Abstract
Charged multivesicular body protein 4B (CHMP4B) functions as a core component of the endosome sorting complex required for transport-III (ESCRT-III) machinery that facilitates diverse membrane remodeling and scission processes in eukaryotes. Mutations in the human CHMP4B gene underlie rare, inherited forms of early-onset lens opacities or cataract. Here we have characterized the lens phenotypes of mutant (knock-in) mice harboring a human cataract-associated mutation (p.D129V) in CHMP4B (Chmp4b-mutant) and conditional knockdown mice deficient in lens CHMP4B (Chmp4b-CKD). In situ hybridization localized Chmp4b transcripts to lens epithelial cells and elongating fiber cells at the lens equator. Heterozygous Chmp4b-mutant (D/V) mice were viable and fertile with lenses grossly similar to those of wild-type. However, homozygous Chmp4b-mutant (V/V) mice died by embryonic day 15.5 (E15.5) with grossly abnormal eye and brain histology. Chmp4b-CKD mice displayed variable degrees of lens dysmorphology including lens ablation. Immuno-localization of aquaporin-0 (AQP0) revealed lens fiber cell degeneration in homozygous Chmp4b-mutant (V/V) mouse embryos and in embryonic and postnatal Chmp4b-CKD mice. DNA fragmentation (TUNEL) analysis revealed global cell death in homozygous Chmp4b-mutant (V/V) embryos, whereas, cell death was confined to the lens of Chmp4b-CKD mice. Immuno-localization of the monocyte/macrophage marker macrosialin (CD68) suggested that severe lens degeneration in Chmp4b-CKD mice resulted in an ocular immune cell response. Collectively, these mouse data suggest that (1) heterozygous, germ-line mutations in Chmp4b may not manifest as cataract, (2) homozygous, germ-line mutations in Chmp4b are embryonic lethal, and (3) conditional loss of Chmp4b results in arrest of lens growth and differentiation.
Keywords: Lens, Cataract, Chmp4b-mutant, Chmp4b-knockdown, Mouse
1. Introduction
First discovered in yeast vacuole protein sorting (vps) mutants, the phylogenetically conserved endosome sorting complex required for transport-III (ESCRT-III) system plays a key role in eukaryotic membrane remodeling and scission processes including multivesicular body/endosome (MVB/E) biogenesis, enveloped virus budding, and cytokinetic abscission (Babst et al., 2002; Hanson and Cashikar, 2012; Christ et al., 2017; Scourfield and Martin-Serrano, 2017; Stoten and Carlton, 2018). In humans, ESCRT-III comprises a structurally conserved family of 12 small α-helical (coiled-coil) proteins (196–453 amino-acids) referred to as charged multivesicular body proteins (CHMPs) that are non-redundant for ESCRT pathway function (Peel et al., 2011). CHMP4 homologs (e.g., Snf7 in S. cerevisiae, Vps32 in C. elegans) constitute the most abundant ESCRT-III core components and ultrastructural studies reveal that they can polymerize into dynamic spiral filaments to facilitate projection of the plasma membrane away from the cytoplasm (negative membrane curvature) forming vesicles with narrow membrane neck-like structures that are subsequently severed (Hanson et al., 2008; Shen et al., 2014; Cashikar et al., 2014; Tang et al., 2015; Chiaruttini et al., 2015). Beyond their classical roles in forming intraluminal vesicles during MVB/E biogenesis, releasing viruses from the cell surface, and abscission of intercellular bridges following mitosis, CHMP4 and other ESCRT-III proteins function in a diverse range of membrane remodeling/scission processes including exosome and ectosome release, plasma membrane repair, neuron pruning, nuclear envelope sealing, and autophagy (Juan and Furthauer, 2018; Jimenez et al., 2014; Loncle et al., 2015; Olmos et al., 2015; Vietri et al., 2015; Lefebvre et al., 2018).
The crystalline lens plays a central role in anterior eye development and refractive vision of vertebrates (Beebe and Coats, 2000; Iribarren, 2015; Donaldson et al., 2017). During mammalian embryogenesis, the lens develops from a placode of head surface ectoderm under the control of a paired-box 6 (PAX6) dependent gene regulatory network and several extracellular signaling pathways (Cvekl and Ashery-Padan, 2014; Cvekl and Zhang, 2017). Invagination of the lens placode produces the lens vesicle of precursor cells that elongate from the posterior pole to fill the vesicle lumen with primary fiber cells forming the embryonic lens nucleus. The anterior lens vesicle cells give rise to the lens epithelium that generates post-mitotic secondary fiber cells at the lens equator. Concentric layers of tightly packed secondary fiber cells are laid-down around the embryonic nucleus to form the refractive mass of the lens and maintain lifelong lens growth. Terminal differentiation of secondary lens fiber cells is characterized by several unique re-modeling processes including accumulation of cytoplasmic crystallins, modification of the cytoskeleton, specialization of the plasma-membrane, programmed degradation of organelles, and formation of a core syncytium (Bassnett et al., 2011; Cheng et al., 2017; Cvekl and Zhang, 2017). Collectively, these fiber cell re-modeling events are designed to establish and maintain lens transparency, minimize light scattering, and generate a high refractive index in order to facilitate the fine focusing of images onto the photosensitive retina.
Heterozygous mutations in the human gene for CHMP4B (CHMP4B) located on the long (q) arm of chromosome 20 have been linked with inherited forms of early-onset, progressive cataract (MIM 605387, CTRCT31) that primarily affects the posterior pole of the lens (Shiels et al., 2007; Zhang et al., 2018; Yamada et al., 2000a; Yamada et al., 2000b). By contrast, germline knockout of the mouse gene for CHMP4B (Chmp4b), also known as mSnf7–2, results in embryonic lethality (Lee et al., 2007). Heterologous overexpression studies in vitro of the CHMP4B cataract mutation p.D129V suggest that the mutant form of human CHMP4B may impair budding from the cell surface and/or inhibit chromatin binding when compared with wild-type CHMP4B (Shiels et al., 2007; Sagona et al., 2014). Here we provide the first report, to our knowledge, comparing the phenotypic effects of a germline Chmp4b mutation (p.D129V) with those of conditional Chmp4b deficiency on lens development in the mouse.
