Abstract
Objective
Treatment of patients with stroke presenting with minor deficits remains controversial, and the recent Potential of rtPA for Ischemic Strokes with Mild Symptoms (PRISMS) trial, which randomized patients to thrombolysis vs aspirin, did not show benefit. We studied the safety and efficacy of thrombolysis in a population of patients with acute stroke presenting with low NIH Stroke Scale (NIHSS) scores screened using MRI.
Methods
The NIH Natural History of Stroke database was reviewed from January 2006 to December 2016 to identify all patients with an initial NIHSS score ≤5 who received thrombolysis within 4.5 hours of symptom onset after being screened with MRI. The 24-hour postthrombolysis MRIs were reviewed for hemorrhagic transformation. Primary outcomes were symptomatic intracranial hemorrhage (sICH) and favorable 90-day outcome modified Rankin Scale score 0–1. Subgroup analysis was performed on patients who would have been eligible for the PRISMS trial, which enrolled patients with a nondisabling neurologic deficit.
Results
A total of 121 patients were included in the study with a median age of 65 and an NIHSS score of 3; 63% were women. The rate of any hemorrhagic transformation was 13%, with 11% of them being limited to petechial hemorrhage. The rate of sICH was <1%. Sixty-six patients had 90-day outcome data; of those, 74% had a favorable outcome. For the subgroup of 81 PRISMS-eligible patients, none experienced sICH. Fifty of these patients had 90-day outcome data; of these, 84% had a favorable outcome.
Conclusions
Thrombolytic therapy was safe in our patients with stroke with minor deficits who were initially evaluated by MRI. Future studies of this population may benefit from MRI selection.
Classification of evidence
This study provides Class IV evidence that for patients with acute ischemic stroke and NIHSS ≤5 screened with MRI, IV tissue plasminogen activator is safe.
Approximately 50% of patients with acute stroke present with a mild deficit (NIH Stroke Scale [NIHSS] score ≤5).1 Current American Heart Association (AHA)/American Stroke Association guidelines for treatment of acute ischemic stroke state that it is reasonable to consider IV thrombolysis treatment for patients presenting with mild and nondisabling deficits and recommend more studies to identify the risk-to-benefit ratio in this population.2 However, mild and nondisabling deficits are the most common reason to withhold thrombolysis in emergency departments for patients with acute stroke who are otherwise eligible to receive thrombolytic therapy.3,4 This is despite multiple studies reporting that approximately 30% of patients who are “too good to treat” have a poor outcome after stroke in the absence of thrombolysis.4–7 Because a majority of previous thrombolysis trials excluded patients with very mild deficits, the potential benefit of thrombolytic therapy in this population is not fully understood.8 However, a meta-analysis of the patients included in thrombolytic trials who had a low NIHSS score found them to have a benefit over placebo.9
To resolve this issue, the Potential of rtPA for Ischemic Strokes with Mild Symptoms (PRISMS) study was initiated to determine if patients with mild and nondisabling deficits would benefit from IV tissue plasminogen activator (tPA) therapy when comparing with oral aspirin.8 However, the PRISMS trial was terminated early due to poor enrollment after one third of the planned population had been recruited. The study found that patients in the aspirin group had better functional outcomes and fewer hemorrhagic complications than patients treated with IV tPA. Although the study was underpowered, the results suggested that the practice of thrombolysis in patients with nondisabling stroke may not be warranted.10
The PRISMS trial screened patients with head CT (HCT). Although HCT meets the standard of care for screening patients for thrombolysis, MRI can also be used to rapidly screen patients, providing more information while still meeting AHA guidelines.11,12 In patients with low NIHSS scores for whom the risk/benefit for thrombolysis may be in question, MRI may offer additional information that shifts the balance. For instance, the AHA guidelines recommend against treating patients with >10 cerebral microbleeds on MRI if the potential benefit is unclear.2 The NIH stroke program has routinely used MRI to screen patients with acute stroke for thrombolysis for more than a decade. The purpose of this study was to investigate the safety and outcome of thrombolysis in patients presenting with NIHSS score ≤5 in an MRI-based practice.
