Abstract
Background:
Pseudomonas aeruginosa is the prominent bacterial pathogen in the cystic fibrosis (CF) lung and contributes to significant morbidity and mortality. Though P. aeruginosa strains initially colonizing the CF lung have a nonmucoid colony morphology, they often mutate into mucoid variants that are associated with clinical deterioration. Both nonmucoid and mucoid P. aeruginosa variants are often co-isolated on microbiological cultures of sputum collected from CF patients. With regional variation in bronchiectasis, tissue damage, inflammation, and microbial colonization, lobar distribution of nonmucoid and mucoid P. aeruginosa variants may impact local microenvironments in the CF lung, but this has not been well-studied.
Methods:
We prospectively collected lobe-specific bronchoalveolar lavage (BAL) fluid from a CF patient cohort (n=14) using a standardized bronchoscopic protocol where collection was performed in 6 lobare regions. The lobar BAL specimens were plated on P. aeruginosa-selective media and proinflammatory cytokines (IL-1, TNF, IL-6 and IL-8) were measured via cytokine array. Correlations between infecting P. aeruginosa variants (nonmucoid, mucoid, or mixed-variant populations), the lobar regions in which these variants were found, and regional proinflammatory cytokine concentrations were measured.
Results:
P. aeruginosa mucoid and nonmucoid variants were homogenously distributed throughout the CF lung. However, infection with mucoid variants variants (found within single-or mixed-variant populations) was associated with significantly greater regional inflammation. The upper and lower lobes of the CF lung did not exhibit differences in inflammatory cytokine concentrations.
Conclusions:
Mucoid P. aeruginosa infection is a microbial determinant of regional inflammation within the CF lung.
Keywords: airway, cystic fibrosis, Pseudomonas aeruginosa, lung, mucoid, inflammation, cytokines
1. Introduction
Cystic fibrosis (CF) is a life-shortening genetic disease that affects approximately 30,000 patients in the United States and more than 70,000 worldwide (1). CF patients suffer from devastating, chronic pulmonary infections associated with hyperinflammation and irreversible damage to the lower airways (2). As CF patients age, Pseudomonas aeruginosa becomes the predominant pathogen in the respiratory tract during the second decade of life for the majority of the patient population (3, 4). During the initial acquisition period, P. aeruginosa strains have a nonmucoid colony morphology (5). Once chronic infection is established, these bacteria may acquire mutations leading to the emergence of mucoid P. aeruginosa variants, which overproduce the exopolysaccharide, alginate, and exhibit enhanced recalcitrance to antimicrobial therapy (6-9). P. aeruginosa mucoid conversion is associated with a worse prognosis in CF, manifested as a precipitous decline in pulmonary function and heightened mortality (10-16).
Mucoid and nonmucoid variants are often co-isolated on microbial culture of CF sputum, suggesting a mixed-variant populations may have selective advantages withstanding endogenous and exogenous factors in the CF lung (i.e. from host immunity and/or antimicrobials, respectively) (17-27). Recent work from our laboratory demonstrates that co-cultures of mucoid and nonmucoid P. aeruginosa strains are more resistant to innate antimicrobials, such as reactive oxygen species (e.g. H2O2) and a cationic antimicrobial peptide, LL-37, compared to mono-cultures of either variant (28).
CF lung disease is heterogeneous with a regional distribution of bronchiectasis, tissue damage, and inflammation. Radiographic and histopathology studies show an upper lobe predominance in the CF lung, which are marred by greater architectural changes (e.g. bronchiectasis, air trapping, and emphysema) compared to the lower lobes (29-36). The anatomic, microbial, and intrinsic immune factors contributing to interlobar variations in CF lung disease are poorly understood. Using bronchoalveolar lavage (BAL) fluid, studies have shed some light on the regional distribution of host and microbial factors contributing to the heterogeneity of CF lung disease (37-40). Neutrophils and neutrophil-derived antimicrobials (e.g. elastase) are at higher concentration levels in BAL fluid from upper lobes compared to lower lobes (37). Regional compartmentalization of bacteria has been reported in the CF lower airways with either homogenous or heterogenous distribution of bacterial species across lung lobes (38, 39, 41-44). However, few studies have examined the effect of regional co-localization of both bacterial and host products within the CF lung to investigate dynamic microbial-immune interactions and local inflammation. More importantly, the lobar distribution and associated regional inflammatory response to mixed communities of mucoid and nonmucoid variants of P. aeruginosa has not been studied.
The goals of this study were two-fold. First, identify if P. aeruginosa mucoid or nonmucoid variants, either in single- or mixed-variant populations, preferentially localize to certain lobes of the CF lung. Second, determine if mucoid and nonmucoid variants are associated with differential patterns of regional inflammation, as measured by proinflammatory cytokines (i.e. IL-1β, TNF-α, IL-6 and IL-8). We collected lobar BAL fluid from CF patients, quantitated microbial density and P. aeruginosa colony variants by culture-based methods, and determined proinflammatory cytokine concentrations via multiplex array. We demonstrate that though mucoid and nonmucoid P. aeruginosa variants are homogenously distributed throughout the CF lung, infection with mucoid variants (in single- or mixed-variant populations) correlates with greater lobar inflammation compared to nonmucoid variants. Our findings herein contribute to a growing understanding of host-pathogen interactions that shape regional microenvironments within the CF lung.
2. Materials and Methods
2.1. Study population and ethics statement.
The study was approved by the Nationwide Children’s Hospital (NCH) Institutional Review Board (IRB07-00396 and IRB15-00800) with informed consent and assent obtained from all patient volunteers.
2.2. Bronchoalveolar lavage (BAL) protocol.
While undergoing clinically-indicated surgical procedures, BAL fluid was collected from 14 patients with CF via flexible fiberoptic bronchoscopy (FFB). All volunteers had clinically stable CF lung disease, had performed spirometry to assess pulmonary function [e.g. forced expiratory volume in 1 second (FEV1)], and were not acutely ill or requiring antimicrobial treatment for pulmonary symptoms. Each subject had an endotracheal tube placed and received mechanical ventilation prior to their surgical procedure and during the FFB. The BAL fluid samples were collected within 10 minutes of initiation of mechanical ventilation for the entire cohort. For each subject, BAL fluid was obtained from subsegmental airway of the right upper lobe (RUL), right middle lobe (RML), right lower lobe (RLL), left upper lobe (LUL), lingula (LIN), and left lower lobe (LLL). Twenty mL of sterile normal saline was instilled sequentially into each subsegmental bronchus and immediately recovered by manual suction with no dwell time. The BAL fluid was placed on ice and transported to the research laboratory. Demographics of all patients in this study are delineated in Table 1.
