Abstract
Rationale & Objective:
Identifying patients who are likely to transfer from peritoneal dialysis (PD) to hemodialysis (HD) prior to transition could improve their subsequent care. This study developed a prediction tool for transition from PD to HD.
Study Design:
Retrospective cohort study.
Setting & participants:
Adults initiating PD between January 2008 and December 2011, followed through June 2015 whose data were available in the US Renal Data System (USRDS).
Predictors:
Clinical characteristics at PD initiation and peritonitis claims.
Outcomes:
Transfer to HD, with the competing outcomes of death and kidney transplantation.
Analytical Approach:
Outcomes were ascertained from USRDS treatment history files. Sub-distribution hazards (competing risk) models were fit using clinical characteristics at PD initiation. A nomogram was developed to classify patient risk at 1, 2, 3, and 4 years. These data were used to generate quartiles of HD transfer risk; this quartile score was incorporated into a cause-specific hazards model that additionally included a time-dependent variable for peritonitis.
Results:
29,573 incident PD patients were followed for a median of 21.6 (interquartile range, 9.0–42.3) months, during which 41.2% transferred to hemodialysis, 25.9% died, 17.1% underwent kidney transplantation; and the remainder were followed to the study end in June 2015. Claims for peritonitis were present in 11,733 (40.2%) of patients. The proportion of patients still receiving PD fell below 50% at 22.6 months and to 14.2% at five years. Peritonitis was associated with a higher rate of HD transfer (HR, 1.82; 95% CI, 1.76–1.89; P<0.001), as were higher quartile scores of HD transfer risk (HRs of 1.31 [95% CI, 1.25–1.37), 1.51 [95% CI, 1.45–1.58], and 1.78 [95% CI, 1.71–1.86] for quartiles 2, 3, and 4 compared to quartile 1 [P<0.001 for all]).
Limitations:
Observational data, reliant on the Medical Evidence Report and Medicare claims.
Conclusions:
A large majority of patients initiating stopped PD discontinue this modality of renal replacement therapy within five years. Transfer to HD was the most common outcome. Patient characteristics and comorbid diseases influenced the probability of HD transfer, death, and transplantation, as did episodes of peritonitis.
Index words: peritoneal dialysis (PD), hemodialysis (HD), HD transfer, competing risks, prediction, nomogram, dialysis modality, modality switch, renal replacement therapy (RRT), prognosis, prediction tool
Introduction
Peritoneal dialysis (PD) is an appealing treatment for patients with good self-care skills or a supportive home environment.1–4 Patients choosing PD can schedule treatments to accommodate work and social responsibilities; dietary regimens are less restrictive and residual kidney function is preserved longer than with hemodialysis (HD). Medicare policy currently encourages the expansion of PD programs, and the number of patients starting PD has increased from 6,376 in 2007 to 11,236 in 2014, rising from 6.0% to 9.3% of incident dialysis patients.5 However, PD utilization among prevalent dialysis patients is only 6.9%, and many patients electing PD ultimately transfer to HD.6–7 Despite regular access to nephrology care, patients transfering from PD to HD in the United States initiate HD with arteriovenous fistulas or grafts half as often than patients starting HD as their first dialysis modality (8.5% vs. 19.8%).7–8 Roughly half of central venous catheters (CVC) placed for transfer from PD to HD remained in place six months after transition.8
These facts suggest that clinicians cannot readily identify which PD patients will transition to HD, and thereby miss opportunities to prepare them for modality change. Although vascular access planning could help patients beginning PD who are likely to transfer to HD, a pre-emptive fistula is of little benefit to a PD patient who receives a kidney transplant or dies without ever receiving HD.
A useful model for transition from PD to HD has been elusive. The risk of transition is to some degree intrinsic to individual patient characteristics, but may also be influenced by events occurring after PD initiation, so we wanted preliminary models that accounted for both types of information. Among patients initiating dialysis in 2001–2009, 44–50% of HD patients and 33–45% of PD patients died within 36 months;5 technique survival in PD must therefore be interpreted alongside the competing risk of death.10 PD patients often have favorable characteristics that make them good candidates for kidney transplantation, and some clinicians prefer PD as a pre-transplantation treatment modality, so transplantation also represents a significant competing outcome for patients on PD.
We built a simple prediction tool based on sequential models for transition from PD to HD, using sub-distribution hazards regression to obtain a patient-specific model for HD transfer, using data obtained at enrollment in the US Renal Data System (USRDS).
Methods
Data Sources and Study Population
The study population consisted of all patients age 18 or older in USRDS who initiated treatment with PD as their first treatment modality between 1/1/2008-12/31/2011. Treatment History files were used to ascertain outcomes. Medicare claims were used to identify peritonitis.
Study Variables
Candidate predictors were selected a priori from the Medical Evidence Form (CMS Form 2728), based on the results of prior analyses. Age, serum albumin, body mass index (BMI, in kg/m2), and estimated glomerular filtration rate (eGFR, in ml/min/1.73m2) were obtained from the Medical Evidence Form. Race was stratified as African-American, Asian, and all others. The primary diagnosis from the USRDS Patients file was categorized as hypertensive, diabetic, and all others. eGFR was stratified at greater than 15 ml/min/1.73m2 versus 15 or less, as a surrogate for dialysis being undertaken for volume overload rather than uremia. We identified episodes of peritonitis as any Medicare claim with ICD-9 codes 567.x and 568.x, occurring during the period in which each patient received PD, constructing a time-dependent variable based on the date of the first claim.