Methods
Magnetic resonance imaging
All patients underwent MRI scanning prior to, and 24 hours after, treatment with IV tPA. The MRI protocol included diffusion-weighted imaging (DWI), gradient echo (GRE) hemosiderin-weighted imaging, fluid-attenuation inversion recovery (FLAIR) imaging, time-of-flight magnetic resonance angiography (MRA), and, in those able to receive gadolinium, perfusion-weighted imaging (PWI). All MRI scans were performed on a 1.5T GE Signa scanner (General Electric Medical Systems, Milwaukee, WI), a 3T Philips Achieva scanner (Philips Healthcare, Best, the Netherlands), or a 3T Siemens Skyra scanner (Siemens AG, Munich, Germany). Scan parameters were similar between scanner types but did evolve over time to maximize diagnostic accuracy.11
Population and outcome measures
The study population was derived from patients consented to the NIH Natural History of Stroke (NHS) Study (identification number NCT00009243), an institutional review board–approved observational cohort study of patients with stroke. Study participants were recruited from 2 regional stroke centers (Medstar Washington Hospital, Washington, DC; and Suburban Hospital, Bethesda, MD) affiliated with the NIH Intramural Stroke program.
Stroke patients enrolled in the NIH NHS study from January 2006 to December 2016 were included in the study if they met the following criteria: (1) pretreatment NIHSS score ≤5, (2) screened with MRI, and (3) treated with IV tPA (0.9 mg/kg) within 4.5 hours from symptom onset. Demographic data, stroke risk factors, initial and 24-hour NIHSS score, type of the neurologic deficit (visual, motor, speech, neglect, sensory), vascular territory, and time from symptom onset to IV tPA administration were collected for all patients. Pretreatment MRI scans were reviewed for the following imaging features: the presence of a stroke on DWI, the presence of a perfusion deficit on PWI (when performed), the presence of a large vessel occlusion (LVO) on MRA, the presence of a likely thrombus on GRE, and the presence of microbleeds, which, when present, were classified as being greater than or less than 10 in number. LVO was defined as a vessel cutoff in the anterior circulation involving the internal carotid artery or the M1 segment of the middle cerebral artery or as a vessel cutoff in the posterior circulation involving the basilar artery or the posterior cerebral artery. If LVO was identified on the pretreatment MRA, the status of its recanalization on the 24-hour MRI was assessed. The presence of a perfusion–diffusion mismatch was assessed visually. The purpose of assessing for a mismatch was to determine the extent of the ischemic event, not to quantify the penumbral salvage, thus quantitative measures were not employed.
The presence of hemorrhagic transformation (HT) on MRI 24 hours post IV tPA was reviewed and graded based on European Cooperative Acute Stroke Study (ECASS) criteria13 by 2 of the authors (S.M. and M.L.) as hemorrhagic infarction type 1 (HI-1), hemorrhagic infarction type 2 (HI-2), parenchymal hematoma type 1, or parenchymal hematoma type 2 (PH-2); disagreements were adjudicated by a third author (R.L.). Although the ECASS ICH grading criteria were developed based on HCT, in this case they were applied to MRI. Symptomatic intracerebral hemorrhage (sICH) was defined as parenchymal hematoma accompanied by a worsening between the pretreatment and 24-hour post-treatment NIHSS of ≥4 or death. Unlike the ECASS classification of sICH, hemorrhagic infarction was not part of the definition for sICH in this study due to the use of MRI, which has an increased sensitivity for detecting blood products compared with HCT. A favorable outcome was defined by a modified Rankin Scale (mRS) score of 0–1 at 90 days. Discharge disposition post-treatment was also reviewed and dichotomized as discharged to home vs discharged to a facility.
We performed a secondary analysis of our study population to investigate the rate of favorable outcome, hemorrhagic transformation, and sICH among patients who would likely have met inclusion/exclusion criteria for the PRISMS study. We used exclusion criteria similar to the PRISMS study,8 which excluded patients with any of the following criteria: (1) complete hemianopia (score of ≥2 on NIHSS vision question), (2) severe aphasia (score of ≥2 on NIHSS language question), (3) severe neglect or extinction (score of ≥2 on NIHSS extinction or inattention question), (4) score of ≥2 on the NIHSS motor questions, or (5) functional disability prior to the index stroke (defined as baseline mRS score ≥2).
