Abstract
Introduction
To determine the influence of white matter hyperintensity (WMH) burden on functional outcome, rate of symptomatic intracerebral hemorrhage (sICH), and procedural success in patients with acute ischemic stroke (AIS) treated by mechanical thrombectomy (MT) with current stentriever/aspiration devices.
Methods
Patients with AIS due to large vessel occlusion (LVO) from the Thrombectomie des Artères Cérébrales (THRACE) trial and prospective cohorts from 2 academic comprehensive stroke centers treated with MT were pooled and retrospectively analyzed. WMH volumes were obtained by semiautomated planimetric segmentation and tested in association with the rate of favorable outcome (90-day functional independence), substantial recanalization after MT, and sICH.
Results
A total of 496 participants were included between 2015 and 2018 (50% female, mean age 68.1 ± 15.0 years). Overall, 434 (88%) patients presented with detectable WMH (mean ± SD 4.93 ± 7.7). Patients demonstrated increasingly worse outcomes with increasing WMH volumes (odds ratio [aOR]1.05 per 1-cm3 increase for unfavorable outcome, 95% confidence interval [CI] 1.01–1.06, p = 0.014). Fifty-seven percent of patients in the first quartile of WMH volume vs 28% in the fourth quartile demonstrated favorable outcome (p < 0.001). WMH severity was not associated with sICH rate (aOR 0.99, 95% CI 0.93–1.04, p = 0.66), nor did it influence recanalization success (aOR 0.99, 95% CI 0.96–1.02, p = 0.84).
Conclusion
Our study provides evidence that in patients with AIS due to LVO and high burden of WMH as assessed by pretreatment MRI, the safety and efficacy profiles of MT are similar to those in patients with lower WMH burden and confirms that they are at higher risk of unfavorable outcome. Because more than a quarter of patients in the highest WMH quartile experienced favorable 3 months outcome, WMH burden may not be a good argument to deny MT.
ClinicalTrials.gov Identifier
Cerebral white matter hyperintensity (WMH) on fluid-attenuated inversion recovery (FLAIR) sequence is a common MRI finding and an imaging manifestation of cerebral small vessel disease in aging individuals. The burden of WMH has been consistently linked to less favorable functional outcomes in patients with acute ischemic stroke (AIS), including those treated with tissue plasminogen activator (tPA),1–3 and to increased rates of symptomatic intracerebral hemorrhage (sICH) in several retrospective cohorts.3–6
Recently, compelling evidence of the efficacy of mechanical thrombectomy (MT) in improving outcome for selected patients with large cerebral vessel occlusion (LVO) was provided by several randomized trials, including the Thrombectomie des Artères Cérébrales (THRACE) study,7,8 changing the landscape of AIS care. To date, the association between WMH burden and risk of poor outcome in patients with AIS treated with MT has been reported with conflicting results,9–15 possibly related to historical trends in the use of thrombectomy devices, inhomogeneous selection criteria for MT, and time to reperfusion considerations. Thus, in patients undergoing MT in modern practice, the influence of WMH on functional outcome, sICH, and procedural success remains largely unexplored.
In this post hoc analysis of the MT arm of the THRACE trial and multicenter retrospective cohorts, we aimed to determine the impact of WMH burden on functional outcome and to assess the effect of WMH on the rate of sICH and substantial recanalization in anterior circulation in patients with AIS undergoing MT with current stentriever/aspiration devices.
Methods
Standard protocol approvals, registrations, and patient consent
The THRACE trial (ClinicalTrials.gov, number NCT01062698) was a randomized controlled trial at 26 French centers. The study design and protocol have been previously detailed elsewhere.7 Briefly, patients with anterior AIS-LVO were randomly assigned 1:1 to receive either IV thrombolysis (IVT) alone or IVT plus MT (IVTMT arm). IVT and MT had to be started within 4 and 5 hours of symptom onset, respectively. Before randomization, written informed consent was obtained from all patients or their legal representatives. The study protocol was approved by the Comité de Protection des Personnes III Nord Est Ethics Committee and the research boards of the participating centers.
Patients from the IVTMT arm of THRACE and retrospective cohorts of consecutive patients from 2 additional academic comprehensive stroke centers using pretreatment MRI as routine imaging in suspected stroke patients (Centre Hospitalier Régional, Lille [center 1] and Centre Hospitalier Sainte Anne, Paris [center 2]) were pooled and retrospectively analyzed. In both centers, MT indication was at the discretion of the treating team. In accordance with French legislation, written informed consent was waived for the retrospective analysis of data collected as part of routine clinical care in these cohorts.