Table 1. Cohort demographics.
BMI- body mass index. FEV1- forced expiratory volume in 1 second.
| CF patients | |
|---|---|
| n= | 14 |
| Females (%) | 30% |
| Age (years) | |
| Median | 23 |
| Range | 14-40 |
| Pancreatic insufficiency (%) | 100 |
| Genotype (%) | |
| F508del homozygous | 57% |
| F508del heterozygous | 29% |
| FEV1 (L) | |
| Median | 2.36 |
| Range | 0.95-3.87 |
| FEV1 (%Predicted) | |
| Median | 71 |
| Range | 22-103 |
| BMI | |
| Median | 19.9 |
| Range | 15.4-30.4 |
2.3. Microbiological analyses.
Patient BAL fluid specimens were maintained on ice immediately following FFB and throughout specimen processing. Within 2 hours of specimen collection, each BAL fluid sample was serially-diluted in Phosphate Buffered Saline [(PBS), Gibco™, pH 7.4] and plated for colony forming units (CFUs/mL) on nonselective [Luria Agar (LA), 10g/L tryptone, 5g/L yeast extract, 10g/L NaCl solidified with 1.5% agar] and P. aeruginosa-selective [Pseudomonas Isolation Agar (PIA)] medium. After 48 hours incubation at 37°C, bacterial colonies were counted on LA and PIA, and total bacterial CFUs/mL and P. aeruginosa CFUs/mL were determined from each type of medium, respectively. A minimum of 10 colonies of growth on LA and PIA was used as the limit-of-detection.
P. aeruginosa variants (nonmucoid, mucoid, or mixed nonmucoid and mucoid) were identified and counted based on colony morphology on PIA. PIA, which contains Triclosan, facilitates growth and maintenance of mucoid P. aeruginosa variants more effectively compared to other culture media (i.e. given the instability of alginate production by clinical isolates grown in vitro) (18, 45-47). As such, PIA was specifically chosen here to identify and differentiate mucoid and nonmucoid P. aeruginosa variants within patient specimens.
Non-Pseudomonas CFUs were calculated by subtracting P. aeruginosa colonies on PIA from all colonies counted on LA. Percentage of P. aeruginosa colonies (%P.a. isolates) was calculated by taking P. aeruginosa growth on PIA as a percentage of total microbial growth on LA. Percent mucoid colonies (%Mucoidy) was calculated by taking mucoid colonies identified on PIA as a percentage of total P. aeruginosa colonies. Each lobar specimen (regardless of patient-of-origin) was stratified into non-overlapping groups based on microbial populations as follows: No P. aeruginosa growth (i.e. only observed microbial growth on LA), and only nonmucoid, only mucoid, or mixed P. aeruginosa morphotypes.
2.4. Proinflammatory cytokine arrays.
On the same day of specimen collection and after plating for microbial growth, BAL fluid was centrifuged and filter-sterilized to remove host and bacterial cells. Cell-free fractions of BAL fluid were subsequently stored at −80°C. Proinflammatory cytokine concentrations within these BAL samples were subsequently quantitated using V-PLEX Proinflammatory Panel 1 Human Cytokine Arrays (Meso Scale Diagnostics, LLC). All cytokine arrays were performed by The Ohio State University Clinical Research Center based on manufacturer’s specifications.
2.5. Statistical analyses.
Statistical analyses were performed using GraphPad Prism v.7 (Graphpad Software). Statistical significance was determined using a p-value <0.05. All assays with patient specimens were performed in duplicate.
3. Results
3.1. Single- and mixed-variant populations of mucoid and nonmucoid P. aeruginosa were isolated from CF patient specimens.
Previous studies had shown that both mucoid and nonmucoid variants of P. aeruginosa are often isolated in mixed communities from chronically-infected CF patients (17-27); however, the anatomic distribution of these variants within the CF lung has not been well-studied.
From multiple patient specimens, we were able to isolate both mucoid and nonmucoid variants of P. aeruginosa, both in single- and mixed-variant populations (Fig. S1A, B, and C). The mixed-variant colony morphologies of one CF patient’s P. aeruginosa infection are shown, visualized directly upon medium surface by handheld camera and with standard light microscopy (10X magnification) (Fig. S1A). Conversely, a proportion of patients were not infected with P. aeruginosa (indicated as “No P.a.”) at the time of specimen collection (Fig. S1B); however, in all instances, specimens from these CF patients did produce microbial growth on LA, suggesting the presence of non-P. aeruginosa species, which were not specifically identified for the purposes of this study.
Of all patients infected with mucoid variants (“mucoid P.a. + patients”), a small majority (56%) showed mixed mucoid/nonmucoid P. aeruginosa populations, whereas others were infected only with mucoid variants (Fig. S1C). In total, these data indicated that mixed-variant P. aeruginosa communities were well-represented within our patient cohort, thereby enabling us to study regional and inflammatory correlates with infection.
3.2. Both mucoid and nonmucoid P. aeruginosa morphotypes were distributed throughout the CF lung.
To determine the lobar distribution of mucoid, nonmucoid, and mixed-variant populations of P. aeruginosa, BAL fluid from 6 lobar regions (RUL, RML, RLL, LUL, LIN, and LLL), regardless of patient-of-origin, were independently examined for total bacterial, total P. aeruginosa, and total mucoid/nonmucoid variant CFUs. This approach was justified because some patients had distinct microbial populations in different lobar specimens (Table S1). For instance, some patients were infected with only mucoid P. aeruginosa in certain lobes but had mixed variant populations in other regions. There were also patients who had no P. aeruginosa isolates in some lobar specimens but nonmucoid isolates in others. As such, each lobar specimen was treated independent of patient-of-origin to determine density and localization of the microbial populations.