Primary Outcome
The primary outcome of interest was a modality change from PD to any form of HD. Death and transplantation were analyzed as competing outcomes. USRDS Treatment History Files were used to define modality changes and deaths. Patients were followed until a first event of transition to HD, kidney transplantation, death, or July 1, 2015. Hemodialysis and transplant events of less than 90 days duration were not considered endpoints, providing patients resumed PD thereafter. Patients were censored for recovery of kidney function or loss to follow-up for more than 90 days. Patients who died within 30 days of the last PD treatment were considered to have died without transition to another treatment type.
Statistical Analysis
Means and medians were used to summarize normally and non-normally distributed continuous variables, respectively, and categorical variables were expressed as frequencies and proportions. Chi-squared tests, one-way analysis of variance, and Kruskal-Wallis tests were used as appropriate to compare baseline values. Competing risks regression, with the methods of Fine and Grey,10,11 was used to obtain sub-distribution hazards for predictor variables on the cumulative incidence functions of all competing outcomes over time. We focused on HD transfer, but also generated subhazards for transplantation and death. Using baseline demographic and clinical variables, we developed the multivariable subdistribution hazards models using iterative backwards and forwards selection among variables with significant Wald tests (P<0.001) in univariable analyses, using likelihood ratio tests and Akaike Information Criteria (AIC) as appropriate to compare nested and non-nested models. All potential two-way interactions were evaluated as additions to the main effects model. Significant interactions were added simultaneously, using backwards selection to eliminate non-contributory interactions. Cumulative incidence plots were used to examine outcomes over time. Proportionality assumptions were evaluated by plotting mean Schoenfeld residuals for each variable over time.
Sensitivity analyses were performed for the competing risks model to evaluate the impact of missing data for the serum albumin level. Missing albumin values were evaluated as a separate category, with single imputation of the mean value, and with a complete-cases analysis.
A nomogram was derived from the competing risks model, using methods described by Zhang, incorporating both main effects and interactions.12 Point scores according to the nomogram were calculated for each patient, and patients were classified into quartiles according to scores, with patients in Quartile 1 having the lowest risks for HD transfer. The quartile score was passed to a cause-specific hazards model for time to transfer to HD, which included a time-dependent variable for peritonitis that could not be incorporated into the initial model. Harrell’s c-index was calculated for the cause-specific model, using methodology described elsewhere.13, 14
The Tufts Medical Center and University of Chicago Institutional Review Boards approved the study and deemed it exempt from requirement for informed consent for the use of de-identified data. All analyses were conducted using SAS, version 9.4 (SAS Institute Inc., Cary NC) and R, version 2.2.7 (R Foundation for Statistical Computing, Vienna, Austria) using the ‘cmprsk’, ‘mstate’ and ‘rms’ packages.
Results
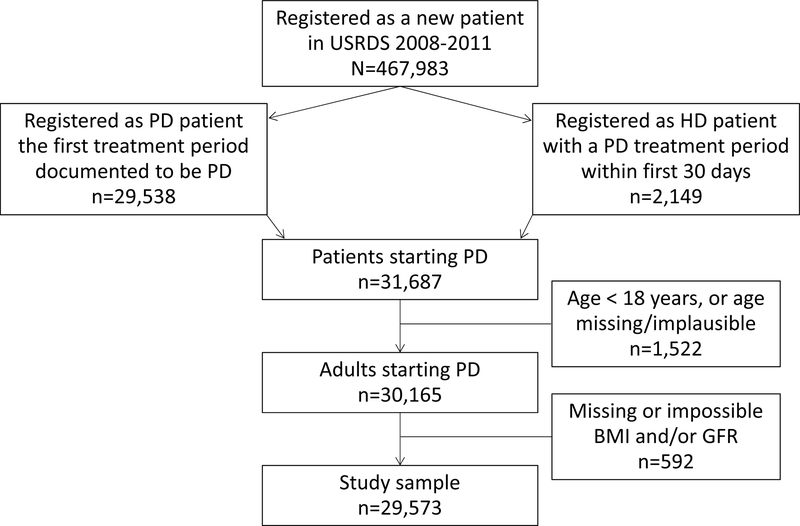
Over the study period, 30,165 adult patients initiated PD as an initial dialysis modality. Of these potential participants, 592 were excluded due to missing or implausible eGFR and/or missing BMI, resulting in a study population of 29,573 patients (Figure 1) with a mean age of 58.0 ± 15.2 years; 43.9% were women; 70.7% were Caucasian, 21.9% were African American, with 7.6% others (Table 1). Exposure to HD occurred in 15,726 patients (53.2%), for a median of 16 (interquartile range [IQR], 3–37) months.
Figure 1.
Flowchart of patients included in the study. Hemodialysis (HD), peritoneal dialysis (PD), glomerular filtration rate (GFR).
Table 1.
