Abstract
The innate and adaptive arms of the immune system tightly regulate immune responses in order to maintain homeostasis and host defense. The interaction between those two systems is critical in the activation and suppression of immune responses which if unchecked may lead to chronic inflammation and autoimmunity. γδ T cells are non-conventional lymphocytes, which express T cell receptor (TCR) γδ chains on their surface and straddle between innate and adaptive immunity. Recent advances in of γδ T cell biology have allowed us to expand our understanding of γδ T cell in the dysregulation of immune responses and the development of autoimmune diseases. In this review, we summarize current knowledge on γδ T cells and their roles in skin and joint inflammation as commonly observed in rheumatic diseases.
Keywords: γδ T cells, T cell receptor (TCR), bone remodeling, autoimmune diseases, skin and joint inflammation, psoriatic arthritis
Introduction
T cells are categorized into distinct types of T cells based on the type of T cell antigen receptors (TCRs) and include αβT and γδT cells, which express αβTCRs and γδTCRs, respectively [1]. αβ T cells are usually found in peripheral tissues and circulatory system whereas γδ T cells may reside in blood and lymphoid tissue as well as epithelial environments such as the skin, gastrointestinal tract, or genitourinary tract, where they have important functions in tissue homeostasis and inflammatory response [1]. Although γδ T cells only comprise a small portion of all T lymphocytes (0.5–5%), they represent a larger proportion of T cells in certain tissues, such as the murine skin and lymph nodes [2,3]. γδ T cells have different functions in distinct pathophysiological conditions as driven by their tissue-specific microenvironments and tropism. These non-conventional T cells bridge the innate and adaptive immune systems by sharing functions with antigen presenting cells, pro-inflammatory and cytotoxic effector cells, and immune-regulatory cells [4,5]. Depending on the particular subset, the stimulus and the microenvironment, γδ T cells are able to produce the effector cytokines of Th1, Th2, and Th17 cells, such as IFN-γ, IL-4 and IL-13, and IL-22 and IL-17, respectively, as well as chemokines including CCL5/RANTES, CXCL10/IP-10, XCL1/lymphotactin [6–9]. They thus have the capacity to regulate both pro-inflammatory and anti-inflammatory responses and orchestrate the specific recruitment of further leukocyte populations. Subsets of γδ T cells may also express FOXP3, a master regulator in the development and function of regulatory T cells, thereby assuming regulatory roles [10]. Moreover, γδ T cells can influence specific antibody responses, and as consequence serum antibody levels including IgG1, IgG2b, and IgE are reduced in γδ T cell deficient (TCRδ−/−) mice [11]. γδ17 T cells have similar features with Th17 cells, which express CC-chemokine receptor 6 (CCR6), IL-23 receptor, retinoic acid receptor-related orphan receptor-γt (RORγt) and aryl hydrocarbon receptor (AhR), as well as the secretion of IL-17 and IL-22 [12]. Since γδ T cells exhibit critical functions in innate and adaptive immunity, their dysregulation has been involved in the pathogenesis of rheumatic diseases [13–16]. The cytokine milieu of the local microenvironment regulates the development and activation of each γδ T cell subtype. In succession, the unique characteristics of each subtype subsequently determine the effectiveness of immune regulation in maintaining homeostasis and self-tolerance or its ineffectiveness and rise of pathologic outcomes resulting in chronic inflammation and autoimmunity. Thus, the molecular events that dictate the development and activation of γδ T cell subtypes are of primary importance. As the role of T cell receptor signaling in γδ T cell development was recently reviewed [17], this review will focus on the human and murine γδ T cell subtypes in the pathogenesis of skin and joint inflammation.
Human and murine γδ T cell subtypes in innate and adaptive immunity
Human γδ T cells
In humans, γδ T cells can be categorized into two major subtypes based on the expression of TCR δ chain: Vδ1 and Vδ2 T cells [18]. The diversity and complexity of γδ T cells are results of specific Vδ/Vγ pairing. Preferentially, the Vδ1 chain is paired with different VγI family members (Vγ2/3/4/5/8) whereas Vδ2 is typically (but not exclusively) paired with the Vγ9 chain [19,20] (Table 1). Although not common, there are descriptions of Vγ9Vδ1 in the literature associated with viral infection and cancer [21]. In addition to these major populations, non-Vδ1 and non-Vδ2 γδT cells are also found in healthy humans. Vδ3+ T cells, are often paired with Vγ2 or Vγ3, and can be found in peripheral blood and liver [22]. Vδ4+, Vδ6+, Vδ7+, and Vδ8+ T cells are detected in the peripheral blood of lymphoma patients but these subtypes have not been well characterized [23]. Vγ9+Vδ2+ γδ T cells are the dominant population in the peripheral blood [24]. Among human γδ T cell subtypes, Vγ9+Vδ2+ γδ T cells have been the most studied, given their abundance in peripheral blood and their ease to be expanded and manipulated in cell culture. These cells possess a ‘phosphoantigen’-reactive semi-invariant TCR and are central to protective host immune responses against microbial pathogens producing the corresponding metabolites [24]. While the antigen specificity of the vast majority of γδ T cells remains elusive [25], the TCR of a Vγ4+Vδ5+ clone directly binds endothelial protein C receptor (EPCR), a major histocompatibility complex-like molecule [26]. Moreover, the recognition of target cells by γδ T cells required a multi-molecular stress signature composed of EPCR and costimulatory ligand(s) demonstrating that γδ TCR mediates recognition of broadly stressed human cells by engaging a stress-regulated self-antigen [26]. While the specificity of most human γδ T cell receptors remains elusive, the breadth of possible ligands appears to span MHC and MHC-related molecules, surface-expressed and soluble proteins as well as small peptides and lipids [25].
Table 1:
| Vγ gene | Paired Vδ gene | Tissue resident |
|---|---|---|
| Human | ||
| Vγ2+, Vγ3+, Vγ4+, Vγ5+, Vγ8+, Vγ9+ | Vδ1+ | Peripheral blood, skin, gut, spleen, liver. |
| Vγ9−/Vγ9+ | Vδ2+ | Peripheral blood and solid tissues. |
| Vγ2+, Vγ3+, Vγ4+ | Vδ3+ | Peripheral blood, liver. |
| Murine | ||
| Vγ1+ | Vδ5+, Vδ6.3+ | Lung, colon. |
| Vγ4+ | Vδ4+ | Skin, brain, lung, colon, joint. |
| Vγ5+ | Vδ1+ | Skin. |
| Vγ6+ | Vδ1+ | Lung, reproductive tract and oral mucosa. |
Human γδ T cells play essential roles in the innate immunity response. Vγ9+Vδ2+ T cells induce monocyte differentiation into antigen presenting cells through release of IFN-γ, TNF-α, GM-CSF, and IL-4, as well as recruitment, activation and differentiation of neutrophils [27–30]. Freshly isolated human peripheral blood γδ T (Vγ9+Vδ2+) cells can function as professional phagocytes via antibody opsonization and CD16 (FcγRIII), leading to antigen processing and presentation on MHC class II [31]. Vγ9+Vδ2+ T cells also efficiently process and display antigens and provide co-stimulatory signals sufficient for strong induction of naïve αβ T cell proliferation and differentiation [32]. The adaptive immune responses of γδ T cells are demonstrated by memory-like Vγ9+Vδ2+ T cells in vaccinated humans which persist for as long as 7 months post the secondary vaccination [33]. Besides the memory function, γδ T cells also regulate adaptive immune through interaction with B cells. Vγ9+Vδ2+ T cells induce the expression of essential B-cell co-stimulatory molecules including CD40L, OX40, CD70, and ICOS, which are important to drive immunoglobulin (Ig) isotype switching in B cells [34–36]. Human Vδ2+ and Vδ3+ γδ T cells both induce expression of maturation markers (CD40, CD86) and secretion of antibodies by B cells [37,36]. Activated Vγ9+Vδ2+ T cells can produce CXCL13, a B cell attracting chemokine, which is key in recruiting B cells to secondary lymphoid tissue and establishing germinal centers and the production and affinity maturation of class-switched antibodies [5,38,39]. Consequently, CXCR5 identifies a unique subset of Vγ9Vδ2 T cells which secrete IL-2, IL-4, and IL-10 and help B cells for antibody production [40]. Collectively, human γδ T cells display a broad array of functional activities as summarized in Figure 1 [5,41].