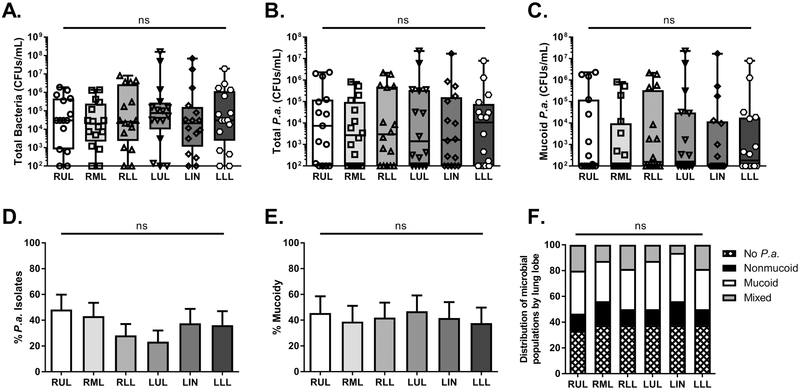
However, there were no statistically significant trends for bacterial density across the different lung lobes of the entire cohort (Fig. 1A, B, and C). Similarly, we examined two other calculated measures of microbial populations: %P. aeruginosa isolates (calculated as a percentage of P. aeruginosa colonies of total microbial growth) and %mucoidy (calculated as a percentage of mucoid P. aeruginosa isolates out of total P. aeruginosa growth). Again, there were no differences for either the relative presence of P. aeruginosa (%P.a.) or mucoid variants (%mucoidy) throughout different regions of the CF lung (Fig. 1D and E). Furthermore, by examining all strata used to categorize each lobar specimen based on microbes isolated- no P. aeruginosa infection (i.e. infection with non-P. aeruginosa species), and infection with nonmucoid, mucoid, and mixed-P. aeruginosa variants, we observed no trends for lobe-dependent frequency of any of these microbial communities (Fig. 1F). These results suggested that within our patient cohort, there was no detectable propensity of P. aeruginosa variants to localize within certain anatomic lobes of the CF lung.
Figure 1. Mucoid, nonmucoid, and mixed-variant P. aeruginosa populations are found throughout the CF lung.
Regional BAL specimens from CF patients (n=14 patients, 84 lobar BAL fluid samples) were plated on nonselective or selective media to quantitate microbial populations. A. Total bacterial colony forming units (CFUs/mL) enumerated on LA (non-selective medium) B. Total P. aeruginosa CFUs/mL enumerated on PIA C. Mucoid variants of P. aeruginosa enumerated on PIA D. Percentage of P. aeruginosa isolates of total microbial growth E. Percentage of mucoid variants of total P. aeruginosa isolates F. Relative frequencies of various microbial populations within regional BAL samples of the CF cohort. Statistical significance was determined via non-parametric, KruskalβWallis one-way analysis of variance (ANOVA). No statistically-significant differences were observed (ns). LA- Luria agar, PIA- Pseudomonas Isolation Agar, RUL- right upper lobe, RML- right middle lobe, RLL- right lower lobe, LUL- left upper lobe, LIN- lingula, LLL- left lower lobe.
3.3. Independent of microbial infection, regional differences in proinflammatory cytokine concentrations were not observed.
Given that we did not observe significant regional differences in bacterial burden and P. aeruginosa variants in our CF patient cohort, we next queried the regional inflammatory status of these patients’ lungs by measuring proinflammatory cytokines within BAL fluid specimens. Based on aforementioned studies, we hypothesized that there would be intrinsic differences between the upper and lower lobes of CF lungs in concentrations of inflammatory indices used here: IL-1β, TNF-α, IL-6, and IL-8. All four cytokines have been quantitated previously within the CF airway, shown to be relevant to the CF disease process (at the host-microbial interface), and found to be reliable indicators of inflammation in other diseases as well (37, 48-52).
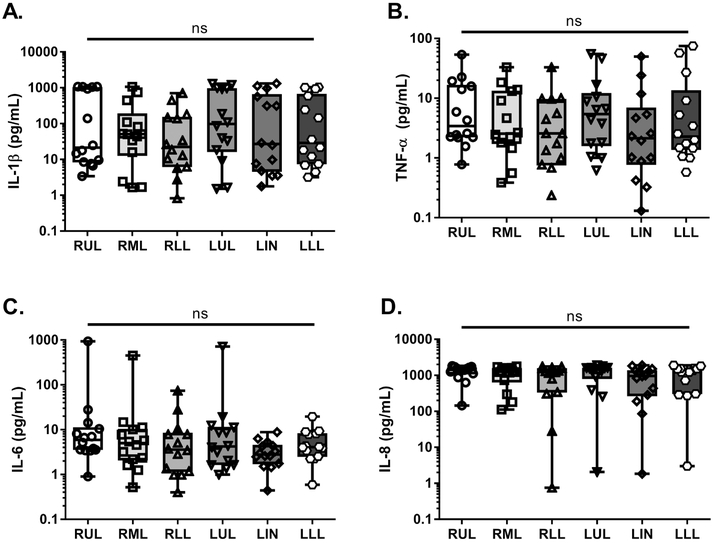
Within our patient cohort, we did not observe any statistically-significant, interlobar differences in proinflammatory cytokine concentrations (Fig. 2A, B, C, and D). There were subtle, but noticeable differences, between the upper and lower lobes, wherein IL-1β concentrations were generally higher in the LUL compared to LLL and TNF-α concentrations were higher in the RUL and LUL compared to RLL and LLL respectively; however, these differences fell short of significance (p>0.05) (Fig. 2A and B). Concentrations of IL-8, a potent neutrophil chemokine, were high across all patient specimens and all lung lobes (Fig. 2D); this observation aligns with previous work demonstrating IL-8 as a hallmark of neutrophil-dominated, CF lung immunopathology (53). These data suggested that independent of microbial infection, there were no regional differences in proinflammatory cytokines within CF patient lungs.
Fig. 2. Independent of infection, significant interlobar differences in proinflammatory cytokines were not observed.