Baseline characteristics of Medicare patients starting PD, by first clinical outcome
| All (N=29,573) |
PD Outcome | |||||
|---|---|---|---|---|---|---|
| HD transfer (n=12,175) |
Death (n=7,646) |
Transplant (n=5,057) |
Other (n=4,695) |
P | ||
| Age years, mean (std) | 58.0 (15.2) | 57.5(14.7) | 66.6(12.9) | 48.7(13.5) | 55.4 (14.3) | <0.001 |
| Female sex, % | 43.9 | 42.9 | 42.5 | 43.2 | 49.3 | 0.001 |
| Race, % | <0.001 | |||||
| Causcasian | 70.7 | 67.5 | 79.5 | 72.4 | 62.6 | |
| African-American | 21.9 | 26.6 | 14.6 | 18.0 | 26.0 | |
| Asian | 5.2 | 3.7 | 4.0 | 7.6 | 8.7 | |
| Other | 2.2 | 2.2 | 1.9 | 2.0 | 2.7 | |
| eGFR, mean (std) | 12.2 (4.9) | 11.9 (4.8) | 13.0 (5.1) | 11.3 (4.6) | 12.4 (5.1) | <0.001 |
| eGFR>15ml/min/1.73 m2, % | 25.2 | 23.1 | 30.9 | 19.4 | 27.9 | <0.001 |
| BMI, kg/m2 mean (std) | 29.3 (7.1) | 30.2 (7.4) | 28.8 (7.2) | 27.8 (6.1) | 29.4 (7.1) | <0.001 |
| BMI > 30 kg/m2, % | 39.2 | 44.9 | 36.1 | 30.2 | 39.0 | <0.001 |
| Albumin (g/dL) mean (std) | 3.59 (0.64) | 3.53 (0.64) | 3.48 (0.62) | 3.76 (0.62) | 3.72 (0.62) | <0.001 |
| Albumin, % | <0.001 | |||||
| >4.0 g/dL | 19.0 | 16.5 | 13.6 | 27.5 | 25.4 | |
| 3.5–4.0 g/dL | 30.6 | 30.1 | 30.9 | 30.3 | 31.8 | |
| < 3.5 g/dL | 29.1 | 31.9 | 34.2 | 21.0 | 21.7 | |
| Missing | 21.3 | 21.4 | 21.3 | 21.3 | 21.1 | |
| Primary ESRD Diagnosis, % | <0.001 | |||||
| Diabetes | 42.6 | 47.1 | 49.5 | 28.3 | 35.0 | |
| Hypertension | 25.9 | 25.9 | 29.1 | 18.1 | 28.9 | |
| All others | 31.5 | 27.0 | 21.4 | 53.6 | 30.1 | |
| Employed, % | 36.0 | 33.4 | 20.2 | 61.2 | 41.3 | <0.001 |
Abbreviations: eGFR, estimated glomerular filtration rate; BMI, body mass index; ESRD, end-stage renal disease; PD, peritoneal dialysis; HD, hemodialysis.
Other: censored due to recovery of kidney function, loss to follow-up, or end of observation period
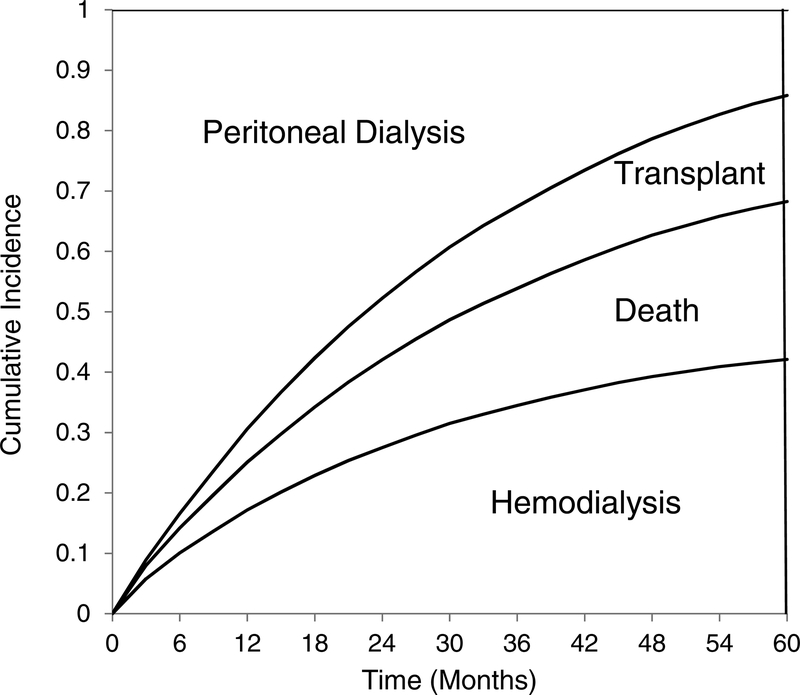
During follow-up, 12,175 (41.2%) patients transferred to hemodialysis, of whom 286 began home HD; 7,646 (25.9%) died, and 5,057 (17.1%) had kidney transplants; the remainder were censored. One or more claims for peritonitis were present in 12,121 (28.5%) of patients. Figure 2 shows the proportions of patients who transferred to hemodialysis, received kidney transplants, died, or remained on PD. Median technique survival was 22.6 months; 14.2% were still receiving PD after five years.
Figure 2.
Hemodialysis transfer, kidney transplantation, and death over time, for all incident PD patients. The prevalence of each outcome is shown as the vertical height of the appropriate area at each time point on the horizontal axis.
The subdistribution hazard model consisted of age, race, and primary diagnosis with eGFR, BMI, serum albumin, and employment status at PD initiation. Pre-dialysis nephrology care, sex, hemoglobin, year of treatment initiation, and the number of days spent training were not significant, nor were any comorbid conditions from the Medical Evidence Form. The frequency of missing albumin levels did not vary by outcome (P=0.7). Sensitivity analyses for missing albumin using complete cases only, with missing albumin as a separate category, and with single imputation of the mean value were essentially identical, so the final model included albumin with single imputation of the mean. Deviations from proportionality were trivial up to 72 months. Subdistribution hazard ratios for HD transfer, death, and kidney transplantation are presented in Table 2.
Table 2.