2. Methods and materials
2.1. Gene-targeted mice and lenses
Mice harboring the p.D129V mutation or flanking loxP (flox) sites in Chmp4b were generated using standard homologous recombination techniques. Briefly, three Chmp4b homology arms were PCR-amplified from the bacterial artificial chromosome (BAC) clone RP24–296E10 (www.chori.org) and directionally inserted into a targeting vector (3LoxPNeo) containing a 5’-LoxP site and two 3’-LoxP sites flanking a neomycin (neo) selection marker cassette (see Fig. 2A). The 5’-homology arm (3.2 kb) ended ~ 300 bp upstream of exon-2 next to the 5’-loxP site. The conditional homology arm (~ 3.9 kb) spanned ~ 300 bp upstream of exon-2 to ~ 300 bp downstream of exon-4 followed by the floxed neo cassette. The p.D129V mutation (c.386A>T) was introduced into exon-3 using the QuikChange II XL site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) according to the manufacturer’s instructions. The 3’-homology arm (~3.2 kb) started ~300 bp downstream from exon-4 and ended ~2.2 kb downstream of exon-5. Sequence-verified Chmp4b-targeting constructs were electroporated into a mouse (129×1/SVJ) embryonic stem (ES) cell-line (SCC10) and following G418 selection, neo resistant clones were validated by PCR-genotyping and karyotyping. Correctly targeted ES cells were injected into blastocysts (C57BL6/J) and implanted into pseudo-pregnant females. Chimeric offspring were crossed with transgenic EIIa-Cre mice (generous gift from Dr. M. Bessler, Washington University). EIIa-Cre mice exhibit varying levels of mosaic Cre expression in the embryo that enables germline deletion of the neo cassette and regeneration of a 3’-LoxP site in intron-4 while leaving the 5’-LoxP site in intron-1 intact (Lakso et al., 1996). Chmp4b-mutant and Chmp4b-floxed mice were maintained on the C57BL/6J (B6J) genetic background as heterozygotes (Chmp4b-D/V) and homozygotes (Chmp4bfx/fx), respectively. Subsequently, Chmp4b-floxed mice were crossed with mice transgenic for lens-specific Cre recombinase (MLR10-Cre, generous gift from Dr. M.L. Robinson, Ohio State University) (Zhao et al., 2004). Throughout, mice were PCR-genotyped from toe, tail, or ear biopsy DNA as described (Zhou et al., 2016) with gene-specific primers (Supplementary Table 1) to confirm correct targeting of Chmp4b, Chmp4b mutant/flox allele status, Cre transgene status, and rescue of a deletion mutation in the gene for lens beaded filament structural protein −2 (Bfsp2) carried by certain inbred mouse strains (Alizadeh et al., 2004; Simirskii et al., 2006). Mice were humanely killed by CO2 asphyxiation or by decapitation. Embryo ages were timed from the appearance of a vaginal plug (E0.5). For histology, embryo heads or enucleated eyes were processed using standard formalin/formaldehyde-fixed-paraffin-embedded (FFPE)-section and histochemical staining (Hematoxylin and Eosin, H&E) techniques then imaged with an upright microscope (BX61, Olympus, Center Valley, PA). Alternatively, lenses were dissected from enucleated eyes in pre-warmed (37°C) phosphate buffered saline (PBS, #P4417–100TAB, Sigma-Aldrich, St. Louis, MO) then imaged under a dissecting microscope (Stemi 2000; Zeiss, Thornwood, NY). All mouse studies were approved by the Institutional Animal Care and Use Committee (IACUC) at Washington University in compliance with the Institute for Laboratory Animal Research (ILAR) guidelines.
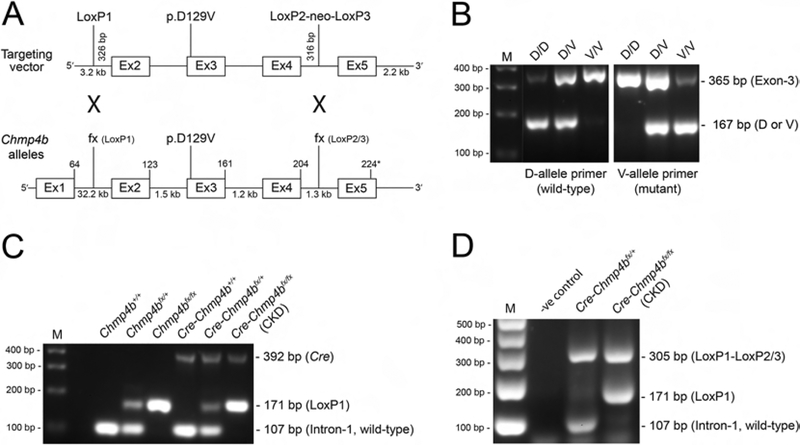
Figure 2. Generation of Chmp4b-mutant and Chmp4b-deficient mice.
(A) Schematic of gene-targeting strategy. Chmp4b is located on mouse chromosome-2 (physical location Chr2:154657026..154694783) spanning ~37.76 kb with 5 coding exons (224 codons) and 4 introns. The 3LoxPNeo recombination vector was used to introduce the p.D129V (c.386A>T) mutation into exon-3 or LoxP sites into intron-1 and intron-4. EIIa-Cre excision removed the neo cassette from intron-4 resulting in LoxP sites flanking exon-2 and exon-4 (fx). MLR10-Cre excision resulted in deletion of exons 2–4 from Chmp4b. (B) PCR-genotyping of Chmp4b-mutant embryonic mice (E12.5) using allele-specific primers showing presence or absence of the wild-type D and mutant V alleles (167 bp) in exon-3 of Chmp4b. (C) PCR-genotyping (ear-DNA) from Chmp4b-floxed mice crossed with MLR10-Cre mice showing presence or absence of the Cre transgene (392 bp) and the LoxP1-site (171 bp) in intron-1 of Chmp4b. (D) PCR-genotyping of lens-DNA from Cre-positive Chmp4b-floxed (fx) mice using intron/exon-specific primers showing that Cre-deletion between the LoxP1 and LoxP2/3 sites (~ 3.9 kb) resulted in a ‘recombination’ product (305 bp) lacking exon-2 through exon-4 of Chmp4b. In some mice with an ear-DNA genotype of Chmp4b-CKD, lens-DNA genotyping revealed an intact LoxP1 site (171 bp) suggesting that Cre-deletion was incomplete. PCR-primer sequences are shown in Supplementary Table 1. M, size markers in base pairs (bp).
2.2. Quantitative (q)RT-PCR
Pairs of lenses from littermates were dissected into RNAlater stabilizing solution (ThermoFisher Scientific, Waltham, MA) and stored at −20°C. Lens total RNA was extracted using the RNeasy Plus Micro kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. RNA was reverse transcribed using the iScript cDNA synthesis kit then PCR-amplified in iQ SYBR Green Supermix with Chmp4b allele-specific primers (Supplementary Table 1) using an iQ5 Real-time PCR cycler running iQ5 software (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Chmp4b transcripts were quantified using the ΔΔCt method and standardized against Rpl19 transcripts.
2.3. In situ hybridization
Lens RNA transcripts were localized in situ using custom-synthesized oligonucleotide probes to Chmp4b (NM_029362.3, target region 136–1586 nt/bp Mm-Chmp4b Cat. # 418331) and the RNAscope 2.0 HD Detection Kit (RED) (P/N 310034, Advanced Cell Diagnostics, Inc, Hayward, CA) as described [Zhou and Shiels, 2018]. Briefly, mouse eyes were fixed (24 hr., 20°C) in neutral buffered formalin (10%, ThermoFisher) and paraffin-embedded. Protease pre-treated FFPE sections (5 μm) were hybridized (2 hr, 40°C) w ith target probe followed by signal amplification (15–30 min, 40°C) and chromogenic lab eling (15–30 min, 20°C) with alkaline phosphatase (AP)-conjugated Fast-Red label probe, than counterstained (Gill’s Hematoxylin-1) and imaged under a bright-field microscope (BX61, Olympus).
2.4. Immunoblot analysis
Lens proteins were subject to immunoblot analysis using anti-CHMP4B (13683–1-AP, Proteintech Group, Rosemont, IL) and visualized with an Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE) as described (Zhou et al., 2016).
