Abstract
Background
Donor brain death duration (BDD) may impact post-transplant graft function and survival in lung transplantation.
Methods
We queried the 2007–2018 United Network for Organ Sharing Registry for adult recipients undergoing first-time isolated lung transplantation. Cox Proportional Hazard modelling with splines enabled identification of three donor brain death intervals for subsequent analysis: short (<24 hours), reference (24–60 hours), and long (>60 hours). The primary outcome was post-transplant survival.
Results
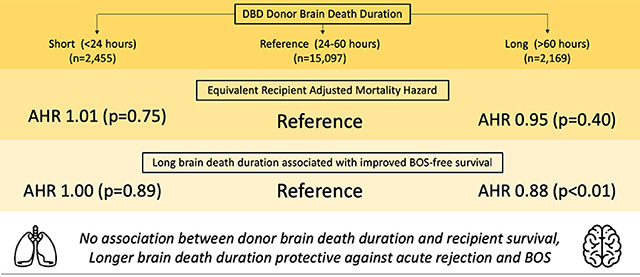
19,721 donors and recipients met inclusion criteria. Median time from donor brain death until cross clamp was 36.6 hours (IQR 19.5). Unadjusted overall survival between cohorts was equivalent (log-rank p=0.42), however longer BDD was associated with improved bronchiolitis obliterans syndrome (BOS)-free survival (log-rank p<0.001). On multivariable Cox Proportional Hazards regression, BDD was not associated with recipient survival (p>0.05). Similarly, logistic regression did not identify an independent association between BDD and primary graft dysfunction (PGD) (p>0.05). Increased BDD was, however, associated with a decreased risk of acute rejection (long vs reference, adjusted OR 0.78, 95% CI 0.64–0.94) and improved BOS-free survival (long vs reference, adjusted HR 0.88, 95% CI 0.81–0.96).
Conclusions
Donor BDD is not associated with post-transplant survival or PGD. Long donor BDD, however, is associated with a decreased risk for acute rejection and improved BOS-free survival. Therefore, lung allografts from donors with a prolonged length of time from brain death until explant should not be viewed less favorably by donor selection centers.
Graphical Abstract
Lung transplantation is increasingly utilized for the treatment of patients with end-stage lung disease. While the number of lung transplants performed each year in the US has increased, a substantial number of transplant candidates die on the waiting list due to a shortage of donor allografts (1,2). As a result, optimizing the allocation and management of the limited donor pool remains vital.
Studies have demonstrated significant physiologic changes associated with brain death including rising intracranial pressure, brain ischemia, and a substantial catecholamine response (3). As a result, careful hemodynamic and metabolic management of the potential organ donor is necessary to minimize end organ damage prior to transplant (4). Several studies have attempted to ascertain the impact of the time interval from donor brain death to subsequent organ procurement on post-transplant allograft function and survival, but with conflicting results and small sample sizes (5,6). Furthermore, prolonged intubation time is associated with an increased risk of developing ventilator associated pneumonia (VAP), aspiration, and barotrauma, which may have implications for graft function and survival. The purpose of this study is to evaluate the impact of donor brain death duration (BDD) on post-lung transplantation outcomes using a large national registry. We hypothesize that increasing BDD will correlate with poorer graft function and recipient survival.
Patients and Methods
Data source
The United Network for Organ Sharing (UNOS) provided deidentified donor and recipient transplant data from October 1987 through March 2018. The database includes prospectively collected clinical data for all organ transplants performed in the US. In addition, UNOS provided a separate file containing donor brain death and cross clamp time information for 98.8% of donors since 2007. This study was deemed exempt by our Institutional Review Board.
Study population
We retrospectively reviewed the UNOS database for all first-time, adult (age ≥18), single or bilateral lung transplant recipients between 2007–2018 and their associated donors. Transplant donors with missing time of brain death or cross clamp along with recipients who underwent multi-organ transplant were excluded. Donors with BDD recorded as less than 1 hour or greater than the 99th percentile were also excluded.