Competing risks model for all PD Outcomes, Main Effects
| Transfer to HD | Death | Transplant | ||||
|---|---|---|---|---|---|---|
| Covariate | HR (95% Cl) | P | HR (95% Cl) | P | HR (95% Cl) | P |
| Age, per 10 y older | 0.96 (0.95, 0.97) | <0.001 | 1.60 (1.57, 1.63) | <0.001 | 0.73 (0.72, 0.74) | <0.001 |
| Race | ||||||
| African American | 1.27 (1.22, 1.32) | <0.001 | 0.73 (0.69, 0.78) | <0.001 | 0.66 (0.61, 0.71) | <0.001 |
| Asian | 0.74 (0.67, 0.81) | <0.001 | 0.65 (0.58, 0.78) | <0.001 | 0.98 (0.88, 1.09) | <0.001 |
| All others | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Primary ESRD Diagnosis | ||||||
| Diabetic | 1.27 (1.21, 1.32) | <0.001 | 1.44 (1.35, 1.52) | <0.001 | 0.60 (0.56, 0.65) | <0.001 |
| Hypertensive | 1.18 (1.13, 1.25) | <0.001 | 1.18 (1.10, 1.26) | <0.001 | 0.62 (0.57, 0.67) | <0.001 |
| All others | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| eGFR>15 ml/min/1.73m2 | 0.92 (0.88, 0.96) | <0.001 | 1.20 (1.14, 1.26) | <0.001 | 0.85 (0.79, 0.91) | <0.001 |
| BMI, per 5 kg/m2 greater | 1.10 (1.09, 1.11) | <0.001 | 0.97 (0.95, 0.99) | 0.002 | 0.85 (0.83, 0.87) | <0.001 |
| Albumin, per 1 mg/dL greater | 0.85 (0.83, 0.88) | <0.001 | 0.72 (0.69, 0.75) | <0.001 | 1.45 (1.38,1.53) | <0.001 |
| Employed | 0.82 (0.79, 0.86) | <0.001 | 0.73 (0.69, 0.77) | <0.001 | 2.07(1.94, 2.20) | <0.001 |
N=29,573. Abbreviations: ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate; BMI, body mass index; PD, peritoneal dialysis; HD, hemodialysis; HR, hazard ratio; CI, confidence interval
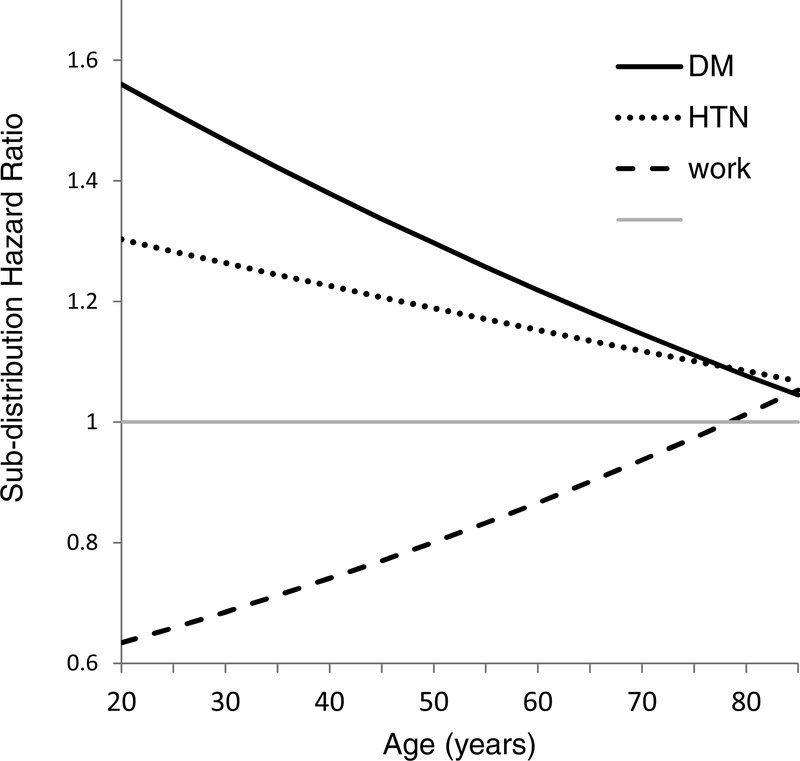
Controlling for other variables, African-American race and higher BMI were associated with higher subhazards for HD transfer, as were diabetic or hypertensive kidney disease. Higher serum albumin, employment, Asian-American race, and PD initiation at eGFR>15 ml/min/1.73m2 were associated with decreased subhazard for HD transfer. Age interacted significantly with primary diagnoses of either diabetic nephropathy or hypertensive nephropathy and also with employment (Figure 3).
Figure 3.
Interactions of the subdistribution hazard ratios for HD transfer with patient age. The dashed line shows the sub-distribution hazard ratios for employment versus unemployment, which increases with increasing age. The solid and dotted lines show that the sub-distribution hazard ratios for primary diabetic nephropathy or hypertensive nephropathy versus other causes of kidney failure decrease with increasing age.