2.5. Immunofluorescence confocal microscopy
Enucleated eyes or embryo heads were processed using standard FFPE-section or cryo-section techniques and immuno-localization performed as described (Zhou et al., 2016; Zhou and Shiels, 2018). Primary antibodies used were, anti-aquaporin 0 (anti-AQP0, AB3071, EMD Millipore, Billerica, MA), and Alexa Fluor 488 anti-mouse cluster of differentiation 68 antigen (anti-CD68, #137011, Biolegend, San Diego, CA). Briefly, for anti-AQP0 localization eyes were fixed (16–24 hr., 4°C) in 4% paraformaldehyde (#157 10, Electron Microscopy Sciences, EMS, Hatfield, PA) diluted in PBS and FFPE-sections (4 μm) cut in the sagittal plane using a microtome (RM2255, Leica Microsystems, Buffalo Grove, IL). For anti-CD68 localization, eyes were fixed as above and cryo-protected by serial incubation (16 hr) in 15% and 30% sucrose/PBS, then embedded in Tissue-Tek O.C.T. compound (#62550–01, EMS) and sagittal cryo-sections (15 μm) cut using a cryostat (Cryotome E, ThermoFisher). Eye sections were permeabilized (0.1% Triton X100/PBS 10 min.), blocked (1 hr., 20°C) in Image-iT FX Signal Enhancer (ThermoFisher) then incubated with primary antibody (16 hr, 4°C). Sections for anti-AQP0 labeling were further incubated in Alexa Fluor 488-conjugated secondary antibody (1 hr., 20°C). Cell nuclei were stained (15 min, 20°C) with DAPI (Sigma). Images were captured with a confocal microscope (FV1000, Olympus) and managed in Photoshop (Adobe Systems, San Jose, CA).
2.6. TUNEL assay
DNA double-strand breaks (3′-OH ends) were detected in FFPE tissue sections using the Click-iT Plus TUNEL assay (ThermoFisher) as described (Zhou et al., 2016).
2.7. Statistical analysis
Student’s t-test was used to determine statistical significance (p) ± standard error (Fig. 4E).
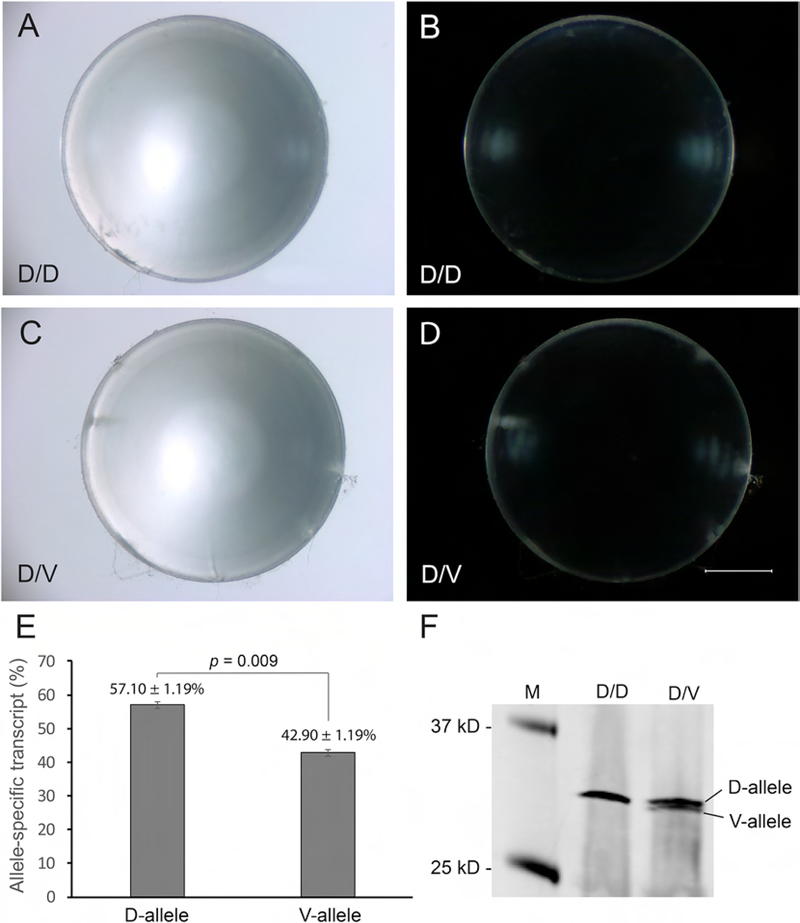
Figure 4. Lens phenotype and CHMP4B expression analysis of Chmp4b-mutant mice.
(AD) Bright-field (A, C) and dark-field (B, D) dissecting microscope images of wild-type (D/D) lenses (A, B) and heterozygous Chmp4b-mutant (D/V) lenses (C, D) at P21 showing lack of gross differences. Scale bar: 500 μm. (E) Allele-specific qPCR analysis showing that the relative level of wild-type Chmp4b-transcript (c.386A, p.D129) was significantly greater than mutant Chmp4b transcript (c.386T, p.V129) in heterozygous Chmp4b-D/V mutant lenses. (F) Immunoblot analysis showing increased electrophoretic mobility of the mutant CHMP4B-V129 protein compared to the wild-type CHMP4B-D129 protein.
3. Results
3.1. Localization of Chmp4b expression in the lens
To determine the distribution of Chmp4b expression in the mouse lens we first undertook immunofluorescent labeling with commercially available antibodies to CHMP4B. While one of these antibodies detected CHMP4B in lens protein lysates using immunoblotting techniques (see Fig. 4F), in our hands, none produced convincing in situ localization of lens Chmp4b expression at the protein level (data not shown). To confirm lens Chmp4b expression at the transcript level we performed in situ hybridization (ISH) using gene-specific, antisense oligo-nucleotide probes. ISH revealed that Chmp4b transcripts were strongly expressed in lens epithelial cells and nascent (peripheral) fiber cells elongating at or near the lens equator (Fig. 1). While we cannot confirm co-localized Chmp4b expression at the protein level, our ISH data suggest that Chmp4b transcripts are enriched in lens cells undergoing epithelial-to-fiber cell differentiation.
Figure 1. In situ hybridization of Chmp4b transcripts in the mouse lens.
(A, B) Chromogenic labeling of Chmp4b transcripts (red-dots) in the mouse lens at P3 (A, inset a) and P22 (B, inset b) showing strong localization to equatorial epithelial cells and nascent, elongating fiber cells at the lens equator. Cell nuclei were counterstained with hematoxylin (blue). Scale bar: 200 μm (A, B), 50 μm (a, b).