Data analysis
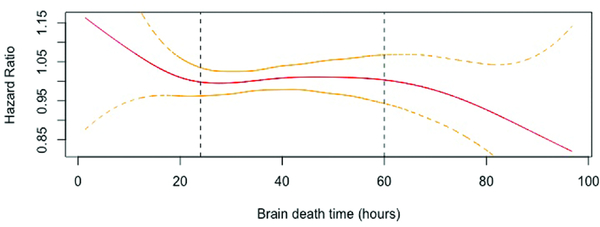
Donor BDD was calculated as the time difference, in hours, between donor declaration of brain death and subsequent cross clamp. To evaluate the impact of BDD on recipient survival, a multivariable Cox Proportional Hazards model was constructed with penalized smooth splines. The use of splines is a well validated technique permitting the creation of regression models using continuous variables through smoothly joined polynomial functions while adjusting for covariates without assuming linearity (7,8). Covariates included in the Cox model were selected a priori and included donor age, gender, race, ischemic time, brain death time, and recipient age, gender, race, BMI, history of diabetes, IV antibiotic requirement in prior two weeks, and lung allocation score (LAS) diagnosis group. The hazard associated with BDD from the Cox model was plotted. BDD intervals were then qualitatively identified for subsequent analyses using graphical inflection points in a similar fashion to prior studies from other medical disciplines (9,10).
Based on the output of the Cox model, donor BDD was divided into three intervals: short (SH, <24 hours), reference (24–60 hours), and long (LG, >60 hours). Comparisons between primary study cohorts were performed using the Wilcoxon rank sum test for continuous variables and the Pearson χ2 test or Fisher’s exact test for categorical variables.
Outcomes
The primary outcome was recipient survival. Secondary outcomes included recipient cause of death, acute rejection prior to discharge, primary graft dysfunction (PGD), and bronchiolitis obliterans syndrome (BOS)-free survival. PGD was defined as PaO2/FiO2 (P/F) ratio <200 (grade 3) or requiring ECMO support at 72 hours post-transplant. In the UNOS database, the 72-hour P/F ratio is only documented in approximately 50% of patients since 2015 so this analysis was restricted to the population of patients with non-missing data during this period.
Unadjusted survival was estimated using the Kaplan-Meier method. Adjusted Cox Proportional Hazard as well as both unadjusted and adjusted logistic regression models were created. Model covariates were selected a priori. Donor brain death duration was entered as a categorical variable divided into reference, short, and long intervals. To account for within-center clustering, transplant center ID was entered into the Cox models as a cluster variable. Models were stratified by year (pre-2012 vs 2012 and later). Multivariable regression was performed as complete case analyses. Multiple imputation was performed on sensitivity analysis. Outcome variables were not imputed.
Two-sided p-values ≤0.05 were considered statistically significant. Statistical analyses were performed using R version 3.5.1 (Vienna, Austria).
Results
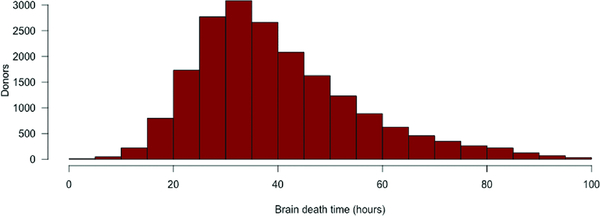
19,721 donors and recipients met inclusion criteria including 6,155 (31.2%) single and 13,566 (68.9%) bilateral lung transplant recipients. Median BDD was 36.6 hours (IQR 19.5) (Figure 1). To qualitatively determine clinically relevant BDD intervals for subsequent analyses, a Cox Proportional Hazards model was created with penalized smooth splines (Figure 2). This yielded three BDD intervals for subsequent analyses: short (SH, <24 hours), reference (24–60 hours), and long (LG, >60 hours).
Figure 1.
Distribution of donor brain death time.
Figure 2.
Adjusted Cox Proportional Hazards model with penalized smooth splines. Vertical lines represent cutoffs for short (SH), reference, and long (LG) cohorts.
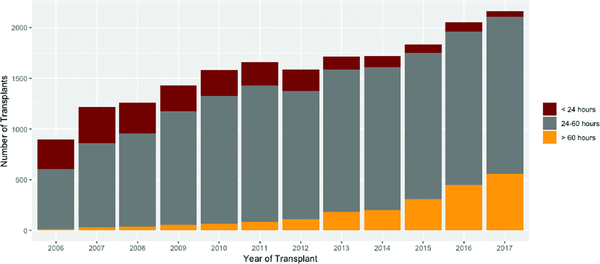
The SH, reference, and LG BDD intervals were comprised of 2,455 (12.3%), 15,097 (76.6%), and 2,169 (11.1%) donor-recipient pairs, respectively. BDD increased annually during the study period (Figure 3). Compared to the other cohorts (Table 1), SH recipients were more likely to be male, younger, white, have a history of IV antibiotics in the preceding two weeks, require ventilatory support prior to transplant, and spend a longer amount of time on the waiting list. These patients also had a lower lung allocation score (LAS) at match and had an earlier year of transplant. SH donors (Table 2) were older, more likely to be white, and have a history of cigarette use and cancer. Increasing BDD was also associated with a greater incidence of pulmonary infection. Donors in all three cohorts donated a similar number of organs for transplant.