Statistical analysis
Descriptive statistics were tabulated for the study population and the subset of PRISMS-eligible patients including demographic characteristics, vascular risk factors, admission medications, presenting NIHSS, premorbid mRS, breakdown of the baseline NIHSS components, stroke subtype, timing intervals based on onset to pretreatment MRI and onset to IV tPA treatment, and imaging markers including DWI lesion, PWI deficit, PWI–DWI mismatch, presence of thrombus on GRE, microbleeds on GRE, and LVO on MRA. The χ2 test and t test were used as appropriate for continuous and categorical variables. Multiple regression and logistic regression models were performed to identify independent predictors of any HT and favorable outcome using significant variables (p < 0.10) from the univariate analysis. There were no missing data for the HT analysis; however, favorable outcome at 90 days was not available for approximately half of the patients. Thus, for the statistical analysis, we used a composite favorable outcome, which was defined as an mRS of 0–1 at 90 days, if available, and when not, as a mRS of 0–1 at 30 days. For patients without mRS data at either of these time points, discharge to home was considered a favorable outcome. Using this composite measure, there were no missing data. SPSS Statistics software v19 (IBM, Armonk, NY) was used for the analysis.
Data availability
Data in this study are monitored by the NIH Office of Human Subjects Research Protections and the Combined NeuroScience Institutional Review Board. Requests for access to the data may be possible if approved by these governing bodies.
Results
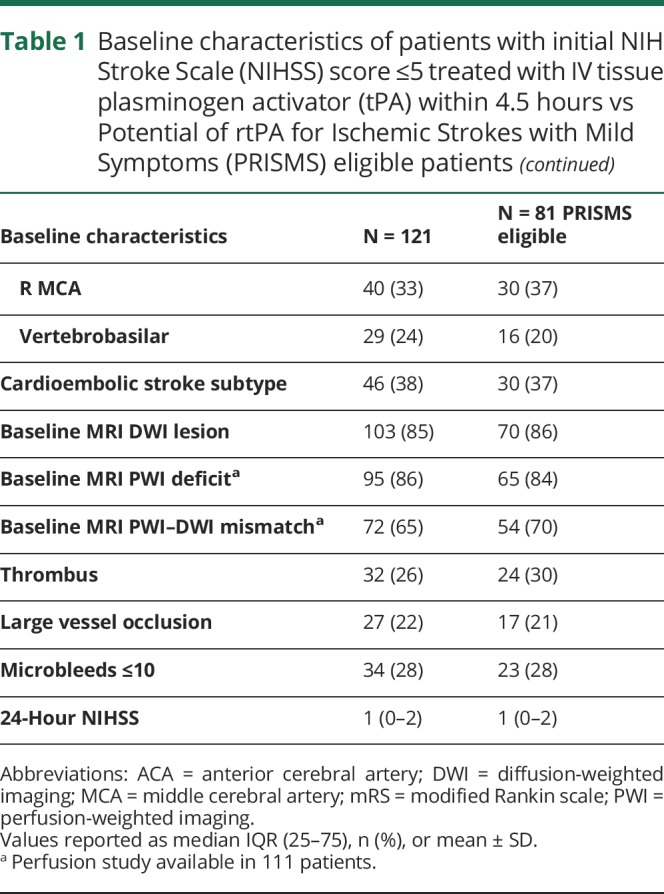
During the study period, 1,130 patients were treated with IV tPA at our 2 sites, of whom 245 (22%) had a pretreatment NIHSS ≤5. During this period, 625 of the treated patients were enrolled in the NIH NHS study, of whom 142 (23%) had an NIHSS ≤5. Of the consented patients with NIHSS ≤5, 121 received IV tPA after screening with MRI within 4.5 hours from last seen normal and were included in this study. Twenty-one patients screened with HCT rather than MRI due to contraindications to MRI or lack of scanner availability at the time were not included in the study. The population characteristics for the 121 treated patients are shown in table 1. The median (interquartile range [IQR]) age was 65 (56–77) years, and 63% of the patients were women. The median (IQR) NIHSS score on admission was 3 (2–4) and the median (IQR) time from symptom onset to IV tPA time was 147 (108–188) minutes. Dysarthria (50%) and motor deficit (33%) were the 2 most common presenting symptoms followed by sensory deficit (27%) and aphasia (23%). For 29 patients (24%), the stroke was in vertebrobasilar territory. Diffusion and perfusion lesions were present in 85% and 86% of patients, respectively. Of the 111 patients who had PWI as part of their screening MRI, 72 (65%) patients had perfusion deficit larger than the diffusion lesion based on visual comparison of the PWI and DWI sequences. Thrombus on GRE was evident in 26%, and LVO visualized in 22% of patients. Eighty-one percent (22/27) of the LVO cases also had hyperintense vessels on the baseline precontrast FLAIR. For the 5 cases without visible hyperintense vessels, 3 had LVO in the posterior circulation and 2 had LVO in the anterior circulation. Microbleeds on GRE were present in 28% with no patients having >10. For the 27 patients with LVO, 70% achieved complete recanalization. We did not identify any mimics in the population as all patients had imaging confirmation of acute ischemia or stroke, represented as PWI or DWI lesion, respectively.