3.2. Chmp4b-mutant mice
To model the phenotypic effects of a human CHMP4B cataract mutation (p.D129V), we generated a Chmp4b-mutant ‘knock-in’ mouse line via homologous recombination techniques (Fig. 2). Adult heterozygous Chmp4b-D/V mutant mice were viable and fertile with no obvious ocular, physical, or behavioral abnormalities. Litters from Chmp4b-D/V parents contained both agouti and black offspring derived from the targeted 129 ES cells and the B6J background strain, respectively. Since Chmp4b is tightly linked (physical distance ~ 256 Kbp, GRCm38) to the non-agouti (a) coat-color locus on mouse chromosome-2, preventing genetic recombination, agouti littermates carried the Chmp4b-D/V mutant genotype, whereas, black littermates were wild-type (Chmp4b-D/D). Since genotyping failed to identify homozygous Chmp4b-V/V mutants in over 90 offspring, we sought to determine that the p.D129V mutation was embryonic lethal in the homozygous state (V/V) by examining littermates during embryogenesis. From embryonic day 12.5 (E12.5) through E16.5, overall embryonic growth and development of the Chmp4b-D/V mutant appeared grossly indistinguishable from wild-type (Fig. 3 and data not shown). However, in the homozygous Chmp4b-V/V mutant head development often appeared grossly abnormal and enlarged at E12.5 (Fig. 3C). Histochemical (H&E) staining of Chmp4b-V/V mutant head sections at E12.5 confirmed brain mal-development showing enlarged, fluid-filled ventricles consistent with hydrocephalus (Supplementary Fig. 1). By E15.5, Chmp4b-V/V mutant embryos were much smaller than wild-type and had usually died (Fig. 3F). By E16.5, Chmp4b-V/V embryos had largely undergone tissue resorption leaving fluid-filled amniotic sacs intact (data not shown). Collectively, these observations indicate that homozygous Chmp4b-V/V mutant mice failed to thrive and were embryonic lethal by E15.5.
Figure 3. Embryonic development of Chmp4b-mutant mice.
(A-F) Representative dissecting microscope images of wild-type (D/D) embryos (A, D), heterozygous (D/V) Chmp4b-mutant embryos (B, E) and homozygous (V/V) Chmp4b-mutant embryos (C, F) at E12.5 (A-C) and E15.5 (D-E) showing the enlarged head at E12.5 (C) and failed growth at E15.5 (F) characteristic of homozygous Chmp4b-mutants. Scale bar: 1000 μm (A-C), 500 μm (D-F).
3.3. Chmp4b-mutant lenses
Dissected lenses from heterozygous Chmp4b-D/V mutant mice at wean-age (P21) were grossly indistinguishable from those of age and sex matched wild-type littermates (Fig. 4A–D). No obvious differences in lens transparency between Chmp4b-D/V mutant and wild-type littermates were observed through one year of age (data not shown). In the absence of an overt cataract phenotype, we sought to determine that the D129 and V129 alleles of CHMP4B were expressed at similar levels in the Chmp4b-D/V mutant lens. Allele-specific qRT-PCR analysis suggested that the expression level of the mutant V-transcript (42.90 ± 1.19%) was significantly lower than (p = 0.009) that of the wild-type D-transcript (57.10 ± 1.19%) in Chmp4b-D/V mutant lenses (Fig. 4E). Immunoblot analysis of wild-type lenses with CHMP4B-antibody detected a protein of expected molecular mass (Mr ~ 32 kDa) that migrated as a doublet on immunoblots of Chmp4b-D/V lenses, consistent with increased electrophoretic mobility of mutant CHMP4B-V129 (Fig. 4F) (Shiels et al., 2007). Overall, these data suggest that a human-like cataract phenotype was non-penetrant in heterozygous Chmp4b-D/V mutant mice on the B6J genetic background consistent with reduced co-expression of the mutant (V) allele compared to the wild-type (D) allele.
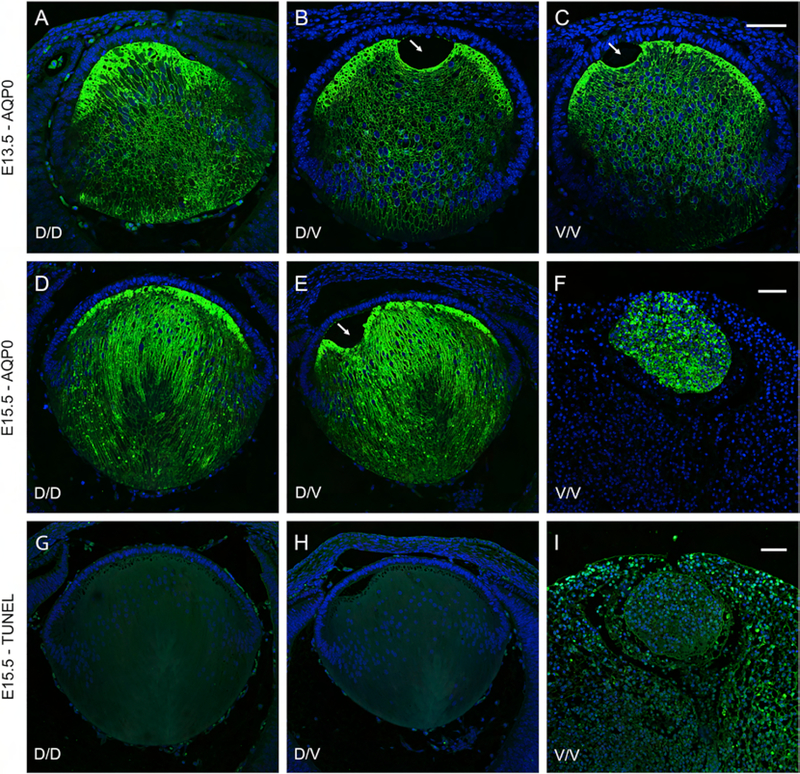
To investigate lens development and pathology in Chmp4b-mutant mice we undertook H&E histology followed by in situ localization of specific markers for lens cell morphology and cell death. First, H&E staining of eye-sections confirmed that lens growth and development was grossly similar in wild-type and heterozygous Chmp4b-D/V mutant embryos between E12.5 and E15.5 (Fig. 5). Although lens development appeared to be grossly normal or mildly delayed in Chmp4b-V/V mutant embryos at E12.5 to E13.5 (Fig. 5C and F), by E15.5 lens growth and development were completely arrested (Fig. 5I) consistent with embryonic lethality of the Chmp4b-V/V mutant. Second, to evaluate lens fiber cell organization we performed immuno-labeling of eye sections with antibody to lens major intrinsic protein (MIP), or aquaporin-0 (AQP0). In wild-type and Chmp4b-D/V lenses, anti-AQP0 labeled plasma-membranes of primary fiber cells that elongate from the posterior of the lens vesicle to fill the vesicle lumen by E13.5 (Fig. 6A and B) followed by concentric over-layering with nascent secondary fiber cells by E15.5 (Fig. 6D and E). At E13.5, anti-AQP0 labelling of primary fiber cell membranes in Chmp4b-V/V lenses appeared similar to wild-type (Fig. 6C). However, by E15.5 anti-AQP0 highlighted random and grossly abnormal intracellular (perinuclear) labeling of amorphous cells- unrecognizable as epithelial or fiber cells - that comprised the small, misshapen Chmp4b-V/V lens, consistent with arrested lens growth and differentiation (Fig. 6F). Third, we performed in situ TUNEL analysis of nuclear DNA (3’-OH) fragmentation to assess levels of embryonic cell death. In E15.5 wild-type and Chmp4b-D/V eye sections, low levels of TUNEL-positive nuclei were evident in vascular and other tissues around the lens but not within the lens itself (Fig. 6G and H). By contrast, much higher levels of TUNEL-positive nuclei were present within Chmp4b-V/V lenses and throughout surrounding ocular and extraocular tissues consistent with global cell death in homozygous Chmp4b-V/V mutant embryos by E15.5 (Fig. 6I).