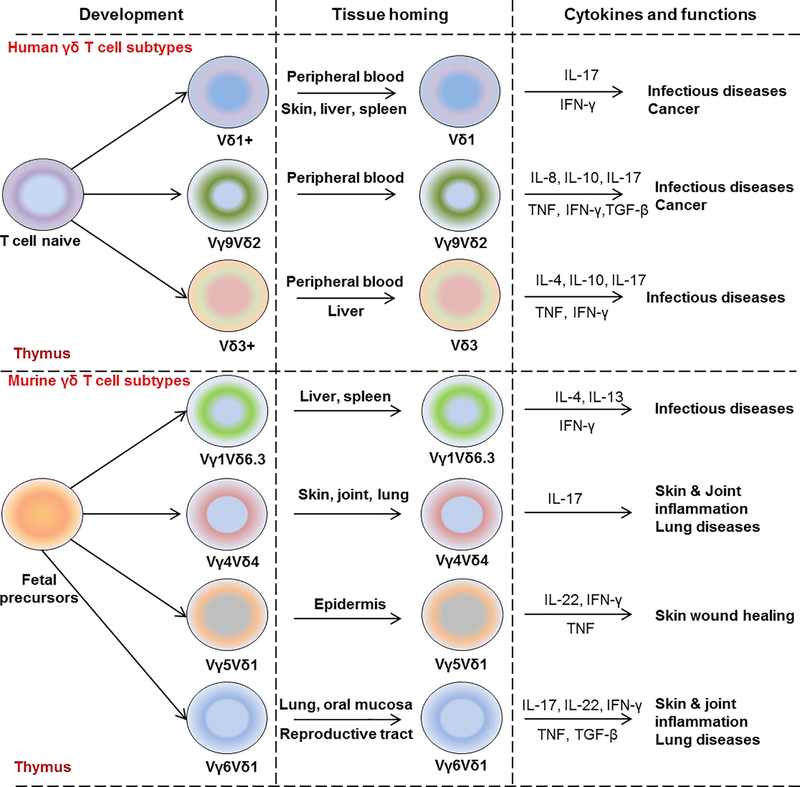
Figure 1: Overview of γδ T cell functional programing in human and mouse.
Schematic illustration of γδ T cell development from naïve fetal precursors in human (upper panel) and mouse (lower panel) thymus. Human naïve γδ T cells differentiate into Vδ1+ γδ T subtypes found in peripheral blood, skin, liver, and spleen and produce IL-17 and IFN-γ; Vγ9+Vδ2+ γδ T cells found in the peripheral blood and produce predominantly TNF and IFN-γ as well as IL-8, IL-10, IL-17; Vδ3+ found in peripheral blood and liver and produce several cytokines such as IL-4, IL-10, IL-17, TNF, and IFN-γ. In mouse, Vγ1+Vδ6.3+ γδ T cells normally resident in lymphoid tissues including spleen and liver and produce IL-4, IL-13, and IFN-γ. Vγ4+Vδ4+ γδ T cells are found in the skin, joint, and lung and known as IL-17-producing γδ T cells. Vγ5+Vδ1+ are residents in epidermis, produce IL-17, TNF, IFN-γ whereas Vγ6+Vδ1+ have been found in the female reproductive tract and oral mucosa and produce IL-17, IL-22, TNF, IFN-γ and TGF-β.
Murine γδ T cells
The murine γδ literature can be confusing due to the various nomenclatures that have been used to number the individual γ and δ receptors. The International Immunogenetics Information System (IMGT) is the most up-to-date resource for TCR genes, although their numbering system does not match with how these cells are historically and most commonly referred to. Although the functions of murine γδ T cell subtypes are only partially understood, at least 2 major functionally distinct γδ T cell subsets have been identified including Vγ1+ and Vγ4+ γδ T cells which have similar features with human peripheral blood γδ T cells [42] Table 1 and Fig. 1. Murine Vγ1+ and Vγ4+ γδ T cells require direct interaction with CD8+ dendritic cells (DCs) in lymphoid tissues for their functional development [43]. IL 23 drives differentiation of peripheral γδ17 T cells from adult bone marrow derived precursors [44]. Moreover, different populations of γδ T have different levels of IL-23R expression as Vγ1+ and Vγ4+ γδ T cells express IL-23R differently in vivo and in vitro [45]. For example, when compared to their IL-23R expression in naïve mice, Vγ4+ γδ T cells express high levels of IL-23R in immunized mice whereas Vγ1+ γδ T cells from either naïve or immunized mice only expressed IL-23R at low or very low levels [45]. In addition, Vγ4+Vδ4+ T cells are found in joints and joint-draining lymph nodes in experimental models of skin and joint inflammation. The vast majority produces IL-17, which contributes to the development of collagen-induced arthritis (CIA) and imiquimod-induced skin inflammation (a model of psoriasis) [46–50].
Dendritic epidermal γδ T cells (DETCs) characteristically express Vγ5+Vδ1+ TCRs and normally reside in the mouse skin (nomenclature according to Heilig and Tonegawa) [51,52]. Vγ6+Vδ4+ T cells (most commonly referred to as Vγ6+Vδ1+ T cells) share the exact same CDR3 a.a sequence (CACWDSSGFHKVF) [53] as the Vγ5+Vδ1+ cells but these cells reside predominantly at mucosal sites. Both subtypes express identical δ1 chains encoding the same CDR3 a.a. sequence (CGSDIGGSSWDTRQMFF). Skin epidermal Vγ5+V1+ DETCs were originally thought to be the only resident γδ T cell population in the skin, although now other γδ T cell populations have also been detected. In addition, Vγ5+ T cells with the same CDR3 sequence have now been detected at very low frequencies in the lymph nodes. However, in general the Vγ5+Vδ1+ DETCs appear to be the major non-circulating skin-resident γδ T cell population in the skin. Skint-1, a thymic epithelial cell determinant, selectively determines the functional phenotype of Vγ5+Vδ1+ fetal thymocytes by inducing an Egr3-mediated pathway, provoking differentiation and IFN-γ production while suppressing the γδ T cell lineage factor, Sox13, and a RORγt transcription factor-associated IL-17-producing capacity [51]. Moreover, Skint-1 is essential for the development in the thymus and the establishment of the DETC population in the skin [54,55]. A recent study showed that signaling via the NF-κB-inducing kinase (NIK) is important for the full development of functional Vγ5+ dendritic epidermal T cell (DETCs) [56]. Vγ6+Vδ1+ T cells are rare in most normal tissues but are the dominant γδ T cell population in the female reproductive tract, oral mucosa and lung [57–59]. Vγ6+Vδ4+ T cells preferentially expand in skin-draining lymph nodes following S. aureus skin infection and mediate long-term immunity to S. aureus [53].
γδ T cells can act as both positive and/or negative regulators of innate immune responses via myeloid cell activation. RNA-Seq analysis of γδ T cells from infected mice demonstrate that γδ T cells highly express several growth factors, chemokines, and other proteins known to control myeloid cell recruitment, activation, and differentiation (Csf1, Ccl3, Ccl4, Ccl5, Ccl6, Ccrl2) [60]. M-CSF (encoded by Csf1) is known to promote development and polarization of macrophages whereas CCL3 and CCL5 are specific ligands of CCR3 and CCR5 receptors [61], which are critical for neutrophil migration [62] (Fig. 2). Consistently, Jiang et al demonstrated that dermal γδ T cells are required for recruitment of Gr-1+CD11b+ neutrophils into skin during skin inflammation [63]. In keeping with these observations, we recently demonstrated that γδ T cells blockade inhibited the expansion and recruitment of neutrophils in blood and spleen as well as neutrophil migration into the joint in a murine experimental arthritis model [64]. Negative regulatory roles of γδ T cells in myeloid cell activity have also been described during wound healing [65]. Specifically, γδ T cells suppress the infiltration of macrophages (F4/80+CD11b+) and myeloid derived suppressor cells (CD11b+Gr1+) during skin wound healing [65]. In addition, Toll-Like receptor 2 (TLRs), which has critical roles in early innate immunity and initiate immune responses, is expressed in freshly isolated γδ T cells although its exact role in γδ T cells is not completely understood [66]. Activated γδ T cells are also capable of expressing MHC class II and co-stimulatory molecules (CD40 and CD80) presenting the specific antigen to other adaptive immune cells [67]. Collectively, murine γδ T cells regulate innate immune responses via multiple pathways including direct activation of TLR pathways in neutrophil and monocytes, and antigen presentation.