Study design, setting, patient population, and variables
The preliminary pooled sample was retrospectively queried to identify adult patients meeting the following criteria: (1) baseline clinical MRI, routinely obtained before MT, with available FLAIR sequence of adequate quality for segmentation; (2) AIS with anterior LVO (internal carotid artery, M1 or proximal M2 segment of middle cerebral artery); (3) 90-day modified Rankin Scale (mRS) score assessed through in-person or telephone interview; and (4) for both retrospective cohorts, treatment between January 1, 2015, and January 1, 2018, when MT became standard of care. Baseline clinical characteristics and laboratory values were collected prospectively at each center and retrospectively queried.
Clinical and treatment-related variables
Age and medical history (including atrial fibrillation, diabetes mellitus, hyperlipidemia, hypertension, and tobacco use) were determined from medical records or patient/surrogate interview. All patients were evaluated in the acute setting by a neurologist, at which time stroke severity was assessed with the NIH Stroke Scale (NIHSS) score. Treatment with IV tPA and procedural endovascular variables were prospectively collected.
WMH volumes
Supratentorial WMH volumes were obtained by the semiautomated planimetric segmentation of axial T2-FLAIR sequences with MRICron (nitrc.org/projects/mricron/) as previously described by a single experienced neuroradiologists (G.B.) blinded to functional outcomes.16 Images at both participating academic centers were obtained with a 1.5T GE magnet (GE, Fairfield, CT). In brief, the segmentation process includes the manual delineation of a region of interest containing WMH, a histogram-based intensity filter applied within the region of interest, visual inspection of the segmented map, and manual correction when needed (figure e-1 available from Dryad, doi.org/10.5061/dryad.b7d9q07). To exclude confounding, hyperintense FLAIR chronic territorial infarcts involving any degree of white matter were manually removed from segmented map. Similarly, to prevent overestimation of WMH lesions, we obtained WMH volume in the unaffected hemisphere and doubled it to obtain final WMH volume in all patients, a validated approach because WMHs have a high interhemispheric severity correlation.17
Outcome assessment
The mRS was assessed, blinded from WMH volumes, through in-person or telephone interview with patients or their surrogates at 3 months after stroke. Favorable outcome, defined as a 3-month mRS score of 0 to 2 or equal to the prestroke mRS score collected prospectively, was chosen as the primary outcome. Secondary outcomes included mortality at 90 days; sICH defined according to the European Cooperative Acute Stroke Study 2 (ECASS II) criteria as an NIHSS score 4 points higher than the value at baseline, the lowest value in the first 7 days, or that led to death or was identified as the predominant cause of the neurologic deterioration18; and rate of substantial recanalization after MT, defined as a modified Treatment in Cerebral Ischemia (mTICI) scale of 2B or 3.
This report was prepared according to the Strengthening the Reporting of Observational Studies in Epidemiology statement.19
Statistics
Descriptive statistics are presented as absolute number (percentage) for discrete variables and mean (SD) or median (25th–75th percentiles) for continuous variables as appropriate. The χ2, Fisher exact test, Student t test, and Mann-Whitney tests were used as appropriate for the univariable analyses, with a value of p < 0.05 as the threshold for statistical significance. Multivariable logistic regression models were used to determine factors that were independently associated with each outcome (functional outcome, recanalization, and sICH). Variables associated with the outcome in univariable analysis (p ≤ 0.1) were entered into binomial logistic regression models (prespecified adjustment for age, sex, NIHSS score, and time to groin puncture, as well as substantial recanalization, based on current known determinants of poor outcome), and then backward elimination was used to remove nonsignificant variables (p ≥ 0.05). WMH volume was then discretized in its quartiles, and we used these transformed values instead of the continuous variables. Finally, the analyses were repeated after stratification by recanalization status. All analyses were done with JMP Pro 13 software (SAS Institute Inc, Cary, NC; 2015).
Data availability
Raw data of this cohort can be made available on request to the corresponding author and after clearance by the local ethics committee.
