Abstract
Background:
The Chinese Great Famine (CGF) caused widespread starvation in 1959–61. Its long-term association with depressive symptoms has not been studied.
Aims:
To estimate the burden of depressive symptoms and the association of famine exposure with depressive symptoms.
Methods:
The China Health and Retirement Longitudinal Study is a nationwide representative survey among 17,708 Chinese adults aged ≥45. Propensity score matching and modified Poisson regression were used to evaluate association between self-reported famine exposure in early life and depressive symptoms among the overall participants. Such associations were also assessed by developmental stages using the modified Poisson regression and logistic regression.
Results:
The prevalence of depressive symptoms was 26.19% (95% confidence interval [CI]: 25.11%-27.27%) in 2011. As defined by loss of family members due to starvation, 11.60% (95% CI: 10.08%-13.12%) of this population experienced severe famine. When compared to participants without starvation, those experienced severe famine during fetal, mid-childhood, young-teen, and early adulthood stages had 1.87 (95% CI: 1.36–2.55), 1.54 (95% CI: 1.23–1.94), 1.47 (95% CI: 1.09–2.00), and 1.77 (95% CI: 1.42–2.21) times higher odds to have depressive symptoms in late adulthood, respectively. The first two trimesters of pregnancy were critical time window during the fetal stage that severe famine had stronger association with depressive symptoms. Famine during the infant, toddler, preschool, or teenager stages was not associated with depressive symptoms. Overall, famine contributed to 13.6% of the depressive symptoms burden in this population.
Conclusions:
The CGF contributed substantially to the burden of depressive symptoms in China.
Keywords: Chinese Great Famine, Depressive symptoms, Middle-aged and older Chinese adult, National survey
Introduction
China has a heavy burden of mental health problems such as schizophrenia and depression (1). The total burden of mental, neurological, and substance use disorders account for 30% of the total disability-adjusted life years (DALYs) (1). Depression comprises the leading component of such mental health disorders in China (1). However, the current estimates of depression prevalence among adults aged 45 years and older are based on regional surveys (1). Large nationally representative surveys targeting at middle-aged and older Chinese adults are needed to accurately estimate the burden of depression and to assist in the better allocation of health care resources in China (1). Many factors have been attributed to the striking burden of mental health disorders in China, such as the one child per family policy implemented in 1980s, unique cultural and social contexts, and dramatic social reforms (1). There is evidence that prenatal exposure to the Chinese Great Famine of 1959 – 1961 contributes to the high rates of adult schizophrenia (2). However, the impact of the Chinese Great Famine on depression has not been documented, although early life nutrition deprivation is an important risk factor for depression (3). The Dutch famine study demonstrated that prenatal exposure to six months’ food shortage increased risk of depression in adult offspring (4, 5). The 1959–1961 Chinese Great Famine lasted for 3 years and affected the entire country (6). It provides an unparalleled opportunity to study the impact of long-term extreme food shortage during different developmental stages on depression risk in late life. Analysis of a nationally representative cohort will improve our understanding of depression risk in a number of ways. We can identify critical time windows for depression prevention, uncover novel mechanisms of depression development, and help explain the heavy burden of mental health disorders in China. Therefore, the current study aims to estimate the prevalence of depressive symptoms and to evaluate the impact of famine exposure overall and during different developmental stages on depressive symptoms in late adulthood among participants of the China Health and Retirement Longitudinal Study (CHARLS).
Methods
Study Population and Design
The CHARLS is a biannual longitudinal survey of a nationally representative sample of Chinese adults aged 45 years and older (7). It is designed to describe the dynamics of retirement and its impact on health, health insurance, and economic well-being in China. The first wave of data was collected between June 2011 and March 2012 by trained interviewers during a face-to-face household encounter. The data consists of comprehensive and detailed information including socioeconomic indicators, biomedical measurements, as well as health status and functional indicators (7).
Detailed sampling design of the CHARLS is available elsewhere (8, 9). Briefly, the survey used a four-stage, stratified, cluster probability sampling design (7). The primary sampling unit (PSU) was administrative villages (cun) in rural areas and neighborhoods (shequ) in urban areas. In the first stage, a random sample of 150 counties was selected to represent the socioeconomic and geographic pattern of all counties. In the second stage, three PSUs were selected in each county with a probability proportional to their population size. In the third stage, a random sample of 24 households was selected among those with residents aged 45 or older in each selected PSU. Finally, for a selected household, one resident was randomly selected as participants of the survey. If spouse of the selected resident was aged 45 years or older, the spouse was also included in the survey. Response rate among eligible households was 80.51% (10). The response rate was higher among rural households than urban households (94.15% vs. 68.63%) (10). Overall, a total of 17,708 individuals within 10,257 households were interviewed in the baseline survey (7, 10). The responses of these participants were used in the current analyses.
The Chinese Great Famine occurred between 1959 and 1961. For purposes of this analysis, we selected the start date as January 1, 1959 and end date as December 31, 1961. We then used this time span to divide the CHARLS participants into 8 age-exposure cohorts based on their developmental stages when experiencing the Chinese Great Famine: Fetal, infant, toddler, preschool, mid-childhood, young teenage, teenage, and adulthood cohorts. Included as the fetal cohort (born or conceived in the famine) were 996 participants born between January 1959 and September 1962. There were 445 participants born in 1958 as the infant cohort (aged 0–1 when famine occurred). Another 1,108 participants born in 1956–57 were included as the toddler cohort (aged 1–2 when famine occurred). Next, there were 1,241 born in 1954–55 as the preschool cohort (aged 3–5 when famine occurred). There were 3,217 born in 1948–53 as the mid-childhood cohort (aged 6–11 when famine occurred). The young teenage cohort (aged 12–14 when famine occurred) included 1,143 born in 1945–47. There were 887 born in 1942–1944 as the teenage cohort (aged 15–17 when famine occurred). Finally, there were 2,486 born before 1941 as the early adulthood cohort (aged 18–30 when famine occurred).