Figure 3.
Distribution of donor brain death time by year.
Table 1.
Recipient characteristics stratified by donor brain death duration
| Time from brain death to cross clamp | ||||
|---|---|---|---|---|
| Variable | < 24 hours | 24–60 hours | > 60 hours | p-value |
| (n=2,455) | (n=15,097) | (n=2,169) | ||
| Male gender | 61.4% (1, 508) | 59.6% (8, 998) | 57.4% (1, 244) | 0.019 |
| Age (median, IQR) | 58 (15) | 60 (14) | 61 (14) | < 0.001 |
| BMI (median, IQR) | 25.4 (7.4) | 25.4 (7.0) | 25.8 (6.8) | 0.087 |
| Ethnicity | < 0.001 | |||
| White | 85.8% (2, 107) | 82.8% (12, 497) | 77.8% (1, 687) | |
| Black | 8.3% (203) | 9.0% (1, 353) | 8.8% (190) | |
| Hispanic | 4.2% (103) | 5.9% (894) | 10.4% (225) | |
| Other | 1.7% (42) | 2.3% (353) | 3.1% (67) | |
| Recipient history | ||||
| Diabetes | 18.5% (453) | 18.7% (2, 822) | 17.5% (380) | 0.419 |
| Malignancy | 6.9% (170) | 7.8% (1, 175) | 7.4% (160) | 0.296 |
| LAS diagnosis group | < 0.001 | |||
| A | 32.1% (789) | 31.0% (4, 677) | 27.7% (601) | |
| B | 3.4% (83) | 3.3% (499) | 3.7% (80) | |
| C | 13.4% (328) | 11.8% (1, 778) | 9.8% (213) | |
| D | 51.1% (1, 255) | 53.9% (8, 143) | 58.8% (1, 275) | |
| Recipient creatinine (median, IQR) | 0.8 (0.3) | 0.8 (0.3) | 0.8 (0.3) | < 0.001 |
| Recipient bilirubin (median, IQR) | 0.5 (0.4) | 0.5 (0.4) | 0.5 (0.4) | 0.027 |
| Pre-transplant status | 0.354 | |||
| Intensive care unit | 11.1% (272) | 10.0% (1, 509) | 9.8% (213) | |
| Hospitalized (non-ICU) | 8.1% (198) | 8.9% (1, 345) | 8.8% (191) | |
| Not hospitalized | 80.9% (1, 985) | 81.1% (12, 243) | 81.4% (1, 765) | |
| Medical therapy | ||||
| IV antibiotics in two weeks before transplant | 12.0% (294) | 10.2% (1, 539) | 8.9% (193) | 0.002 |
| Ventilator support at transplant | 6.7% (165) | 6.1% (922) | 4.4% (96) | 0.002 |
| ECMO support at transplant | 2.6% (65) | 3.0% (455) | 3.7% (80) | 0.110 |
| ABO blood type | 0.057 | |||
| A | 42.0% (1, 032) | 40.4% (6, 103) | 37.7% (818) | |
| B | 11.5% (282) | 11.0% (1, 659) | 11.3% (246) | |
| AB | 4.0% (98) | 3.8% (578) | 3.7% (80) | |
| O | 42.5% (1, 043) | 44.8% (6, 757) | 47.3% (1, 025) | |
| Days on waitlist (median, IQR) | 65 (187.5) | 62 (171) | 53 (156) | 0.016 |
| LAS at match (median, IQR) | 39.6 (15.3) | 40.6 (16.8) | 40.0 (16.0) | 0.019 |
| Year of transplant (median, IQR) | 2009 (5) | 2012 (5) | 2015 (4) | < 0.001 |
IQR, interquartile range; BMI, body mass index; LAS, lung allocation score; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation
Table 2.