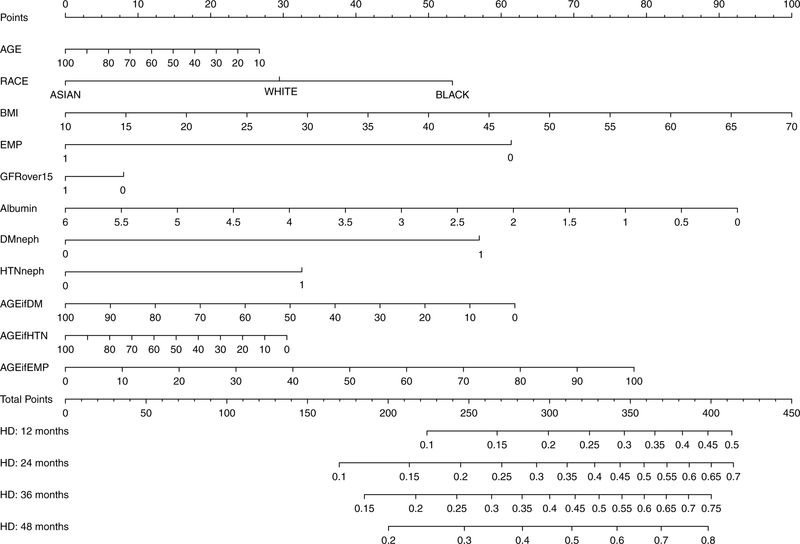
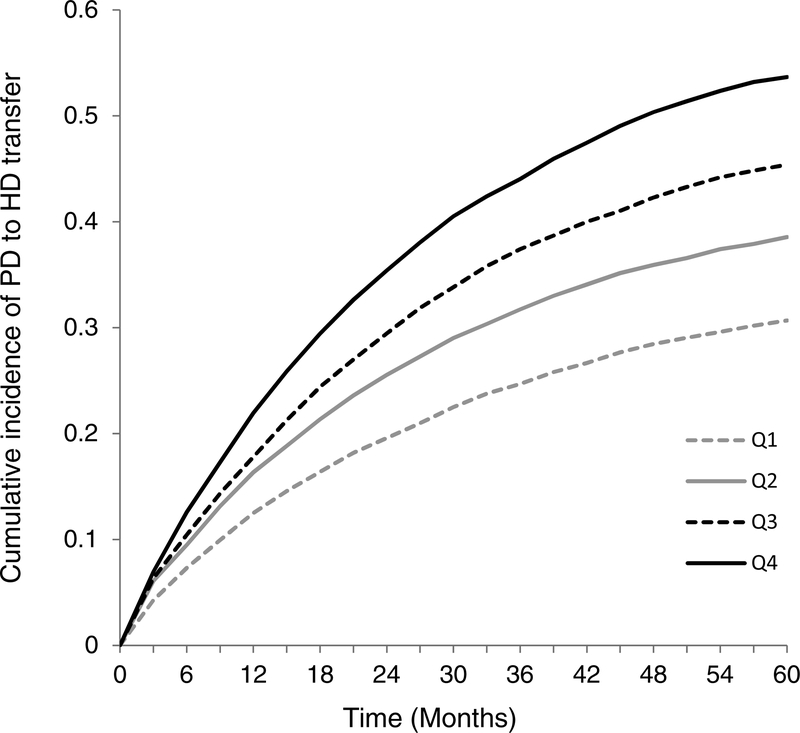
A nomogram to permit practical application of this model is shown in Figure 4. Points are assigned by extending a vertical line from each axis up to the axis for “Points” at the top of the nomogram. The last three axes represent the interaction variables with age, and should be used for patients who have primary diabetic nephropathy, primary hypertensive nephropathy, or are employed. For patients who do not meet these interaction conditions, points are assessed by drawing a vertical line from the zero-point of that axis. Using the total points, the cumulative incidence of HD transfer at 12, 24, 36, and 48 months can be obtained from the bottom of the nomogram. Quartile score was derived from total points. Quartile 1 (lowest risk) patients had ≤ 260 points; Quartile 2 patients had 261–277 points; Quartile 3 patients had 278–296 points, and Quartile 4 (highest risk) patients had ≥ 297 points. Cumulative incidences of HD transfer over time for each of the quartiles are shown in Figure 5, with clear separation of all 4 groups within six months. Table 3 shows the cause-specific hazards associated with quartile score and the time-dependent variable for peritonitis, all of which had significant associations with HD transfer. No interaction was observed between the two variables. Harrell’s c-index for the cause-specific hazards model incorporating the peritonitis variable was 0.767 (95% CI, 0.754–0.779). Among patients in the lowest risk quartile (Quartile 1) with no exposure to peritonitis (n=4,898), 23.9% transitioned to HD, at a median of 13.8 (IQR, 4.5–30.4) months.
Figure 4.
Competing risks nomogram. To calculate points for an individual patient, the patient’s value for each characteristic should be traced upwards to the ‘Points’ axis. For example, a BMI of 40 kg/m2 would receive 50 points. The last three axes (AGE_if_DM, AGE_if_HTN, and AGE_if_employed) represent interaction variables. For patients who meet the conditions specified (diabetic nephropathy, hypertensive nephropathy, employment), points are assessed based on age. For patients who do not meet these conditions (e.g. non-diabetic, not hypertensive nephropathy, unemployed), points are assigned based on the zero-point of the axis. Total score is the sum of points from each patient axis, which is used to draw a vertical line in the lower pane of the nomogram. Predicted cumulative incidences of HD transfer at 12, 24, 36, and 48 months are read from where this vertical line intersects the axes in the lower pane.
Figure 5.
The dotted, dashed, solid black and grey lines represent the cumulative incidences of HD transfer over time among the four risk quartiles assigned in the subdistribution hazards model. Quartile 1 (Q1) has the lowest risk for HD transfer.
Table 3.