Results
Study population
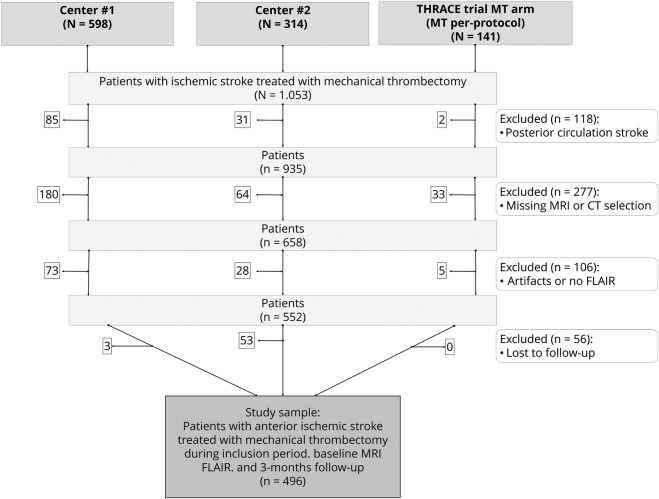
Among 1,053 patients treated with MT during trial inclusion period (141 in the THRACE trial) or between January 2015 and 2018 (598 in center 1, and 313 in center 2), a total of 496 patients met inclusion criteria (49.6% female sex, mean age 68.09 ±15.3 years; figure 1 presents details on patients selection), of whom 209 (42.1%) achieved a favorable functional outcome at 90 days. Baseline characteristics of study population and its stratification per functional outcome are detailed in table 1. There was no difference between included and excluded patients in terms of clinical severity or medical history. Excluded patients more frequently had a contraindication for IVT and had higher diffusion-weighted imaging (DWI) Alberta Stroke Program Early CT (ASPECT) scores (both p < 0.05; table e-1 available from Dryad, doi.org/10.5061/dryad.b7d9q07). Baseline characteristics of patients differed among cohorts for the following points: use of tPA before MT (73%, 57%, and 100% in centers 1 and 2 and THRACE, respectively, p < 0.001), current smoke (39%, 18%, and 23%, p < 0.001), mean age (70 ± 15 years in both centers and 63 ± 13 years in THRACE, p = 0.01 for pairwise center-THRACE comparisons), history of dyslipidemia (44%, 29%, and 42%, p < 0.01), diabetes mellitus (17%, 10%, and 7%, p = 0.02), and favorable outcome (37%, 43%, and 57%, p < 0.05).
Figure 1. Flowchart of patient selection.
FLAIR = fluid-attenuated inversion recovery; MT = mechanical thrombectomy; THRACE = Thrombectomie des Artères Cérébrales.
Table 1.
Baseline characteristics of included patients
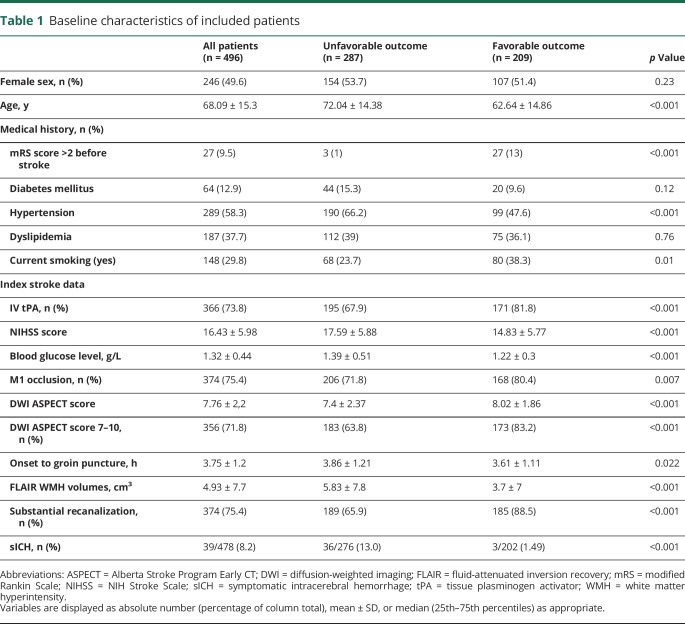
Patients with unfavorable outcome (287%–57.8%) were significantly older (72 ± 14.4 vs 62.6 ± 14.9 years, p < 0.001) and more frequently had a history of hypertension (66.2% vs 47.6%, p < 0.001), a contraindication for tPA (32.1% vs 19.2% p < 0.001), and prestroke mRS score >2 (12.7% vs 1.2%, p < 0.001). Clinical and imaging baseline characteristics were more severe in patients with unfavorable outcome (lower DWI ASPECT score, higher NIHSS score, less frequent M1 occlusions, longer delay between onset and groin puncture, all p < 0.02).
WMH prevalence, severity, and outcomes
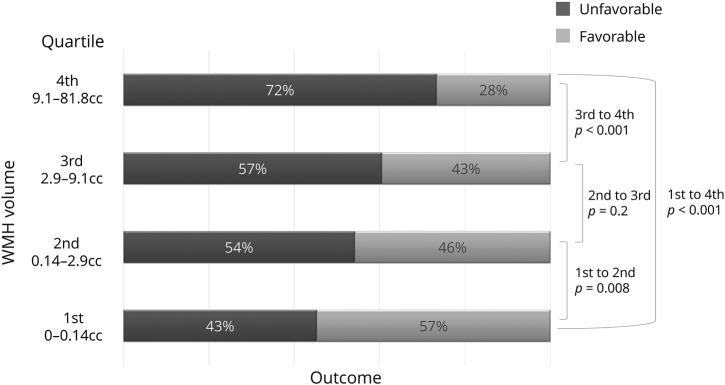
Overall, 369 (69%) patients presented with detectable WMH with a median volume of 4.99 (interquartile range 2.7–12.5) cm3. Patients demonstrated increasingly worse outcomes with increasing WMH volumes (p < 0.01 overall, for trend, and for first-second, first-fourth, first-third, and third-fourth quartiles transitions) as displayed in a histogram graph of functional outcomes per increasing quartile of WMH volume (figure 2), as well as in univariable analysis with WMH volume as a continuous variable (5.83 ± 7.8 in patients with poor outcome vs 3.7 ± 7, p < 0.001;, table 1 and table e-2 available from Dryad, doi.org/10.5061/dryad.b7d9q07).