Exposure to the Chinese Great Famine
In the third wave survey (2014–2015), exposure to the Chinese Great Famine was measured using three items:
Did you and your family experience starvation in 1958–1962?
During those days, did you and your family move away from the famine-stricken area?
During those days, did any of your family members starved to death?
By combining responses to these questions, participants were categorized as exposed to severe famine if they reported death of family member(s) to starvation, or moderate famine if they reported starvation or moving away from famine-stricken areas, or no famine if they reported having no starvation. For all analyses, the reference group consisted of persons responding no starvation.
Depressive symptoms
The Center for Epidemiological Studies Depression Scale (CES-D) short form was used to measure depressive symptoms in the baseline survey (2011–2012) of the CHARLS (11). The CES-D short form contains the following ten items: 1) being bothered by things that do not usually bother, 2) having trouble concentrating, 3) feeling depressed, 4) feeling that everything you did was an effort, 5) feeling hopeful about the future, 6) feeling fearful, 7) being sleepless, 8) feeling happy, 9) feeling lonely, and 10) inability to “get going”. For each item, respondents reported the frequency of occurrence for the item during the past week. To create the CES-D summary, each item was scored on a four-point scale ranging from 0 (rarely or none of the time) to 3 (most or all of the time). Before analysis, item 5 and 8 were reversely scored to match the polarity of the other items. The summed range of CES-D item scores varied from 0 to 30 with higher scores indicating higher levels of depressive symptoms (11). The CES-D short form has been validated among a subsample of 742 CHARLS participants aged 60 years and older showing adequate psychometric properties (12). In the validation study, a two-factor model fitted the best. The comparative fit index (CFI) and Tucker-Lewis fit index (TLI) for the two-factor model were 0.99 and 0.98, respectively, and the completely standardized factor loadings were of 0.30 and above (12). To be consistent with the validation study, a score of 12 or higher was used to define depressive symptoms in the current analysis (12).
Covariates in the baseline survey
Demographic and social economic variables, including age, gender, education level, childhood living areas (rural vs. urban), current living areas (rural vs. urban), marital status, self-perceived family income level, employment status, and individual income sources, were measured by self-report. Education categories included “illiterate”, “less than elementary school”, “elementary school”, “middle school”, and “high school or above”. The question “Where did you mainly live before you were 16 years old? Is it in village or city/town?” formed the basis for categories of childhood living areas. Marital status included the following categories: currently married (including “married and live together” and “married but temporarily separated”) and currently not married (including “separated”, “divorced”, “widowed”, and “never married”). Self-perceived family income categories included “average and above”, “relatively poor”, and “poor”. Respondents were either “currently employed” or “currently not employed”. Finally, individual income sources included three categories “wage, bonus and others”, “others”, and “none”.
Lifestyle and personal health behaviors, including drinking and smoking, were collected using a standardized questionnaire (7). There were four drinking status categories: currently regular drinkers, currently occasional drinkers, former drinker, and never drinkers. To distinguish between regular and occasional drinkers, we calculated the average standard glasses of alcoholic beverage per week. One standard glass of alcoholic beverage was equivalent to 15 grams of alcohol, corresponding to approximately 500 ml of beer, 150 ml of wine, and 50 ml of liquor. A summary amount of alcohol consumed was calculated by combining drinking frequency and volume per week. Currently regular drinkers consumed more than two standard glasses of alcoholic beverage per week. Those having two or fewer standard glasses of alcoholic beverage per week were currently occasional drinkers. Smoking categories included current smokers, former smokers and never smokers.
Biomedical measures included blood pressure, body weight, and height. Blood pressure was measured three times for each participant by trained observers according to standard protocol after the participant rested for 30 minutes (10). We used the mean of three measures for analyses. With participants in light indoor clothes, body weight and height were measured using a health meter (Omron™ HN-286) and a stadiometer (Seca™ 213), respectively. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
Comorbidities were measured by self-reported responses to the question “Have you EVER been told by a doctor or other health professional that you have xx?” Choices for chronic conditions included hypertension, dyslipidemia, diabetes, cancer or malignant tumor (excluding minor skin cancers), chronic lung diseases (except for tumors or cancer), liver diseases (except for fatty liver disease, tumors or cancer), cardiovascular diseases (including heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems), stoke, kidney disease (except for cancer or tumor), stomach or other gastrointestinal diseases (except for tumor or cancer), arthritis, and asthma.
Statistical Analysis
Prevalence of depressive symptoms and severe famine
When estimating prevalence and their corresponding standard errors (SEs), we took into consideration the complex survey design and nonresponse rate in both estimates. We also created 5-year age groups for purposes of analyses. The SAS PROC SURVEYFREQ procedure generated the overall and gender specific prevalence of depressive symptoms and severe famine exposure among all the participants and for the 5-year age groups.
Associations between famine exposure and depressive symptoms
Continuous variables were shown as means [standard deviations (SDs)] and categorical variables were shown as percentages. Variables of interest included demographics factors, social economic status, self-reported diseases, biomedical measures, and health related behaviors. Bivariate association analyses were conducted between famine severity levels and these variables among the overall participants using Chi-square tests for categorical variables and one-way analysis of variance (ANOVA) for continuous variables.