Table 1.
Baseline characteristics of patients with initial NIH Stroke Scale (NIHSS) score ≤5 treated with IV tissue plasminogen activator (tPA) within 4.5 hours vs Potential of rtPA for Ischemic Strokes with Mild Symptoms (PRISMS) eligible patients
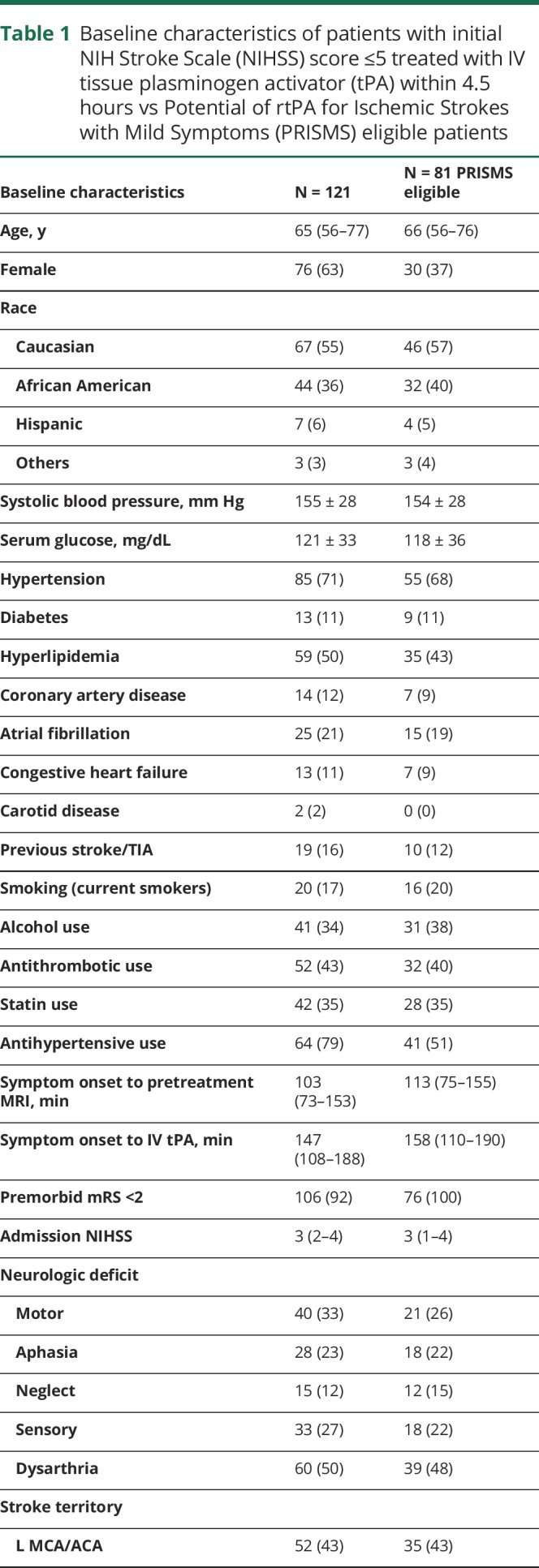
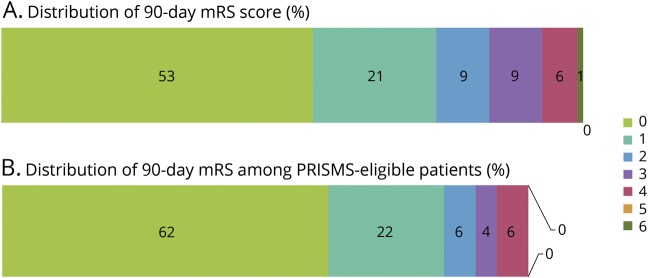
Hemorrhagic transformation on 24-hour post-treatment MRI was identified in 16 (13%) patients, which consisted of 7 (6%) HI-1, 6 (5%) HI-2, and 3 (2%) PH-2. Symptomatic ICH occurred in only one patient (0.8%) who had a left middle cerebral artery distribution stroke and experienced a PH-2 with an increase in NIHSS from 4 to 13. The patient who had an sICH survived to 90 days, having an mRS of 3 at that time. Outcome mRS was available for 78 patients at 30 days and 66 patients at 90 days. Discharge disposition was available in all 121 patients. The rate of favorable outcome (mRS 0–1) was 77% at 30 days, and 74% at 90 days with 78% discharged to home (table 2 and figure, A). The percentage of patients with a PWI–DWI mismatch was similar between the patients with (70%) and without (69%) a favorable outcome. Comparing stroke location, patients with anterior circulation stroke had a favorable outcome at a rate of 74% compared to the posterior circulation, which had a rate of 83%. Separating patients into those treated within the 3-hour window from those treated in the 3- to 4.5-hour window resulted in 78% vs 75% who had favorable outcomes.
