. [DOI] [PubMed] [Google Scholar]
- 12.Berg K, Wood-Dauphinee S, Williams JI, Gayton D. Measuring balance in elderly: preliminary development of an instrument. Physiother Can 1989;41:304–311. [Google Scholar]
- 13.Lessard I, Brais B, Côté I, et al. Assessing mobility in autosomal recessive spastic ataxia of Charlevoix-Saguenay population: validity and reliability of four outcome measures. J Neurol Sci 2018;390:4–9. [DOI] [PubMed] [Google Scholar]
- 14.Wade DT, Wood VA, Heller A, Maggs J, Langton Hewer R. Walking after stroke: measurement and recovery over the first 3 months. Scand J Rehabil Med 1987;19:25–30. [PubMed] [Google Scholar]
- 15.Balke B. A simple field test for the assessment of physical fitness. Rep Civ Aeromed Res Inst US 1963:1–8. [PubMed] [Google Scholar]
- 16.ATS. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 17.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61–63. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 19.Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to Their Development and Use. 4th ed. New York: Oxford University Press; 2008. [Google Scholar]
- 20.Mokkink LB, Terwee CB, Patrick DL, et al. COSMIN checklist manual [online]. Available at: cosmin.nl. Accessed September 20, 2018.
- 21.McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res 1995;4:293–307. [DOI] [PubMed] [Google Scholar]
- 22.Bulmer MG. Principles of Statistics. Mineola: Dover Publications; 1979. [Google Scholar]
- 23.Pallant J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using IBM SPSS. 6th ed. Maidenhead: Open University Press; 2016. [Google Scholar]
- 24.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 2nd ed. New Jersey: Prentice Hall Health; 2000. [Google Scholar]
- 25.Desrosiers J, Rochette A, Corriveau H. Validation of a new lower-extremity motor coordination test. Arch Phys Med Rehabil 2005;86:993–998. [DOI] [PubMed] [Google Scholar]
- 26.Lessard I, Lavoie C, Côté I, Mathieu J, Brais B, Gagnon C. Validity and reliability of the LEMOCOT in the adult ARSACS population: a measure of lower limb coordination. J Neurol Sci 2017;377:193–196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.