Proinflammatory cytokine concentrations were measured in regional BAL specimens from all CF patients (n=14 patients= 84 lobar BALs) via V-PLEX array. Data from each specimen were stratified based upon lung lobe of origin. A. IL-1β (pg/mL) B. TNF-α (pg/mL) C. IL-6 (pg/mL) D. IL-8 (pg/mL). Statistical significance was determined via non-parametric, Kruskal–Wallis one-way analysis of variance (ANOVA). No statistically-significant differences were observed (ns). RUL- right upper lobe, RML- right middle lobe, RLL- right lower lobe, LUL- left upper lobe, LIN- lingula, LLL- left lower lobe.
3.4. Mucoid and mixed-variant P. aeruginosa infections were associated with elevated proinflammatory cytokine concentrations within regional BAL fluid.
Multiple laboratory and patient studies have demonstrated that bacterial infection is a critical driver of the inflammatory process in CF (54-56). Though, we did not find significant interlobar variations in inflammation or in the density and distribution of microbial populations independently, we postulated that regional inflammation and microbes may still be interrelated in our cohort. Specifically, we hypothesized that P. aeruginosa colony variants may be driving inflammation differentially, independent of their location within the CF lung.
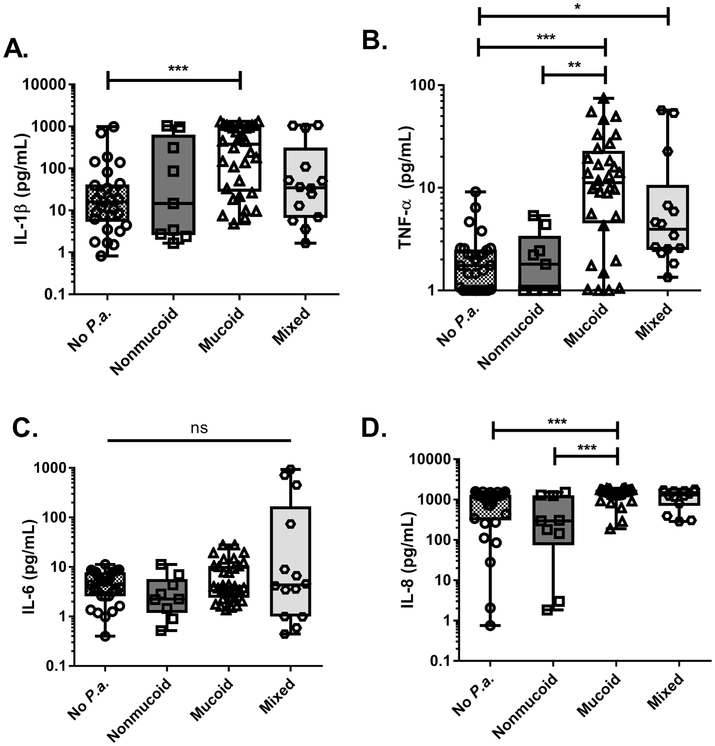
As described earlier, each lobar BAL specimen was grouped only based on microbes isolated therein (i.e. infected with no P. aeruginosa or with nonmucoid, mucoid, or mixed-variants of P. aeruginosa). Subsequently, proinflammatory cytokine concentrations in each of these groups were analyzed. Indeed, regional BAL specimens from which we isolated P. aeruginosa mucoid or mixed variants contained higher concentrations of IL-1β, TNF-α, and IL-8 compared to specimens that were culture-negative for P. aeruginosa or contained nonmucoid P. aeruginosa variants only (Fig. 3A, B, and D). We did not observe any statistically-significant differences among these groups for IL-6 concentration (Fig. 3C).
Fig. 3. Mucoid or mixed-variant P. aeruginosa infections were associated with higher proinflammatory cytokines within regional BAL fluid.
Proinflammatory cytokine concentrations were measured in regional BAL specimens from all CF patients (n=14 patients= 84 lobar BALs) via V-PLEX array. Data from each specimen were stratified based upon type of microbial infection [No P.a. (n=29), Nonmucoid (n=9), Mucoid (n=32), Mixed (n=14)]. A. IL-1β (pg/mL) B. TNF-α (pg/mL) C. IL-6 (pg/mL) D. IL-8 (pg/mL). Statistical significance was determined via non-parametric, Kruskal–Wallis one-way analysis of variance (ANOVA). *p<0.05, **p<0.01, ***p<0.001, ns- not statistically significant.
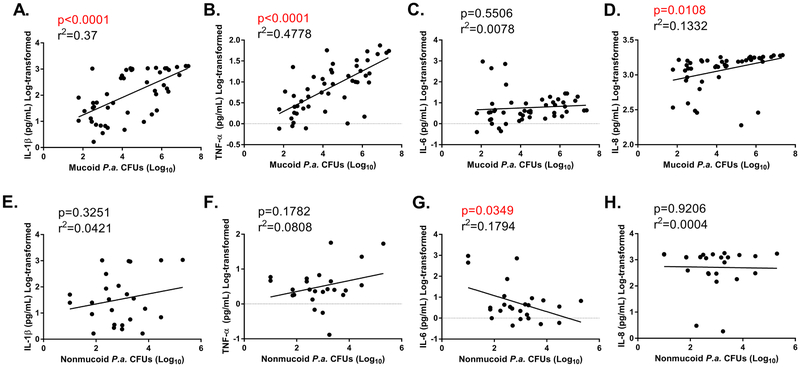
BAL fluid samples containing mixed-variant P. aeruginosa isolates differed in the relative density of mucoid and nonmucoid constituents (i.e. CFUs of mucoid and nonmucoid variants or “%mucoidy” for these specimens varied significantly); some specimens within this group showed predominantly mucoid isolates, whereas others contained mostly nonmucoid P. aeruginosa. As such, we performed linear regression analyses to determine associations between total CFUs of mucoid/nonmucoid isolates and proinflammatory indices across all BAL specimens. We found statistically-significant, positive correlations between mucoid P. aeruginosa CFUs and IL-1, TNFα, and IL-8 (Fig. 4A, B, C, and D); in contrast, density of nonmucoid P. aeruginosa variants was not positively correlated with any proinflammatory cytokine (Fig. 4E, F, G, and H). However, there was a negative correlation between nonmucoid P. aeruginosa CFUs and IL-6 concentration (Fig. 4G).