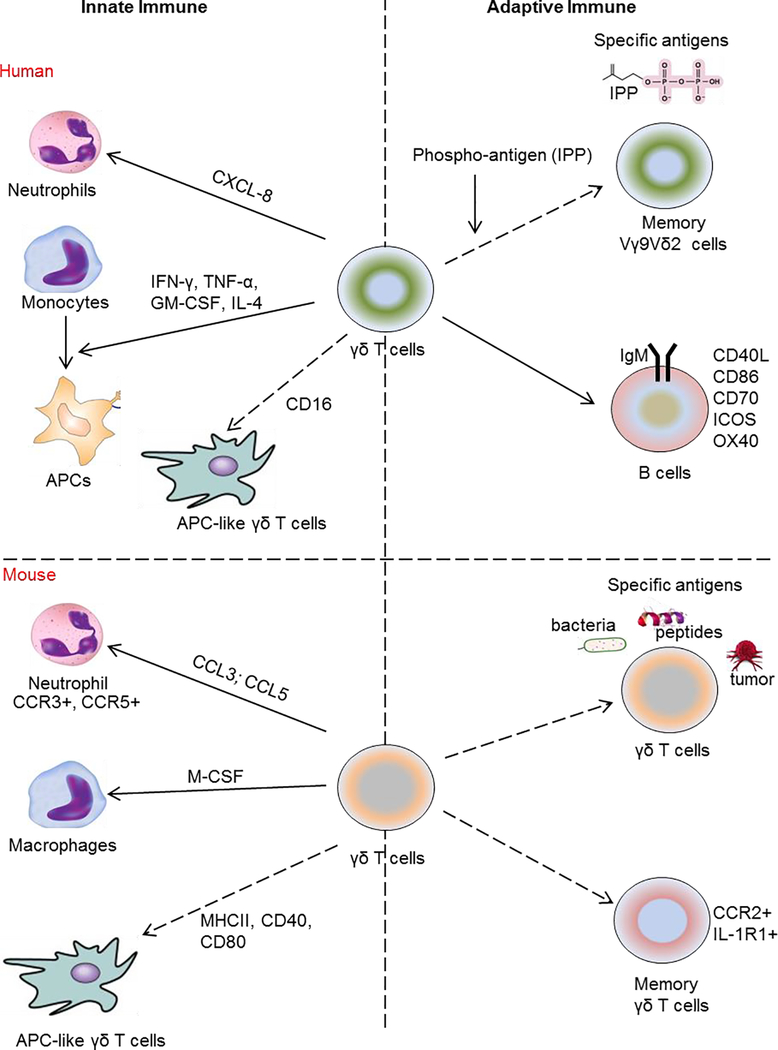
Figure 2: Functional roles of human/murine γδ T cells in immune responses.
The figure illustrates the roles of human (upper panel) and mouse (lower panel) γδ T cells in innate and adaptive immune responses. In human, γδ T cells induce neutrophil migration through regulation of CXCL8 production, monocyte differentiation into antigen presenting cells (APCs) through release of IFN-γ, TNF-α, GM-CSF, and IL-4 and function as a professional phagocyte via antibody opsonization and CD16 (FcγRIII), leading to antigen processing and presentation on MHC class II. For adaptive immune responses, non-peptide phospho-antigens are specific antigens for Vγ9+Vδ2+ γδ T and also induce robust expansion of memory Vγ9+Vδ2+. γδ T cells induce the expression of essential B-cell co-stimulatory molecules including CD40L, CD86, CD70, OX40, and ICOS as well as secretion of IgM by B cells.
In mouse, γδ T cells regulate macrophages and neutrophils through release of M-CSF (responsible for macrophages polarization), CCL3 and CCL5 (chemokines for neutrophil migration). Activated γδ T cells are also capable of expressing MHC class II and co-stimulatory molecules (CD40 and CD80) presenting the specific antigen to other immune cells. For adaptive immune responses, γδ T cells have the ability to recognize and are specifically stimulated by a different antigens derived from bacteria, small peptides, and tumor cells and function as memory γδ T cells (CCR2+ and IL-1R+).
Key features of the adaptive immune system are antigen specificity and generation of immunologic memory which provides a rapid and robust immune response [68]. Although specific antigens for murine γδ T cells have not been well characterized, γδ T cells have the ability to recognize and are specifically stimulated by a different repertoire of antigens derived from bacteria [69], small peptides [67], and tumor cells [25]. Interestingly, a memory feature is observed in murine γδ T cells where memory-like Vγ4+ γδT17 cells are detected, respond more rapidly, and produce more IL-17 which leading to a faster skin inflammatory response in a murine skin inflammation model [47]. IL-17-producing memory γδ T cells promote inflammation in both involved and uninvolved psoriatic-like lesions in a murine model of psoriasis [70,71]. In addition, γδ T cells modulate systemic antibody levels including all major subclasses and especially IgE antibodies as well as affect IL-4 production, B-cell activation, and B-cell tolerance [72]. Moreover, intraepithelial-resident γδ T cells have a unique role in initiating and regulating IgE production, driving an early innate-like response, which directs a subsequent adaptive response [73]. High-throughput antibody sequencing revealed that γδ T cells shape the IgE repertoire by supporting specific variable-diversity-joining (VDJ) rearrangements [73]. Also, γδ T cells control humoral immune response by inducing T follicular helper (Tfh) cell differentiation [74]. In summary, γδ T cells can regulate a plethora of innate and adaptive immune responses (Fig. 2).
Pathophysiology of γδT cells in autoimmunity
γδ T cell subsets contribute to tissue damage and development of experimental autoimmune diseases including psoriasis-like disease [13], collagen-induced arthritis [46], colitis [75], autoimmune uveitis [76] and experimental autoimmune encephalomyelitis (EAE) [77]. Inflammatory functions of γδ T cells are defined by their cytokine production, including IL-17, IFN-γ, and TNF-α, which are commonly involved in autoimmunity (Table 2). Different subsets of γδ T cells are associated with different autoimmune diseases as depending on their tissue expression, and function may contribute to pathogenicity. Apart from the implication of γδ T cells in psoriasis, which is well established [78,79]; several studies suggest that γδ T cells are involved in the pathogenesis of rheumatoid arthritis (RA) [80–82]. In RA patients, peripheral Vγ9+Vδ2+ T cells, which express high levels of chemokine receptors CCR5 and CXCR3, migrate into the synovium and secrete IFN-γ and IL-17 [81]. Also in juvenile idiopathic arthritis (JIA), Vγ9+Vδ2+ T cells are a major synovial fluid T cell population and their proliferation is regulated by CD4+CD25+FOXP3+ T cells, thus controlling synovial inflammation [83]. Vγ9+Vδ2+ T cells could play a critical negative-feedback role in ameliorating disease in JIA patients by inducing apoptosis of rheumatoid synovial fibroblasts [83]. The specific subtypes and tissues involved in these pathologies are considered below.
Table 2:
Functions of murine γδ T cell subsets in some inflammatory diseases.
| γδT subsets | Functions | References |
|---|---|---|
| Vγ1+ | Pathogenesis of airway hyper responsiveness | [108] |
| Vγ4+ | Development of collagen-induced arthritis Pathogenesis of autoimmune uveitis Disruption of intestinal homeostasis (colitis) Development of psoriasis induced by IL-17, IL-22 |
[46] [76] [109] [110] |
| Vγ5+ | Protective roles in skin wound healing (Vγ5+Vδ1+) | [94] |
| Vγ6+ | IL-17-mediated inflammation of joint (arthritis) Pathogenic roles in psoriasis (Vγ6+Vδ1+) Protective roles in pulmonary fibrosis (Vγ6+ Vδ1+) |
[85] [97] [111] |
Inflammatory arthritis and bone remodeling
In collagen-induced arthritis (CIA), IL 17–producing γδ T cells are detected in the joint, and their numbers are significant higher than Th17 cells suggesting that γδ T cells are the major source of IL-17A in the joint [15]. Using the same model, Roark et al found an increased number of Vγ1+ and Vγ4+ T cells in the joints but only the Vγ4+ cells were activated and produced IL-17 during CIA [46]. Moreover, depletion of Vγ4+ T cells showed a significant reduction in disease incidence and severity that correlated with a reduction of total IgG and IgG2a anti-collagen antibodies [46]. Vγ4+ γδ T cells increase rapidly and appear to be specifically responsive to the collagen/CFA injections, whereas the Vγ1+ subset does not, suggesting that antigen-driven clonal and/or memory response is predominantly via the Vγ4+ γδ T cell subset [46]. In the CIA model, treatment with IL-28A dramatically reduces numbers of pro-inflammatory IL-17–producing γδ T cells in the joints and inguinal lymph nodes, to exert an anti-inflammatory effect further highlighting the importance of the γδ T cells in joint inflammation and inflammatory arthritis [84]. Apart from the CIA model, γδ T cells have been important modulators of inflammatory arthritis in the experimental models using IL-1Ra-deficient mice and IL-23 gene transfer. In the IL-1Ra-deficient mice, both Vγ6+ and Vγ4+ γδ T cells were observed to the joints, but only the Vγ6+ subset efficiently produced IL-17 [85] whereas functional depletion of γδ T cells showed protective effects by preventing neutrophil accumulation in the blood, spleen and bone marrow as well as by reducing neutrophil infiltration into the joints of the IL-23 gene transfer mice [64]. Collectively, these results suggest functional specific roles of each murine γδ T cell subtypes in the different disease models.