Cause-specific hazard ratios for HD transfer, death, and kidney transplant
| HD transfer | Death | Kidney Transplant | ||||
|---|---|---|---|---|---|---|
| Covariate | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Peritonitis claim | 1.83 (1.76, 1.89) | <0.001 | 1.47 (1.41, 1.54) | <0.001 | 0.70 (0.65, 0.75) | <0.001 |
| Risk Quartile | ||||||
| Lowest risk | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| 2nd Quartile | 1.31 (1.25, 1.37) | <0.001 | 1.49 (1.41, 1.57) | <0.001 | 0.60 (0.56, 0.64) | <0.001 |
| 3rd Quartile | 1.51 (1.45, 1.58) | <0.001 | 1.31 (1.24, 1.39) | <0.001 | 0.52 (0.48, 0.55) | <0.001 |
| Highest risk | 1.78 (1.71, 1.86) | <0.001 | 1.09 (1.03, 1.15) | 0.004 | 0.40 (0.37, 0.43) | <0.001 |
PD, peritoneal dialysis; HD, hemodialysis; HR, hazard ratio; CI, confidence interval
The competing risk nomogram can be illustrated with two examples of patients initiating PD, based on patients from the data set. Detailed instructions for use of the nomogram, along with a third illustrative case, is included in Item S1.
Patient 1 is a 77-year-old retiree with hypertensive nephropathy. She is Caucasian, with BMI of 24 kg/m2; albumin, 3.5 g/dL; and eGFR, 16 ml/min/1.73m2. She receives 7 points for age, 29 points for race, 23 points for BMI, and 61 points for retirement, but zero points for GFR 16 ml/min/1.73m2. She has 38 points for albumin, 32 points for hypertensive nephropathy, and 7 points on the “AGE_if_HTN” axis. Ignoring the “DM” and “AGE_if_employed” (zero points), the “AGE_if_DM”axis is marked at the zero-point, for 62 points. This totals 259 points, which places her in Quartile 1, with low risk of HD transfer but high competing risk of death. Retirement, age, hypertensive nephropathy, and initiation at eGFR>15 ml/min/1.73m2 all imply excess cardiovascular risk. Among 3,440 patients in our data with scores of 254–264 points, 33.8% transferred to HD. The model for this case died after 7 months of PD. Using the nomogram, Patient 1’s probability of HD transfer is 14%, 23%, 29% and 35% at 1,2,3, and 4 years.
Patient 2 is a 46 year old executive. He is African-American, with BMI=40 kg/m2, albumin=3.7 g/dL, and eGFR=9 ml/min/1.73 m2. He receives 16 points for age, 53 points for race, 50 points for BMI, 8 points for low eGFR, and 36 points for albumin. He receives 57 points for diabetic nephropathy, but zero points for hypertensive nephropathy. Based on his age, the “AGE_if_DM” axis yields another 33 points. The 0 on the “AGE_if_HTN” (because he does not have hypertensive nephropathy), gives 30 points. He gets zero points on the “employed” axis, but 36 points on the “AGE_if_employed” axis. In total, he has 319 points, and occupies Quartile 4. Among 1411 patients with scores of 314–324 points, 53.5% transferred to HD. Race, obesity, and youth all decrease competing risk of death; race and obesity reduce transplantation. HD transfer becomes more likely with extended survival and fewer competing events. The model for this case transferred to HD after 45 months of PD. Patient 2’s probability of HD transfer from the nomogram is 24%, 37%, 46%, and 52% at 1,2,3, and 4 years.
Discussion
Fewer than 12% of patients initiating PD in the United States still used PD after five years. This study used a competing risks framework to develop a novel prototype tool to assess the risk for HD transfer among patients initiating PD. Peritoneal exchange capacity is not infinite, but death and kidney transplantation were both substantial competing outcomes that prevented HD outcomes. Despite this, the cumulative incidence of HD transfer was as high as the combination of death and transplantation. HD transfer, transplantation and death were influenced in different ways by patient characteristics, and the patient-specific risk model needed to account for death or transplantation occurring prior to exhaustion of peritoneal transport. This model, with eight readily available enrollment characteristics, could roughly stratify the risk of transition to HD, largely by modeling the probability of competing events.
The quartile score represents the subhazard of HD transfer, which depends upon the subhazards of death and transplantation. Variables could become important due to their associations with competing outcomes, as shown by the effects of age. Age was marginally significant in the main effects model, but interacted with primary ESRD diagnosis and employment (Figure 3), in ways that were clinically plausible. For example, employment improves access to insurance, medications, and transportation, which increases the opportunity for kidney transplantation and reduces HD transfer among young patients. However, suitability for transplantation decreases with age. An employed patient who is too old for a kidney transplant still has financial advantages that decrease the risk of death, so the hazard of HD transfer increases. Similarly, diabetes is usually not fatal in younger dialysis patients, but reduces the opportunity for transplantation, so transfer to HD is increased. Older dialysis patients with diabetic nephropathy have a high risks of cardiovascular death and therefore less chance of requiring transfer to HD.
The patient-specific model provides proof of concept that the risk of HD transfer can be assessed, and could be used to assess individual risks over time using the nomogram. The quartile score serves as a basic risk stratification at PD initiation that can be combined with other variables. The outcomes of individual patients are influenced by events occurring after the initiation of PD. A time-dependent variable for peritonitis events occurring after PD initiation, which could not be incorporated into a competing risk regression, provided further discrimination among patients, suggesting that a peritonitis or suspected peritonitis event increased the hazard of HD transfer by 83%. An episode occurring in a patient with high baseline risk should therefore prompt serious consideration of vascular access planning.