Donor characteristics stratified by donor brain death duration
| Time from brain death to cross clamp | ||||
|---|---|---|---|---|
| Variable | < 24 hours | 24–60 hours | > 60 hours | p-value |
| (n=2,455) | (n=15,097) | (n=2,169) | ||
| Donor male gender | 60.0% (1, 472) | 59.9% (9, 043) | 61.7% (1, 339) | 0.261 |
| Donor age (median, IQR) | 35 (26.5) | 32 (24) | 32 (23) | < 0.001 |
| Donor BMI (median, IQR) | 24.9 (6.1) | 25.2 (6.4) | 25.7 (6.6) | < 0.001 |
| Donor ethnicity | < 0.001 | |||
| White | 73.0% (1, 792) | 62.3% (9, 403) | 42.9% (931) | |
| Black | 15.2% (372) | 19.8% (2, 991) | 20.6% (446) | |
| Hispanic | 8.6% (210) | 13.9% (2, 100) | 29.3% (636) | |
| Other | 3.3% (81) | 4.0% (603) | 7.2% (156) | |
| Donor history | ||||
| Cigarette use | 11.6% (286) | 9.2% (1, 396) | 6.1% (132) | < 0.001 |
| Cocaine use | 10.3% (254) | 13.5% (2, 041) | 14.8% (321) | < 0.001 |
| Alcohol abuse | 12.4% (304) | 13.9% (2, 105) | 14.6% (316) | 0.065 |
| Diabetes | 7.5% (185) | 7.0% (1, 058) | 7.1% (153) | 0.639 |
| Hypertension | 24.6% (604) | 23.3% (3, 511) | 21.7% (471) | 0.068 |
| Cancer | 2.6% (64) | 1.9% (290) | 1.1% (23) | 0.001 |
| Pulmonary infection | 40.7% (998) | 56.4% (8, 522) | 72.5% (1, 573) | < 0.001 |
| Donor creatinine (median, IQR) | 1.0 (0.6) | 1.0 (0.7) | 1.0 (0.9) | < 0.001 |
| Donor bilirubin (median, IQR) | 0.8 (0.7) | 0.7 (0.6) | 0.8 (0.7) | < 0.001 |
| Donor cause of death | < 0.001 | |||
| Anoxia | 14.8% (363) | 18.5% (2, 790) | 23.7% (515) | |
| Cerebrovascular/stroke | 40.1% (984) | 33.0% (4, 985) | 29.2% (634) | |
| Head trauma | 42.6% (1, 046) | 45.4% (6, 848) | 44.1% (957) | |
| CNS tumor | 0.5% (12) | 0.7% (100) | 0.7% (15) | |
| Other | 2.0% (50) | 2.5% (374) | 2.2% (48) | |
| Organs recovered for transplant (median, IQR) | 6 (2) | 6 (2) | 6 (1) | < 0.001 |
| ABO blood type | 0.004 | |||
| A | 37.4% (917) | 36.6% (5, 529) | 33.4% (724) | |
| B | 11.1% (273) | 10.8% (1, 630) | 10.6% (230) | |
| AB | 2.4% (60) | 2.2% (337) | 1.6% (34) | |
| O | 49.1% (1, 205) | 50.3% (7, 601) | 54.4% (1, 181) | |
| Graft ischemic time (hours, median, IQR) | 5.0 (2.1) | 5.0 (2.1) | 4.9 (2.2) | < 0.001 |
IQR, interquartile range; BMI, body mass index; CNS, central nervous system
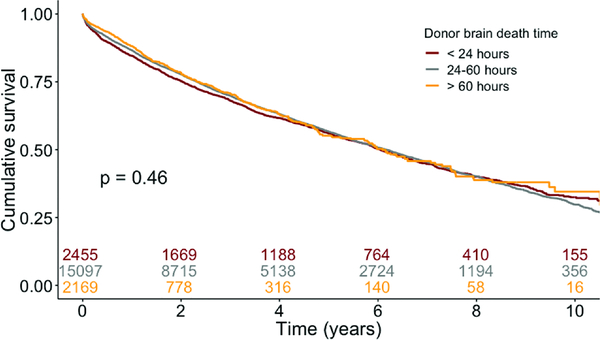
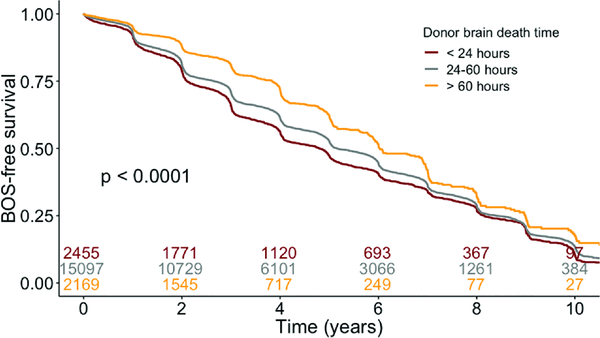
Recipients from all cohorts had similar unadjusted lengths of hospital stay, rates of grade 3 primary graft dysfunction (PGD) 72 hours post-transplant, and cause of death (Table 3). LG interval recipients had lower rates of acute rejection prior to discharge (6.4% vs. 8.3% in reference and 9.6% in SH; p<0.001) compared with reference and SH recipient cohorts. There were no differences in mortality due to acute rejection amongst the groups. Recipients with SH, reference, and LG BDD intervals had similar survival (Figure 4, logrank p=0.46). Increasing BDD, however, was associated with improved BOS-free survival on unadjusted analysis (Figure 5).