A common denominator between γδ T cell subtypes is IL-17 expression, and IL-17+γδ T cell subtypes in both human and mouse affect physiological bone remodeling. IL-17 induces RANKL from stromal cells as well as RANK receptor expression and thus can modulate bone resorption via the osteoclasts [86,87]. Recent studies have also shown that IL-17 can induce osteogenic activity in vitro in both murine and human cells [88,89]. A role of IL-17 in bone formation is also supported by recent evidence observed in SpA patient derived human cells and an SpA experimental model [90]. Another study demonstrated that the IL-17A+Vγ6+ T cell subtype modulates bone regeneration and bone fracture healing through stimulating the proliferation and differentiation of osteoblasts in the drill-hole injury murine model [91].
Although the human and murine data are in agreement regarding IL-17 actions, there are other cytokines produced by γδ T cells that affect bone remodeling and need to be considered. In humans, the effects of γδ T cells on osteoclastogenesis differ between “activated” and “freshly isolated” γδ T cells [92]. Specifically, “activated” γδ T cells inhibit osteoclastogenesis through secretion of high levels of IFN-γ (anti-osteoclastogenic) whereas “freshly isolated” γδ T cells enhance osteoclast differentiation by production of high levels of IL-6 [92]. In addition, human Vγ9+Vδ2+ T cells inhibit immature dendritic cells (DCs) trans-differentiation into osteoclasts [93]. Microarray analysis of human immature dendritic cells (iDCs) identified that expression of osteoclast related genes including c-Fos, ATPase H+ transporting V0 subunit d (ATP6V0D2), RANK and cathepsin K was decreased when iDCs were co-cultured with γδ T cells, indicating that γδ T cells inhibited osteoclastogenesis through the RANK/c-Fos/ATP6V0D2 signaling pathway [93].
Skin inflammation
In the murine skin, there are distinct populations of γδ T cells including Vγ5+Vδ1+ T cell subsets which localize in the epidermis [94] whereas Vγ4+ and Vγ6+ T cell subsets are resident in the dermis [95]. Vγ5+Vδ1+ DETCs are responsible for wound healing by secreting keratinocyte growth factors and inflammatory cytokines (IFN-γ, TNF, and IL-13) [94,96] whereas the Vγ4+ and Vγ6+ T cell subsets contribute to the development of skin inflammatory disease by production of IL-17 [13,97]. A spontaneous mutation in Sox13, a developmental transcription factor, causes defect in development of dermal Vγ4+ γδ17 T cells in mice and protects the mice from psoriasis-like skin inflammation, suggesting that dermal Vγ4+ γδ17 T cells mature in the neonatal thymus in a Sox13-dependent manner [98]. In the imiquimod (IQM)-induced skin inflammation model, Vγ4+Vδ4+ T cells are long lived and persist in the skin long after the initial inflammation, thus memory Vγ4+Vδ4+ T cells mediate the severity of IQM secondary challenge [71]. Follow up studies demonstrated that Vγ4+ T cells predominantly induce skin inflammation and are the major IL-17 producers [99]. Specifically, adiponectin, a mediator of insulin metabolism, inhibits production of IL-17 by murine dermal Vγ4+ γδ T cells through binding of AdipoR1, and Adiponectin deficient mice showed severe skin inflammation with elevated infiltration of Vγ4+ γδ17 T cells in the epidermis [99]. In addition, dermal γδ T cells are regulated by CD69, an activation marker that regulates secretion of IL-22 through aryl hydrocarbon receptor (AhR). CD69-deficient mice had lower expression of epidermal IL-22 and STAT3 which attenuated skin inflammation, compared with wild-type mice [79]. In humans, dermal Vγ9+Vδ2+ T cells express CCR6 and produce inflammatory mediators including IL-17A, TNF-α, IFN-γ, CXCL8, and CCL4 upon activation with specific antigen [78]. Collectively, these results show that dermal γδ17 T cells are regulators of skin inflammation, and their modulation could prevent skin inflammation.
Enthesitis
Vγ6+ γδ T cells have been detected in the murine enthesis [100], suggesting that they may play a role in the development of enthesitis. However, other groups have previously demonstrated that enthesitis can occur in the absence of γδ T cells [101]. Notably, the mice used in these studies were of different backgrounds and thus the difference in MHC complex may account for additional immune activation signals that may be required to induce an inflammatory response. Follow up studies in human enthesis detected the presence of γδ T cells (on the basis of TCR expression) and constituted a very small fraction of the total lymphocyte population [102,103].
Concluding remarks
In different conditions, γδ T cells have multiple distinct functions and may act as antigen presenting cells, pro-inflammatory and/or immune-regulatory cells. Moreover, translation of murine experimental models to human disease can be challenging. Recent RNA-Seq analysis of healthy murine and human skin transcriptomes demonstrated that γδ T cells which are highly expressed in murine skin are relatively rare in human skin, and the ratio of γδ T cells to αβ T cells increases only modestly in the setting of psoriasis [104]. These data partly reflect the differences of various γδ T cell subtypes between mouse and human as there is no equivalent of mouse DETCs (Vγ5+Vδ1+) in healthy human epidermis and only around 4% of dermal leukocytes expresses γδ TCR as reviewed previously [105]. Similar observations may be possible for other tissues implicated in the pathogenesis of rheumatic diseases given the multifunctionality of γδ T cell subsets and/or clonal plasticity combined with the plethora of effector functions exhibited by γδ T cells that govern autoimmunity. Nevertheless as our imaging and genetic tools increase [106], we are moving closer to a detailed understanding of individual γδ T cell subtypes and/or their cytokines that could be a promising therapy for modulation of immune responses in multiple autoimmune diseases.
Acknowledgments
Financial support
This work was supported by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01AR062173, and a National Psoriasis Foundation Translational Research grant to IEA. EM was supported by (1DP2OD008752).