Figure 5. Lens histology of embryonic Chmp4b-mutant mice.
(A-I) Histochemical (H&E) staining of sagittal eye sections from wild-type (D/D) mice (A, D, G), heterozygous Chmp4b-mutant (D/V) mice (B, E, H), and homozygous Chmp4b-mutant (V/V) mice (C, F, I) at E12.5 (AC), 13.5 (D-F), and E15.5 (G-I) showing complete arrest of lens growth and differentiation in V/V embryos (I). Arrowheads (D-G) indicate a lens fixation defect. Scale bar: 50 μm (A-C), 100 μm (D-F), 100 μm (G-I).
Figure 6. Lens dysmorphology and cell death in embryonic Chmp4b-mutant mice.
(A-I) Fluorescence confocal microscopy of sagittal eye sections from wild-type (D/D) mice (A, D, G), heterozygous Chmp4b-mutant (D/V) mice (B, E, H), and homozygous Chmp4b-mutant (V/V) mice (C, F, I) at E13.5 (A-C) and E15.5 (D-I) showing anti-AQP0 labeling (A-F) and TUNEL assay (G-I). Anti-AQP0 localization revealed that normal lens growth and fiber cell differentiation in the V/V lens at E13.5 (C) are completely arrested by E15.5 (F). Arrows (B, C, E) indicate a lens fixation defect. TUNEL analysis revealed TUNEL-positive cell death in the V/V lens and surrounding tissue at E15.5 (I). Cell nuclei were stained blue with DAPI. Scale bar: 50 μm.
3.4. Chmp4b-floxed mice
To overcome the embryonic lethal effects of a homozygous, germline mutation in Chmp4b-V/V mice we generated Chmp4b-floxed mice to facilitate conditional Chmp4b loss-of-function in the lens using Cre-loxP recombination (Fig. 2). Adult Chmp4b-floxed mice were viable and fertile without obvious ocular, physical, or behavioral abnormalities. We first crossed Chmp4b-floxed (Chmp4bfx/fx) mice with transgenic MLR10-Cre mice that express Cre-recombinase, under the control of a modified αA-crystallin gene promoter, commencing in the lens vesicle and primary fiber cells at E10.5 then extending to all lens epithelial cells and secondary fiber cells by E12.5 (Zhao et al., 2004). We then crossed Cre-positive heterozygous-floxed offspring (Cre-Chmp4bfx/+) with Chmp4bfx/fx mice in order to abolish Chmp4b expression, by deletion of exon-2 through exon-4 (~ 3.9 kb), specifically in the lens (Fig. 2). Resulting offspring displayed grossly similar embryonic development between E12.5 and E15.5 regardless of Cre-transgene and/or Chmp4b-floxed allele status (Supplementary Fig. 2).
3.5. Chmp4b-deficient lenses
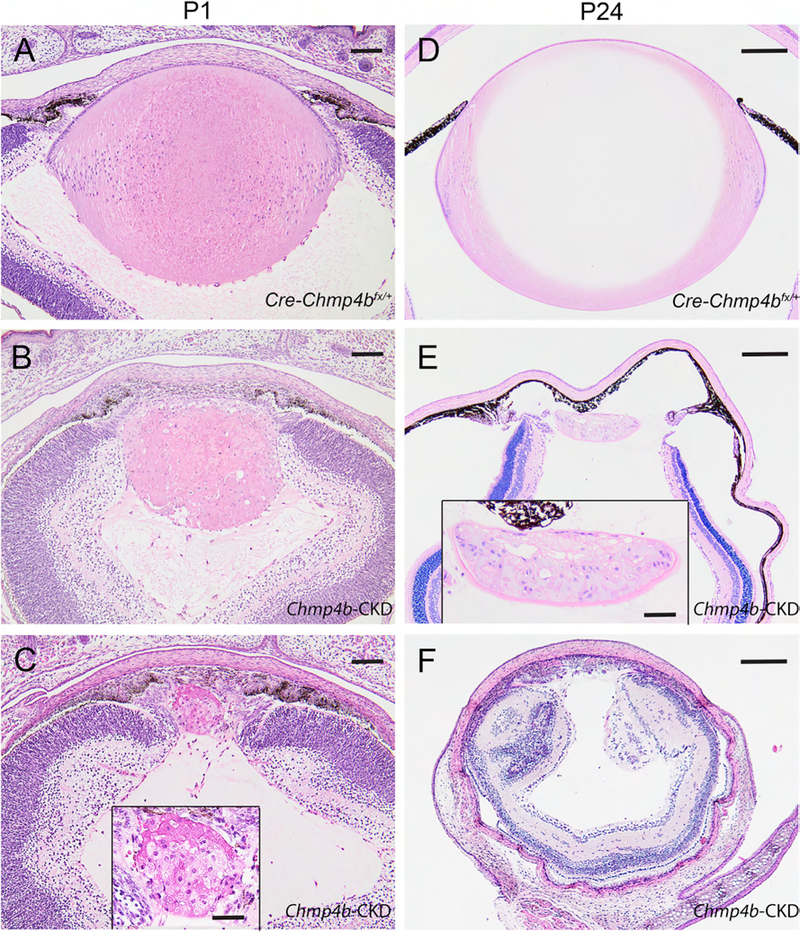
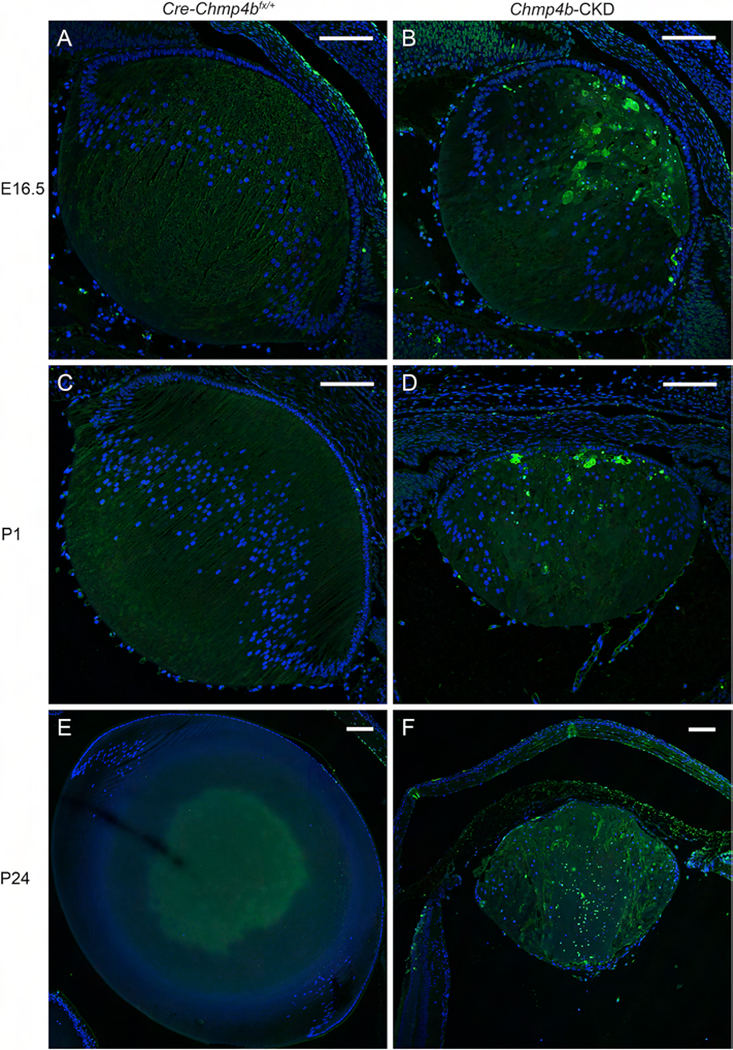
To investigate lens development and pathology in Chmp4b-deficient mice we performed H&E histology followed by in situ localization of specific markers for lens cell morphology, cell death, and an immune-cell response. First, H&E staining of embryonic eye sections revealed that lenses of control mice that were Cre-positive and heterozygous-floxed (Cre-Chmp4bfx/+) were grossly indistinguishable from wild-type (Chmp4b+/+) at E12.5 (Fig. 7A and B), E14.5 (Fig. 7D and E), and E16.5 (Fig. 7G and H). At E12.5, lenses of conditional deletion (Cre-Chmp4bfx/fx) mice appeared similar to those of control (Cre-Chmp4bfx/+) mice (Fig. 7C). By E14.5, Cre-Chmp4bfx/fx lenses displayed a grossly disturbed ‘bow-region’ of cell nuclei near the lens equator accompanied by progressive degeneration of secondary fiber cells at E16.5 particularly toward the anterior pole (Fig. 7F and I). At postnatal ages (P1 and P24), H&E staining confirmed that lenses of control (Cre-Chmp4bfx/+) mice (Fig. 8A and D) remained grossly indistinguishable from wild-type (not shown). By contrast, postnatal Cre-Chmp4bfx/fx mice displayed a variable lens phenotype ranging from a small dysmorphic lens to whole lens ablation with severe microphthalmia (Fig. 8B, C, E, and F). Such variability in lens phenotype suggested that the temporal and/or spatial pattern of Cre-mediated Chmp4b deletion was non-uniform, or mosaic, across the lens during embryonic development even amongst littermates. We were unable to confirm the presence of CHMP4B antigen in small lenses from mice with a Cre-Chmp4bfx/fx genotype from ear DNA since several commercial CHMP4B antibodies failed, in our hands, to detect specific CHMP4B immuno-labeling even in wild-type lens sections (data not shown). However, PCR-genotyping of lens DNA suggested that Cre-deletion of Chmp4b was incomplete in some mice with a Cre-Chmp4bfx/fx ear DNA genotype (Fig. 2D). Overall, these data were consistent with a conditional knockdown, rather than a conditional knockout, of Chmp4b expression in the lens (Chmp4b-CKD).