Multinomial propensity score matching was used to generate weights which estimated the average treatment effect by balancing the included covariates across multiple groups and allowed pairwise comparisons among the three famine severity groups (13–15). The weights were the reciprocal of the probability that a study participant was exposed to the corresponding famine severity based on covariates included in the matching. We generated two sets of weights. The first weight was generated using age, sex, education, and childhood living areas, and the second weight using all covariates mentioned above. After weighting, the covariate distributions in all the three famine severity groups were more similar compared to the original sample. Since the prevalence of depressive symptoms was more than 10% in the study population, odds ratio from a logistic regression model overestimates risk ratio (RR) (16). Therefore, we used the modified Poisson regression models to estimate RRs and the corresponding 95% confidence intervals (CIs) associated with moderate and severe famine exposure, respectively (16), and incorporated the calculated weights into the Poisson models.
To identify important time windows that famine had stronger impact on late adulthood depressive symptoms, we further evaluated associations between moderate and severe famine exposure and depressive symptoms in each age-exposure cohort. We built two models in each age-exposure cohort using the modified Poisson regression. Model 1 adjusted for demographic variables, including age, gender, education, and childhood living areas. Model 2 was the fully adjusted model with all variables controlled. The modified Poisson regression did not converge for Model 2; therefore, Model 2 was a multivariate logistic regression. Odds ratios (OR) and 95% CI were presented for variables in the logistic regression models.
Impact of severe famine exposure at different fetal ages
Food shortage gradually occurred in the end of 1958. Therefore, we used January 1, 1959 as the start-point of the Chinese Great Famine. To identify critical periods of exposure during pregnancy, we tested the association of severe famine with subsequent depressive symptoms by trimesters among participants born in 1959. Individuals born in the first 4 months of 1959 was treated as exposed to severe famine in the third trimester, those born in May, June, and July as exposed since the second trimester, and those born between August and December as exposed since the first trimester. To evaluate whether longer duration of severe famine exposure during the fetal stage had greater impact on depressive symptoms, we further calculated associations of severe famine with depressive symptoms according to length of severe famine exposure during the fetal stage, from ≤3 months to ≤10 months in 1959. Due to limited number of participants born in 1959, we only adjusted for age, sex, and childhood living areas in these analyses using logistic regression models.
We also evaluated associations of severe famine exposed during the entire pregnancy and at least the first 3 months of pregnancy, respectively, with depressive symptoms in late adulthood. To assess the joint effect of parents’ long-term exposure to severe famine and fetus’ exposure to famine during the first 3 months, we tested famine-depressive symptoms associations among participants who were conceived during the last 3 months of the Chinese Great Famine. For all these analyses, we built the same two models as in our main analyses.
Percentage population attributable risk of depressive symptoms due to the Chinese Great Famine
We estimated the prevalence of severe and moderate famine exposure, respectively, among the overall participants using the SAS PROC SURVEYFREQ procedure taking into account the complex study design and response rate. The percentage population attributable risk of depressive symptoms due to the Chinese Great Famine in the middle-aged and older Chinese population was estimated using the formula as seen below (17),
, where P1 and P2 are prevalence of moderate and severe famine exposure, respectively, and RR1 and RR2 are the corresponding RRs estimated from the modified Poisson regression models weighted by propensity scores matching on all covariates.
We used SAS 9.3 (SAS Institute Inc., Cary, North Carolina) to perform data analyses. All P values were two-sided, and P<0.05 was considered significant.
Ethics Statement
The CHARLS study data is publicly available and open to researchers all over the world. The current study is secondary analyses to the de-identified CHARLS study data. The Institutional Review Boards at the University of Georgia granted the current study exemption from review.
Results
Characteristics of the CHARLS participants are shown by levels of famine exposure (Supplementary Table S1), and variables that significantly differed by famine severity levels are demonstrated in Table 1. In the overall cohort, participants were more likely to be female and had an average age of 61.5 years old. Most of the participants lived in rural areas (80.6%) and were married (87.8%). Only 11.4% of the participants had high school or above education. The proportion of current smokers was 29.8%, and 16.0% were currently regular drinkers. Participants had an average normal weight (mean BMI=23.3 kg/m2), and over 60% reported having at least one chronic conditions.
Table 1.