Table 2.
Primary outcome measures in patients treated with IV tissue plasminogen activator
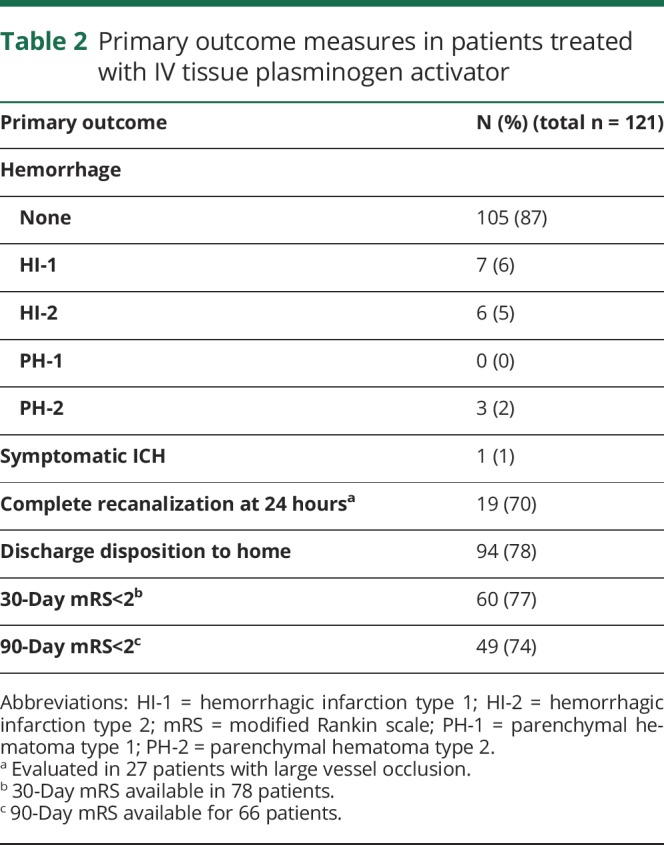
Figure. Distribution of 90-day modified Rankin Scale (mRS) score.
(A) Distribution of 90-day mRS score among patients with initial NIH Stroke Scale (NIHSS) score ≤5 treated with IV tissue plasminogen activator (tPA) within 4.5 hours from the time of symptom onset. (B) Distribution of 90-day mRS score among patients with NIHSS score ≤5 and nondisabling deficit (similar to Potential of rtPA for Ischemic Strokes with Mild Symptoms [PRISMS] selection criteria) treated with IV tPA.
Age (p < 0.0001, odds ratio [OR] 0.9, 95% confidence interval [CI] 0.89–0.96), baseline NIHSS (p = 0.009, OR 0.6, 95% CI 0.40–0.88), and sex (p = 0.032, OR 3.2, 95% CI 1.1–9.5) were independent predictors of favorable outcome. Younger (median of 63 vs 80 years) male (68% vs female 33%) patients with less severe baseline NIHSS (median of 3 vs 4) were more likely to have favorable outcomes. There were no independent predictors for incidence of any HT. The multivariate analysis included age, sex, presence of congestive heart failure, baseline NIHSS score, and HT.