Figure 7. Lens histology of embryonic Chmp4b-CKD mice.
(A-I) Histochemical (H&E) staining of sagittal eye sections from wild-type mice (A, D, G), control (Cre-Chmp4bfx/+) mice (B, E, H), and Chmp4b-CKD mice (C, F, I) at E12.5 (A-C), E14.5 (D-F), and E16.5 (G-I). In Chmp4b-CKD mice (C, F, I), disturbance of the nuclear bow-region at the lens equator (F, arrows) and fiber cell degeneration with failed de-nucleation toward the lens anterior pole (F and I, arrows) was evident. Scale bar 50 μm.
Figure 8. Lens histology of post-natal Chmp4b-CKD mice.
(A-F) Histochemical (H&E) staining of sagittal eye sections from control (Cre-Chmp4bfx/+) mice (A, D) and Chmp4b-CKD mice (B, C, E, F) at P1 (A-C) and P24 (D-F) showing lens phenotype variation, including ablation (F), associated with transgenic MLR10-Cre activation. Scale bar: 100 μm (A-C), 200 μm (D-F), 50 μm (C and E inset).
Second, anti-AQP0 localization highlighted the concentric organization of tightly packed layers of fiber cell membranes in control lenses from Cre-Chmp4bfx/+ mice at birth (Fig. 9A). By contrast, anti-AQP0 localization revealed that fiber cell organization in developing lenses from Chmp4b-CKD mice at birth was variably disturbed with regions of large vesicle-like structures or fiber cell degeneration (Fig. 9B–D) suggesting that CHMP4B was required for lens growth and differentiation.
Figure 9. Lens dysmorphology in Chmp4b-CKD mice.
(A-D) Immunofluorescence confocal microscopy of sagittal eye sections (P1) from control (Cre-Chmp4bfx/+) mice (A) and Chmp4b-CKD mice (B-D) labeled with anti-AQP0 showing variably impaired lens growth and fiber cell differentiation in the latter. Cell nuclei were stained blue with DAPI. Scale bar: 100 μm.
Third, we performed TUNEL analysis of nuclear DNA (3’-OH) fragmentation to assess levels of lens cell death. As expected, control (Cre-Chmp4bfx/+) lenses were largely devoid of TUNEL-positive nuclei at embryonic (E16.5) and post-natal ages between birth and wean-age (Fig. 10A, C, and E). By contrast, TUNEL-positive nuclei were abundant within age-matched Chmp4b-CKD lenses (Fig. 10B, D, and F) consistent with lens-restricted cell death following activation of Chmp4b-deletion by MLR10-Cre between E10.5 and E12.5. Finally, to explore the possibility that TUNEL-positive cell-death within the Chmp4b-CKD lens leads to an immune-cell response, we performed in situ localization of an antibody to the macrophage-lineage marker macrosialin (CD68). In control (Cre-Chmp4bfx/+) lenses, CD68-positive cells were localized to embryonic vasculature surrounding (but not within) the lens - known as the tunica vasculosa lentis (TVL) - that undergoes complete regression soon after birth (Fig. 11A, C, and E). However, CD68-positive cells accumulated within and around Chmp4b-CKD lenses at embryonic (E16.5) and post-natal ages between birth and wean-age (Fig. 11B, D, and F). Collectively, these observations suggest that lens cell death, resulting from functional loss of Chmp4b, elicited macrophage infiltration from the TVL and/or other ocular or extraocular tissues consistent with an immune cell response.
Figure 10. Lens cell death in Chmp4b-CKD mice.
(A-F) Fluorescence confocal microscopy of sagittal eye sections from control (Cre-Chmp4bfx/+) mice (A, C, E) and Chmp4b-CKD mice (B, D, F) at E16.5 (A, B), P1 (C, D), and P24 (E, F) showing TUNEL-positive cell death in the Chmp4b-CKD lens (B, D, F). Cell nuclei were stained blue with DAPI. Scale bar: 100 μm.
Figure 11. Immune-cell response in Chmp4b-CKD lenses.
(A-F) Immunofluorescence confocal microscopy of sagittal eye sections from control (Cre-Chmp4bfx/+) mice (A, C, E) and Chmp4b-CKD mice (B, D, F) at E16.5 (A, B), P1 (C, D), and P24 (E, F) labeled with anti-CD68. CD68-positive cells were present in the embryonic and postnatal Chmp4b-CKD lens (B, D, F) and surrounding embryonic vasculature or TVL (B) but were absent from wild-type lens (A, C, E) and confined to the embryonic TVL (A). Cell nuclei were stained blue with DAPI. Scale bar: 100 μm (A-F), 50 μm (F inset).