Figure 2. Outcome distribution per quartile of WMH volume.
The p values are derived from a log-linear model. WMH = white matter hyperintensity.
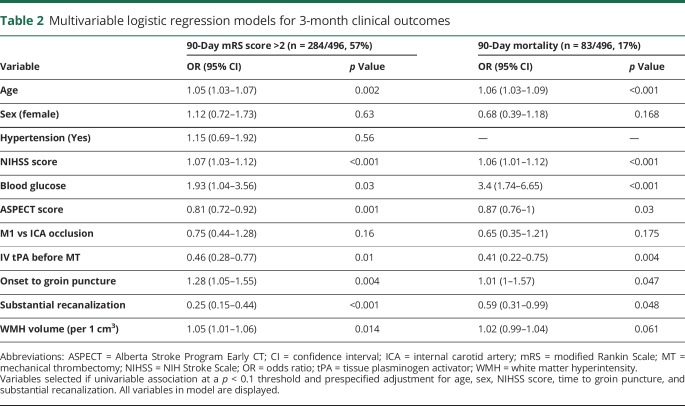
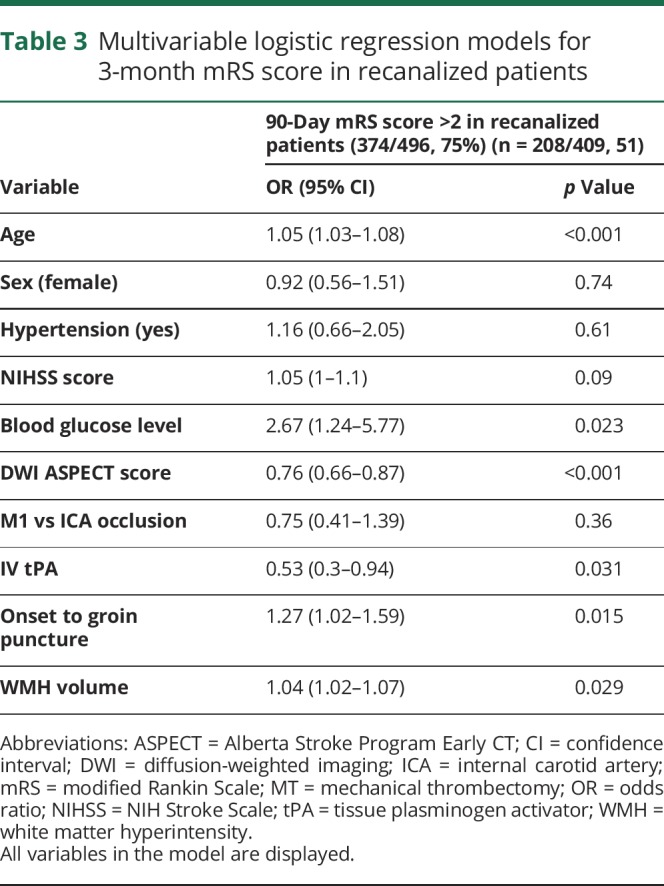
After adjustment for age, sex, hypertension, NIHSS score, blood glucose level, ASPECT score, occlusion level, IV tPA, delay from onset to groin puncture, and recanalization success, WMH volumes showed a significant association with 3-month unfavorable outcome (adjusted odds ratio [aOR]1.05 per 1-cm3 increase for unfavorable outcome, 95% confidence interval [CI] 1.01–1.06, p = 0.014) and a trend toward an association with mortality at 90 days (aOR = 1.02, 95% CI 0.99–1.04, p = 0.061). Table 2 provides details. When stratified by recanalization status, WMH volume remained independently associated with poor functional outcome in patients with substantial recanalization (aOR 1.04, 95% CI 1.02–1.07, p = 0.029; table 3 provides full model). Table e-3 (available from Dryad, doi.org/10.5061/dryad.b7d9q07) presents results from the same model with an interaction term added between revascularization and WMH volumes, which was not significant. In sensitivity analyses, using WMH volume quartiles, we found similar effects, directions, and significance for associations with functional outcome and mortality (tables e-4 and e-5 available from Dryad, doi.org/10.5061/dryad.b7d9q07, with adjustment per cohort of origin).