Fig. 4. Infection with mucoid P. aeruginosa directly correlates with higher proinflammatory cytokine concentrations within regional BAL specimens.
Proinflammatory cytokine concentrations were measured in regional BAL specimens from all CF patients (n=14 patients= 84 lobar BALs) via V-PLEX array. Cytokine concentrations are plotted versus mucoid P. aeruginosa CFUs/mL (A.-D.) or nonmucoid P. aeruginosa CFUs/mL (E.-H.) for each patient specimen. Correlation plots for A./E. IL-1β (pg/mL), B./F. TNF-α (pg/mL), C./G. IL-6 (pg/mL), D./H. IL-8 (pg/mL). R2 and slope p-values are shown. Statistically significant correlations (p<0.05) are highlighted in red text.
Additional associations between microbial variables (e.g. total bacterial CFUs, non-P. aeruginosa CFUs, %mucoidy, etc.) and proinflammatory cytokines were documented as well (Table S2). There were positive correlations between total bacterial burden as well as non-P. aeruginosa CFUs with cytokine concentrations, suggesting that future studies should examine the regional impact of other bacterial and fungal species upon the CF lung as well. Importantly, however, only %mucoidy was positively correlated with all four proinflammatory cytokines examined (Table S2). In total, these data suggested that P. aeruginosa variants differentially impact the inflammatory microenvironment of the CF lung, and specifically, mucoid variants are associated with greater regional inflammation than nonmucoid variants.
4. Discussion
The regional heterogeneity of the CF lung with respect to bronchiectasis, tissue damage, and microbial colonization has been well described with the pathophysiologic mechanisms being poorly understood (29-36). The etiology of interlobar variations in the CF lung often manifested with upper lobes predominance may be attributable to host-pathogen interactions. However, few studies have addressed co-localization of bacterial pathogens and host immune factors as an influencing factor in this clinical scenario of the CF airway. Given the importance of P. aeruginosa in the CF and the important clinical implications of mucoid conversion, we successfully examined the regional distribution of mucoid and nonmucoid variants of P. aeruginosa to improve our understanding how these bacterial morphotypes may differentially impact the inflammatory microenvironment of the CF lung.
This study made three important observations: 1) Mucoid and nonmucoid variants of P. aeruginosa (including mixed populations) were distributed throughout the CF lung, without preferential localization to an anatomic lobe/region; 2) Independent of bacterial infection, the upper lobes of the CF lung showed marginally greater proinflammatory cytokine concentrations compared to the lower lobes, though these differences did not meet statistical significance; 3) Mucoid variants of P. aeruginosa (and to a lesser extent, mixed-variant populations) were associated with greater regional inflammation compared to nonmucoid variants.
As indicated by published studies, more airway destruction is seen in the CF lung where greater concentrations of neutrophils and neutrophil-derived products exist (37). Similarly, mixed variant populations of mucoid and nonmucoid P. aeruginosa exhibit enhanced resistance to neutrophil antimicrobials (i.e. LL-37 and H2O2) in vitro (28). Although the isolation frequency of mucoid, nonmucoid, and mixed P. aeruginosa variants among different lobes did not achieve statistical significance in the current study, we believe further analysis is needed in larger CF cohorts as mixed communities of both P. aeruginosa variants may be an important factor why an upper lobe predominance occurs in CF.
Recent work investigated interlobar variations in microbial communities in the CF lung that examined regionality of genotypic and phenotypic variants of bacterial pathogens. While some studies showed regional compartmentalization of clonally-related variants of P. aeruginosa (and of other bacterial species), others found a more homogenous distribution of microbes (38, 39, 41-44). To our knowledge, the lobar distribution of mucoid and nonmucoid variants of P. aeruginosa has not been previously explored. Bjarnsholt et al. showed differences in the presence of mucoid and mixed mucoid/nonmucoid-variant populations within the conductive and respiratory zones of explanted CF lungs (21); however, both zones are represented in each lung lobe [i.e. the conductive zones within proximal, larger airways (e.g. segmental bronchi), and the respiratory zones within the terminal bronchioles/alveoli]. The current study examined P. aeruginosa morphotypes isolated from all lobes of the lung including the lingula to better understand aforementioned evidence of local interlobar differences in the CF lung and found mucoid and nonmucoid variants were not geographically restricted by the lobe.
No significant differences in concentrations of proinflammatory cytokines, IL-1β, TNF-α, IL-6, and IL-8, across different lung lobes were found in our analysis (Fig. 2A, B, C, and D). However, there were slight trends towards higher concentrations of some of these inflammatory indices within the upper lobes. Independent of lung lobe, P. aeruginosa variants may differentially influence local inflammation in the CF lung. Regional BAL fluid specimens containing mucoid (and also mixed-mucoid/nonmucoid) isolates had higher concentrations of proinflammatory cytokines compared to specimens that were culture-negative for P. aeruginosa or contained nonmucoid variants only (Fig. 3A, B, and D). Linear regression analyses demonstrated a direct relationship between the burden of mucoid variants and cytokine concentrations, indicating that the mucoid phenotype was the likely driver of inflammation in both single- and mixed-variant P. aeruginosa infections. These findings add further insight to a large body of clinical literature demonstrating deleterious impacts of mucoid conversion upon CF patients, thereby necessitating the development of therapeutics specifically targeting mucoid P. aeruginosa strains.
Surprisingly, we found that nonmucoid variants of P. aeruginosa were inversely correlated with IL-6 concentration in the BAL fluid analyzed (Fig. 4G). Bonfield et al. also reported that CF patients colonized with P. aeruginosa had lower concentrations of IL-6 in BAL fluid samples compared to patients not colonized (48). Based on these observations, we postulate that nonmucoid P. aeruginosa suppresses synthesis or abrogates stability of IL-6. To that end, a recent study reported that LasB protease of P. aeruginosa specifically degrades IL-6 in vitro as well as within a murine model of disease, thereby compromising IL-6-mediated tissue-protective and antimicrobial responses; in contrast, overexpression of IL-6 protects animals from lethal P. aeruginosa lung infection (57). Our findings and the aforementioned mechanistic study provide a rationale to further examine P. aeruginosa/IL-6 interactions, as these may shed light on P. aeruginosa evasion of innate immunity and illuminate avenues for novel therapeutic modalities.