4. Discussion
Currently, at least three heterozygous missense mutations in CHMP4B (p.H57R, p.D129V, p.E161K) have been linked with autosomal dominant forms of pediatric cataract in humans (Shiels et al., 2007; Zhang et al., 2018). In this study, we generated gene-targeted mice to model the pathogenic effects of one of these germline cataract mutations (p.D129V) in Chmp4b compared with those of conditional Chmp4b-deficient lenses. Whereas heterozygous Chmp4b-D/V mutant mice did not develop early-onset cataract, homozygous Chmp4b-V/V mutants underwent global embryonic cell-death (Fig. 3, 5, and 6). Similarly, lenses heterozygous for Chmp4b-deficiency did not develop early-onset cataract. However, conditional loss of Chmp4b function resulted in variable degrees of lens dysmorphology associated with cell death and an immune cell response (Fig. 8–11). Recently, accumulation of immune cells has been reported in mouse lenses conditionally lacking N-cadherin (Logan et al., 2017). Overall, while cataract was not associated with heterozygous mutation or deficiency of CHMP4B in mice, these data support the notion that CHMP4B plays a critical role in lens growth and fiber cell differentiation.
In humans, CHMP4B-related cataract is described as posterior-polar with disc-like opacities located at the posterior pole of the lens on the optical axis of the eye (Shiels et al., 2007; Yamada et al., 2000a; Yamada et al., 2000b). We note that posterior-polar cataract associated with the heterozygous p.D129V mutation in CHMP4B exhibited a variable age-at-diagnosis (4 −20 years) and age-at-surgery (4 – 40 years) with evidence of incomplete penetrance (Shiels et al., 2007). In mice, cataract was non-penetrant in heterozygous Chmp4b-D/V mutant lenses consistent with reduced expression levels of mutant (V) versus wild-type (D) Chmp4b alleles on the B6J genetic background (Fig. 4). Factors that may influence cataract penetrance in mice versus humans include genetic background of the mouse strain and morphological differences between the spheroidal mouse lens versus the ellipsoidal (biconvex) human lens. While we cannot exclude penetrance of Chmp4b-D/V related cataract on other genetic backgrounds (e.g., 129, FVB), we observed that cataract was also non-penetrant in heterozygous Chmp4b-deficient lenses (Fig. 7 and 8) further suggesting that Chmp4b expression at approximately 50% of wild-type levels was sufficient for normal lens growth and transparency in mice with the B6J genetic background.
In contrast to heterozygous Chmp4b-D/V mutant mice, homozygous Chmp4b-V/V mutants were non-viable beyond E15.5 (Fig. 3) consistent with in silico predictions that the non-conservative p.D129V substitution of aspartate-129 with valine was damaging at the protein function level (PolyPhen-2 score = 0.999). Similarly, germline Chmp4b knockout (null) mice were also reported to be embryonic lethal; however, such Chmp4b-null embryos died around E8.5 prior to the commencement of eye development at E9.5 (Lee et al., 2007). While we did not examine very early Chmp4b-V/V mutant embryos, gross brain abnormalities were evident by E12.5 (Supplementary Fig. 1). The apparent extended longevity of Chmp4b-V/V mutant embryos over Chmp4b-null embryos suggested that the Chmp4b-V mutant allele retained some functionality compared with the Chmp4b-null allele. Although we cannot confirm that lens cell-death in the Chmp4b-V/V embryo was a direct result of the mutant protein, we note that in the Chmp4b-CKD embryo lens cell death and macrophage infiltration were in progress soon after conditional activation of MLR10-Cre in the lens (Fig. 10 and 11). Collectively, these observations suggest that CHMP4B function is required for mammalian cell and tissue viability.
Functional over-expression studies in transfected mammalian cells have attempted to determine the effects of mutant CHMP4B-V dysfunction on the canonical ESCRT-III membrane-scission processes of viral-budding and cytokinesis in vitro. First, a truncated form of recombinant CHMP4B harboring the p.D129V substitution (D129V-CHMP4B1–150) displayed a different sub-cellular localization pattern to wild-type (CHMP4B1–150) and a reduced (~ 50%) capacity to release virus-like-particles (HIV-1 Gag polyprotein, Pr55) from the cell surface - consistent with deleterious effects on the endosome-lysosome pathway (Shiels et al., 2007). Second, full-length mutant CHMP4B-D129V exhibited reduced (~ 50%) ability to localize with chromatin found in chromosomal bridges and derived micronuclei compared with wild-type CHMP4B (Sagona et al., 2014). These abnormal chromatin structures arise during failed cytokinesis, particularly in transformed cells, and CHMP4B-positive micronuclei were often co-localized with markers for lysosomes and autophagosomes suggesting that CHMP4B may participate in autophagolysosomal degradation of micronuclei and other extranuclear chromatin during lens cell differentiation (Sagona et al., 2014). Further studies of the Chmp4b-mutant and Chmp4b-deficient mice described here may help to elucidate the role of the endosome-lysosome and autophagy pathways in lens growth and differentiation.
Supplementary Material
Highlights.
First report of germline Chmp4b-mutant and lens conditional Chmp4b-knockdown mice
Heterozygous Chmp4b-mutant and Chmp4b-deficient mice did not develop cataract
Homozygous Chmp4b-mutant mice were embryonic lethal
Conditional Chmp4b-knockdown caused lens cell death and an immune cell response
Acknowledgements
We thank M. Casey for gene-targeting support, B. McMahan and G. Ling for histology support, and Dr. M.L. Robinson for sharing MLR10-Cre mice. This work was supported by NIH/NEI grants EY012284 (to A.S.) and EY02687 (Core Grant for Vision Research) and an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness (RPB).
Abbreviations:
- ESCRT
endosome sorting complex required for transport
- CHMP
charged multi-vesicular body protein
- Snf7
sucrose non-fermenting 7
- Vps32
vacuole protein sorting 32
- MIM
Mendelian Inheritance in Man
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- Rpl19
ribosomal protein L 19
- ISH
in situ hybridization
- Cre
causes recombination or cyclization recombinase
- loxP
locus of crossing-over (x) P1
- TUNEL
terminal deoxynucleotidal transferase [TdT]-mediated deoxyuridine triphosphate (dUTP) nick-end labeling
- AQP0
aquaporin-0
- CD68
cluster of differentiation antigen 68
- DAPI
4′,6-diamidino-2-phenylindole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supporting information
Supplementary Table 1. PCR amplification primers for genotyping Chmp4b-mutant and Chmp4b-deficient mice.
Supplementary Figure 1. Brain development in Chmp4b-mutant embryos. (A, B) Histochemical (H&E) staining of coronal head sections from wild-type (A) and homozygous (V/V) Chmp4b-mutant (B) embryos at E12.5 showing abnormal enlargement of the third and fourth ventricles in the latter. Scale bar: 200 μm.