Based on inclusion/exclusion criteria, 81/121 patients in our study met the approximated eligibility criteria for the PRISMS trial. The median initial NIHSS score was 3 (IQR 1–4), and there was PWI–DWI mismatch in 54 (71%) of these patients. Ten patients had hemorrhagic transformation (12%); 6 of them were HI-1, 3 were HI-2, and 1 was PH-2. No sICH was seen in this group of patients. The rate of favorable 90-day outcome (mRS score 0–1) was 84% of 50 patients with data available, while 86% of the 81 patients were discharged to home. The figure, B, depicts the distribution of 90-day mRS scores for patients presenting with nondisabling deficits. Favorable 90-day outcome rates were 79% vs 78% in patients with PWI–DWI mismatch vs without mismatch.
Discussion
This study evaluates the safety and efficacy of MRI-guided thrombolysis in a population of patients with acute ischemic stroke who presented with a low NIHSS score, the majority of whom (67%) had a nondisabling deficit. The rate of sICH was exceptionally low in this study and did not occur in patients with a nondisabling deficit. The rate of favorable outcome was in the range of 74%–77% for this study, which is higher than the 69% rate found in the meta-analysis of the thrombolytic trials of patients with NIHSS of 0–4.9 For the patients with nondisabling deficits in our population, the rate of favorable outcome was 84%, which is higher than either the treatment (78%) or the placebo (81%) arms of the PRISMS trial.10
The primary difference between this study and all previous studies looking at thrombolysis of patients with low NIHSS scores is the use of MRI instead of HCT to screen patients prior to treatment. HCT is used to rule out hemorrhagic stroke but rarely contributes to the decision-making process beyond this exclusion. MRI, on the other hand, provides a wealth of information about the brain and the stroke which, particularly in a situation of questionable risk–benefit, may greatly affect the decision-making process. For instance, it has long been the practice of the NIH stroke team to consider >10 microbleeds on GRE to be a relative contraindication to tPA, and, in the case of a nondisabling deficit, would be a sufficient finding for which to withhold thrombolysis. This practice was recently endorsed by the AHA guidelines.2 Meta-analysis of the sICH for patients with an NIHSS of 0–4 in the thrombolytic trials found a sICH rate of 1.7% 14; analysis of the Get With The Guidelines–Stroke Registry found an sICH rate of 1.8% for patients with an NIHSS of 0–5.15 The rate of sICH in our MRI-selected population was 50% less than either of these CT-selected populations and markedly less than the 3.3% rate seen in the PRISMS trial. Although our MRI hemorrhage rate of 0.8% falls on the lower bound of the CI for the rate in the PRISMS trial, when the population is limited to those who would likely be included in the PRISMS trial, the rate of sICH drops to zero. The PRISMS trial also reported the sICH rate based on SITS-MOST criteria,16 which is more similar to the definition of sICH used in this study, and was found to be 1.3%.
One of the challenges to showing the benefit of thrombolysis in patients with a nondisabling deficit is that the outcome measures used are based on the presence of disability. Thus, in order to demonstrate benefit, either there must be an initially unrecognized deficit (such as difficulty with ambulation),7 or there must be a worsening of the initial deficit,6 in which case thrombolysis has the opportunity to exert a benefit on outcome. It is difficult based purely on clinical assessment to determine which patients are at highest risk of worsening. This may be why patients who are “too good to treat” appear to have a 30% chance of a poor outcome. In our study, patients presenting with nondisabling deficits had a 16% chance of poor outcome, possibly reflecting the benefit of tPA. Rapidly improving symptoms in one study of minor stroke were associated with an increased likelihood of subsequent deterioration,6 while another study did not find any clinical predictors of worsening other than a high NIHSS on presentation.17 MRI, however, provides several biomarkers that could help assess this risk of deterioration, including thrombus imaging on GRE, vessel cutoff on MRA, hyperintense vessels on FLAIR, and mismatch between DWI and PWI lesions. A prior study looking at MRI predictors of clinical deterioration found that vessel cutoff on MRA and PWI–DWI mismatch were associated with subsequent worsening.18 In our study, two-thirds of the patients had a PWI–DWI mismatch, and it was even higher in the subgroup with nondisabling deficit, suggesting that such a finding may influence the clinician to treat with thrombolysis despite the otherwise questionable benefit.