Table 2.
Multivariable logistic regression models for 3-month clinical outcomes
Table 3.
Multivariable logistic regression models for 3-month mRS score in recanalized patients
Secondary outcomes
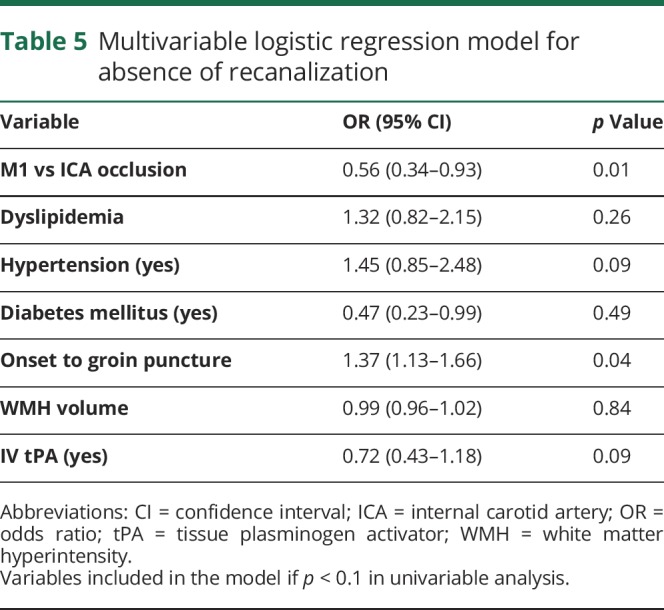
sICH occurred in 39 of 478 (8.2%) and was significantly more frequent in patients with unfavorable outcome (36 of 276 [13.0%] vs 3 of 202 [1.49%], p < 0.001; table 4 gives the multivariable model). WMH volume was not associated with sICH in both univariable and multivariable analyses (aOR 0.99, 95% CI 0.93–1.04, p = 0.60) and after stratification per recanalization status (all p > 0.5). Similarly, we found no association between WMH volume and substantial recanalization, obtained in 75.4% of the sample (aOR 0.99, 95% CI 0.96–1.02, p = 0.84; table 5).
Table 4.
Multivariable logistic regression model for sICH
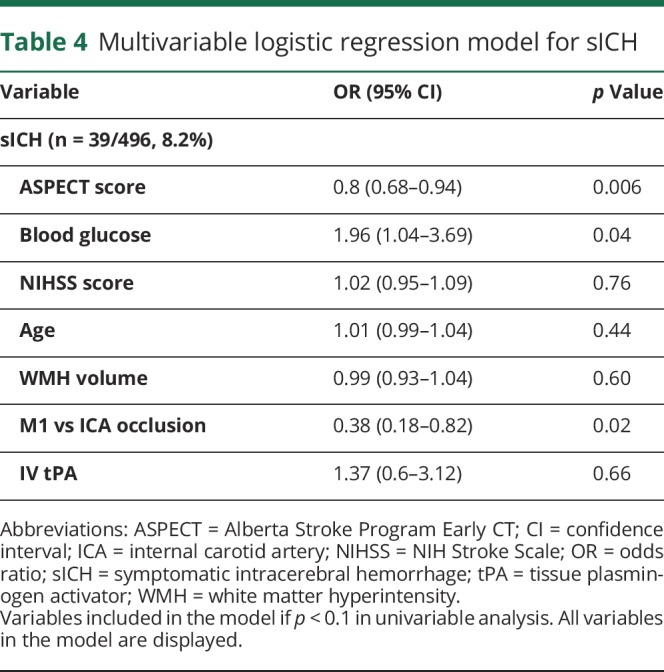
Table 5.
Multivariable logistic regression model for absence of recanalization
Discussion
In this multicenter study, we found that WMH volume measured on the baseline MRI obtained during emergency evaluation for AIS due to LVO is associated with worse functional outcome after MT independently of age, initial clinical imaging severity, or vascular risk factors. Conversely, we found no association between WMH severity and the rate of sICH or procedural success.