Conflict of interest
IEA has received grants, salary, consulting fees from Schering Plough Biopharma/Merck, Novartis, Pfizer and Tanabe Research Labs USA. The authors have no other conflicts of interest to declare.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Roberts S, Girardi M (2008) Conventional and Unconventional T Cells In: Gaspari AA, Tyring SK (eds) Clinical and Basic Immunodermatology. Springer London, London, pp 85–104. doi: 10.1007/978-1-84800-165-7_6 [DOI] [Google Scholar]
- 2.Nielsen MM, Witherden DA, Havran WL (2017) γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol 17 (12):733–745. doi: 10.1038/nri.2017.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vrieling M, Santema W, Van Rhijn I, Rutten V, Koets A (2012) γδ T Cell Homing to Skin and Migration to Skin-Draining Lymph Nodes Is CCR7 Independent. J Immunol 188 (2):578–584. doi: 10.4049/jimmunol.1101972 [DOI] [PubMed] [Google Scholar]
- 4.Su D, Shen M, Li X, Sun L (2013) Roles of γδ T cells in the pathogenesis of autoimmune diseases. Clin Dev Immunol 2013:985753–985753. doi: 10.1155/2013/985753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyler CJ, Doherty DG, Moser B, Eberl M (2015) Human Vγ9/Vδ2 T cells: Innate adaptors of the immune system. Cell Immunol 296 (1):10–21. doi: 10.1016/j.cellimm.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Das H, Kamath A, Bukowski JF (2001) Human Vγ2Vδ2 T Cells Produce IFN-γ and TNF-α with an On/Off/On Cycling Pattern in Response to Live Bacterial Products. J Immunol 167 (11):6195–6201. doi: 10.4049/jimmunol.167.11.6195 [DOI] [PubMed] [Google Scholar]
- 7.Glatzel A, Wesch D, Schiemann F, Brandt E, Janssen O, Kabelitz D (2002) Patterns of Chemokine Receptor Expression on Peripheral Blood γδ T Lymphocytes: Strong Expression of CCR5 Is a Selective Feature of Vδ2/Vγ9 γδ T Cells. J Immunol 168 (10):4920–4929. doi: 10.4049/jimmunol.168.10.4920 [DOI] [PubMed] [Google Scholar]
- 8.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B (2011) Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 12 (3):255–263. doi: 10.1038/ni.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik S, Want MY, Awasthi A (2016) The Emerging Roles of Gamma-Delta T Cells in Tissue Inflammation in Experimental Autoimmune Encephalomyelitis. Frontiers in immunology 7:14–14. doi: 10.3389/fimmu.2016.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casetti R, Agrati C, Wallace M, Sacchi A, Martini F, Martino A, Rinaldi A, Malkovsky M (2009) Cutting edge: TGF-beta1 and IL-15 Induce FOXP3+ gammadelta regulatory T cells in the presence of antigen stimulation. J Immunol 183 (6):3574–3577. doi: 10.4049/jimmunol.0901334 [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Jin N, Roark CL, Aydintug MK, Wands JM, Huang H, O’Brien RL, Born WK (2009) The influence of IgE-enhancing and IgE-suppressive gammadelta T cells changes with exposure to inhaled ovalbumin. J Immunol 183 (2):849–855. doi: 10.4049/jimmunol.0804104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A (2010) Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol 130 (5):1373–1383. doi: 10.1038/jid.2009.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala Venkatakrishna R, Zhang H-g, Wang T, Zheng J, Yan J (2011) Pivotal Role of Dermal IL-17-Producing γδ T Cells in Skin Inflammation. Immunity 35 (4):596–610. doi: 10.1016/j.immuni.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guggino G, Ciccia F, Di Liberto D, Lo Pizzo M, Ruscitti P, Cipriani P, Ferrante A, Sireci G, Dieli F, Fourniè JJ, Giacomelli R, Triolo G (2016) Interleukin (IL)-9/IL-9R axis drives γδ T cells activation in psoriatic arthritis patients. Clin Exp Immunol 186 (3):277–283. doi: 10.1111/cei.12853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito Y, Usui T, Kobayashi S, Iguchi-Hashimoto M, Ito H, Yoshitomi H, Nakamura T, Shimizu M, Kawabata D, Yukawa N, Hashimoto M, Sakaguchi N, Sakaguchi S, Yoshifuji H, Nojima T, Ohmura K, Fujii T, Mimori T (2009) γδ T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum 60 (8):2294–2303. doi:doi: 10.1002/art.24687 [DOI] [PubMed] [Google Scholar]
- 16.Bank I, Cohen L, Mouallem M, Farfel Z, Grossman E, Ben-Nun A (2002) gammadelta T cell subsets in patients with arthritis and chronic neutropenia. Ann Rheum Dis 61 (5):438–443. doi: 10.1136/ard.61.5.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muro R, Takayanagi H, Nitta T (2019) T cell receptor signaling for γδT cell development. Inflamm Regen 39 (1):6. doi: 10.1186/s41232-019-0095-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottino C, Tambussi G, Ferrini S, Ciccone E, Varese P, Mingari MC, Moretta L, Moretta A (1988) Two subsets of human T lymphocytes expressing gamma/delta antigen receptor are identifiable by monoclonal antibodies directed to two distinct molecular forms of the receptor. J Exp Med 168 (2):491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.8Wesch D, Hinz T, Kabelitz D (1998) Analysis of the TCR Vgamma repertoire in healthy donors and HIV-1-infected individuals. Int Immunol 10 (8):1067–1075 [DOI] [PubMed] [Google Scholar]
- 20.Davey MS, Willcox CR, Hunter S, Kasatskaya SA, Remmerswaal EBM, Salim M, Mohammed F, Bemelman FJ, Chudakov DM, Oo YH, Willcox BE (2018) The human Vδ2(+) Tcell compartment comprises distinct innate-like Vγ9(+) and adaptive Vγ9(−) subsets. Nat Commun 9 (1):1760–1760. doi: 10.1038/s41467-018-04076-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davey MS, Willcox CR, Joyce SP, Ladell K, Kasatskaya SA, McLaren JE, Hunter S, Salim M, Mohammed F, Price DA, Chudakov DM, Willcox BE (2017) Clonal selection in the human Vδ1 T cell repertoire indicates γδ TCR-dependent adaptive immune surveillance. Nat Commun 8:14760–14760. doi: 10.1038/ncomms14760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangan BA, Dunne MR, O’Reilly VP, Dunne PJ, Exley MA, O’Shea D, Scotet E, Hogan AE, Doherty DG (2013) Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vδ3 T cells. J Immunol 191 (1):30–34. doi: 10.4049/jimmunol.1300121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Xu M, Wang C, Zhu L, Hu J, Chen S, Wu X, Li B, Li Y (2014) The feature of distribution and clonality of TCR γ/δ subfamilies T cells in patients with B-cell non-Hodgkin lymphoma. J Immunol Res 2014:241246–241246. doi: 10.1155/2014/241246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimova T, Brouwer M, Gosselin F, Tassignon J, Leo O, Donner C, Marchant A, Vermijlen D (2015) Effector Vγ9Vδ2 T cells dominate the human fetal γδ T-cell repertoire. Proc Natl Acad Sci U S A 112 (6):E556–E565. doi: 10.1073/pnas.1412058112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermijlen D, Gatti D, Kouzeli A, Rus T, Eberl M (2018) γδ T cell responses: How many ligands will it take till we know? Semin Cell Dev Biol 84:75–86. doi: 10.1016/j.semcdb.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 26.Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, Moreau J-F, Hayday AC, Willcox BE, Déchanet-Merville J (2012) Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat Immunol 13:872. doi: 10.1038/ni.2394 [DOI] [PubMed] [Google Scholar]
- 27.Caccamo N, La Mendola C, Orlando V, Meraviglia S, Todaro M, Stassi G, Sireci G, Fournié JJ, Dieli F (2011) Differentiation, phenotype, and function of interleukin-17–producing human Vγ9Vδ2 T cells. Blood 118 (1):129–138. doi: 10.1182/blood-2011-01-331298 [DOI] [PubMed] [Google Scholar]