Another aspect of MRI-guided treatment that likely influenced this study is the exclusion of stroke mimics. Patients presenting with an MRI that is negative for an ischemic process are generally not treated, and certainly in the situation of a mild deficit, such a patient would not receive thrombolysis. On the one hand, when screening a patient based on HCT, mild deficits may not be convincing enough to conclude that a patient is having a stroke, leading to such a patient going untreated.19 On the other hand, in everyday practice, stroke mimics are routinely treated with thrombolysis when screened with HCT.20 The mimic rate in the PRISMS trial was 13%,10 which was evenly divided between arms, and presumably worked to obscure any potential benefit in the target population. The patients studied in our MRI-screened cohort had confirmation of an ischemic event prior to treatment.
The multivariate analysis did not identify any associations between imaging findings and outcome. This is likely due to the MRIs being the basis for selecting patients in the first place. The association of good functional outcome with lower age and less stroke severity was not surprising; however, the increased benefit for male patients was not expected. Further studies would be needed to determine if this is a meaningful finding.
There are several limitations to this study. Because the use of HCT in screening patients with stroke is rare at our institutions, we are not able to provide a comparison group. Our definition of sICH was different from that of the PRISMS trial, which defined it as “any neurologic decline within 36 hours attributed to ICH by local investigators.” 10 Due to the retrospective nature of our study, we could not replicate this definition and instead defined sICH as parenchymal hematoma with neurologic decline ≥4 points on the NIHSS during the first 24 hours. However, the definition of sICH used in this study is similar to that of the SITS-MOST criteria, and was lower in this study compared to when these criteria were applied to the PRISMS trial. The study population is a retrospective assessment of patients prospectively enrolled in an observational cohort study and is subject to sampling bias. The patients were not treated as part of a clinical trial, and thus it is not known if similar outcomes would be achieved in a prospective study. It is not possible in this study to exactly reproduce the selection bias that likely occurred in the PRISMS trial with regards to what constitutes a nondisabling deficit. Only patients enrolled in the NIH NHS study were included in the analysis; however, the rate of low NIHSS in all thrombolysed patients (22%) was similar to that of the patients included in the study (23%), suggesting a representative sample. Patients are generally approached for enrollment in the NHS study immediately after treatment; however, it is possible that a patient approached at a later time point, possibly after a complication occurred, may be less likely to consent, which could artificially inflate the rate of good outcome. However, every attempt is made by the NIH stroke team to consent all treated patients to the NHS regardless of outcome. In addition, missing outcome data could be biased toward patients with a poor outcome. We also do not have data on patients who were not treated based on MRI findings, which would be a useful comparison. Although the use of MRI to select patients for thrombolysis has been standardized at the NIH by a clinical pathway throughout the duration of the study period, practices have changed over time and may vary somewhat between clinicians. Although the results of this study support the practice of MRI-selected treatment of patients with a low NIHSS, considering the above-mentioned limitations, they are not conclusive and should be interpreted as preliminary findings that should be followed up with randomized studies testing this practice. In addition, the practice of screening possible patients with stroke with low NIHSS for treatment will be limited to hospitals with well-established MRI protocols and to patients who are able to safely have an MRI performed.
The use of MRI-guided thrombolysis of patients with a low or nondisabling NIHSS score appears to have been safe and effective at our institutions over the last decade. Future studies investigating the treatment of this population may benefit from MRI-based selection.