Supplementary Figure 2. Embryonic development of Chmp4b-CKD mice. (A-F) Representative images of embryos derived from breeding transgenic MLR10-Cre mice with Chmp4b-floxed mice (Chmp4bfx/fx) showing grossly similar development in Cre-negative heterozygous floxed (Chmp4bfx/+) embryos (A, D), Cre-positive heterozygous floxed (Cre-Chmp4bfx/+) embryos (B, E), and Cre-positive homozygous floxed (Chmp4b-CKD) embryos (C, F) at E12.5 (A-C) and E15.5 (D-F). Scale bar: 1 mm.
References
- Alizadeh A, Clark J, Seeberger T, Hess J, Blankenship T, FitzGerald PG, 2004. Characterization of a mutation in the lens-specific CP49 in the 129 strain of mouse. Invest Ophthalmol Vis Sci 45, 884–891. [DOI] [PubMed] [Google Scholar]
- Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD, 2002. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell 3, 271–282. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Shi Y, Vrensen GF, 2011. Biological glass: structural determinants of eye lens transparency. Philos Trans R Soc Lond B Biol Sci 366, 1250–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DC, Coats JM, 2000. The lens organizes the anterior segment: specification of neural crest cell differentiation in the avian eye. Dev Biol 220, 424–431. [DOI] [PubMed] [Google Scholar]
- Cashikar AG, Shim S, Roth R, Maldazys MR, Heuser JE, Hanson PI, 2014. Structure of cellular ESCRT-III spirals and their relationship to HIV budding. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Nowak RB, Fowler VM, 2017. The lens actin filament cytoskeleton: Diverse structures for complex functions. Exp Eye Res 156, 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaruttini N, Redondo-Morata L, Colom A, Humbert F, Lenz M, Scheuring S, Roux A, 2015. Relaxation of Loaded ESCRT-III Spiral Springs Drives Membrane Deformation. Cell 163, 866–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ L, Raiborg C, Wenzel EM, Campsteijn C, Stenmark H, 2017. Cellular Functions and Molecular Mechanisms of the ESCRT Membrane-Scission Machinery. Trends Biochem Sci 42, 42–56. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Ashery-Padan R, 2014. The cellular and molecular mechanisms of vertebrate lens development. Development 141, 4432–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Zhang X, 2017. Signaling and Gene Regulatory Networks in Mammalian Lens Development. Trends Genet 33, 677–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson PJ, Grey AC, Maceo Heilman B, Lim JC, Vaghefi E, 2017. The physiological optics of the lens. Prog Retin Eye Res 56, e1–e24. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Cashikar A, 2012. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol 28, 337–362. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Roth R, Lin Y, Heuser JE, 2008. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J Cell Biol 180, 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iribarren R, 2015. Crystalline lens and refractive development. Prog Retin Eye Res 47, 86–106. [DOI] [PubMed] [Google Scholar]
- Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, Perez F, 2014. ESCRT machinery is required for plasma membrane repair. Science 343, 1247136. [DOI] [PubMed] [Google Scholar]
- Juan T, Furthauer M, 2018. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol 74, 66–77. [DOI] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H, 1996. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A 93, 5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Beigneux A, Ahmad ST, Young SG, Gao FB, 2007. ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr Biol 17, 1561–1567. [DOI] [PubMed] [Google Scholar]
- Lefebvre C, Legouis R, Culetto E, 2018. ESCRT and autophagies: Endosomal functions and beyond. Semin Cell Dev Biol 74, 21–28. [DOI] [PubMed] [Google Scholar]
- Logan CM, Bowen CJ, Menko AS, 2017. Induction of Immune Surveillance of the Dysmorphogenic Lens. Sci Rep 7, 16235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncle N, Agromayor M, Martin-Serrano J, Williams DW, 2015. An ESCRT module is required for neuron pruning. Sci Rep 5, 8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos Y, Hodgson L, Mantell J, Verkade P, Carlton JG, 2015. ESCRT-III controls nuclear envelope reformation. Nature 522, 236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel S, Macheboeuf P, Martinelli N, Weissenhorn W, 2011. Divergent pathways lead to ESCRT-III-catalyzed membrane fission. Trends Biochem Sci 36, 199–210. [DOI] [PubMed] [Google Scholar]
- Sagona AP, Nezis IP, Stenmark H, 2014. Association of CHMP4B and autophagy with micronuclei: implications for cataract formation. Biomed Res Int 2014, 974393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scourfield EJ, Martin-Serrano J, 2017. Growing functions of the ESCRT machinery in cell biology and viral replication. Biochem Soc Trans 45, 613–634. [DOI] [PubMed] [Google Scholar]
- Shen QT, Schuh AL, Zheng Y, Quinney K, Wang L, Hanna M, Mitchell JC, Otegui MS, Ahlquist P, Cui Q, Audhya A, 2014. Structural analysis and modeling reveals new mechanisms governing ESCRT-III spiral filament assembly. J Cell Biol 206, 763–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Knopf HL, Yamada K, Yoshiura K, Niikawa N, Shim S, Hanson PI, 2007. CHMP4B, a novel gene for autosomal dominant cataracts linked to chromosome 20q. Am J Hum Genet 81, 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simirskii VN, Lee RS, Wawrousek EF, Duncan MK, 2006. Inbred FVB/N mice are mutant at the cp49/Bfsp2 locus and lack beaded filament proteins in the lens. Invest Ophthalmol Vis Sci 47, 4931–4934. [DOI] [PubMed] [Google Scholar]
- Stoten CL, Carlton JG, 2018. ESCRT-dependent control of membrane remodelling during cell division. Semin Cell Dev Biol 74, 50–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Henne WM, Borbat PP, Buchkovich NJ, Freed JH, Mao Y, Fromme JC, Emr SD, 2015. Structural basis for activation, assembly and membrane binding of ESCRTIII Snf7 filaments. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vietri M, Schink KO, Campsteijn C, Wegner CS, Schultz SW, Christ L, Thoresen SB, Brech A, Raiborg C, Stenmark H, 2015. Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature 522, 231–235. [DOI] [PubMed] [Google Scholar]
- Yamada K, Tomita H, Yoshiura K, Kondo S, Wakui K, Fukushima Y, Ikegawa S, Nakamura Y, Amemiya T, Niikawa N, 2000a. An autosomal dominant posterior polar cataract locus maps to human chromosome 20p12-q12. Eur J Hum Genet 8, 535–539. [DOI] [PubMed] [Google Scholar]
- Yamada K, Tomita HA, Kanazawa S, Mera A, Amemiya T, Niikawa N, 2000b. Genetically distinct autosomal dominant posterior polar cataract in a four-generation Japanese family. Am J Ophthalmol 129, 159–165. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Da Wang J, Jia HY, Zhang JS, Li Y, Xiong Y, Li J, Li XX, Huang Y, Zhu GY, Rong SS, Wormstone M, Wan XH, 2018. Mutation profiles of congenital cataract genes in 21 northern Chinese families. Mol Vis 24, 471–477. [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang Y, Rizo CM, Overbeek PA, Robinson ML, 2004. Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Invest Ophthalmol Vis Sci 45, 1930–1939. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bennett TM, Shiels A, 2016. Lens ER-stress response during cataract development in Mip-mutant mice. Biochim Biophys Acta 1862, 1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Shiels A, 2018. Epha2 and Efna5 participate in lens cell pattern-formation. Differentiation 102, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.