Table 3.
Unadjusted outcomes stratified by donor brain death duration
| Time from brain death to cross clamp | |||||
|---|---|---|---|---|---|
| Variable | < 24 hours | 24–60 hours | > 60 hours | Missing (%) | p-value |
| (n=2,375) | (n=14,766) | (n=2,137) | |||
| Length of stay, transplant to discharge, days (IQR) | 16 (17) | 16 (15) | 15 (13) | 1.5% | 0.012 |
| Acute rejection between transplant and discharge | 9.6% (235) | 8.3% (1, 258) | 6.4% (138) | - | < 0.001 |
| Treated for rejection within 1 year* | 30.5% (600) | 28.1% (3, 174) | 22.3% (301) | 15.9% | < 0.001 |
| Primary graft dysfunction, grade 3** | 29.7% (38) | 31.8% (809) | 30.3% (229) | 48.9% | 0.670 |
| Recipient cause of death*** | 0.3% | ||||
| Primary failure | 3.0% (35) | 2.6% (141) | 1.9% (9) | 0.436 | |
| Acute rejection | 1.3% (15) | 1.2% (66) | 2.1% (10) | 0.269 | |
| Chronic rejection | 13.7% (160) | 15.1% (815) | 16.9% (81) | 0.221 | |
| Infection | 21.0% (245) | 20.3% (1, 096) | 16.7% (80) | 0.137 | |
| Cardiovascular | 6.1% (71) | 8.0% (433) | 6.9% (33) | 0.065 | |
| Pulmonary | 21.2% (248) | 20.1% (1, 088) | 20.3% (97) | 0.696 | |
| Cerebrovascular | 3.7% (43) | 3.3% (178) | 4.4% (21) | 0.391 | |
| Multiple-organ failure | 5.8% (68) | 5.1% (275) | 6.3% (30) | 0.361 | |
Prior to 2017
2015–2018, of patients with documented P/F ratio and/or ECMO at 72 hours
of patients that died during follow up
Figure 4.
Kaplan-Meier survival analysis. Survival curves stratified by donor brain death duration.
Figure 5.
Kaplan-Meier bronchiolitis obliterans syndrome (BOS)-free survival. Survival curves stratified by donor brain death duration.
Factors associated with improved recipient survival on Cox Proportional Hazards analysis (Table 4) included donor white race, shorter ischemic times, as well as younger recipient age, female gender, and group C lung disease diagnosis. Increasing donor age above 40, donor black and Hispanic race, recipient BMI less than 20 or greater than 30, recipient diabetes, and requiring IV antibiotics in the two weeks prior to transplant all predicted worsened survival. Compared with the reference cohort, neither SH (HR 1.01, 95% CI 0.94–1.009) nor LG (HR 0.95, 95% CI 0.86–1.06) intervals were associated with different post-transplant recipient survival. Results remained unchanged after multiple imputation was performed for missing covariate data on sensitivity analysis.
Table 4.