Characteristics significantly differed by famine status among the CHARLS participants in the 2011 baseline survey
| Overall (n=13,005) |
None (n=2,313) |
Moderate famine (n=9,123) |
Severe Famine (n=1,569) |
P | |
|---|---|---|---|---|---|
| Depression, % | 28.3 | 22.7 | 28.5 | 37.8 | <0.001 |
| Male, % | 48.0 | 43.1 | 49.9 | 47.0 | <0.001 |
| Age, years, mean (SD) | 61.5 (8.5) | 62.1 (9.4) | 61.5 (8.1) | 62.8 (8.0) | <0.001 |
| Living in rural areas, % | |||||
| During childhood | 91.2 | ||||
| Currently | 80.6 | 74.3 | 81.0 | 86.0 | <0.001 |
| Currently married, % | 87.8 | 85.9 | 88.4 | 85.7 | <0.001 |
| Education, % | <0.001 | ||||
| Illiterate | 27.9 | 27.2 | 27.2 | 37.1 | |
| <elementary school | 18.4 | 17.5 | 18.2 | 22.8 | |
| Elementary school | 21.9 | 19.5 | 23.0 | 19.2 | |
| Middle school | 20.5 | 21.8 | 20.6 | 14.0 | |
| High school or above | 11.4 | 14.0 | 11.2 | 6.8 | |
| Self-reported family income, % | <0.001 | ||||
| Average and above | 55.6 | 59.8 | 55.3 | 52.4 | |
| Relatively poor | 31.7 | 28.5 | 32.0 | 34.3 | |
| Poor | 12.7 | 11.7 | 12.7 | 13.3 | |
| Individual income sources, % | <0.001 | ||||
| Wage and bonus | 16.8 | 18.0 | 16.4 | 11.5 | |
| Others | 20.5 | 23.2 | 21.0 | 22.1 | |
| None | 62.7 | 58.8 | 62.6 | 66.4 | |
| Currently employed, % | 70.7 | 65.6 | 70.8 | 73.3 | <0.001 |
| Drinking status, % | <0.001 | ||||
| Never | 73.4 | 77.2 | 72.3 | 72.2 | |
| Former | 6.3 | 5.3 | 6.2 | 8.0 | |
| Occasional drinker | 4.3 | 3.6 | 4.2 | 4.7 | |
| Regular drinker | 16.0 | 13.9 | 17.2 | 15.2 | |
| Smoking status, % | <0.001 | ||||
| Never | 61.9 | 66.3 | 60.0 | 62.0 | |
| Former | 8.3 | 7.3 | 8.7 | 8.4 | |
| Current smoker | 29.8 | 26.4 | 31.3 | 29.6 | |
| BMI, kg/m2, mean (SD) | 23.3 (4.0) | 23.3 (3.9) | 23.4 (4.0) | 23.2 (4.1) | 0.006 |
| DBP, mmHg, mean (SD) | 75.8 (12.1) | 75.4 (12.1) | 76.0 (12.1) | 74.8 (12.1) | 0.003 |
| Self-reported chronic conditions, % | |||||
| Lung disease | 10.2 | 10.2 | 10.1 | 13.4 | <0.001 |
| Kidney disease | 6.4 | 4.9 | 6.8 | 7.0 | 0.003 |
| Digestive disease | 22.8 | 19.6 | 22.8 | 28.2 | <0.001 |
| Arthritis | 34.0 | 29.2 | 34.2 | 43.7 | <0.001 |
Note. Severe Famine = ‘One or more member of the family died from starvation between 1959 and 1961’. Depressive symptoms = CESD10 >= 12. BMI=body mass index in kg/m2. DBP=diastolic blood pressure in mmHg. SBP=systolic blood pressure in mmHg. SD=standard deviation.
Prevalence of depressive symptoms
There were 15,541 participants (87.8% of the CHARLS cohort) with no missing measurement of CESD-10 scores. As shown in Table 2 and supplementary Figure S1, the overall prevalence of depressive symptoms was 26.2% (95% CI: 25.1%-27.3%), and there was significant gender difference (males vs females: 20.3% vs 31.5%, P < 0.001). Respondents aged 80 years and older had the highest prevalence of depressive symptoms, with 30.8% (95% CI: 26.4%-35.2%) of men and 40.5% (95% CI: 34.4%-46.6%) of women having depressive symptoms. In addition, prevalence rates were greater among rural residents compared to urban residents for both men and women.
Table 2.
Prevalence of depressive symptoms (CHARLS, 2011) and exposure to severe famine (CHARLS, 2014) by gender and living areas
| Overall | Rural | Urban | |
|---|---|---|---|
| Depressive symptoms | |||
| n | 15,541 | 11,870 | 3,468 |
| Total | 26.2 (25.1 – 27.3) | 30.5 (29.2 – 31.8) | 17.7 (16.0 – 19.3) |
| Male | 20.3 (19.2 – 21.4) | 24.1 (22.7 – 25.4) | 12.5 (11.0 – 14.1) |
| Female | 31.5 (30.0 – 32.9) | 36.4 (34.8 – 38.1) | 22.0 (19.5 – 24.4) |
| Severe Famine | |||
| n | 12,910 | 10,372 | 2,519 |
| Total | 11.6 (10.1 – 13.1) | 12.5 (11.5 – 13.5) | 9.2 (4.5 – 13.9) |
| Male | 11.3 (9.5 – 13.0) | 12.1 (10.8 – 13.3) | 9.1 (3.8 – 14.4) |
| Female | 11.9 (10.4 – 13.4) | 12.9 (11.7 – 14.1) | 9.3 (5.0 – 13.5) |
Note. Prevalence is presented as percentage (95% confidence interval). CHARLS = Chinese Health and Retirement Longitudinal Study. Depressive symptom was defined as CESD10>= 12, and Severe Famine as one or more family members died from starvation in 1959–61.
Prevalence of exposure to severe famine
There were 12,910 participants (83.1%) in the third wave survey in 2014–15, which contained items of exposure to the Chinese Great Famine. As shown in Tables 2 and supplementary Table S2, the overall prevalence of exposure to severe famine was 11.6% (95% CI: 10.1%-13.1%). Participants aged 65–70 years old had the highest prevalence of exposure to severe famine (16.6%, 95% CI: 12.0%-21.2%). There were significant gender differences in three age groups: Male participants had higher prevalence of severe famine exposure in the 65–70 age groups, while female participants had much higher prevalence in the 50–55 and 70–75 age groups.