We provide preliminary evidence for the safety of MT with regard to the risk of sICH in patients with increased WMH burden. This finding is of importance because previous reports have linked WMH or leukoaraiosis (on CT) burden with higher risk of hemorrhagic transformation, more so after IVT,3,20 potentially influencing therapeutic decision-making, even in patients with AIS due to LVO who also are eligible for MT. The sICH risk increase in severe WMH has been hypothesized to result from complex processes involving chronic endothelial dysfunction in patients with severe small vessel disease, worsened by acute blood brain-barrier disruption, as well as matrix metalloproteinase upregulation responsible for modifications of cerebral tissue response to thrombolysis in the acute phase of AIS, potentially promoting hemorrhagic transformations.3,21 We did not find an association between WMH and sICH, despite the fact that 3 of 4 patients were treated with IVT before MT in our pooled sample. Of note, patients treated with tPA in our sample experienced a more favorable outcome, likely explained by shorter delays from onset and a trend toward more frequent recanalization. Possible explanations might include inherent selection bias in our cohorts, which included only patients eligible and who received MT for AIS due to LVO. Patients who receive MT experience earlier and more frequent arterial recanalization8 and hence reductions in final infarct core volumes, a major risk factor for sICH, especially after IVT.22 The modern nature of our sample with current revascularization techniques may also explain the absence of association with sICH in patients likely recanalized sooner than in earlier populations with inhomogeneous indications for MT. This hypothesis is further supported by the fact that IVT was associated with a strong trend toward more frequent recanalization (78% vs 70%, p = 0.07). A previous report4 found higher odds of hemorrhagic transformation in patients with moderate to severe WMH and treated with MT, but those authors did not assess the rate of sICH among hemorrhagic transformations, limiting comparability with our findings. Furthermore, patients were included before 2008, at a time when MT was not standard of care or performed with current devices, raising concerns for generalizability in patients treated after MT-related practice guidelines in 2015. Finally, the authors used a minimal Thrombolysis in Myocardial Infarction score of 2 (partial recanalization) to define technical success, a scoring system that is less accurate than the mTICI score most commonly used for MT, raising the possibility that patients experienced important infarct growth, mirroring populations of patients treated with IVT alone, and associations with more frequent sICH in patients with severe leukoaraiosis. Furthermore, none of the recent reports using CT or MRI to assess white matter found an association with increased sICH after MT.6,9,23 Together, our results suggest that in modern cohorts of patients with AIS-LVO treated with current devices, WMH burden is not associated with increased risks of sICH.
A complementary result is the absence of differences in the rate of technical success (e.g., recanalization) in patients with or without severe WMH in this study. Patients with tortuous vessels are known to have risk factors similar to those with cerebral microvascular insult, including aging, diabetes mellitus, hypertension, and smoking, and both macrovascular and microvascular modifications are known to coexist in the elderly.24 WMHs could therefore be an indirect indicator of larger vessels atherosclerosis and forebode a complex aortic and cervical access for MT. Leptomeningeal collateral status is also known to play a key role with regard to recanalization in patients with LVO, and some have hypothesized that chronic alterations of microvascular units due to small vessel disease might influence collateral function or development, with inconclusive results thus far.9,25 We did not find an association between WMH and recanalization, suggesting that if it exists, it is likely of limited pertinence with regard to clinical benefit derived from MT. These results taken together demonstrate no decrease in technical success of MT in patients with severe WMHs.
Another key result of this study is the association between WMH burden and worse functional outcome, which was not unexpected. This association has indeed been described in several cohorts of patients with AIS2,11,23,26 but never, to the best of our knowledge, in patients with AIS due to LVO selected for MT with MRI. Cerebral small vessel disease MRI markers, including WMH, have consistently been shown to strongly influence outcome in patients with ischemic stroke, hypothetically due to their cumulative impact on brain networks efficiency27,28 and global modifications in white matter structural integrity,29 limiting recovery potential, but also by their associations with recurrence risk and independent higher susceptibility to ischemia.30,31
Our results add to the current literature and confirm an association that had previously been demonstrated, mostly with noncontrast CT scales, but in patients selected for MT at a time when it was not standard of care and when devices were of earlier generations.1,11,23,32 A recent analysis11 found that patients treated by MT with most severe leukoaraiosis burden (38 of 251, 15%, as per the visual Van Swieten CT scale) demonstrated more severe clinical evolution (aOR 0.27, 95% CI 0.10 to 0.77 for favorable outcome) after adjustment for age, baseline clinical severity, and procedural success. To date, however, no study has reported such an association when MRI is used to quantify white matter anomalies. Another study,10 for instance, in a cohort of 56 patients, using FLAIR imaging and an approach similar to that used in the current analysis, did not show an association between WMH volumes and functional outcomes. A potential explanation for previously inconclusive results is that CT is known to be less sensitive to chronic white matter changes than magnetic resonance–FLAIR sequences, notably with a decreased sensitivity for patients with mild leukoaraisosis.33 A floor effect, by which CT would classify those patients with low but present white matter changes using FLAIR as being unscathed with visual CT rating scales, would strongly increase the sample size needed to demonstrate an effect using magnetic resonance–assessed WMH volumes due to the more stretched linear distribution of WMH volumes and a lowered threshold for white matter changes detection. In our study, for instance, WMH severity was mild with a median WMH volume of 2.94 (interquartile range 0.14–9.1) cm3.34 In our sample, in a fully adjusted model, patients in the lowest quartile of WMH volumes (vs highest) demonstrated the strongest association with decreased rates of unfavorable outcomes (aOR 0.42, 95% CI 0.21–0.84, p = 0.001), further reinforcing the above assertion.