To our knowledge, this is the first working model that provides clinicians with a risk assessment for HD transfer among patients initiating PD. Our data indicate that HD transfer is a common endpoint among PD patients. In this sample, HD transfer occurred in > 20% of Quartile 1 patients who never developed peritonitis, suggesting that clinicians should consider this possibility in all new PD patients. Multiple studies have shown that patients who transition from PD to HD frequently require CVCs,6–8, 15 suggesting that clinicians cannot anticipate the impending need for HD in many PD patients. In many cases, transition may happen precipitously. Still, appreciating the patient-specific risk of HD and the impact of peritonitis episodes could improve outcomes for PD patients. Expectant placement of AV fistulas or grafts in high-risk patients might allow them to transition to HD without CVCs.16 Conversely, clinicians may be able to avoid referring patients for vascular access surgery who are unlikely to require HD. Finally, awareness of a high risk for PD failure might motivate a living kidney donor to expedite his or her diagnostic testing.
Our data are consistent with other reports on the impacts of age, race, comorbid conditions, and serum albumin on all-cause mortality and kidney transplantation among dialysis patients.17–24 Increased transfer to HD among patients with higher BMI probably reflects a combination of longer survival and higher solute clearance requirements.25,26 Lower survival among incident dialysis patients with eGFR>15 mL/min/1.73 m2 has also been reported.27–29 Initiation of PD for refractory volume overload, rather than uremia, often suggests underlying heart disease. Use of the eGFR variable and the primary ESRD diagnosis provided a rough approximation of cardiovascular risk without the use of ICD-9 codes or the comorbid conditions from Medical Evidence forms.
Our findings of 17% HD transfer and 8% death at one year are comparable to other reports. In a large dialysis provider database, 21% of patients transferred to HD and 9% had died over the first year after initiation.6 These findings are similar to one year outcomes observed in three large PD cohorts derived from an equipment supplier’s database, in which 20% transferred to HD and 14% died.30 A logistic model that examined risks for HD transfer at six months and twelve months among incident PD patients in Australia noted similar associations with clinical covariates.31 In the United states, implementation of the 2011 bundled Medicare payment rules, which increase financial incentives for PD, may cause changes in population selection that affect outcomes.
The strengths of our study include the use of a large national data set, inclusion of long-term outcomes, and methodology accounting for the competing risks of death and kidney transplantation. Our US PD population included African-American patients, a demographic with significantly increased risk of transfer to HD, and our methodology allowed us to detect important interactions between age and other covariates. While this model illustrates the possibility for prediction of HD transfer, certain limitations are worth noting. Our data consisted of single observations taken at the time of dialysis initiation. Addition of clinical and laboratory covariates captured longitudinally during clinical follow up after the initiation of PD would strengthen future models by allowing time-varying prediction of short-term PD modality failure. Variables that might reasonably be expected to be helpful in assessing technique longevity include: measurements of residual kidney function, peritoneal transport as measured by peritoneal equilibration tests, and the changes in dialysis prescription which may compensate for declining transport kinetics or diminished ultrafiltration. Hospitalizations and surgical procedures also likely affect patients’ capacity to continue PD, even when these occur for reasons unrelated to PD. Longitudinal changes in laboratory values might also provide evidence of declining residual or dialytic clearance. The current model describes risk of HD transfer over time from PD initiation; prediction of PD failure from other time points would be useful for clinicians seeking lead time to plan for HD. The ascertainment of the peritonitis variable via claims was a major limitation. We could not rule out the possibility of missing peritonitis claims due to the changes in Medicare billing that occurred over the course of the study, misclassification of claims representing suspected peritonitis associated with exit site or tunnel infections that were not truly complicated by peritonitis or for other intraabdominal processes, or less severe episodes of peritonitis that were treated entirely by the dialysis facility and did not result in a specific claim. Similarly, these claims lacked any information about microbiology. The success of this exploratory variable provides proof of concept that future models would benefit from inclusion of variables indicating events that occur after the initiation of dialysis. A peritonitis variable built upon a more reliable assessment, such as the database of a large dialysis provider, would be a more valid way to assess the association between peritonitis and modality transfer. Additionally, the reasons for PD to HD transfer could not be ascertained, and we could not rule out residual confounding by other comorbid conditions or unmeasured factors. Despite these limitations, this model shows that PD to HD transfer can be modeled if competing risks and intercurrent events are taken into account, and provides a foundation for future models. Further work is needed to evaluate model performance prospectively and discover additional factors that can refine prediction of HD transfer over a shorter timeline.
Only one in seven patients initiating PD in the United States still receives PD after five years. Kidney transplantation, HD transfer, and death are all common outcomes for PD patients. Predictive models for PD failure that incorporate the hazards of competing outcomes may help improve the care of PD patients. Transition to HD needs to be considered for all new PD patients with favorable survival prognoses, especially when there is no plan for expedited kidney transplantation. Future studies incorporating longitudinal clinical information into prognostic models may further aid clinicians in determining the appropriate time table for implementing vascular access plans.
Supplementary Material
Support:
The project described was supported by NIH/NIDDK T32 DK007777 and NIH/National Center for Advancing Translational Sciences, UL1TR001064. The funders had no role in study design, data collection, analysis, reporting, but the US Renal Data System approved the decision to submit for publication.
Financial Disclosure: D.E.W., D.C.M., and K.B.M. all receive salary support from Dialysis Clinic, Inc., paid to their institution. E.L. is employed by Dialysis Clinic, Inc. R.L.M. has been compensated for giving a lecture to Dialysis Clinic, Inc. personnel on this topic. R.R. declares that she has no relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Item S1. Use of the competing risks nomogram
Supplementary Material Descriptive Text for Online Delivery
Supplementary File (PDF).