Associations between famine and depressive symptoms
As shown inTable 1, the prevalence of depressive symptoms increased with the severity of famine (P<0.001). After propensity score matching on age, gender, education level, and childhood living areas, participants exposed to moderate and severe famine were 1.22 (95% CI: 1.17–1.27) and 1.48 (95% CI: 1.42–1.54) times more likely to have depressive symptoms in late adulthood. When further matching on all covariates, individuals who had moderate and severe famine exposure were 1.17 (95% CI: 1.08–1.29) and 1.34 (95% CI: 1.20–1.51) times more likely to have depressive symptoms.
Table 3 and supplementary Figure S2 shows the results of analyses by developmental stages. After adjustment for age, sex, education level, and childhood living areas, modified Poisson regression analyses demonstrate that participants exposed to moderate and severe famine during the fetal stage were 1.16 (95% CI: 0.90–1.49) and 1.87 (95% CI: 1.36–2.55) times more likely to have depressive symptoms in late adulthood. There was significant linear trend of depressive symptoms prevalence across severities of famine (P=0.002) in the fetal cohort. Significant linear trends were also identified in mid-childhood (P<0.001), young teenage (P=0.01), and early adulthood (P<0.001) cohorts. In these cohorts, moderate and severe famine exposure were associated with 1.32 (95% CI: 1.08–1.61) and 1.54 (95% CI: 1.23–1.94), 1.17 (95% CI: 0.89–1.52) and 1.47 (95% CI: 1.09–2.00), and 1.37 (95% CI: 1.14–1.66) and 1.77 (95% CI: 1.42–2.21) times higher likelihood to have depressive symptoms in late adult life, respectively. In the fully adjusted logistic regression models (Table 3 and Table S4), significant linear trends between famine severity and prevalence of depressive symptoms remained for the fetal (P=0.03), mid-childhood (P=0.02), young teenage (P=0.02), and early adulthood cohorts (P<0.001). There was no significant association between famine and depressive symptoms in infant (P=0.76), toddler (P=0.44), preschool (P=0.12), or teenage (P=0.89) cohorts.
Table 3.
Associations of exposure to the Chinese Great Famine with depressive symptoms in late adulthood overall and by developmental stages among participants of the CHARLS study.
| Development Stages (ages when famine occurred) |
Famine Severity |
Age, sex, education, and childhood living area adjusted robust Poisson model |
Fully adjusted model* | ||
|---|---|---|---|---|---|
| RR (95% CI) | P | RR/OR (95% CI) | P | ||
| Overall participants | None | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 |
| (Propensity score | Moderate | 1.22 (1.17–1.27) | 1.17 (1.08–1.29) | ||
| matching analyses) | Severe | 1.48 (1.42–1.54) | 1.34 (1.20–1.51) | ||
| Overall participants | None | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 |
| (non-matching | Moderate | 1.23 (1.13–1.35) | 1.30 (1.12–1.52) | ||
| analyses) | Severe | 1.48 (1.33–1.65) | 1.64 (1.35–2.00) | ||
| Fetal (born or | None | 1.00 (reference) | 0.002 | 1.00 (reference) | 0.03 |
| conceived in famine) | Moderate | 1.16 (0.90–1.49) | 1.03 (0.68–1.58) | ||
| Severe | 1.87 (1.36–2.55) | 2.37 (1.25–4.48) | |||
| Infant | None | 1.00 (reference) | 0.76 | 1.00 (reference) | 0.55 |
| (0–1 year) | Moderate | 1.32 (0.79–2.19) | 2.58 (0.99–6.69) | ||
| Severe | 0.96 (0.41–2.27) | 0.86 (0.18–4.03) | |||
| Toddler | None | 1.00 (reference) | 0.44 | 1.00 (reference) | 0.96 |
| (1–2 years) | Moderate | 1.20 (0.88–1.65) | 0.99 (0.59–1.67) | ||
| Severe | 1.11 (0.69–1.79) | 1.03 (0.47–2.26) | |||
| Preschool | None | 1.00 (reference) | 0.12 | 1.00 (reference) | 0.57 |
| (3–5 years) | Moderate | 1.12 (0.84–1.48) | 1.18 (0.73–1.88) | ||
| Severe | 1.30 (0.93–1.83) | 1.19 (0.64–2.20) | |||
| Mid-childhood | None | 1.00 (reference) | <0.001 | 1.00 (reference) | 0.02 |
| (6–11 years) | Moderate | 1.32 (1.08–1.61) | 1.32 (0.95–1.85) | ||
| Severe | 1.54 (1.23–1.94) | 1.63 (1.10–2.44) | |||
| Young Teen | None | 1.00 (reference) | 0.01 | 1.00 (reference) | 0.02 |
| (12–14 years) | Moderate | 1.17 (0.89–1.52) | 1.56 (0.95–2.58) | ||
| Severe | 1.47 (1.09–2.00) | 2.07 (1.12–3.82) | |||
| Teenagers | None | 1.00 (reference) | 0.89 | 1.00 (reference) | 0.42 |
| (15–17 years) | Moderate | 0.99 (0.75–1.32) | 1.10 (0.62–1.95) | ||
| Severe | 1.02 (0.72–1.44) | 1.30 (0.66–2.58) | |||
| Adult | None | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 |
| (18–30 years) | Moderate | 1.37 (1.14–1.66) | 1.63 (1.17–2.27) | ||
| Severe | 1.77 (1.42–2.21) | 2.20 (1.42–3.41) | |||
Note.CI=confidence interval, OR=odds ratio, RR=risk ratio.