We acknowledge the following limitations. First, we did not differentiate between deep and periventricular WMH, precluding an analysis of the influence of either component on functional outcome or sICH risk. Similarly, we did not adjust our analyses for other MRI markers of small vessel disease, including cerebral microbleeds, lacunes, or brain atrophy. Furthermore, our results are derived from multiple cohorts, including the MT arm of the THRACE trial with patients recruited before 2015, and was designed retrospectively, with an inherent yet undemonstrated cohort effect. Procedural and follow-up imaging and treatment delays were prospectively recorded at each center, but no core laboratory reviewed imaging data beyond baseline MR findings and WMH segmentation. As a consequence, there was no normalization for head size, a known confounder of WMH effect. Strengths include a large sample of well-phenotyped patients with homogeneous and current indication for MT, converging and stable statistical analyses on WMH pertinence in AIS-LVO, and novelty in this subset of patients with AIS. Another key strength of this cohort is that it is derived from centers using MRI as a first-line imaging selection tool for MT, limiting selection biases for this study.
Our study provides evidence that patients with AIS due to LVO with an important WMH burden are at higher risk for poor functional outcome after MT but demonstrates similar rates of sICH and procedural success. However, because 27% of patients with severe WMH in our sample experienced favorable outcome at 3 months and because no pragmatic trial would randomize patients for MT vs medical management on the basis of WMH burden, these data represent a possibly definite argument that WMH burden, while being associated with poorer outcomes, is not a good argument to deny MT.
Glossary
- AIS
acute ischemic stroke
- aOR
adjusted odds ratio
- ASPECT
Alberta Stroke Program Early CT
- CI
confidence interval
- DWI
diffusion-weighted imaging
- ECASS II
European Cooperative Acute Stroke Study 2
- FLAIR
fluid-attenuated inversion recovery
- IVT
IV thrombolysis
- LVO
large vessel occlusion
- mRS
modified Rankin Scale
- MT
mechanical thrombectomy
- mTICI
modified Treatment in Cerebral Ischemia
- NIHSS
NIH Stroke Scale
- sICH
symptomatic intracerebral hemorrhage
- THRACE
Thrombectomie des Artères Cérébrales
- tPA
tissue plasminogen activator
- WMH
white matter hyperintensity
Appendix. Authors
Footnotes
Editorial, page 691
CME Course: NPub.org/cmelist
Study funding
The THRACE trial received funding from the French Ministry for Health.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Henninger N, Lin E, Baker SP, Wakhloo AK, Takhtani D, Moonis M. Leukoaraiosis predicts poor 90-day outcome after acute large cerebral artery occlusion. Cerebrovasc Dis Basel Switz 2012;33:525–531. [DOI] [PubMed] [Google Scholar]
- 2.Arsava EM, Rahman R, Rosand J, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology 2009;72:1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charidimou A, Pasi M, Fiorelli M, et al. Leukoaraiosis, cerebral hemorrhage, and outcome after intravenous thrombolysis for acute ischemic stroke: a meta-analysis (v1). Stroke 2016;47:2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi ZS, Loh Y, Liebeskind DS, et al. Leukoaraiosis predicts parenchymal hematoma after mechanical thrombectomy in acute ischemic stroke. Stroke 2012;43:1806–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raychev R, Jahan R, Liebeskind D, Clark W, Nogueira RG, Saver J. Determinants of intracranial hemorrhage occurrence and outcome after neurothrombectomy therapy: insights from the Solitaire FR With Intention for Thrombectomy randomized trial. AJNR Am J Neuroradiol 2015;36:2303–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang S, Fei A, Peng Y, et al. Predictors of outcome and hemorrhage in patients undergoing endovascular therapy with Solitaire stent for acute ischemic stroke. PLoS One 2015;10:e0144452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016;15:1138–1147. [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet Lond Engl 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 9.Giurgiutiu D-V, Yoo AJ, Fitzpatrick K, et al. Severity of leukoaraiosis, leptomeningeal collaterals, and clinical outcomes after intra-arterial therapy in patients with acute ischemic stroke. J Neurointerventional Surg 2015;7:326–330. [DOI] [PubMed] [Google Scholar]
- 10.Atchaneeyasakul K, Leslie-Mazwi T, Donahue K, Giese AK, Rost NS. White matter hyperintensity volume and outcome of mechanical thrombectomy with stentriever in acute ischemic stroke. Stroke 2017;48:2892–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y, Zi W, Wan Y, et al. Leukoaraiosis severity and outcomes after mechanical thrombectomy with stent-retriever devices in acute ischemic stroke. J Neurointerventional Surg 2019;11:137–140. [DOI] [PubMed] [Google Scholar]