Cox Proportional Hazards model of recipient survival
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Predictor | Hazard Ratio | Lower | Upper | p-value |
| Donor/graft characteristics | ||||
| Age < 40 (per 5 years) | 0.99 | 0.98 | 1.01 | 0.238 |
| Age ≥ 40 (per 5 years) | 1.06 | 1.04 | 1.08 | < 0.001 |
| Male gender (vs female) | 0.96 | 0.91 | 1.02 | 0.211 |
| Ethnicity | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 1.19 | 1.12 | 1.27 | < 0.001 |
| Hispanic | 1.09 | 1.01 | 1.18 | 0.019 |
| Other | 1.02 | 0.88 | 1.18 | 0.814 |
| Ischemic time < 6 hours (per hour) | 0.96 | 0.93 | 0.99 | 0.017 |
| Ischemic time ≥ 6 hours (per hour) | 1.06 | 1.02 | 1.11 | 0.004 |
| Brain death time | ||||
| 24–60 hours | Ref | Ref | Ref | Ref |
| < 24 hours | 1.01 | 0.94 | 1.09 | 0.745 |
| > 60 hours | 0.95 | 0.86 | 1.06 | 0.397 |
| Recipient characteristics | ||||
| Age < 45 (per 5 years) | 0.88 | 0.86 | 0.91 | < 0.001 |
| Age ≥ 45 (per 5 years) | 1.17 | 1.15 | 1.20 | < 0.001 |
| Male gender (vs female) | 1.08 | 1.02 | 1.15 | 0.010 |
| Ethnicity | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 1.08 | 0.99 | 1.19 | 0.100 |
| Hispanic | 0.93 | 0.82 | 1.05 | 0.223 |
| Other | 0.86 | 0.71 | 1.04 | 0.128 |
| BMI | ||||
| 20–25 | Ref | Ref | Ref | Ref |
| <20 | 1.09 | 1.02 | 1.18 | 0.015 |
| 25–30 | 1.03 | 0.97 | 1.10 | 0.267 |
| >30 | 1.14 | 1.05 | 1.23 | 0.001 |
| Diabetes | 1.06 | 1.00 | 1.13 | 0.040 |
| IV antibiotics in two weeks before transplant | 1.21 | 1.12 | 1.32 | < 0.001 |
| LAS diagnosis group | ||||
| D | Ref | Ref | Ref | Ref |
| A | 0.95 | 0.89 | 1.01 | 0.125 |
| B | 1.14 | 0.97 | 1.34 | 0.102 |
| C | 0.77 | 0.66 | 0.89 | < 0.001 |
BMI, body mass index; LAS, lung allocation score
The association between BDD and grade 3 PGD 72 hours post-transplant as well as acute rejection prior to discharge was modeled with logistic regression (Table 5). On both unadjusted and adjusted analyses, BDD was not associated with 72-hour grade 3 PGD. However, LG BDD was associated with decreased risk of acute rejection prior to discharge (unadjusted OR 0.75, 95% CI 0.62–0.90; adjusted OR 0.78, 95% CI 0.64–0.94). Similarly, LG BDD was associated with a decreased risk of treatment for rejection within 1-year post-transplant (unadjusted OR 0.73, 95% CI 0.64–0.84; adjusted OR 0.84, 95% CI 0.73–0.96). Compared with the reference cohort, LG BDD was associated with improved BOS-free survival (adjusted HR 0.88, 95% CI 0.81–0.96).
Table 5.
Unadjusted and adjusted logistic regression
| Unadjusted | Adjusted* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% Confidence Interval | 95% Confidence Interval | ||||||||
| Outcome | Brain death time | Odds Ratio | Lower | Upper | p-value | Odds Ratio | Lower | Upper | p-value |
| Primary graft dysfunction | 24–60 hours | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| < 24 hours | 0.90 | 0.60 | 1.36 | 0.578 | 0.84 | 0.55 | 1.29 | 0.428 | |
| > 60 hours | 0.95 | 0.79 | 1.13 | 0.602 | 1.01 | 0.84 | 1.22 | 0.910 | |
| Acute rejection episode prior to discharge | 24–60 hours | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| < 24 hours | 1.16 | 1.01 | 1.35 | 0.041 | 1.10 | 0.94 | 1.29 | 0.224 | |
| > 60 hours | 0.75 | 0.62 | 0.9 | 0.002 | 0.78 | 0.64 | 0.94 | 0.010 | |
| Treated for rejection within 1 year |
24–60 hours | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| < 24 hours | 1.12 | 1.01 | 1.25 | 0.031 | 1.03 | 0.92 | 1.15 | 0.604 | |
| > 60 hours | 0.73 | 0.64 | 0.84 | < 0.001 | 0.84 | 0.73 | 0.96 | 0.012 | |
adjusted for donor age, gender, race, ischemic time, brain death time, and recipient age, gender, race, BMI, history of diabetes, IV antibiotic requirement in the prior two weeks, medical condition at transplant (not hospitalized, hospitalized, or in the intensive care unit), and year of transplant
Comment
In this UNOS analysis, we examined the impact of donor BDD on recipient outcomes. While increasing BDD was associated with a greater incidence of donor pulmonary infection, we found no association between BDD and recipient survival or the incidence of PGD. We demonstrated that longer BDD intervals were associated with a lower risk of acute rejection and improved overall BOS-free survival.