Glossary
- AHA
American Heart Association
- CI
confidence interval
- DWI
diffusion-weighted imaging
- ECASS
European Cooperative Acute Stroke Study
- FLAIR
fluid-attenuation inversion recovery
- GRE
gradient echo
- HCT
head CT
- HI-1
hemorrhagic infarction type 1
- HI-2
hemorrhagic infarction type 2
- HT
hemorrhagic transformation
- IQR
interquartile range
- LVO
large vessel occlusion
- MRA
magnetic resonance angiography
- mRS
modified Rankin Scale
- NHS
NIH Natural History of Stroke
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- PH-2
parenchymal hematoma type 2
- PRISMS
Potential of rtPA for Ischemic Strokes with Mild Symptoms
- PWI
perfusion-weighted imaging
- sICH
symptomatic intracerebral hemorrhage
- SITS-MOST
Safe Implementation of Thrombolysis in Stroke–Monitoring Study
- tPA
tissue plasminogen activator
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study funding
All of the authors are supported by the Intramural Program of the NIH, NINDS.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Dhamoon MS, Moon YP, Paik MC, et al. Long-term functional recovery after first ischemic stroke: the Northern Manhattan Study. Stroke 2009;40:2805–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 3.Kleindorfer D, Kissela B, Schneider A, et al. Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: a population-based study. Stroke 2004;35:e27–e29. [DOI] [PubMed] [Google Scholar]
- 4.Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from tPA therapy? An analysis of patient eligibility. Neurology 2001;56:1015–1020. [DOI] [PubMed] [Google Scholar]
- 5.Khatri P, Conaway MR, Johnston KC; Acute Stroke Accurate Prediction Study Investigators. Ninety-day outcome rates of a prospective cohort of consecutive patients with mild ischemic stroke. Stroke 2012;43:560–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith EE, Abdullah AR, Petkovska I, Rosenthal E, Koroshetz WJ, Schwamm LH. Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke 2005;36:2497–2499. [DOI] [PubMed] [Google Scholar]
- 7.Smith EE, Fonarow GC, Reeves MJ, et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: findings from Get With the Guidelines–Stroke. Stroke 2011;42:3110–3115. [DOI] [PubMed] [Google Scholar]
- 8.Yeatts SD, Broderick JP, Chatterjee A, et al. Alteplase for the treatment of acute ischemic stroke in patients with low National Institutes of Health Stroke Scale and not clearly disabling deficits (potential of rtPA for ischemic strokes with mild symptoms [PRISMS]): rationale and design. Int J Stroke 2018;13:654–661. [DOI] [PubMed] [Google Scholar]
- 9.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khatri P, Kleindorfer DO, Devlin T, et al. Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the PRISMS randomized clinical trial. JAMA 2018;320:156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah S, Luby M, Poole K, et al. Screening with MRI for accurate and rapid stroke treatment: SMART. Neurology 2015;84:2438–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sablot D, Ion I, Khlifa K, et al. Target door-to-needle time for tissue plasminogen activator treatment with magnetic resonance imaging screening can be reduced to 45 min. Cerebrovasc Dis 2018;45:245–251. [DOI] [PubMed] [Google Scholar]
- 13.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II): Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–1251. [DOI] [PubMed] [Google Scholar]
- 14.Whiteley WN, Emberson J, Lees KR, et al. Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol 2016;15:925–933. [DOI] [PubMed] [Google Scholar]
- 15.Romano JG, Smith EE, Liang L, et al. Outcomes in mild acute ischemic stroke treated with intravenous thrombolysis: a retrospective analysis of the Get With the Guidelines–Stroke registry. JAMA Neurol 2015;72:423–431. [DOI] [PubMed] [Google Scholar]
- 16.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke–Monitoring Study (SITS-MOST): an observational study. Lancet 2007;369:275–282. [DOI] [PubMed] [Google Scholar]
- 17.Nedeltchev K, Schwegler B, Haefeli T, et al. Outcome of stroke with mild or rapidly improving symptoms. Stroke 2007;38:2531–2535. [DOI] [PubMed] [Google Scholar]
- 18.Rajajee V, Kidwell C, Starkman S, et al. Early MRI and outcomes of untreated patients with mild or improving ischemic stroke. Neurology 2006;67:980–984. [DOI] [PubMed] [Google Scholar]
- 19.Richoz B, Hugli O, Dami F, Carron PN, Faouzi M, Michel P. Acute stroke chameleons in a university hospital: risk factors, circumstances, and outcomes. Neurology 2015;85:505–511. [DOI] [PubMed] [Google Scholar]
- 20.Burton TM, Luby M, Nadareishvili Z, et al. Effects of increasing IV tPA-treated stroke mimic rates at CT-based centers on clinical outcomes. Neurology 2017;89:343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data in this study are monitored by the NIH Office of Human Subjects Research Protections and the Combined NeuroScience Institutional Review Board. Requests for access to the data may be possible if approved by these governing bodies.