Publisher's Disclaimer: Disclaimer: The data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Prior Presentation: Aspects of this work were presented in abstract form at the American Society of Nephrology Kidney Week 2016 (November 15–20, Chicago, IL).
References
- 1.Devoe DJ, Wong B, James MT, Ravani P, Oliver MJ, Barnieh L, et al. Patient education and peritoneal dialysis modality selection: A systematic review and meta-analysis. Am J Kidney Dis 68(3): 422–33, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Robinski M, Mau W, Wienke A, Girndt M. The choice of renal replacement therapy (CORETH) project: dialysis patients’ psychosocial characteristic and treatment satisfaction. Nephrol Dial Transplant 32(2): 315–24, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Bieber SD, Mehrotra R. Patient and technique survival of older adults with ESRD treated with peritoneal dialysis. Perit Dial Int 35(6): 612–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahlerus C, Quinn M, Messersmith E, Lachance L, Subramanian L, Perry E, et al. Patient perspectives on the choice of dialysis modality: Results from the Empowering Patients on Choices for Renal Replacement Therapy (EPOCH-RRT) Study. Am J Kidney Dis 68(6): 901–10, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3)(suppl 1):S1–S434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pulliam J, Li NC, Maddux F, Hakim R, Finkelstein FO, Lacson E Jr. First-year outcomes of incident peritoneal dialysis patients in the United States. Am J Kidney Dis 64(5): 761–9, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Al-Jaishi AA, Jain AK, Garg AX, Zhang JC, Moist LM. Hemodialysis vascular access creation in patients switching from peritoneal dialysis to hemodialysis: A population-based retrospective cohort. Am J Kidney Dis 67(5): 811–6, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Lee T, Thamer M, Zhang Y, Zhang Q, Allon M. Association of peritonitis with hemodialysis catheter dependence after modality switch. Clin J Am Soc Nephrol 11(11): 1999–2004, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3)(suppl 1):S1–S434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noordzij M, Leffondre K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant 28(11): 2670–2677, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94(446): 496–509, 1999 [Google Scholar]
- 12.Zhang Z, Geskus RB, Kattan MW, Zhang H, Liu T. Nomogram for survival analysis in the presence of competing risk. Ann Transl Med 5(20):403–8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 23(13):2109–2123, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Forman S, Barton B. Fitting Cox model using PROC PHREG and beyond in SAS. SAS Global Forum Paper 236–2009: 1–10, 2009 [Google Scholar]
- 15.Szeto C, Kwan BC, Chow K, Pang W, Kwong VW, Leung C, et al. Outcome of hemodialysis patients who had failed peritoneal dialysis. Nephron Clin Pract 116(4): c300–6, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Nezagatgoo N, Ndzengue A, Ramaiah M, Gosmanova EO. Outcomes of simultaneous peritoneal dialysis and arteriovenous fistula placement in end-stage renal disease patients. Perit Dial Int 37(6): 658–61, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, et al. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis 56(4): 415–25, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jialin W, Yi Z, Weijie Y. Relationship between body mass index and mortality in hemodialysis patients: a meta-analysis. Nephron Clin Pract 121(3–4): c102–11, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Streja E, Rhee CM, Soohoo M, Feng M, Brunelli SM, et al. Lean body mass and survival in hemodialysis patients and the roles of race and ethnicity. J Ren Nutr 26(1): 26–37, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 63(3): 793–808, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: The Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 14(12): 3270–7, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2(1): 89–99, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Rhee CM, Lertdumrongluk P, Streja E, Park J, Moradi J, Lau WL, et al. Impact of age, race, and ethnicity on dialysis patient survival and kidney transplantation disparities. Am J Nephrol 39(3): 183–94, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill J, Dong J, Rose C, Johnston O, Landsberg D, Gill J. The effect of race and income on living kidney donation in the United States. J Am Soc Nephrol 24(11): 1872–9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int 64(5): 1838–44, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Salim SA, Akula Y, Kandhuri S, Afshan S, Zsom L, Dixit MP, et al. Successful peritoneal dialysis in large-weight subjects: Clinical features and comparisons with normal-weight subjects. Adv Perit Dial 2016; 32: 61–7 [PubMed] [Google Scholar]
- 27.US Renal Data System, 2004. Annual Report. Chapter 3: Patient Characteristics. Available at: https://www.usrds.org/2004/pdf/03_pt_char_04.pdf. Accessed February 15, 2019
- 28.Perez Fontan M, Remon Rodriguez C, da Cunha Naveira M, Borras Sans M, Rodriguez Suarez C, Quiros Ganga P,et al. Baseline residual kidney function and its ensuing rate of decline interact to predict mortality of peritoneal dialysis patients. PLoS One 2016; 11(7):e0158696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He L, Liu X, Li Z, Abreu Z, Malavade T, Lok CE,et al. Rate of decline of residual kidney function before and after the start of peritoneal dialysis. Perit Dial Int 2016; 36(3): 334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo A, Mujais S. Patient and technique survival on peritoneal dialysis in the United States: Evaluation in large incident cohorts. Kidney Int 2013; 64 (Suppl 88): S3–S12. [DOI] [PubMed] [Google Scholar]
- 31.Lan PG, Clayton PA, Saunders J, Polkinghorne KR, Snelling PL. Predictors and outcomes of transfers from peritoneal dialysis to hemodialysis. Perit Dial Int 2013; 35(3): 306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.