Our study was limited by the use of a small, single-center cohort. There were also limitations regarding the majority of male patients undergoing surgery during the study period. Therefore, further studies with larger patient cohorts are needed to confirm associations between P. aeruginosa colony variants and inflammatory indices. Though not within the immediate scope of this work, correlations between microbial populations/variants, inflammatory indices, and patient demographics (e.g. age, sex, race, genotype, etc.) could also be investigated. Here, we briefly considered an association between patient age, mucoidy, and inflammation, finding that advanced patient age correlated with higher overall bacterial density (CFUs), greater relative presence of mucoid variants (%mucoidy), as well as higher concentrations of IL-1β and TNF-α in BAL specimens (Table S3). These data validate previous studies, demonstrating that mucoid P. aeruginosa becomes more prevalent with age and that CF lung inflammation is likely progressive throughout the course of a patient’s lifetime (11, 27, 55, 58-60).
The lung microbiome of the CF lung is very dynamic, so we cannot report on any effect of anesthesia, endotracheal tube placement, and mechanical ventilation on our findings. Given that all of these factors were limited to a short duration (less than 10 minutes for the entire cohort), we believe the effect is not significant. We also showed that total bacterial burden (as well as CFUs of non P. aeruginosa species, which were enumerated on non-selective medium but not specifically identified) also correlated with inflammatory cytokine concentrations.
In conclusion, mucoid and nonmucoid variants of P. aeruginosa are regionally distributed throughout the CF lung, and lobar infection with mucoid variants is correlated with greater inflammation than is infection with nonmucoid variants or non-Pseudomonas species. In addition to expanding the cohort size to optimize statistical power, future work in this area will include longitudinal studies to define dynamic host-pathogen interactions and account for non-Pseudomonad species, including Staphylococcus aureus and Burkholderia cenocepacia, which are also important bacterial pathogens in the CF population (61).
Supplementary Material
Highlights:
P. aeruginosa mucoid/nonmucoid variants are distributed throughout the CF lung
CF lung upper lobes are slightly more inflamed compared to the lower lobes
P. aeruginosa mucoid variants are associated with greater regional inflammation
Acknowledgements
The authors would like to thank the patients who volunteered for the study as well as the respiratory therapy and operating room staff at Nationwide Children’s Hospital who kindly allowed our team to collect samples for analysis. These studies were supported by a TL1 pre-doctoral fellowship awarded to S.M. by the Center for Clinical and Translational Science (CCTS), The Ohio State University College of Medicine (TL1TR001069) and by NIH grants awarded to D.J.W. (R01AI34895 and R01AI097511). The funders did not have any role in study design, data collection and interpretation, decision to publish, or preparation of the manuscript. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foundation CF. 2017. Annual Data Report 2016 Cystic Fibrosis Foundation Patient Registry. [Google Scholar]
- 2.Strausbaugh SD, Davis PB. 2007. Cystic Fibrosis: A Review of Epidemiology and Pathobiology. Clin Chest Med 28:279–288. [DOI] [PubMed] [Google Scholar]
- 3.Sanders DB, Fink A. 2016. Background and Epidemiology. Pediatr Clin North Am 63:567–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surette MG. 2014. The cystic fibrosis lung microbiome. Ann Am Thorac Soc 11:61–65. [DOI] [PubMed] [Google Scholar]
- 5.Govan JR, Deretic V. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60:539–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodges NA, Gordon CA. 1991. Protection of Pseudomonas aeruginosa against Ciprofloxacin and 1-Lactams by Homologous Alginate. Antimicrob Agents Chemother 35:2450–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hentzer M, Teitzel GM, Balzer GJ, Molin S, Givskov M, Matthew R, Heydorn A, Parsek MR. 2001. Alginate Overproduction Affects Pseudomonas aeruginosa Biofilm Structure and Function Alginate Overproduction Affects Pseudomonas aeruginosa Biofilm Structure and Function. J Bacteriol 183:5395–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goltermann L, Tolker-Nielsen T. 2017. Importance of the exopolysaccharide matrix in antimicrobial tolerance of Pseudomonas aeruginosa aggregates. Antimicrob Agents Chemother 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hengzhuang W, Wu H, Ciofu O, Song Z, Høiby N. 2011. Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 55:4469–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langan KM, Kotsimbos T, Peleg AY. 2015. Managing Pseudomonas aeruginosa respiratory infections in cystic fibrosis. Curr Opin Infect Dis 28:547–556. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Kosorok MR, Farrell PM, Laxova A, West SEH, Green CG, Rock MJ, Splaingard ML. 2005. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 293:581–588. [DOI] [PubMed] [Google Scholar]
- 12.Henry RL, Mellis CM, Petrovic L. 1992. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr Pulmonol 12:158–61. [DOI] [PubMed] [Google Scholar]
- 13.Farrell PM, Collins J, Broderick LS, Rock MJ, Li Z, Kosorok MR, Laxova A, Gershan WM, Brody AS. 2009. Association between Mucoid Pseudomonas Infection and Bronchiectasis in Children with Cystic Fibrosis. Radiology 252:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konig B. Friedl P. Pederson SS. Konig W. 1992. Alginate- Its role in neutrophil responses and signal transduction toward mucoid Pseudomonas aeruginosa bacteria. Int Arch Allergy Immunol 99:98–106. [DOI] [PubMed] [Google Scholar]