RR was from robust Poisson regression for the overall participants after propensity score matching. All other estimates were ORs from logistic regression. Age, sex, education, childhood living area, current living area, marital status, self-perceived family income level, individual income sources, employment status, smoking, drinking, body mass index, diastolic blood pressure, lung disease, chronic kidney disease, digestive diseases, and arthritis were matched on or controlled for in the models.
Impact of famine at different fetal ages
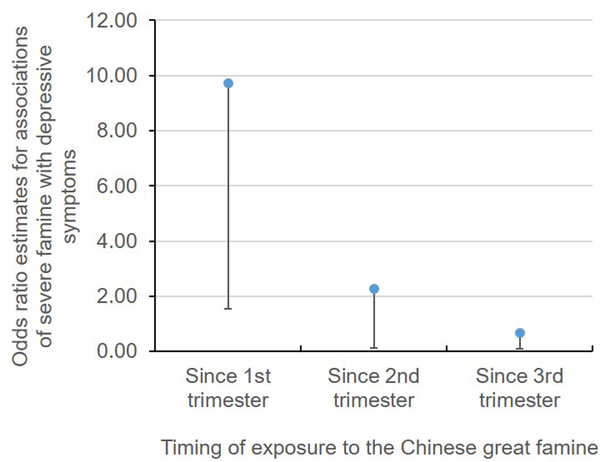
As shown in Figure, exposure to severe famine since the first trimester was associated with 9.7 greater odds of having depressive symptoms in late adulthood. If exposed since the second trimester, severe famine was associated with 2.3 greater odds of having depressive symptoms. Among those exposed in the last trimester, the association of severe famine with depressive symptoms was very weak (OR≈1). In addition, individuals experiencing severe famine for at least the entire first trimester had 2.86 (95% CI: 1.61–5.08) greater odds of getting depressive symptoms in late adulthood among the overall participants. For those who were exposed to severe famine during the entire pregnancy, there was 3.39 (95% CI: 1.65–6.97) greater odds of having depressive symptoms in late adulthood. We further demonstrated that (supplementary Table S3), for individuals conceived in the last 3 months of the Chinese Great Famine, severe famine had even larger effect on depressive symptoms (OR=5.92, 95% CI: 1.59–22.09).
Figure.
Associations of severe famine defined as having family members starved to death during the Chinese Great Famine with depressive symptoms in late adulthood by pregnancy trimesters when the famine occurred. Associations were adjusted for age, sex, education, and living areas (rural vs. urban). Dots represent associations, and the vertical lines represent the lower limit of the 95% confidence intervals for the association estimates.
Population attributable risk due to famine exposure
The prevalence of exposure to moderate famine was 69.2% and exposure to severe famine was 11.6% in the overall sample. Meanwhile, participants who were exposed to moderate famine and severe famine were 1.17 (95% CI: 1.08–1.29) and 1.34 (95% CI: 1.20–1.51) times more likely to develop depressive symptoms in late adulthood in the fully adjusted propensity score matching analyses. Therefore, 13.6% of the overall depressive symptoms cases can be attributable to early life famine exposure in the middle-aged and older Chinese population.
Discussion
In the largest ever study on early life exposure to the Chinese Great Famine and late adulthood prevalence of depressive symptoms in a middle-aged and older Chinese population using individual level data, we identified that exposure to the famine was associated with depressive symptoms in late adulthood. We further revealed that famine experience during fetal, mid-childhood, young teenage and early adulthood was positively associated with depressive symptoms in late adult life, while famine experience during infant, toddler, preschool, and teenager stages was not associated with depressive symptoms in late adulthood. Sensitivity analysis revealed that in the fetal stage, the first and second trimesters, not the third trimester, were critical time windows that famine had stronger association with depressive symptoms in late adulthood. In addition, we demonstrated that 11.6% of the middle-aged and older Chinese population experienced severe famine in 1959–1961, and 26.2% had depressive symptoms. Overall, 13.6% of the depressive symptoms among the middle-aged and older Chinese adults can be attributed to the Chinese Great Famine.
We demonstrated that the first and second trimesters were the key time windows during the fetal stage that exposure to severe famine was strongly associated with depressive symptoms in late adulthood, while famine in the last trimester had very weak association with depressive symptoms. The Dutch famine study has reported that individuals born in or around the 6 months’ Hunger Winter increased risk for depressive symptoms in late adulthood (4, 5). Our study contributes further robust evidence that long-term exposure to famine (3 years) was strongly associated with depressive symptoms in late adulthood in a Chinese population. More importantly, our study was able to demonstrate the first two trimesters, particularly the first trimester, were critical time window that famine exerted effect on depressive symptoms in late adulthood. In addition, our study also identified that famine had the greatest effect on depressive symptoms among individuals who were conceived in the last three months of the Chinese Great Famine. This indicates that long-term maternal and fetal experience of severe famine may have a joint impact on the offspring’s risk for depressive symptoms in late adulthood. Similar to our study, St Clair and colleagues reported that prenatal exposure to the Chinese Great Famine of 1959–61 had higher rate of adult schizophrenia through a regional survey in the Wuhu region of Anhui province, one of the most affected provinces (2). Our study provided additional evidence that famine experience may impact other mental disorders in this population. Fetal stage is critical in human development. In this stage, genome-wide demethylation occurs, and human embryonic develops (18). Famine may have affected the demethylation process (19–21). In addition, methylations in certain regions of the human genome can persist for life long time and may have been involved in the etiology and clinical manifestation of depression (22, 23). However, the role of DNA methylation in famine and depression association is rarely investigated. Further studies in this area may help to find novel mechanisms underlying depression risk.