- 28.Eberl M, Roberts GW, Meuter S, Williams JD, Topley N, Moser B (2009) A Rapid Crosstalk of Human γδ T Cells and Monocytes Drives the Acute Inflammation in Bacterial Infections. PLOS Pathog 5 (2):e1000308. doi: 10.1371/journal.ppat.1000308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eberl M, Moser B (2009) Monocytes and γδ T cells: close encounters in microbial infection. Trends in Immunology 30 (12):562–568. doi: 10.1016/j.it.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 30.Davey MS, Lin C-Y, Roberts GW, Heuston S, Brown AC, Chess JA, Toleman MA, Gahan CGM, Hill C, Parish T, Williams JD, Davies SJ, Johnson DW, Topley N, Moser B, Eberl M (2011) Human Neutrophil Clearance of Bacterial Pathogens Triggers Anti-Microbial γδ T Cell Responses in Early Infection. PLoS Pathog 7 (5):e1002040. doi: 10.1371/journal.ppat.1002040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Wu W, Wong WM, Ward E, Thrasher AJ, Goldblatt D, Osman M, Digard P, Canaday DH, Gustafsson K (2009) Human γδ T Cells: A Lymphoid Lineage Cell Capable of Professional Phagocytosis. J Immunol 183 (9):5622–5629. doi: 10.4049/jimmunol.0901772 [DOI] [PubMed] [Google Scholar]
- 32.Brandes M, Willimann K, Moser B (2005) Professional Antigen-Presentation Function by Human γδ T Cells. Science 309 (5732):264–268. doi: 10.1126/science.1110267 [DOI] [PubMed] [Google Scholar]
- 33.Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, Hunt R, Clagett M, Sehgal PK, Li Y, Zeng X, Morita CT, Brenner MB, Letvin NL, Chen ZW (2002) Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science (New York, NY) 295 (5563):2255–2258. doi: 10.1126/science.1068819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandes M, Willimann K, Lang AB, Nam K-H, Jin C, Brenner MB, Morita CT, Moser B (2003) Flexible migration program regulates γδ T-cell involvement in humoral immunity. Blood 102 (10):3693–3701. doi: 10.1182/blood-2003-04-1016 [DOI] [PubMed] [Google Scholar]
- 35.Horner AA, Jabara H, Ramesh N, Geha RS (1995) gamma/delta T lymphocytes express CD40 ligand and induce isotype switching in B lymphocytes. J Exp Med 181 (3):1239–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bansal RR, Mackay CR, Moser B, Eberl M (2012) IL-21 enhances the potential of human γδ T cells to provide B-cell help. Eur J Immunol 42 (1):110–119. doi: 10.1002/eji.201142017 [DOI] [PubMed] [Google Scholar]
- 37.Petrasca A, Melo AM, Breen EP, Doherty DG (2018) Human Vδ3+ γδ T cells induce maturation and IgM secretion by B cells. Immunol Lett 196:126–134. doi: 10.1016/j.imlet.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 38.Vermijlen D, Ellis P, Langford C, Klein A, Engel R, Willimann K, Jomaa H, Hayday AC, Eberl M (2007) Distinct cytokine-driven responses of activated blood gammadelta T cells: insights into unconventional T cell pleiotropy. J Immunol 178 (7):4304–4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Born WK, Huang Y, Reinhardt RL, Huang H, Sun D, O’Brien RL (2017) γδ T Cells and B Cells In: Alt FW (ed) Adv Immunol, vol 134 Academic Press, pp 1–45. doi: 10.1016/bs.ai.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 40.Caccamo N, Battistini L, Bonneville M, Poccia F, Fournié JJ, Meraviglia S, Borsellino G, Kroczek RA, La Mendola C, Scotet E, Dieli F, Salerno A (2006) CXCR5 Identifies a Subset of Vγ9Vδ2 T Cells which Secrete IL-4 and IL-10 and Help B Cells for Antibody Production. J Immunol 177 (8):5290–5295. doi: 10.4049/jimmunol.177.8.5290 [DOI] [PubMed] [Google Scholar]
- 41.McCarthy NE, Eberl M (2018) Human γδ T-Cell Control of Mucosal Immunity and Inflammation. Front Immunol 9:985–985. doi: 10.3389/fimmu.2018.00985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Brien RL, Born WK (2010) gammadelta T cell subsets: a link between TCR and function? Semin Immunol 22 (4):193–198. doi: 10.1016/j.smim.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cook L, Miyahara N, Jin N, Wands JM, Taube C, Roark CL, Potter TA, Gelfand EW, O’Brien RL, Born WK (2008) Evidence that CD8+ dendritic cells enable the development of gammadelta T cells that modulate airway hyperresponsiveness. J Immunol 181 (1):309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papotto PH, Gonçalves‐Sousa N, Schmolka N, Iseppon A, Mensurado S, Stockinger B, Ribot JC, Silva‐Santos B (2017) IL‐23 drives differentiation of peripheral γδ17 T cells from adult bone marrow ‐ derived precursors. EMBO reports:e201744200. doi: 10.15252/embr.201744200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang D, Zuo A, Shao H, Born WK, O’Brien RL, Kaplan HJ, Sun D (2013) IL-23 receptor expression on γδ T cells correlates with their enhancing or suppressive effects on autoreactive T cells in experimental autoimmune uveitis. J Immunol 191 (3):1118–1125. doi: 10.4049/jimmunol.1300626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL (2007) Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J Immunol 179 (8):5576–5583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramírez-Valle F, Gray EE, Cyster JG (2015) Inflammation induces dermal Vγ4+ γδT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc Natl Acad Sci U S A 112 (26):8046–8051. doi: 10.1073/pnas.1508990112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adamopoulos IE, Suzuki E, Chao C-C, Gorman D, Adda S, Maverakis E, Zarbalis K, Geissler R, Asio A, Blumenschein WM, McClanahan T, De Waal Malefyt R, Gershwin ME, Bowman EP (2015) IL-17A gene transfer induces bone loss and epidermal hyperplasia associated with psoriatic arthritis. Ann Rheum Dis 74 (6):1284–1292. doi: 10.1136/annrheumdis-2013-204782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki E, Maverakis E, Sarin R, Bouchareychas L, Kuchroo VK, Nestle FO, Adamopoulos IE (2016) T Cell-Independent Mechanisms Associated with Neutrophil Extracellular Trap Formation and Selective Autophagy in IL-17A-Mediated Epidermal Hyperplasia. J Immunol 197 (11):4403–4412. doi: 10.4049/jimmunol.1600383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KHG (2009) Interleukin1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31 (2):331–341. doi: 10.1016/j.immuni.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 51.Turchinovich G, Hayday Adrian C (2011) Skint-1 Identifies a Common Molecular Mechanism for the Development of Interferon-γ-Secreting versus Interleukin-17-Secreting γδ T Cells. Immunity 35 (1):59–68. doi: 10.1016/j.immuni.2011.04.018 [DOI] [PubMed] [Google Scholar]
- 52.Heilig JS, Tonegawa S (1986) Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature 322 (6082):836–840. doi: 10.1038/322836a0 [DOI] [PubMed] [Google Scholar]
- 53.Dillen CA, Pinsker BL, Marusina AI, Merleev AA, Farber ON, Liu H, Archer NK, Lee DB, Wang Y, Ortines RV, Lee SK, Marchitto MC, Cai SS, Ashbaugh AG, May LS, Holland SM, Freeman AF, Miller LG, Yeaman MR, Simon SI, Milner JD, Maverakis E, Miller LS (2018) Clonally expanded γδ T cells protect against Staphylococcus aureus skin reinfection. J Clin Invest 128 (3):1026–1042. doi: 10.1172/JCI96481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, Tigelaar RE, Lifton RP (2008) Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat Genet 40 (5):656–662. doi: 10.1038/ng.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chodaczek G, Papanna V, Zal MA, Zal T (2012) Body-barrier surveillance by epidermal γδ TCRs. Nat Immunol 13 (3):272–282. doi: 10.1038/ni.2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mair F, Joller S, Hoeppli R, Onder L, Hahn M, Ludewig B, Waisman A, Becher B (2015) The NFκB-inducing kinase is essential for the developmental programming of skin-resident and IL-17-producing γδ T cells. eLife 4:e10087. doi: 10.7554/eLife.10087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itohara S, Farr AG, Lafaille JJ, Bonneville M, Takagaki Y, Haas W, Tonegawa S (1990) Homing of a γδ thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature 343:754. doi: 10.1038/343754a0 [DOI] [PubMed] [Google Scholar]