This is the first study, to our knowledge, to analyze the impact of BDD on lung transplant outcomes using a large national database. Previous studies have used transplant models. Van der Hoeven and colleagues examined the impact of BDD in a rat model on donor kidney function (11). They found evidence of donor organ injury caused by a brain death-associated inflammatory response, which was magnified by donor instability prior to procurement. Using a rat lung transplant model, Avlonitis and colleagues demonstrated more severe reperfusion injury in lungs retrieved early from brain dead donors compared with those retrieved late (6). In this study, we did not find an association between BDD and PGD rates, although our analysis was limited to allografts deemed suitable for transplantation.
Several studies, the majority of which are single-institutional analyses, have examined the impact of lung transplant BDD on recipient outcomes. Wauters and colleagues demonstrated a recipient survival benefit associated with donor BDD exceeding 10 hours (12). This survival benefit was not observed until several years post-transplant, suggesting causes other than early graft dysfunction. Pecoraro and colleagues, however, found no impact on long-term outcomes or perioperative complications with varying lengths of BDD, although their study had a small sample size(13).
The significance of BDD has also been examined in other organ transplants, with conflicting results. In a review of 500 heart transplants, Cantin and colleagues concluded that BDD longer than 72 hours was associated with decreased survival (5). However, the time from traumatic injury to brain death was included in their definition of management time, confounding this analysis. Similarly, a single center retrospective review of 157 heart transplants by Ramjug and colleagues found an association between longer BDD and decreased recipient survival, although the median BDD in their population was 13 hours, compared to the 36 hour median interval in the present study (14). In 2013, Marasco and colleagues found no association between BDD and recipient survival in heart transplants performed at their institution (15). Nijboer and colleagues, in a UNOS kidney transplant database study in 2011, found an association between longer BDD intervals and a decreased risk of delayed graft function as well as 1- and 3-year graft failure (16).
In the context of the published literature, this study represents the largest analysis of recipient outcomes stratified by donor BDD in lung transplantation. Our primary finding, the lack of an association between BDD and recipient survival, corroborates many previous studies. However, the median BDD in our study (~37 hours) is longer than the majority of the single center retrospective analyses. This difference may be the result of the more modern cohort analyzed in the present study, especially since donor BDD has been increasing over time, as well as differences in donor management times between the US and Europe. In their analysis of the UNOS kidney registry, Nijboer and colleagues also highlighted the stark difference in BDD between the US and Europe (16). They speculated that the difference stemmed from the often-lengthy consent process for organ donation in the US as well as more expedient scheduling of organ retrieval in Europe. We suspect this finding may also be related to procurement delays associated with the organ procurement organization (OPO) system in the US.
Our findings demonstrating an association between donor BDD and lung transplant acute rejection and BOS, but not PGD are novel among published studies. Donor brain death and the resulting inflammatory response illustrated by previous studies may provide a possible mechanism for these findings (5,6). Donor brain death induced hemodynamic instability likely leads to increased allograft inflammation and upregulation of cytokines (11). Increased BDD may be protective against the development of subsequent acute cellular rejection by allowing greater time for homeostatic mechanisms and donor hemodynamic stabilization prior to procurement. While long BDD intervals were associated with reduced acute rejection and improved BOS-free survival, a concomitant decrease in overall mortality was not observed; therefore, its clinical significance is unclear. Additional research is needed to elucidate the mechanism of this effect and to determine if donor management and allocation strategies should be altered as a result. At a minimum, these results support the practice of allowing time for the donor brain death inflammatory response to settle, increasing the yield of transplantable organs (17).
There are several limitations to this study. As a retrospective review of a large national registry, we are limited by the quantity and quality of available variables which weakens our ability to control for potential confounders. The UNOS/OPTN Registry is an ideal data source for this analysis, however, as it captures 100% of transplants performed in the US and provides a sample size that is able to generate meaningful trends that were deficient in single-center studies. Furthermore, less than 2% of lung transplant donors had missing brain death time recorded in the database limiting the introduction of selection bias. However, that time from donor declaration of brain death until cross clamp was used as a surrogate for BDD, as some donors likely had an unknown period of brain death prior to clinical assessment. Similarly, the total duration of donor mechanical ventilation was also not available. Furthermore, an important limitation was the lack of comprehensive data regarding PGD. While >3,000 patients were included in the PGD analysis from 2015, this only represents approximately 50% of all lung transplants during this period due to missing data, which introduces the possibility of bias. The lack of availability of other diagnostic criteria for PGD including infiltrates on chest radiography is another limitation. In addition, while donor BDD did not seem to correlate with the number of organs recovered for transplant in this study, this analysis was limited to lung allograft donors and further investigation is warranted to elucidate the impact of BDD on procurement practices. Perhaps most importantly, this analysis was limited to the subset of donor allografts deemed suitable for transplantation, introducing the potential for selection bias. Despite these limitations, the design of the present study is likely the best possible way to address this question.