Besides the fetal stage, our study revealed that famine was also associated with depressive symptoms among those exposed to famine during mid-childhood, young teenage or early adulthood. Interestingly, these periods correspond to recent studies of human brain development in early life. During these ages, structural brain development consists of increased white matter volume and enhanced functional brain development as measured by fMRI (24). The Chinese Great Famine represents extreme stress due to nutrition deprivation. Findings from the current study suggest that nutrition, stress, or the combination of them, are more important in these stages. Intervention strategies aiming at reducing stress or improving nutrition supply during these developmental stages may help to reduce risk for depressive symptoms. Mid-childhood is a key period when crucial shift in cognitive skills occurs (25). It is also the period that children develop skills in learning, reasoning and understanding (25). These skills are essential in social and academic success. Hayden and colleagues showed that meaningful and stable vulnerability to depression occurred as early as in mid-childhood age (26). Furthermore, nutrition has an important role in children’s behavioral outcomes at this stage (27). Our study demonstrated the first evidence that mid-childhood nutrition deprivation has long-term effect on depressive symptoms in late adulthood. Early teen years are marked by rapid changes in physical, cognitive, and emotional functions (28). Stress and the ways in which young teens cope with stress have significant long-term consequences such as depression (28). In addition, stress increased risk of depression in adolescence which in turn predicts a range of mental health disorder in adulthood (29).
It is surprising that exposure to famine during infant, toddler, preschool, and teenager stages were not associated with depressive symptoms in late adulthood. For the infants, toddlers, and preschool children, this may be because psychosocial skills were not developed during these stages (1–5 years old), and with care from their parents, they were less likely to sense stress from the Chinese Great Famine. It is unclear why famine experienced during teenager stage was not associated with depressive symptoms in late adulthood. Future studies are warranted to confirm our finding and delineate potential mechanisms.
Our study also provided accurate estimates of the prevalence of depressive symptoms and severe famine experience among the middle-aged and older population in China. The current prevalence estimate for depressive symptoms is much higher than that of 6% reported in a four-province survey in 2009 (30), but is similar to findings from a meta-analyses by Li and colleagues in which the prevalence of depressive symptoms was estimated to be 23.6% in Chinese older adults (31). A systematic review by Gu and colleagues also reported a similar lifetime prevalence of depressive symptoms of 33% and a 12-month prevalence of 23% (32). The CHARLS is a nationally representative survey conducted in all 28 provinces in China, therefore, our study provided a direct estimate of depressive symptoms burden in middle-aged and older Chinese adults, and indicated that depressive symptoms were a major public health challenge in China. More health care resources should be allocated to address this issue.
Our study has important strengthens. First, it is the largest famine study using a nationwide representative sample in China. Therefore, findings from the current study are generalizable to the entire country. Second, it is the first study on the Chinese Great Famine to use individual reports as famine exposure data. Previous investigations on the Chinese Great Famine were all ecological studies which compared participants from areas of different famine severities. Ecological fallacy in those studies could not be ruled out. Our study has collected famine severity data from individual participants, and ecological fallacy was avoided. Third, we are the first study that focused on famine experience in different birth cohorts, from fetal to early adulthood. Previous famine studies have focused on prenatal exposure to famine, and very few studies have reported famine’s impact on older children and younger adults. This is mainly because famine in other countries was short and in small scale. The Chinese Great Famine lasted for more than 3 years and affected the entire main land China, which provides an unparalleled opportunity to study famine’s impact during different developmental stages on depressive symptoms in late life. Certain limitations should also be acknowledged. Famine experience was based on self-report. For younger cohorts, such as those born during famine, famine experience is an intergenerational story told by family survivors. Therefore, there might be some recall bias on famine experience. However, recall of dramatic life events, such as starving to death or moving to other areas due to famine should be reliable.
In conclusion, 26.2% of the middle-aged and older Chinese adults had depressive symptoms, and 11.6% of this population experienced severe famine during 1959–1961. Famine experienced during the fetal, mid-childhood, young teenage, and early adulthood stages was associated with an increased prevalence of depressive symptoms in late adulthood, which partly explain the high burden of mental disorders in older Chinese adults.
Supplementary Material
Acknowledgment
We thank the Peking University National Center for Economic Research for providing the CHARLS data. Shengxu Li is partly supported by National Institute of General Medical Sciences (1P20GM109036-01A1).
Footnotes
Financial Disclosure
The authors declare no conflict of interest.
Declaration of interest: None.