- 58.Roark CL, Aydintug MK, Lewis J, Yin X, Lahn M, Hahn Y-S, Born WK, Tigelaar RE, O’Brien RL (2004) Subset-specific, uniform activation among Vγ6/Vδ1+ γδ T cells elicited by inflammation. J Leukoc Biol 75 (1):68–75. doi: 10.1189/jlb.0703326 [DOI] [PubMed] [Google Scholar]
- 59.Hayes SM, Sirr A, Jacob S, Sim GK, Augustin A (1996) Role of IL-7 in the shaping of the pulmonary gamma delta T cell repertoire. J Immunol 156 (8):2723–2729 [PubMed] [Google Scholar]
- 60.Mamedov MR, Scholzen A, Nair RV, Cumnock K, Kenkel JA, Oliveira JHM, Trujillo DL, Saligrama N, Zhang Y, Rubelt F, Schneider DS, Chien Y-h, Sauerwein RW, Davis MM (2018) A Macrophage Colony-Stimulating-Factor-Producing γδ T Cell Subset Prevents Malarial Parasitemic Recurrence. Immunity 48 (2):350–363.e357. doi: 10.1016/j.immuni.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartl D, Krauss-Etschmann S, Koller B, Hordijk PL, Kuijpers TW, Hoffmann F, Hector A, Eber E, Marcos V, Bittmann I, Eickelberg O, Griese M, Roos D (2008) Infiltrated Neutrophils Acquire Novel Chemokine Receptor Expression and Chemokine Responsiveness in Chronic Inflammatory Lung Diseases. J Immunol 181 (11):8053–8067. doi: 10.4049/jimmunol.181.11.8053 [DOI] [PubMed] [Google Scholar]
- 62.Jennifer MR, Sivasami P, Harshini KA, Jerry WR, Timothy AS, Jerry RM, Montelongo M, Vincent TC, Teluguakula N (2019) Neutrophils induce a novel chemokine receptors repertoire during influenza pneumonia. Front Cell Infect Microbiol:In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang X, Park CO, Geddes Sweeney J, Yoo MJ, Gaide O, Kupper TS (2017) Dermal γδ T Cells Do Not Freely Re-Circulate Out of Skin and Produce IL-17 to Promote Neutrophil Infiltration during Primary Contact Hypersensitivity. PLOS ONE 12 (1):e0169397. doi: 10.1371/journal.pone.0169397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bouchareychas L, Grössinger EM, Kang M, Adamopoulos IE (2018) γδTCR regulates production of interleukin-27 by neutrophils and attenuates inflammatory arthritis. Sci Rep 8 (1):7590–7590. doi: 10.1038/s41598-018-25988-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rani M, Zhang Q, Schwacha MG (2014) Gamma delta (γδ) T-cells regulate wound myeloid cell activity after burn. Shock 42 (2):133–141. doi: 10.1097/SHK.0000000000000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mokuno Y, Matsuguchi T, Takano M, Nishimura H, Washizu J, Ogawa T, Takeuchi O, Akira S, Nimura Y, Yoshikai Y (2000) Expression of toll-like receptor 2 on gamma delta T cells bearing invariant V gamma 6/V delta 1 induced by Escherichia coli infection in mice. J Immunol 165 (2):931–940. doi: 10.4049/jimmunol.165.2.931 [DOI] [PubMed] [Google Scholar]
- 67.Cheng L, Cui Y, Shao H, Han G, Zhu L, Huang Y, O’Brien RL, Born WK, Kaplan HJ, Sun D (2008) Mouse gammadelta T cells are capable of expressing MHC class II molecules, and of functioning as antigen-presenting cells. J Neuroimmunol 203 (1):3–11. doi: 10.1016/j.jneuroim.2008.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lanier LL, Sun JC (2009) Do the terms innate and adaptive immunity create conceptual barriers? Nat Rev Immunol 9 (5):302–303. doi: 10.1038/nri2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Brien RL, Happ MP, Dallas A, Palmer E, Kubo R, Born WK (1989) Stimulation of a major subset of lymphocytes expressing T cell receptor γδ by an antigen derived from mycobacterium tuberculosis. Cell 57 (4):667–674. doi: 10.1016/0092-8674(89)90135-9 [DOI] [PubMed] [Google Scholar]
- 70.Lalor SJ, McLoughlin RM (2016) Memory γδ T Cells–Newly Appreciated Protagonists in Infection and Immunity. Trends Immuno 37 (10):690–702. doi: 10.1016/j.it.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 71.Hartwig T, Pantelyushin S, Croxford AL, Kulig P, Becher B (2015) Dermal IL-17-producing γδ T cells establish long-lived memory in the skin. Eur J Immunol 45 (11):3022–3033. doi:doi: 10.1002/eji.201545883 [DOI] [PubMed] [Google Scholar]
- 72.Huang Y, Heiser RA, Detanico TO, Getahun A, Kirchenbaum GA, Casper TL, Aydintug MK, Carding SR, Ikuta K, Huang H, Cambier JC, Wysocki LJ, O’Brien RL, Born WK (2015) γδ T cells affect IL-4 production and B-cell tolerance. Proc Natl Acad Sci U S A 112 (1):E39–E48. doi: 10.1073/pnas.1415107111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crawford G, Hayes MD, Seoane RC, Ward S, Dalessandri T, Lai C, Healy E, Kipling D, Proby C, Moyes C, Green K, Best K, Haniffa M, Botto M, Dunn-Walters D, Strid J (2018) Epithelial damage and tissue γδ T cells promote a unique tumor-protective IgE response. Nat Immunol 19 (8):859–870. doi: 10.1038/s41590-018-0161-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rezende RM, Lanser AJ, Rubino S, Kuhn C, Skillin N, Moreira TG, Liu S, Gabriely G, David BA, Menezes GB, Weiner HL (2018) γδ T cells control humoral immune response by inducing T follicular helper cell differentiation. Nat Commun 9 (1):3151–3151. doi: 10.1038/s41467-018-05487-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Do J-s, Visperas A, Dong C, Baldwin WM 3rd, Min B (2011) Cutting edge: Generation of colitogenic Th17 CD4 T cells is enhanced by IL-17+ γδ T cells. J Immunol 186 (8):4546–4550. doi: 10.4049/jimmunol.1004021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cui Y, Shao H, Lan C, Nian H, O’Brien RL, Born WK, Kaplan HJ, Sun D (2009) Major role of gamma delta T cells in the generation of IL-17+ uveitogenic T cells. J Immunol 183 (1):560–567. doi: 10.4049/jimmunol.0900241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KHG (2009) Interleukin-1 and IL-23 Induce Innate IL-17 Production from γδ T Cells, Amplifying Th17 Responses and Autoimmunity. Immunity 31 (2):331–341. doi: 10.1016/j.immuni.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 78.Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, Smith CH, Hayday AC, Nickoloff BJ, Nestle FO (2011) Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J Immunol 187 (5):2783–2793. doi: 10.4049/jimmunol.1100804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cibrian D, Saiz ML, de la Fuente H, Sánchez-Díaz R, Moreno-Gonzalo O, Jorge I, Ferrarini A, Vázquez J, Punzón C, Fresno M, Vicente-Manzanares M, Daudén E, Fernández-Salguero PM, Martín P, Sánchez-Madrid F (2016) CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis. Nat Immunol 17 (8):985–996. doi: 10.1038/ni.3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brennan FM, Londei M, Jackson AM, Hercend T, Brenner MB, Maini RN, Feldmann M (1988) T cells expressing γδ chain receptors in rheumatoid arthritis. J Autoimmun 1 (4):319–326. doi: 10.1016/0896-8411(88)90002-9 [DOI] [PubMed] [Google Scholar]
- 81.Mo W-X, Yin S-S, Chen H, Zhou C, Zhou J-X, Zhao L-D, Fei Y-Y, Yang H-X, Guo J-B, Mao Y-J, Huang L-F, Zheng W-J, Zhang W, Zhang J-M, He W, Zhang X (2017) Chemotaxis of Vδ2 T cells to the joints contributes to the pathogenesis of rheumatoid arthritis. Ann Rheum Dis 76 (12):2075–2084. doi: 10.1136/annrheumdis-2016-211069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keystone EC, Rittershaus C, Wood N, Snow KM, Flatow J, Purvis JC, Poplonski L, Kung PC (1991) Elevation of a gamma delta T cell subset in peripheral blood and synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol 84 (1):78–82 [PMC free article] [PubMed] [Google Scholar]
- 83.Bendersky A, Marcu-Malina V, Berkun Y, Gerstein M, Nagar M, Goldstein I, Padeh S, Bank I (2012) Cellular Interactions of Synovial Fluid γδ T Cells in Juvenile Idiopathic Arthritis. J Immunol 188 (9):4349–4359. doi: 10.4049/jimmunol.1102403 [DOI] [PubMed] [Google Scholar]