Conclusion
Increased time from donor declaration of brain death until cross clamp does not impact post-lung transplant recipient survival or primary graft dysfunction but is associated with decreased rates of acute rejection and improved BOS-free survival. Therefore, donor selection centers should not exclude lung transplant donors solely based upon prolonged brain death and intubation times.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Valapour M, Lehr CJ, Skeans MA et al. OPTN/SRTR 2016 Annual Data Report: Lung. Am J Transplant 2018;18 Suppl 1:363–433. [DOI] [PubMed] [Google Scholar]
- 2.Chambers DC, Yusen RD, Cherikh WS et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1047–1059. [DOI] [PubMed] [Google Scholar]
- 3.Smith M Physiologic changes during brain stem death--lessons for management of the organ donor. J Heart Lung Transplant 2004;23:S217–22. [DOI] [PubMed] [Google Scholar]
- 4.Citerio G, Cypel M, Dobb GJ et al. Organ donation in adults: a critical care perspective. Intensive Care Med 2016;42:305–15. [DOI] [PubMed] [Google Scholar]
- 5.Cantin B, Kwok BW, Chan MC et al. The impact of brain death on survival after heart transplantation: time is of the essence. Transplantation 2003;76:1275–9. [DOI] [PubMed] [Google Scholar]
- 6.Avlonitis VS, Wigfield CH, Golledge HD, Kirby JA, Dark JH. Early hemodynamic injury during donor brain death determines the severity of primary graft dysfunction after lung transplantation. Am J Transplant 2007;7:83–90. [DOI] [PubMed] [Google Scholar]
- 7.Molinari N, Daures JP, Durand JF. Regression splines for threshold selection in survival data analysis. Stat Med 2001;20:237–47. [DOI] [PubMed] [Google Scholar]
- 8.Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed 1997;54:201–8. [DOI] [PubMed] [Google Scholar]
- 9.Sun Z, Adam MA, Kim J et al. Determining the Optimal Timing for Initiation of Adjuvant Chemotherapy After Resection for Stage II and III Colon Cancer. Dis Colon Rectum 2016;59:87–93. [DOI] [PubMed] [Google Scholar]
- 10.Salazar MC, Rosen JE, Wang Z et al. Association of Delayed Adjuvant Chemotherapy With Survival After Lung Cancer Surgery. JAMA Oncol 2017;3:610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Hoeven JA, Molema G, Ter Horst GJ et al. Relationship between duration of brain death and hemodynamic (in)stability on progressive dysfunction and increased immunologic activation of donor kidneys. Kidney Int 2003;64:1874–82. [DOI] [PubMed] [Google Scholar]
- 12.Wauters S, Verleden GM, Belmans A et al. Donor cause of brain death and related time intervals: does it affect outcome after lung transplantation? Eur J Cardiothorac Surg 2011;39:e68–76. [DOI] [PubMed] [Google Scholar]
- 13.Pecoraro Y, Tsushima Y, Opitz I et al. Impact of time interval between donor brain death and cold preservation on long-term outcome in lung transplantation. Eur J Cardiothorac Surg 2016;50:264–8. [DOI] [PubMed] [Google Scholar]
- 14.Ramjug S, Hussain N, Yonan N. Prolonged time between donor brain death and organ retrieval results in an increased risk of mortality in cardiac transplant recipients. Interact Cardiovasc Thorac Surg 2011;12:938–42. [DOI] [PubMed] [Google Scholar]
- 15.Marasco S, Kras A, Schulberg E et al. Donor brain death time and impact on outcomes in heart transplantation. Transplant Proc 2013;45:33–7. [DOI] [PubMed] [Google Scholar]
- 16.Nijboer WN, Moers C, Leuvenink HG, Ploeg RJ. How important is the duration of the brain death period for the outcome in kidney transplantation? Transpl Int 2011;24:14–20. [DOI] [PubMed] [Google Scholar]
- 17.Munshi L, Keshavjee S, Cypel M. Donor management and lung preservation for lung transplantation. Lancet Respir Med 2013;1:318–28. [DOI] [PubMed] [Google Scholar]