References:
- 1.Charlson FJ, Baxter AJ, Cheng HG, Shidhaye R, Whiteford HA. The burden of mental, neurological, and substance use disorders in China and India: a systematic analysis of community representative epidemiological studies. Lancet. 2016. [DOI] [PubMed] [Google Scholar]
- 2.St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA. 2005; 294(5): 557–62. [DOI] [PubMed] [Google Scholar]
- 3.Whitaker RC, Phillips SM, Orzol SM. Food insecurity and the risks of depression and anxiety in mothers and behavior problems in their preschool-aged children. Pediatrics. 2006; 118(3): e859–68. [DOI] [PubMed] [Google Scholar]
- 4.de Rooij SR, Painter RC, Phillips DI, Räikkönen K, Schene AH, Roseboom TJ. Self-reported depression and anxiety after prenatal famine exposure: mediation by cardio-metabolic pathology? J Dev Orig Health Dis. 2011; 2(3): 136–43. [DOI] [PubMed] [Google Scholar]
- 5.Stein AD, Pierik FH, Verrips GH, Susser ES, Lumey LH. Maternal exposure to the Dutch famine before conception and during pregnancy: quality of life and depressive symptoms in adult offspring. Epidemiology. 2009; 20(6): 909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Guo C, Nichols C, Chen S, Martorell R. Elevated levels of protein in urine in adulthood after exposure to the Chinese famine of 1959–61 during gestation and the early postnatal period. Int J Epidemiol. 2014; 43(6): 1806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). International journal of epidemiology. 2014; 43(1): 61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Liu T, Sun W, Wu L, Zou ZY, Li C, et al. Prevalence and risk factors of arthritis in a middle-aged and older Chinese population: the China health and retirement longitudinal study. Rheumatology. 2015; 54(4): 697–706. [DOI] [PubMed] [Google Scholar]
- 9.Feng XL, Pang M, Beard J. Health system strengthening and hypertension awareness, treatment and control: data from the China Health and Retirement Longitudinal Study. Bulletin of the World Health Organization. 2014; 92(1): 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Strauss J, Yang G, Giles J, Hu P, Hu Y, et al. China Health and Retirement Longitudinal Study-2011–2012 National Baseline Users’ Guide. Peking University, 2013. [Google Scholar]
- 11.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993; 5(2): 179–93. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Mui AC. Factorial validity of the Center for Epidemiologic Studies Depression Scale short form in older population in China. International Psychogeriatrics. 2014; 26: 49–57. [DOI] [PubMed] [Google Scholar]
- 13.Stuart EA. Matching methods for causal inference: A review and a look forward. Statistical science: a review journal of the Institute of Mathematical Statistics. 2010; 25(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgette L, Griffin BA, McCaffrey D. Propensity scores for multiple treatments: A tutorial for the mnps function in the twang package. R package Rand Corporation. 2017. [Google Scholar]
- 15.McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A Tutorial on Propensity Score Estimation for Multiple Treatments Using Generalized Boosted Models. Statistics in medicine. 2013; 32(19): 3388–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004; 159(7): 702–6. [DOI] [PubMed] [Google Scholar]
- 17.Walter SD. The estimation and interpretation of attributable risk in health research. Biometrics. 1976; 32(4): 829–49. [PubMed] [Google Scholar]
- 18.Guo H, Zhu P, Yan L, Li R, Hu B, Lian Y, et al. The DNA methylation landscape of human early embryos. Nature. 2014; 511(7511): 606–10. [DOI] [PubMed] [Google Scholar]
- 19.Tobi EW, Goeman JJ, Monajemi R, Gu H, Putter H, Zhang Y, et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat Commun. 2014; 5: 5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, et al. DNA methylation differences after exposure to prenatal famine are common and timing-and sex-specific. Hum Mol Genet. 2009; 18(21): 4046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008; 105(44): 17046–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Córdova-Palomera A, Fatjó-Vilas M, Gastó C, Navarro V, Krebs MO, Fañanás L. Genome-wide methylation study on depression: differential methylation and variable methylation in monozygotic twins. Transl Psychiatry. 2015; 5: e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemoda Z, Massart R, Suderman M, Hallett M, Li T, Coote M, et al. Maternal depression is associated with DNA methylation changes in cord blood T lymphocytes and adult hippocampi. Transl Psychiatry. 2015; 5: e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haartsen R, Jones EJ, Johnson MH. Human brain development over the early years. Current Opinion in Behavioral Sciences. 2016; 10: 149–54. [Google Scholar]
- 25.Eccles JS. The development of children ages 6 to 14. Future Child. 1999; 9(2): 30–44. [PubMed] [Google Scholar]
- 26.Hayden EP, Olino TM, Mackrell SV, Jordan PL, Desjardins J, Katsiroumbas P. Cognitive vulnerability to depression during middle childhood: Stability and associations with maternal affective styles and parental depression. Pers Individ Dif. 2013; 55(8): 892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gispert-Llaurado M, Perez-Garcia M, Escribano J, Closa-Monasterolo R, Luque V, Grote V, et al. Fish consumption in mid-childhood and its relationship to neuropsychological outcomes measured in 7–9 year old children using a NUTRIMENTHE neuropsychological battery. Clin Nutr. 2016. [DOI] [PubMed] [Google Scholar]
- 28.McNeely C, Blanchard J. The Teen Years Explained; A Guide to Healthy Adolescent Development. Johns Hopkins University, 2010. [Google Scholar]
- 29.Fergusson DM, Horwood LJ, Ridder EM, Beautrais AL. Subthreshold depression in adolescence and mental health outcomes in adulthood. Arch Gen Psychiatry. 2005; 62(1): 66–72. [DOI] [PubMed] [Google Scholar]
- 30.Phillips MR, Zhang J, Shi Q, Song Z, Ding Z, Pang S, et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001–05: an epidemiological survey. Lancet. 2009; 373(9680): 2041–53. [DOI] [PubMed] [Google Scholar]
- 31.Li D, Zhang DJ, Shao JJ, Qi XD, Tian L. A meta-analysis of the prevalence of depressive symptoms in Chinese older adults. Arch Gerontol Geriatr. 2014; 58(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 32.Gu L, Xie J, Long J, Chen Q, Pan R, Yan Y, et al. Epidemiology of major depressive disorder in mainland china: a systematic review. PLoS One. 2013; 8(6): e65356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.