- 84.Blazek K, Eames HL, Weiss M, Byrne AJ, Perocheau D, Pease JE, Doyle S, McCann F, Williams RO, Udalova IA (2015) IFN-λ resolves inflammation via suppression of neutrophil infiltration and IL-1β production. J Exp Med 212 (6):845–853. doi: 10.1084/jem.20140995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akitsu A, Ishigame H, Kakuta S, Chung S-H, Ikeda S, Shimizu K, Kubo S, Liu Y, Umemura M, Matsuzaki G, Yoshikai Y, Saijo S, Iwakura Y (2015) IL-1 receptor antagonist-deficient mice develop autoimmune arthritis due to intrinsic activation of IL-17-producing CCR2(+)Vγ6(+)γδ T cells. Nat Commun 6:7464–7464. doi: 10.1038/ncomms8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T (1999) IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 103 (9):1345–1352. doi: 10.1172/JCI5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adamopoulos IE, Chao C-C, Geissler R, Laface D, Blumenschein W, Iwakura Y, McClanahan T, Bowman EP (2010) Interleukin-17A upregulates receptor activator of NF-kappaB on osteoclast precursors. Arthritis Res Ther 12 (1):R29–R29. doi: 10.1186/ar2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Osta B, Roux J-P, Lavocat F, Pierre M, Ndongo-Thiam N, Boivin G, Miossec P (2015) Differential Effects of IL-17A and TNF-α on Osteoblastic Differentiation of Isolated Synoviocytes and on Bone Explants from Arthritis Patients. Front Immunol 6:151–151. doi: 10.3389/fimmu.2015.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jo S, Wang SE, Lee YL, Kang S, Lee B, Han J, Sung I-H, Park Y-S, Bae S-C, Kim T-H (2018) IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res Ther 20 (1):115–115. doi: 10.1186/s13075-018-1582-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Tok MN, van Duivenvoorde LM, Kramer I, Ingold P, Pfister S, Roth L, Blijdorp IC, van de Sande MGH, Taurog JD, Kolbinger F, Baeten DL (2019) Interleukin-17A Inhibition Diminishes Inflammation and New Bone Formation in Experimental Spondyloarthritis. Arthritis Rheumatol 71 (4):612–625. doi: 10.1002/art.40770 [DOI] [PubMed] [Google Scholar]
- 91.Ono T, Okamoto K, Nakashima T, Nitta T, Hori S, Iwakura Y, Takayanagi H (2016) IL-17-producing γδ T cells enhance bone regeneration. Nat Commun 7:10928. doi: 10.1038/ncomms10928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Phalke SP, Chiplunkar SV (2015) Activation status of γδ T cells dictates their effect on osteoclast generation and bone resorption. Bone Rep 3:95–103. doi: 10.1016/j.bonr.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu X, Zeng Z, Qiu D, Chen J (2018) Vγ9Vδ2 T cells inhibit immature dendritic cell transdifferentiation into osteoclasts through downregulation of RANK, c‑Fos and ATP6V0D2. Int J Mol Med 42 (4):2071–2079. doi: 10.3892/ijmm.2018.3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL (2002) A Role for Skin γδ T Cells in Wound Repair. Science 296 (5568):747–749. doi: 10.1126/science.1069639 [DOI] [PubMed] [Google Scholar]
- 95.Gray EE, Suzuki K, Cyster JG (2011) Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol 186 (11):6091–6095. doi: 10.4049/jimmunol.1100427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dalessandri T, Crawford G, Hayes M, Castro Seoane R, Strid J (2016) IL-13 from intraepithelial lymphocytes regulates tissue homeostasis and protects against carcinogenesis in the skin. Nat Commun 7:12080–12080. doi: 10.1038/ncomms12080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kulig P, Musiol S, Freiberger SN, Schreiner B, Gyülveszi G, Russo G, Pantelyushin S, Kishihara K, Alessandrini F, Kündig T, Sallusto F, Hofbauer GFL, Haak S, Becher B (2016) IL-12 protects from psoriasiform skin inflammation. Nat Commun 7:13466–13466. doi: 10.1038/ncomms13466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gray EE, Ramírez-Valle F, Xu Y, Wu S, Wu Z, Karjalainen KE, Cyster JG (2013) Deficiency in IL-17-committed Vγ4(+) γδ T cells in a spontaneous Sox13-mutant CD45.1(+) congenic mouse substrain provides protection from dermatitis. Nat Immunol 14 (6):584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shibata S, Tada Y, Hau CS, Mitsui A, Kamata M, Asano Y, Sugaya M, Kadono T, Masamoto Y, Kurokawa M, Yamauchi T, Kubota N, Kadowaki T, Sato S (2015) Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from γδ-T cells. Nat Commun 6:7687. doi: 10.1038/ncomms8687 [DOI] [PubMed] [Google Scholar]
- 100.Reinhardt A, Yevsa T, Worbs T, Lienenklaus S, Sandrock I, Oberdörfer L, Korn T, Weiss S, Förster R, Prinz I (2016) Interleukin-23–Dependent γ/δ T Cells Produce Interleukin-17 and Accumulate in the Enthesis, Aortic Valve, and Ciliary Body in Mice. Arthritis Rheumatol 68 (10):2476–2486. doi: 10.1002/art.39732 [DOI] [PubMed] [Google Scholar]
- 101.Corthay A, Hansson A-S, Holmdahl R (2000) T lymphocytes are not required for the spontaneous development of entheseal ossification leading to marginal ankylosis in the DBA/1 mouse. Arthritis Rheum 43 (4):844–851. doi: [DOI] [PubMed] [Google Scholar]
- 102.Cuthbert RJ, Fragkakis EM, Dunsmuir R, Li Z, Coles M, Marzo-Ortega H, Giannoudis PV, Jones E, El-Sherbiny YM, McGonagle D (2017) Brief Report: Group 3 Innate Lymphoid Cells in Human Enthesis. Arthritis Rheumatol 69 (9):1816–1822. doi: 10.1002/art.40150 [DOI] [PubMed] [Google Scholar]
- 103.Reinhardt A, Prinz I (2018) Whodunit? The Contribution of Interleukin (IL)-17/IL-22-Producing γδ T Cells, αβ T Cells, and Innate Lymphoid Cells to the Pathogenesis of Spondyloarthritis. Front Immunol 9:885–885. doi: 10.3389/fimmu.2018.00885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Merleev AA, Marusina AI, Ma C, Elder JT, Tsoi LC, Raychaudhuri SP, Weidinger S, Wang EA, Adamopoulos IE, Luxardi G, Gudjonsson JE, Shimoda M, Maverakis E (2018) Meta-analysis of RNA sequencing datasets reveals an association between TRAJ23, psoriasis, and IL-17A. JCI insight 3 (13):e120682. doi: 10.1172/jci.insight.120682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai Y, Fleming C, Yan J (2013) Dermal γδ T cells — A new player in the pathogenesis of psoriasis. Int Immunopharmacol 16 (3):388–391. doi: 10.1016/j.intimp.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 106.Man F, Lim L, Volpe A, Gabizon A, Shmeeda H, Draper B, Parente-Pereira AC, Maher J, Blower PJ, Fruhwirth GO, de Rosales RTM (2019) In Vivo PET Tracking of 89Zr-Labeled Vγ9Vδ2 T Cells to Mouse Xenograft Breast Tumors Activated with Liposomal Alendronate. Mol Ther 27 (1):219–229. doi: 10.1016/j.ymthe.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu D, Wu P, Qiu F, Wei Q, Huang J (2017) Human γδT-cell subsets and their involvement in tumor immunity. Cellular & molecular immunology 14 (3):245–253. doi: 10.1038/cmi.2016.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hahn Y-S, Taube C, Jin N, Sharp L, Wands JM, Aydintug MK, Lahn M, Huber SA, O’Brien RL, Gelfand EW, Born WK (2004) Different potentials of gamma delta T cell subsets in regulating airway responsiveness: V gamma 1+ cells, but not V gamma 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. The Journal of Immunology 172 (5):2894–2902. doi: 10.4049/jimmunol.172.5.2894 [DOI] [PubMed] [Google Scholar]
- 109.Park S-G, Mathur R, Long M, Hosh N, Hao L, Hayden MS, Ghosh S (2010) T regulatory cells maintain intestinal homeostasis by suppressing γδ T cells. Immunity 33 (5):791–803. doi: 10.1016/j.immuni.2010.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Imai Y, Ayithan N, Wu X, Yuan Y, Wang L, Hwang ST (2015) Cutting Edge: PD-1 Regulates Imiquimod-Induced Psoriasiform Dermatitis through Inhibition of IL-17A Expression by Innate γδ-Low T Cells. J Immunol 195 (2):421–425. doi: 10.4049/jimmunol.1500448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Simonian PL, Roark CL, Diaz del Valle F, Palmer BE, Douglas IS, Ikuta K, Born WK, O’Brien RL, Fontenot AP (2006) Regulatory Role of γδ T Cells in the Recruitment of CD4+ and CD8+ T Cells to Lung and Subsequent Pulmonary Fibrosis. J Immunol 177 (7):4436–4443. doi: 10.4049/jimmunol.177.7.4436 [DOI] [PubMed] [Google Scholar]