- 15.Demko CA, Byard PJ, Davis PB. 1995. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol 48:1041–1049. [DOI] [PubMed] [Google Scholar]
- 16.Parad RB, Gerard CJ, Zurakowski D, Nichols DP, Pier GB. 1999. Pulmonary outcome in cystic fibrosis is influenced primarily by mucoid Pseudomonas aeruginosa infection and immune status and only modestly by genotype. Infect Immun 67:4744–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damkiaer S, Yang L, Molin S, Jelsbak L. 2013. Evolutionary remodeling of global regulatory networks during long-term bacterial adaptation to human hosts. Proc Natl Acad Sci U S A 110:7766–7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciofu O, Lee B, Johannesson M, Hermansen NO, Meyer P, Hoiby N. 2008. Investigation of the algT operon sequence in mucoid and non-mucoid Pseudomonas aeruginosa isolates from 115 Scandinavian patients with cystic fibrosis and in 88 in vitro non-mucoid revertants. Microbiology 154:103–113. [DOI] [PubMed] [Google Scholar]
- 19.Clark ST, Diaz Caballero J, Cheang M, Coburn B, Wang PW, Donaldson SL, Zhang Y, Liu M, Keshavjee S, Yau YCW, Waters VJ, Elizabeth Tullis D, Guttman DS, Hwang DM. 2015. Phenotypic diversity within a Pseudomonas aeruginosa population infecting an adult with cystic fibrosis. Sci Rep 5:10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Haagensen JAJ, Jelsbak L, Johansen HK, Sternberg C, Hoiby N, Molin S. 2008. In Situ Growth Rates and Biofilm Development of Pseudomonas aeruginosa Populations in Chronic Lung Infections. J Bacteriol 190:2767–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjarnsholt T, Jensen PO, Fiandaca MJ, Pedersen J, Hansen CR, Andersen CB, Pressler T, Givskov M, Hoiby N. 2009. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol 44:547–558. [DOI] [PubMed] [Google Scholar]
- 22.Hoiby N, Ciofu O, Bjarnsholt T. 2010. Pseudomonas aeruginosa biofilms in Cystic Fibrosis. Future Microbiol 5:1663–1674. [DOI] [PubMed] [Google Scholar]
- 23.Bragonzi A, Wiehlmann L, Klockgether J, Cramer N, Worlitzsch D, Döning G, Tümmler B. 2006. Sequence diversity of the mucABD locus in Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiology 152:3261–3269. [DOI] [PubMed] [Google Scholar]
- 24.Dobrindt U, Hacker JH, Svanborg C. 2013. Current Topics in Microbiology and Immunology Between Pathogenicity and Commensalism. [DOI] [PubMed] [Google Scholar]
- 25.Seale TW, Thirkill H, Tarpay M, Flux M, Rennert OM. 1979. Serotypes and antibiotic susceptibilities of Pseudomonas aeruginosa isolates from single sputa of cystic fibrosis patients. J Clin Microbiol 9:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tai AS, Sherrard LJ, Kidd TJ, Ramsay KA, Buckley C, Syrmis M, Grimwood K, Bell SC, Whiley DM. 2017. Antibiotic perturbation of mixed-strain Pseudomonas aeruginosa infection in patients with cystic fibrosis. BMC Pulm Med 17:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Troxler BR, Hoover WC, Britton LJ, Gerwin AM, Rowe SM. 2012. Clearance of initial mucoid Pseudomonas aeruginosa in patients with cystic fibrosis. Pediatr Pulmonol 47:1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malhotra S, Limoli DH, English AE, Parsek MR, Wozniak DJ. 2018. Mixed Communities of Mucoid and Nonmucoid Pseudomonas aeruginosa Exhibit Enhanced Resistance to Host Antimicrobials. MBio 9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goddard M. 2011. Histopathology of bronchiectasis. Bronchiectasis 22–31. [Google Scholar]
- 30.Dasenbrook EC, Lu L, Donnola S, Weaver DE, Gulani V, Jakob PM, Konstan MW, Flask CA. 2013. Normalized T1 Magnetic Resonance Imaging for Assessment of Regional Lung Function in Adult Cystic Fibrosis Patients - A Cross-Sectional Study. PLoS One 8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Sanders DB, Rock MJ, Kosorok MR, Collins J, Green CG, Brody AS, Farrell PM. 2012. Regional differences in the evolution of lung disease in children with cystic fibrosis. Pediatr Pulmonol 47:635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maffessanti M, Candusso M, Brizzi F, Piovesana F. 1996. Cystic Fibrosis in Children: HRCT Findings and Distribution of Disease. J Thorac Imaging. [DOI] [PubMed] [Google Scholar]
- 33.Mets OM, Roothaan SM, Bronsveld I, Luijk B, Van De Graaf EA, Vink A, De Jong PA. 2015. Emphysema is common in lungs of cystic fibrosis lung transplantation patients: A histopathological and computed tomography study. PLoS One 10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mott LS, Park J, Gangell CL, De Klerk NH, Sly PD, Murray CP, Stick SM. 2013. Distribution of early structural lung changes due to cystic fibrosis detected with chest computed tomography. J Pediatr 163:243–248.e3. [DOI] [PubMed] [Google Scholar]
- 35.Nemec SF, Bankier AA, Eisenberg RL. 2013. Upper LobeβPredominant Diseases of the Lung. Am J Roentgenol 200:W222–W237. [DOI] [PubMed] [Google Scholar]
- 36.Perera PL, Screaton NJ. 2011. Radiological features of bronchiectasis. Bronchiectasis 44–67. [Google Scholar]
- 37.Meyer KC, Sharma A, Assistance T, Peterson K, Brennan L. 1997. Regional Variability of Lung Inflammation in Cystic Fibrosis. Am J Respir Crit Care Med 156:1536–1540. [DOI] [PubMed] [Google Scholar]
- 38.Gutierrez JP, Grimwood K, Armstrong DS, Carlin JB, Carzino R, Olinsky A, Robertson CF, Phelan PD. 2001. Interlobar differences in bronchoalveolar lavage fluid from children with cystic fibrosis. Eur Respir J 17:281–6. [DOI] [PubMed] [Google Scholar]