- 12.Rost NS, Fitzpatrick K, Biffi A, et al. White matter hyperintensity burden and susceptibility to cerebral ischemia. Stroke J Cereb Circ 2010;41:2807–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arsava EM, Bayrlee A, Vangel M, et al. Severity of leukoaraiosis determines clinical phenotype after brain infarction. Neurology 2011;77:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busl KM, Nogueira RG, Yoo AJ, Hirsch JA, Schwamm LH, Rost NS. Prestroke dementia is associated with poor outcomes after reperfusion therapy among elderly stroke patients. J Stroke Cerebrovasc Dis 2013;22:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra RV, Leslie-Mazwi TM, Oh DC, et al. Elderly patients are at higher risk for poor outcomes after intra-arterial therapy. Stroke 2012;43:2356–2361. [DOI] [PubMed] [Google Scholar]
- 16.Rost NS, Rahman RM, Biffi A, et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology 2010;75:1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology 2006;66:23–29. [DOI] [PubMed] [Google Scholar]
- 18.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Yan S, Xu M, Zhong G, Liebeskind DS, Lou M. More extensive white matter hyperintensity is linked with higher risk of remote intracerebral hemorrhage after intravenous thrombolysis. Eur J Neurol 2018;25:380–e15. [DOI] [PubMed] [Google Scholar]
- 21.Montaner J, Alvarez-Sabín J, Molina CA, et al. Matrix metalloproteinase expression is related to hemorrhagic transformation after cardioembolic stroke. Stroke 2001;32:2762–2767. [DOI] [PubMed] [Google Scholar]
- 22.Marsh EB, Llinas RH, Schneider ALC, et al. Predicting hemorrhagic transformation of acute ischemic stroke. medicine (Baltimore) [online serial]. 2016;95. Accessed at: ncbi.nlm.nih.gov/pmc/articles/PMC4718251/. Accessed October 18, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Puri AS, Khan MA, Goddeau RP, Henninger N. Leukoaraiosis predicts a poor 90-day outcome after endovascular stroke therapy. Am J Neuroradiol 2014;35:2070–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boulouis G, Charidimou A, Auriel E, et al. Intracranial atherosclerosis and cerebral small vessel disease in intracerebral hemorrhage patients. J Neurol Sci 2016;369:324–329. [DOI] [PubMed] [Google Scholar]
- 25.Sanossian N, Ovbiagele B, Saver JL, et al. Leukoaraiosis and collaterals in acute ischemic stroke. J Neuroimaging 2011;21:232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu W-S, Woo SH, Schellingerhout D, et al. Stroke outcomes are worse with larger leukoaraiosis volumes. Brain 2017:140:158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence AJ, Chung AW, Morris RG, Markus HS, Barrick TR. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology 2014;83:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bournonville C, Hénon H, Dondaine T, et al. Identification of a specific functional network altered in poststroke cognitive impairment. Neurology 2018;90:e1879–e1888. [DOI] [PubMed] [Google Scholar]
- 29.Etherton MR, Wu O, Cougo P, et al. Integrity of normal-appearing white matter and functional outcomes after acute ischemic stroke. Neurology 2017;88:1701–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau KK, Li L, Schulz U, et al. Total small vessel disease score and risk of recurrent stroke: validation in 2 large cohorts. Neurology 2017;88:2260–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz UG, Grüter BE, Briley D, Rothwell PM. Leukoaraiosis and increased cerebral susceptibility to ischemia: lack of confounding by carotid disease. J Am Heart Assoc 2013;2:e000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilberti N, Gamba M, Premi E, et al. Leukoaraiosis is a predictor of futile recanalization in acute ischemic stroke. J Neurol 2017;264:448–452. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson KJ, Cvoro V, MacLullich AMJ, et al. Visual rating scales of white matter hyperintensities and atrophy: comparison of computed tomography and magnetic resonance imaging. J Stroke Cerebrovasc Dis 2018;27:1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rost NS, Sadaghiani S, Biffi A, et al. Setting a gold standard for quantification of leukoaraiosis burden in patients with ischemic stroke: the Atherosclerosis Risk in Communities Study. J Neurosci Methods 2014;221:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data of this cohort can be made available on request to the corresponding author and after clearance by the local ethics committee.