- 39.Hogan DA, Willger SD, Dolben EL, Hampton TH, Stanton B, Morrison HG, Sogin ML, Czum J, Ashare A. 2016. Analysis of lung microbiota in bronchoalveolar lavage, protected brush and sputum samples from subjects with Mild-To- Moderate cystic fibrosis lung disease. PLoS One 11:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jorth P, Staudinger BJ, Wu X, Hisert KB, Hayden H, Garudathri J, Harding CL, Radey MC, Rezayat A, Bautista G, Berrington WR, Goddard AF, Zheng C, Angermeyer A, Brittnacher MJ, Kitzman J, Shendure J, Fligner CL, Mittler J, Aitken ML, Manoil C, Bruce JE, Yahr TL, Singh PK. 2015. Regional Isolation Drives Bacterial Diversification within Cystic Fibrosis Lungs. Cell Host Microbe 18:307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willner D, Haynes MR, Furlan M, Schmieder R, Lim YW, Rainey PB, Rohwer F, Conrad D. 2012. Spatial distribution of microbial communities in the cystic fibrosis lung. ISME J 6:471–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garg N, Wang M, Hyde E, da Silva RR, Melnik AV., Protsyuk I, Bouslimani A, Lim YW, Wong R, Humphrey G, Ackermann G, Spivey T, Brouha SS, Bandeira N, Lin GY, Rohwer F, Conrad DJ, Alexandrov T, Knight R, Dorrestein PC. 2017. Three-Dimensional Microbiome and Metabolome Cartography of a Diseased Human Lung. Cell Host Microbe 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung H, Lieberman TD, Vargas SO, Flett KB, McAdam AJ, Priebe GP, Kishony R. 2017. Global and local selection acting on the pathogen Stenotrophomonas maltophilia in the human lung. Nat Commun 8:14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goddard AF, Staudinger BJ, Dowd SE, Joshi-Datar A, Wolcott RD, Aitken ML, Fligner CL, Singh PK. 2012. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc Natl Acad Sci 109:13769–13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pugashetti BK, Metzger HM, Vadas L, David S. 1982. Phenotypic Differences Among Clinically Isolated Mucoid Pseudomonas aeruginosa Strains. J Clin Microbiol 16:686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wood LF, Leech AJ, Ohman DE. 2006. Cell wall-inhibitory antibiotics activate the alginate biosynthesis operon in Pseudomonas aeruginosa: Roles of σ22 (AlgT) and the AlgW and Prc proteases. Mol Microbiol 62:412–426. [DOI] [PubMed] [Google Scholar]
- 47.Candido Caçador N, Ciofu O, Galetti R, da Costa Darini AL, Gomes Monteiro Marin Torres LA, Paulino da Costa Capizzani C, Høiby N. 2018. Adaptation of Pseudomonas aeruginosa to the chronic phenotype by mutations in the algTmucABD operon in isolates from Brazilian cystic fibrosis patients. PLoS One 13:e0208013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. 1995. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med 152:2111–2118. [DOI] [PubMed] [Google Scholar]
- 49.Colombo C, Costantini D, Rocchi A, Cariani L, Garlaschi ML, Tirelli S, Calori G, Copreni E, Conese M. 2005. Cytokine levels in sputum of cystic fibrosis patients before and after antibiotic therapy. Pediatr Pulmonol 40:15–21. [DOI] [PubMed] [Google Scholar]
- 50.Lawrence T. 2009. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sagel SD, Chmiel JF, Konstan MW. 2007. Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc 4:406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Courtney JM, Ennis M, Elborn JS. 2004. Cytokines and inflammatory mediators in cystic fibrosis. J Cyst Fibros 3:223–231. [DOI] [PubMed] [Google Scholar]
- 53.Hartl D, Gaggar A, Bruscia E, Hector A, Marcos V, Jung A, Greene C, McElvaney G, Mall M, Döring G. 2012. Innate immunity in cystic fibrosis lung disease. J Cyst Fibros 11:363–82. [DOI] [PubMed] [Google Scholar]
- 54.Armstrong DS, Grimwood K, Carzino R, Carlin JB, Olinsky A, Phelan PD. 1995. Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ 310:1571–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armstrong DS, Grimwood K, Carlin JB, Carzino R, Gutierrez JP, Hull J, Olinsky A, Phelan EM, Robertson CF, Phelan PD. 1997. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 156:1197–1204. [DOI] [PubMed] [Google Scholar]
- 56.Armstrong DS, Hook SM, Jamsen KM, Nixon GM, Carzino R, Carlin JB, Robertson CF, Grimwood K. 2005. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol 40:500–510. [DOI] [PubMed] [Google Scholar]
- 57.Saint-Criq V, Villeret B, Bastaert F, Kheir S, Hatton A, Cazes A, Xing Z, Sermet-Gaudelus I, Garcia-Verdugo I, Edelman A, Sallenave J-M. 2017. Pseudomonas aeruginosa LasB protease impairs innate immunity in mice and humans by targeting a lung epithelial cystic fibrosis transmembrane regulator–IL-6–antimicrobial–repair pathway. Thorax thoraxjnl-2017-210298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levy H, Kalish LA, Cannon CL, García KC, Gerard C, Goldmann D, Pier GB, Weiss ST, Colin AA. 2008. Predictors of mucoid Pseudomonas colonization in cystic fibrosis patients. Pediatr Pulmonol 43:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pittman JE, Wylie KM, Akers K, Storch GA, Hatch J, Quante J, Frayman KB, Clarke N, Davis M, Stick SM, Hall GL, Montgomery G, Ranganathan S, Davis SD, Ferkol TW, AREST CF. 2017. Association of Antibiotics, Airway Microbiome and Inflammation in Infants with Cystic Fibrosis. Ann Am Thorac Soc 14:AnnalsATS.201702-121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Starner TD, McCray PB Jr. 2005. Pathogenesis of early lung disease in cystic fibrosis: A window of opportunity to eradicate bacteria. Ann Intern Med 143:816–822. [DOI] [PubMed] [Google Scholar]
- 61.Chmiel JF, Aksamit TR, Chotirmall SH, Dasenbrook EC, Elborn JS, LiPuma JJ, Ranganathan SC, Waters VJ, Ratjen FA. 2014. Antibiotic management of lung infections in cystic fibrosis: I. The microbiome, methicillin-resistant Staphylococcus aureus, gram-negative bacteria, and multiple infections. Ann Am Thorac Soc 11:1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.